Abstract
The analysis of complex genetic determinants that control the ability of a fungus to colonize its host has been impaired by the lack of sophisticated genetic tools for characterizing important pathogens. We have developed a system for the genetic transformation of Magnaporthe grisea, the causal agent of rice blast disease, to overcome this limitation. A M. grisea arginine auxotroph was shown to contain a mutation (arg3-12) that abolishes ornithine carbamoyltransferase activity. M. grisea strains that contain arg3-12 were used as recipients in transformation experiments with plasmid pMA2, which carries the ArgB+ gene from Aspergillus nidulans. Stable prototrophic transformants arose at a frequency of about 35 per microgram of plasmid DNA. Integration of single or multiple plasmid copies occurred at a single site in the genome of each transformant; rearrangements were often created during integration. When M. grisea genomic segments were incorporated into pMA2, the presence of any one of five different M. grisea segments did not greatly affect the efficiency of transformation. Integration via homologous recombination occurred when the donor plasmid was linearized by cleaving at a unique restriction site within the M. grisea segment.
Keywords: plant pathology, Pyricularia, Magnaporthe grisea
Full text
PDF
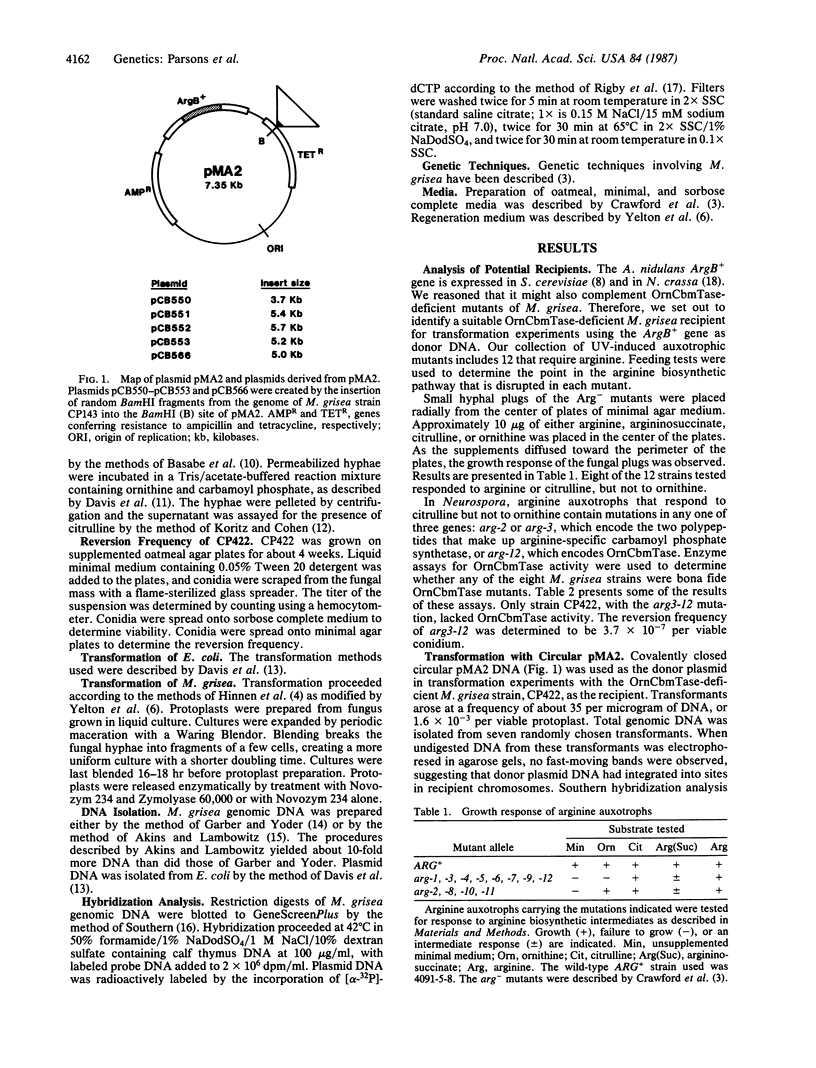

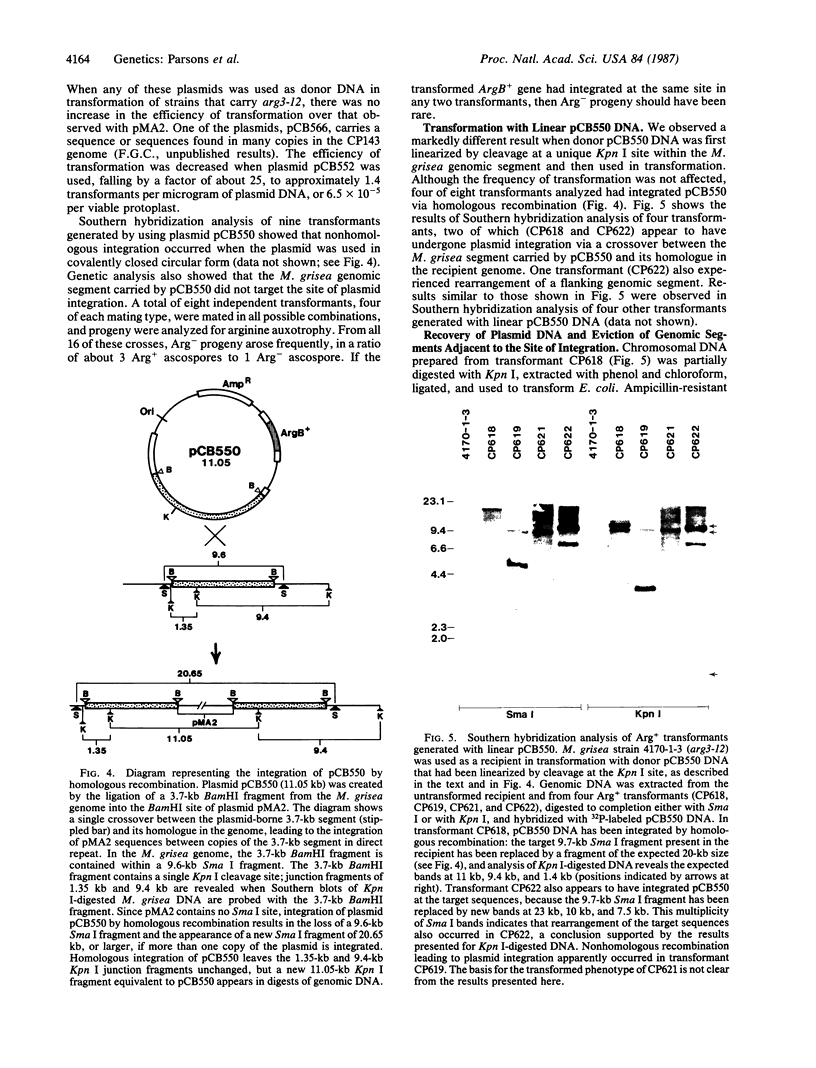

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akins R. A., Lambowitz A. M. General method for cloning Neurospora crassa nuclear genes by complementation of mutants. Mol Cell Biol. 1985 Sep;5(9):2272–2278. doi: 10.1128/mcb.5.9.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basabe J. R., Lee C. A., Weiss R. L. Enzyme assays using permeabilized cells of Neurospora. Anal Biochem. 1979 Jan 15;92(2):356–360. doi: 10.1016/0003-2697(79)90670-5. [DOI] [PubMed] [Google Scholar]
- Berse B., Dmochowska A., Skrzypek M., Wegleński P., Bates M. A., Weiss R. L. Cloning and characterization of the ornithine carbamoyltransferase gene from Aspergillus nidulans. Gene. 1983 Nov;25(1):109–117. doi: 10.1016/0378-1119(83)90173-7. [DOI] [PubMed] [Google Scholar]
- Boylan M. T., Holland M. J., Timberlake W. E. Saccharomyces cerevisiae centromere CEN11 does not induce chromosome instability when integrated into the Aspergillus nidulans genome. Mol Cell Biol. 1986 Nov;6(11):3621–3625. doi: 10.1128/mcb.6.11.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case M. E., Schweizer M., Kushner S. R., Giles N. H. Efficient transformation of Neurospora crassa by utilizing hybrid plasmid DNA. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5259–5263. doi: 10.1073/pnas.76.10.5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford M. S., Chumley F. G., Weaver C. G., Valent B. Characterization of the Heterokaryotic and Vegetative Diploid Phases of MAGNAPORTHE GRISEA. Genetics. 1986 Dec;114(4):1111–1129. doi: 10.1093/genetics/114.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. H., Ristow J. L., Ginsburgh C. L. Independent localization and regulation of carbamyl phosphate synthetase A polypeptides of Neurospora crassa. Mol Gen Genet. 1981;181(2):215–221. doi: 10.1007/BF00268429. [DOI] [PubMed] [Google Scholar]
- Garber R. C., Yoder O. C. Isolation of DNA from filamentous fungi and separation into nuclear, mitochondrial, ribosomal, and plasmid components. Anal Biochem. 1983 Dec;135(2):416–422. doi: 10.1016/0003-2697(83)90704-2. [DOI] [PubMed] [Google Scholar]
- Hinnen A., Hicks J. B., Fink G. R. Transformation of yeast. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1929–1933. doi: 10.1073/pnas.75.4.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORITZ S. B., COHEN P. P. Colorimetric determination of carbamylamino acids and related compounds. J Biol Chem. 1954 Jul;209(1):145–150. [PubMed] [Google Scholar]
- Orr-Weaver T. L., Szostak J. W., Rothstein R. J. Yeast transformation: a model system for the study of recombination. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6354–6358. doi: 10.1073/pnas.78.10.6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paszkowski J., Shillito R. D., Saul M., Mandák V., Hohn T., Hohn B., Potrykus I. Direct gene transfer to plants. EMBO J. 1984 Dec 1;3(12):2717–2722. doi: 10.1002/j.1460-2075.1984.tb02201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perucho M., Hanahan D., Wigler M. Genetic and physical linkage of exogenous sequences in transformed cells. Cell. 1980 Nov;22(1 Pt 1):309–317. doi: 10.1016/0092-8674(80)90178-6. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Winston F., Chumley F., Fink G. R. Eviction and transplacement of mutant genes in yeast. Methods Enzymol. 1983;101:211–228. doi: 10.1016/0076-6879(83)01016-2. [DOI] [PubMed] [Google Scholar]
- Yelton M. M., Hamer J. E., Timberlake W. E. Transformation of Aspergillus nidulans by using a trpC plasmid. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1470–1474. doi: 10.1073/pnas.81.5.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]