Abstract
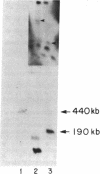
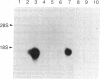
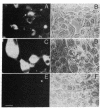
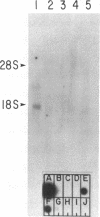
A class I gene distinct from HLA-A, -B, or -C was identified in a cosmid clone and transfected into mouse L cells. The gene, placed adjacent to the polyoma enhancer, produced a full-length class I mRNA and high levels of a 43-kDa protein in the cytoplasm. The surface expression of the gene product required its association with human beta 2-microglobulin. The protein was recognized by a xenoantiserum raised against a mixture of human B- and T-cell lines. The product was also serologically reactive with the HLA framework monoclonal antibodies. The complete nucleotide sequence of the gene was determined and a specific oligonucleotide probe was synthesized. This probe was used to identify a full-length mRNA transcript in a B-lymphoblastoid cell line (JY). The gene was mapped within a 190-kilobase Not I restriction fragment located in the telomeric portion of the human major histocompatibility complex. Distinct features of the gene include the structure of the promoter, the position of the translation initiation site, a frameshift mutation at the carboxyl terminus, the insertion of an Alu repeat element in the eighth exon, divergence in the derived amino acid sequence, and the lack of expression of the gene in some cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bushkin Y., Posnett D. N., Pernis B., Wang C. Y. A new HLA-linked T cell membrane molecule, related to the beta chain of the clonotypic receptor, is associated with T3. J Exp Med. 1986 Aug 1;164(2):458–473. doi: 10.1084/jem.164.2.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson W. F., Kress M., Khoury G., Jay G. Comparison of HLA class I gene sequences. Derivation of locus-specific oligonucleotide probes specific for HLA-A, HLA-B, and HLA-C genes. J Biol Chem. 1985 Nov 5;260(25):13414–13423. [PubMed] [Google Scholar]
- Devlin J. J., Lew A. M., Flavell R. A., Coligan J. E. Secretion of a soluble class I molecule encoded by the Q10 gene of the C57BL/10 mouse. EMBO J. 1985 Feb;4(2):369–374. doi: 10.1002/j.1460-2075.1985.tb03638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duceman B. W., Ness D., Rende R., Chorney M. J., Srivastava R., Greenspan D. S., Pan J., Weissman S. M., Grumet F. C. HLA-JY328: mapping studies and expression of a polymorphic HLA class I gene. Immunogenetics. 1986;23(2):90–99. doi: 10.1007/BF00377967. [DOI] [PubMed] [Google Scholar]
- Duncan C. H., Jagadeeswaran P., Wang R. R., Weissman S. M. Structural analysis of templates and RNA polymerase III transcripts of Alu family sequences interspersed among the human beta-like globin genes. Gene. 1981 Mar;13(2):185–196. doi: 10.1016/0378-1119(81)90007-x. [DOI] [PubMed] [Google Scholar]
- Favaloro J., Treisman R., Kamen R. Transcription maps of polyoma virus-specific RNA: analysis by two-dimensional nuclease S1 gel mapping. Methods Enzymol. 1980;65(1):718–749. doi: 10.1016/s0076-6879(80)65070-8. [DOI] [PubMed] [Google Scholar]
- Fisher D. A., Hunt S. W., 3rd, Hood L. Structure of a gene encoding a murine thymus leukemia antigen, and organization of Tla genes in the BALB/c mouse. J Exp Med. 1985 Aug 1;162(2):528–545. doi: 10.1084/jem.162.2.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumaki Y., Collins F., Kole R., Stoeckert C. J., Jr, Jagadeeswaran P., Duncan C. H., Weissman S. M., Jagadeeswaran P., Pan J., Forget B. G. Sequences of human repetitive DNA, non-alpha-globin genes, and major histocompatibility locus genes. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):1079–1086. [PubMed] [Google Scholar]
- Gladstone P., Fueresz L., Pious D. Gene dosage and gene expression in the HLA region: evidence from deletion variants. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1235–1239. doi: 10.1073/pnas.79.4.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guild B. C., Strominger J. L. HLA-A2 antigen phosphorylation in vitro by cyclic AMP-dependent protein kinase. Sites of phosphorylation and segmentation in class i major histocompatibility complex gene structure. J Biol Chem. 1984 Nov 10;259(21):13504–13510. [PubMed] [Google Scholar]
- Hood L., Steinmetz M., Malissen B. Genes of the major histocompatibility complex of the mouse. Annu Rev Immunol. 1983;1:529–568. doi: 10.1146/annurev.iy.01.040183.002525. [DOI] [PubMed] [Google Scholar]
- Kahn-Perles B., Wietzerbin J., Caillol D. H., Lemonnier F. Delineation of three subsets of class I human T antigens (HTA) on Molt-4 cells: serologic and regulatory relationship to HLA class I antigens. J Immunol. 1985 Mar;134(3):1759–1765. [PubMed] [Google Scholar]
- Kavathas P., Herzenberg L. A. Stable transformation of mouse L cells for human membrane T-cell differentiation antigens, HLA and beta 2-microglobulin: selection by fluorescence-activated cell sorting. Proc Natl Acad Sci U S A. 1983 Jan;80(2):524–528. doi: 10.1073/pnas.80.2.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller B. H., Orr H. T. Cloning and complete sequence of an HLA-A2 gene: analysis of two HLA-A alleles at the nucleotide level. J Immunol. 1985 Apr;134(4):2727–2733. [PubMed] [Google Scholar]
- Kress M., Barra Y., Seidman J. G., Khoury G., Jay G. Functional insertion of an Alu type 2 (B2 SINE) repetitive sequence in murine class I genes. Science. 1984 Nov 23;226(4677):974–977. doi: 10.1126/science.6095445. [DOI] [PubMed] [Google Scholar]
- Lawrance S. K., Das H. K., Pan J., Weissman S. M. The genomic organisation and nucleotide sequence of the HLA-SB(DP) alpha gene. Nucleic Acids Res. 1985 Oct 25;13(20):7515–7528. doi: 10.1093/nar/13.20.7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrance S. K., Smith C. L., Srivastava R., Cantor C. R., Weissman S. M. Megabase-scale mapping of the HLA gene complex by pulsed field gel electrophoresis. Science. 1987 Mar 13;235(4794):1387–1390. doi: 10.1126/science.3029868. [DOI] [PubMed] [Google Scholar]
- Malissen M., Malissen B., Jordan B. R. Exon/intron organization and complete nucleotide sequence of an HLA gene. Proc Natl Acad Sci U S A. 1982 Feb;79(3):893–897. doi: 10.1073/pnas.79.3.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin L. H., Calabi F., Milstein C. Isolation of CD1 genes: a family of major histocompatibility complex-related differentiation antigens. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9154–9158. doi: 10.1073/pnas.83.23.9154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathenson S. G., Geliebter J., Pfaffenbach G. M., Zeff R. A. Murine major histocompatibility complex class-I mutants: molecular analysis and structure-function implications. Annu Rev Immunol. 1986;4:471–502. doi: 10.1146/annurev.iy.04.040186.002351. [DOI] [PubMed] [Google Scholar]
- Orr H. T., DeMars R. Class I-like HLA genes map telomeric to the HLA-A2 locus in human cells. Nature. 1983 Apr 7;302(5908):534–536. doi: 10.1038/302534a0. [DOI] [PubMed] [Google Scholar]
- Pontarotti P. A., Mashimo H., Zeff R. A., Fisher D. A., Hood L., Mellor A., Flavell R. A., Nathenson S. G. Conservation and diversity in the class I genes of the major histocompatibility complex: sequence analysis of a Tlab gene and comparison with a Tlac gene. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1782–1786. doi: 10.1073/pnas.83.6.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronne H., Widmark E., Rask L., Peterson P. A. Intron sequences reveal evolutionary relationships among major histocompatibility complex class I genes. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5860–5864. doi: 10.1073/pnas.82.17.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood A. K., Pan J., Biro P. A., Pereira D., Srivastava R., Reddy V. B., Duceman B. W., Weissman S. M. Structure and polymorphism of class I MHC antigen mRNA. Immunogenetics. 1985;22(2):101–121. doi: 10.1007/BF00563508. [DOI] [PubMed] [Google Scholar]
- Sood A. K., Pereira D., Weissman S. M. Isolation and partial nucleotide sequence of a cDNA clone for human histocompatibility antigen HLA-B by use of an oligodeoxynucleotide primer. Proc Natl Acad Sci U S A. 1981 Jan;78(1):616–620. doi: 10.1073/pnas.78.1.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava R., Duceman B. W., Biro P. A., Sood A. K., Weissman S. M. Molecular organization of the class I genes of human major histocompatibility complex. Immunol Rev. 1985 Jul;84:93–121. doi: 10.1111/j.1600-065x.1985.tb01127.x. [DOI] [PubMed] [Google Scholar]
- Srivastava R., Lawrance S. K., Smith C. L., Cantor C. R., Weissman S. M. Long-range and molecular mapping of the human major histocompatibility complex. Trans Assoc Am Physicians. 1986;99:1–12. [PubMed] [Google Scholar]
- Szöts H., Riethmüller G., Weiss E., Meo T. Complete sequence of HLA-B27 cDNA identified through the characterization of structural markers unique to the HLA-A, -B, and -C allelic series. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1428–1432. doi: 10.1073/pnas.83.5.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terhorst C., Parham P., Mann D. L., Strominger J. L. Structure of HLA antigens: amino-acid and carbohydrate compositions and NH2-terminal sequences of four antigen preparations. Proc Natl Acad Sci U S A. 1976 Mar;73(3):910–914. doi: 10.1073/pnas.73.3.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]