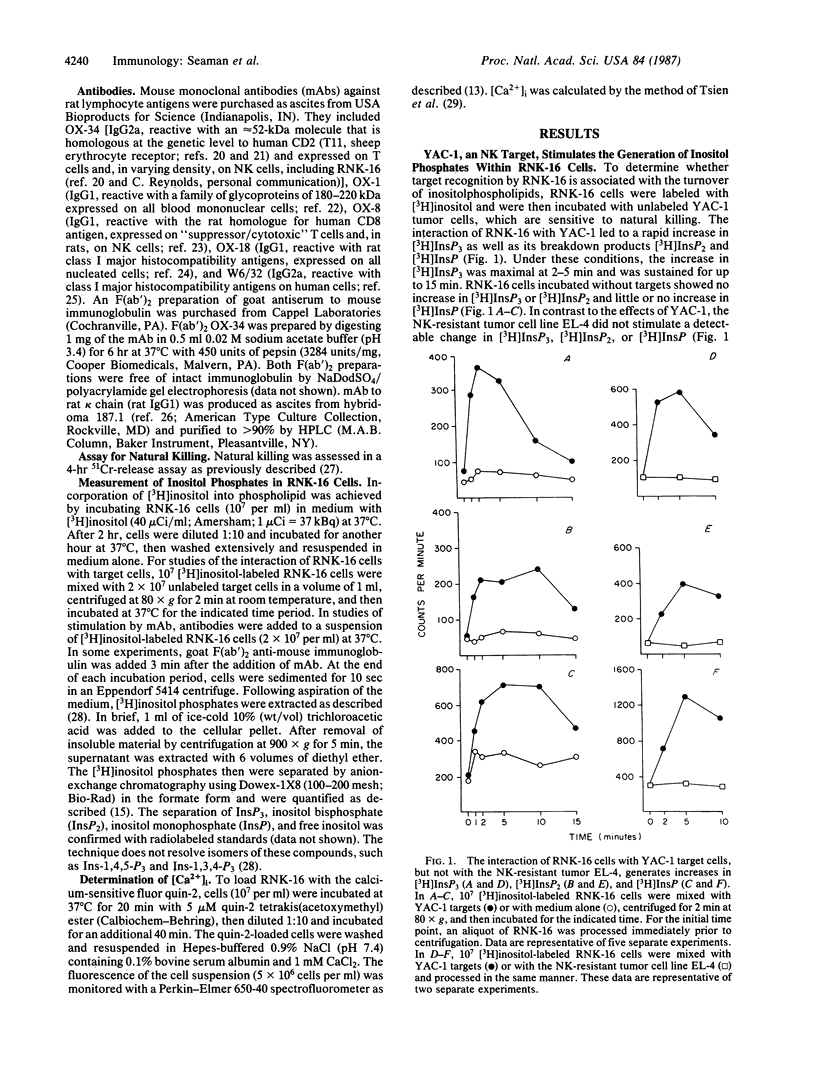
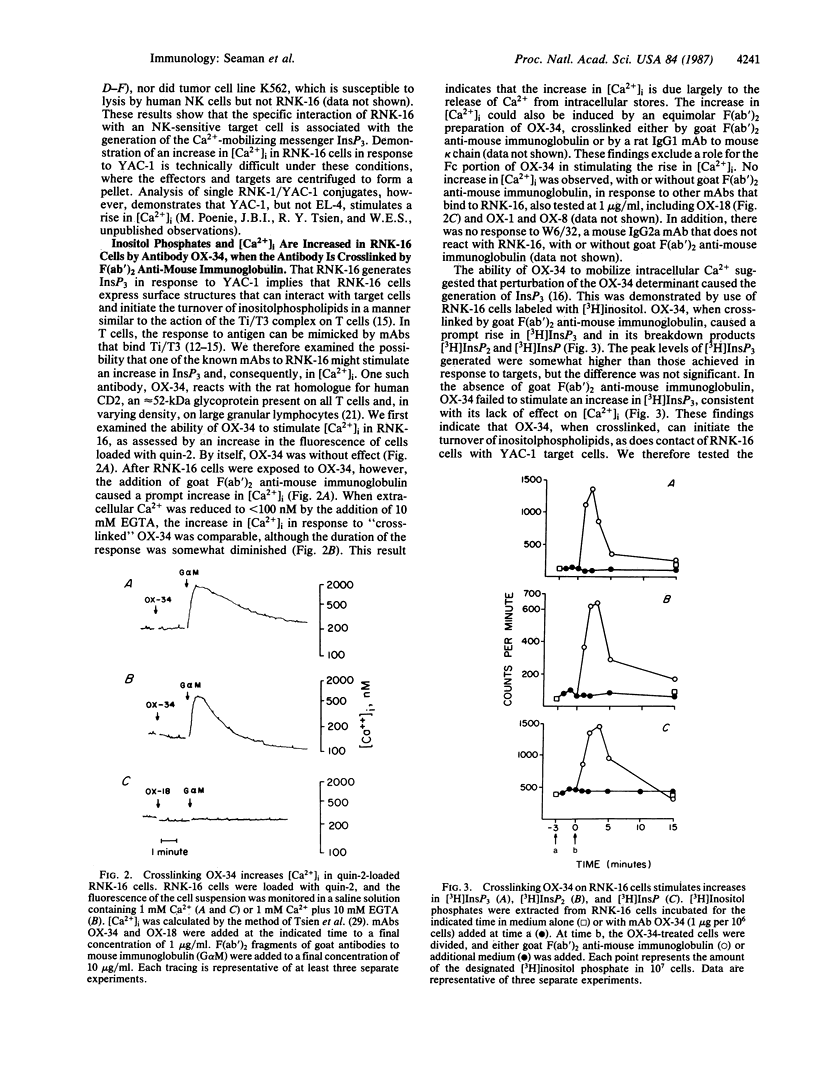
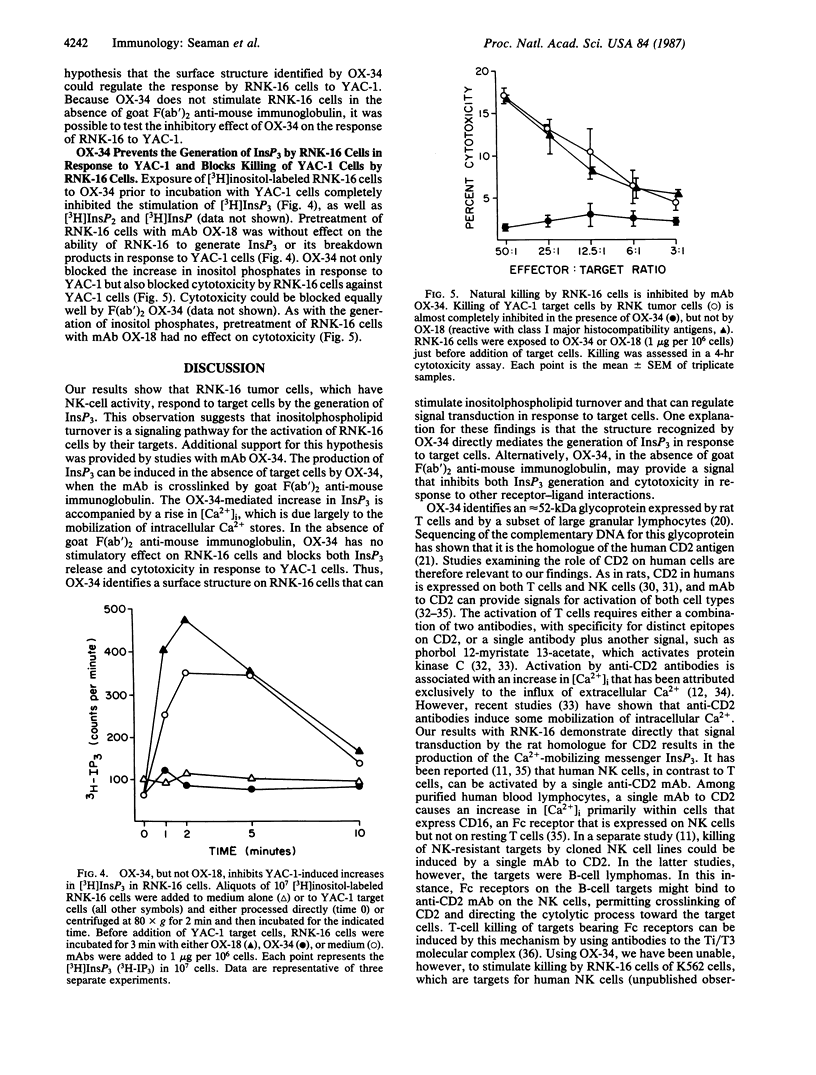
Abstract
RNK-16 cells, rat leukemia cells with features of natural killer (NK) cells, were adapted for growth in vitro and used to examine the mechanism of NK-cell activation. Contact of RNK-16 cells with tumor cells (YAC-1) that are lysed by NK cells, but not with resistant tumor cells (EL-4, K562), led to an increase in inositol trisphosphate (InsP3), a Ca2+-mobilizing messenger. A similar increase in InsP3 could be elicited in RNK-16 cells by monoclonal antibody OX-34, when the antibody was crosslinked by F(ab')2 fragments of goat antibodies to mouse immunoglobulin. This reaction was accompanied by an increase in the concentration of cytoplasmic free calcium Ca2+, due primarily to the release of Ca2+ from intracellular stores. In contrast to the stimulatory effects of crosslinked OX-34, OX-34 alone did not affect the levels of either InsP3 or cytoplasmic free Ca2+. Moreover, OX-34 alone blocked the generation of InsP3 by RNK-16 cells in response to YAC-1 cells and prevented target-cell killing. These findings demonstrate that OX-34 identifies a structure on the surface of RNK-16 cells that can stimulate the generation of InsP3, and they suggest that this structure can regulate signal transduction during target-cell recognition by NK cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcover A., Weiss M. J., Daley J. F., Reinherz E. L. The T11 glycoprotein is functionally linked to a calcium channel in precursor and mature T-lineage cells. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2614–2618. doi: 10.1073/pnas.83.8.2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allavena P., Ortaldo J. R. Characteristics of human NK clones: target specificity and phenotype. J Immunol. 1984 May;132(5):2363–2369. [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Bolhuis R. L., Roozemond R. C., van de Griend R. J. Induction and blocking of cytolysis in CD2+, CD3- NK and CD2+, CD3+ cytotoxic T lymphocytes via CD2 50 KD sheep erythrocyte receptor. J Immunol. 1986 Jun 1;136(11):3939–3944. [PubMed] [Google Scholar]
- Brenner M. B., McLean J., Dialynas D. P., Strominger J. L., Smith J. A., Owen F. L., Seidman J. G., Ip S., Rosen F., Krangel M. S. Identification of a putative second T-cell receptor. Nature. 1986 Jul 10;322(6075):145–149. doi: 10.1038/322145a0. [DOI] [PubMed] [Google Scholar]
- Brodsky F. M., Parham P. Monomorphic anti-HLA-A,B,C monoclonal antibodies detecting molecular subunits and combinatorial determinants. J Immunol. 1982 Jan;128(1):129–135. [PubMed] [Google Scholar]
- Fukumoto T., McMaster W. R., Williams A. F. Mouse monoclonal antibodies against rat major histocompatibility antigens. Two Ia antigens and expression of Ia and class I antigens in rat thymus. Eur J Immunol. 1982 Mar;12(3):237–243. doi: 10.1002/eji.1830120313. [DOI] [PubMed] [Google Scholar]
- Henkart P. A. Mechanism of lymphocyte-mediated cytotoxicity. Annu Rev Immunol. 1985;3:31–58. doi: 10.1146/annurev.iy.03.040185.000335. [DOI] [PubMed] [Google Scholar]
- Herberman R. B., Reynolds C. W., Ortaldo J. R. Mechanism of cytotoxicity by natural killer (NK) cells. Annu Rev Immunol. 1986;4:651–680. doi: 10.1146/annurev.iy.04.040186.003251. [DOI] [PubMed] [Google Scholar]
- Holter W., Fischer G. F., Majdic O., Stockinger H., Knapp W. T cell stimulation via the erythrocyte receptor. Synergism between monoclonal antibodies and phorbol myristate acetate without changes of free cytoplasmic Ca++ levels. J Exp Med. 1986 Mar 1;163(3):654–664. doi: 10.1084/jem.163.3.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard F. D., Ledbetter J. A., Wong J., Bieber C. P., Stinson E. B., Herzenberg L. A. A human T lymphocyte differentiation marker defined by monoclonal antibodies that block E-rosette formation. J Immunol. 1981 Jun;126(6):2117–2122. [PubMed] [Google Scholar]
- Ikuta K., Hattori M., Wake K., Kano S., Honjo T., Yodoi J., Minato N. Expression and rearrangement of the alpha, beta, and gamma chain genes of the T cell receptor in cloned murine large granular lymphocyte lines. No correlation with the cytotoxic spectrum. J Exp Med. 1986 Aug 1;164(2):428–442. doi: 10.1084/jem.164.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imboden J. B., Stobo J. D. Transmembrane signalling by the T cell antigen receptor. Perturbation of the T3-antigen receptor complex generates inositol phosphates and releases calcium ions from intracellular stores. J Exp Med. 1985 Mar 1;161(3):446–456. doi: 10.1084/jem.161.3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imboden J. B., Weiss A., Stobo J. D. The antigen receptor on a human T cell line initiates activation by increasing cytoplasmic free calcium. J Immunol. 1985 Feb;134(2):663–665. [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Heslop J. P., Berridge M. J. The inositol tris/tetrakisphosphate pathway--demonstration of Ins(1,4,5)P3 3-kinase activity in animal tissues. Nature. 1986 Apr 17;320(6063):631–634. doi: 10.1038/320631a0. [DOI] [PubMed] [Google Scholar]
- Jefferies W. A., Green J. R., Williams A. F. Authentic T helper CD4 (W3/25) antigen on rat peritoneal macrophages. J Exp Med. 1985 Jul 1;162(1):117–127. doi: 10.1084/jem.162.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P., Gagnon J., Barclay A. N., Williams A. F. Purification, chain separation and sequence of the MRC OX-8 antigen, a marker of rat cytotoxic T lymphocytes. EMBO J. 1985 Oct;4(10):2539–2545. doi: 10.1002/j.1460-2075.1985.tb03968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June C. H., Ledbetter J. A., Rabinovitch P. S., Martin P. J., Beatty P. G., Hansen J. A. Distinct patterns of transmembrane calcium flux and intracellular calcium mobilization after differentiation antigen cluster 2 (E rosette receptor) or 3 (T3) stimulation of human lymphocytes. J Clin Invest. 1986 Apr;77(4):1224–1232. doi: 10.1172/JCI112425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer A., Dennert G. Reorientation of the microtubule-organizing center and the Golgi apparatus in cloned cytotoxic lymphocytes triggered by binding to lysable target cells. J Immunol. 1984 Nov;133(5):2762–2766. [PubMed] [Google Scholar]
- Kupfer A., Singer S. J., Dennert G. On the mechanism of unidirectional killing in mixtures of two cytotoxic T lymphocytes. Unidirectional polarization of cytoplasmic organelles and the membrane-associated cytoskeleton in the effector cell. J Exp Med. 1986 Mar 1;163(3):489–498. doi: 10.1084/jem.163.3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuer S. C., Hussey R. E., Fabbi M., Fox D., Acuto O., Fitzgerald K. A., Hodgdon J. C., Protentis J. P., Schlossman S. F., Reinherz E. L. An alternative pathway of T-cell activation: a functional role for the 50 kd T11 sheep erythrocyte receptor protein. Cell. 1984 Apr;36(4):897–906. doi: 10.1016/0092-8674(84)90039-4. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Nunn M. E., Herberman R. B., Holden H. T. Natural cell-mediated cytotoxicity in mice against non-lymphoid tumor cells and some normal cells. Int J Cancer. 1977 Sep 15;20(3):381–387. doi: 10.1002/ijc.2910200309. [DOI] [PubMed] [Google Scholar]
- Ortaldo J. R., Herberman R. B. Heterogeneity of natural killer cells. Annu Rev Immunol. 1984;2:359–394. doi: 10.1146/annurev.iy.02.040184.002043. [DOI] [PubMed] [Google Scholar]
- Podack E. R., Dennert G. Assembly of two types of tubules with putative cytolytic function by cloned natural killer cells. 1983 Mar 31-Apr 6Nature. 302(5907):442–445. doi: 10.1038/302442a0. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Levey R. H., Schlossman S. F. Discrete stages of human intrathymic differentiation: analysis of normal thymocytes and leukemic lymphoblasts of T-cell lineage. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1588–1592. doi: 10.1073/pnas.77.3.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds C. W., Bere E. W., Jr, Ward J. M. Natural killer activity in the rat. III. Characterization of transplantable large granular lymphocyte (LGL) leukemias in the F344 rat. J Immunol. 1984 Jan;132(1):534–540. [PubMed] [Google Scholar]
- Reynolds C. W., Bonyhadi M., Herberman R. B., Young H. A., Hedrick S. M. Lack of gene rearrangement and mRNA expression of the beta chain of the T cell receptor in spontaneous rat large granular lymphocyte leukemia lines. J Exp Med. 1985 May 1;161(5):1249–1254. doi: 10.1084/jem.161.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz J., Campen T. J., Schmidt R. E., Royer H. D., Hercend T., Hussey R. E., Reinherz E. L. Analysis of T-cell receptor gene rearrangement and expression in human natural killer clones. Science. 1985 Jun 28;228(4707):1540–1543. doi: 10.1126/science.2409597. [DOI] [PubMed] [Google Scholar]
- Seaman W. E., Blackman M. A., Gindhart T. D., Roubinian J. R., Loeb J. M., Talal N. beta-Estradiol reduces natural killer cells in mice. J Immunol. 1978 Dec;121(6):2193–2198. [PubMed] [Google Scholar]
- Siliciano R. F., Pratt J. C., Schmidt R. E., Ritz J., Reinherz E. L. Activation of cytolytic T lymphocyte and natural killer cell function through the T11 sheep erythrocyte binding protein. Nature. 1985 Oct 3;317(6036):428–430. doi: 10.1038/317428a0. [DOI] [PubMed] [Google Scholar]
- Staerz U. D., Bevan M. J. Cytotoxic T lymphocyte-mediated lysis via the Fc receptor of target cells. Eur J Immunol. 1985 Dec;15(12):1172–1177. doi: 10.1002/eji.1830151206. [DOI] [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983 Nov 3;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tutt M. M., Kuziel W. A., Hackett J., Jr, Bennett M., Tucker P. W., Kumar V. Murine natural killer cells do not express functional transcripts of the alpha-, beta-, or gamma-chain genes of the T cell receptor. J Immunol. 1986 Nov 1;137(9):2998–3001. [PubMed] [Google Scholar]
- Weiss A., Imboden J., Shoback D., Stobo J. Role of T3 surface molecules in human T-cell activation: T3-dependent activation results in an increase in cytoplasmic free calcium. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4169–4173. doi: 10.1073/pnas.81.13.4169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A., Newton M., Crommie D. Expression of T3 in association with a molecule distinct from the T-cell antigen receptor heterodimer. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6998–7002. doi: 10.1073/pnas.83.18.6998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss M. J., Daley J. F., Hodgdon J. C., Reinherz E. L. Calcium dependency of antigen-specific (T3-Ti) and alternative (T11) pathways of human T-cell activation. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6836–6840. doi: 10.1073/pnas.81.21.6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A. F., Barclay A. N., Clark S. J., Paterson D. J., Willis A. C. Similarities in sequences and cellular expression between rat CD2 and CD4 antigens. J Exp Med. 1987 Feb 1;165(2):368–380. doi: 10.1084/jem.165.2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woollett G. R., Barclay A. N., Puklavec M., Williams A. F. Molecular and antigenic heterogeneity of the rat leukocyte-common antigen from thymocytes and T and B lymphocytes. Eur J Immunol. 1985 Feb;15(2):168–173. doi: 10.1002/eji.1830150211. [DOI] [PubMed] [Google Scholar]
- Wright S. C., Bonavida B. Studies on the mechanism of natural killer (NK) cell-mediated cytotoxicity (CMC). I. Release of cytotoxic factors specific for NK-sensitive target cells (NKCF) during co-culture of NK effector cells with NK target cells. J Immunol. 1982 Jul;129(1):433–439. [PubMed] [Google Scholar]
- Yelton D. E., Desaymard C., Scharff M. D. Use of monoclonal anti-mouse immunoglobulin to detect mouse antibodies. Hybridoma. 1981;1(1):5–11. doi: 10.1089/hyb.1.1981.1.5. [DOI] [PubMed] [Google Scholar]
- Young H. A., Ortaldo J. R., Herberman R. B., Reynolds C. W. Analysis of T cell receptors in highly purified rat and human large granular lymphocytes (LGL): lack of functional 1.3 kb beta-chain mRNA. J Immunol. 1986 Apr 1;136(7):2701–2704. [PubMed] [Google Scholar]
- Young J. D., Hengartner H., Podack E. R., Cohn Z. A. Purification and characterization of a cytolytic pore-forming protein from granules of cloned lymphocytes with natural killer activity. Cell. 1986 Mar 28;44(6):849–859. doi: 10.1016/0092-8674(86)90007-3. [DOI] [PubMed] [Google Scholar]


