Abstract
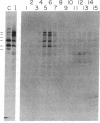
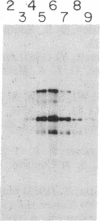
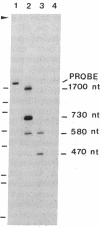
Protein 4.1 is an extrinsic membrane protein that facilitates the interaction of spectrin and actin in the erythroid membrane skeleton and exists as several structurally related polypeptides in chickens. The ratio of protein 4.1 variants is developmentally regulated during terminal differentiation of chicken erythroid and lenticular cells. To examine the mechanisms by which multiple chicken protein 4.1 variants are differentially expressed, we have isolated cDNA clones specific for chicken erythroid protein 4.1. We show that a single protein 4.1 gene gives rise to multiple 6.6-kilobase mRNAs by differential RNA processing. Furthermore, the ratios of protein 4.1 mRNAs change during chicken embryonic erythropoiesis. We observe a quantitative difference in variant ratios when protein 4.1 is synthesized in vivo or in a rabbit reticulocyte lysate in vitro. Our results show that the expression of multiple protein 4.1 polypeptides is regulated at the levels of translation and RNA processing.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amara S. G., Jonas V., Rosenfeld M. G., Ong E. S., Evans R. M. Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature. 1982 Jul 15;298(5871):240–244. doi: 10.1038/298240a0. [DOI] [PubMed] [Google Scholar]
- Aster J. C., Brewer G. J., Maisel H. The 4.1-like proteins of the bovine lens: spectrin-binding proteins closely related in structure to red blood cell protein 4.1. J Cell Biol. 1986 Jul;103(1):115–122. doi: 10.1083/jcb.103.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aster J. C., Welsh M. J., Brewer G. J., Maisel H. Identification of spectrin and protein 4.1-like proteins in mammalian lens. Biochem Biophys Res Commun. 1984 Mar 15;119(2):726–734. doi: 10.1016/s0006-291x(84)80311-3. [DOI] [PubMed] [Google Scholar]
- Axelrod V. D., Kramer F. R. Transcription from bacteriophage T7 and SP6 RNA polymerase promoters in the presence of 3'-deoxyribonucleoside 5'-triphosphate chain terminators. Biochemistry. 1985 Oct 8;24(21):5716–5723. doi: 10.1021/bi00342a005. [DOI] [PubMed] [Google Scholar]
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Baines A. J., Bennett V. Synapsin I is a spectrin-binding protein immunologically related to erythrocyte protein 4.1. 1985 May 30-Jun 5Nature. 315(6018):410–413. doi: 10.1038/315410a0. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Branton D., Cohen C. M., Tyler J. Interaction of cytoskeletal proteins on the human erythrocyte membrane. Cell. 1981 Apr;24(1):24–32. doi: 10.1016/0092-8674(81)90497-9. [DOI] [PubMed] [Google Scholar]
- Breitbart R. E., Nguyen H. T., Medford R. M., Destree A. T., Mahdavi V., Nadal-Ginard B. Intricate combinatorial patterns of exon splicing generate multiple regulated troponin T isoforms from a single gene. Cell. 1985 May;41(1):67–82. doi: 10.1016/0092-8674(85)90062-5. [DOI] [PubMed] [Google Scholar]
- Bruns G. A., Ingram V. M. The erythroid cells and haemoglobins of the chick embryo. Philos Trans R Soc Lond B Biol Sci. 1973 Oct 25;266(877):225–305. doi: 10.1098/rstb.1973.0050. [DOI] [PubMed] [Google Scholar]
- Capetanaki Y. G., Ngai J., Flytzanis C. N., Lazarides E. Tissue-specific expression of two mRNA species transcribed from a single vimentin gene. Cell. 1983 Dec;35(2 Pt 1):411–420. doi: 10.1016/0092-8674(83)90174-5. [DOI] [PubMed] [Google Scholar]
- Cohen C. M., Foley S. F., Korsgren C. A protein immunologically related to erythrocyte band 4.1 is found on stress fibres on non-erythroid cells. Nature. 1982 Oct 14;299(5884):648–650. doi: 10.1038/299648a0. [DOI] [PubMed] [Google Scholar]
- Conboy J., Kan Y. W., Shohet S. B., Mohandas N. Molecular cloning of protein 4.1, a major structural element of the human erythrocyte membrane skeleton. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9512–9516. doi: 10.1073/pnas.83.24.9512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree G. R., Kant J. A. Organization of the rat gamma-fibrinogen gene: alternative mRNA splice patterns produce the gamma A and gamma B (gamma ') chains of fibrinogen. Cell. 1982 Nov;31(1):159–166. doi: 10.1016/0092-8674(82)90415-9. [DOI] [PubMed] [Google Scholar]
- Dodgson J. B., Strommer J., Engel J. D. Isolation of the chicken beta-globin gene and a linked embryonic beta-like globin gene from a chicken DNA recombinant library. Cell. 1979 Aug;17(4):879–887. doi: 10.1016/0092-8674(79)90328-3. [DOI] [PubMed] [Google Scholar]
- Early P., Rogers J., Davis M., Calame K., Bond M., Wall R., Hood L. Two mRNAs can be produced from a single immunoglobulin mu gene by alternative RNA processing pathways. Cell. 1980 Jun;20(2):313–319. doi: 10.1016/0092-8674(80)90617-0. [DOI] [PubMed] [Google Scholar]
- Goldberg D. A. Isolation and partial characterization of the Drosophila alcohol dehydrogenase gene. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5794–5798. doi: 10.1073/pnas.77.10.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman S. R., Casoria L. A., Coleman D. B., Zagon I. S. Identification and location of brain protein 4.1. Science. 1984 Jun 29;224(4656):1433–1436. doi: 10.1126/science.6374897. [DOI] [PubMed] [Google Scholar]
- Goodman S. R., Yu J., Whitfield C. F., Culp E. N., Posnak E. J. Erythrocyte membrane skeletal protein bands 4.1 a and b are sequence-related phosphoproteins. J Biol Chem. 1982 Apr 25;257(8):4564–4569. [PubMed] [Google Scholar]
- Granger B. L., Lazarides E. Appearance of new variants of membrane skeletal protein 4.1 during terminal differentiation of avian erythroid and lenticular cells. Nature. 1985 Jan 17;313(5999):238–241. doi: 10.1038/313238a0. [DOI] [PubMed] [Google Scholar]
- Granger B. L., Lazarides E. Membrane skeletal protein 4.1 of avian erythrocytes is composed of multiple variants that exhibit tissue-specific expression. Cell. 1984 Jun;37(2):595–607. doi: 10.1016/0092-8674(84)90390-8. [DOI] [PubMed] [Google Scholar]
- Jackson R. J., Hunt T. Preparation and use of nuclease-treated rabbit reticulocyte lysates for the translation of eukaryotic messenger RNA. Methods Enzymol. 1983;96:50–74. doi: 10.1016/s0076-6879(83)96008-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leto T. L., Marchesi V. T. A structural model of human erythrocyte protein 4.1. J Biol Chem. 1984 Apr 10;259(7):4603–4608. [PubMed] [Google Scholar]
- Marchesi V. T. Stabilizing infrastructure of cell membranes. Annu Rev Cell Biol. 1985;1:531–561. doi: 10.1146/annurev.cb.01.110185.002531. [DOI] [PubMed] [Google Scholar]
- Moon R. T., Ngai J., Wold B. J., Lazarides E. Tissue-specific expression of distinct spectrin and ankyrin transcripts in erythroid and nonerythroid cells. J Cell Biol. 1985 Jan;100(1):152–160. doi: 10.1083/jcb.100.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Periasamy M., Strehler E. E., Garfinkel L. I., Gubits R. M., Ruiz-Opazo N., Nadal-Ginard B. Fast skeletal muscle myosin light chains 1 and 3 are produced from a single gene by a combined process of differential RNA transcription and splicing. J Biol Chem. 1984 Nov 10;259(21):13595–13604. [PubMed] [Google Scholar]
- Ricciardi R. P., Miller J. S., Roberts B. E. Purification and mapping of specific mRNAs by hybridization-selection and cell-free translation. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4927–4931. doi: 10.1073/pnas.76.10.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spiegel J. E., Beardsley D. S., Southwick F. S., Lux S. E. An analogue of the erythroid membrane skeletal protein 4.1 in nonerythroid cells. J Cell Biol. 1984 Sep;99(3):886–893. doi: 10.1083/jcb.99.3.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staufenbiel M., Lazarides E. Assembly of protein 4.1 during chicken erythroid differentiation. J Cell Biol. 1986 Apr;102(4):1157–1163. doi: 10.1083/jcb.102.4.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Hagenbüchle O., Schibler U. A single mouse alpha-amylase gene specifies two different tissue-specific mRNAs. Cell. 1981 Feb;23(2):451–458. doi: 10.1016/0092-8674(81)90140-9. [DOI] [PubMed] [Google Scholar]