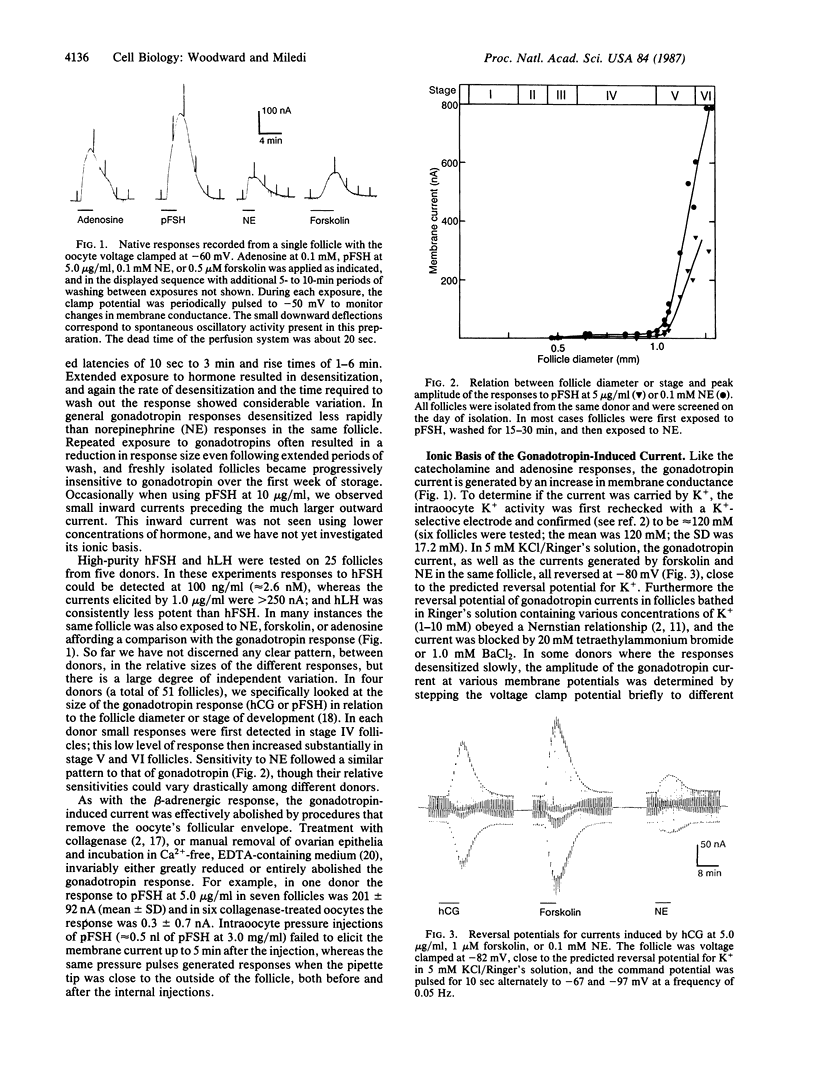
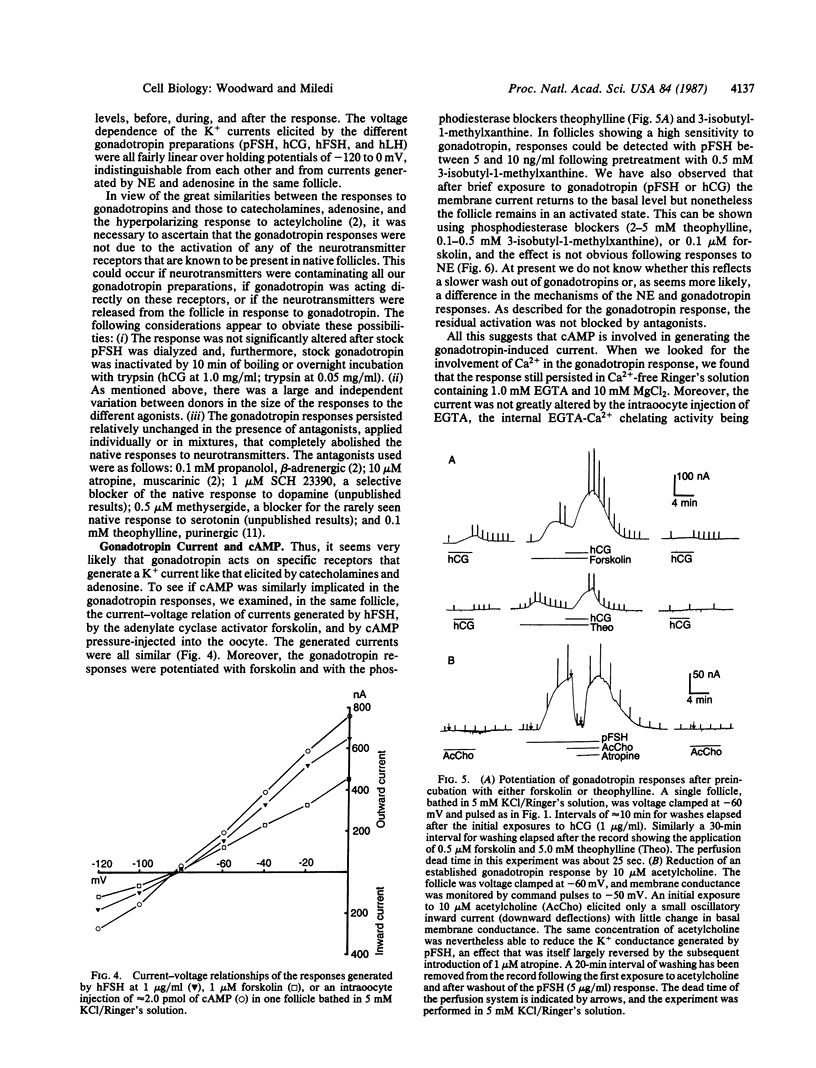
Abstract
Membrane currents were recorded, using the voltage clamp technique, from Xenopus laevis oocytes still surrounded by their enveloping follicular and epithelial cells. Exposure of the follicles to mammalian gonadotropins elicited a current generated largely by an increase in membrane K+ conductance. The gonadotropin response resembled responses elicited by adenosine and catecholamines in the same follicle, but was not blocked by purinergic or catecholaminergic antagonists. The gonadotropin-induced currents were potentiated by the adenylate cyclase activator forskolin and by phosphodiesterase inhibitors; similar currents were elicited in the same follicle by intraoocyte injection of cAMP, which indicates a role for this second messenger in the response mechanism. Gonadotropin responses were either abolished or substantially reduced after treatments that remove the ovarian epithelial and follicular cells. Our experiments suggest that the gonadotropin receptors, and the K+ channels they regulate, reside in the follicular cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Browne C. L., Werner W. Intercellular junctions between the follicle cells and oocytes of Xenopus laevis. J Exp Zool. 1984 Apr;230(1):105–113. doi: 10.1002/jez.1402300114. [DOI] [PubMed] [Google Scholar]
- Browne C. L., Wiley H. S., Dumont J. N. Oocyte-follicle cell gap junctions in Xenopus laevis and the effects of gonadotropin on their permeability. Science. 1979 Jan 12;203(4376):182–183. doi: 10.1126/science.569364. [DOI] [PubMed] [Google Scholar]
- Dascal N., Lotan I., Gillo B., Lester H. A., Lass Y. Acetylcholine and phorbol esters inhibit potassium currents evoked by adenosine and cAMP in Xenopus oocytes. Proc Natl Acad Sci U S A. 1985 Sep;82(17):6001–6005. doi: 10.1073/pnas.82.17.6001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont J. N., Brummett A. R. Oogenesis in Xenopus laevis (Daudin). V. Relationships between developing oocytes and their investing follicular tissues. J Morphol. 1978 Jan;155(1):73–97. doi: 10.1002/jmor.1051550106. [DOI] [PubMed] [Google Scholar]
- Fortune J. E. Steroid production by Xenopus ovarian follicles at different developmental stages. Dev Biol. 1983 Oct;99(2):502–509. doi: 10.1016/0012-1606(83)90299-3. [DOI] [PubMed] [Google Scholar]
- Gundersen C. B., Miledi R., Parker I. Serotonin receptors induced by exogenous messenger RNA in Xenopus oocytes. Proc R Soc Lond B Biol Sci. 1983 Aug 22;219(1214):103–109. doi: 10.1098/rspb.1983.0062. [DOI] [PubMed] [Google Scholar]
- Hallberg R. L., Smith D. C. In vivo and in vitro hormonal effects on the metabolism of immature oocytes of Xenopus laevis. Dev Biol. 1976 Feb;48(2):308–316. doi: 10.1016/0012-1606(76)90092-0. [DOI] [PubMed] [Google Scholar]
- Jared D. W., Wallace R. A. Protein uptake in vitro by amphibian oocytes. Exp Cell Res. 1969 Oct;57(2):454–457. doi: 10.1016/0014-4827(69)90175-x. [DOI] [PubMed] [Google Scholar]
- Jordana X., Allende C. C., Allende J. E. Differential inhibition by progesterone of the adenylate cyclase of oocytes and follicle cells of Xenopus laevis. FEBS Lett. 1982 Jun 21;143(1):124–128. doi: 10.1016/0014-5793(82)80287-1. [DOI] [PubMed] [Google Scholar]
- Kusano K., Miledi R., Stinnakre J. Acetylcholine receptors in the oocyte membrane. Nature. 1977 Dec 22;270(5639):739–741. doi: 10.1038/270739a0. [DOI] [PubMed] [Google Scholar]
- Kusano K., Miledi R., Stinnakre J. Cholinergic and catecholaminergic receptors in the Xenopus oocyte membrane. J Physiol. 1982 Jul;328:143–170. doi: 10.1113/jphysiol.1982.sp014257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licht P., Papkoff H. Separation of two distinct gonadotropins from the pituitary gland of the bullfrog Rana catesbeiana. Endocrinology. 1974 Jun;94(6):1587–1594. doi: 10.1210/endo-94-6-1587. [DOI] [PubMed] [Google Scholar]
- Licht P., Papkoff H. Species specificity in the response of an in vitro amphibian (Xenopus laevis) ovulation assay to mammalian luteinizing hormones. Gen Comp Endocrinol. 1976 Aug;29(4):552–555. doi: 10.1016/0016-6480(76)90039-3. [DOI] [PubMed] [Google Scholar]
- Licht P. Reproductive endocrinology of reptiles and amphibians: gonadotropins. Annu Rev Physiol. 1979;41:337–351. doi: 10.1146/annurev.ph.41.030179.002005. [DOI] [PubMed] [Google Scholar]
- Lotan I., Dascal N., Cohen S., Lass Y. Adenosine-induced slow ionic currents in the Xenopus oocyte. Nature. 1982 Aug 5;298(5874):572–574. doi: 10.1038/298572a0. [DOI] [PubMed] [Google Scholar]
- Lotan I., Dascal N., Oron Y., Cohen S., Lass Y. Adenosine-induced K+ current in Xenopus oocyte and the role of adenosine 3',5'-monophosphate. Mol Pharmacol. 1985 Aug;28(2):170–177. [PubMed] [Google Scholar]
- Marsh J. M. The role of cyclic AMP in gonadal function. Adv Cyclic Nucleotide Res. 1975;6:137–199. [PubMed] [Google Scholar]
- Masui Y., Clarke H. J. Oocyte maturation. Int Rev Cytol. 1979;57:185–282. doi: 10.1016/s0074-7696(08)61464-3. [DOI] [PubMed] [Google Scholar]
- Masui Y. Relative roles of the pituitary, follicle cells, and progesterone in the induction of oocyte maturation in Rana pipiens. J Exp Zool. 1967 Dec;166(3):365–375. doi: 10.1002/jez.1401660309. [DOI] [PubMed] [Google Scholar]
- Miledi R. A calcium-dependent transient outward current in Xenopus laevis oocytes. Proc R Soc Lond B Biol Sci. 1982 Jul 22;215(1201):491–497. doi: 10.1098/rspb.1982.0056. [DOI] [PubMed] [Google Scholar]
- Miledi R., Parker I. Chloride current induced by injection of calcium into Xenopus oocytes. J Physiol. 1984 Dec;357:173–183. doi: 10.1113/jphysiol.1984.sp015495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Parker I., Sumikawa K. Synthesis of chick brain GABA receptors by frog oocytes. Proc R Soc Lond B Biol Sci. 1982 Nov 22;216(1205):509–515. doi: 10.1098/rspb.1982.0089. [DOI] [PubMed] [Google Scholar]
- Mulner O., Ozon R. The roles of follicular envelopes in the initiation of Xenopus oocyte maturation. Gen Comp Endocrinol. 1981 Jul;44(3):335–343. doi: 10.1016/0016-6480(81)90010-1. [DOI] [PubMed] [Google Scholar]
- Mulner O., Thibier C., Ozon R. Steroid biosynthesis by ovarian follicles of Xenopus laevis in vitro during oogenesis. Gen Comp Endocrinol. 1978 Mar;34(3):287–295. doi: 10.1016/0016-6480(78)90250-2. [DOI] [PubMed] [Google Scholar]
- Otero C., Bravo R., Rodriguez C., Paz B., Allende J. E. The stimulatory effect of human chorionic gonadotropin on amino acid uptake by amphibian follicles. Dev Biol. 1978 Mar;63(1):213–223. doi: 10.1016/0012-1606(78)90126-4. [DOI] [PubMed] [Google Scholar]
- Smith L. D., Ecker R. E., Subtelny S. In vitro induction of physiological maturation in Rana pipiens oocytes removed from their ovarian follicles. Dev Biol. 1968 Jun;17(6):627–643. doi: 10.1016/0012-1606(68)90010-9. [DOI] [PubMed] [Google Scholar]
- Stinnakre J., Van Renterghem C. Cyclic adenosine monophosphate, calcium, acetylcholine and the current induced by adenosine in the Xenopus oocyte. J Physiol. 1986 May;374:551–569. doi: 10.1113/jphysiol.1986.sp016097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumikawa K., Parker I., Miledi R. Partial purification and functional expression of brain mRNAs coding for neurotransmitter receptors and voltage-operated channels. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7994–7998. doi: 10.1073/pnas.81.24.7994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Renterghem C., Penit-Soria J., Stinnakre J. Beta-adrenergic induced potassium current in Xenopus oocyte: involvement of cyclic AMP. Biochimie. 1984 Feb;66(2):135–138. doi: 10.1016/0300-9084(84)90202-5. [DOI] [PubMed] [Google Scholar]
- Van Renterghem C., Renit-Soria J., Stinnakre J. beta-Adrenergic induced K+ current in Xenopus oocytes: role of cAMP, inhibition by muscarinic agents. Proc R Soc Lond B Biol Sci. 1985 Jan 22;223(1232):389–402. doi: 10.1098/rspb.1985.0008. [DOI] [PubMed] [Google Scholar]
- Wallace R. A., Steinhardt R. A. Maturation of Xenopus oocytes. II. Observations on membrane potential. Dev Biol. 1977 Jun;57(2):305–316. doi: 10.1016/0012-1606(77)90217-2. [DOI] [PubMed] [Google Scholar]
- van den Hoef M. H., Dictus W. J., Hage W. J., Bluemink J. G. The ultrastructural organization of gap junctions between follicle cells and the oocyte in Xenopus laevis. Eur J Cell Biol. 1984 Mar;33(2):242–247. [PubMed] [Google Scholar]



