Abstract
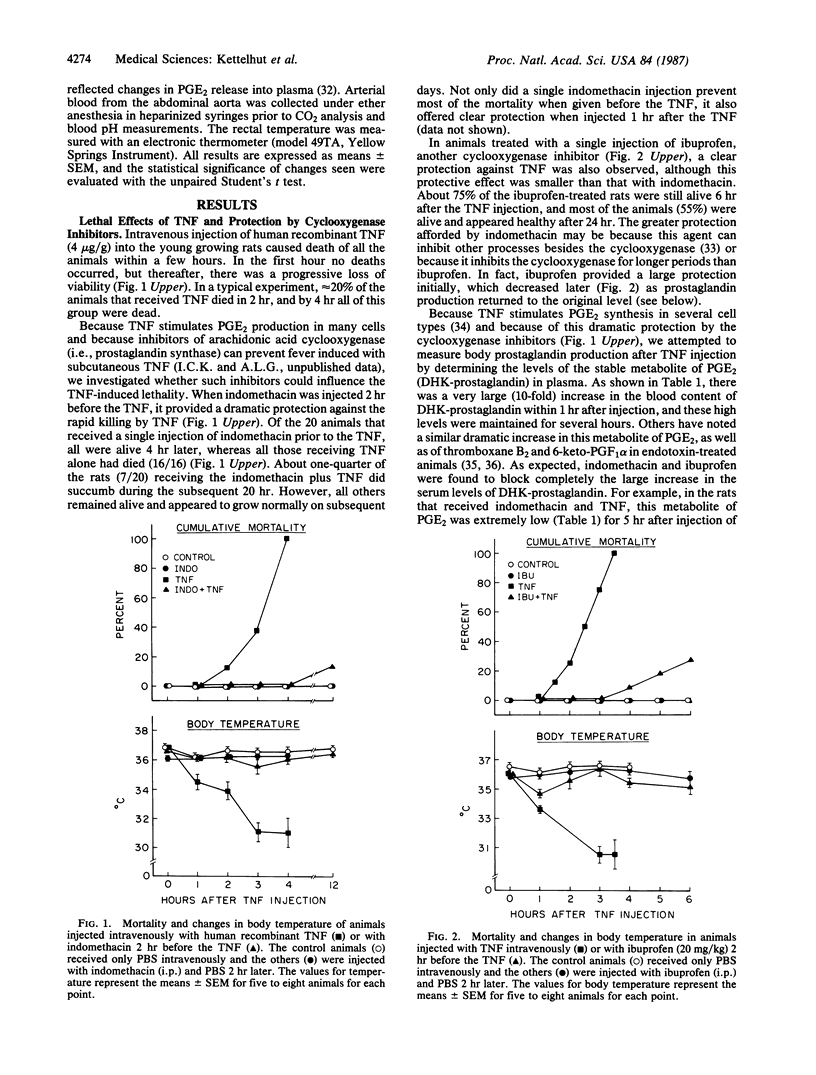
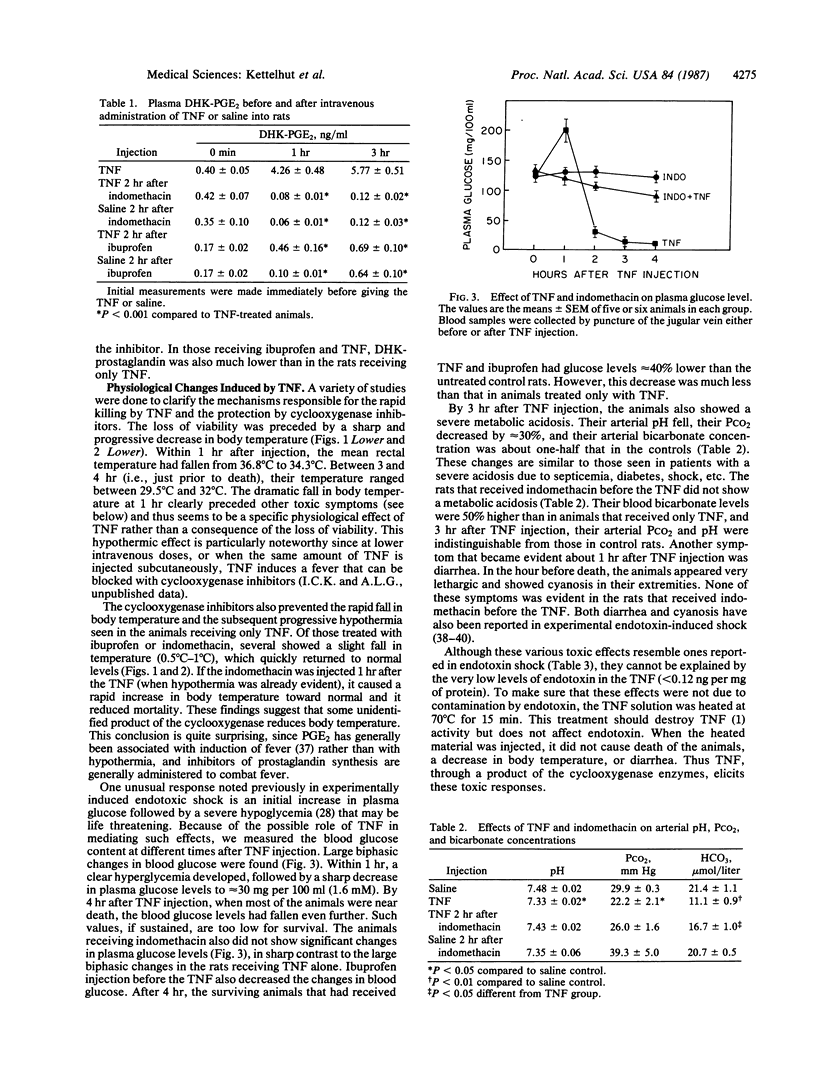
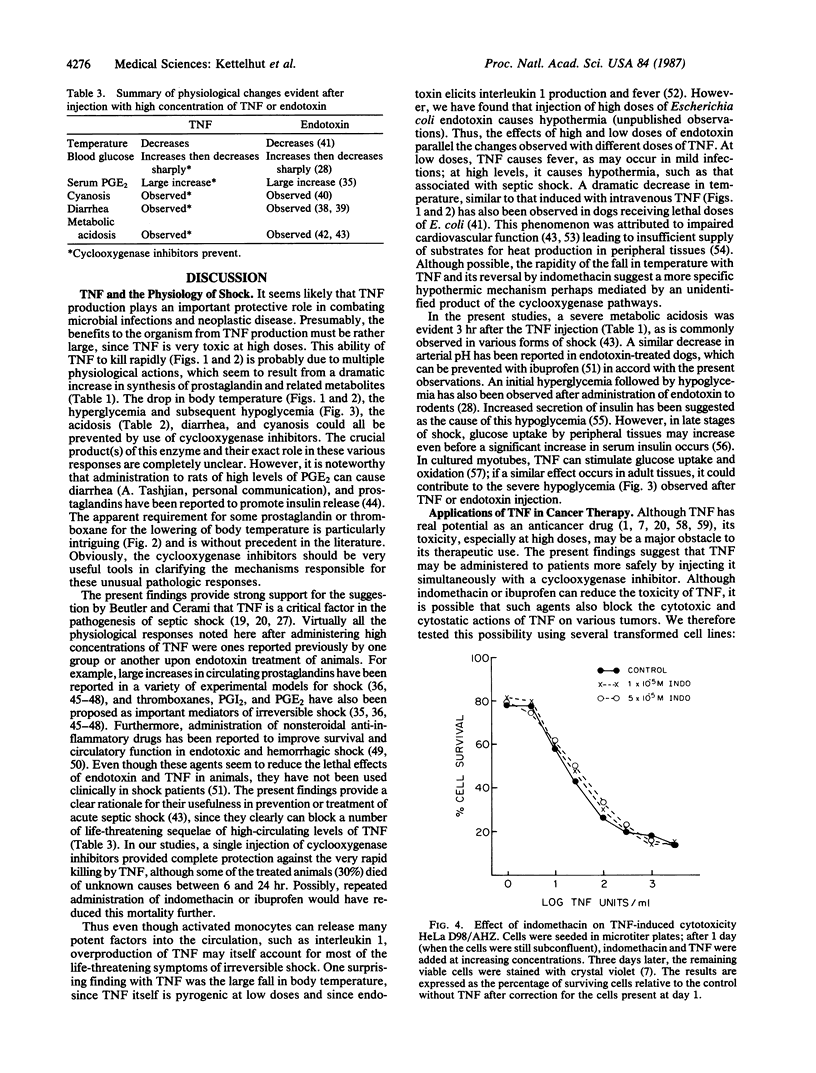
Tumor necrosis factor (TNF) is a macrophage product under active study as an anticancer drug. However, this agent can be very toxic and has been implicated in the pathogenesis of endotoxic shock. After intravenous injection of human recombinant TNF (4 micrograms/g), growing rats showed an unusual constellation of physiological responses, and all died within 2-4 hr. In 1 hr, TNF caused a sharp fall (2.5 degrees C) in body temperature and a large increase in plasma prostaglandin E2 levels. Blood glucose initially increased, but then a profound hypoglycemia developed by 2 hr. The TNF-treated animals also showed diarrhea, cyanosis, and a severe metabolic acidosis. A single injection of the cyclooxygenase inhibitors indomethacin or ibuprofen before the TNF treatment completely prevented the rapid killing and reduced eventual lethality by 70%. These agents blocked prostaglandin E2 production and prevented the hypothermia, changes in blood glucose, acidosis, and other symptoms. Since similar physiological changes have been reported after endotoxin injection, our data support the suggestion that TNF production is a critical factor in the development of septic shock. These findings also indicate that increased production of prostaglandins or thromboxanes is important in endotoxic shock and argue that cyclooxygenase inhibitors should be useful in its therapy. Indomethacin did not block the cytotoxic effects of TNF in vitro on several transformed cell lines (HeLa, Me 180, or L929). Therefore, combined use of TNF with a cyclooxygenase inhibitor may allow safer administration of high doses of this polypeptide to cancer patients.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggarwal B. B., Eessalu T. E., Hass P. E. Characterization of receptors for human tumour necrosis factor and their regulation by gamma-interferon. Nature. 1985 Dec 19;318(6047):665–667. doi: 10.1038/318665a0. [DOI] [PubMed] [Google Scholar]
- Almqvist P. M., Kuenzig M., Schwartz S. I. Treatment of experimental canine endotoxin shock with ibuprofen, a cyclooxygenase inhibitor. Circ Shock. 1984;13(3):227–232. [PubMed] [Google Scholar]
- Axelrod L., Levine L. Plasma prostaglandin levels in rats with diabetes mellitus and diabetic ketoacidosis. Diabetes. 1982 Nov;31(11):994–1001. doi: 10.2337/diacare.31.11.994. [DOI] [PubMed] [Google Scholar]
- Balkwill F. R., Lee A., Aldam G., Moodie E., Thomas J. A., Tavernier J., Fiers W. Human tumor xenografts treated with recombinant human tumor necrosis factor alone or in combination with interferons. Cancer Res. 1986 Aug;46(8):3990–3993. [PubMed] [Google Scholar]
- Baracos V., Rodemann H. P., Dinarello C. A., Goldberg A. L. Stimulation of muscle protein degradation and prostaglandin E2 release by leukocytic pyrogen (interleukin-1). A mechanism for the increased degradation of muscle proteins during fever. N Engl J Med. 1983 Mar 10;308(10):553–558. doi: 10.1056/NEJM198303103081002. [DOI] [PubMed] [Google Scholar]
- Bastiaans J. C., Vleeming W. The effect of compensation of acidosis on survival in endotoxin shock. Adv Shock Res. 1981;6:163–174. [PubMed] [Google Scholar]
- Bernheim H. A., Gilbert T. M., Stitt J. T. Prostaglandin E levels in third ventricular cerebrospinal fluid of rabbits during fever and changes in body temperature. J Physiol. 1980 Apr;301:69–78. doi: 10.1113/jphysiol.1980.sp013189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler B., Cerami A. Cachectin and tumour necrosis factor as two sides of the same biological coin. Nature. 1986 Apr 17;320(6063):584–588. doi: 10.1038/320584a0. [DOI] [PubMed] [Google Scholar]
- Beutler B., Greenwald D., Hulmes J. D., Chang M., Pan Y. C., Mathison J., Ulevitch R., Cerami A. Identity of tumour necrosis factor and the macrophage-secreted factor cachectin. Nature. 1985 Aug 8;316(6028):552–554. doi: 10.1038/316552a0. [DOI] [PubMed] [Google Scholar]
- Beutler B., Mahoney J., Le Trang N., Pekala P., Cerami A. Purification of cachectin, a lipoprotein lipase-suppressing hormone secreted by endotoxin-induced RAW 264.7 cells. J Exp Med. 1985 May 1;161(5):984–995. doi: 10.1084/jem.161.5.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler B., Milsark I. W., Cerami A. C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985 Aug 30;229(4716):869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- Bond R. F. Peripheral vascular adrenergic depression during hypotension induced by E coli endotoxin. Adv Shock Res. 1983;9:157–169. [PubMed] [Google Scholar]
- Brouckaert P. G., Leroux-Roels G. G., Guisez Y., Tavernier J., Fiers W. In vivo anti-tumour activity of recombinant human and murine TNF, alone and in combination with murine IFN-gamma, on a syngeneic murine melanoma. Int J Cancer. 1986 Nov 15;38(5):763–769. doi: 10.1002/ijc.2910380521. [DOI] [PubMed] [Google Scholar]
- Bult H., Beetens J., Vercruysse P., Herman A. G. Blood levels of 6-keto-PGF1alpha, the stable metabolite of prostacyclin during endotoxin-induced hypotension. Arch Int Pharmacodyn Ther. 1978 Dec;236(2):285–286. [PubMed] [Google Scholar]
- Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerami A., Ikeda Y., Le Trang N., Hotez P. J., Beutler B. Weight loss associated with an endotoxin-induced mediator from peritoneal macrophages: the role of cachectin (tumor necrosis factor). Immunol Lett. 1985;11(3-4):173–177. doi: 10.1016/0165-2478(85)90165-8. [DOI] [PubMed] [Google Scholar]
- Cook J. A., Wise W. C., Halushka P. V. Elevated thromboxane levels in the rat during endotoxic shock: protective effects of imidazole, 13-azaprostanoic acid, or essential fatty acid deficiency. J Clin Invest. 1980 Jan;65(1):227–230. doi: 10.1172/JCI109655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer J. M., Beutler B., Cerami A. Cachectin/tumor necrosis factor stimulates collagenase and prostaglandin E2 production by human synovial cells and dermal fibroblasts. J Exp Med. 1985 Dec 1;162(6):2163–2168. doi: 10.1084/jem.162.6.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demling R. H., Smith M., Gunther R., Flynn J. T., Gee M. H. Pulmonary injury and prostaglandin production during endotoxemia in conscious sheep. Am J Physiol. 1981 Mar;240(3):H348–H353. doi: 10.1152/ajpheart.1981.240.3.H348. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1. Rev Infect Dis. 1984 Jan-Feb;6(1):51–95. doi: 10.1093/clinids/6.1.51. [DOI] [PubMed] [Google Scholar]
- Falk A., Redfors S., Myrvold H., Haglund U. Small intestinal mucosal lesions in feline septic shock: a study on the pathogenesis. Circ Shock. 1985;17(4):327–337. [PubMed] [Google Scholar]
- Feinman R., Henriksen-DeStefano D., Tsujimoto M., Vilcek J. Tumor necrosis factor is an important mediator of tumor cell killing by human monocytes. J Immunol. 1987 Jan 15;138(2):635–640. [PubMed] [Google Scholar]
- Filkins J. P. Monokines and the metabolic pathophysiology of septic shock. Fed Proc. 1985 Feb;44(2):300–304. [PubMed] [Google Scholar]
- Filkins J. P. Reticuloendothelial system function and glucose-insulin dyshomeostasis in sepsis. Am J Emerg Med. 1984 Jan;2(1):70–73. doi: 10.1016/0735-6757(84)90111-6. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick F. A., Aguirre R., Pike J. E., Lincoln F. H. The stability of 13,14-dihydro-15 keto-PGE2. Prostaglandins. 1980 Jun;19(6):917–931. doi: 10.1016/0090-6980(80)90126-4. [DOI] [PubMed] [Google Scholar]
- Fransen L., Van der Heyden J., Ruysschaert R., Fiers W. Recombinant tumor necrosis factor: its effect and its synergism with interferon-gamma on a variety of normal and transformed human cell lines. Eur J Cancer Clin Oncol. 1986 Apr;22(4):419–426. doi: 10.1016/0277-5379(86)90107-0. [DOI] [PubMed] [Google Scholar]
- Granström E., Hamberg M., Hansson G., Kindahl H. Chemical instability of 15-keto-13,14-dihydro-PGE2: the reason for low assay reliability. Prostaglandins. 1980 Jun;19(6):933–957. doi: 10.1016/0090-6980(80)90127-6. [DOI] [PubMed] [Google Scholar]
- Hand M. S., Fettman M. J., Chandrasena L. G., Cleek J. L., Mason R. A., Phillips R. W. Persistent hyperinsulinemia due to induced euglycemia in awake endotoxic minipigs. Circ Shock. 1984;14(3):169–187. [PubMed] [Google Scholar]
- Haranaka K., Satomi N. Cytotoxic activity of tumor necrosis factor (TNF) on human cancer cells in vitro. Jpn J Exp Med. 1981 Jun;51(3):191–194. [PubMed] [Google Scholar]
- Haranaka K., Satomi N., Sakurai A. Antitumor activity of murine tumor necrosis factor (TNF) against transplanted murine tumors and heterotransplanted human tumors in nude mice. Int J Cancer. 1984 Aug 15;34(2):263–267. doi: 10.1002/ijc.2910340219. [DOI] [PubMed] [Google Scholar]
- Helson L., Green S., Carswell E., Old L. J. Effect of tumour necrosis factor on cultured human melanoma cells. Nature. 1975 Dec 25;258(5537):731–732. doi: 10.1038/258731a0. [DOI] [PubMed] [Google Scholar]
- Hinshaw L. B., Peyton M. D., Archer L. T., Black M. R., Coalson J. J., Greenfield L. J. Prevention of death in endotoxin shock by glucose administration. Surg Gynecol Obstet. 1974 Dec;139(6):851–859. [PubMed] [Google Scholar]
- Jacobs E. R., Soulsby M. E., Bone R. C., Wilson F. J., Jr, Hiller F. C. Ibuprofen in canine endotoxin shock. J Clin Invest. 1982 Sep;70(3):536–541. doi: 10.1172/JCI110645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan L., Weiss J., Elsbach P. Low concentrations of indomethacin inhibit phospholipase A2 of rabbit polymorphonuclear leukocytes. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2955–2958. doi: 10.1073/pnas.75.6.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmenout A., Fransen L., Tavernier J., Van der Heyden J., Tizard R., Kawashima E., Shaw A., Johnson M. J., Semon D., Müller R. Molecular cloning and expression of human tumor necrosis factor and comparison with mouse tumor necrosis factor. Eur J Biochem. 1985 Nov 4;152(3):515–522. doi: 10.1111/j.1432-1033.1985.tb09226.x. [DOI] [PubMed] [Google Scholar]
- Old L. J. Tumor necrosis factor (TNF). Science. 1985 Nov 8;230(4726):630–632. doi: 10.1126/science.2413547. [DOI] [PubMed] [Google Scholar]
- Ortaldo J. R., Mason L. H., Mathieson B. J., Liang S. M., Flick D. A., Herberman R. B. Mediation of mouse natural cytotoxic activity by tumour necrosis factor. Nature. 1986 Jun 12;321(6071):700–702. doi: 10.1038/321700a0. [DOI] [PubMed] [Google Scholar]
- Pennica D., Nedwin G. E., Hayflick J. S., Seeburg P. H., Derynck R., Palladino M. A., Kohr W. J., Aggarwal B. B., Goeddel D. V. Human tumour necrosis factor: precursor structure, expression and homology to lymphotoxin. Nature. 1984 Dec 20;312(5996):724–729. doi: 10.1038/312724a0. [DOI] [PubMed] [Google Scholar]
- Phair R. D., Pek S. B., Lands W. E. Arachidonic acid induced release of insulin and glucagon: role of endogenous prostaglandins in pancreatic hormone secretion. Diabete Metab. 1984 May;10(2):71–77. [PubMed] [Google Scholar]
- Philip R., Epstein L. B. Tumour necrosis factor as immunomodulator and mediator of monocyte cytotoxicity induced by itself, gamma-interferon and interleukin-1. Nature. 1986 Sep 4;323(6083):86–89. doi: 10.1038/323086a0. [DOI] [PubMed] [Google Scholar]
- Ruggiero V., Tavernier J., Fiers W., Baglioni C. Induction of the synthesis of tumor necrosis factor receptors by interferon-gamma. J Immunol. 1986 Apr 1;136(7):2445–2450. [PubMed] [Google Scholar]
- Sato T., Isoyama T., Tanaka J., Jones R. T., Cowley R. A., Trump B. F. The pathophysiology of septic shock: changes in hemodynamics in rats following live E coli injection. An application of the thermodilution method for measurement of cardiac output. Adv Shock Res. 1982;7:25–42. [PubMed] [Google Scholar]
- Shine K. I., Kuhn M., Young L. S., Tillisch J. H. Aspects of the management of shock. Ann Intern Med. 1980 Nov;93(5):723–734. doi: 10.7326/0003-4819-93-5-723. [DOI] [PubMed] [Google Scholar]
- Shirai T., Yamaguchi H., Ito H., Todd C. W., Wallace R. B. Cloning and expression in Escherichia coli of the gene for human tumour necrosis factor. 1985 Feb 28-Mar 6Nature. 313(6005):803–806. doi: 10.1038/313803a0. [DOI] [PubMed] [Google Scholar]
- Short B. L., Gardiner M., Walker R. I., Jones S. R., Fletcher J. R. Indomethacin improves survival in gram-negative sepsis. Adv Shock Res. 1981;6:27–36. [PubMed] [Google Scholar]
- Sugarman B. J., Aggarwal B. B., Hass P. E., Figari I. S., Palladino M. A., Jr, Shepard H. M. Recombinant human tumor necrosis factor-alpha: effects on proliferation of normal and transformed cells in vitro. Science. 1985 Nov 22;230(4728):943–945. doi: 10.1126/science.3933111. [DOI] [PubMed] [Google Scholar]
- Tanaka J., Sato T., Jones R. T., Trump B. F., Cowley R. A. The pathophysiology of septic shock: responses to different doses of live Escherichia coli injection in rats. Adv Shock Res. 1983;9:101–114. [PubMed] [Google Scholar]
- Torti F. M., Dieckmann B., Beutler B., Cerami A., Ringold G. M. A macrophage factor inhibits adipocyte gene expression: an in vitro model of cachexia. Science. 1985 Aug 30;229(4716):867–869. doi: 10.1126/science.3839597. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Beutler B., Lowry S. F., Merryweather J., Wolpe S., Milsark I. W., Hariri R. J., Fahey T. J., 3rd, Zentella A., Albert J. D. Shock and tissue injury induced by recombinant human cachectin. Science. 1986 Oct 24;234(4775):470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- Tsujimoto M., Vilcek J. Tumor necrosis factor receptors in HeLa cells and their regulation by interferon-gamma. J Biol Chem. 1986 Apr 25;261(12):5384–5388. [PubMed] [Google Scholar]
- Wang A. M., Creasey A. A., Ladner M. B., Lin L. S., Strickler J., Van Arsdell J. N., Yamamoto R., Mark D. F. Molecular cloning of the complementary DNA for human tumor necrosis factor. Science. 1985 Apr 12;228(4696):149–154. doi: 10.1126/science.3856324. [DOI] [PubMed] [Google Scholar]
- Williamson B. D., Carswell E. A., Rubin B. Y., Prendergast J. S., Old L. J. Human tumor necrosis factor produced by human B-cell lines: synergistic cytotoxic interaction with human interferon. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5397–5401. doi: 10.1073/pnas.80.17.5397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise W. C., Cook J. A., Halushka P. V. Implications for thromboxane A2 in the pathogenesis of endotoxic shock. Adv Shock Res. 1981;6:83–91. [PubMed] [Google Scholar]
- Yelich M. R., Filkins J. P. Insulin hypersecretion and potentiation of endotoxin shock in the rat. Circ Shock. 1982;9(6):589–603. [PubMed] [Google Scholar]


