Abstract
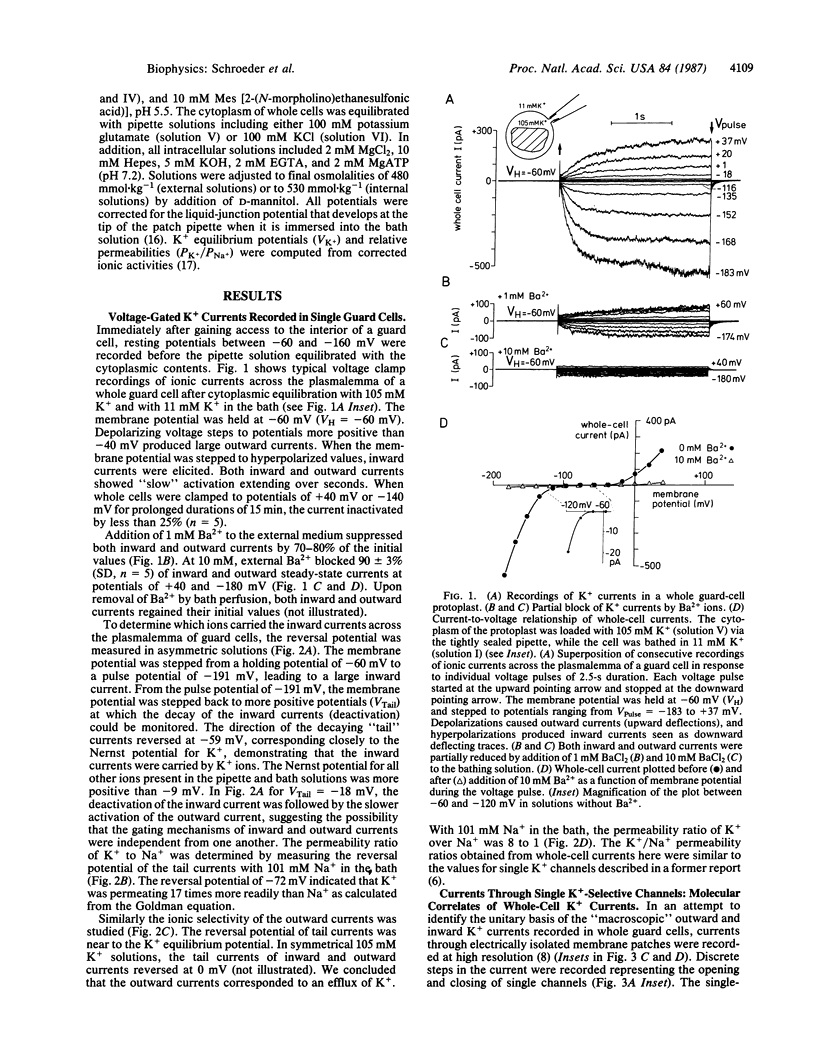
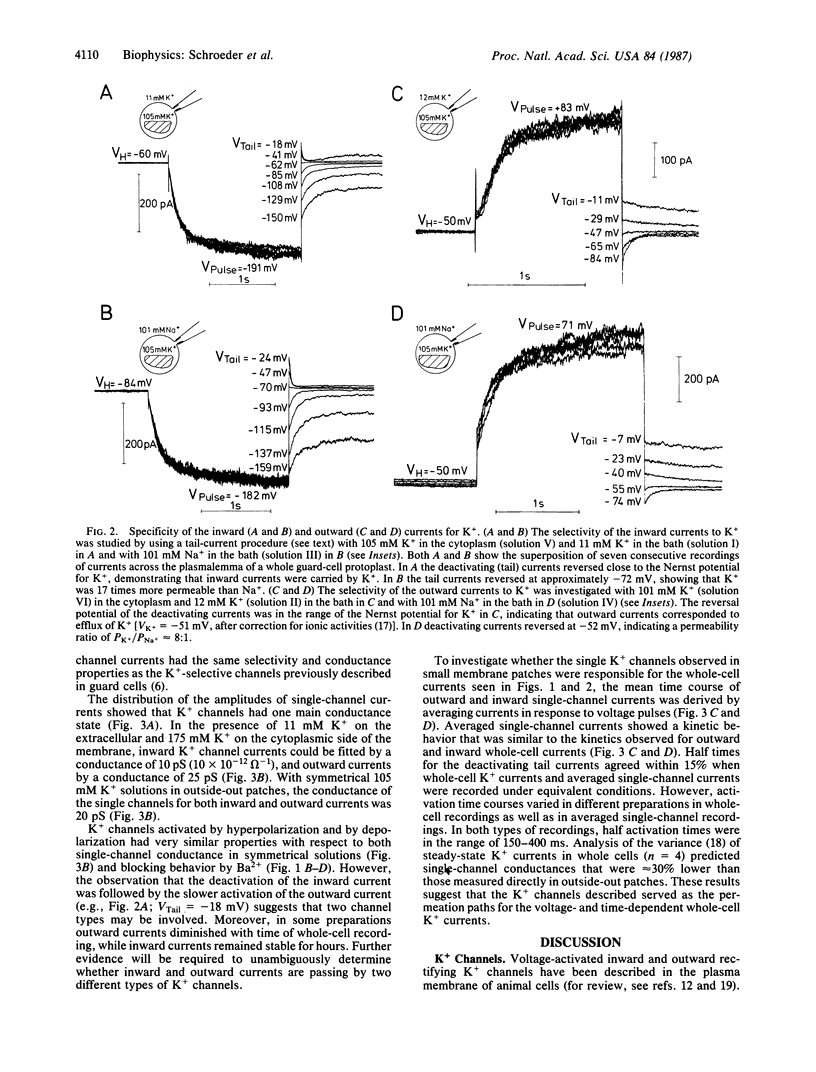
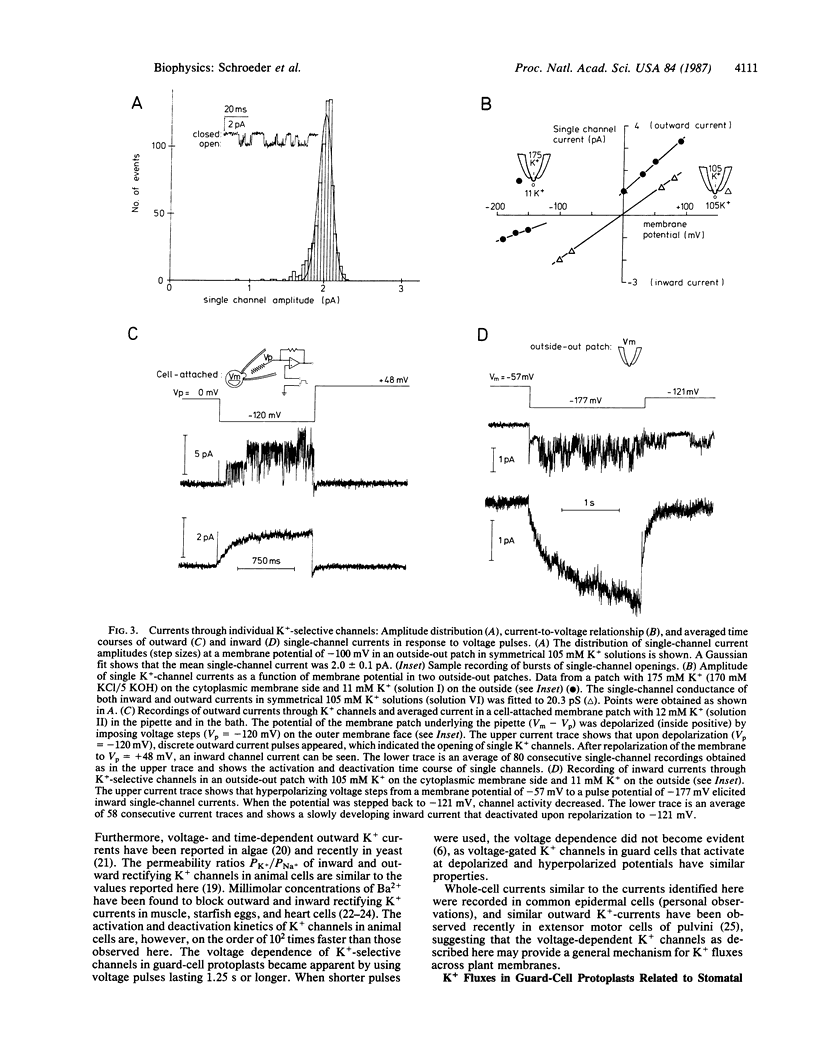
Stomatal pores in leaves enable plants to regulate the exchange of gases with their environment. Variations of the pore aperture are mediated by controlled changes of potassium salt concentrations in the surrounding guard cells. The voltage-dependent gating of K+-selective channels in the plasma membrane (plasmalemma) of cell-wall-free guard cells (protoplasts) was studied at the molecular level in order to investigate the regulation of K+ fluxes during stomatal movements. Inward and outward K+ currents across the plasmalemma of guard cells were identified by using the whole-cell configuration of the patch-clamp technique. Depolarizations of the membrane potential from a holding potential of -60 mV to values more positive than -40 mV produced outward currents that were shown to be carried by K+. Hyperpolarizations elicited inward K+ currents. Inward and outward currents were selective for K+ over Na+ and could be partially blocked by exposure to extracellular Ba2+. In cell-attached and excised membrane patches, previously identified K+-selective single channels in guard cells were studied. Averaging of single-channel currents during voltage pulses resulted in activation and deactivation kinetics that were similar to corresponding kinetics of inward and outward currents in whole cells, showing that K+-selective channels were the molecular pathways for the K+ currents recorded across the plasmalemma of single guard-cell protoplasts. Estimates demonstrate that K+ currents through the voltage-gated K+ channels recorded in whole guard cells can account for physiological K+ fluxes reported to occur during stomatal movements in leaves.
Keywords: patch clamp, K+ current, ion channel, rectifier, stomata
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong C. M., Bezanilla F. Inactivation of the sodium channel. II. Gating current experiments. J Gen Physiol. 1977 Nov;70(5):567–590. doi: 10.1085/jgp.70.5.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F. A high capacity data recording device based on a digital audio processor and a video cassette recorder. Biophys J. 1985 Mar;47(3):437–441. doi: 10.1016/S0006-3495(85)83935-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol. 1982 Oct;331:577–597. doi: 10.1113/jphysiol.1982.sp014393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustin M. C., Martinac B., Saimi Y., Culbertson M. R., Kung C. Ion channels in yeast. Science. 1986 Sep 12;233(4769):1195–1197. doi: 10.1126/science.2426783. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Miyazaki S., Moody W., Patlak J. Blocking effects of barium and hydrogen ions on the potassium current during anomalous rectification in the starfish egg. J Physiol. 1978 Jun;279:167–185. doi: 10.1113/jphysiol.1978.sp012338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Latorre R., Miller C. Conduction and selectivity in potassium channels. J Membr Biol. 1983;71(1-2):11–30. doi: 10.1007/BF01870671. [DOI] [PubMed] [Google Scholar]
- Saftner R. A., Raschke K. Electrical potentials in stomatal complexes. Plant Physiol. 1981 Jun;67(6):1124–1132. doi: 10.1104/pp.67.6.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakmann B., Trube G. Voltage-dependent inactivation of inward-rectifying single-channel currents in the guinea-pig heart cell membrane. J Physiol. 1984 Feb;347:659–683. doi: 10.1113/jphysiol.1984.sp015089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder-Hartwig K. Gesünder leben--bewusster pflegen. Pflegeplanung, Pflegedokumentation, Qualitätssicherung--Chancen für die Pflege?/Blick in die Geschichte der beruflichen Pflege. Krankenpflege (Frankf) 1987 Feb;41(2):49–52. [PubMed] [Google Scholar]
- Van Kirk C. A., Raschke K. Release of Malate from Epidermal Strips during Stomatal Closure. Plant Physiol. 1978 Mar;61(3):474–475. doi: 10.1104/pp.61.3.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WERMAN R., GRUNDFEST H. Graded and all-or-none electrogenesis in arthropod muscle. II. The effects of alkali-earth and onium ions on lobster muscle fibers. J Gen Physiol. 1961 May;44:997–1027. doi: 10.1085/jgp.44.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]


