Abstract
Summary
Tumor cell metastasis is facilitated by “pre-metastatic niches” formed in destination organs by invading bone marrow-derived cells (BMDCs). Lysyl oxidase (LOX) is critical for pre-metastatic niche formation. LOX secreted by hypoxic breast tumor cells accumulates at pre-metastatic sites, cross-links collagen-IV in the basement membrane, and is essential for CD11b+ myeloid cell recruitment. CD11b+ cells adhere to cross-linked collagen-IV and produce matrix metalloproteinase-2 which cleaves collagen, enhancing the invasion and recruitment of BMDCs and metastasizing tumor cells. LOX inhibition prevents CD11b+ cell recruitment and metastatic growth. CD11b+ cells and LOX also co-localize in biopsies of human metastases. Our findings demonstrate a critical role for LOX in pre-metastatic niche formation and support targeting LOX for the treatment and prevention of metastatic disease.
Introduction
During tumor progression, cells can acquire the capability for invasion and metastasis to escape the primary tumor mass and colonize nutrient-rich new organs (Gupta and Massague, 2006; Hanahan and Weinberg, 2000). There are few effective treatment options for patients with metastatic disease (Steeg, 2006) and over 90% of cancer-related deaths can be attributed to tumor metastases (Gupta and Massague, 2006). Increased metastases, enhanced tumor progression, and decreased patient survival have been associated with primary tumors that contain large numbers of poorly oxygenated (hypoxic) tumor cells (Cairns et al., 2003; Hockel and Vaupel, 2001; Pouyssegur et al., 2006). Improved understanding of the role of tumor hypoxia in the metastatic process is clearly needed so that more effective therapeutic strategies can be devised to treat metastatic cancer.
Tumor cell metastasis is facilitated by formation of “pre-metastatic niches” in destination organs (Kaplan et al., 2005) that consist of clusters of bone marrow-derived cells (BMDCs). These BMDCs are thought to create an environment that is permissive for the subsequent invasion and growth of tumor cells (Condeelis and Pollard, 2006; Coussens and Werb, 2002). The main BMDCs identified at pre-metastatic sites are haematopoietic progenitor cells that express vascular endothelial growth factor receptor-1 (VEGFR-1), along with BMDCs expressing CD133, CD34, and c-Kit (Kaplan et al., 2005). CD11b+ (Mac-1+) cells have also been identified in metastatic target organs (Hiratsuka et al., 2006), and primary tumors are known to recruit CD11b+ Gr-1+ myeloid cells (Yang et al., 2008) and CD45+ monocytic lineage cells (including VEGFR-1+ and CD11b+ cells; (Du et al., 2008). CD11b+ cells have a variety of functions that may enhance metastatic tumor growth. CD11b+ Gr-1+ cells are known as myeloid suppressor cells that are capable of inhibiting T-cell and NK cell-mediated immune responses (Liu et al., 2007; Serafini et al., 2006). CD11b+ Gr-1+ cells also incorporate into tumor endothelium and enhance angiogenesis (Yang et al., 2004), while CD11b+ myeloid cells enhance tumor growth through vasculogenesis (Ahn and Brown, 2008). The presence of CD11b+ cells at pre-metastatic sites may have important implications for using anti-VEGF therapy to disrupt the pre-metastatic niche (Kaplan et al., 2005) since tumors containing CD11b+ Gr-1+ cells show decreased response to anti-VEGF therapy (Shojaei and Ferrara, 2008). Thus myeloid lineage cells may be important components of the pre-metastatic niche.
The mechanism by which BMDCs are recruited to pre-metastatic sites is poorly understood. Unidentified tumor-secreted factors are thought to induce elevated fibronectin expression at pre-metastatic sites and increase the recruitment of VEGFR1+ cells (Kaplan et al., 2005). The recruitment of CD11b+ myeloid cells to pre-metastatic sites may be influenced by VEGF-A and by the TGF-β and/or TNF-α pathways (Hiratsuka et al., 2006). However, tumor-secreted proteins that are essential for formation of the pre-metastatic niche and that could potentially be targeted therapeutically are still largely unknown.
Lysyl oxidase (LOX) is an amine oxidase that cross-links collagens and elastins in the extracellular matrix (Kagan and Li, 2003). LOX expression is increased in tumor cells exposed to physiologically relevant levels of hypoxia (Denko et al., 2003), and LOX is associated with metastasis and poor survival in patients with breast cancer or head and neck cancer (Erler et al., 2006). LOX has been shown to enhance tumor cell invasion in vitro (Erler et al., 2006; Kirschmann et al., 2002), and inhibition of the expression or the enzymatic activity of secreted LOX eliminated metastases in an orthotopic model of breast cancer (Erler et al., 2006). Based on the marked decreases in metastatic growth we previously observed with therapeutic LOX inhibition and on the ability of LOX to remodel the extracellular matrix, we hypothesized that LOX may influence multiple steps in the metastatic process. We therefore studied the role of LOX in the recruitment and invasion of BMDCs to pre-metastatic sites and in formation of the pre-metastatic niche.
Results and Discussion
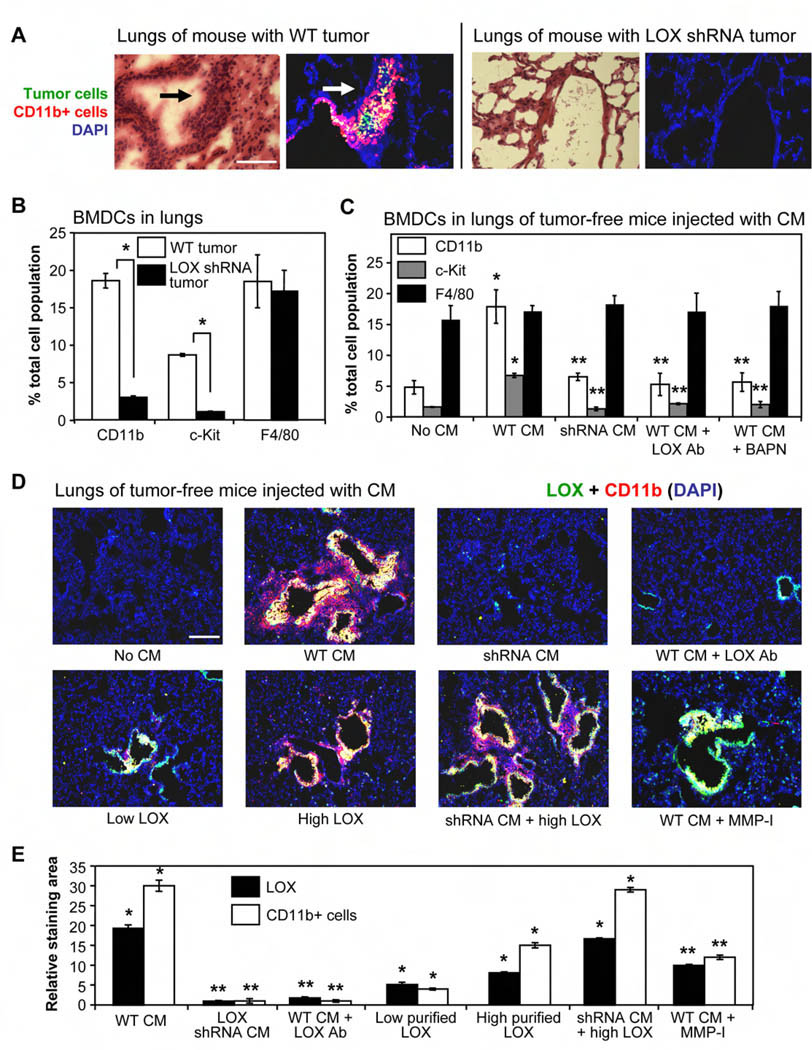
To investigate the role of LOX in formation of the pre-metastatic niche, we orthotopically implanted mice with either wild-type MDA-MB-231 human breast tumor cells (Wt), or MDA-MB-231 cells expressing a LOX-targeting shRNA with significantly reduced LOX protein expression and secretion (Supplemental Figure 1A). Analysis of lungs harvested 6 weeks after tumor implantation indicated that mice bearing LOX shRNA tumors had significantly reduced numbers of pulmonary metastatic lesions compared to Wt tumor-bearing mice (Figure 1A H&E panels, Supplemental Figure 1B). These data are in agreement with our previous results indicating that inhibition of LOX decreased tumor cell invasion and metastasis (Erler et al., 2006). Interestingly, when lungs from these mice were analyzed for the presence of BMDCs by flow cytometry (Supplemental Figure 1C), we found that the lungs of Wt tumor-bearing mice had significantly more CD11b+ myeloid cells and c-Kit+ (CD117+) myeloid progenitor cells than mice with LOX shRNA tumors (Figure 1B). We did not observe a significant increase in the numbers of F4/80+ mature macrophages, and 95% of the CD11b+ cells found in the lungs of Wt tumor-bearing mice were negative for F4/80. Since monocytes have the potential to mature into macrophages once in tissues, the increased numbers of CD11b+ F4/80− cells indicates that CD11b+ BMDCs recruited to metastatic sites are largely immature progenitor cells from the myeloid lineage. These data are in agreement with the increased numbers of immature myeloid cells relative to mature myeloid cells observed during tumor progression in mouse tumor models and in cancer patients (Kusmartsev et al., 2008). We also found that CD11b+ cells co-localized with tumor cells in pulmonary foci of Wt tumor-bearing mice (Figure 1A immunofluorescent panels), while clusters of CD11b+ cells and tumor cells were not observed in lungs of mice with LOX shRNA tumors. The bone marrow-derived origin of cells in pulmonary pre-metastatic sites was confirmed by transplanting male bone marrow cells into lethally-irradiated female mice prior to tumor implant, and staining the excised lungs with a Y-chromosome-specific fluorescent DNA probe (Supplemental Figure 1D). Taken together, these data demonstrate a role for LOX in the development of pulmonary foci in tumor-bearing mice that contain tumor cells and CD11b+ myeloid cells.
Figure 1. LOX secreted from hypoxic tumor cells co-localizes with CD11b+ cells in the lungs and increases CD11b+ cell recruitment and invasion.
(A) Nude mice were orthotopically implanted with 107 wild-type (Wt) or LOX shRNA-expressing MDA-MB-231 human breast tumor cells. Lungs were excised 6 weeks later, and frozen serial sections were stained with either H&E or with pan-cytokeratin (green) to identify tumor cells and CD11b (red) to identify myeloid cells. Arrows indicate pulmonary cell clusters (foci). Scale bar = 75µm.
(B) Lungs from mice bearing Wt or LOX shRNA-expressing tumors were homogenized and analyzed by flow cytometry for numbers of CD11b+ myeloid cells, c-Kit+ (CD117) myeloid progenitor cells, or F4/80+ mature macrophages. Data are mean ± SEM. * = p<0.05.
(C) Tumor-free mice were injected with the indicated CM daily for 3 weeks. Homogenized lungs were analyzed for CD11b, c-Kit, and F4/80 positive cells by flow cytometry. LOX Ab = LOX-specific antibody; BAPN = small molecule inhibitor of LOX. Data are mean ± SEM. * = p<0.05 relative to control; ** = p<0.05 relative to mice injected with Wt CM.
(D) Merged immunofluorescent staining of LOX (green) and CD11b+ cells (red) in representative lung sections from tumor-free mice injected daily for 3 weeks with the indicated CM. Co-localization is indicated in yellow. Low/high LOX = relative concentration of purified LOX; MMP-I = matrix metalloproteinase inhibitor. Scale bar = 150µm.
(E) Image analysis of lung sections from mice in Figure 1D. Data indicate the relative area of LOX (green) or CD11b (red) staining relative to control mice. Data are mean ± SEM. * = p<0.05 relative to control; ** = p<0.05 relative to mice injected with Wt CM.
We then determined how LOX integrates into the kinetics of pulmonary foci formation by excising lungs from mice bearing orthotopic Wt MDA-MB-231 tumors at various times after tumor implant. Consistent with previous reports (Kaplan et al., 2005), we found focal areas of fibronectin (FN) staining 3 days after tumor implantation surrounding terminal bronchioles and distal alveoli in the lungs (Supplemental Figure 1E), which are common areas of pulmonary metastasis. We used a human-specific FN antibody, indicating that at least some of the pulmonary FN was secreted from the primary tumor. Interestingly, LOX co-localized exclusively with FN at these sites within 7 days of tumor implantation, and both FN and LOX staining intensified over the next week. We found recruitment of CD11b+ cells to areas of LOX staining by 14 days after tumor implantation, and observed increased numbers of CD11b+ cells over the next few weeks. Tumor cells were observed in regions of CD11b+ cell accumulation by 5 weeks after tumor implant. Importantly, we did not observe pulmonary LOX staining, CD11b+ cell clusters, or tumor cells in mice bearing LOX shRNA-expressing tumors. These data indicate that LOX secreted by the primary tumor binds to regions of FN accumulation in the lungs of Wt tumor-bearing mice before the recruitment of CD11b+ cells or tumor cells.
We then wanted to validate the role of LOX in BMDC recruitment in a model system without tumor cells present. LOX is known to be secreted by hypoxic tumor cells (Erler et al., 2006), and we therefore used Wt or LOX shRNA-expressing MDA-MB-231 cells exposed to hypoxia in vitro (2% O2 for 24hr) to produce conditioned media (CM) with or without LOX. A fluorescence-based enzymatic activity assay (Palamakumbura and Trackman, 2002) was used to verify the presence of enzymatically active LOX in CM from Wt cells (Supplemental Figure 2A). We injected LOX-containing CM into tumor-free mice daily for 3 weeks, which is consistent with the timeframe of BMDC recruitment to pre-metastatic sites (Kaplan et al., 2005). Interestingly, tumor-free mice injected with CM from hypoxic Wt cells (high LOX activity) had increased pulmonary accumulation of CD11b+ myeloid cells and c-Kit+ myeloid progenitor cells (Figure 1C). These data are in agreement with previous reports suggesting involvement of progenitor cells and myeloid lineage cells in the pre-metastatic niche (Hiratsuka et al., 2006; Kaplan et al., 2005). Importantly, the numbers of pulmonary BMDCs were not increased by injection of CM derived from hypoxic cells expressing LOX shRNA, or when LOX-containing CM was combined with either an antibody that binds to the active site of LOX and blocks enzymatic function (Erler et al., 2006) or a small molecule inhibitor of LOX (β-aminoproprionitrile; BAPN). These data indicate that LOX secreted by hypoxic tumor cells significantly increases the number of myeloid lineage cells in the lungs without requiring the presence of pulmonary metastatic tumor cells.
We also analyzed sections of lung tissue excised after 3 weeks of LOX-containing CM or purified LOX protein injections. Tumor-free mice injected with LOX-containing CM had intense LOX staining around terminal bronchioles and distal alveoli in the lungs. Interestingly, CD11b+ cells were found co-localized with LOX at these sites and were also observed in lung tissue directly adjacent to areas of LOX staining (Figure 1D). This pattern of CD11b staining suggests CD11b+ cell invasion into lung tissue surrounding areas of LOX staining, which is consistent with reports describing a role for LOX in enhancing monocyte migration (Denholm et al., 1989; Lazarus et al., 1995). Total LOX or CD11b staining relative to naïve mouse lungs is quantified in Figure 1E; the proportion of CD11b+ cells that invaded into lung tissue surrounding areas of LOX staining is quantified in Supplemental Figure 2B. Invading BMDCs were observed as quantifiable clusters of densely-stained cells surrounding bronchioles in H&E-stained lung tissue (Supplemental Figure 2C–D). In agreement with the flow cytometry data in Figure 1C, CD11b+ cell accumulation was dramatically reduced in the lungs of mice injected with CM from LOX shRNA-expressing cells or when a LOX-inhibitory antibody was administered with the Wt CM (Figure 1D–E). Taken together, these data indicate that LOX secreted by hypoxic tumor cells accumulates in the lungs and is essential for the recruitment of CD11b+ cells.
We also purified LOX from the CM of hypoxic Wt cells for subsequent administration to tumor-free mice. The LOX purity was verified and LOX functional activity was validated by inducing migration of LOX shRNA-expressing cells in vitro (Supplemental Figure 2E). We used two concentrations of LOX protein, with the higher LOX concentration having about half the enzymatic activity of LOX typically found in hypoxic Wt CM (Supplemental Figure 2A). Importantly, the recruitment of CD11b+ cells induced by injection of LOX-containing CM could be replicated in tumor-free mice injected only with purified LOX (Figure 1D), but the overall levels of LOX and CD11b+ cell staining in the lungs were less than in mice injected with hypoxic Wt CM (Figure 1E). Interestingly, addition of CM from hypoxic LOX shRNA-expressing cells to injections of purified LOX significantly increased the levels of pulmonary LOX and CD11b+ cell accumulation (Figure 1D–E), and also stimulated CD11b+ cell invasion into the surrounding lung tissue to similar levels as observed with hypoxic Wt CM (Figure 1D, Supplemental Figure 2B). These data indicate that while accumulation of LOX secreted by hypoxic tumor cells is essential for pulmonary recruitment of CD11b+ cells, other factor(s) present in CM from hypoxic (Wt or shRNA-expressing) tumor cells are capable of enhancing the effects of LOX. It is worth noting that fibronectin is a hypoxia-induced secreted protein (Caniggia et al., 2000) that is known to be elevated at pre-metastatic sites (Kaplan et al., 2005) and has been reported to increase LOX enzymatic activity (Fogelgren et al., 2005). Indeed, we have found that LOX co-localizes exclusively with fibronectin at pre-metastatic sites (Supplemental Figure 1E) prior to recruitment of CD11b+ cells. The precise role of fibronectin in LOX-mediated formation of the pre-metastatic niche is currently under investigation.
Since invasion of BMDCs is increased by activation of matrix metalloproteinases (Coussens and Werb, 2002), we added a chemical matrix metalloproteinase (MMP) inhibitor (Koivunen et al., 1999) to LOX-containing Wt CM prior to injection. The MMP inhibitor (MMP-I) induced modest decreases in relative LOX and CD11b+ cell staining in the lungs (Figure 1E), but dramatically changed the pattern of CD11b+ cell accumulation. CD11b+ cells were recruited to areas of LOX staining, but did not invade into the surrounding lung tissue to form cell clusters as had occurred with injection of hypoxic Wt CM alone (Figure 1D, Supplemental Figure 2B and 2D). These data indicate that the ability of CD11b+ cells to invade into lung tissue beyond regions of LOX accumulation is dependent on MMP activity.
To establish a mechanistic role for LOX in the recruitment and MMP-dependent invasion of BMDCs, we first pretreated growth factor-reduced Matrigel (reconstituted basement membrane) with LOX prior to contact with CD11b+ cells isolated from whole bone marrow by magnetic bead-assisted cell sorting (Liu et al., 2007). We found increased adhesion of CD11b+ cells to Matrigel pre-incubated with LOX that could be inhibited with BAPN during the pre-incubation step (Figure 2A). LOX is known to crosslink collagens and elastins in the extracellular matrix (Kagan and Li, 2003) thereby increasing the tensile strength of basement membranes (Maki et al., 2002). Chemically cross-linking Matrigel by pre-incubation with a high concentration of glucose (Kent et al., 1985) recapitulated the increased CD11b+ cell adhesion observed on Matrigel pre-incubated with LOX. We also found increased adhesion of CD11b+ cells (Figure 2B) and c-Kit+ cells (Supplemental Figure 3A) to naïve mouse lung tissue pre-incubated with LOX ex vivo. CD11b+ cells adhered to the ex vivo lung tissue in areas that stained positively for LOX (Figure 2C). These data indicate that CD11b+ cells and c-Kit+ cells readily adhere to basement membrane and lung tissue that has been cross-linked by LOX.
Figure 2. LOX secreted from hypoxic tumor cells promotes BMDC invasion by cross-linking collagen IV, increasing BMDC adhesion, and enhancing MMP-2 activity of the invading BMDCs.
(A) Matrigel-coated wells were incubated with the indicated additives for 24hr; Matrigel cross-linked with glucose was included for comparison. Solutions were removed and CD11b+ cells isolated from murine whole bone marrow were added. Numbers of CD11b+ cells remaining in solution were quantified after 2.5hr. Data are mean ± SEM. * = p<0.05 relative to control; ** = p<0.05 relative to matrix pre-incubated with LOX.
(B) Naïve mouse lung tissue was excised and a 2cm3 piece was incubated in serum-free media containing either LOX or glucose for 6hr. Media was changed, CD11b+ cells were added, and the numbers of cells remaining in solution after 12hr were quantified. Data are mean ± SEM.
(C) Lung tissue from B was frozen, sectioned, and stained for LOX (green) and CD11b+ cells (red). Scale bar = 300µm.
(D) Gelatin zymography showing MMP-2 activity of monocytes in contact with Matrigel or collagen IV pre-incubated with the indicated CM.
(E) Gelatin zymography showing MMP-2 activity of freshly harvested BMDCs in contact with collagen IV pre-incubated with the indicated CM.
(F) Matrigel filters were incubated with the indicated CM or purified protein for 24hr. The CM was then removed and freshly harvested whole murine bone marrow cells were allowed to invade through the “modified” Matrigel. BMDCs that invaded through the modified Matrigel were also stained for CD11b and c-Kit. Data are mean ± SEM. * = p<0.05 relative to control; ** = p<0.05 relative to matrices pre-incubated with Wt CM.
(G) Matrigel filters were pre-incubated as in 2F, and invasion of isolated CD11b+ cells or c-Kit+ cells through the modified matrix was quantified. Data are mean ± SEM. * = p<0.05 relative to control; ** = p<0.05 relative to matrices pre-incubated with LOX.
(H) Mice with wild-type bone marrow or MMP-2 knockout bone marrow were injected daily with the indicated CM for 3 weeks prior to flow cytometry analysis of lungs for CD11b, c-Kit, or F4/80 positive cells. Data are mean ± SEM. * = p<0.05 relative to no CM mice; ** = p<0.05 relative to mice with Wt bone marrow injected with Wt CM.
(I) Immunofluorescent staining for LOX, CD11b+ cells, and MMP-2 in representative frozen serial sections of lungs from mice with wild-type or MMP-2 knockout bone marrow injected with Wt CM. Scale bar = 75µm.
To determine how the increased adhesion of BMDCs to matrices cross-linked by LOX would affect the MMP-dependent invasion of BMDCs observed in Figure 1D–E, we assayed the MMP activity of BMDCs and monocytes after contact with matrices pre-incubated with LOX. Since collagen IV is a major constituent of Matrigel and LOX is known to cross-link collagen IV, we incorporated collagen IV matrices into our studies. We chose to focus on MMP-2 and MMP-9 because these MMPs are selectively inhibited by the MMP-I used in Figure 1D–E (Koivunen et al., 1999). Interestingly, monocytes in contact with Matrigel (or collagen IV) pre-incubated with LOX-containing Wt CM (but not CM from LOX shRNA-expressing cells) had elevated MMP-2 activity (Figure 2D). Monocyte MMP-2 activity was also increased by contact with chemically cross-linked Matrigel. Increases in MMP-9 activity were observed in monocytes in contact with Matrigel or collagen IV pre-incubated with hypoxic CM from either Wt or shRNA-expressing tumor cells (Supplemental Figure 3B), indicating the increase in MMP-9 activity was LOX-independent. Consistently, MMP-9 activity was also not increased in monocytes in contact with chemically cross-linked Matrigel. We found that MMP-2 activity was increased in freshly isolated BMDCs in contact with collagen IV matrices pre-incubated with LOX-containing Wt CM, purified LOX, or with matrix that was chemically cross-linked (Figure 2E). BMDC MMP-2 activity was reduced by the presence of LOX antibody or BAPN during matrix pre-incubation, or by MMP- inhibition. Thus pre-incubation of Matrigel or collagen IV with enzymatically active LOX increases the MMP-2 activity of BMDCs and monocytes that are subsequently in contact with the modified matrices.
To further define the role of LOX and MMPs in BMDC invasion, we performed in vitro transwell invasion assays using freshly harvested murine bone marrow, the established RAW monocyte cell line, and freshly isolated CD11b+ cells or c-Kit+ cells. We placed CM from hypoxic Wt or LOX shRNA-expressing tumor cells into transwell chambers containing filters coated with Matrigel. The CM was removed after 24 hours, replaced with freshly harvested murine BMDCs, and the numbers of BMDCs that invaded through the “modified” Matrigel were quantified 24 hours later (Figure 2F). Pre-incubation of Matrigel with LOX-containing Wt CM dramatically increased the subsequent invasion of BMDCs compared to Matrigel pre-incubated with LOX shRNA CM or Wt CM containing the LOX-targeting antibody. We also stained the bone marrow cells that invaded through the modified Matrigel and found that 94%±1% were CD11b-positive and 47%±7% were c-Kit-positive. Increased BMDC invasion was also observed when the Matrigel was pre-incubated with purified LOX protein alone or in combination with hypoxic shRNA CM. The MMP-dependence of BMDC invasion was confirmed by addition of the MMP inhibitor to the cells during invasion (Figure 2F). These data are consistent with the decreased CD11b+ cell invasion observed in the lungs of mice injected with LOX-containing CM and the MMP-I (Figure 1D, Supplemental Figure 2B). Chemical cross-linking of Matrigel with glucose also increased the subsequent invasion of BMDCs. Similar increases in the invasion of freshly harvested BMDCs were observed through collagen IV matrices pre-incubated with LOX-containing CM (Supplemental Figure 3C), but not with matrices composed of laminin (the other main component of Matrigel). We obtained similar results using isolated CD11b+ cells, c-Kit+ cells (Figure 2G), or an established monocyte cell line (Supplemental Figure 3D). Taken together, these data indicate that enzymatically active LOX modifies the collagen IV component of basement membrane (Matrigel) in a manner that is functionally similar to chemical cross-linking. CD11b+ cells adhere more readily to matrices cross-linked by LOX (Figure 2A–C), and respond with increased MMP-2 expression (Figure 2D–E). The action of LOX and MMP-2 remodel the matrix such that it is more permissive for subsequent invasion of CD11b+ cells and c-Kit+ cells (Figure 2F–G).
To further validate the role of BMDC MMP-2 activity in LOX-mediated recruitment and invasion of CD11b+ cells in vivo, we injected LOX-containing CM into female mice transplanted with bone marrow from either male wild-type mice or MMP-2 knockout (KO) mice. As expected, LOX-containing CM induced pulmonary recruitment of CD11b+ and c-Kit+ cells in mice with wild-type bone marrow. However, mice with MMP-2 KO bone marrow injected with Wt CM had significantly decreased numbers of CD11b+ cells and c-Kit+ cells in the lungs relative to mice with Wt bone marrow injected with Wt CM (Figure 2H). We also found that CD11b+ cells in areas of pulmonary LOX staining expressed MMP-2 (Figure 2I), consistent with the increased MMP-2 activity observed in BMDCs in contact with LOX-modified matrices in vitro. Fewer CD11b+ cells were observed in lungs of MMP-2 KO mice injected with Wt CM, and the CD11b+ cells co-localized only partially with LOX staining. Taken together, these data indicate that MMP-2 activity in BMDCs is required for the invasion and maximal LOX-mediated recruitment of CD11b+ cells to areas of pulmonary LOX accumulation for formation of the pre-metastatic niche. These results are consistent with recent identification of MMP-2 as a tumor progression gene associated with breast cancer metastasis to the lung (Gupta and Massague, 2006), with our previous findings that LOX is strongly associated with MMP-2 expression in breast cancer patients (Erler et al., 2006), and with the MMP-I data in Figure 1D–E. We hypothesized that while LOX-mediated cross-linking of basement membrane was required for adhesion of CD11b+ cells and initiation of the pre-metastatic niche, an additional MMP-dependent mechanism may increase CD11b+ cell recruitment to pre-metastatic sites.
MMP-2 is known to cleave collagen IV into peptides (Egeblad and Werb, 2002), and some collagen IV peptides have chemo-attractant properties (Cameron et al., 1991; Shahan et al., 2000). Using a collagen IV ELISA, we observed the release of collagen IV peptides during invasion of CD11b+ cells through Matrigel pre-incubated with purified LOX (Figure 3A). Peptide release was decreased when LOX enzymatic activity was inhibited with BAPN during the pre-incubation step or when MMP-2 activity from the CD11b+ cells was inhibited. We obtained similar findings using collagen IV matrices (data not shown), but did not detect the release of laminin peptides from laminin matrices pre-incubated with LOX-containing CM and contacted with BMDCs (Supplemental Figure 3E). Taken together, these data indicate that collagen IV peptides are released by the MMP-2 activity of CD11b+ cells in contact with Matrigel cross-linked by LOX.
Figure 3. Collagen IV remodeling mediated by LOX and BMDC MMP-2 activity promotes pulmonary metastatic growth.
(A) ELISA to detect collagen IV remodeling via peptide formation. Collagen IV peptides exogenously added to media (white bars) are provided for comparison. Matrigel pre-incubated for 24hr with the indicated additives (grey bars) were subsequently contacted with CD11b+ cells for 24hr. Peptides released into the surrounding media were quantified with the ELISA. Plasma samples from the indicated tumor-bearing mice (black bars) were also analyzed. Data are mean ± SEM. * = p<0.05 relative to control; ** = p<0.05 relative to matrices pre-incubated with LOX (grey) or relative to Wt tumor-bearing mice (black).
(B) Sections of lungs from Wt tumor-bearing mouse illustrating loss of collagen IV antibody epitope in some areas through LOX-mediated collagen IV remodeling. Collagen I staining was not affected. Scale bar = 150µm.
(C) Numbers of isolated CD11b+ cells or c-Kit+ cells invading through naïve Matrigel toward collagen IV peptides in the bottom of the transwell. Data are mean ± SEM. * = p<0.05 relative to control (no peptides).
(D) Flow cytometric quantification of CD11b+ cells and tumor cells (human pan-cytokeratin-positive) in lungs of mice bearing LOX shRNA-expressing tumors. Mice were “pre-conditioned” by injection of Wt CM or purified LOX protein for 2 weeks after tumor implant. Lungs were harvested 6 weeks after tumor implant. Data are mean ± SEM. * = p<0.05 relative to control mice; ** = p<0.05 relative to mice pre-conditioned with LOX protein.
(E) Flow cytometric quantification of CD11b+ cells and tumor cells in lungs of mice with LOX shRNA-expressing tumors treated with the indicated solutions. Data are mean ± SEM. Clod = clodronate; * = p<0.05 relative to control shRNA tumor-bearing mice; ** = p<0.05 relative to shRNA tumor-bearing mice injected with LOX protein.
(F) Same experiment as E, using 4T1 murine mammary tumor cells in Balb/c mice. Pulmonary cell foci (clusters) were quantified from H&E-stained lung tissue. Data are mean ± SEM. * = p<0.05 relative to control 4T1 shRNA tumor-bearing mice; ** = p<0.05 relative to 4T1 shRNA tumor-bearing mice injected with LOX protein.
(G) Flow cytometry analysis for CD11b+ cells in lungs of tumor-free Balb/c mice or Balb/c mice bearing LOX shRNA-expressing 4T1 tumors injected with the indicated CM daily for 3 weeks. Pulmonary cell foci were quantified from H&E-stained lung tissue. Data are mean ± SEM. * = p<0.05 relative to control mice; ** = p<0.05 relative to mice injected with Wt CM.
(H) Lungs of Balb/c mice implanted with LOX shRNA-expressing 4T1 tumors and injected with the indicated CM daily for 3 weeks. Arrows indicate macroscopic lung metastases.
We also observed increased collagen IV peptides in the plasma of mice bearing Wt tumors (Figure 3A), and a decrease in collagen IV staining in some lung regions from Wt tumor-bearing mice relative to LOX shRNA tumor-bearing mice (Figure 3B). These data are indicative of collagen remodeling and a breakdown of antibody-recognizable triple-helical collagen IV in the basement membrane (Harrison et al., 2006; Jemal et al., 2008; Liu et al., 2007) in lungs of mice bearing tumors that express LOX. Pulmonary collagen I staining was similar in both Wt and LOX shRNA tumor-bearing mice.
To determine the ability of collagen IV peptides to attract BMDCs, we quantified the numbers of isolated CD11b+ cells and c-Kit+ cells that invaded through naïve Matrigel toward exogenously added collagen IV peptides (Figure 3C). We found that collagen IV peptides enhanced the invasion of myeloid lineage cells. Thus the generation of chemo-attractive collagen IV peptides in lung regions that have CD11b+ cells in contact with LOX cross-linked basement membrane will induce further recruitment and invasion of BMDCs to these sites.
Increased MMP-2 activity in BMDCs also increases the invasion of tumor cells (Hagemann et al., 2004), and collagen cross-linking leads to increased stiffness of the extracellular matrix which enhances growth of tumor foci (Paszek et al., 2005). Taken together with our data, these observations suggest that LOX-mediated increases in BMDC MMP-2 activity may increase the subsequent invasion of metastatic tumor cells to the pre-metastatic niche and enhance metastatic growth. Indeed, we have previously observed a role for LOX in enhancing the metastatic growth of breast tumors (Erler et al., 2006), but we wanted to distinguish between the role of LOX in the dissemination of metastatic tumor cells and the role of LOX in formation of the pre-metastatic niche. We therefore “pre-conditioned” lungs with LOX for a fixed period of time before the arrival of metastatic tumor cells. It is worth noting that pre-conditioning lungs with LOX in vivo is analogous to pre-incubating Matrigel (or collagen IV) matrices with LOX in vitro (Figure 2). MDA-MB-231 tumor cell metastases are detectable in lungs from 3–4 weeks after primary tumor implantation (Erler et al., 2006), and BMDCs are recruited to pre-metastatic sites within 2 weeks of primary tumor implantation (Supplemental Figure 1E). We therefore pre-conditioned the lungs of mice bearing orthotopic LOX shRNA-expressing tumors by injecting LOX-containing CM or purified LOX for only the first 2 weeks after tumor implant. Lungs were analyzed for CD11b+ cells and tumor cells 4 weeks later. We observed increased CD11b+ cell recruitment in the lungs of shRNA tumor-bearing mice pre-conditioned with LOX or with LOX-containing CM (Figure 3D), despite the fact that LOX shRNA-expressing tumors are largely non-metastatic (Supplemental Figure 1B). Importantly, CD11b+ cell recruitment was diminished by inhibition of LOX with BAPN or a LOX-targeting antibody. The presence of densely-stained pulmonary cell clusters was verified and quantified in H&E stained lung sections (Supplemental Figure 4A). These data indicate that the action of enzymatically active LOX secreted by hypoxic tumor cells is a critical pre-requisite to pulmonary CD11b+ cell recruitment, pre-metastatic niche formation, and the enhanced development of lung metastases.
We then wanted to determine if LOX was sufficient to increase metastases of breast tumors to the lungs in the absence of myeloid cells. We therefore used clodronate to deplete myeloid cells, monocytes, and macrophages (Van Rooijen and Sanders, 1994; Zeisberger et al., 2006) from mice bearing shRNA tumors and supplemented with LOX injections. We found that clodronate significantly decreased the numbers of CD11b+ cells and the numbers of metastatic tumor cells in the lungs of MDA-MB-231 tumor-bearing mice (Figure 3E).
We also investigated CD11b+ cell recruitment in mice bearing highly aggressive 4T1 murine mammary tumors. 4T1 cells expressing LOX shRNA secreted minimal detectable enzymatically active LOX compared to wild-type 4T1 cells (Supplemental Figure 4B). Clodronate decreased CD11b+ cell recruitment and metastatic tumor cell foci formation in the lungs of 4T1 tumor-bearing mice (Figure 3F). These data indicate that depletion of BMDCs decreases the metastatic growth of breast tumor cells in the lung. However, clodronate is not specific for CD11b+ cells and pleiotropic effects associated with BMDC depletion would preclude therapeutic CD11b+ cell depletion to target the pre-metastatic niche. These data highlight the utility of inhibiting a tumor-secreted protein such as LOX to target the pre-metastatic niche and metastatic growth.
We also investigated the influence of LOX-mediated CD11b+ cell recruitment and pre-metastatic niche formation in the growth of pulmonary metastatic foci from 4T1 tumors. CD11b+ cell recruitment was observed in the lungs of tumor-free Balb/c mice injected with hypoxic Wt 4T1 CM, but not with CM from hypoxic LOX shRNA-expressing 4T1 cells (Figure 3G). Balb/c mice were also orthotopically implanted with Wt or shRNA-expressing 4T1 tumor cells and given daily injections of CM from hypoxic Wt or shRNA-expressing 4T1 cells for 3 weeks. Increased CD11b+ cells and tumor cell foci were observed in the lungs of mice bearing Wt 4T1 tumors regardless of the daily injections of LOX-containing CM (Supplemental Figure 4C–D). Mice with LOX shRNA-expressing 4T1 tumors exhibited increased pulmonary CD11b+ cells when LOX-containing Wt CM was provided (Figure 3G). Similar to our observations in mice with MDA-MB-231 tumors expressing LOX shRNA, 4T1 tumors that expressed LOX shRNA were virtually non-metastatic unless LOX-containing CM was provided, which increased the numbers of microscopic metastatic foci (Figure 3G, Supplemental Figure 4E) and macroscopic metastatic tumors (Figure 3H). These data demonstrate a role for LOX secreted by hypoxic tumor cells in pulmonary CD11b+ cell recruitment and metastatic growth of 4T1 tumors in an immunocompetent mouse strain.
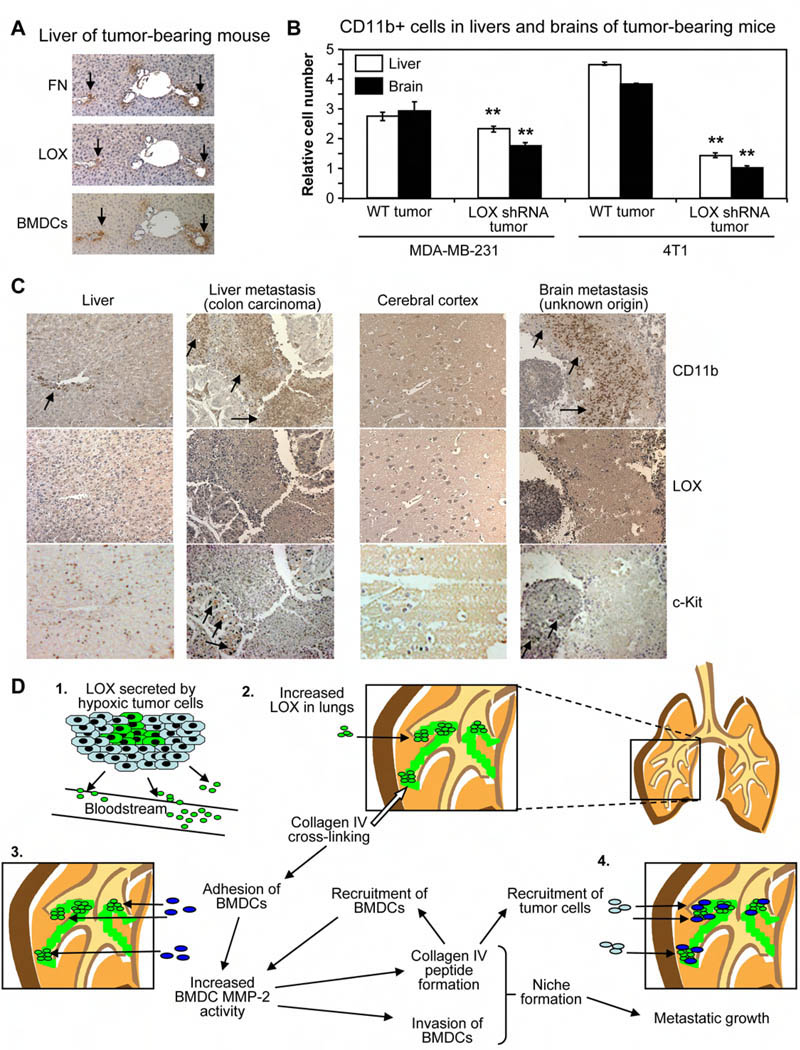
We also studied the role of LOX in CD11b+ cell recruitment to tissues other than the lung. We found LOX co-localized with fibronectin and BMDCs in the livers of Wt tumor-bearing mice (Figure 4A), consistent with our lung data. We observed modest increases in CD11b+ cell recruitment to the livers and brains of Wt MDA-MB-231 tumor-bearing mice relative to mice with LOX shRNA tumors (Figure 4B), and found large LOX-dependent increases in CD11b+ cell recruitment to the livers and brains of 4T1 tumor-bearing Balb/c mice. Importantly, 4T1 tumors readily metastasize to the liver and brain, and we have observed metastatic MDA-MB-231 tumor cells in the liver 10 weeks after tumor implant. These data indicate that LOX also affects the recruitment of CD11b+ cells to the livers and brains of tumor-bearing mice.
Figure 4. CD11b+ cells and LOX are associated with liver and brain metastases in patients.
(A) Serial sections from liver tissue of Wt tumor-bearing mouse stained for fibronectin, LOX, and BMDCs. Scale bar = 50µm.
(B) Flow cytometry analysis for CD11b+ cells in livers and brains of Nude mice bearing MDA-MB-231 human breast tumors or Balb/c mice with 4T1 murine mammary tumors. Data are mean ± SEM. ** = p<0.05 relative to Wt tumor-bearing mice.
(C) Tissue microarrays (TMAs) of clinical metastases stained for CD11b+ cells, LOX, or c-Kit+ cells. Samples from normal cerebral cortex and liver are provided as negative controls. Metastatic and normal TMAs were stained simultaneously, and were photographed with identical microscope and camera settings. Arrows indicate regions of CD11b+ cells or c-Kit+ cells. Scale bar = 150µm.
(D) (1) Hypoxic primary tumor cells secrete LOX into the bloodstream. (2) LOX accumulates in the lungs of tumor-bearing mice and cross-links collagen IV. (3) Adhesion of CD11b+ cells to cross-linked matrix increases BMDC MMP-2 activity. Collagen IV remodeling by LOX and MMP-2 leads to peptide formation, invasion of CD11b+ cells, and increased recruitment of BMDCs. (4) LOX-dependent formation of the pre-metastatic niche enhances metastatic growth.
In order to assess the relevance of LOX and CD11b+ cell recruitment to human metastases, we stained tissue microarrays (TMAs) containing samples of clinical metastatic nodules from a variety of different sites for CD11b+ cells, c-Kit+ cells, or LOX (Figure 4C, Supplemental Figure 4F). Interestingly, we found that 51 out of 95 human metastatic lesions contained CD11b+ cells, including metastases sampled from the brain, liver, neck, ovary, greater omentum, and lymph nodes. The primary tumors that gave rise to metastases on the TMA varied, with carcinomas of the breast, colon, stomach, thyroid, esophagus, or nasopharynx producing metastases that were associated with CD11b+ cells. Importantly, CD11b+ cells were not found in significant numbers in most normal tissues apart from the spleen, indicating that the presence of large clusters of CD11b+ cells in metastatic target organs such as the liver or brain is stimulated by tumor-derived factors (Figure 4C). We also found that CD11b+ cells and c-Kit+ cells in human metastases were typically found in areas that stained positively for LOX. These data establish that myeloid lineage cells are associated with tumor metastases in a wide variety of cancer patients and also suggest that targeting LOX-mediated recruitment of CD11b+ cells to metastatic sites represents a viable therapeutic strategy for the clinic.
Elucidating the micro-environmental influences on metastatic growth are paramount to understanding how to inhibit this lethal multistep process in cancer patients (Steeg, 2006). Formation of the pre-metastatic niche has been shown to enhance the establishment and growth of metastatic foci (Kaplan et al., 2005), and we have identified LOX as a tumor-secreted protein that is critically involved in pre-metastatic niche formation (Figure 4D). Our data show that LOX secreted by hypoxic primary tumor cells accumulates with fibronectin at sites of future metastasis, cross-links collagen IV in the basement membrane, and increases adhesion of CD11b+ cells. Adherent CD11b+ cells produce MMP-2 which degrades collagen IV, increasing CD11b+ cell invasion into the lung tissue and releasing chemo-attractive collagen IV peptides. The collagen IV peptides enhance further recruitment of CD11b+ cells, generating a positive feed-forward loop for increased accumulation of BMDCs, increased extracellular matrix remodeling, and creation of the pre-metastatic niche. Importantly, formation of the pre-metastatic niche is critically dependent on the accumulation of enzymatically active LOX. Taken together, our data demonstrate a crucial role for LOX secreted by hypoxic tumor cells in formation of the pre-metastatic niche and in the enhancement of metastatic tumor growth. These data support targeting hypoxia-induced secreted LOX for the treatment and prevention of metastatic cancer.
Experimental Procedures
Cell lines and tumor implants
MDA-MB-231 wt and LOX shRNA-expressing cells were previously described (Erler et al., 2006). 107 tumor cells were implanted orthotopically in the mammary fat pad for in vivo experiments. 4T1 murine mammary cells (ATCC) were infected with retrovirus to stably express murine LOX shRNA (TCTCTCCTCCTCCTTCTAC). All animal work was approved by and conformed to the regulatory standards of the Stanford University Administrative Panel on Laboratory Animal Care (APLAC) in accordance with US federal law.
Immunological studies
For immunofluorescent studies and selected H&E stained sections, lungs were perfused with a 1:1 mixture of PBS/OCT post-excision before embedding in OCT (Tissue-Tek). For paraffin-embedded samples, lungs were perfused with formalin prior to formalin fixation. Western blots were performed as previously described (Erler et al., 2006). Collagen IV ELISAs were performed with the DELPHIA assay (Perkin Elmer) according to manufacturer’s instructions.
Antibodies included
CD11b (eBioscience), F4/80 (Abcam), c-Kit/CD117 (ACK2; eBioscience), collagen IV (Millipore), laminin (Chemicon), pan-cytokeratin (ICN), and a LOX antibody that recognizes a peptide sequence from the active site of both human and murine LOX (Erler et al., 2006). Alexa 488 and 594 fluorescent secondary antibodies were used to visualize immunofluorescent staining. Images were photographed using a Nikon 360 microscope camera and analyzed using Q-capture software. Flow cytometry analysis for BMDCs was performed as described (Kaplan et al., 2005).
Conditioned media assays
Conditioned media (CM) consisted of serum-free, phenol red-free Modified Eagle’s Medium cultured on Wt or LOX shRNA-expressing MDA-MB-231 cells incubated in hypoxia (2% O2) for 24 hours. CM was passed through a 0.2-µm filter and 300µl was intraperitoneally injected daily into mice (Kaplan et al., 2005). For LOX inhibition, β-aminoproprionitrile (BAPN; 100mg/kg) was added daily to CM, and the LOX-targeting antibody (purified; 1mg/kg) was added twice weekly (Erler et al., 2006). CTT gelatinase inhibitor of MMP-2 and MMP-9 activities (Biomol International) was added to CM twice weekly and dosed at 50µg/mouse (Koivunen et al., 1999). Purified LOX protein was obtained by Nickel agarose extraction from Wt hypoxic CM and was injected twice weekly at either 2µg/mouse (low dose) or 5µg/mouse (high dose). A fluorescence-based assay was used to assess LOX enzymatic activity as previously described (Palamakumbura and Trackman, 2002).
Adhesion assay, invasion assays, and MMP gelatin enzymography
CD11b+ cells and c-Kit+ cells were isolated from whole bone marrow using magnetic bead-assisted flow cytometry according to manufacturer’s instructions (Miltenyi Biotec). Matrices were incubated with CM or LOX for 24 hours prior to removal of the CM and addition of BMDCs for the adhesion or invasion assays. BAPN was used at 200µM, and 200mg/ml glucose for 24hr was used to chemically cross-link matrices (Kent et al., 1985). In vitro invasion of whole bone marrow cells, RAW monocytes, CD11b+ cells, or c-Kit+ cells were measured in a transwell assay (BD Biosciences) as previously described (Erler et al., 2006). Cell migration (“scratch” assays) were performed as previously described (Erler et al., 2006). Gelatin enzymography was performed to assess MMP activity as described (Hagemann et al., 2004).
Ex vivo assays and clodronate encapsulation
A 2cm3 piece of lung tissue was maintained in 0.5ml serum-free media (Hiratsuka et al., 2006) and cross-linked by incubating with LOX or glucose (Kent et al., 1985) for 6hr. Isolated CD11b+ cells or c-Kit+ cells were added and the numbers of cells remaining in the media were counted.
Clodronate was encapsulated in liposomes of cholesterol and phosphatidylcholine (Sigma) prepared under nitrogen (Van Rooijen and Sanders, 1994; van Rooijen and van Kesteren-Hendrikx, 2003).
Human samples
Tissue microarrays (TMAs) were purchased from Pantomics Inc or from Tissue Array Networks. The TMAs contained human tissues obtained with informed consent according to US federal law, and are exempt from consideration by the Stanford Administrative Panel on Human Subjects in Medical Research. TMAs were stained with a rabbit monoclonal anti-human CD11b (AbCam) or an anti-LOX (Erler et al., 2006) antibody. Metastatic and normal TMAs were stained simultaneously and images were captured with identical settings using a Nikon 360 microscope camera.
Statistical analyses
Data were analyzed by Student's t-test; p-values <0.05 were considered significant. Error bars depict standard error of the mean.
Significance
Understanding the metastatic process is central to the development of improved therapies to treat cancer patients. Pre-metastatic niches are comprised of largely unidentified proteins and bone marrow-derived cells, and are thought to prepare target organs for the subsequent arrival of metastatic tumor cells. We have found that lysyl oxidase (LOX) secreted by hypoxic tumor cells is a critical mediator of pre-metastatic niche formation. LOX modifies the extracellular matrix and induces the recruitment and invasion of CD11b+ myeloid cells to pre-metastatic sites, thereby promoting a cascade of events that increases metastatic tumor growth. The central role of LOX in pre-metastatic niche formation identifies LOX as a viable therapeutic target for the treatment and prevention of metastatic disease.
Supplementary Material
Acknowledgements
This research was supported by funds from the National Institutes of Health (JTE and AJG), the Canadian Institutes of Health Research (KLB), and the Institute of Cancer Research and Cancer Research UK (JTE, TRC, GL, and DB). We thank Pauline Chu for immunohistochemistry and immunofluorescent staining, Fredrik Wallberg for cell sorting of CD11b+ cells and c-Kit+ cells, Zena Werb and Andrew Ewald for MMP-2 KO mice, and also David Lyden, Shaheen Rafii, Rosie Kaplan, Peter Marinkovich, Val Weaver, Adam Krieg, Scott Welford, G-One Ahn and Marlene Rabinovich for useful discussions. JTE, KLB, and AJG designed the experiments, analyzed the data, and wrote the paper. JTE, KLB, TRC, GL, and DB performed the experiments. AJG supervised the project.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn GO, Brown JM. Matrix metalloproteinase-9 is required for tumor vasculogenesis but not for angiogenesis: role of bone marrow-derived myelomonocytic cells. Cancer Cell. 2008;13:193–205. doi: 10.1016/j.ccr.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns RA, Khokha R, Hill RP. Molecular mechanisms of tumor invasion and metastasis: an integrated view. Curr Mol Med. 2003;3:659–671. doi: 10.2174/1566524033479447. [DOI] [PubMed] [Google Scholar]
- Cameron JD, Skubitz AP, Furcht LT. Type IV collagen and corneal epithelial adhesion and migration. Effects of type IV collagen fragments and synthetic peptides on rabbit corneal epithelial cell adhesion and migration in vitro. Invest Ophthalmol Vis Sci. 1991;32:2766–2773. [PubMed] [Google Scholar]
- Caniggia I, Mostachfi H, Winter J, Gassmann M, Lye SJ, Kuliszewski M, Post M. Hypoxia-inducible factor-1 mediates the biological effects of oxygen on human trophoblast differentiation through TGFbeta(3) J Clin Invest. 2000;105:577–587. doi: 10.1172/JCI8316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denholm EM, Wolber FM, Phan SH. Secretion of monocyte chemotactic activity by alveolar macrophages. Am J Pathol. 1989;135:571–580. [PMC free article] [PubMed] [Google Scholar]
- Denko NC, Fontana LA, Hudson KM, Sutphin PD, Raychaudhuri S, Altman R, Giaccia AJ. Investigating hypoxic tumor physiology through gene expression patterns. Oncogene. 2003;22:5907–5914. doi: 10.1038/sj.onc.1206703. [DOI] [PubMed] [Google Scholar]
- Du R, Lu KV, Petritsch C, Liu P, Ganss R, Passegue E, Song H, Vandenberg S, Johnson RS, Werb Z, Bergers G. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13:206–220. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- Erler JT, Bennewith KL, Nicolau M, Dornhofer N, Kong C, Le QT, Chi JT, Jeffrey SS, Giaccia AJ. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440:1222–1226. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- Fogelgren B, Polgar N, Szauter KM, Ujfaludi Z, Laczko R, Fong KS, Csiszar K. Cellular fibronectin binds to lysyl oxidase with high affinity and is critical for its proteolytic activation. J Biol Chem. 2005;280:24690–24697. doi: 10.1074/jbc.M412979200. [DOI] [PubMed] [Google Scholar]
- Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Hagemann T, Robinson SC, Schulz M, Trumper L, Balkwill FR, Binder C. Enhanced invasiveness of breast cancer cell lines upon co-cultivation with macrophages is due to TNF-alpha dependent up-regulation of matrix metalloproteases. Carcinogenesis. 2004;25:1543–1549. doi: 10.1093/carcin/bgh146. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Harrison CA, Gossiel F, Layton CM, Bullock AJ, Johnson T, Blumsohn A, MacNeil S. Use of an in vitro model of tissue-engineered skin to investigate the mechanism of skin graft contraction. Tissue Eng. 2006;12:3119–3133. doi: 10.1089/ten.2006.12.3119. [DOI] [PubMed] [Google Scholar]
- Hiratsuka S, Watanabe A, Aburatani H, Maru Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol. 2006;8:1369–1375. doi: 10.1038/ncb1507. [DOI] [PubMed] [Google Scholar]
- Hockel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst. 2001;93:266–276. doi: 10.1093/jnci/93.4.266. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- Kagan HM, Li W. Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell. J Cell Biochem. 2003;88:660–672. doi: 10.1002/jcb.10413. [DOI] [PubMed] [Google Scholar]
- Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent MJ, Light ND, Bailey AJ. Evidence for glucose-mediated covalent cross-linking of collagen after glycosylation in vitro. Biochem J. 1985;225:745–752. doi: 10.1042/bj2250745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschmann DA, Seftor EA, Fong SF, Nieva DR, Sullivan CM, Edwards EM, Sommer P, Csiszar K, Hendrix MJ. A molecular role for lysyl oxidase in breast cancer invasion. Cancer Res. 2002;62:4478–4483. [PubMed] [Google Scholar]
- Koivunen E, Arap W, Valtanen H, Rainisalo A, Medina OP, Heikkila P, Kantor C, Gahmberg CG, Salo T, Konttinen YT, et al. Tumor targeting with a selective gelatinase inhibitor. Nat Biotechnol. 1999;17:768–774. doi: 10.1038/11703. [DOI] [PubMed] [Google Scholar]
- Kusmartsev S, Eruslanov E, Kubler H, Tseng T, Sakai Y, Su Z, Kaliberov S, Heiser A, Rosser C, Dahm P, et al. Oxidative Stress Regulates Expression of VEGFR1 in Myeloid Cells: Link to Tumor-Induced Immune Suppression in Renal Cell Carcinoma. J Immunol. 2008;181:346–353. doi: 10.4049/jimmunol.181.1.346. [DOI] [PubMed] [Google Scholar]
- Lazarus HM, Cruikshank WW, Narasimhan N, Kagan HM, Center DM. Induction of human monocyte motility by lysyl oxidase. Matrix Biol. 1995;14:727–731. doi: 10.1016/s0945-053x(05)80015-0. [DOI] [PubMed] [Google Scholar]
- Liu C, Yu S, Kappes J, Wang J, Grizzle WE, Zinn KR, Zhang HG. Expansion of spleen myeloid suppressor cells represses NK cell cytotoxicity in tumor-bearing host. Blood. 2007;109:4336–4342. doi: 10.1182/blood-2006-09-046201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki JM, Rasanen J, Tikkanen H, Sormunen R, Makikallio K, Kivirikko KI, Soininen R. Inactivation of the lysyl oxidase gene Lox leads to aortic aneurysms, cardiovascular dysfunction, and perinatal death in mice. Circulation. 2002;106:2503–2509. doi: 10.1161/01.cir.0000038109.84500.1e. [DOI] [PubMed] [Google Scholar]
- Palamakumbura AH, Trackman PC. A fluorometric assay for detection of lysyl oxidase enzyme activity in biological samples. Anal Biochem. 2002;300:245–251. doi: 10.1006/abio.2001.5464. [DOI] [PubMed] [Google Scholar]
- Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Pouyssegur J, Dayan F, Mazure NM. Hypoxia signalling in cancer and approaches to enforce tumour regression. Nature. 2006;441:437–443. doi: 10.1038/nature04871. [DOI] [PubMed] [Google Scholar]
- Serafini P, Borrello I, Bronte V. Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin Cancer Biol. 2006;16:53–65. doi: 10.1016/j.semcancer.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Shahan TA, Fawzi A, Bellon G, Monboisse JC, Kefalides NA. Regulation of tumor cell chemotaxis by type IV collagen is mediated by a Ca(2+)- dependent mechanism requiring CD47 and the integrin alpha(V)beta(3) J Biol Chem. 2000;275:4796–4802. doi: 10.1074/jbc.275.7.4796. [DOI] [PubMed] [Google Scholar]
- Shojaei F, Ferrara N. Refractoriness to Antivascular Endothelial Growth Factor Treatment: Role of Myeloid Cells. Cancer Res. 2008;68:5501–5504. doi: 10.1158/0008-5472.CAN-08-0925. [DOI] [PubMed] [Google Scholar]
- Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;12:895–904. doi: 10.1038/nm1469. [DOI] [PubMed] [Google Scholar]
- Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- van Rooijen N, van Kesteren-Hendrikx E. "In vivo" depletion of macrophages by liposome-mediated "suicide". Methods Enzymol. 2003;373:3–16. doi: 10.1016/s0076-6879(03)73001-8. [DOI] [PubMed] [Google Scholar]
- Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, Matrisian LM, Carbone DP, Lin PC. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6:409–421. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- Yang L, Huang J, Ren X, Gorska AE, Chytil A, Aakre M, Carbone DP, Matrisian LM, Richmond A, Lin PC, Moses HL. Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell. 2008;13:23–35. doi: 10.1016/j.ccr.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisberger SM, Odermatt B, Marty C, Zehnder-Fjallman AH, Ballmer-Hofer K, Schwendener RA. Clodronate-liposome-mediated depletion of tumour-associated macrophages: a new and highly effective antiangiogenic therapy approach. Br J Cancer. 2006;95:272–281. doi: 10.1038/sj.bjc.6603240. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.