Abstract
Background
HF-ACTION assigned 2331 outpatients with medically stable heart failure to exercise training or usual care. We compared medical resource use and costs incurred by these patients during follow-up.
Methods and Results
Extensive data on medical resource use and hospital bills were collected throughout the trial for estimates of direct medical costs. Intervention costs were estimated using patient-level trial data, administrative records, and published unit costs. Mean follow-up was 2.5 years. There were 2297 hospitalizations in the exercise group and 2332 in the usual care group (P = .92). The mean number of inpatient days was 13.6 (SD, 27.0) in the exercise group and 15.0 (SD, 31.4) in the usual care group (P = .23). Other measures of resource use were similar between groups, except for trends indicating that fewer patients in the exercise group underwent high-cost inpatient procedures. Total direct medical costs per participant were an estimated $50,857 (SD, $81,488) in the exercise group and $56,177 (SD, $92,749) in the usual care group (95% confidence interval for the difference, $–12,755 to $1547; P = .10). The direct cost of exercise training was an estimated $1006 (SD, $337). Patient time costs were an estimated $5018 (SD, $4600).
Conclusions
The cost of exercise training was relatively low for the health care system, but patients incurred significant time costs. In this economic evaluation, there was little systematic benefit in terms of overall medical resource use with this intervention.
Trial Registration
clinicaltrials.gov Identifier: NCT00047437
Keywords: Costs and Cost Analysis, Exercise Therapy, Heart Failure
Introduction
More than 4 million Medicare beneficiaries have a diagnosis of heart failure.1 After hospitalization for heart failure, approximately 1 in 4 patients are readmitted within 30 days and two thirds are readmitted within 1 year.1 Postdischarge mortality is 10% at 30 days, 22% at 1 year, and 42% at 5 years.2 In 2009, total direct costs of heart failure in the United States will be an estimated $33.7 billion, of which approximately 60% will be attributable to inpatient care.3 Lower-cost interventions that improve outcomes and quality of life among patients with heart failure could help to mitigate the upward trend in health care expenditures.
Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION) was a multicenter study of exercise training plus usual care versus usual care alone in medically stable outpatients with heart failure and reduced ejection fraction. The trial randomly assigned 1159 patients to 36 supervised sessions of exercise training followed by home-based exercise, in addition to usual care, and 1172 patients to usual care alone. In the first 3 months, the exercise training group experienced statistically significant but modest improvements in walk distance, exercise time, peak oxygen consumption, and self-reported health status.4 During a mean follow-up of 2.5 years, 65% of patients in the exercise training group and 68% in the usual care group died or were hospitalized for any reason.5 After adjustment for highly prognostic baseline characteristics, exercise training was associated with modest significant reductions in all-cause mortality or all-cause hospitalization and cardiovascular mortality or heart failure hospitalization. Exercise training had nonsignificant effects on cardiovascular mortality or cardiovascular hospitalization.
Although the clinical benefits of exercise training in HF-ACTION were modest, the hazard ratios represented the timing of first events and did not fully reflect the experiences of patients over time. Furthermore, the exercise regimen required a cost commitment by health care payers and a time commitment by patients. Because health care resources and patients’ time are limited, a wise investment is one in which the value of benefits realized with an intervention is commensurate with or exceeds the time and resources required. A secondary objective of HF-ACTION was to test the hypothesis that exercise training would be economically attractive (ie, cost-saving or a reasonable value). Therefore, we estimated the costs of exercise training and the cumulative medical resource use and costs incurred by patients enrolled in HF-ACTION from the societal perspective. We also calculated the cost-effectiveness of exercise training observed during the trial.
Methods
The design of HF-ACTION has been described previously.6 Eligible patients had left ventricular ejection fraction (LVEF) of 35% or less and New York Heart Association class II to IV symptoms. Patients were randomly assigned to either exercise training plus usual care or usual care alone, and both groups received approximately 30 minutes of education from a nurse and printed materials to promote self-management of heart failure, including a recommendation of 30 minutes of moderate-intensity activity on most days of the week.7
Exercise Training
Patients in the exercise training group were prescribed a regimen of 36 supervised exercise training sessions with a goal of 3 sessions per week over 12 weeks. After the first 18 sessions, patients were to begin transition to home-based exercise with a goal of 5 sessions per week. Home exercise equipment (ie, heart rate monitor and either stationary bicycle or treadmill) was provided to patients in the exercise training group. During supervised training, the duration of time spent exercising was recorded, exclusive of warm-up and cool-down.
Data Collection
Patients in both groups were scheduled for study visits every 3 months for the first 24 months, then once every 12 months thereafter, with a maximum follow-up of 4 years. At the 3-month, 12-month, and 24-month visits, patients were to undergo cardiopulmonary exercise testing and a 6-minute walk test, the latter of which was also to be performed at the 36-month and final visits. Patients in both groups were also to receive “monitoring” telephone calls every 2 weeks for the first 9 months, then monthly until 24 months, and quarterly calls thereafter. Time spent on home exercise by patients in the exercise training group was to be recorded in adherence logs completed quarterly through 24 months, then at the 36-month and final visits.
The EQ-5D, a 5-item questionnaire used to obtain utility weights for use in cost-effectiveness analysis, was completed by patients quarterly through the first year of follow-up, then annually and at the final visit.8 In addition, detailed information on medical resource use was collected from patients and verified through available sources. These resources included 27 types of medication, urgent and nonurgent outpatient visits and procedures, days of home intravenous therapy, days in skilled nursing facilities and rehabilitation centers, and inpatient care, including admission and discharge dates, reason for admission, cardiac procedures, and discharge destination. For patients enrolled at US sites, hospital billing data were collected for inpatient admissions and urgent care provided in emergency departments and observation units.
A time survey was developed to obtain estimates of patient travel time, and the time exercise trainers (eg, exercise physiologists, nurses) spent before and after each session on activities with patients (eg, scheduling, warm-up) and without patients (eg, cleaning equipment, documentation) from 9 sites. These sites were also asked to report the number of patients supervised during each training session. Patients also reported the number of miles they lived from the exercise facility.
The institutional review board of the Duke University Health System approved this study. All patients provided written informed consent.
Cost Estimation of Medical Resources
In accordance with standard methods for economic evaluation in health care,9 we valued costs from the societal perspective and reported costs in 2008 US dollars. We discounted costs incurred beyond the first year of follow-up at 3% per annum. Professional fees for inpatient services, outpatient visits, and inpatient and outpatient procedures were based on the 2008 Medicare Physician Fee Schedule. Medication costs were based on average wholesale prices and estimated duration of treatment.10 Costs for skilled nursing and rehabilitative care were based on Medicare reimbursement rates.11,12 Costs for home intravenous therapy were derived from drug costs11 and government reimbursement rates.11,13
We converted hospital charges to costs by multiplying department-level cost-to-charge ratios from each hospital’s annual Medicare cost report and department-specific charges. We applied the same method to estimate costs of care in emergency departments and observation units. When bills for these visits were not available, we applied median cost estimates from available bills.
To impute costs for the 815 (17.6%) hospitalizations for which complete hospital bills were not available, we multiplied the median daily costs from available bills for 47 categories representing the primary reason for admission by the length of stay. To avoid overestimating costs when patients were hospitalized for procedures characterized by high costs and short lengths of stay (see Table 1), we modified this method. When the length of stay for an individual admission was longer than the median length of stay associated with the procedure, we estimated costs by assigning the median cost estimate. For each additional day beyond the median, we applied the daily cost for a heart failure admission ($1202).
Table 1.
Counts of Inpatient Tests and Procedures
| Test or Procedure | No. (%) |
P Value* | |
|---|---|---|---|
| Usual Care (n = 1172) | Exercise Training (n = 1159) | ||
| Cardiac catheterization | 171 (14.6) | 153 (13.2) | .33 |
| Coronary artery bypass surgery | 15 (1.3) | 9 (0.8) | .23 |
| PCI without stent† | 5 (0.4) | 6 (0.5) | .75 |
| PCI with non-drug-coated stent† | 10 (0.9) | 16 (1.4) | .22 |
| PCI with drug-coated stent† | 26 (2.2) | 31 (2.7) | .48 |
| Heart transplantation | 34 (2.9) | 22 (1.9) | .11 |
| Valve surgery | 8 (0.7) | 3 (0.3) | .14 |
| Intra-aortic balloon pump placement | 6 (0.5) | 9 (0.8) | .42 |
| Left ventricular assist device placement or removal | 23 (2.0) | 14 (1.2) | .14 |
| Thoracentesis | 4 (0.3) | 5 (0.4) | .73 |
| Pulmonary artery catheter placement (eg, Swan-Ganz catheter) | 41 (3.5) | 41 (3.5) | .96 |
| Venous catheter placement (eg, Hickman line) | 18 (1.5) | 24 (2.1) | .33 |
| ICD placement or removal† | 128 (10.9) | 85 (7.3) | .003 |
| Combination ICD/pacemaker† | 66 (5.6) | 66 (5.7) | .95 |
| Pacemaker† | 12 (1.0) | 8 (0.7) | .38 |
| Biventricular pacemaker or cardiac resynchronization therapy† | 55 (4.7) | 57 (4.9) | .80 |
| Programmed electrophysiology test | 45 (3.8) | 39 (3.4) | .54 |
| Electrocardiogram | 486 (41.5) | 475 (41.0) | .81 |
| Multigated acquisition scan, rest | 3 (0.3) | 7 (0.6) | .20 |
| Transthoracic echocardiogram | 293 (25.0) | 279 (24.1) | .60 |
| Transesophageal echocardiogram | 43 (3.7) | 35 (3.0) | .38 |
| Cardiac exercise stress test with imaging | 21 (1.8) | 22 (1.9) | .85 |
| Cardiac exercise stress test without imaging | 11 (0.9) | 3 (0.3) | .03 |
| Cardiac pharmacologic stress test with imaging | 54 (4.6) | 49 (4.2) | .66 |
| Cardiac pharmacologic stress test without imaging | 2 (0.2) | 4 (0.3) | .41 |
| ICD firing | 44 (3.8) | 42 (3.6) | .87 |
| Magnetic resonance imaging scan, cardiac | 9 (0.8) | 5 (0.4) | .29 |
| Computed tomography scan, cardiac | 31 (2.6) | 34 (2.9) | .67 |
Abbreviations: PCI, percutaneous coronary intervention; ICD, implantable cardioverter-defibrillator.
P values were not adjusted for multiple comparisons.
Procedure characterized by high costs and short length of stay.
Protocol-Driven Costs
We also assigned costs to educational resources, tests, procedures, and study visits required by the protocol. Although both study groups incurred these costs, we included them in the analysis to better reflect the total costs required to achieve the levels of adherence and monitoring observed in HF-ACTION.
Exercise Training
From the perspective of the health care system, direct costs for supervised exercise training include personnel and fixed facility costs. Direct costs for home-based exercise include equipment costs and the costs of personnel for “monitoring” calls (assumed to last 15 minutes) to promote adherence. The Appendix shows the unit costs and assumptions we applied in the cost calculations. The time exercise trainers spent on each session represents the time before and after each session plus the time each patient spent exercising.
We conducted sensitivity analyses of the impact of study assumptions on the direct costs of supervised exercise training. First, instead of the salary of an exercise physiologist, we applied the salary of a registered nurse. Second, we used a “top-down” cost-estimation approach in which we assigned the average 2008 reimbursement rate for cardiac rehabilitation ($36.21) to each exercise session instead of our cost estimate.14 Finally, we varied the number of patients simultaneously supervised by one trainer and the fixed cost applied to each session.
Patient Time
Recommendations for economic evaluations in health care also call for estimation of patients’ time-related costs.9,15 For supervised exercise sessions, these costs included travel time, time with the trainer before and after exercise, and time spent exercising; the latter component varied across sessions and patients. For home exercise, time was limited to the reported time spent exercising.
Cost-Effectiveness Analysis
We estimated the cost-effectiveness of exercise training by calculating the ratio of the difference in mean costs between the exercise training and usual care groups to the difference in mean quality-adjusted life-years (QALYs).9 To estimate QALYs, we used utility weights from the United States16 derived from the EQ-5D from baseline through the final visit to calculate the area under the quality-adjusted survival curve using the trapezoidal rule. For patients who died, we extrapolated the most recent EQ-5D score to zero on the death date. In other cases with missing data, we applied the patient’s most recent utility score.
Statistical Analysis
We used chi-square tests to compare proportions. Using generalized estimating equations, we compared counts of medical resource use by applying log links and negative binomial distributions and we compared costs by applying log links and gamma distributions. In adjusted comparisons of total costs, we included the 5 prognostic variables included in the adjusted analyses of the clinical end points5 (ie, duration of cardiopulmonary exercise testing, LVEF, Beck Depression Inventory II score, history of atrial fibrillation or flutter, and heart failure etiology) plus the number of hospitalizations during the 6-month period before randomization. We used nonparametric bootstrapping to calculate bias-adjusted 95% confidence intervals (CIs) for differences in costs and QALYs and their joint distributions.17 We used SAS version 9.1 (SAS Institute Inc, Cary, North Carolina) for all statistical analyses.
Results
From April 2003 through February 2007, 2331 patients were enrolled at 82 sites in the United States (n = 2068), Canada (n = 188), and France (n = 75). Median age at baseline was 59 years, 28% were women, and 40% were racial/ethnic minorities. Median follow-up was 2.5 years in both groups.
Resource Use
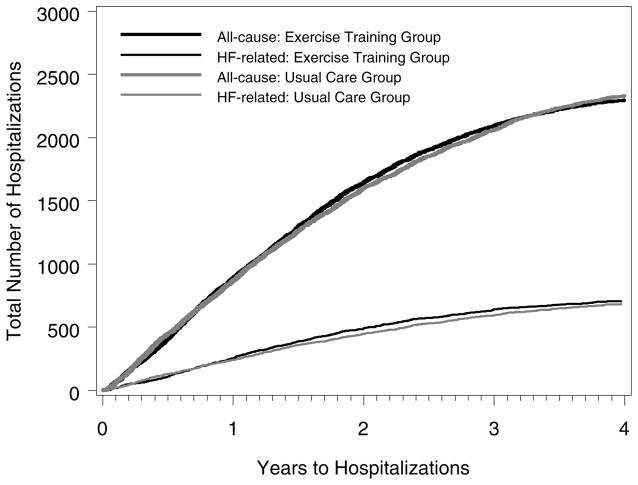
A total of 4629 hospitalizations occurred during the follow-up period: 2297 in the exercise training group and 2332 in the usual care group (P =.92). Figure 1 shows the cumulative number of all-cause and heart failure hospitalizations. The mean number of inpatient days was 13.6 (SD, 27.0) in the exercise training group and 15.0 (SD, 31.4) in the usual care group (P = .23).
Figure 1.
Cumulative Counts of All-Cause and Heart Failure Hospitalizations
Table 1 shows the proportions of patients who underwent inpatient cardiac procedures. Significantly fewer patients in the exercise training group received an implantable cardioverter-defibrillator (ICD), pacemaker, combination ICD/pacemaker, biventricular pacemaker, or cardiac resynchronization therapy (P = .03). However, among patients who received an ICD, pacemaker, or biventricular pacemaker before or during the study, the proportions were similar (P = .73). Fewer patients in the exercise group underwent heart transplantation or placement of a left ventricular assist device (LVAD).
Table 2 shows the mean number of urgent and nonurgent care visits during follow-up. Urgent care visits were similar between groups. Patients in the exercise training group had significantly more nonurgent care visits to specialists other than cardiologists or orthopedic surgeons and to primary care physicians, compared with patients in the usual care group. Other medical resource use was similar between the groups on average but varied considerably across patients.
Table 2.
Medical Resource Use
| Resource | Mean (SD) |
P Value* | |
|---|---|---|---|
| Usual Care (n = 1172) | Exercise Training (n = 1159) | ||
| Urgent care visit | 1.92 (3.30) | 1.83 (2.60) | .31 |
| Emergency department or observation unit | 1.54 (2.83) | 1.46 (2.24) | .37 |
| Heart failure clinic or office | 0.26 (1.11) | 0.23 (0.90) | .37 |
| Standalone urgent care facility | 0.12 (0.44) | 0.14 (0.58) | .15 |
| Outpatient cardiac or orthopedic procedure | 4.17 (4.15) | 4.48 (4.17) | .02 |
| Nonurgent outpatient visit | 29.45 (33.62) | 31.69 (31.62) | .01 |
| Cardiologist | 7.26 (7.69) | 7.46 (7.66) | .48 |
| Orthopedic surgeon | 0.44 (1.48) | 0.50 (1.49) | .40 |
| Other specialist | 4.91 (7.59) | 5.70 (8.42) | .01 |
| Primary care physician | 6.54 (8.21) | 7.26 (7.96) | .01 |
| Physician extender | 1.83 (4.44) | 2.23 (6.23) | .01 |
| Occupational or physical therapy | 1.81 (7.58) | 2.02 (7.87) | .39 |
| Mental health provider | 0.88 (6.11) | 0.79 (8.04) | .73 |
| Nurse | 3.63 (12.26) | 3.82 (11.01) | .67 |
| Other | 2.13 (8.19) | 1.91 (6.30) | .36 |
| Home intravenous therapy, d | 4.10 (29.51) | 4.94 (27.65) | .51 |
| Skilled nursing facility, d | 1.99 (19.08) | 1.64 (12.77) | .53 |
| Rehabilitation center, d | 1.48 (15.15) | 0.85 (6.56) | .15 |
P values are from generalized estimating equations regression with binomial distributions. Estimates represent the mean number of visits or days per patient during the follow-up period. P values were not adjusted for multiple comparisons.
Direct Medical Costs
Table 3 shows that mean direct medical costs, excluding the cost of exercise training, were $5320 lower in the exercise group compared with the usual care group (95% CI for the difference, $–12,755 to $1547; P = .10). This difference was attributable to lower mean costs for inpatient care. Although relatively few patients underwent high-cost inpatient procedures, median inpatient costs were $2720 lower in the exercise training group than in the usual care group (95% CI for the difference, $–5551 to $2).
Table 3.
Estimated Medical Costs, Intervention Costs, and Patient Time Costs
| Cost Component | Usual Care (n = 1172) | Exercise Training (n = 1159) |
|---|---|---|
| Baseline costs, $ | ||
| Patient education | 25 (0) | 26 (0) |
| Cardiopulmonary exercise test | 105 (0) | 105 (0) |
| Six-minute walk test | 59 (0) | 59 (0) |
| Echocardiogram | 212 (0) | 212 (0) |
| Study visit | 91 (0) | 91 (0) |
| Protocol costs after baseline, mean (SD), $ | ||
| Study visits | 625 (244) | 647 (221) |
| Cardiopulmonary exercise tests at 3, 12, and 24 months | 229 (96) | 242 (83) |
| Six-minute walk tests at 3, 12, 24, and 36 months | 201 (69) | 208 (63) |
| Monitoring telephone calls* | 225 (59) | 227 (54) |
| Total nonintervention protocol costs, including baseline | 1769 (449) | 1817 (401) |
| Medical resources | ||
| Concomitant medications | 8869 (5730) | 8847 (5627) |
| Nonurgent outpatient visits | 2196 (2650) | 2349 (2382) |
| Outpatient cardiac and orthopedic procedures | 1761 (4769) | 1729 (4400) |
| Urgent/emergent care visits | 1622 (4046) | 1400 (2996) |
| Intravenous infusion | 422 (3007) | 508 (2830) |
| Skilled nursing facility | 565 (5673) | 501 (3928) |
| Rehabilitation | 514 (5302) | 299 (2290) |
| Hospitalizations | ||
| Inpatient hospital costs | 36,308 (83,935) | 31,511 (73,892) |
| Physician rounding fees | 1491 (2901) | 1367 (2570) |
| Physician procedure fees | 659 (1310) | 529 (1120) |
| Total, mean | 38,459 (87,432) | 33,407 (76,854) |
| Total, median (interquartile range) | 10,528 (0–41,706) | 7807 (0–37,137) |
| Total direct medical costs | ||
| Mean (SD) | 56,177 (92,749) | 50,857 (81,488) |
| Median (interquartile range) | 28,245 (13,067–60,942) | 25,904 (13,574–56,725) |
| Exercise intervention costs, $ | ||
| Direct medical costs | ||
| Supervised exercise training* | 0 | 632 (259) |
| Home exercise training† | 0 | 374 (120) |
| Total‡ | 0 | 1006 (337) |
| Patient time | ||
| Supervised exercise training | 0 | 1045 (418) |
| Home exercise training | 0 | 3974 (4395) |
| Total§ | 0 | 5018 (4600) |
| Direct nonmedical costs | ||
| Travel and parking | 0 | 457 (176) |
| Total exercise intervention costs | 0 | 6482 (4884) |
| Total costs, excluding patient time and direct nonmedical costs, $ | ||
| Mean (SD) | 56,177 (92,749) | 51,863 (81,469) |
| Median (interquartile range) | 28,245 (13,067–60,942) | 26,933 (14,470–57,875) |
| Total costs, including patient time and direct nonmedical costs, $ | ||
| Mean (SD) | 56,177 (92,749) | 57,338 (81,343) |
| Median (interquartile range) | 28,245 (13,067–60,942) | 34,228 (19,840–64,493) |
Values are expressed as mean (SD) unless otherwise indicated.
Based on the salary of an exercise physiologist ($27.83 per hour).
Includes cost for bike or treadmill, heart rate monitor, shipping and set-up.
Does not include $227 for telephone calls to promote adherence.
Does not include patient time for telephone calls to promote adherence.
Exercise Training Costs
In 1104 responses to the 9-site time survey, the mean number of patients supervised during each training session was 1.7 (SD, 1.0). Trainers reported spending a total of 35 minutes on non-exercise activities for each session, representing the sum of 4 median estimates of time before and after each session with and without patients.
On average, patients completed 32.7 (SD, 12.7) supervised exercise sessions representing 17.1 (SD, 7.9) hours of exercise. When including time before and after each session, exercise trainers spent an average of 36.0 (SD, 14.8) hours per patient (assuming a 1:1 patient-to-trainer ratio) and patients spent an average of 52.8 (SD, 21.2) hours including travel time. In the base-case analysis with a patient-to-trainer ratio of 1.7 and the salary of an exercise physiologist, the direct total cost of supervised exercise training was an estimated $632 per patient ($588 for personnel and $44 for facility costs). Mean patient time costs for supervised exercise training were an estimated $1045 per patient.
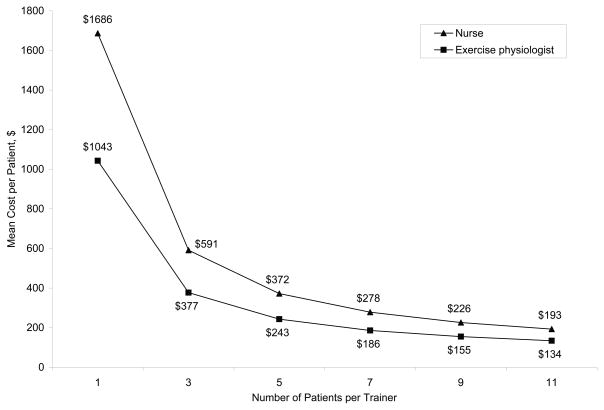
Figure 2 shows the results of the sensitivity analyses. Costs decreased as the patient-to-trainer ratio increased, but the relationship was not linear because facility costs remained constant. At a ratio of 1.7, the estimated cost with a nurse’s salary ($1009) was closer to the cost derived from the top-down method of $1183 (SD, $457) per patient. When hourly fixed facility costs increased by $1.22 per patient, the mean cost of supervised exercise increased linearly by approximately $43.
Figure 2.
Direct Costs for Supervised Exercise Training for Various Patient-to-Trainer Ratios
Eighty-six percent (997/1159) of patients in the exercise training group provided exercise logs indicating some home exercise. Patients spent a mean of 204.7 (SD, 228.0) hours on home exercise and a median 125.7 hours. Direct costs to facilitate home exercise training averaged $601, including $374 in exercise equipment costs and $227 in personnel costs to promote adherence. Patient time costs for home exercise were an estimated $3974 per patient.
Total Costs
Total costs in the exercise training group, including direct medical costs and intervention costs, were an estimated $51,863, approximately $4300 lower than in the usual care group (95% CI for the difference, $–11,690 to $2631; P = .19). Adjustment for baseline differences in prognostic variables and previous hospitalizations narrowed the difference in expected total costs between groups to $2636 ($52,917 in the exercise training group and $55,553 in the usual care group; P = .43). With the inclusion of patient time costs and out-of-pocket costs for travel and parking, total costs in the exercise training group increased to $57,338, $1161 higher than the usual care group (95% CI for the difference, $–6205 to $8404; P = .73 without adjustment; P = .36 with adjustment).
Cost-Effectiveness
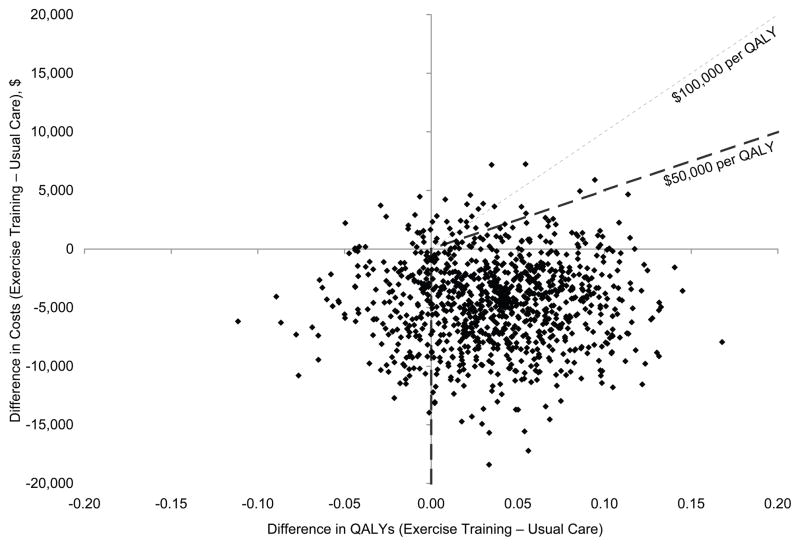
Among patients who completed the EQ-5D at baseline, mean undiscounted QALYs were an estimated 2.02 (SD, 1.00) among 1150 patients in the exercise training group, compared with 1.99 (SD, 1.01) among 1158 patients in the usual care group (95% CI for the difference, −0.06 to 0.11). Figure 3 shows a scatterplot of 1000 bootstrap samples representing the joint distribution of differences in mean direct medical costs (including intervention costs, excluding patient time, parking, and travel costs) and mean QALYs. The figure indicates that most estimates were consistent with a decrease in costs (89.9%) and an increase in QALYs (76.5%) and that 73.2% of the bootstrap replications were either associated with lower costs and increased QALYs or were associated with increased QALYs at or below the $50,000 per QALY threshold (upper right quadrant below the line for $50,000 per QALY). With a $100,000 per QALY threshold, 74.4% of the estimates would meet the cost-effectiveness criterion. When we included patient time, parking, and travel costs, 47.9% of the estimates were at or below the $50,000 per QALY threshold and 59.2% of the estimates were at or below the $100,000 per QALY threshold.
Figure 3.
Scatterplot of 1000 Bootstrap Replications Representing Incremental Costs (Including Intervention Costs and Excluding Patient Time, Travel, and Parking Costs) and Quality-Adjusted Life Years (QALYs)
Discussion
During 2.5 years of follow-up, patients in both study groups incurred an average of more than $50,000 in direct medical costs, reflecting the high rates of interaction that patients with heart failure have with the health care system. More than half of the costs were attributable to inpatient care, and about one sixth were attributable to medications. Based on point estimates, mean unadjusted direct medical costs in the exercise training group were $4300 lower than in the usual care group. However, with patient time, travel, and parking costs, the cost difference disappeared and there was no statistically significant difference in costs between the groups.
Unlike the adjusted time-to-event analyses in the clinical study, which found a modest clinical benefit with exercise training,5 our examination of the cumulative experience of patients revealed no significant group differences in rates of all-cause or heart failure hospitalizations. This finding reflects a different approach to examining clinical outcomes. Time-to-event analyses account for the benefit of delaying a first event, whereas cumulative event analyses do not consider the timing of events but account for all events during the follow-up period. Some trialists recommend routine reporting of the total number of events and the proportions of patients with first events and their timing.18 Previous trials of heart failure therapies have also found more modest effects when examining all events rather than time-to-event end points.19,20
The estimates of cost and cost-effectiveness were also influenced by the difference in the number of patients who underwent high-cost inpatient procedures, particularly heart transplantations and LVAD and ICD implantations. The finding that significantly fewer patients in the exercise training group received an ICD during the study may be attributable to the larger number of patients in the exercise training group who had an ICD at baseline (42.3%) compared with the usual care group (38.2%, P = .05). Also, despite nonsignificant differences in the proportions of patients who underwent heart transplantation or LVAD placement, the high cost of hospitalization for these procedures (approximately $200,000 for heart transplantation and $225,000 for LVAD placement) accounted for approximately 20% of mean inpatient costs overall. When we excluded costs for these hospitalizations, mean inpatient costs were similar between the groups ($23,060 in the exercise training group and $23,169 in the usual care group). When we applied these costs in the cost-effectiveness analysis, the proportion of bootstrap replications consistent with increased QALYs at or below the $50,000 per QALY threshold decreased from approximately 73% in the primary analysis to 57%. Using a threshold of $100,000 per QALY, the proportion increased to 66%. These findings indicate that the cost-effectiveness of exercise training is relatively uncertain, given that approximately one third of the bootstrap samples were consistent with decreased QALYs or costs greater than $100,000 per QALY. In light of this information, decision makers should consider whether the lower incidence of high-cost procedures in the exercise training group would likely reoccur if this trial were conducted again. Such results could be due to chance, considering the high number of comparisons performed.
On the one hand, most measures of resource use were remarkably similar between the groups, indicating that it is unlikely that systematic benefits of exercise training influenced resource use for most patients. On the other hand, it is possible that exercise training attenuated the disease process in patients with more severe heart failure such that fewer end-stage procedures were necessary. A post hoc analysis revealed that, among patients with baseline cardiopulmonary exercise duration of less than 10 minutes, patients in the exercise training group were less likely than patients in the usual care group to undergo heart transplantation or LVAD placement (2.6% vs. 5.0%; P = .03) and incurred significantly lower direct medical costs ($58,846 vs $70,228; P = .03). Furthermore, despite the lack of a difference in hospital admissions, median inpatient costs were $2300 lower in the exercise training group. On average, hospital stays were 0.7 days shorter in the exercise training group (7.55 vs 6.86; P = .15).
Many of the tests and procedures required by the study protocol would not necessarily be required for patients to undertake an exercise training program. Although there may be a relationship between protocol fidelity in the trial and the modest clinical and quality-of-life benefits observed,4,5 clinicians may wish to consider ways to reduce costs associated with exercise training. For example, trainers could supervise more patients per exercise session. In our base-case analysis, we assumed that 1 trainer would oversee a mean of 1.7 patients per session. Although this may have been the case at the 9 sites that participated in the time survey, the representativeness of this estimate is unclear. If exercise training for patients with heart failure is integrated into existing cardiovascular rehabilitation programs, a 4:1 patient-to-trainer ratio may be appropriate, assuming there is no impact on patient outcomes or adherence. With this ratio, estimated costs for exercise training would be $293 and $454 per patient when applying salaries for exercise physiologists and nurses, respectively. Determining the optimal patient-to-trainer ratio to increase efficiency while maintaining adequate monitoring and adherence will be a critical factor in evaluations of exercise training from a payer’s perspective.
HF-ACTION is relatively unique in considering the translation of trial results to clinical practice (ie, efficacy to effectiveness). Adherence in the trial was suboptimal, with approximately 30% or more of patients training at or above the target number of minutes per week,5 despite a number of support structures in place and patients self-identifying for participation. Without such support systems, adherence levels would likely be lower, as would expenses for exercise training by payers. Another factor that may have influenced comparisons between treatment groups was a potential crossover effect resulting from patients exercising in the usual care group. Based on self-reported information collected quarterly during the first 2 years, between 22% and 28% of patients in the usual care group consistently reported some level of physical activity. Thus, it is clear that identifying or allowing for self-selection of individuals most likely to adhere to exercise training may be a key determination in its ultimate value, as exploratory evidence from the study indicates that adherence may be related to clinical outcomes.
Limitations
Although we believe the resource use and hospital billing data collected in this study are among the most comprehensive of any trial-based economic evaluation in heart failure, resource use data were only collected annually beyond the 24-month visit. Participants in HF-ACTION may also not be representative of the heart failure population as a whole. They were generally younger, had systolic dysfunction, and self-selected for participation. Finally, the multiple hypothesis tests of resource use and costs may have increased the probability of false-positive findings. Appropriate interpretation of the data requires consideration of the totality of the evidence presented.
Conclusion
Relative to the overall cost of heart failure to the health care system, costs associated with exercise training are small. However, in this economic evaluation, we found little systematic benefit in terms of overall medical resource use with this intervention.
Supplementary Material
Acknowledgments
We thank Betsy O’Neal and Ann Burnette, Duke University, for acquisition of hospital billing data; and Damon Seils, Duke University, for assistance with manuscript preparation. Mss O’Neal and Burnette and Mr Seils did not receive compensation for their assistance apart from their employment at the institution where the study was conducted. HF-ACTION Investigators: The HF-ACTION Investigators are listed in the Supplemental Material - Appendix B.
Sources of Funding: HF-ACTION was funded by grants 5U01HL063747, 5U01HL066461, 5U01HL068973, 5U01HL066501, 5U01HL066482, 5U01HL064250, 5U01HL066494, 5U01HL064257, 5U01HL066497, 5U01HL068980, 5U01HL064265, 5U01HL066491, and 5U01HL064264 from the National Heart, Lung, and Blood Institute; and grants R37AG018915 and P60AG010484 from the National Institute on Aging.
Footnotes
Disclosures: None of the authors reported financial disclosures relevant to the subject matter of the manuscript. Drs Reed and Schulman have made available online detailed listings of financial disclosures (http://www.dcri.duke.edu/research/coi.jsp).
References
- 1.Curtis LH, Whellan DJ, Hammill BG, Hernandez AF, Anstrom KJ, Shea AM, Schulman KA. Incidence and prevalence of heart failure in elderly persons, 1994–2003. Arch Intern Med. 2008;168:418–424. doi: 10.1001/archinternmed.2007.80. [DOI] [PubMed] [Google Scholar]
- 2.Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study) Am J Cardiol. 2008;101:1016–1022. doi: 10.1016/j.amjcard.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 3.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O’Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics -- 2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–e181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 4.Flynn KE, Piña IL, Whellan DJ, Lin L, Blumenthal JA, Ellis SJ, Fine LJ, Howlett JG, Keteyian SJ, Kitzman DW, Kraus WE, Miller NH, Schulman KA, Spertus JA, O’Connor CM, Weinfurt KP. Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1451–1459. doi: 10.1001/jama.2009.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS, Miller NH, Fleg JL, Schulman KA, McKelvie RS, Zannad F, Piña IL HF-ACTION Investigators. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1439–50. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Whellan DJ, O’Connor CM, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS, Houston-Miller N, Fleg JL, Schulman KA, Piña IL HF-ACTION Trial Investigators. Heart failure and a controlled trial investigating outcomes of exercise training (HF-ACTION): design and rationale. Am Heart J. 2007;153:201–211. doi: 10.1016/j.ahj.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 7.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B American College of Cardiology; American Heart Association Task Force on Practice Guidelines; American College of Chest Physicians; International Society for Heart and Lung Transplantation; Heart Rhythm Society. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–e235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 8.Szende A, Oppe M, Devlin N, editors. EQ-5D Value Sets: Inventory, Comparative Review and User Guide. Vol. 2. Dordrecht, The Netherlands: Springer; 2007. [Google Scholar]
- 9.Gold MR, Siegel JE, Russell LB, Weinstein MC, editors. Cost-Effectiveness in Health and Medicine. New York, NY: Oxford University Press; 1996. [Google Scholar]
- 10.Red Book. Montvale, NJ: Thomson Healthcare; 2006. [Google Scholar]
- 11.Medicare program; home health prospective payment system refinement and rate update for calendar year 2008; proposed rule. Fed Regist. 2007;72:23356–25481. [PubMed] [Google Scholar]
- 12.Medicare program; prospective payment system and consolidated billing for skilled nursing facilities. Fed Regist. 2007;72:25526–25600. [PubMed] [Google Scholar]
- 13.Maryland Medicaid Pharmacy Program. [Accessed December 8, 2008];On-Line Billing Instructions for Compounded Home Intravenous Therapy (HIT) Claims. http://www.dhmh.state.md.us/mma/mpap/pdf/IVCompoundBillingInstructionsApr07.pdf.
- 14.Centers for Medicare & Medicaid Services. [Accessed February 3, 2009];Outpatient PPS Pricer: 3rd Quarter 2008. Available at: http://www.cms.hhs.gov/PCPricer/08_OPPS.asp.
- 15.Russell LB. Completing costs: patients’ time. Med Care. 2009;7:S89–S93. doi: 10.1097/MLR.0b013e31819bc077. [DOI] [PubMed] [Google Scholar]
- 16.US Valuation of the EuroQol EQ-5D Health States. Agency for Healthcare Research and Quality; Rockville, MD: Dec, 2005. [Accessed December 16, 2009]. http://www.ahrq.gov/rice/EQ5Dproj.htm. [Google Scholar]
- 17.Efron B, Tibshirani RJ. Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Stat Sci. 1986;1:54–77. [Google Scholar]
- 18.Poole-Wilson PA, Kirwan BA, Vokó Z, de Brouwer S, Dunselman PH, van Dalen FJ, Lubsen J ACTION (A Coronary disease Trial Investigating Outcome with Nifedipine GITS) Investigators. Resource utilization implications of treatment were able to be assessed from appropriately reported clinical trial data. J Clin Epidemiol. 2007;60:727–733. doi: 10.1016/j.jclinepi.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 19.Cohn JN, Tognoni G Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345:1667–1675. doi: 10.1056/NEJMoa010713. [DOI] [PubMed] [Google Scholar]
- 20.Reed SD, Friedman JY, Velazquez EJ, Gnanasakthy A, Califf RM, Schulman KA. Multinational economic evaluation of valsartan in patients with chronic heart failure: results from the Valsartan Heart Failure Trial (Val-HeFT) Am Heart J. 2004;148:122–128. doi: 10.1016/j.ahj.2003.12.040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.