Abstract
Since their discovery by Steinman and Cohn in 1973, dendritic cells (DCs) have become increasingly recognized for their crucial role as regulators of innate and adaptive immunity. DCs are exquisitely adept at acquiring, processing and presenting antigens to T cells. They also adjust the context (and hence the outcome) of antigen presentation in response to a plethora of environmental inputs that signal the occurence of pathogens or tissue damage. Such signals generally boost DC maturation, which promotes their migration from peripheral tissues into and within secondary lymphoid organs and their capacity to induce and regulate effector T cell responses. Conversely, more recent observations indicate that DCs are also crucial to ensure immunological peace. Indeed, DCs constantly present innocuous self and non-self antigens in a fashion that promotes tolerance, at least in part, through the control of regulatory T cells (Tregs). Tregs are specialized T cells that exert their immuno-suppressive function through a variety of mechanisms affecting both DCs and effector cells. Here, we review recent advances in our understanding of the relationship between tolerogenic DCs and Tregs.
1. Introduction
Dendritic cells (DCs) are a family of leukocytes that have mostly been studied as potent stimulators of adaptive immunity, but there is mounting evidence that DCs also establish and maintain immunological tolerance (1). Indeed, DCs can prevent, inhibit or modulate T cell-mediated effector responses through a variety of mechanisms, ranging from the production of pleiotropic anti-inflammatory factors that exert broadly attenuating effects to the induction of antigen-specific T cell responses resulting in anergy, deletion or instruction of regulatory T cells (Tregs, Figure 1). Here, we will focus on the mechanisms by which DCs induce and control tolerance, particularly the function and differentiation of Tregs, which are crucial to contain autoimmunity and chronic inflammation. Failure of Treg function has been implicated in the development of many autoimmune processes, whereas cellular therapy by adoptive transfer of Tregs has shown efficacy in these disorders (2). On the other hand, Treg-mediated suppressive activity can also contribute to the immune escape of pathogens or tumors. Indeed, elimination of Tregs in mice carrying malignancies can improve anti-tumor immune responses and survival (3). Therefore, understanding the role of DCs in Treg activation and differentiation is critical for the development of therapeutic strategies in many disease settings.
Figure 1. Types of tolerogenic DCs and their mechanisms of action.
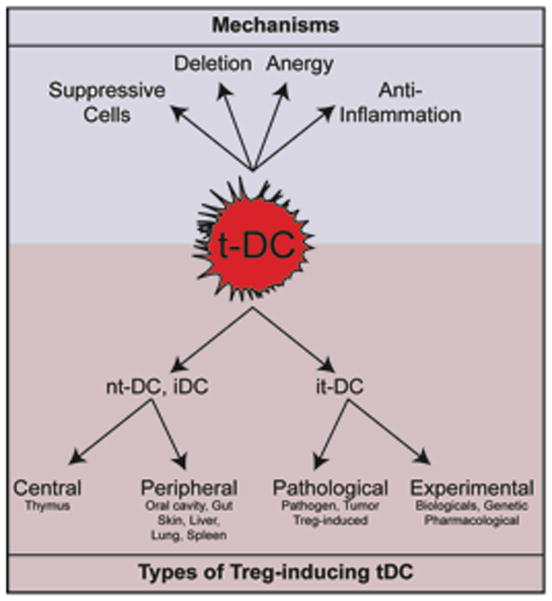
Tolerogenic DCs (tDCs) participate to the establishment of T cell tolerance by a variety of mechanisms, including the induction of anergy, deletion of antigen-reactive T cells, stimulation of suppressive regulatory T cells (Tregs) either by activation of existing Tregs or de novo differentiation of Tregs from Tns and production of anti-inflammatory cytokines and other factors. Depending on the differentiation state of the DC and the site of tolerogenic instruction, tDCs can be separated in natural tolerogenic DCs (ntDCs) and induced tolerogenic DCs (itDCs). The steady state environment instructs ntDCs (and includes iDCs) while itDCs arise during pathologies or after manipulation.
At steady-state, tissue-resident DCs are immature (henceforth called iDCs); these cells are poised to acquire antigenic material from their environment but they are poorly immunogenic because they express only modest levels of MHC molecules and little or no costimulatory molecules and proinflammatory cytokines. iDCs sense the presence of infectious microbes using specific receptors that detect pathogen associated molecular patterns (PAMPs) or damage associated molecular patterns (DAMPs) that are released within tissues as a consequence of cellular distress. These “danger” signals trigger signaling cascades in iDCs that result in their maturation, a profound phenotypic and functional metamorphosis driven by changes in gene expression (4, 5). During the maturation process, DCs loose their capacity to acquire soluble antigen but gain T cell stimulatory capacity due to increased antigen processing and upregulation of MHC, costimulatory molecules and cytokines (6). Maturation signals also trigger in iDCs a profound change in their repertoire of traffic molecules, such as the upregulation of CCR7, a chemokine receptor that enables DCs in peripheral tissues to access local lymph vessels and migrate to the draining lymph nodes (7). Here, the now fully mature DCs (mDCs) report the inflammatory and antigenic status of their source tissue to recirculating lymphocytes (6). Whereas newly generated mDCs are generally believed to possess primarily immunogenic functions, the role of iDCs is less well defined as they are not in a final differentiation state and can give rise to both immunogenic, pro-inflammatory mDCs as well as semi-mature DCs that share some phenotypic features of mDCs, such as CCR7 expression, but possess the capacity to establish and maintain tolerance.
Clues that iDCs themselves can either convert conventional naïve T cells (Tns) to assume a Treg phenotype and/or promote the function of existing Tregs have been gleaned from experiments in which antigen was administered to mice without a concomitant maturation signal (8–14). Under these conditions, antigen accumulated on DCs in secondary lymphoid organs (SLOs) and triggered the differentiation and/or proliferation of Tregs resulting in antigen-specific tolerance that could prevent or reverse autoimmune processes (Table 1). Animals that lack functional iDCs develop severe autoimmunity possibly due, at least in part, to reduced numbers of circulating Tregs (15–18). Similarly, a DC-restricted genetic deficiency in αvβ8 integrin, which activates TGFβ, a key cytokine for the induction and maintenance of Tregs (19), or disruption of DC-expressed TGFβ receptor (TGFβR) impairs the tolerogenic function of DCs and fosters autoimmunity (20). On the other hand, increased DC numbers are accompanied by a concomitant increase in Tregs, whereas elimination of Tregs elevates the number of DCs (16, 21, 22), suggesting that DCs and Tregs regulate each other’s homeostasis.
Table 1.
| Mechanism of t-DC induction | Treg phenotype | Origin of DC | DC phenotype | Mechanism of Treg induction | Disease Model | Reference |
|---|---|---|---|---|---|---|
|
Central suppressive tolerance | ||||||
| TSLP | CD4+CD25+Foxp3+ | Thymus | mDC | Watanabe et al., 2005 (81) | ||
| CD4+CD25+Foxp3+ | Thymus | pDC | Proietto et al., 2008 (76) Proietto et al., 2009 (77) |
|||
| Peripheral suppressive tolerance | ||||||
|
Dermal tolerance | ||||||
| Retinoic acid | CD4+Foxp3+ | Skin DC | CD103− iDC | IBD | Guilliams et al., 2010 (71) | |
| CD4+CTLA4+Foxp3+IL-10+TGFβ+ | Skin LN | DEC-205+ iDC | T1D | Bruder et al., 2005 (300) | ||
| CD4+CD25+CTLA4+ | Skin LN | DEC-205+ iDC | Mahnke et al., 2003 (301) | |||
|
Oral tolerance | ||||||
| CD4+CD25+* | Peyer’s Patches | CD11c+ CD11b+ | CIA | Min et al., 2006 (302) | ||
| CD25+* | Peyer’s Patches | pDC-like- CD8α+ | Bilsborough et al., 2003 (303) | |||
| CD25+IL-10+INFγ+* | Oral cavity | CD11c+ | Mascarell et al., 2008 (304) | |||
| CD25+Foxp3+* | Peyer’s Patches | CD11c+ IDO+ | CIA | Park et al., 2008 (305) | ||
| CD25+CD103+Foxp3+ | LP | (RA, TGFβ) | Sun et al., 2007 (93) | |||
| CD4+Foxp3+ | MLN and LP | CD103+ | (RA, TGFβ) | Coombes et al., 2007 (92) | ||
| CD4+Foxp3+ | MLN | CD103+ | IDO | IBD | Matteoli et al., 2010 (70) | |
| IEC secreting TGFβ, RA | CD4+CD25+Foxp3+* | BMDC or SpDC | CD103+ | IBD | Iliev et al., 2009 (106) | |
| IEC secreting TGFβ, RA | CD4+CD25+Foxp3+* | MLN | CD103+ | Iliev et al., 2009 (104) | ||
|
Systemic tolerance | ||||||
| CD4+IL-10+ | Spleen | CD11clow CD45RB+ |
Wakkach et al., 2003 (250) | |||
| CD4+* | Spleen | pDCs | Martín et al., 2002 (306) | |||
| CD4+Foxp3+* | Spleen | CD8α+ | EAE | Smith et al., 2010 (307) | ||
| CD4+CD25+Foxp3+* | Spleen | DEC-205+ | Kretschmer et al., 2005 (11) | |||
| CD4+CD25−* | hu-PBMC- pDC | BDCA4+Lin− CD123+ | IDO | Chen et al., 2008 (308) | ||
| CD4+CD25+Fopx3+ IL-10+TGFβ+ | hu-PBMC- pDC | BDCA4+Lin− CD123+ | Moseman et al., 2004 (309) | |||
| IL-10+* | hu-PBMC-pDC | BDCA4+Lin− CD123+ | CD275 | Ito et al., 2007 (310) | ||
| CCR4+CD25+Foxp3+* | Allograft draining LN | pDCs | HA | Ochando et al., 2006 (68) | ||
| CD4+CD25+Foxp3+ | Spleen and LN | pDC CCR9+ | aGVHD | Hadeiba et al., 2008 (311) | ||
|
Inhaled Tolerance | ||||||
| CD4+IL-10+* | Lung LN | IL-10 | Akbari et al., 2001 (251) | |||
| CD4+IL-10+* | Lung LN | IL-10 CD275 |
EA | Akbari et al., 2002 (312) | ||
| In vitro immature | ||||||
| CD4+CTLA-4+IL-10+* | huMoDC | CD83− | Jonuleit et al., 2000 (313) | |||
| CD4+IL-10+ | huMoDC | CD83− | Dhodapkar et al., 2001 (314) | |||
| CD8+IL-10+* | huMoDC | Dhodapkar et al., 2002 (315) | ||||
| CD4+IL-10+* | huMoDC | CD1a+CD83−ILT3+ILT4+ | IL-10 | Levings et al., 2005 (316) | ||
| CD4+IL-10+ | huMoDC | iDC | CD275 | Tuettenberg et al., 2009 (317) | ||
| CD4+CD25+Foxp3+IL-10+TGFβ+ | huMoDC | Cools et al., 2008 (318) | ||||
| CD4+CD25+Foxp3+ | BMDC | PA | Stepkowski et al., 2006 (319) | |||
It must be noted that neither iDCs nor mDCs are homogenous cell populations. Several distinct subsets that express discrete surface markers have been identified nearly two decades ago (23). The phenotypic diversity of the DC family is reflected in distinct functional properties that are rooted, in part, in the expression of different PAMP and DAMP receptors, divergent antigen presentation and crosspresentation capacities, as well as differential propensities to induce tolerance and Treg differentiation.
It is thus apparent that DCs encompass a heterogeneous mix of antigen presenting cells that differ not only with regard to phenotype, differentiation and maturation status but also with regard to tolerance-inducing capacity. For the purpose of this article, we will functionally (rather than phenotypically) define two subsets of DCs based on their net effect on T cells: one subset is represented by immunogenic DCs that induce effector responses, while the other subset induces or enhances tolerance (Figure 2). We will refer to the former as stimulatory DCs (sDCs) and the latter as tolerogenic DCs (tDCs). tDCs not only comprise most iDCs, but also include other DCs covering a spectrum of different maturation states. This review will summarize current knowledge of the origins and phenotypes of tDCs, the factors maintaining or inducing their tolerogenicity and how these cells promote the expansion, function or differentiation of Tregs.
Figure 2. Relationship of maturation status, tolerogenicity and immunogenicity among DC subsets.
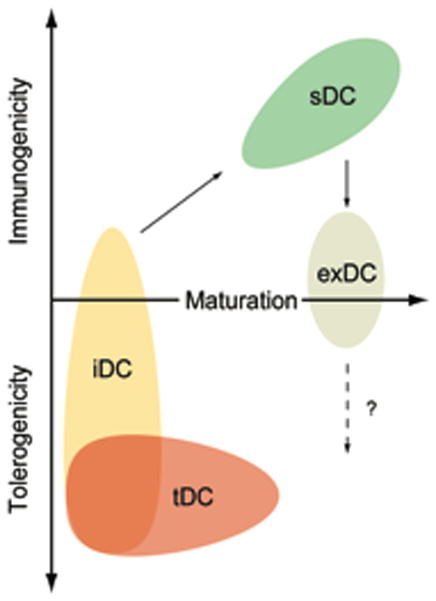
Immature DCs (iDCs) receive activation signals from microbial byproducts or tissue distress to acquire a mature phenotype, including the ability to migrate to lymph nodes and enhanced antigen presentation and costimulatory capacities. These mature DCs are highly stimulatory (sDC) and induce effector responses. Tolerogenic DCs (tDCs) include most iDCs but also comprise some cells with advanced maturation status. Only iDCs can give rise to mDCs. mDCs may loose their immunostimulatory capacity to become exhausted (exDC), however, their role in the induction of Tregs remains uncertain.
2. What is the origin of Treg-inducing tDCs?
2.1 Tregs induction sites
Mammals, including humans, that lack functional Tregs succumb to fatal autoimmune disorders (24), highlighting the importance of Tregs in controlling immune responses. In general, we discriminate between two major types of Tregs based on their origin (25). Natural Tregs (nTregs) originate during thymic development and first appear in the fetal circulation (26–28). The phenotype and suppressive program of CD4+ nTregs is controlled by the transcription factor Foxp3, which is upregulated in developing T cells upon recognition of self-antigens in the thymus (29–31). Innocuous self and non-self antigens that appear postnatally (like hormones, food and commensal flora) can drive the differentiation of additional Tregs (32). Some of these antigens may be transported into the thymus by migratory iDCs (33) that may then induce new nTregs. In addition, conventional Tns can be converted to so-called adaptive Tregs (aTregs) in extrathymic sites such as SLOs. aTregs are phenotypically heterogeneous and include both CD4+ and CD8+ T cells most (but not all) of which also express Foxp3 (Table 1). A common trait of all Tregs is the expression of one or more anti-inflammatory molecules, such as IL-10, TGFβ or IL-35 and/or inhibitory receptors, such as cytotoxic T-lymphocyte antigen 4 (CTLA4), lymphocyte-activation gene 3 (LAG-3), Glucocorticoid-induced tumor necrosis factor receptor (GITR), CD39 or CD73, among others (34, 35).
2.2. The phenotype of tDCs
The mechanisms by which tDCs exert their activity are varied and incompletely understood. As mentioned above, iDCs are typically tolerogenic (1), so the maturation status, or rather, the absence of maturation provides a hint for the tolerogenic capacity of DCs. However, iDCs are comprised of several different subsets that possess distinct abilities to present antigen, secrete cytokines and induce tolerance (36). Thus, the various subsets of iDCs and mDCs do not fill a well-defined functional niche, but cover a spectrum of immunological properties, wherein iDCs primarily maintain tolerance, whereas mDCs initiate and control predominantly (but not exclusively) effector responses (Figure 2).
Maturation phenotype
DCs receive maturation signals by a variety of inputs, including PAMP and DAMP receptors that sense certain microbial and tissue damage signatures. Such sensors include toll-like receptors (TLRs), NOD like receptors (NLRs), RIG-I–like receptors (RLRs) and others (37–40). Additionally, inflammatory cytokines (e.g. TNFα and IL-1β) or the ligation of surface-expressed activating receptors such as CD40 can trigger DC maturation (41–43). One key consequence of DC recognition of “danger” signals is the activation of members of the nuclear factor kappa B (NFκB) and interferon responsive factor (IRF) families (38, 44, 45). Upon maturation, DCs upregulate a plethora of gene products involved in antigen presentation and costimulation including MHC-II, CD40, CD80, CD86, OX40L and inducible T cell co-stimulator ligand (ICOSL or CD275), as well as cytokines that promote and modulate inflammation and effector cell functions, including IL-1β, IL-2, IL-6, IL-8, IL-12 and IL-18 (6). These changes are necessary for DCs to initiate T cell responses because Tns require three concomitant inputs to differentiate into full-fledged effector cells (Teffs): signal 1 is the antigenic stimulus provided by MHC molecules displaying a cognate peptide; signal 2 is provided by costimulatory molecules; and signal 3 is provided by cytokines produced by DCs or other microenvironmental sources (46). Since many tDCs have an immature phenotype (Tables 1, 2, Figure 2) it has been suggested that a major mechanism of their tolerogenicity is a consequence of their presentation of an antigen (signal 1) to T cells without concomitant costimulation or cytokines (signal 2 and 3). However, when iDCs are subjected to certain in vitro manipulations, such as exposure to TNFα or IFNγ or inhibition of E-cadherin, they assume phenotypic features of mDCs, including high levels of MHC and costimulatory molecules [(47, 48) and our unpublished results]. Nevertheless, Tns that are exposed to such treated DCs preferentially differentiate into aTregs (Table 3). Moreover, although CCR7 is usually considered an indicator of DC maturation, some iDCs in peripheral tissues can also upregulate CCR7, which allows them to migrate to lymph nodes without assuming a fully mature phenotype. These migratory DCs favor the induction of aTregs rather than effector cells (49–52). CCR7 deficiency impairs lymphatic migration of iDCs and compromises the induction of inhaled and oral tolerance (53, 54).
Table 2.
| Mechanism of t-DC induction | Treg phenotype | Origin of DC | DC phenotype | Mechanism of Treg induction | Disease Model | Reference |
|---|---|---|---|---|---|---|
| Pathologically-induced tolerogenic DC | ||||||
|
Pathogen-induced tolerogenic DC | ||||||
| F. hepatica products | CD4+CD25+Foxp3+ | BMDC | i-DC | Falcón et al., 2010 (320) | ||
| S. japonicum SJMHE1 peptide | CD4+CD25+* | BMDC | i-DC | DTH | Wang et al., 2009 (321) | |
| C. albicans | CD4+Foxp3+IL-10+ | BMDC | IBD | Bonifazi et al., 2009 (322) | ||
| Monophosphoryl Lipid A | CD4+Foxp3+IL-10+TGFβ+ | Oral cavity | Oral-m-LC | Allam et al., 2008 (323) | ||
| LPS | CD4+CD25+Foxp3+ | BMDC | EAU | Lau et al., 2008 (324) | ||
| Cryptococcus neoformans Glucuronoxylomannan | CD4+Foxp3+ | BMDC | Liu et al., 2008 (325) | |||
| Curcuma longa L. products (Curcumin) | CD4+CD25+Foxp3+IL10+* | BMDC | IBD | Cong et al., 2009 (208) | ||
| Yersinia virulence factor | CD4+IL-10+ | BMDC | Depaolo et al., 2008 (326) | |||
|
Tumor-induced tolerogenic DC | ||||||
| Pancreatic tumor-derived Mucins | * | huMoDC | Monti et al., 2004 (327) | |||
| B16 Melanoma | CD4+CD25+Foxp3+* | Spleen | iDC | TGFβ | TI | Ghiringhelli et al., 2005 (328) |
| P815 Mastocytoma | CD4+IL-10+ | Tumor-infiltrating | CD4−CD8− | TI | Liu et al., 2005 (154) | |
| MO4 Carcinoma | CD4+IL-10+* | Spleen | CD4−CD8− | TI | Zhang et al., 2005 (155) | |
| Necrotic myeloma cells | CD4+IL-10+ | huMoDC | Fiore et al., 2005 (156) | |||
| Ovarian carcinoma | CD8+CCR7+CD45RO+IL-10+* | Ovary ascite | pDC | CP | Wei et al., 2005 (157) | |
| Lung carcinoma cells | CD4+CD25+Foxp3+* | huMoDC | iDC | Dumitriu et al., 2009 (158) | ||
|
Retrocontrol-induced tolerogenic DC | ||||||
| CD8+CD28− suppressor | CD4+ | huMoDC | ILT3+ILT4+ | Chang et al., 2002 (329) | ||
| CD8+CD28− suppressor | CD4+CD45RO+CD25+ | huMoDC | ILT3+ILT4+ | Manavalan et al., 2003 (330) | ||
| CD4+ Tregs | CD4+* | BMDC | Martin et al., 2003 (231) | |||
Table 3.
| Mechanism of t-DC induction | Treg phenotype | Origin of DC | DC phenotype | Mechanism of Treg induction | Disease Model | Reference |
|---|---|---|---|---|---|---|
| Experimentally-induced tolerogenic DC | ||||||
|
Biological-induced tolerogenic DC | ||||||
| Galectin 1 | CD4+IL-10+ | BMDC | IL-27 | EAE | Ilarregui et al., 2009 (331) | |
| CD40L+IL-3 | CD4+CD25+Foxp3+IL-10+TGFβ+ | Thymus | pDC | Martín-Gayo et al., 2010 (78) | ||
| IL-10+IFNα | CD8+CD28− | huMoDC | Qin et al., 2008 (332) | |||
| Blocking CD200R | CD4+CD25+* | BMDC | SA | Gorczynski et al., 2004 (199) | ||
| Thymosin α1+TLR9 | CD4+CD25+* IL-10+* | BMDC | Romani et al., 2006 (333) | |||
| In vivo-inducedGM-CSF DC | CD4+Foxp3+IL-10+* | Spleen | iCD8α− | EAT | Ganesh et al., 2009 (334) | |
| Vitamin D3 | CD4+IL-10+* | huMoDC | smDC | PD-L1 | Unger et al., 2009 (115) | |
| Vitamin D3+ Dexamethasone +LPS | CD4+IL-10+ | huMoDC | CCR7+ | Anderson et al., 2009 (116) | ||
| Vitamin D3 | CD4+Foxp3+* | huMoDC | Penna et al., 2005 (335) | |||
| Vitamin D3 | CD4+CD25+Foxp3+CD62L+ | BMDC | iDC | Ureta et al., 2007 (118) | ||
| P-selectin | CD4+CD25+CD25+Foxp3+* | huMoDC | Urzainqui et al., 2007 (336) | |||
| CTLA4-Ig fusion protein | CD4+CD25+Foxp3+* | Spleen | TGFβ | CIA | Ko et al., 2010 (337) | |
| Estrogen | CD28−* | Spleen | EAE | Pettersson et al., 2004 (338) | ||
| VIP | CD4+TGFβ+IL-10+* | BMDC | IBD | Gonzalez-Rey et al., 2006 (339) | ||
| VIP | CD4+TGFβ+IL-10+* CD8+CD28−* | huMoDC | iDC | Gonzalez-Rey et al., 2006 (340) | ||
| VIP | CD4+IL-10+ | BMDC | iDC IL-10+ | DTH | Delgado et al., 2005 (341) | |
| VIP | CD4+TGFβ+IL-10+* | BMDC | iDC IL-10+ | EAE RA |
Chorny et al., 2005 (342) | |
| VIP | CD4+IL-10+* | BMDC | iDC | GVHD | Chorny et al., 2006 (343) | |
| BiP | CD4+CD25+CD27+* | huMoDC | IDO, IL10 | Corrigall et al., 2009 (344) | ||
| HGF | CD4+CD25+Foxp3+IL-10+ | Spleen | EAE | Benkhoucha et al., 2010 (345) | ||
| HGF | CD4+CD25+Foxp3+IL-10+* | huMoDC | ILT3, IL-10 | Rutella et al., 2006 (196) | ||
| TSLP | CD25+Foxp3+* | BMDC | iDC | T1D | Besin et al., 2008 (40) | |
| HLA-G | CD25+CTLA-4+* | huMoDC | Ristich et al., 2005 (197) | |||
| ILT3 | CD8+CD28−* | BMDC | Vlad et al., 2010 (272) 2010 | |||
| IL-10 | CD4+CTLA-4+* CD8+* | huMoDC | Steinbrink et al., 2002 (346) | |||
| IL-10 | CD25+Foxp3+LAG3+CTLA4+* | huMoDC | iDC ILT2+ IL-10+ | Li et al., 2010 (347) | ||
| IL-10 | IL-10+Vα24+iNKT* | huMoDC | smDC | Yamaura et al., 2008 (348) | ||
| IL-10 | CD4+CD25+IL-10+* | huMoDC | iDC | xGVHD | Sato et al., 2003 (349) | |
| IL-10 | CD4+IL-10+ | PBMC | DC-10 | ILT4 | Gregori et al., 2010 (271) | |
| IL-10 | CD4+* | huMoDC | Pacciani et al., 2010 (350) | |||
| IL-10 | CD4+* | huMoDC | iDC IL-10+ | Torres-Aguilar et al., 2010 (351) | ||
| IL-10 | CD4+IL-10+ | BMDC | CD11clow CD45RB+ |
Wakkach et al., 2003 (250) | ||
| IL-10 | CD4+* | huMoDC | Kubsch et al., 2003 (352) | |||
| IL-10+TGFβ | CD4+CD25+Foxp3+ | BMDC | CD200R3+ CD49+ |
cGVHD | Sato et al., 2009 (353) | |
| IL-10+TGFβ | CD4+CD25+Foxp3+* | BMDC | iDC | Fujita et al., 2007 (354) | ||
| IL-10+TGFβ | CD4+* | huMoDC | iDC IL-10+ | Torres-Aguilar et al., 2010 (351) | ||
| TGFβ | CD4+CD25+CTLA-4+* | BMDC | iDC | aGVHD | Sato et al., 2003 (355) | |
| TNFα | CD4+CD25+* | BMDC | smDC | SA | Fu et al., 2010 (219) | |
| TNFα | CD4+CD25+* | BMDC | smDC IL-10+ |
SA | Fu et al., 2009 (356) | |
| TNFα | CD4+Foxp3+* | BMDC | smDC | EAE | Zozulya et al., 2009 (357) | |
| TNFα | CD4+CD25+IL-10+CTLA4+GITR+Foxp3+* | BMDC | smDC | EAT | Verginis et al., 2005 (358) | |
| TNFα | CD4+IL-10+ | BMDC | smDC | EAE | Menges et al., 2002 (359) | |
| IFNγ | CD4+Foxp3+* | huMoDC | smDC | Eljaafari et al., 2009 (314) | ||
| Anti-CD45RB+LF15 0195 | CD25+* | Spleen | iDC | HA | Min et al., 2003 (360) | |
| E-cadherin | CD4+IL-10+ | BMDC | mDC | EAE | Jiang et al., 2007 (361) | |
|
Pharmacologically-induced tolerogenic DC | ||||||
| Aspirin | CD25+Foxp3+* | huMoDC | iDC | Buckland et al., 2006 (211) | ||
| Dexamethasone | CD4+IL-10+* | huMoDC | smDC | Unger et al., 2009 (115) | ||
| Dexamethasone | CD4+IL-10+* | huMoDC | smDC | Anderson et al., 2008 (119) | ||
| Resveratrol | CD4+IL-10+ | huMoDC | iDC | Svajger et al., 2010 (212) | ||
| Rosiglitazone (NFkB inhibitor) | Foxp3+ | BMDC | iDC | EAE | Iruretagoyena et al., 2006 (206) | |
| LF 15-0195 (IKK inhibitor) | CD4+CD25+CTLA4+Foxp3+* | BMDC | iDC | HA | Zhang et al., 2008 (207) | |
| Curcumin | CD4+CD25+Foxp3+IL10+* | BMDC | IL-10, TGFβ, RA | IBD | Cong et al., 2009 (208) | |
| Prednisolone | * | huMoDC | iDC | MG | Luther et al., 2009 (362) | |
|
Genetically-induced tolerogenic DC | ||||||
| SOCS3KO | CD25+Foxp3+* | BMDC | iDC | TGFβ | EAE | Matsumura et al., 2007 (363) |
| Dominant negative IKK2 transduction | * | BMDC | iDC | Tomasoni et al., 2005 (234) | ||
| Foxp3 transduction | CD25+* | huMoDC | TGFβ | Lipscomb et al., 2010 (237) | ||
| IL-10 transduced | CD4+CD25+ Foxp3+IL-10+* | BMDC | smDC | IL-10 | EA | Henry et al., 2008 (203) |
| CD40/80/86 KD | * | BMDC | CIA | Zheng et al., 2010 (235) | ||
| RelB KD | Foxp3+ | BMDC | EAMG | Yang et al., 2010 (232) | ||
| RelBKO | CD4+IL-10+* | BMDC | iDC | Martin et al., 2003 (231) | ||
| RelB KD | CD4+Foxp3+ | iDC | Zhang et al., 2009 (233) | |||
| CD40 KD | IL-10+* | BMDC | IL-10 | EAMG | Martin et al., 2003 (231) | |
IBD: Intestinal Bowel Disease, CIA: Collagen-Induced Arthritis, CLP: Cecal Ligation and Puncture, EAE: Experimental Autoimmune Encephalomyelitis, EAMG: Experimental Autoimmune Myasthenia Gravis, MG: Myasthenia Gravis, CAV: Chronic Allograft Vasculopathy, GVHD: Graft Versus Host Disease, cGVHD: chronic Graft Versus Host Disease, aGVHD: acute Graft Versus Host Disease, EAT: Experimental Autoimmune Thyroiditis, LA: Laryngeal Allograft, T1D: Type 1 Diabetes, SA: Skin Allograft, HA: Heart Allograft, AIH: Allergen-induced Immediate Hypersensitivity, EAU: Experimental Autoimmune Uveoretinitis, KA: Kidney Allograft, XGVHD: xenogeneic graft-versus-host disease, ABPA: Allergic bronchopulmonary aspergillosis, EA: Experimental asthma, PA: Pancreatic Allograft, DTH: delayed-type hypersensitivity, TI: Tumor implantation, CP: Cancer-bearing patients,
with suppressive activity
Thus, while immaturity appears to be a good indicator of DC tolerogenicity, phenotypically mature DCs not always induce immunity but, depending upon prior exposure to certain differentiation signals, may retain their tolerogenic function. This suggests that tolerance is not always a mere consequence of T cells perceiving insufficient signals 2 or 3, but additional DC-derived tolerance-promoting factors are likely to play a role. A case in point are so-called exhausted DCs (exDCs), which were observed to arise in vitro following an extended interval after exposure to maturation signals, such as bacterial lipopolysaccharide (LPS). The term ‘exhaustion’ was proposed because exDCs, unlike freshly activated mDCs, have lost their initial capacity to induce Tn differentiation into T helper (Th)-1 cells. Instead, exDCs secrete immunosuppressive IL-10 and elicit nonpolarized memory cells and/or Th2 responses (55, 56). Whether exDCs can also induce Tregs in vivo remains to be determined.
tDC subsets
In mice at least seven different DC subpopulations can be identified, which are distinguishable by both surface and intracellular markers that govern their function (36, 57–64). Murine lymphoid tissue-resident DC subsets include CD8α+, CD4+, CD8α− CD4− (DN) and plasmacytoid DCs (pDCs). Migratory DCs that carry antigen from peripheral organs to SLOs include CD103+DCs that have been identified in the lung, the gastrointestinal tract and the skin, CD11b+ “myeloid DCs” and epidermal Langerhans cells (LCs). In vitro assays suggest that there may be a hierarchy of tolerogenic potential that is highest for pDCs followed by CD103+ DCs and CD8α+ DCs with CD11b+ DCs having low activity in most assays.
It should be cautioned, however, that the tolerogenicity of DC subsets is context dependent. For instance, CD8α+DCs preferentially promote aTreg differentiation in the presence of TGFβ (58, 65), although it should be noted that addition of TGFβ to activated Tns induces aTreg differentiation even in absence of DCs (66). pDCs are key participants in the establishment of oral and transplant tolerance (59, 67, 68), presumably owing to their expression of indoleamine 2,3-dioxygenase (IDO), an enzyme that inhibits effector T cell proliferation (69). Intestinal CD103+ DCs also express IDO and secrete all-trans retinoic acid (RA), which promotes Tn differentiation into aTreg (57, 70). Some skin-derived CD103− DCs and other DCs can also produce RA (71) while IDO expression is inducible in DCs by a variety of signals, including TGFβ, interferons (69, 72) and engagement of GITR (73), among others. Therefore, although DCs subpopulations have different tolerogenic capacities a priori, they can adapt their function according to environmental inputs.
3. Instructive signals for Treg-inducing tDCs
In addition to the fact that immature tDCs present little or no signals 2 and 3 (see above), they can receive tolerance-promoting molecular ‘reminders’ that counteract sDC differentiation in response to maturation stimuli (Figure 3). These signals can be mimicked in vitro to induce tDCs under tissue culture conditions. Thus, we can differentiate between tDCs that arise naturally from hematopoietic precursors and tDCs that have received instructive signals that may cement or modulate their tolerogenic phenotype. To facilitate discussion, we will refer to natural versus induced tDCs as ntDCs and itDCs, respectively. (Figure 1). While ntDCs maintain tolerance constitutively within a steady-state environment, itDCs have received inputs from their environment, such as experimental or pharmacological interventions, infectious agents or other pathophysiological conditions. It should be emphasized that this terminology is merely meant to offer a conceptual frame of reference and does not imply that ntDCs and itDCs are strictly separate populations. Both subsets overlap and likely coexist and cooperate within tissues, making a real-life distinction between them often difficult.
Figure 3. Education of immunogenic or tolerogenic DCs by environmental signals.
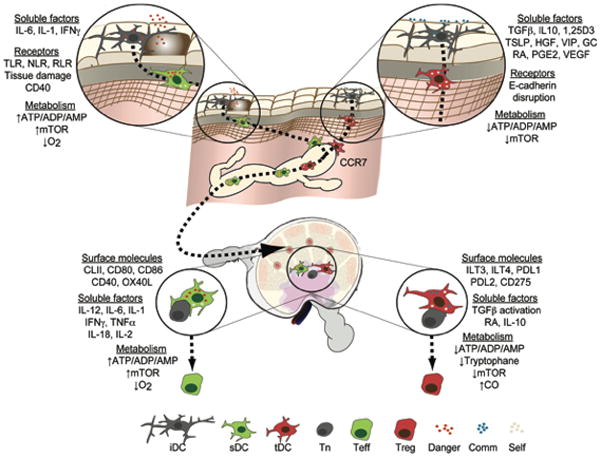
Immature DCs (iDCs) perceive a myriad of inputs leading to their differentiation into sDCs or tDCs. Upon engagement of danger signal receptors by microbes or cellular distress, the presence of activating cytokines or changes in the abundance of certain metabolites, these cells mature and become sDCs that migrate to the draining secondary lymphoid organs (SLOs) using CCR7. Through presentation of cognate antigen and costimulatory surface receptors as well as production of cytokines and the regulation of metabolites, sDCs coerce naïve T cells (Tns) to become effector cells (Teffs). On the other hand, at steady state, commensals and structural cells produce anti-inflammatory cytokines that in combination with regular levels of metabolites and minute quantities of danger signals imprint tDCs to migrate to SLOs using CCR7. Upon contact with antigen specific cells, tDCs induce the differentiation of regulatory T cells (Tregs) through a variety of mechanisms. Toll-like receptors (TLR), Nod-like receptors (NLR), RigI-like receptors (RLR), mammalian target of rapamycin (mTOR), 1,25-dihydroxyvitamin D3 (1,25D3), thymic stromal lymphoietin (TSLP), hepatocyte growth factor (HGF), vasoactive intestinal peptide (VIP), Glucocorticoid (GC), all-trans retinoic acid (RA), prostaglandin E2 (PGE2), vascular endothelial growth factor (VEGF), programmed death-1 ligand (PDL), carbon monoxide (CO), Commensal (Comm).
3.1. Natural tolerogenic DCs
As discussed above, nTreg and aTreg originate from different anatomic compartments and in response to distinct immunological processes. The rules governing the function of tDCs in the thymus where central tolerance is established by selection of Tns and generation of nTregs and in peripheral tissues where tDCs convert Tns into aTregs are only beginning to be understood.
Central suppressive tolerance
Although thymic epithelial cells contribute to self antigen-reactive nTreg commitment (31, 74, 75), thymic DCs and, in particular, thymic pDCs also promote the induction of Foxp3+ nTreg (Table 1, (76–79)). The mechanism(s) by which the thymic environment promotes this capacity on DCs involves IL-7-related thymic stromal lymphoietin (TSLP) produced by Hassall’s corpuscles in the thymic medulla (80–84). By contrast, in extra-thymic sites, such as the lung and skin (85), TSLP biases DCs and Tns toward a Th2 response, suggesting that other, as yet unknown, factors may contribute to tDC instruction or function in the thymus.
Peripheral suppressive tolerance
Oral intake of antigenic material, such as food and commensal microorganisms, efficiently generates antigen-specific systemic tolerance (10). Recent reviews have summarized the current knowledge of intestinal tract-associated Tregs and DCs and their role in oral tolerance (57, 62, 86, 87). DCs within the intestinal mucosa directly sample the lumen of the intestinal tract (88) and transport antigen to mesenteric lymph nodes (MLNs) in a CCR7-dependent manner. Here, the antigen-laden DCs promote the differentiation of Tns into Foxp3+ aTregs (89–92). DCs from the lamina propria (LP) are also thought to induce Foxp3+ aTregs (93). This tolerogenic ability of intestinal DCs is presumably controlled by the mucosal environment, which is rich in anti-inflammatory factors such as TGFβ, retinoic acid (RA), IL-10, vasoactive intestinal peptide (VIP), TSLP and hepatocyte growth factor (HGF). When these agents are added to iDCs in vitro, they promote the differentiation of itDC, which elicit more efficient Tn-to-aTreg conversion than iDCs (Table 3 (94–98)). Intestinal tDCs with the most potent aTreg inductive capacity express CD103 (aE), an integrin chain whose expression is regulated by TGFβ signaling (99). In addition, TGFβ and RA also act directly on activated Tns and promote aTreg differentiation, even in the absence of DCs (100–102).
Intestinal epithelial cells (IECs) are central for the local milieu that fosters tolerogenic responses by both DCs and activated T cells. IEC are not only a rich source of TSLP, TGFβ and RA (103–107) but also IEC-derived RA induces in DCs the expression of retinal dehydrogenases (RALDH). This presumably enables intestinal DCs to metabolise food-derived vitamin A to produce RA by themselves. However, RA- and/or TGFβ-conditioned splenic DCs fail to promote significant Foxp3+ aTreg differentiation in vitro (our unpublished results), suggesting that other instructive elements are necessary for full-fledged tDC induction in the intestine.
Like intestinal DCs, lung DCs, which capture antigens from the airways, are tasked with balancing immune responses to pathogens with those to the regular microbial flora and harmless inhaled antigens (8). Pulmonary DCs traffic continuously from the lungs to the draining mediastinal and peribronchial LNs, but to do so they are thought to require subtle maturation signals presumably from the local flora (108). Thus, DCs surveilling the airways acquire a semi-mature phenotype whereby they upregulate CCR7, which enables their migration to lymph nodes (51) and induction of aTregs that control pulmonary tolerance and homeostasis (9, 109–111). Similar to IECs, resting pulmonary stromal cells promote TGFβ-dependent differentiation of tDCs that promote the differentiation of Tregs in vitro (112). On the other hand, upon exposure to TLR ligands, lung stroma cells are critical initiators of inflammatory responses to infections by generating cytokines that instruct immunogenic sDCs (113).
In the skin, DCs function is influenced by vitamin D3, which is activated by ultraviolet radiation and then enzymatically converted to 1,25-dihydroxyvitamin D3 (1,25D3). Ex-vivo treatment of DCs with vitamin D receptor agonists elicits Treg-inducing tDC (114–122). Of note, vitamin D signaling appears to engage an autonomous transcriptional program in DCs that is distinct and independent from the transcriptional pathways that underlie DC maturation (123, 124). Some DCs in skin-draining lymph nodes induce Foxp3+ aTregs through the production of RA (71), but dermal lymph nodes contain much fewer RA-producing DCs (which are CD103−) than the intestinal tract (125).
The liver arguably provides the quintessential tolerogenic environment for T cells and DCs (126). Thus, liver allografts typically require much less immunosuppression for long-term survival (127), and targeted expression of antigens in the liver can establish tolerance by inducing antigen-specific Foxp3+ Tregs (128–130). Although the liver is a major reservoir for RA, vitamin D3 and TSLP (131), the role of these factors in hepatic tDC function is unclear. Liver sinusoidal endothelial cells elicit tolerogenic functions in cocultured DCs in vitro (132), and they are also implicated in the conversion of adoptively transferred DC precursors into hepatic tDCs in vivo (133). Hepatic DCs can induce both T cell anergy and deletional tolerance (67). They also regulate inflammatory processes during liver fibrosis and hepatic ischemia by producing cytokines, such as TNFα or IL-10 (134–137).
In summary, while the factors implicated in DC instruction to promote Treg differentiation seem to possess organ-specific flavors, TGFβ, RA and vitamin D3 appear to play a major role. Moreover, the balance of tDCs and sDCs in peripheral organs is the result of continuous intimate crosstalk between iDCs and their local surroundings. Stromal, epithelial and endothelial cells are particularly well positioned to perceive homeostatic changes at body surfaces, the extracellular environment and the blood stream. Therefore, it makes sense that these cells communicate with DCs through cytokines and direct contact and apparently contribute to the regulation of DC function and tolerance.
3.2. Induced tolerogenic DCs
A variety of inputs have been implicated in the induction of tDCs, including pathological conditions and specific molecular manipulations of iDCs or DC precursors. For example, many pathogens and tumors can mimic or produce tolerogenic factors and instruct tDCs as an immune escape mechanism. Pre-existing Tregs can also educate iDCs to become tolerogenic and induce more Tregs, a phenomenon termed “infectious tolerance”. The tolerogenic potential of DCs has also been harnessed by modifying their biology using compounds and introducing genetic alterations.
3.2.1 Disease-induced tolerogenic DC
Pathogen-induced tolerogenic DC
Certain pathogens have evolved immune escape mechanisms that exploit Tregs (138–140). In most cases, the contribution of tDCs to these infectious settings is still unclear, although different modalities have been described by which pathogens can modify DCs. For example, products from F. hepatica, C. albincans, S. japonicum, S. mansoni, B. pertussis and V. cholerae all promote DC tolerogenicity and induce Treg differentiation (Table 2), but the molecular basis for their recognition and signaling remain largely unknown. One mechanism involves microbial and parasite byproducts or toxins that prompt DCs to produce anti-inflammatory cytokines, like IL-10 and TGFβ. Examples for these compounds include cyclosporin, FK506 (Tacrolimus), FK520, ISA247 (voclosporin) and rapamycin (Sirolimus), which have been harnessed as immunosuppressive drugs to treat immune disorders and transplant rejection (141, 142). Cholera toxin (CTx), an exotoxin secreted by V. Cholerae, is a multimeric complex of six protein subunits recognized and internalized by membrane-bound gangliosides. Within the cell it increases cytosolic cyclic AMP levels (143). DC treatment with CTx B subunit (CTB) inhibits their maturation and production of IL-12 while increasing IL-10 secretion and aTreg differentiation (144, 145). Other pathogens, such as helminths, also release factors that mimic immunosuppressive molecules like TGFβ and promote itDCs, thereby staging a permissive microenvironment. Helminth infection in vivo is associated with increased numbers of Tregs whose depletion enhances parasite clearance (140, 146). However, whether and how helminth-derived products act on DCs to induce Tregs has not been determined. Similarly, some viruses encode analogs of IL-10 that are produced by infected cells (147–149) and attenuate DCs immunogenicity (150, 151), however, a direct effect on Treg differentiation remains to be demonstrated.
Tumor-induced tolerogenic DC
Cancer cells as well as the associated tumor stroma can confer tolerogenic properties on DCs resulting in differentiation and accumulation of aTregs within the tumor mass and in the draining lymph nodes (Table 2, (152–158)). Remarkably, the presence of DCs is crucial for the vascularization of some tumors, and DC depletion can enhance the elimination of malignant cells in animal models (159, 160). The mechanisms by tumors instruct DCs to become itDCs involve the production of IL-10, vascular endothelial growth factor (VEGF), prostaglandin E2, TGFβ and other tolerogenic factors by cancerous cells (152, 161–165).
Treg-induced tolerogenic DC
Even immune challenges that induce a potent effector response can trigger concomitant differentiation of aTregs (21, 110, 166, 167). The role of these inflammation-induced aTregs remains unclear but might limit immunopathology, suppress autoaggressive responses and/or promote restitution of tissue homeostasis (via TGFβ) or T and B cell memory generation (via IL-10). Antigen-specific Tregs, either activated nTregs that expand when exposed to cognate antigen (168) or newly converted aTregs, can spread their tolerance-promoting message to local DCs and Tns through a mechanism termed “infectious tolerance”. This has been elegantly demonstrated by Waldmann and colleagues who transferred CD4+ T cells from tolerized animals to new recipients which, in turn, developed tolerance. Tregs contributed directly to Tn differentiation into aTreg by producing IL-10 and TGFβ and retained this capacity during multiple transfers to successive hosts (169–173). Similarly, McGuirk et al. showed that conditioning of DCs by Tregs confers them the ability to induce Tregs in an IL-10-dependent manner (174), suggesting that tDCs may be key players during Treg-induced “infectious tolerance”.
3.2.2. Experimentally-induced tolerogenic DC
Given their potent activity, researchers have attempted to emulate the conditions leading to tDC differentiation and function in order to understand the underlying biology and to utilize tDCs for immune therapy (1, 175–177). Indeed, tDCs have be induced in vitro by 1) anti-inflammatory biologicals, 2) pharmacologic agents and 3) genetic modification (Table 3). Reports on this subject are dominated by work with murine or human DCs that were differentiated in vitro from blood or bone marrow progenitors (178) or blood monocytes (179), respectively.
Induction of tolerogenic DCs using biologics
A number of biomolecules that are physiologically encountered in tolerogenic situations can induce tDC differentiation in vitro (Figure 4). For example, incubation of murine splenic or bone marrow-derived DCs (BMDCs), or of human monocyte-derived DCs (huMoDC) or rat BMDC with IL-10 alone or in combination with other cytokines confers a certain capacity to induce suppressive lymphocytes, including CD4+CD25+, CD8+ and Valpha24+ invariant natural killer T (iNKT). The suppressive capacity of these cells has been extensively tested in models allograft rejection, allergies and xenogeneic, acute and chromic allogenic graft-versus-host disease (Table 3). Signaling through the IL-10 receptor (IL10R) maintains iDCs in their immature state even in the presence of maturation signals (180, 181). IL10R ligation triggers janus kinases (JAK)-mediated phosphorylation of Stat3 (signal transducer and activator of transcription 3 (182)). Activated phospho-Stat3 is translocated to the nucleus where it represses genes associated with DC maturation and immunogenicity (181, 183). A few genes are specifically induced by IL-10, including suppressor of cytokine signaling 3 (SOCS3) and signaling lymphocytic activation molecule (SLAM (184)). SOCS3 negatively regulates Stat-dependent signaling of inflammatory cytokines (185), particularly IL-6, which can inhibit Tregs-mediated suppression (186). SLAM signaling activates src homology 2 domain-containing protein tyrosine phosphatase 1 (SHP-1), which inactivates costimulatory receptors by dephosphorylating their cytoplasmic tail (187, 188). More studies will be necessary to elucidate the effects of IL-10 on DCs in vivo.
Figure 4. Induced-tolerogenic DCs.
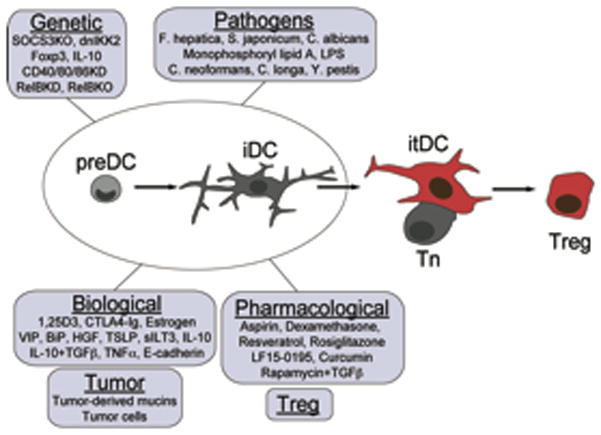
DCs progenitors (preDCs) and immature DCs (iDCs) from multiple sources are susceptible to tolerogenic instruction by multiple strategies. These cells can be used as therapeutic tools for the induction of antigen specific tolerance.
TGFβ, a cytokine produced by Tregs and other sources in many tissues, has also profound effects on DCs in vitro. Using animals that express a dominant negative form of the TGFβ receptor complex (dnTGFβR) specifically on DCs, the Flavell group has shown that the action of TGFβ allows DCs to attenuate the neuropathology associated with EAE (20). Functional TGFβR (and TGFβ-producing Tregs (189)) is also required on NK cells to restrain their pro-inflammatory activity (190). Thus, the TGFβ pathway is a major mechanism by which Tregs control both NK cells and DCs. Ligation of TGFβR leads to heterodimerization of Smad2 and Smad4, which regulate gene expression in the nucleus (191, 192). The downstream consequences appear similar to those of IL-10 and include inhibition of DC the maturation through blockade of NFκB signaling. However, in contrast to IL-10, TGFβ signaling induces a much larger set of genes in DCs (193). The TGFβ-induced transcriptional program in tDCs includes TGFβ production itself as well as TGFβR, CXCL14, IL-18, the transcription factors peroxisome proliferator-activated receptor γ (PPARγ) and plasminogen activator inhibitor 1 (194, 195). The specific role of each of these factors in tDC function remains to be analyzed.
Other bioderivatives instructing itDCs are HGF and the vitamin D3 metabolite, 1,25D3. When treated in vitro with these compounds DCs initiate the expression of gene products that have been implicated immune tolerance, including indoleamine 2,3-dioxygenase (IDO), C5R1, CCL2, IL-10, TGFβ, TRAIL, inhibin and the inhibitory receptors CD300LF and CYP24A1 (124, 196). Several other factors, such as estrogen, vasoactive intestinal peptide (VIP), binding immunoglobulin protein (BiP), TSLP, GM-CSF, G-CSF, IFNα/β/γ, IL-6, PGE2 and TNFα, may also promote Treg-inducing capacities on tDCs.
Antibodies and synthetic soluble ligands of specific surface receptors have also been used to produce itDCs. For example, human MoDC treated with HLA-G, a non-classical histocompatibility molecule associated with tolerance, induced suppressive autologous T cells that expressed CD25 and cytotoxic T-lymphocyte antigen 4 (CTLA-4), two markers commonly found on Tregs (197, 198). Similarly, the antibody-mediated activation of the suppressive receptor CD200R boosts the tolerogenicity of mouse BMDCs by activating Tregs in vivo (199–202).
Pharmacologically-induced tolerogenic DCs
The use of immunosuppresive drugs has been crucial for the treatment of many diseases. Not surprisingly, immunosuppressants frequently affect DC immunogenicity often by intervening with their maturation, although the specific contribution of such drug effects on DCs relative to their influence over other target cells is not known. Nevertheless, immunosuppressive compounds have been successfully employed to manipulate DC function in many disease models (175).
Glucocorticoids (GCs) were the first immunosuppressants to be used in a clinical setting (203). Treatment of human MoDC or mouse BMDC with prednisolone or dexamethasone conditions these cells for tolerogenic instruction of aTregs (Table 3). GC binding to the glucocorticoid receptor (GR) regulates DC activation through nuclear glucocorticoid response elements (GRE) that negatively regulate promoters for members of the canonical NFκB pathway, inflammatory cytokines, chemokines, their receptors and antigen presentation molecules (203). In addition to repressing DC maturation, dexamethasone also induces a discrete set of anti-inflammatory gene products and chemoattractants, including IL-10, GITRL, IDO, CCL2 (MCP-1), CCL8 (MCP-2), CCR2, CCL9 (MIP-1c) and CCLl2 (MIP-2) (73, 204). This impairs the DCs’ ability to migrate and provokes them to assume a tolerogenic phenotype capable of instructing Tns to express CD25, Foxp3 and IL-10.
Many maturation signals for DCs induce phosphorylation and proteolysis of the inhibitor of NFκBα (IκBα) by the inhibitor kinase-β (IKKβ), thereby releasing Rel-A (or p65; a subunit of NFκB) for nuclear translocation. In contrast, the non-canonical pathway operational during tolerogenic instruction activates NFκB-inducing kinase (NIK) and IKKα resulting in the formation of Rel-B dimers (69, 205). The inhibitory effect of GCs on the canonical NFκB pathway likely plays a key role in the conversion of DCs to itDCs. Accordingly, inhibition of NFκB or IKKβ by small molecule antagonists produces itDCs with the capacity to stimulate Foxp3+CD25+ aTregs that alleviate disease symptoms in EAE, heart allograft rejection, and intestinal bowel disease (IBD (206–211)).
Recent observations suggest that cellular metabolism also plays a role in DC immunogenicity. For example, treatment of human MoDCs with resveratrol induces tDCs that stimulate IL-10-secreting aTregs (212, 213). Resveratrol activates sirtuin 1 (SIRT-1) and PPARγ coactivator (PGC)-1α, which are involved in energy metabolism (214). Another pathway affecting metabolism and DC immunogenicity is represented by the serine/threonine kinase mammalian target of rapamycin (mTOR). This kinase forms signaling complexes that sense oxygen supply, free amino acids, ATP levels, growth factors, cytokines and cellular stress (215). Inhibition of mTOR by rapamycin, a macrolide from Streptomyces hygroscopicus, exerts immunosuppressive effects in humans and animals (216) and has shown efficacy in both clinical and preclinical settings of autoimmunity and inflammatory disease (217–225). Treatment of DCs with rapamycin stimulates Treg expansion in vivo and in vitro (226–230). We will further discuss this subject in section 4.2 below,
Genetically-induced tolerogenic DCs
Various genetic manipulations have been used, including gene knock-out, knockdown and transgenic over-expression of active or dominant negative mutants of molecules involved in DC maturation to enhance or inhibit DC tolerogenicity (176). Genetically induced tDCs can induce hyporesponsiveness and prolong allograft survival when transferred to transplant recipients, but a mechanistic role for tDC-induced Treg differentiation has only been established in a few cases. For instance, RelB deficient DCs induce CD40+ Tregs that suppressed delayed-type hypersensitivity (DTH) and experimental autoimmune myasthenia gravis (EAMG)(231–233). This provides yet another example for the importance of NFκB (and presumably CD40) activation in a DC’s decision on whether to exert immunogenic or Treg-inducing effects. Similarly, BMDCs that over-expressed dominant negative IKKβ were refractory to maturation and prone to induce Tregs that enhanced kidney allograft survival (234). Another approach to target NFκB-dependent effects in maturing DCs is to eliminate the expression of downstream target genes. Silencing of IL-12, CD80, CD86 and/or CD40 results in DCs that stimulate Treg differentiation and alleviates disease symptoms in collagen-induced arthritis (CIA) and EAMG (231, 235).
An alternative approach to silencing immunogenic molecules is the forced expression of tolerogenic factors. For example, treatment with IL-10-transduced DCs prevents the development of experimental asthma (EA) by boosting CD4+CD25+Foxp3+ IL-10 secreting Tregs that effectively transfer tolerance to naïve animals. IL-10 produced by recipient cells is required to establish this infectious tolerance demonstrating that Tregs require other supporting cell populations to suppress immune responses (236). Remarkably, transduction of DCs with ectopic Foxp3 also results in itDCs that stimulate CD4+Foxp3+ aTregs (237). The mechanism by which Foxp3 controls the tolerogenic potential of DCs remains unknown but likely involve pathways similar to those that induce Tregs (29).
4. How are tDCs inducing Tregs?
tDCs can induce Tregs by several different pathways that may act either alone or in combination. As discussed above (section 2.2), a relatively simple Treg promoting condition involves presentation of modest levels of a cognate antigen in the absence of signals 2 and 3, which is thought to be employed by iDCs but probably applies also to tDCs (Figure 2). In addition, tDCs can produce anti-inflammatory molecules that may be secreted, membrane bound, or both. Such signals may act directly on T cells and/or modify environmental conditions, such as the metabolic state of a tissue to fine-tune T cell differentiation.
4.1. Influence of the maturation status of DC in the induction of Tregs
Studies by several laboratories have shown that presentation of very low levels of antigen in the absence of other stimuli promotes Treg differentiation in vitro and in vivo (11, 12, 238–240). Another key factor for efficient differentiation of aTregs and function of nTregs is a milieu containing little or no inflammatory cytokines, such as IL-6 and IL-12, or costimulatory membrane receptors (CD80/86/40), which counteract the tolerogenic effect of iDCs and enhances effector differentiation of Tns (186, 241, 242). TCR signals in conjunction with costimulation precipitates a signaling cascade resulting in intracellular calcium (Ca2+) flux and the activation of the transcription factors nuclear factor of activated T cells (NFAT), activator protein 1 (AP-1) and NFκB that coordinate gene expression in nascent Teffs (243). While activated T cells that acquire effector functions express IL-2, IL-4, IL-17, T-bet, Edg3 and CD69 among others (244), differentiating Tregs present a different transcriptional signature (244–247) driven by NFAT, Foxp3 and runt-related transcription factor 1 (Runx-1 or myeloid leukemia factor, AML1 (240, 248, 249)). Indeed, the Treg transcriptome is enriched with gene products implicated in their suppressive function like IL-10, CD103, Killer cell lectin-like receptor subfamily G member 1 (Klrg1), Neuropilin 1 (Nrp1), GITR, ICOS (CD278), Fibrinogen-like protein 2 (Fgl2), Probable G-protein coupled receptor 83 (Gpr83) and CTLA-4. However, it is still unclear how exactly iDCs or tDCs skew the TCR signaling cascade in Tns to accomplish the subsequent selection of Treg-associated transcription factors. Furthermore, as discussed above, some mature and semi-mature DC expressing high levels of costimulatory molecules can also induce suppressive function on T cells (47). Thus, the magnitude of antigen presentation/costimulation or activating cytokines alone can not fully explain the function of all tDCs subsets.
4.1 Tolerogenic factors produced by tDC
The presence of IL-10 has been identified in numerous settings of tolerance (Tables 1–3). Indeed, secretion of IL-10 by tDCs is necessary for tolerance in a variety of models of Treg differentiation (174, 250, 251). IL-10 can initiate a powerful anti-inflammatory positive feedback loop because can both modify and be produced by leukocytes and structural cells within tissues (e.g. IECs, AECs and LSECs). Thus, when tDCs are induced by IL-10 in peripheral tissues they acquire the ability to secrete IL-10 themselves and migrate to lymphoid organs where tDC-derived IL-10 then contributes to Treg differentiation and proliferation. Having been instructed by tDCs, the activated Tregs enter the blood stream and home to the peripheral organ where antigen recognition triggers their production of even more IL-10 (252–255). In the presence of this cytokine proliferation, cytokine production and migratory capacities of effector T cells are impaired (181). Mechanistically, the Akdis and Blaser groups have shown that ligation of IL10R overrides costimulatory signaling via activation of SHP-1, which dephosphorylates the cytoplasmic tails of CD28, ICOS and CD2, thus inhibiting the recruitment of phosphatidylinositol 3-kinase (PI3K (188, 256–259)). Additionally, IL-10 signaling is also required for the stabilization of the suppressive phenotype of Tregs in the face of strong inflammatory signals (260).
TGFβ is unique among cytokines in that it can induce Foxp3 expression and aTreg differentiation in the absence of DCs (102). However, it is not clear whether and to what extent the tolerogenic capacity of tDCs relies on TGFβ production. Exploring this question is complicated by the fact that TGFβ effects are highly pleiotropic, and genetic mutants present complex phenotypes with multiple immune disorders and poor survival (192). A strong argument for the importance of TGFβ production by tDCs has come from animals with a DC-restricted deletion of the TGFβ-activating integrin, αvβ8. These mutant mice develop autoimmunity similar to animals in which DCs are chronically depleted or TGFβR signaling is dysfunctional in T cells, suggesting that DCs are important to ensure the bioavailability of active TGFβ (17–19, 261, 262). Antigen presentation by DCs in the presence of TGFβ results in the differentiation of Foxp3+ aTregs (65), which present a transcriptional signature that is similar to, but distinct from that of nTregs (192, 245, 263). A recent study has shown that activation of Foxo3a and Foxo1 by TGFβ signaling precedes Foxp3 expression in aTregs (264). However, we are only beginning to understand how Treg differentiation is controlled upstream of Foxp3.
Some DCs can synthesize RA, a metabolite of vitamin A that is generated by RALDH. Most intestinal DCs express at least one of the three isoforms of this enzyme, while most DCs in other lymphoid tissues express little or no RALDH (125). When T or B cells are activated in the presence of DC-derived RA, they are “imprinted” to express gut homing receptors (125, 265). In addition, exposure of activated CD4 T cells to RA promotes their differentiation into Foxp3+ aTregs (57, 87, 100, 101, 122, 266–268). RA binds the nuclear RA receptor α (RARα) and regulates the expression of Foxp3 and Smad3 in T cells (101, 269), but whether RARα is necessary for differentiation of Tregs in vivo is unclear. It has been suggested that RA is particularly relevant in aTreg differentiation in mucosal environments because the continuous exposure to commensal antigens requires a fine balance between tolerance and immunity (270). Recent observations suggest that some DCs in the skin also express RALDH and may produce RA for dermal Treg differentiation (71). More experimentation will be necessary to evaluate the exact role of RA-producing DCs for tolerance versus immunity in vivo.
tDCs also express several membrane receptors that may instruct antigen-specific Tns during their activation. Among these are the immunoglobulin-like transcript (ILT) receptors, which are found on tDCs that stimulate Treg differentiation (271–273). The proximal signaling cascade for ILTs is not known and the impact of ILT recognition by T cells is also not well established. However, multiple groups have shown an important role for these molecules in cancer, transplantation and autoimmunity by using animals deficient for the expression of ILTs, blocking antibodies and recombinant ILT3 (272, 274, 275). DCs also express programmed death-1 ligands (PD-Ls), PD-L1 and PD-L-2, which control T cell activation through engagement of PD-1 and (in case of PD-L1) CD80 (276). PD-1 is a critical determinant of “exhausted” T cells that arise during chronic viral infections, and it also contributes to Treg differentiation (276–279). The effects of PD-1 signaling resemble those of the IL10R by limiting PI3K activation and shutting down costimulatory signaling through SHP-1. However, PD-1 is not thought to be expressed by Tns, but is only upregulated during activation, so its role (if any) in the initial phase of Treg education is uncertain.
4.2. DCs and metabolism
Immune responses precipitate dramatic changes in the metabolic state of many cells. Changes in intra- and extracellular metabolites are becoming increasingly recognized as integral part of the ‘information content’ of tissues in which immune responses are induced. For example, differentiation of inflammatory cells and the induction of T cell memory in vivo can be modified by the dietary abundance of amino acid and fatty acid metabolism (280–282). DCs also modulate T cell differentiation by modifying metabolic parameters surrounding T cells. DCs can release IDO and heme oxygenase-1 (HO-1) to control the abundance of environmental tryptophan and carbon monoxide (CO), respectively. In the presence of extracellular IDO, T cells proliferation is compromised and aTregs differentiation is enhanced, although the precise molecular basis for this effect is unclear (171, 283–286). IDO expression by DCs is induced by IFNγ and TGFβ suggesting that this enzyme may represent a feedback mechanism by which DCs modulate their own immunogenicity during inflammation (287, 288). HO-1 degrades heme, thereby producing CO which inhibits DC immunogenicity (289). Indeed, HO-1 has a potent anti-inflammatory effect that may be mediated through Treg activity (290, 291), but the mechanisms are still incompletely understood.
The serine/threonine kinase mTOR plays a pivotal role in DC immunogenicity and the control Treg differentiation. Activation of TLR signaling stimulates mTOR and promotes sDC function (292, 293), whereas blockade of mTOR activity by hypoxia, amino acid starvation or rapamycin enhances Tregs (226, 294–297). mTOR is involved in the regulation of numerous essential cellular processes, such as cell cycle progression, protein synthesis, lipid metabolism and mitochondrial biogenesis (226, 298, 299). Treatment of DCs with the mTOR inhibitor rapamycin interferes with antigen processing and presentation, partly by regulating autophagy and production of MHC complexes, and also alters the response to cytokines, chemokines, growth factors and TLRs agonists (226). It has been reported that rapamycin-treated DCs do not directly induce aTreg differentiation (230), however, DC exposure to a combination of rapamycin and TGFβ effectively potentiates the capacity of DCs to induce aTreg differentiation (our unpublished results). It will be important to assess whether and how maturation and differentiation signal alter the metabolic state (e.g. oxidative versus glycolytic) of iDCs that give rise to either sDCs or tDCs, and how such metabolic changes may be linked to the phenotypic and functional characteristics of these versatile cells.
5. Concluding remarks
It is becoming increasingly clear that both mature and immature DC subsets can support immunological tolerance through Tregs and other mechanisms. A variety of environmental cues that may arise naturally or by pharmacological or experimental intervention can coerce iDCs to acquire a stable tolerogenic disposition that is preserved even in the face of concomitant maturation signals. These tDCs can induce or enhance the suppressive function of existing Tregs and convert activated Tns into aTregs. At present, we have only rudimentary knowledge of the rules that govern tolerogenic versus immunogenic functions of DCs, and the signals that tDCs use to transmit their suppressive message to T cells are also still incompletely understood. A better understanding of these issues may offer new opportunities for the treatment of autoimmunity, allograft rejection, allergy, asthma and various forms of hypersensitivity. Therapeutic applications of tDCs, either by cellular therapy or by targeting of endogenous DCs with novel drugs, could accomplish effects that elude traditional strategies for immune suppression. Specifically, while systemic immunosuppressants exert broadly paralyzing effects on immune cells, tDCs can induce tolerance to the specific antigens that elicit pathologic immune responses in a patient without compromising the immune defense against pathogens or tumors. While the prospect of clinical translation is exciting and seems almost within reach, substantial gaps in our knowledge remain to be filled before we will be able to exploit the full potential of tDC-based therapy.
Acknowledgments
We thank Dr. F. Vascotto for comments on the manuscript and Dr. D. Alvarez for help with figures. This work was supported by grants from the National Institutes of Health (AI069259, AI072252, AI078897, HL56949 and AR42689 to UVA) and through a grant from the Vertex-Harvard sponsored research program. RAM is a recipient of a NIH T32 Training Grant from the Joint Program of Transfusion Medicine at Harvard Medical School.
Bibliography
- 1.Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 2.Roncarolo M, Battaglia M. Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans. Nat Rev Immunol. 2007;7:585–598. doi: 10.1038/nri2138. [DOI] [PubMed] [Google Scholar]
- 3.Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6:295–307. doi: 10.1038/nri1806. [DOI] [PubMed] [Google Scholar]
- 4.Türeci O, Bian H, Nestle FO, Raddrizzani L, Rosinski JA, Tassis A, Hilton H, Walstead M, Sahin U, Hammer J. Cascades of transcriptional induction during dendritic cell maturation revealed by genome-wide expression analysis. The FASEB Journal. 2003;17:836–847. doi: 10.1096/fj.02-0724com. [DOI] [PubMed] [Google Scholar]
- 5.McIlroy D, Tanguy-Royer S, Le Meur N, Guisle I, Royer P-J, Léger J, Meflah K, Grégoire M. Profiling dendritic cell maturation with dedicated microarrays. J Leukoc Biol. 2005;78:794–803. doi: 10.1189/jlb.0105029. [DOI] [PubMed] [Google Scholar]
- 6.Banchereau J, Briere F, Caux C, Davoust J. Immunobiology of dendritic cells. Annu Rev Immunol. 2000 doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 7.Alvarez D, Vollmann EH, von Andrian UH. Mechanisms and consequences of dendritic cell migration. Immunity. 2008;29:325–342. doi: 10.1016/j.immuni.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lambrecht BN, Hammad H. Biology of lung dendritic cells at the origin of asthma. Immunity. 2009;31:412–424. doi: 10.1016/j.immuni.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 9.Ostroukhova M, Seguin-Devaux C, Oriss TB, Dixon-McCarthy B, Yang L, Ameredes BT, Corcoran TE, Ray A. Tolerance induced by inhaled antigen involves CD4(+) T cells expressing membrane-bound TGF-beta and FOXP3. J Clin Invest. 2004;114:28–38. doi: 10.1172/JCI20509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsuji NM, Kosaka A. Oral tolerance: intestinal homeostasis and antigen-specific regulatory T cells. Trends Immunol. 2008;29:532–540. doi: 10.1016/j.it.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6:1219–1227. doi: 10.1038/ni1265. [DOI] [PubMed] [Google Scholar]
- 12.Apostolou I, von Boehmer H. In Vivo Instruction of Suppressor Commitment in Naive T Cells. J Exp Med. 2004;199:1401–1408. doi: 10.1084/jem.20040249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vermaelen KY, Carro-Muino I, Lambrecht BN, Pauwels RA. Specific migratory dendritic cells rapidly transport antigen from the airways to the thoracic lymph nodes. J Exp Med. 2001;193:51–60. doi: 10.1084/jem.193.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Heer HJ, Hammad H, Soullié T, Hijdra D, Vos N, Willart MAM, Hoogsteden HC, Lambrecht BN. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J Exp Med. 2004;200:89–98. doi: 10.1084/jem.20040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bar-On L, Jung S. Defining dendritic cells by conditional and constitutive cell ablation. Immunol Rev. 2010;234:76–89. doi: 10.1111/j.0105-2896.2009.00875.x. [DOI] [PubMed] [Google Scholar]
- 16.Darrasse-Jeze G, Deroubaix S, Mouquet H, Victora GD, Eisenreich T, Yao K-H, Masilamani RF, Dustin ML, Rudensky A, Liu K, Nussenzweig MC. Feedback control of regulatory T cell homeostasis by dendritic cells in vivo. Journal of Experimental Medicine. 2009:1–14. doi: 10.1084/jem.20090746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Birnberg T, Bar-On L, Sapoznikov A, Caton ML, Cervantes-Barragán L, Makia D, Krauthgamer R, Brenner O, Ludewig B, Brockschnieder D, Riethmacher D, Reizis B, Jung S. Lack of conventional dendritic cells is compatible with normal development and T cell homeostasis, but causes myeloid proliferative syndrome. Immunity. 2008;29:986–997. doi: 10.1016/j.immuni.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 18.Ohnmacht C, Pullner A, King SBS, Drexler I, Meier S, Brocker T, Voehringer D. Constitutive ablation of dendritic cells breaks self-tolerance of CD4 T cells and results in spontaneous fatal autoimmunity. J Exp Med. 2009;206:549–559. doi: 10.1084/jem.20082394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Travis MA, Reizis B, Melton AC, Masteller E, Tang Q, Proctor JM, Wang Y, Bernstein X, Huang X, Reichardt LF, Bluestone JA, Sheppard D. Loss of integrin alpha(v)beta8 on dendritic cells causes autoimmunity and colitis in mice. Nature. 2007;449:361–365. doi: 10.1038/nature06110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laouar Y, Town T, Jeng D, Tran E, Wan Y, Kuchroo VK, Flavell RA. TGF-beta signaling in dendritic cells is a prerequisite for the control of autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2008;105:10865–10870. doi: 10.1073/pnas.0805058105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lund J, Hsing L, Pham T, Rudensky A. Coordination of Early Protective Immunity to Viral Infection by Regulatory T Cells. Science. 2008 doi: 10.1126/science.1155209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu K, Victora G, Schwickert T, Guermonprez P, Meredith M, Yao K, Chu F, Randolph G, Rudensky A, Nussenzweig M. In Vivo Analysis of Dendritic Cell Development and Homeostasis. Science. 2009 doi: 10.1126/science.1170540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vremec D, Zorbas M, Scollay R, Saunders DJ, Ardavin CF, Wu L, Shortman K. The surface phenotype of dendritic cells purified from mouse thymus and spleen: investigation of the CD8 expression by a subpopulation of dendritic cells. J Exp Med. 1992;176:47–58. doi: 10.1084/jem.176.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paust S, Cantor H. Regulatory T cells and autoimmune disease. Immunol Rev. 2005;204:195–207. doi: 10.1111/j.0105-2896.2005.00247.x. [DOI] [PubMed] [Google Scholar]
- 25.Bluestone J, Abbas A. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3:253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 26.Mold JE, Michaelsson J, Burt TD, Muench MO, Beckerman KP, Busch MP, Lee T-H, Nixon DF, Mccune JM. Maternal Alloantigens Promote the Development of Tolerogenic Fetal Regulatory T Cells in Utero. Science. 2008;322:1562–1565. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lio C-WJ, Hsieh C-S. A two-step process for thymic regulatory T cell development. Immunity. 2008;28:100–111. doi: 10.1016/j.immuni.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Min B, Thornton A, Caucheteux SM, Younes S-A, Oh K, Hu-Li J, Paul WE. Gut flora antigens are not important in the maintenance of regulatory T cell heterogeneity and homeostasis. Eur J Immunol. 2007;37:1916–1923. doi: 10.1002/eji.200737236. [DOI] [PubMed] [Google Scholar]
- 29.Kim JM, Rudensky A. The role of the transcription factor Foxp3 in the development of regulatory T cells. Immunol Rev. 2006;212:86–98. doi: 10.1111/j.0105-2896.2006.00426.x. [DOI] [PubMed] [Google Scholar]
- 30.Ribot J, Romagnoli P, van Meerwijk JPM. Agonist ligands expressed by thymic epithelium enhance positive selection of regulatory T lymphocytes from precursors with a normally diverse TCR repertoire. J Immunol. 2006;177:1101–1107. doi: 10.4049/jimmunol.177.2.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bensinger S, Bandeira A, Jordan M, Caton A, Laufer T. Major histocompatibility complex class II-positive cortical epithelium mediates the selection of CD4(+)25(+) immunoregulatory T cells. J Exp Med. 2001;194:427–438. doi: 10.1084/jem.194.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vigouroux S, Yvon E, Biagi E, Brenner MK. Antigen-induced regulatory T cells. Blood. 2004;104:26–33. doi: 10.1182/blood-2004-01-0182. [DOI] [PubMed] [Google Scholar]
- 33.Bonasio R, Scimone ML, Schaerli P, Grabie N, Lichtman AH, von Andrian UH. Clonal deletion of thymocytes by circulating dendritic cells homing to the thymus. Nat Immunol. 2006;7:1092–1100. doi: 10.1038/ni1385. [DOI] [PubMed] [Google Scholar]
- 34.Tang Q, Bluestone JA. The Foxp3+ regulatory T cell: a jack of all trades, master of regulation. Nat Immunol. 2008;9:239–244. doi: 10.1038/ni1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vignali D, Collison L, Workman C. How regulatory T cells work. Nat Rev Immunol. 2008;8:523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ueno H, Klechevsky E, Morita R, Aspord C, Cao T, Matsui T, Di Pucchio T, Connolly J, Fay JW, Pascual V, Palucka AK, Banchereau J. Dendritic cell subsets in health and disease. Immunological Reviews. 2007;219:118–142. doi: 10.1111/j.1600-065X.2007.00551.x. [DOI] [PubMed] [Google Scholar]
- 37.Barton GM, Medzhitov R. Toll-like receptor signaling pathways. Science. 2003;300:1524–1525. doi: 10.1126/science.1085536. [DOI] [PubMed] [Google Scholar]
- 38.Re F, Strominger JL. Heterogeneity of TLR-induced responses in dendritic cells: from innate to adaptive immunity. Immunobiology. 2004;209:191–198. doi: 10.1016/j.imbio.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 39.Franchi L, Muñoz-Planillo R, Reimer T, Eigenbrod T, Núñez G. Inflammasomes as microbial sensors. Eur J Immunol. 2010;40:611–615. doi: 10.1002/eji.200940180. [DOI] [PubMed] [Google Scholar]
- 40.Pétrilli V, Dostert C, Muruve DA, Tschopp J. The inflammasome: a danger sensing complex triggering innate immunity. Curr Opin Immunol. 2007;19:615–622. doi: 10.1016/j.coi.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 41.Sims JE, Smith DE. The IL-1 family: regulators of immunity. Nature Reviews Immunology. 2010;10:89–102. doi: 10.1038/nri2691. [DOI] [PubMed] [Google Scholar]
- 42.Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3:745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 43.Elgueta R, Benson MJ, de Vries VC, Wasiuk A, Guo Y, Noelle RJ. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev. 2009;229:152–172. doi: 10.1111/j.1600-065X.2009.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meylan E, Tschopp J. Toll-like receptors and RNA helicases: two parallel ways to trigger antiviral responses. Mol Cell. 2006;22:561–569. doi: 10.1016/j.molcel.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 45.Salter RD, Watkins SC. Dendritic cell altered states: what role for calcium? Immunol Rev. 2009;231:278–288. doi: 10.1111/j.1600-065X.2009.00806.x. [DOI] [PubMed] [Google Scholar]
- 46.Cronin SJF, Penninger JM. From T-cell activation signals to signaling control of anti-cancer immunity. Immunol Rev. 2007;220:151–168. doi: 10.1111/j.1600-065X.2007.00570.x. [DOI] [PubMed] [Google Scholar]
- 47.Reis E, Sousa C. Dendritic cells in a mature age. Nature Reviews Immunology. 2006;6:476–483. doi: 10.1038/nri1845. [DOI] [PubMed] [Google Scholar]
- 48.Tisch R. Immunogenic versus tolerogenic dendritic cells: a matter of maturation. Int Rev Immunol. 2010;29:111–118. doi: 10.3109/08830181003602515. [DOI] [PubMed] [Google Scholar]
- 49.Worbs T, Bode U, Yan S, Hoffmann MW, Hintzen G, Bernhardt G, Förster R, Pabst O. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. The Journal of experimental medicine. 2006;203:519–527. doi: 10.1084/jem.20052016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jang MH, Sougawa N, Tanaka T, Hirata T, Hiroi T, Tohya K, Guo Z, Umemoto E, Ebisuno Y, Yang BG, Seoh JY, Lipp M, Kiyono H, Miyasaka M. CCR7 Is Critically Important for Migration of Dendritic Cells in Intestinal Lamina Propria to Mesenteric Lymph Nodes. J Immunol. 2006;176:803–810. doi: 10.4049/jimmunol.176.2.803. [DOI] [PubMed] [Google Scholar]
- 51.Hintzen G, Ohl L, del Rio M-L, Rodriguez-Barbosa J-I, Pabst O, Kocks JR, Krege J, Hardtke S, Förster R. Induction of tolerance to innocuous inhaled antigen relies on a CCR7-dependent dendritic cell-mediated antigen transport to the bronchial lymph node. J Immunol. 2006;177:7346–7354. doi: 10.4049/jimmunol.177.10.7346. [DOI] [PubMed] [Google Scholar]
- 52.Ohl L, Mohaupt M, Czeloth N, Hintzen G, Kiafard Z, Zwirner J, Blankenstein T, Henning G, Forster R. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity. 2004;21:279–288. doi: 10.1016/j.immuni.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 53.Martin-Fontecha A, Sebastiani S, Hopken UE, Uguccioni M, Lipp M, Lanzavecchia A, Sallusto F. Regulation of dendritic cell migration to the draining lymph node: impact on T lymphocyte traffic and priming. J Exp Med. 2003;198:615–621. doi: 10.1084/jem.20030448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Förster R, Davalos-Misslitz A, Rot A. CCR7 and its ligands: balancing immunity and tolerance. Nat Rev Immunol. 2008;8:362–371. doi: 10.1038/nri2297. [DOI] [PubMed] [Google Scholar]
- 55.Langenkamp A, Casorati G, Garavaglia C, Dellabona P, Lanzavecchia A, Sallusto F. T cell priming by dendritic cells: thresholds for proliferation, differentiation and death and intraclonal functional diversification. Eur J Immunol. 2002;32:2046–2054. doi: 10.1002/1521-4141(200207)32:7<2046::AID-IMMU2046>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 56.Langenkamp A, Messi M, Lanzavecchia A, Sallusto F. Kinetics of dendritic cell activation: impact on priming of TH1, TH2 and nonpolarized T cells. Nat Immunol. 2000;1:311–316. doi: 10.1038/79758. [DOI] [PubMed] [Google Scholar]
- 57.Siddiqui KRR, Powrie F. CD103+ GALT DCs promote Foxp3+ regulatory T cells. Mucosal immunology. 2008;1(Suppl 1):S34–38. doi: 10.1038/mi.2008.43. [DOI] [PubMed] [Google Scholar]
- 58.Shortman K, Heath WR. The CD8+ dendritic cell subset. Immunol Rev. 2010;234:18–31. doi: 10.1111/j.0105-2896.2009.00870.x. [DOI] [PubMed] [Google Scholar]
- 59.Swiecki M, Colonna M. Unraveling the functions of plasmacytoid dendritic cells during viral infections, autoimmunity, and tolerance. Immunol Rev. 2010;234:142–162. doi: 10.1111/j.0105-2896.2009.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pulendran B, Tang H, Denning T. Division of labor, plasticity, and crosstalk between dendritic cell subsets. Current Opinion in Immunology. 2008;20:61–67. doi: 10.1016/j.coi.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Coquerelle C, Moser M. DC subsets in positive and negative regulation of immunity. Immunol Rev. 2010;234:317–334. doi: 10.1111/j.0105-2896.2009.00887.x. [DOI] [PubMed] [Google Scholar]
- 62.Milling S, Yrlid U, Cerovic V, MacPherson G. Subsets of migrating intestinal dendritic cells. Immunol Rev. 2010;234:259–267. doi: 10.1111/j.0105-2896.2009.00866.x. [DOI] [PubMed] [Google Scholar]
- 63.Steinman RM, Idoyaga J. Features of the dendritic cell lineage. Immunol Rev. 2010;234:5–17. doi: 10.1111/j.0105-2896.2009.00888.x. [DOI] [PubMed] [Google Scholar]
- 64.Liu K, Nussenzweig MC. Origin and development of dendritic cells. Immunol Rev. 2010;234:45–54. doi: 10.1111/j.0105-2896.2009.00879.x. [DOI] [PubMed] [Google Scholar]
- 65.Yamazaki S, Dudziak D, Heidkamp GF, Fiorese C, Bonito AJ, Inaba K, Nussenzweig MC, Steinman RM. CD8+ CD205+ splenic dendritic cells are specialized to induce Foxp3+ regulatory T cells. Journal of immunology (Baltimore, Md: 1950) 2008;181:6923–6933. doi: 10.4049/jimmunol.181.10.6923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen W, Jin W, Hardegen N, Lei K, Li L, Marinos N, McGrady G, Wahl S. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goubier A, Dubois B, Gheit H, Joubert G, Villard-Truc F, Asselin-Paturel C, Trinchieri G, Kaiserlian D. Plasmacytoid dendritic cells mediate oral tolerance. Immunity. 2008;29:464–475. doi: 10.1016/j.immuni.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ochando JC, Homma C, Yang Y, Hidalgo A, Garin A, Tacke F, Angeli V, Li Y, Boros P, Ding Y, Jessberger R, Trinchieri G, Lira SA, Randolph GJ, Bromberg JS. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nature Immunology. 2006;7:652–662. doi: 10.1038/ni1333. [DOI] [PubMed] [Google Scholar]
- 69.Puccetti P, Grohmann U. IDO and regulatory T cells: a role for reverse signalling and non-canonical NF-kappaB activation. Nat Rev Immunol. 2007;7:817–823. doi: 10.1038/nri2163. [DOI] [PubMed] [Google Scholar]
- 70.Matteoli G, Mazzini E, Iliev ID, Mileti E, Fallarino F, Puccetti P, Chieppa M, Rescigno M. Gut CD103+ dendritic cells express indoleamine 2,3-dioxygenase which influences T regulatory/T effector cell balance and oral tolerance induction. Gut. 2010;59:595–604. doi: 10.1136/gut.2009.185108. [DOI] [PubMed] [Google Scholar]
- 71.Guilliams M, Crozat K, Henri S, Tamoutounour S, Grenot P, Devilard E, De Bovis B, Alexopoulou L, Dalod M, Malissen B. Skin-draining lymph nodes contain dermis-derived CD103− dendritic cells that constitutively produce retinoic acid and induce Foxp3+ regulatory T cells. Blood. 2010;115:1958–1968. doi: 10.1182/blood-2009-09-245274. [DOI] [PubMed] [Google Scholar]
- 72.Belladonna ML, Volpi C, Bianchi R, Vacca C, Orabona C, Pallotta MT, Boon L, Gizzi S, Fioretti MC, Grohmann U, Puccetti P. Cutting edge: Autocrine TGF-beta sustains default tolerogenesis by IDO-competent dendritic cells. Journal of immunology (Baltimore, Md: 1950) 2008;181:5194–5198. doi: 10.4049/jimmunol.181.8.5194. [DOI] [PubMed] [Google Scholar]
- 73.Grohmann U, Volpi C, Fallarino F, Bozza S, Bianchi R, Vacca C, Orabona C, Belladonna ML, Ayroldi E, Nocentini G, Boon L, Bistoni F, Fioretti MC, Romani L, Riccardi C, Puccetti P. Reverse signaling through GITR ligand enables dexamethasone to activate IDO in allergy. Nat Med. 2007;13:579–586. doi: 10.1038/nm1563. [DOI] [PubMed] [Google Scholar]
- 74.Liston A, Nutsch KM, Farr AG, Lund JM, Rasmussen JP, Koni PA, Rudensky AY. Differentiation of regulatory Foxp3+ T cells in the thymic cortex. Proc Natl Acad Sci USA. 2008;105:11903–11908. doi: 10.1073/pnas.0801506105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Aschenbrenner K, D’Cruz LM, Vollmann EH, Hinterberger M, Emmerich J, Swee LK, Rolink A, Klein L. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nature Immunology. 2007;8:351–358. doi: 10.1038/ni1444. [DOI] [PubMed] [Google Scholar]
- 76.Proietto AI, van Dommelen S, Zhou P, Rizzitelli A, D’Amico A, Steptoe RJ, Naik SH, Lahoud MH, Liu Y, Zheng P, Shortman K, Wu L. Dendritic cells in the thymus contribute to T-regulatory cell induction. Proc Natl Acad Sci USA. 2008;105:19869–19874. doi: 10.1073/pnas.0810268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Proietto AI, van Dommelen S, Wu L. The impact of circulating dendritic cells on the development and differentiation of thymocytes. Immunol Cell Biol. 2009;87:39–45. doi: 10.1038/icb.2008.86. [DOI] [PubMed] [Google Scholar]
- 78.Martín-Gayo E, Sierra-Filardi E, Corbí AL, Toribio ML. Plasmacytoid dendritic cells resident in human thymus drive natural Treg cell development. Blood. 2010 doi: 10.1182/blood-2009-10-248260. [DOI] [PubMed] [Google Scholar]
- 79.Atibalentja DF, Byersdorfer CA, Unanue ER. Thymus-blood protein interactions are highly effective in negative selection and regulatory T cell induction. J Immunol. 2009;183:7909–7918. doi: 10.4049/jimmunol.0902632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang J, Xing F. Human TSLP-educated DCs. Cell Mol Immunol. 2008;5:99–106. doi: 10.1038/cmi.2008.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Watanabe N, Wang Y-H, Lee HK, Ito T, Wang Y-H, Cao W, Liu Y-J. Hassall’s corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature. 2005;436:1181–1185. doi: 10.1038/nature03886. [DOI] [PubMed] [Google Scholar]
- 82.Liu Y-J, Soumelis V, Watanabe N, Ito T, Wang Y-H, Malefyt RdW, Omori M, Zhou B, Ziegler SF. TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu Rev Immunol. 2007;25:193–219. doi: 10.1146/annurev.immunol.25.022106.141718. [DOI] [PubMed] [Google Scholar]
- 83.Mazzucchelli R, Hixon J, Spolski R, Chen X, Li W, Hall V, Willette-Brown J, Hurwitz A, Leonard W, Durum S. Development of regulatory T cells requires IL-7R{alpha} stimulation by IL-7 or TSLP. Blood. 2008 doi: 10.1182/blood-2008-02-137414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Besin G, Gaudreau S, Ménard M, Guindi C, Dupuis G, Amrani A. Thymic stromal lymphopoietin and thymic stromal lymphopoietin-conditioned dendritic cells induce regulatory T-cell differentiation and protection of NOD mice against diabetes. Diabetes. 2008;57:2107–2117. doi: 10.2337/db08-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ziegler SF, Artis D. Sensing the outside world: TSLP regulates barrier immunity. Nature Immunology. 2010;11:289–293. doi: 10.1038/ni.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Coombes JL, Powrie F. Dendritic cells in intestinal immune regulation. Nat Rev Immunol. 2008;8:435–446. doi: 10.1038/nri2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Belkaid Y, Oldenhove G. Tuning microenvironments: induction of regulatory T cells by dendritic cells. Immunity. 2008;29:362–371. doi: 10.1016/j.immuni.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chieppa M, Rescigno M, Huang AYC, Germain RN. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J Exp Med. 2006;203:2841–2852. doi: 10.1084/jem.20061884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang X, Izikson L, Liu L, Weiner HL. Activation of CD25(+)CD4(+) regulatory T cells by oral antigen administration. J Immunol. 2001;167:4245–4253. doi: 10.4049/jimmunol.167.8.4245. [DOI] [PubMed] [Google Scholar]
- 90.Miyamoto K, Kingsley CI, Zhang X, Jabs C, Izikson L, Sobel RA, Weiner HL, Kuchroo VK, Sharpe AH. The ICOS molecule plays a crucial role in the development of mucosal tolerance. J Immunol. 2005;175:7341–7347. doi: 10.4049/jimmunol.175.11.7341. [DOI] [PubMed] [Google Scholar]
- 91.Hultkrantz S, Ostman S, Telemo E. Induction of antigen-specific regulatory T cells in the liver-draining celiac lymph node following oral antigen administration. Immunology. 2005;116:362–372. doi: 10.1111/j.1365-2567.2005.02236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Coombes J, Siddiqui K, Arancibia-Carcamo C, Hall J, Sun C, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-{beta} and retinoic acid dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sun C, Hall J, Blank R, Bouladoux N, Oukka M, Mora J, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nilsen EM, Johansen FE, Jahnsen FL, Lundin KE, Scholz T, Brandtzaeg P, Haraldsen G. Cytokine profiles of cultured microvascular endothelial cells from the human intestine. Gut. 1998;42:635–642. doi: 10.1136/gut.42.5.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Iwata M. Retinoic acid production by intestinal dendritic cells and its role in T-cell trafficking. Seminars in Immunology. 2009;21:8–13. doi: 10.1016/j.smim.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 96.Grider JR, Rivier JR. Vasoactive intestinal peptide (VIP) as transmitter of inhibitory motor neurons of the gut: evidence from the use of selective VIP antagonists and VIP antiserum. J Pharmacol Exp Ther. 1990;253:738–742. [PubMed] [Google Scholar]
- 97.Taylor BC, Zaph C, Troy AE, Du Y, Guild KJ, Comeau MR, Artis D. TSLP regulates intestinal immunity and inflammation in mouse models of helminth infection and colitis. Journal of Experimental Medicine. 2009;206:655–667. doi: 10.1084/jem.20081499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Göke M, Kanai M, Podolsky DK. Intestinal fibroblasts regulate intestinal epithelial cell proliferation via hepatocyte growth factor. Am J Physiol. 1998;274:G809–818. doi: 10.1152/ajpgi.1998.274.5.G809. [DOI] [PubMed] [Google Scholar]
- 99.Robinson PW, Green SJ, Carter C, Coadwell J, Kilshaw PJ. Studies on transcriptional regulation of the mucosal T-cell integrin alphaEbeta7 (CD103) Immunology. 2001;103:146–154. doi: 10.1046/j.1365-2567.2001.01232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mucida D, Pino-Lagos K, Kim G, Nowak E, Benson MJ, Kronenberg M, Noelle RJ, Cheroutre H. Retinoic acid can directly promote TGF-beta-mediated Foxp3(+) Treg cell conversion of naive T cells. Immunity. 2009;30:471–472. doi: 10.1016/j.immuni.2009.03.008. author reply 472–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nolting J, Daniel C, Reuter S, Stuelten C, Li P, Sucov H, Kim B, Letterio J, Kretschmer K, Kim H, von Boehmer H. Retinoic acid can enhance conversion of naive into regulatory T cells independently of secreted cytokines. J Exp Med. 2009 doi: 10.1084/jem.20090639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dignass AU, Podolsky DK. Cytokine modulation of intestinal epithelial cell restitution: central role of transforming growth factor beta. Gastroenterology. 1993;105:1323–1332. doi: 10.1016/0016-5085(93)90136-z. [DOI] [PubMed] [Google Scholar]
- 104.Iliev ID, Spadoni I, Mileti E, Matteoli G, Sonzogni A, Sampietro GM, Foschi D, Caprioli F, Viale G, Rescigno M. Human intestinal epithelial cells promote the differentiation of tolerogenic dendritic cells. Gut. 2009;58:1481–1489. doi: 10.1136/gut.2008.175166. [DOI] [PubMed] [Google Scholar]
- 105.Shale M, Ghosh S. How intestinal epithelial cells tolerise dendritic cells and its relevance to inflammatory bowel disease. Gut. 2009;58:1291–1299. doi: 10.1136/gut.2006.098475. [DOI] [PubMed] [Google Scholar]
- 106.Iliev ID, Mileti E, Matteoli G, Chieppa M, Rescigno M. Intestinal epithelial cells promote colitis-protective regulatory T-cell differentiation through dendritic cell conditioning. Mucosal immunology. 2009;2:340–350. doi: 10.1038/mi.2009.13. [DOI] [PubMed] [Google Scholar]
- 107.Rimoldi M, Chieppa M, Salucci V, Avogadri F, Sonzogni A, Sampietro GM, Nespoli A, Viale G, Allavena P, Rescigno M. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat Immunol. 2005;6:507–514. doi: 10.1038/ni1192. [DOI] [PubMed] [Google Scholar]
- 108.Jakubzick C, Bogunovic M, Bonito AJ, Kuan EL, Merad M, Randolph GJ. Lymph-migrating, tissue-derived dendritic cells are minor constituents within steady-state lymph nodes. The Journal of experimental medicine. 2008;205:2839–2850. doi: 10.1084/jem.20081430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bakocević N, Worbs T, Davalos-Misslitz A, Förster R. T cell-dendritic cell interaction dynamics during the induction of respiratory tolerance and immunity. J Immunol. 2010;184:1317–1327. doi: 10.4049/jimmunol.0902277. [DOI] [PubMed] [Google Scholar]
- 110.Curotto de Lafaille MA, Kutchukhidze N, Shen S, Ding Y, Yee H, Lafaille JJ. Adaptive Foxp3+ regulatory T cell-dependent and -independent control of allergic inflammation. Immunity. 2008;29:114–126. doi: 10.1016/j.immuni.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 111.Lloyd CM, Hawrylowicz CM. Regulatory T cells in asthma. Immunity. 2009;31:438–449. doi: 10.1016/j.immuni.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li Q, Guo Z, Xu X, Xia S, Cao X. Pulmonary stromal cells induce the generation of regulatory DC attenuating T-cell-mediated lung inflammation. Eur J Immunol. 2008;38:2751–2761. doi: 10.1002/eji.200838542. [DOI] [PubMed] [Google Scholar]
- 113.Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat Med. 2009;15:410–416. doi: 10.1038/nm.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Farquhar CA, Paterson AM, Cobbold SP, Rueda HG, Fairchild PJ, Yates SF, Adams E, Saunders NJ, Waldmann H, Nolan KF. Tolerogenicity is not an absolute property of a dendritic cell. Eur J Immunol. 2010 doi: 10.1002/eji.200939974. [DOI] [PubMed] [Google Scholar]
- 115.Unger WWJ, Laban S, Kleijwegt FS, van der Slik AR, Roep BO. Induction of Treg by monocyte-derived DC modulated by vitamin D3 or dexamethasone: differential role for PD-L1. Eur J Immunol. 2009;39:3147–3159. doi: 10.1002/eji.200839103. [DOI] [PubMed] [Google Scholar]
- 116.Anderson AE, Swan DJ, Sayers BL, Harry RA, Patterson AM, von Delwig A, Robinson JH, Isaacs JD, Hilkens CMU. LPS activation is required for migratory activity and antigen presentation by tolerogenic dendritic cells. Journal of Leukocyte Biology. 2009;85:243–250. doi: 10.1189/jlb.0608374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Penna G, Giarratana N, Amuchastegui S, Mariani R, Daniel KC, Adorini L. Manipulating dendritic cells to induce regulatory T cells. Microbes Infect. 2005;7:1033–1039. doi: 10.1016/j.micinf.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 118.Ureta G, Osorio F, Morales J, Rosemblatt M, Bono MR, Fierro JA. Generation of dendritic cells with regulatory properties. Transplant Proc. 2007;39:633–637. doi: 10.1016/j.transproceed.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 119.Anderson AE, Sayers BL, Haniffa MA, Swan DJ, Diboll J, Wang X-N, Isaacs JD, Hilkens CMU. Differential regulation of naïve and memory CD4+ T cells by alternatively activated dendritic cells. Journal of leukocyte biology. 2008;84:124–133. doi: 10.1189/jlb.1107744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Penna G, Amuchastegui S, Laverny G, Adorini L. Vitamin D receptor agonists in the treatment of autoimmune diseases: selective targeting of myeloid but not plasmacytoid dendritic cells. J Bone Miner Res. 2007;22(Suppl 2):V69–73. doi: 10.1359/jbmr.07s217. [DOI] [PubMed] [Google Scholar]
- 121.Adorini L, Penna G. Induction of tolerogenic dendritic cells by vitamin D receptor agonists. Handb Exp Pharmacol. 2009:251–273. doi: 10.1007/978-3-540-71029-5_12. [DOI] [PubMed] [Google Scholar]
- 122.Mora J, Iwata M, Von Andrian U. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol. 2008 doi: 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Griffin M, Lutz W, Phan V, Bachman L, McKean D, Kumar R. Dendritic cell modulation by 1alpha,25 dihydroxyvitamin D3 and its analogs: a vitamin D receptor-dependent pathway that promotes a persistent state of immaturity in vitro and in vivo. Proc Natl Acad Sci U S A. 2001;98:6800–6805. doi: 10.1073/pnas.121172198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Széles L, Keresztes G, Töröcsik D, Balajthy Z, Krenács L, Póliska S, Steinmeyer A, Zuegel U, Pruenster M, Rot A, Nagy L. 1,25-dihydroxyvitamin D3 is an autonomous regulator of the transcriptional changes leading to a tolerogenic dendritic cell phenotype. Journal of immunology (Baltimore, Md: 1950) 2009;182:2074–2083. doi: 10.4049/jimmunol.0803345. [DOI] [PubMed] [Google Scholar]
- 125.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 126.Tiegs G, Lohse AW. Immune tolerance: what is unique about the liver. J Autoimmun. 2010;34:1–6. doi: 10.1016/j.jaut.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 127.Crispe IN, Giannandrea M, Klein I, John B, Sampson B, Wuensch S. Cellular and molecular mechanisms of liver tolerance. Immunol Rev. 2006;213:101–118. doi: 10.1111/j.1600-065X.2006.00435.x. [DOI] [PubMed] [Google Scholar]
- 128.Lüth S, Huber S, Schramm C, Buch T, Zander S, Stadelmann C, Brück W, Wraith DC, Herkel J, Lohse AW. Ectopic expression of neural autoantigen in mouse liver suppresses experimental autoimmune neuroinflammation by inducing antigen-specific Tregs. J Clin Invest. 2008:8. doi: 10.1172/JCI32132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Cao O, Dobrzynski E, Wang L, Nayak S, Mingle B, Terhorst C, Herzog RW. Induction and role of regulatory CD4+CD25+ T cells in tolerance to the transgene product following hepatic in vivo gene transfer. Blood. 2007;110:1132–1140. doi: 10.1182/blood-2007-02-073304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Martino AT, Nayak S, Hoffman BE, Cooper M, Liao G, Markusic DM, Byrne BJ, Terhorst C, Herzog RW. Tolerance induction to cytoplasmic beta-galactosidase by hepatic AAV gene transfer: implications for antigen presentation and immunotoxicity. PLoS ONE. 2009;4:e6376. doi: 10.1371/journal.pone.0006376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Friedman SL, Rockey DC, McGuire RF, Maher JJ, Boyles JK, Yamasaki G. Isolated hepatic lipocytes and Kupffer cells from normal human liver: morphological and functional characteristics in primary culture. Hepatology. 1992;15:234–243. doi: 10.1002/hep.1840150211. [DOI] [PubMed] [Google Scholar]
- 132.Schildberg FA, Hegenbarth SI, Schumak B, Scholz K, Limmer A, Knolle PA. Liver sinusoidal endothelial cells veto CD8 T cell activation by antigen-presenting dendritic cells. Eur J Immunol. 2008;38:957–967. doi: 10.1002/eji.200738060. [DOI] [PubMed] [Google Scholar]
- 133.Xia S, Guo Z, Xu X, Yi H, Wang Q, Cao X. Hepatic microenvironment programs hematopoietic progenitor differentiation into regulatory dendritic cells maintaining liver tolerance. Blood. 2008 doi: 10.1182/blood-2008-05-159921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bamboat ZM, Stableford JA, Plitas G, Burt BM, Nguyen HM, Welles AP, Gonen M, Young JW, DeMatteo RP. Human liver dendritic cells promote T cell hyporesponsiveness. J Immunol. 2009;182:1901–1911. doi: 10.4049/jimmunol.0803404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Connolly MK, Bedrosian AS, Mallen-St Clair J, Mitchell AP, Ibrahim J, Stroud A, Pachter HL, Bar-Sagi D, Frey AB, Miller G. In liver fibrosis, dendritic cells govern hepatic inflammation in mice via TNF-alpha. J Clin Invest. 2009;119:3213–3225. doi: 10.1172/JCI37581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bamboat ZM, Ocuin LM, Balachandran VP, Obaid H, Plitas G, DeMatteo RP. Conventional DCs reduce liver ischemia/reperfusion injury in mice via IL-10 secretion. J Clin Invest. 2010;120:559–569. doi: 10.1172/JCI40008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Goddard S, Youster J, Morgan E, Adams DH. Interleukin-10 secretion differentiates dendritic cells from human liver and skin. Am J Pathol. 2004;164:511–519. doi: 10.1016/S0002-9440(10)63141-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Belkaid Y. Regulatory T cells and infection: a dangerous necessity. Nature Reviews Immunology. 2007;7:875–888. doi: 10.1038/nri2189. [DOI] [PubMed] [Google Scholar]
- 139.Mills KHG, McGuirk P. Antigen-specific regulatory T cells--their induction and role in infection. Semin Immunol. 2004;16:107–117. doi: 10.1016/j.smim.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 140.Grainger JR, Hall JA, Bouladoux N, Oldenhove G, Belkaid Y. Microbe-dendritic cell dialog controls regulatory T-cell fate. Immunol Rev. 2010;234:305–316. doi: 10.1111/j.0105-2896.2009.00880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cooper JE, Wiseman AC. Novel immunosuppressive agents in kidney transplantation. Clin Nephrol. 2010;73:333–343. doi: 10.5414/cnp73333. [DOI] [PubMed] [Google Scholar]
- 142.Korom S, Boehler A, Weder W. Immunosuppressive therapy in lung transplantation: state of the art. Eur J Cardiothorac Surg. 2009;35:1045–1055. doi: 10.1016/j.ejcts.2009.02.035. [DOI] [PubMed] [Google Scholar]
- 143.Fishman PH, Orlandi PA. Cholera toxin internalization and intoxication. J Cell Sci. 2003;116:431–432. doi: 10.1242/jcs.00199. author reply 432–433. [DOI] [PubMed] [Google Scholar]
- 144.D’Ambrosio A, Colucci M, Pugliese O, Quintieri F, Boirivant M. Cholera toxin B subunit promotes the induction of regulatory T cells by preventing human dendritic cell maturation. Journal of Leukocyte Biology. 2008;84:661–668. doi: 10.1189/jlb.1207850. [DOI] [PubMed] [Google Scholar]
- 145.Lavelle E, McNeela E, Armstrong M, Leavy O, Higgins S, Mills K. Cholera toxin promotes the induction of regulatory T cells specific for bystander antigens by modulating dendritic cell activation. J Immunol. 2003;171:2384–2392. doi: 10.4049/jimmunol.171.5.2384. [DOI] [PubMed] [Google Scholar]
- 146.Gomez-Escobar N, Gregory WF, Maizels RM. Identification of tgh-2, a filarial nematode homolog of Caenorhabditis elegans daf-7 and human transforming growth factor beta, expressed in microfilarial and adult stages of Brugia malayi. Infection and Immunity. 2000;68:6402–6410. doi: 10.1128/iai.68.11.6402-6410.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fleming SB, Haig DM, Nettleton P, Reid HW, McCaughan CA, Wise LM, Mercer A. Sequence and functional analysis of a homolog of interleukin-10 encoded by the parapoxvirus orf virus. Virus Genes. 2000;21:85–95. doi: 10.1023/b:viru.0000018443.19040.99. [DOI] [PubMed] [Google Scholar]
- 148.Hsu DH, de Waal Malefyt R, Fiorentino DF, Dang MN, Vieira P, de Vries J, Spits H, Mosmann TR, Moore KW. Expression of interleukin-10 activity by Epstein-Barr virus protein BCRF1. Science. 1990;250:830–832. doi: 10.1126/science.2173142. [DOI] [PubMed] [Google Scholar]
- 149.Kotenko SV, Saccani S, Izotova LS, Mirochnitchenko OV, Pestka S. Human cytomegalovirus harbors its own unique IL-10 homolog (cmvIL-10) Proc Natl Acad Sci USA. 2000;97:1695–1700. doi: 10.1073/pnas.97.4.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chang WLW, Baumgarth N, Yu D, Barry PA. Human cytomegalovirus-encoded interleukin-10 homolog inhibits maturation of dendritic cells and alters their functionality. J Virol. 2004;78:8720–8731. doi: 10.1128/JVI.78.16.8720-8731.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chang WLW, Barry PA, Szubin R, Wang D, Baumgarth N. Human cytomegalovirus suppresses type I interferon secretion by plasmacytoid dendritic cells through its interleukin 10 homolog. Virology. 2009;390:330–337. doi: 10.1016/j.virol.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nature Reviews Immunology. 2004;4:941–952. doi: 10.1038/nri1498. [DOI] [PubMed] [Google Scholar]
- 153.Ghiringhelli F, Puig PE, Roux S, Parcellier A, Schmitt E, Solary E, Kroemer G, Martin F, Chauffert B, Zitvogel L. Tumor cells convert immature myeloid dendritic cells into TGF-{beta}-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J Exp Med. 2005;202:919–929. doi: 10.1084/jem.20050463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Liu Y, Bi X, Xu S, Xiang J. Tumor-infiltrating dendritic cell subsets of progressive or regressive tumors induce suppressive or protective immune responses. Cancer Res. 2005;65:4955–4962. doi: 10.1158/0008-5472.CAN-04-3957. [DOI] [PubMed] [Google Scholar]
- 155.Zhang X, Huang H, Yuan J, Sun D, Hou W-S, Gordon J, Xiang J. CD4−8− dendritic cells prime CD4+ T regulatory 1 cells to suppress antitumor immunity. Journal of immunology (Baltimore, Md: 1950) 2005;175:2931–2937. doi: 10.4049/jimmunol.175.5.2931. [DOI] [PubMed] [Google Scholar]
- 156.Fiore F, Nuschak B, Peola S, Mariani S, Muraro M, Foglietta M, Coscia M, Bruno B, Boccadoro M, Massaia M. Exposure to myeloma cell lysates affects the immune competence of dendritic cells and favors the induction of Tr1-like regulatory T cells. Eur J Immunol. 2005;35:1155–1163. doi: 10.1002/eji.200425093. [DOI] [PubMed] [Google Scholar]
- 157.Wei S, Kryczek I, Zou L, Daniel B, Cheng P, Mottram P, Curiel T, Lange A, Zou W. Plasmacytoid dendritic cells induce CD8+ regulatory T cells in human ovarian carcinoma. Cancer Res. 2005;65:5020–5026. doi: 10.1158/0008-5472.CAN-04-4043. [DOI] [PubMed] [Google Scholar]
- 158.Dumitriu IE, Dunbar DR, Howie SE, Sethi T, Gregory CD. Human Dendritic Cells Produce TGF− 1 under the Influence of Lung Carcinoma Cells and Prime the Differentiation of CD4+CD25+Foxp3+ Regulatory T Cells. The Journal of Immunology. 2009;182:2795–2807. doi: 10.4049/jimmunol.0712671. [DOI] [PubMed] [Google Scholar]
- 159.Fainaru O, Adini A, Benny O, Adini I, Short S, Bazinet L, Nakai K, Pravda E, Hornstein MD, D’Amato RJ, Folkman J. Dendritic cells support angiogenesis and promote lesion growth in a murine model of endometriosis. The FASEB Journal. 2008;22:522–529. doi: 10.1096/fj.07-9034com. [DOI] [PubMed] [Google Scholar]
- 160.Fainaru O, Almog N, Yung CW, Nakai K, Montoya-Zavala M, Abdollahi A, D’Amato R, Ingber DE. Tumor growth and angiogenesis are dependent on the presence of immature dendritic cells. The FASEB Journal. 2010;24:1411–1418. doi: 10.1096/fj.09-147025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ikushima H, Miyazono K. TGFbeta signalling: a complex web in cancer progression. Nat Rev Cancer. 2010;10:415–424. doi: 10.1038/nrc2853. [DOI] [PubMed] [Google Scholar]
- 162.Yigit R, Massuger LFAG, Figdor CG, Torensma R. Ovarian cancer creates a suppressive microenvironment to escape immune elimination. Gynecol Oncol. 2010;117:366–372. doi: 10.1016/j.ygyno.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 163.Kelly RJ, Morris JC. Transforming growth factor-beta: a target for cancer therapy. J Immunotoxicol. 2010;7:15–26. doi: 10.3109/15476910903389920. [DOI] [PubMed] [Google Scholar]
- 164.Bierie B, Moses HL. Transforming growth factor beta (TGF-beta) and inflammation in cancer. Cytokine Growth Factor Rev. 2010;21:49–59. doi: 10.1016/j.cytogfr.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bernabeu C, Lopez-Novoa JM, Quintanilla M. The emerging role of TGF-beta superfamily coreceptors in cancer. Biochim Biophys Acta. 2009;1792:954–973. doi: 10.1016/j.bbadis.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 166.Lanteri MC, O’Brien KM, Purtha WE, Cameron MJ, Lund JM, Owen RE, Heitman JW, Custer B, Hirschkorn DF, Tobler LH, Kiely N, Prince HE, Ndhlovu LC, Nixon DF, Kamel HT, Kelvin DJ, Busch MP, Rudensky AY, Diamond MS, Norris PJ. Tregs control the development of symptomatic West Nile virus infection in humans and mice. J Clin Invest. 2009;119:3266–3277. doi: 10.1172/JCI39387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bilenki L, Gao X, Wang S, Yang J, Fan Y, Han X, Qiu H, Yang X. Dendritic cells from mycobacteria-infected mice inhibits established allergic airway inflammatory responses to ragweed via IL-10- and IL-12-secreting mechanisms. J Immunol. 2010;184:7288–7296. doi: 10.4049/jimmunol.0902829. [DOI] [PubMed] [Google Scholar]
- 168.Fisson S, Darrasse-Jeze G, Litvinova E, Septier F, Klatzmann D, Liblau R, Salomon B. Continuous activation of autoreactive CD4+ CD25+ regulatory T cells in the steady state. J Exp Med. 2003;198:737–746. doi: 10.1084/jem.20030686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jonuleit H, Schmitt E, Kakirman H, Stassen M, Knop J, Enk AH. Infectious tolerance: human CD25(+) regulatory T cells convey suppressor activity to conventional CD4(+) T helper cells. J Exp Med. 2002;196:255–260. doi: 10.1084/jem.20020394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Waldmann H, Adams E, Fairchild P, Cobbold S. Infectious tolerance and the long-term acceptance of transplanted tissue. Immunol Rev. 2006;212:301–313. doi: 10.1111/j.0105-2896.2006.00406.x. [DOI] [PubMed] [Google Scholar]
- 171.Belladonna ML, Orabona C, Grohmann U, Puccetti P. TGF-beta and kynurenines as the key to infectious tolerance. Trends in Molecular Medicine. 2009;15:41–49. doi: 10.1016/j.molmed.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 172.Mekala DJ, Alli RS, Geiger TL. IL-10-dependent infectious tolerance after the treatment of experimental allergic encephalomyelitis with redirected CD4+CD25+ T lymphocytes. Proc Natl Acad Sci USA. 2005;102:11817–11822. doi: 10.1073/pnas.0505445102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Andersson J, Tran D, Pesu M, Davidson T, Ramsey H, O’Shea J, Shevach E. CD4+FoxP3+ regulatory T cells confer infectious tolerance in a TGF-{beta}-dependent manner. J Exp Med. 2008 doi: 10.1084/jem.20080308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.McGuirk P, McCann C, Mills KHG. Pathogen-specific T regulatory 1 cells induced in the respiratory tract by a bacterial molecule that stimulates interleukin 10 production by dendritic cells: a novel strategy for evasion of protective T helper type 1 responses by Bordetella pertussis. J Exp Med. 2002;195:221–231. doi: 10.1084/jem.20011288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hackstein H, Thomson AW. Dendritic cells: emerging pharmacological targets of immunosuppressive drugs. Nature Reviews Immunology. 2004;4:24–34. doi: 10.1038/nri1256. [DOI] [PubMed] [Google Scholar]
- 176.Morelli A, Thomson A. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat Rev Immunol. 2007;7:610–621. doi: 10.1038/nri2132. [DOI] [PubMed] [Google Scholar]
- 177.Morelli AE, Thomson AW. Dendritic cells: regulators of alloimmunity and opportunities for tolerance induction. Immunol Rev. 2003;196:125–146. doi: 10.1046/j.1600-065x.2003.00079.x. [DOI] [PubMed] [Google Scholar]
- 178.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by GM-CSF plus IL-4 and downregulated by TNF-α. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lang R, Patel D, Morris JJ, Rutschman RL, Murray PJ. Shaping gene expression in activated and resting primary macrophages by IL-10. Journal of immunology (Baltimore, Md: 1950) 2002;169:2253–2263. doi: 10.4049/jimmunol.169.5.2253. [DOI] [PubMed] [Google Scholar]
- 181.Moore K, de Waal Malefyt R, Coffman R, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 182.Murray PJ. Understanding and exploiting the endogenous interleukin-10/STAT3-mediated anti-inflammatory response. Current opinion in pharmacology. 2006;6:379–386. doi: 10.1016/j.coph.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 183.Murray PJ. The primary mechanism of the IL-10-regulated antiinflammatory response is to selectively inhibit transcription. Proc Natl Acad Sci USA. 2005;102:8686–8691. doi: 10.1073/pnas.0500419102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Perrier P, Martinez FO, Locati M, Bianchi G, Nebuloni M, Vago G, Bazzoni F, Sozzani S, Allavena P, Mantovani A. Distinct transcriptional programs activated by interleukin-10 with or without lipopolysaccharide in dendritic cells: induction of the B cell-activating chemokine, CXC chemokine ligand 13. J Immunol. 2004;172:7031–7042. doi: 10.4049/jimmunol.172.11.7031. [DOI] [PubMed] [Google Scholar]
- 185.Croker BA, Krebs DL, Zhang J-G, Wormald S, Willson TA, Stanley EG, Robb L, Greenhalgh CJ, Förster I, Clausen BE, Nicola NA, Metcalf D, Hilton DJ, Roberts AW, Alexander WS. SOCS3 negatively regulates IL-6 signaling in vivo. Nat Immunol. 2003;4:540–545. doi: 10.1038/ni931. [DOI] [PubMed] [Google Scholar]
- 186.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 187.Veillette A, Latour S. The SLAM family of immune-cell receptors. Curr Opin Immunol. 2003;15:277–285. doi: 10.1016/s0952-7915(03)00041-4. [DOI] [PubMed] [Google Scholar]
- 188.Akdis CA, Blaser K. Mechanisms of interleukin-10-mediated immune suppression. Immunology. 2001;103:131–136. doi: 10.1046/j.1365-2567.2001.01235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Feuerer M, Shen Y, Littman DR, Benoist C, Mathis D. How punctual ablation of regulatory T cells unleashes an autoimmune lesion within the pancreatic islets. Immunity. 2009;31:654–664. doi: 10.1016/j.immuni.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Laouar Y, Sutterwala FS, Gorelik L, Flavell RA. Transforming growth factor-beta controls T helper type 1 cell development through regulation of natural killer cell interferon-gamma. Nature Immunology. 2005;6:600–607. doi: 10.1038/ni1197. [DOI] [PubMed] [Google Scholar]
- 191.Miyazono K. Positive and negative regulation of TGF-beta signaling. J Cell Sci. 2000;113(Pt 7):1101–1109. doi: 10.1242/jcs.113.7.1101. [DOI] [PubMed] [Google Scholar]
- 192.Rubtsov YP, Rudensky AY. TGFbeta signalling in control of T-cell-mediated self-reactivity. Nature Reviews Immunology. 2007;7:443–453. doi: 10.1038/nri2095. [DOI] [PubMed] [Google Scholar]
- 193.Karlsson G, Liu Y, Larsson J, Goumans M-J, Lee J-S, Thorgeirsson SS, Ringnér M, Karlsson S. Gene expression profiling demonstrates that TGF-beta1 signals exclusively through receptor complexes involving Alk5 and identifies targets of TGF-beta signaling. Physiol Genomics. 2005;21:396–403. doi: 10.1152/physiolgenomics.00303.2004. [DOI] [PubMed] [Google Scholar]
- 194.Fainaru O, Shay T, Hantisteanu S, Goldenberg D, Domany E, Groner Y. TGF[beta]-dependent gene expression profile during maturation of dendritic cells. Genes Immun. 2007;8:239–244. doi: 10.1038/sj.gene.6364380. [DOI] [PubMed] [Google Scholar]
- 195.Sargent JL, Milano A, Bhattacharyya S, Varga J, Connolly MK, Chang HY, Whitfield ML. A TGFbeta-responsive gene signature is associated with a subset of diffuse scleroderma with increased disease severity. J Invest Dermatol. 2010;130:694–705. doi: 10.1038/jid.2009.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Rutella S, Bonanno G, Procoli A, Mariotti A, De Ritis DG, Curti A, Danese S, Pessina G, Pandolfi S, Natoni F, Di Febo A, Scambia G, Manfredini R, Salati S, Ferrari S, Pierelli L, Leone G, Lemoli RM. Hepatocyte growth factor favors monocyte differentiation into regulatory interleukin (IL)-10++IL-12low/neg accessory cells with dendritic-cell features. Blood. 2006;108:218–227. doi: 10.1182/blood-2005-08-3141. [DOI] [PubMed] [Google Scholar]
- 197.Ristich V, Liang S, Zhang W, Wu J, Horuzsko A. Tolerization of dendritic cells by HLA-G. Eur J Immunol. 2005;35:1133–1142. doi: 10.1002/eji.200425741. [DOI] [PubMed] [Google Scholar]
- 198.Liang S, Ristich V, Arase H, Dausset J, Carosella ED, Horuzsko A. Modulation of dendritic cell differentiation by HLA-G and ILT4 requires the IL-6--STAT3 signaling pathway. Proc Natl Acad Sci USA. 2008;105:8357–8362. doi: 10.1073/pnas.0803341105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Gorczynski RM, Chen Z, Kai Y, Wong S, Lee L. Induction of tolerance-inducing antigen-presenting cells in bone marrow cultures in vitro using monoclonal antibodies to CD200R. Transplantation. 2004;77:1138–1144. doi: 10.1097/01.tp.0000121773.18476.1c. [DOI] [PubMed] [Google Scholar]
- 200.Gorczynski R, Khatri I, Lee L, Boudakov I. An interaction between CD200 and monoclonal antibody agonists to CD200R2 in development of dendritic cells that preferentially induce populations of CD4+CD25+ T regulatory cells. Journal of immunology (Baltimore, Md: 1950) 2008;180:5946–5955. doi: 10.4049/jimmunol.180.9.5946. [DOI] [PubMed] [Google Scholar]
- 201.Gorczynski RM, Lee L, Boudakov I. Augmented Induction of CD4+CD25+ Treg using monoclonal antibodies to CD200R. Transplantation. 2005;79:1180–1183. [PubMed] [Google Scholar]
- 202.Gorczynski RM. Thymocyte/splenocyte-derived CD4+CD25+Treg stimulated by anti-CD200R2 derived dendritic cells suppress mixed leukocyte cultures and skin graft rejection. Transplantation. 2006;81:1027–1034. doi: 10.1097/01.tp.0000214984.65520.50. [DOI] [PubMed] [Google Scholar]
- 203.Leung DYM, Bloom JW. Update on glucocorticoid action and resistance. J Allergy Clin Immunol. 2003;111:3–22. doi: 10.1067/mai.2003.97. quiz 23. [DOI] [PubMed] [Google Scholar]
- 204.Roca L, Di Paolo S, Petruzzelli V, Grandaliano G, Ranieri E, Schena FP, Gesualdo L. Dexamethasone modulates interleukin-12 production by inducing monocyte chemoattractant protein-1 in human dendritic cells. Immunol Cell Biol. 2007;85:610–616. doi: 10.1038/sj.icb.7100108. [DOI] [PubMed] [Google Scholar]
- 205.Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280–288. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 206.Iruretagoyena MI, Sepúlveda SE, Lezana JP, Hermoso M, Bronfman M, Gutiérrez MA, Jacobelli SH, Kalergis AM. Inhibition of nuclear factor-kappa B enhances the capacity of immature dendritic cells to induce antigen-specific tolerance in experimental autoimmune encephalomyelitis. J Pharmacol Exp Ther. 2006;318:59–67. doi: 10.1124/jpet.106.103259. [DOI] [PubMed] [Google Scholar]
- 207.Zhang X, Li M, Lian D, Zheng X, Zhang Z-X, Ichim TE, Xia X, Huang X, Vladau C, Suzuki M, Garcia B, Jevnikar AM, Min W-P. Generation of therapeutic dendritic cells and regulatory T cells for preventing allogeneic cardiac graft rejection. Clin Immunol. 2008;127:313–321. doi: 10.1016/j.clim.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 208.Cong Y, Wang L, Konrad A, Schoeb T, Elson C. Curcumin induces the tolerogenic dendritic cell that promotes differentiation of intestine-protective regulatory T cells. Eur J Immunol. 2009;39:3134–3146. doi: 10.1002/eji.200939052. [DOI] [PubMed] [Google Scholar]
- 209.Buckland M, Lombardi G. Aspirin and the induction of tolerance by dendritic cells. Handb Exp Pharmacol. 2009:197–213. doi: 10.1007/978-3-540-71029-5_9. [DOI] [PubMed] [Google Scholar]
- 210.Buckland M, Jago C, Fazekesova H, George A, Lechler R, Lombardi G. Aspirin modified dendritic cells are potent inducers of allo-specific regulatory T-cells. Int Immunopharmacol. 2006;6:1895–1901. [PubMed] [Google Scholar]
- 211.Buckland M, Jago CB, Fazekasova H, Scott K, Tan PH, George AJT, Lechler R, Lombardi G. Aspirin-treated human DCs up-regulate ILT-3 and induce hyporesponsiveness and regulatory activity in responder T cells. Am J Transplant. 2006;6:2046–2059. doi: 10.1111/j.1600-6143.2006.01450.x. [DOI] [PubMed] [Google Scholar]
- 212.Svajger U, Obermajer N, Jeras M. Dendritic cells treated with resveratrol during differentiation from monocytes gain substantial tolerogenic properties upon activation. Immunology. 2010;129:525–535. doi: 10.1111/j.1365-2567.2009.03205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kim G-Y, Cho H, Ahn S-C, Oh Y-H, Lee C-M, Park Y-M. Resveratrol inhibits phenotypic and functional maturation of murine bone marrow-derived dendritic cells. Int Immunopharmacol. 2004;4:245–253. doi: 10.1016/j.intimp.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 214.Pervaiz S, Holme AL. Resveratrol: its biologic targets and functional activity. Antioxid Redox Signal. 2009;11:2851–2897. doi: 10.1089/ars.2008.2412. [DOI] [PubMed] [Google Scholar]
- 215.Hay N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 216.Augustine JJ, Bodziak KA, Hricik DE. Use of sirolimus in solid organ transplantation. Drugs. 2007;67:369–391. doi: 10.2165/00003495-200767030-00004. [DOI] [PubMed] [Google Scholar]
- 217.Raimondi G, Sumpter TL, Matta BM, Pillai M, Corbitt N, Vodovotz Y, Wang Z, Thomson AW. Mammalian target of rapamycin inhibition and alloantigen-specific regulatory T cells synergize to promote long-term graft survival in immunocompetent recipients. Journal of immunology (Baltimore, Md: 1950) 2010;184:624–636. doi: 10.4049/jimmunol.0900936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Esposito M, Ruffini F, Bellone M, Gagliani N, Battaglia M, Martino G, Furlan R. Rapamycin inhibits relapsing experimental autoimmune encephalomyelitis by both effector and regulatory T cells modulation. Journal of Neuroimmunology. 2010:1–12. doi: 10.1016/j.jneuroim.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 219.Fu B-m, He X-s, Yu S, Hu A-b, Zhang J, Ma Y, Tam N-l, Huang J-f. A tolerogenic semimature dendritic cells induce effector T-cell hyporesponsiveness by activation of antigen-specific CD4+CD25+ T regulatory cells that promotes skin allograft survival in mice. Cell Immunol. 2010;261:69–76. doi: 10.1016/j.cellimm.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 220.Ge W, Jiang J, Baroja ML, Arp J, Zassoko R, Liu W, Bartholomew A, Garcia B, Wang H. Infusion of Mesenchymal Stem Cells and Rapamycin Synergize to Attenuate Alloimmune Responses and Promote Cardiac Allograft Tolerance. American Journal of Transplantation. 2009;9:1760–1772. doi: 10.1111/j.1600-6143.2009.02721.x. [DOI] [PubMed] [Google Scholar]
- 221.Valle A, Jofra T, Stabilini A, Atkinson M, Roncarolo M-G, Battaglia M. Rapamycin prevents and breaks the anti-CD3-induced tolerance in NOD mice. Diabetes. 2009;58:875–881. doi: 10.2337/db08-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Zang W, Lin M, Kalache S, Zhang N, Krüger B, Waaga-Gasser AM, Grimm M, Hancock W, Heeger P, Schröppel B, Murphy B. Inhibition of the alloimmune response through the generation of regulatory T cells by a MHC class II-derived peptide. J Immunol. 2008;181:7499–7506. doi: 10.4049/jimmunol.181.11.7499. [DOI] [PubMed] [Google Scholar]
- 223.Massey DCO, Bredin F, Parkes M. Use of sirolimus (rapamycin) to treat refractory Crohn’s disease. Gut. 2008;57:1294–1296. doi: 10.1136/gut.2008.157297. [DOI] [PubMed] [Google Scholar]
- 224.Monti P, Scirpoli M, Maffi P, Piemonti L, Secchi A, Bonifacio E, Roncarolo M-G, Battaglia M. Rapamycin monotherapy in patients with type 1 diabetes modifies CD4+CD25+FOXP3+ regulatory T-cells. Diabetes. 2008;57:2341–2347. doi: 10.2337/db08-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Battaglia M, Stabilini A, Migliavacca B, Horejs-Hoeck J, Kaupper T, Roncarolo M. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J Immunol. 2006;177:8338–8347. doi: 10.4049/jimmunol.177.12.8338. [DOI] [PubMed] [Google Scholar]
- 226.Thomson AW, Turnquist HR, Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol. 2009;9:324–337. doi: 10.1038/nri2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Battaglia M, Stabilini A, Roncarolo M. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105:4743–4748. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- 228.Ohtani M, Nagai S, Kondo S, Mizuno S, Nakamura K, Tanabe M, Takeuchi T, Matsuda S, Koyasu S. mTOR and GSK3 differentially regulate LPS-induced IL-12 production in dendritic cells. Blood. 2008 doi: 10.1182/blood-2008-02-137430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Horibe EK, Sacks J, Unadkat J, Raimondi G, Wang Z, Ikeguchi R, Marsteller D, Ferreira LM, Thomson AW, Lee WPA, Feili-Hariri M. Rapamycin-conditioned, alloantigen-pulsed dendritic cells promote indefinite survival of vascularized skin allografts in association with T regulatory cell expansion. Transplant Immunology. 2008;18:307–318. doi: 10.1016/j.trim.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 230.Turnquist HR, Raimondi G, Zahorchak AF, Fischer RT, Wang Z, Thomson A. Rapamycin-conditioned dendritic cells are poor stimulators of allogeneic CD4+ T cells, but enrich for antigen-specific Foxp3+ T regulatory cells and promote organ transplant tolerance. J Immunol. 2007;178:7018–7031. doi: 10.4049/jimmunol.178.11.7018. [DOI] [PubMed] [Google Scholar]
- 231.Martin E, O’Sullivan B, Low P, Thomas R. Antigen-specific suppression of a primed immune response by dendritic cells mediated by regulatory T cells secreting interleukin-10. Immunity. 2003;18:155–167. doi: 10.1016/s1074-7613(02)00503-4. [DOI] [PubMed] [Google Scholar]
- 232.Yang H, Zhang Y, Wu M, Li J, Zhou W, Li G, Li X, Xiao B, Christadoss P. Suppression of ongoing experimental autoimmune myasthenia gravis by transfer of RelB-silenced bone marrow dentritic cells is associated with a change from a T helper Th17/Th1 to a Th2 and FoxP3+ regulatory T-cell profile. Inflamm Res. 2010;59:197–205. doi: 10.1007/s00011-009-0087-6. [DOI] [PubMed] [Google Scholar]
- 233.Zhang Y, Yang H, Xiao B, Wu M, Zhou W, Li J, Li G, Christadoss P. Dendritic cells transduced with lentiviral-mediated RelB-specific ShRNAs inhibit the development of experimental autoimmune myasthenia gravis. Molecular immunology. 2009;46:657–667. doi: 10.1016/j.molimm.2008.08.274. [DOI] [PubMed] [Google Scholar]
- 234.Tomasoni S, Aiello S, Cassis L, Noris M, Longaretti L, Cavinato RA, Azzollini N, Pezzotta A, Remuzzi G, Benigni A. Dendritic cells genetically engineered with adenoviral vector encoding dnIKK2 induce the formation of potent CD4+ T-regulatory cells. Transplantation. 2005;79:1056–1061. doi: 10.1097/01.tp.0000161252.17163.31. [DOI] [PubMed] [Google Scholar]
- 235.Zheng X, Suzuki M, Ichim TE, Zhang X, Sun H, Zhu F, Shunnar A, Garcia B, Inman RD, Min W. Treatment of Autoimmune Arthritis Using RNA Interference-Modulated Dendritic Cells. Journal of immunology (Baltimore, Md: 1950) 2010 doi: 10.4049/jimmunol.0901717. [DOI] [PubMed] [Google Scholar]
- 236.Henry E, Desmet CJ, Garzé V, Fiévez L, Bedoret D, Heirman C, Faisca P, Jaspar FJ, Gosset P, Jacquet APA, Desmecht D, Thielemans K, Lekeux P, Moser M, Bureau F. Dendritic cells genetically engineered to express IL-10 induce long-lasting antigen-specific tolerance in experimental asthma. J Immunol. 2008;181:7230–7242. doi: 10.4049/jimmunol.181.10.7230. [DOI] [PubMed] [Google Scholar]
- 237.Lipscomb MW, Taylor JL, Goldbach CJ, Watkins SC, Wesa AK, Storkus WJ. DC expressing transgene Foxp3 are regulatory APC. Eur J Immunol. 2010;40:480–493. doi: 10.1002/eji.200939667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Kretschmer K, Apostolou I, Jaeckel E, Khazaie K, von Boehmer H. Making regulatory T cells with defined antigen specificity: role in autoimmunity and cancer. Immunol Rev. 2006;212:163–169. doi: 10.1111/j.0105-2896.2006.00411.x. [DOI] [PubMed] [Google Scholar]
- 239.Picca CC, Larkin J, Boesteanu A, Lerman MA, Rankin AL, Caton AJ. Role of TCR specificity in CD4+ CD25+ regulatory T-cell selection. Immunol Rev. 2006;212:74–85. doi: 10.1111/j.0105-2896.2006.00416.x. [DOI] [PubMed] [Google Scholar]
- 240.Hermann-Kleiter N, Baier G. NFAT pulls the strings during CD4+ T helper cell effector functions. Blood. 2010;115:2989–2997. doi: 10.1182/blood-2009-10-233585. [DOI] [PubMed] [Google Scholar]
- 241.King IL, Segal BM. Cutting edge: IL-12 induces CD4+CD25− T cell activation in the presence of T regulatory cells. Journal of immunology (Baltimore, Md: 1950) 2005;175:641–645. doi: 10.4049/jimmunol.175.2.641. [DOI] [PubMed] [Google Scholar]
- 242.Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nature Immunology. 2007;8:1353–1362. doi: 10.1038/ni1536. [DOI] [PubMed] [Google Scholar]
- 243.Hogan PG, Lewis RS, Rao A. Molecular basis of calcium signaling in lymphocytes: STIM and ORAI. Annu Rev Immunol. 2010;28:491–533. doi: 10.1146/annurev.immunol.021908.132550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky A. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 245.Feuerer M, Hill JA, Kretschmer K, Von Boehmer H, Mathis D, Benoist C. Genomic definition of multiple ex vivo regulatory T cell subphenotypes. Proceedings of the National Academy of Sciences. 2010;107:5919–5924. doi: 10.1073/pnas.1002006107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Hill JA, Feuerer M, Tash K, Haxhinasto S, Perez J, Melamed R, Mathis D, Benoist C. Foxp3 transcription-factor-dependent and -independent regulation of the regulatory T cell transcriptional signature. Immunity. 2007;27:786–800. doi: 10.1016/j.immuni.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 247.Sadlon TJ, Wilkinson BG, Pederson S, Brown CY, Bresatz S, Gargett T, Melville EL, Peng K, D’Andrea RJ, Glonek GG, Goodall GJ, Zola H, Shannon MF, Barry SC. Genome-Wide Identification of Human FOXP3 Target Genes in Natural Regulatory T Cells. J Immunol. 2010 doi: 10.4049/jimmunol.1000082. [DOI] [PubMed] [Google Scholar]
- 248.Hu H, Djuretic I, Sundrud M, Rao A. Transcriptional partners in regulatory T cells: Foxp3, Runx and NFAT. Trends in Immunology. 2007;28:329–332. doi: 10.1016/j.it.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 249.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T Cells and Immune Tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 250.Wakkach A, Fournier N, Brun V, Breittmayer J, Cottrez F, Groux H. Characterization of dendritic cells that induce tolerance and T regulatory 1 cell differentiation in vivo. Immunity. 2003;18:605–617. doi: 10.1016/s1074-7613(03)00113-4. [DOI] [PubMed] [Google Scholar]
- 251.Akbari O, DeKruyff RH, Umetsu DT. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nature Immunology. 2001;2:725–731. doi: 10.1038/90667. [DOI] [PubMed] [Google Scholar]
- 252.Scott-Browne JP, Shafiani S, Tucker-Heard G, Ishida-Tsubota K, Fontenot JD, Rudensky A, Bevan MJ, Urdahl KB. Expansion and function of Foxp3-expressing T regulatory cells during tuberculosis. J Exp Med. 2007;204:2159–2169. doi: 10.1084/jem.20062105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Sharma PK, Saha PK, Singh A, Sharma SK, Ghosh B, Mitra DK. FoxP3+ regulatory T cells suppress effector T-cell function at pathologic site in miliary tuberculosis. Am J Respir Crit Care Med. 2009;179:1061–1070. doi: 10.1164/rccm.200804-529OC. [DOI] [PubMed] [Google Scholar]
- 254.Shafiani S, Tucker-Heard G, Kariyone A, Takatsu K, Urdahl KB. Pathogen-specific regulatory T cells delay the arrival of effector T cells in the lung during early tuberculosis. The Journal of experimental medicine. 2010 doi: 10.1084/jem.20091885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Zhang N, Schröppel B, Lal G, Jakubzick C, Mao X, Chen D, Yin N, Jessberger R, Ochando JC, Ding Y, Bromberg JS. Regulatory T cells sequentially migrate from inflamed tissues to draining lymph nodes to suppress the alloimmune response. Immunity. 2009;30:458–469. doi: 10.1016/j.immuni.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Akdis CA, Joss A, Akdis M, Faith A, Blaser K. A molecular basis for T cell suppression by IL-10: CD28-associated IL-10 receptor inhibits CD28 tyrosine phosphorylation and phosphatidylinositol 3-kinase binding. FASEB J. 2000;14:1666–1668. doi: 10.1096/fj.99-0874fje. [DOI] [PubMed] [Google Scholar]
- 257.Taylor A, Akdis M, Joss A, Akkoç T, Wenig R, Colonna M, Daigle I, Flory E, Blaser K, Akdis CA. IL-10 inhibits CD28 and ICOS costimulations of T cells via src homology 2 domain-containing protein tyrosine phosphatase 1. J Allergy Clin Immunol. 2007;120:76–83. doi: 10.1016/j.jaci.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 258.Taylor A, Verhagen J, Akkoç T, Wenig R, Flory E, Blaser K, Akdis M, Akdis CA. IL-10 suppresses CD2-mediated T cell activation via SHP-1. Mol Immunol. 2009;46:622–629. doi: 10.1016/j.molimm.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 259.Akdis CA, Joss A, Akdis M, Blaser K. Mechanism of IL-10-induced T cell inactivation in allergic inflammation and normal response to allergens. Int Arch Allergy Immunol. 2001;124:180–182. doi: 10.1159/000053704. [DOI] [PubMed] [Google Scholar]
- 260.Murai M, Turovskaya O, Kim G, Madan R, Karp CL, Cheroutre H, Kronenberg M. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat Immunol. 2009;10:1178–1184. doi: 10.1038/ni.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Gorelik L, Flavell R. Abrogation of TGFbeta signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12:171–181. doi: 10.1016/s1074-7613(00)80170-3. [DOI] [PubMed] [Google Scholar]
- 262.Kim B-G, Li C, Qiao W, Mamura M, Kasprzak B, Kasperczak B, Anver M, Wolfraim L, Hong S, Mushinski E, Potter M, Kim S-J, Fu X-Y, Deng C, Letterio JJ. Smad4 signalling in T cells is required for suppression of gastrointestinal cancer. Nature. 2006;441:1015–1019. doi: 10.1038/nature04846. [DOI] [PubMed] [Google Scholar]
- 263.Chen W, Konkel JE. TGF-beta and ‘adaptive’ Foxp3(+) regulatory T cells. J Mol Cell Biol. 2010;2:30–36. doi: 10.1093/jmcb/mjp004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Harada Y, Harada Y, Elly C, Ying G, Paik J-H, Depinho RA, Liu Y-C. Transcription factors Foxo3a and Foxo1 couple the E3 ligase Cbl-b to the induction of Foxp3 expression in induced regulatory T cells. The Journal of experimental medicine. 2010 doi: 10.1084/jem.20100004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B, Otipoby KL, Yokota A, Takeuchi H, Ricciardi-Castagnoli P, Rajewsky K, Adams DH, von Andrian UH. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–1160. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 266.von Boehmer H. Oral tolerance: is it all retinoic acid? J Exp Med. 2007;204:1737–1739. doi: 10.1084/jem.20071251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Hill JA, Hall JA, Sun C-M, Cai Q, Ghyselinck N, Chambon P, Belkaid Y, Mathis D, Benoist C. Retinoic acid enhances Foxp3 induction indirectly by relieving inhibition from CD4+CD44hi Cells. Immunity. 2008;29:758–770. doi: 10.1016/j.immuni.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Benson M, Pino-Lagos K, Rosemblatt M, Noelle R. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med. 2007;204:1765–1774. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Takaki H, Ichiyama K, Koga K, Chinen T, Takaesu G, Sugiyama Y, Kato S, Yoshimura A, Kobayashi T. STAT6 inhibits TGF-beta 1-mediated Foxp3 induction through direct binding to the Foxp3 promoter, which is reverted by retinoic acid receptor. Journal of Biological Chemistry. 2008:15. doi: 10.1074/jbc.M801123200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Manicassamy S, Pulendran B. Retinoic acid-dependent regulation of immune responses by dendritic cells and macrophages. Seminars in Immunology. 2009;21:22–27. doi: 10.1016/j.smim.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Gregori S, Tomasoni D, Pacciani V, Scirpoli M, Battaglia M, Magnani CF, Hauben E, Roncarolo M-G. Differentiation of type 1 T regulatory (Tr1) cells by tolerogenic DC-10 requires the IL-10-dependent ILT4/HLA-G pathway. Blood. 2010 doi: 10.1182/blood-2009-07-234872. [DOI] [PubMed] [Google Scholar]
- 272.Vlad G, Chang C-C, Colovai AI, Vasilescu ER, Cortesini R, Suciu-Foca N. Membrane and soluble ILT3 are critical to the generation of T suppressor cells and induction of immunological tolerance. Int Rev Immunol. 2010;29:119–132. doi: 10.3109/08830180903281185. [DOI] [PubMed] [Google Scholar]
- 273.Gregori S, Magnani CF, Roncarolo M-G. Role of human leukocyte antigen-G in the induction of adaptive type 1 regulatory T cells. Hum Immunol. 2009;70:966–969. doi: 10.1016/j.humimm.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 274.Vlad G, Chang C-C, Colovai AI, Berloco P, Cortesini R, Suciu-Foca N. Immunoglobulin-like transcript 3: A crucial regulator of dendritic cell function. Hum Immunol. 2009;70:340–344. doi: 10.1016/j.humimm.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 275.Wu J, Horuzsko A. Expression and function of immunoglobulin-like transcripts on tolerogenic dendritic cells. Hum Immunol. 2009;70:353–356. doi: 10.1016/j.humimm.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Keir ME, Francisco LM, Sharpe AH. PD-1 and its ligands in T-cell immunity. Current Opinion in Immunology. 2007;19:309–314. doi: 10.1016/j.coi.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 277.Riley JL. PD-1 signaling in primary T cells. Immunol Rev. 2009;229:114–125. doi: 10.1111/j.1600-065X.2009.00767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Wang L, Pino-Lagos K, de Vries V, Guleria I, Sayegh M, Noelle R. Programmed death 1 ligand signaling regulates the generation of adaptive Foxp3+CD4+ regulatory T cells. Proc Natl Acad Sci USA. 2008 doi: 10.1073/pnas.0710441105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Sundrud MS, Koralov SB, Feuerer M, Calado DP, Kozhaya AE, Rhule-Smith A, Lefebvre RE, Unutmaz D, Mazitschek R, Waldner H, Whitman M, Keller T, Rao A. Halofuginone inhibits TH17 cell differentiation by activating the amino acid starvation response. Science. 2009;324:1334–1338. doi: 10.1126/science.1172638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang L-S, Jones RG, Choi Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Pearce EL. Metabolism in T cell activation and differentiation. Current Opinion in Immunology. 2010 doi: 10.1016/j.coi.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nature Reviews Immunology. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 284.Curti A, Trabanelli S, Salvestrini V, Baccarani M, Lemoli RM. The role of indoleamine 2,3-dioxygenase in the induction of immune tolerance: focus on hematology. Blood. 2009;113:2394–2401. doi: 10.1182/blood-2008-07-144485. [DOI] [PubMed] [Google Scholar]
- 285.Löb S, Königsrainer A. Role of IDO in organ transplantation: promises and difficulties. Int Rev Immunol. 2009;28:185–206. doi: 10.1080/08830180902989119. [DOI] [PubMed] [Google Scholar]
- 286.Katz JB, Muller AJ, Prendergast GC. Indoleamine 2,3-dioxygenase in T-cell tolerance and tumoral immune escape. Immunol Rev. 2008;222:206–221. doi: 10.1111/j.1600-065X.2008.00610.x. [DOI] [PubMed] [Google Scholar]
- 287.Jurgens B, Hainz U, Fuchs D, Felzmann T, Heitger A. Interferon-gamma triggered indoleamine 2,3-dioxygenase competence in human monocyte-derived dendritic cells induces regulatory activity in allogeneic T cells. Blood. 2009 doi: 10.1182/blood-2008-12-195073. [DOI] [PubMed] [Google Scholar]
- 288.Orabona C, Puccetti P, Vacca C, Bicciato S, Luchini A, Fallarino F, Bianchi R, Velardi E, Perruccio K, Velardi A, Bronte V, Fioretti MC, Grohmann U. Toward the identification of a tolerogenic signature in IDO-competent dendritic cells. Blood. 2006;107:2846–2854. doi: 10.1182/blood-2005-10-4077. [DOI] [PubMed] [Google Scholar]
- 289.Rémy S, Blancou P, Tesson L, Tardif V, Brion R, Royer PJ, Motterlini R, Foresti R, Painchaut M, Pogu S, Gregoire M, Bach JM, Anegon I, Chauveau C. Carbon monoxide inhibits TLR-induced dendritic cell immunogenicity. J Immunol. 2009;182:1877–1884. doi: 10.4049/jimmunol.0802436. [DOI] [PubMed] [Google Scholar]
- 290.Chora AA, Fontoura P, Cunha A, Pais TF, Cardoso S, Ho PP, Lee LY, Sobel RA, Steinman L, Soares MP. Heme oxygenase-1 and carbon monoxide suppress autoimmune neuroinflammation. J Clin Invest. 2007;117:438–447. doi: 10.1172/JCI28844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Yamashita K, Ollinger R, McDaid J, Sakahama H, Wang H, Tyagi S, Csizmadia E, Smith NR, Soares MP, Bach FH. Heme oxygenase-1 is essential for and promotes tolerance to transplanted organs. The FASEB Journal. 2006;20:776–778. doi: 10.1096/fj.05-4791fje. [DOI] [PubMed] [Google Scholar]
- 292.Schmitz F, Heit A, Dreher S, Eisenächer K, Mages J, Haas T, Krug A, Janssen K-P, Kirschning CJ, Wagner H. Mammalian target of rapamycin (mTOR) orchestrates the defense program of innate immune cells. Eur J Immunol. 2008;38:2981–2992. doi: 10.1002/eji.200838761. [DOI] [PubMed] [Google Scholar]
- 293.Cao W, Manicassamy S, Tang H, Kasturi SP, Pirani A, Murthy N, Pulendran B. Toll-like receptor-mediated induction of type I interferon in plasmacytoid dendritic cells requires the rapamycin-sensitive PI(3)K-mTOR-p70S6K pathway. Nat Immunol. 2008;9:1157–1164. doi: 10.1038/ni.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Haxhinasto S, Mathis D, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. Journal of Experimental Medicine. 2008;205:565–574. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Cobbold SP, Adams E, Farquhar CA, Nolan KF, Howie D, Lui KO, Fairchild PJ, Mellor AL, Ron D, Waldmann H. Infectious tolerance via the consumption of essential amino acids and mTOR signaling. Proc Natl Acad Sci USA. 2009;106:12055–12060. doi: 10.1073/pnas.0903919106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Sauer S, Bruno L, Hertweck A, Finlay D, Leleu M, Spivakov M, Knight ZA, Cobb BS, Cantrell D, O’Connor E, Shokat KM, Fisher AG, Merkenschlager M. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc Natl Acad Sci USA. 2008;105:7797–7802. doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Ben-Shoshan J, Maysel-Auslender S, Mor A, Keren G, George J. Hypoxia controls CD4 +CD25 +regulatory T-cell homeostasis viahypoxia-inducible factor-1α. Eur J Immunol. 2008;38:2412–2418. doi: 10.1002/eji.200838318. [DOI] [PubMed] [Google Scholar]
- 298.Delgoffe GM, Powell JD. mTOR: taking cues from the immune microenvironment. Immunology. 2009;127:459–465. doi: 10.1111/j.1365-2567.2009.03125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Laplante M, Sabatini DM. mTOR signaling at a glance. J Cell Sci. 2009;122:3589–3594. doi: 10.1242/jcs.051011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Bruder D, Westendorf AM, Hansen W, Prettin S, Gruber AD, Qian Y, von Boehmer H, Mahnke K, Buer J. On the edge of autoimmunity: T-cell stimulation by steady-state dendritic cells prevents autoimmune diabetes. Diabetes. 2005;54:3395–3401. doi: 10.2337/diabetes.54.12.3395. [DOI] [PubMed] [Google Scholar]
- 301.Mahnke K, Qian Y, Knop J, Enk AH. Induction of CD4+/CD25+ regulatory T cells by targeting of antigens to immature dendritic cells. Blood. 2003;101:4862–4869. doi: 10.1182/blood-2002-10-3229. [DOI] [PubMed] [Google Scholar]
- 302.Min S-Y, Park K-S, Cho M-L, Kang J-W, Cho Y-G, Hwang S-Y, Park M-J, Yoon C-H, Min J-K, Lee S-H, Park S-H, Kim H-Y. Antigen-induced, tolerogenic CD11c+,CD11b+ dendritic cells are abundant in Peyer’s patches during the induction of oral tolerance to type II collagen and suppress experimental collagen-induced arthritis. Arthritis Rheum. 2006;54:887–898. doi: 10.1002/art.21647. [DOI] [PubMed] [Google Scholar]
- 303.Bilsborough J, George TC, Norment A, Viney JL. Mucosal CD8alpha+ DC, with a plasmacytoid phenotype, induce differentiation and support function of T cells with regulatory properties. Immunology. 2003;108:481–492. doi: 10.1046/j.1365-2567.2003.01606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Mascarell L, Lombardi V, Louise A, Saint-Lu N, Chabre H, Moussu H, Betbeder D, Balazuc A-M, Van Overtvelt L, Moingeon P. Oral dendritic cells mediate antigen-specific tolerance by stimulating TH1 and regulatory CD4+ T cells. J Allergy Clin Immunol. 2008;122:603–609.e605. doi: 10.1016/j.jaci.2008.06.034. [DOI] [PubMed] [Google Scholar]
- 305.Park M-J, Min S-Y, Park K-S, Cho Y-G, Cho M-L, Jung Y-O, Park H-S, Chang S-H, Cho SG, Min J-K, Park S-H, Kim H-Y. Indoleamine 2,3-dioxygenase-expressing dendritic cells are involved in the generation of CD4+CD25+ regulatory T cells in Peyer’s patches in an orally tolerized, collagen-induced arthritis mouse model. Arthritis Res Ther. 2008;10:R11. doi: 10.1186/ar2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.
- 307.Smith TRF, Maricic I, Ria F, Schneider S, Kumar V. CD8á+ Dendritic cells prime TCR-peptide-reactive regulatory CD4+FOXP3− T cells. Eur J Immunol. 2010 doi: 10.1002/eji.200939608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Chen W, Liang X, Peterson AJ, Munn DH, Blazar BR. The indoleamine 2,3-dioxygenase pathway is essential for human plasmacytoid dendritic cell-induced adaptive T regulatory cell generation. Journal of immunology (Baltimore, Md: 1950) 2008;181:5396–5404. doi: 10.4049/jimmunol.181.8.5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Moseman EA, Liang X, Dawson AJ, Panoskaltsis-Mortari A, Krieg AM, Liu Y-J, Blazar BR, Chen W. Human plasmacytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+CD25+ regulatory T cells. J Immunol. 2004;173:4433–4442. doi: 10.4049/jimmunol.173.7.4433. [DOI] [PubMed] [Google Scholar]
- 310.Ito T, Yang M, Wang Y-H, Lande R, Gregorio J, Perng OA, Qin X-F, Liu Y-J, Gilliet M. Plasmacytoid dendritic cells prime IL-10-producing T regulatory cells by inducible costimulator ligand. J Exp Med. 2007;204:105–115. doi: 10.1084/jem.20061660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Hadeiba H, Sato T, Habtezion A, Oderup C, Pan J, Butcher EC. CCR9 expression defines tolerogenic plasmacytoid dendritic cells able to suppress acute graft-versus-host disease. Nature Immunology. 2008;9:1253–1260. doi: 10.1038/ni.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Akbari O, Freeman G, Meyer E, Greenfield E, Chang T, Sharpe A, Berry G, DeKruyff R, Umetsu D. Antigen-specific regulatory T cells develop via the ICOS-ICOS-ligand pathway and inhibit allergen-induced airway hyperreactivity. Nat Med. 2002;8:1024–1032. doi: 10.1038/nm745. [DOI] [PubMed] [Google Scholar]
- 313.Jonuleit H, Schmitt E, Schuler G, Knop J, Enk AH. Induction of interleukin 10-producing, nonproliferating CD4(+) T cells with regulatory properties by repetitive stimulation with allogeneic immature human dendritic cells. J Exp Med. 2000;192:1213–1222. doi: 10.1084/jem.192.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Dhodapkar MV, Steinman RM, Krasovsky J, Munz C, Bhardwaj N. Antigen-specific inhibition of effector T cell function in humans after injection of immature dendritic cells. J Exp Med. 2001;193:233–238. doi: 10.1084/jem.193.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Dhodapkar M, Steinman R. Antigen-bearing immature dendritic cells induce peptide-specific CD8(+) regulatory T cells in vivo in humans. Blood. 2002;100:174–177. doi: 10.1182/blood.v100.1.174. [DOI] [PubMed] [Google Scholar]
- 316.Levings M, Gregori S, Tresoldi E, Cazzaniga S, Bonini C, Roncarolo M. Differentiation of Tr1 cells by immature dendritic cells requires IL-10 but not CD25+CD4+ Tr cells. Blood. 2005;105:1162–1169. doi: 10.1182/blood-2004-03-1211. [DOI] [PubMed] [Google Scholar]
- 317.Tuettenberg A, Huter E, Hubo M, Horn J, Knop J, Grimbacher B, Kroczek RA, Stoll S, Jonuleit H. The role of ICOS in directing T cell responses: ICOS-dependent induction of T cell anergy by tolerogenic dendritic cells. Journal of immunology (Baltimore, Md: 1950) 2009;182:3349–3356. doi: 10.4049/jimmunol.0802733. [DOI] [PubMed] [Google Scholar]
- 318.Cools N, Van Tendeloo VFI, Smits ELJM, Lenjou M, Nijs G, Van Bockstaele DR, Berneman ZN, Ponsaerts P. Immunosuppression induced by immature dendritic cells is mediated by TGF-beta/IL-10 double-positive CD4+ regulatory T cells. J Cell Mol Med. 2008;12:690–700. doi: 10.1111/j.1582-4934.2007.00084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Stepkowski SM, Phan T, Zhang H, Bilinski S, Kloc M, Qi Y, Katz SM, Rutzky LP. Immature syngeneic dendritic cells potentiate tolerance to pancreatic islet allografts depleted of donor dendritic cells in microgravity culture condition. Transplantation. 2006;82:1756–1763. doi: 10.1097/01.tp.0000250732.30273.9b. [DOI] [PubMed] [Google Scholar]
- 320.Falcón C, Carranza F, Martínez FF, Knubel CP, Masih DT, Motrán CC, Cervi L. Excretory-secretory products (ESP) from Fasciola hepatica induce tolerogenic properties in myeloid dendritic cells. Veterinary immunology and immunopathology. 2010 doi: 10.1016/j.vetimm.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 321.Wang X, Zhou S, Chi Y, Wen X, Hoellwarth J, He L, Liu F, Wu C, Dhesi S, Zhao J, Hu W, Su C. CD4+CD25+ Treg induction by an HSP60-derived peptide SJMHE1 from Schistosoma japonicum is TLR2 dependent. Eur J Immunol. 2009;39:3052–3065. doi: 10.1002/eji.200939335. [DOI] [PubMed] [Google Scholar]
- 322.Bonifazi P, Zelante T, D’Angelo C, De Luca A, Moretti S, Bozza S, Perruccio K, Iannitti RG, Giovannini G, Volpi C, Fallarino F, Puccetti P, Romani L. Balancing inflammation and tolerance in vivo through dendritic cells by the commensal Candida albicans. Mucosal immunology. 2009;2:362–374. doi: 10.1038/mi.2009.17. [DOI] [PubMed] [Google Scholar]
- 323.Allam J-P, Peng W-M, Appel T, Wenghoefer M, Niederhagen B, Bieber T, Bergé S, Novak N. Toll-like receptor 4 ligation enforces tolerogenic properties of oral mucosal Langerhans cells. J Allergy Clin Immunol. 2008;121:368–374. e361. doi: 10.1016/j.jaci.2007.09.045. [DOI] [PubMed] [Google Scholar]
- 324.Lau AWT, Biester S, Cornall RJ, Forrester JV. Lipopolysaccharide-activated IL-10-secreting dendritic cells suppress experimental autoimmune uveoretinitis by MHCII-dependent activation of CD62L-expressing regulatory T cells. Journal of immunology (Baltimore, Md: 1950) 2008;180:3889–3899. doi: 10.4049/jimmunol.180.6.3889. [DOI] [PubMed] [Google Scholar]
- 325.Liu T, Chen X, Feng B-S, He S-H, Zhang T-Y, Wang B-Q, Yang P-C. Glucuronoxylomannan promotes the generation of antigen-specific T regulatory cell that suppresses the antigen specific Th2 response upon activation. J Cell Mol Med. 2008 doi: 10.1111/j.1582-4934.2008.00583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Depaolo RW, Tang F, Kim I, Han M, Levin N, Ciletti N, Lin A, Anderson D, Schneewind O, Jabri B. Toll-like receptor 6 drives differentiation of tolerogenic dendritic cells and contributes to LcrV-mediated plague pathogenesis. Cell Host Microbe. 2008;4:350–361. doi: 10.1016/j.chom.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Monti P, Leone BE, Zerbi A, Balzano G, Cainarca S, Sordi V, Pontillo M, Mercalli A, Di Carlo V, Allavena P, Piemonti L. Tumor-derived MUC1 mucins interact with differentiating monocytes and induce IL-10highIL-12low regulatory dendritic cell. Journal of immunology (Baltimore, Md: 1950) 2004;172:7341–7349. doi: 10.4049/jimmunol.172.12.7341. [DOI] [PubMed] [Google Scholar]
- 328.Ghiringhelli F, Puig P, Roux S, Parcellier A, Schmitt E, Solary E, Kroemer G, Martin F, Chauffert B, Zitvogel L. Tumor cells convert immature myeloid dendritic cells into TGF-{beta}-secreting cells inducing CD4+CD25+ regulatory T cell proliferation. J Exp Med. 2005;202:919–929. doi: 10.1084/jem.20050463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Chang CC, Ciubotariu R, Manavalan JS, Yuan J, Colovai AI, Piazza F, Lederman S, Colonna M, Cortesini R, Dalla-Favera R, Suciu-Foca N. Tolerization of dendritic cells by T(S) cells: the crucial role of inhibitory receptors ILT3 and ILT4. Nature Immunology. 2002;3:237–243. doi: 10.1038/ni760. [DOI] [PubMed] [Google Scholar]
- 330.Manavalan JS, Rossi PC, Vlad G, Piazza F, Yarilina A, Cortesini R, Mancini D, Suciu-Foca N. High expression of ILT3 and ILT4 is a general feature of tolerogenic dendritic cells. Transpl Immunol. 2003;11:245–258. doi: 10.1016/S0966-3274(03)00058-3. [DOI] [PubMed] [Google Scholar]
- 331.Ilarregui JM, Croci DO, Bianco GA, Toscano MA, Salatino M, Vermeulen ME, Geffner JR, Rabinovich GA. Tolerogenic signals delivered by dendritic cells to T cells through a galectin-1-driven immunoregulatory circuit involving interleukin 27 and interleukin 10. Nat Immunol. 2009:1–13. doi: 10.1038/ni.1772. [DOI] [PubMed] [Google Scholar]
- 332.Qin H, Vlad G, Cortesini R, Suciu-Foca N, Manavalan JS. CD8+ suppressor and cytotoxic T cells recognize the same human leukocyte antigen-A2 restricted cytomegalovirus peptide. Hum Immunol. 2008;69:776–780. doi: 10.1016/j.humimm.2008.08.287. [DOI] [PubMed] [Google Scholar]
- 333.Romani L, Bistoni F, Perruccio K, Montagnoli C, Gaziano R, Bozza S, Bonifazi P, Bistoni G, Rasi G, Velardi A, Fallarino F, Garaci E, Puccetti P. Thymosin alpha1 activates dendritic cell tryptophan catabolism and establishes a regulatory environment for balance of inflammation and tolerance. Blood. 2006;108:2265–2274. doi: 10.1182/blood-2006-02-004762. [DOI] [PubMed] [Google Scholar]
- 334.Ganesh BB, Cheatem DM, Sheng JR, Vasu C, Prabhakar BS. GM-CSF-induced CD11c+CD8a--dendritic cells facilitate Foxp3+ and IL-10+ regulatory T cell expansion resulting in suppression of autoimmune thyroiditis. Int Immunol. 2009;21:269–282. doi: 10.1093/intimm/dxn147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Penna G, Roncari A, Amuchastegui S, Daniel KC, Berti E, Colonna M, Adorini L. Expression of the inhibitory receptor ILT3 on dendritic cells is dispensable for induction of CD4+Foxp3+ regulatory T cells by 1,25-dihydroxyvitamin D3. Blood. 2005;106:3490–3497. doi: 10.1182/blood-2005-05-2044. [DOI] [PubMed] [Google Scholar]
- 336.Urzainqui A, Martínez del Hoyo G, Lamana A, de la Fuente H, Barreiro O, Olazabal IM, Martin P, Wild MK, Vestweber D, González-Amaro R, Sánchez-Madrid F. Functional role of P-selectin glycoprotein ligand 1/P-selectin interaction in the generation of tolerogenic dendritic cells. J Immunol. 2007;179:7457–7465. doi: 10.4049/jimmunol.179.11.7457. [DOI] [PubMed] [Google Scholar]
- 337.Ko H-J, Cho M-L, Lee S-Y, Oh H-J, Heo Y-J, Moon Y-M, Kang C-M, Kwok S-K, Ju JH, Park S-H, Park K-S, Kim H-Y. CTLA4-Ig modifies dendritic cells from mice with collagen-induced arthritis to increase the CD4+CD25+Foxp3+ regulatory T cell population. J Autoimmun. 2010;34:111–120. doi: 10.1016/j.jaut.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 338.Pettersson A, Ciumas C, Chirsky V, Link H, Huang Y-M, Xiao B-G. Dendritic cells exposed to estrogen in vitro exhibit therapeutic effects in ongoing experimental allergic encephalomyelitis. Journal of Neuroimmunology. 2004;156:58–65. doi: 10.1016/j.jneuroim.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 339.Gonzalez-Rey E, Delgado M. Therapeutic treatment of experimental colitis with regulatory dendritic cells generated with vasoactive intestinal peptide. Gastroenterology. 2006;131:1799–1811. doi: 10.1053/j.gastro.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 340.Gonzalez-Rey E, Chorny A, Fernandez-Martin A, Ganea D, Delgado M. Vasoactive intestinal peptide generates human tolerogenic dendritic cells that induce CD4 and CD8 regulatory T cells. Blood. 2006;107:3632–3638. doi: 10.1182/blood-2005-11-4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Delgado M, Gonzalez-Rey E, Ganea D. The neuropeptide vasoactive intestinal peptide generates tolerogenic dendritic cells. Journal of immunology (Baltimore, Md: 1950) 2005;175:7311–7324. doi: 10.4049/jimmunol.175.11.7311. [DOI] [PubMed] [Google Scholar]
- 342.Chorny A, Gonzalez-Rey E, Fernandez-Martin A, Pozo D, Ganea D, Delgado M. Vasoactive intestinal peptide induces regulatory dendritic cells with therapeutic effects on autoimmune disorders. Proc Natl Acad Sci USA. 2005;102:13562–13567. doi: 10.1073/pnas.0504484102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Chorny A, Gonzalez-Rey E, Fernandez-Martin A, Ganea D, Delgado M. Vasoactive intestinal peptide induces regulatory dendritic cells that prevent acute graft-versus-host disease while maintaining the graft-versus-tumor response. Blood. 2006;107:3787–3794. doi: 10.1182/blood-2005-11-4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Corrigall VM, Vittecoq O, Panayi GS. Binding immunoglobulin protein-treated peripheral blood monocyte-derived dendritic cells are refractory to maturation and induce regulatory T-cell development. Immunology. 2009;128:218–226. doi: 10.1111/j.1365-2567.2009.03103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Benkhoucha M, Santiago-Raber M-L, Schneiter G, Chofflon M, Funakoshi H, Nakamura T, Lalive PH. Hepatocyte growth factor inhibits CNS autoimmunity by inducing tolerogenic dendritic cells and CD25+Foxp3+ regulatory T cells. Proceedings of the National Academy of Sciences. 2010;107:6424–6429. doi: 10.1073/pnas.0912437107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Steinbrink K, Graulich E, Kubsch S, Knop J, Enk AH. CD4(+) and CD8(+) anergic T cells induced by interleukin-10-treated human dendritic cells display antigen-specific suppressor activity. Blood. 2002;99:2468–2476. doi: 10.1182/blood.v99.7.2468. [DOI] [PubMed] [Google Scholar]
- 347.Li X, Yang A, Huang H, Zhang X, Town J, Davis B, Cockcroft DW, Gordon JR. Induction of type 2 T helper cell allergen tolerance by IL-10-differentiated regulatory dendritic cells. Am J Respir Cell Mol Biol. 2010;42:190–199. doi: 10.1165/rcmb.2009-0023OC. [DOI] [PubMed] [Google Scholar]
- 348.Yamaura A, Hotta C, Nakazawa M, Van Kaer L, Minami M. Human invariant Valpha24+ natural killer T cells acquire regulatory functions by interacting with IL-10-treated dendritic cells. Blood. 2008;111:4254–4263. doi: 10.1182/blood-2007-04-085142. [DOI] [PubMed] [Google Scholar]
- 349.Sato K, Yamashita N, Baba M, Matsuyama T. Modified myeloid dendritic cells act as regulatory dendritic cells to induce anergic and regulatory T cells. Blood. 2003;101:3581–3589. doi: 10.1182/blood-2002-09-2712. [DOI] [PubMed] [Google Scholar]
- 350.Pacciani V, Gregori S, Chini L, Corrente S, Chianca M, Moschese V, Rossi P, Roncarolo MG, Angelini F. Induction of anergic allergen-specific suppressor T cells using tolerogenic dendritic cells derived from children with allergies to house dust mites. J Allergy Clin Immunol. 2010;125:727–736. doi: 10.1016/j.jaci.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 351.Torres-Aguilar H, Aguilar-Ruiz SR, González-Pérez G, Munguía R, Bajaña S, Meraz-Ríos MA, Sánchez-Torres C. Tolerogenic dendritic cells generated with different immunosuppressive cytokines induce antigen-specific anergy and regulatory properties in memory CD4+ T cells. Journal of immunology (Baltimore, Md: 1950) 2010;184:1765–1775. doi: 10.4049/jimmunol.0902133. [DOI] [PubMed] [Google Scholar]
- 352.Kubsch S, Graulich E, Knop J, Steinbrink K. Suppressor activity of anergic T cells induced by IL-10-treated human dendritic cells: association with IL-2- and CTLA-4-dependent G1 arrest of the cell cycle regulated by p27Kip1. Eur J Immunol. 2003;33:1988–1997. doi: 10.1002/eji.200323600. [DOI] [PubMed] [Google Scholar]
- 353.Sato K, Eizumi K, Fukaya T, Fujita S, Sato Y, Takagi H, Yamamoto M, Yamashita N, Hijikata A, Kitamura H, Ohara O, Yamasaki S, Saito T, Sato K. Naturally occurring regulatory dendritic cells regulate murine cutaneous chronic graft-versus-host disease. Blood. 2009;113:4780–4789. doi: 10.1182/blood-2008-10-183145. [DOI] [PubMed] [Google Scholar]
- 354.Fujita S, Sato Y, Sato K, Eizumi K, Fukaya T, Kubo M, Yamashita N, Sato K. Regulatory dendritic cells protect against cutaneous chronic graft-versus-host disease mediated through CD4+CD25+Foxp3+ regulatory T cells. Blood. 2007;110:3793–3803. doi: 10.1182/blood-2007-04-086470. [DOI] [PubMed] [Google Scholar]
- 355.Sato K, Yamashita N, Yamashita N, Baba M, Matsuyama T. Regulatory dendritic cells protect mice from murine acute graft-versus-host disease and leukemia relapse. Immunity. 2003;18:367–379. doi: 10.1016/s1074-7613(03)00055-4. [DOI] [PubMed] [Google Scholar]
- 356.Fu B-m, He X-s, Yu S, Hu A-b, Ma Y, Wu L-w, Tam N-l, Huang J-f. Tolerogenic semimature dendritic cells induce effector T-cell hyporesponsiveness by the activation of antigen-specific CD4+ CD25+ T-regulatory cells. Experimental and clinical transplantation: official journal of the Middle East Society for Organ Transplantation. 2009;7:149–156. [PubMed] [Google Scholar]
- 357.Zozulya AL, Ortler S, Lee J, Weidenfeller C, Sandor M, Wiendl H, Fabry Z. Intracerebral dendritic cells critically modulate encephalitogenic versus regulatory immune responses in the CNS. J Neurosci. 2009;29:140–152. doi: 10.1523/JNEUROSCI.2199-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Verginis P, Li HS, Carayanniotis G. Tolerogenic semimature dendritic cells suppress experimental autoimmune thyroiditis by activation of thyroglobulin-specific CD4+CD25+ T cells. Journal of immunology (Baltimore, Md: 1950) 2005;174:7433–7439. doi: 10.4049/jimmunol.174.11.7433. [DOI] [PubMed] [Google Scholar]
- 359.Menges M, Rössner S, Voigtländer C, Schindler H, Kukutsch NA, Bogdan C, Erb K, Schuler G, Lutz MB. Repetitive injections of dendritic cells matured with tumor necrosis factor alpha induce antigen-specific protection of mice from autoimmunity. J Exp Med. 2002;195:15–21. doi: 10.1084/jem.20011341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Min W-P, Zhou D, Ichim TE, Strejan GH, Xia X, Yang J, Huang X, Garcia B, White D, Dutartre P, Jevnikar AM, Zhong R. Inhibitory feedback loop between tolerogenic dendritic cells and regulatory T cells in transplant tolerance. J Immunol. 2003;170:1304–1312. doi: 10.4049/jimmunol.170.3.1304. [DOI] [PubMed] [Google Scholar]
- 361.Jiang A, Bloom O, Ono S, Cui W, Unternaehrer J, Jiang S, Whitney JA, Connolly J, Banchereau J, Mellman I. Disruption of E-cadherin-mediated adhesion induces a functionally distinct pathway of dendritic cell maturation. Immunity. 2007;27:610–624. doi: 10.1016/j.immuni.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Luther C, Adamopoulou E, Stoeckle C, Brucklacher-Waldert V, Rosenkranz D, Stoltze L, Lauer S, Poeschel S, Melms A, Tolosa E. Prednisolone Treatment Induces Tolerogenic Dendritic Cells and a Regulatory Milieu in Myasthenia Gravis Patients. The Journal of Immunology. 2009;183:841–848. doi: 10.4049/jimmunol.0802046. [DOI] [PubMed] [Google Scholar]
- 363.Matsumura Y, Kobayashi T, Ichiyama K, Yoshida R, Hashimoto M, Takimoto T, Tanaka K, Chinen T, Shichita T, Wyss-Coray T, Sato K, Yoshimura A. Selective expansion of foxp3-positive regulatory T cells and immunosuppression by suppressors of cytokine signaling 3-deficient dendritic cells. Journal of immunology (Baltimore, Md: 1950) 2007;179:2170–2179. doi: 10.4049/jimmunol.179.4.2170. [DOI] [PubMed] [Google Scholar]


