Abstract
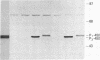
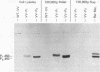
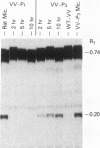
Two cDNA clones representing the mRNA coding sequences for mouse cytochromes P1-450 and P3-450 were inserted into the thymidine kinase gene of the wild-type vaccinia virus under the control of the vaccinia virus promoter. Murine and human cells infected with each of the resulting infectious recombinant viruses efficiently expressed their respective P-450 proteins. The newly synthesized protein products are translocated into the microsomes, and their characterization by immunochemical analysis indicates that the sizes of the polypeptides expressed were indistinguishable from their cytochrome P-450 counterparts found in mammalian liver microsomes. Functional analysis of each of the proteins by spectral and enzymatic analysis indicates that the expressed proteins have incorporated heme, and the holoenzymes displayed catalytic activities characteristic of their respective cytochrome P-450 enzymes. Thus, this system can be used to produce properly processed and catalytically active P-450 gene products in a wide variety of cells. The remarkable fidelity of expression and processing of these enzymes suggests that the vaccinia virus recombinants can be used for a wide variety of studies, including analysis of the effects of defined mutations produced in vitro, and directly correlate the structure/activity relationships of the cytochrome P-450 enzymes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adesnik M., Atchison M. Genes for cytochrome P-450 and their regulation. CRC Crit Rev Biochem. 1986;19(3):247–305. doi: 10.3109/10409238609084657. [DOI] [PubMed] [Google Scholar]
- Chakrabarti S., Brechling K., Moss B. Vaccinia virus expression vector: coexpression of beta-galactosidase provides visual screening of recombinant virus plaques. Mol Cell Biol. 1985 Dec;5(12):3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conney A. H. Pharmacological implications of microsomal enzyme induction. Pharmacol Rev. 1967 Sep;19(3):317–366. [PubMed] [Google Scholar]
- Friedman F. K., Park S. S., Gelboin H. V. The application of monoclonal antibodies for studies on cytochrome P-450. Rev Drug Metab Drug Interact. 1985;5(2-3):159–192. doi: 10.1515/dmdi.1985.5.2-3.159. [DOI] [PubMed] [Google Scholar]
- Garon C. F., Barbosa E., Moss B. Visualization of an inverted terminal repetition in vaccinia virus DNA. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4863–4867. doi: 10.1073/pnas.75.10.4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelboin H. V. Benzo[alpha]pyrene metabolism, activation and carcinogenesis: role and regulation of mixed-function oxidases and related enzymes. Physiol Rev. 1980 Oct;60(4):1107–1166. doi: 10.1152/physrev.1980.60.4.1107. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. J., Kimura S., Song B. J., Pastewka J., Gelboin H. V., Hardwick J. P. Sequence of two related P-450 mRNAs transcriptionally increased during rat development. An R.dre.1 sequence occupies the complete 3' untranslated region of a liver mRNA. J Biol Chem. 1986 Aug 15;261(23):10667–10672. [PubMed] [Google Scholar]
- Gonzalez F. J., Mackenzie P. I., Kimura S., Nebert D. W. Isolation and characterization of full-length mouse cDNA and genomic clones of 3-methylcholanthrene-inducible cytochrome P1-450 and P3-450. Gene. 1984 Sep;29(3):281–292. doi: 10.1016/0378-1119(84)90057-x. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K. The purification fo four strains of poxvirus. Virology. 1962 Sep;18:9–18. doi: 10.1016/0042-6822(62)90172-1. [DOI] [PubMed] [Google Scholar]
- Kimura S., Gonzalez F. J., Nebert D. W. The murine Ah locus. Comparison of the complete cytochrome P1-450 and P3-450 cDNA nucleotide and amino acid sequences. J Biol Chem. 1984 Sep 10;259(17):10705–10713. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lu A. Y., West S. B. Multiplicity of mammalian microsomal cytochromes P-45. Pharmacol Rev. 1979 Dec;31(4):277–295. [PubMed] [Google Scholar]
- Mackett M., Smith G. L., Moss B. General method for production and selection of infectious vaccinia virus recombinants expressing foreign genes. J Virol. 1984 Mar;49(3):857–864. doi: 10.1128/jvi.49.3.857-864.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackett M., Smith G. L., Moss B. Vaccinia virus: a selectable eukaryotic cloning and expression vector. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7415–7419. doi: 10.1073/pnas.79.23.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebert D. W., Gelboin H. V. Substrate-inducible microsomal aryl hydroxylase in mammalian cell culture. I. Assay and properties of induced enzyme. J Biol Chem. 1968 Dec 10;243(23):6242–6249. [PubMed] [Google Scholar]
- Negishi M., Nebert D. W. Structural gene products of the Ah locus. Genetic and immunochemical evidence for two forms of mouse liver cytochrome P-450 induced by 3-methylcholanthrene. J Biol Chem. 1979 Nov 10;254(21):11015–11023. [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- Oeda K., Sakaki T., Ohkawa H. Expression of rat liver cytochrome P-450MC cDNA in Saccharomyces cerevisiae. DNA. 1985 Jun;4(3):203–210. doi: 10.1089/dna.1985.4.203. [DOI] [PubMed] [Google Scholar]
- Reik L. M., Levin W., Ryan D. E., Thomas P. E. Immunochemical relatedness of rat hepatic microsomal cytochromes P-450c and P-450d. J Biol Chem. 1982 Apr 10;257(7):3950–3957. [PubMed] [Google Scholar]
- Song B. J., Gelboin H. V., Park S. S., Yang C. S., Gonzalez F. J. Complementary DNA and protein sequences of ethanol-inducible rat and human cytochrome P-450s. Transcriptional and post-transcriptional regulation of the rat enzyme. J Biol Chem. 1986 Dec 15;261(35):16689–16697. [PubMed] [Google Scholar]
- Thomas P. E., Reik L. M., Ryan D. E., Levin W. Induction of two immunochemically related rat liver cytochrome P-450 isozymes, cytochromes P-450c and P-450d, by structurally diverse xenobiotics. J Biol Chem. 1983 Apr 10;258(7):4590–4598. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuber M. X., Simpson E. R., Waterman M. R. Expression of bovine 17 alpha-hydroxylase cytochrome P-450 cDNA in nonsteroidogenic (COS 1) cells. Science. 1986 Dec 5;234(4781):1258–1261. doi: 10.1126/science.3535074. [DOI] [PubMed] [Google Scholar]