Abstract
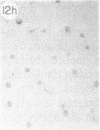
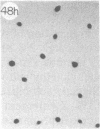
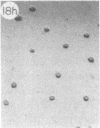
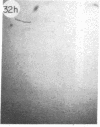
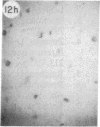
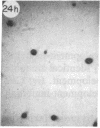
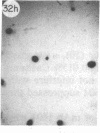
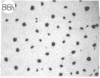
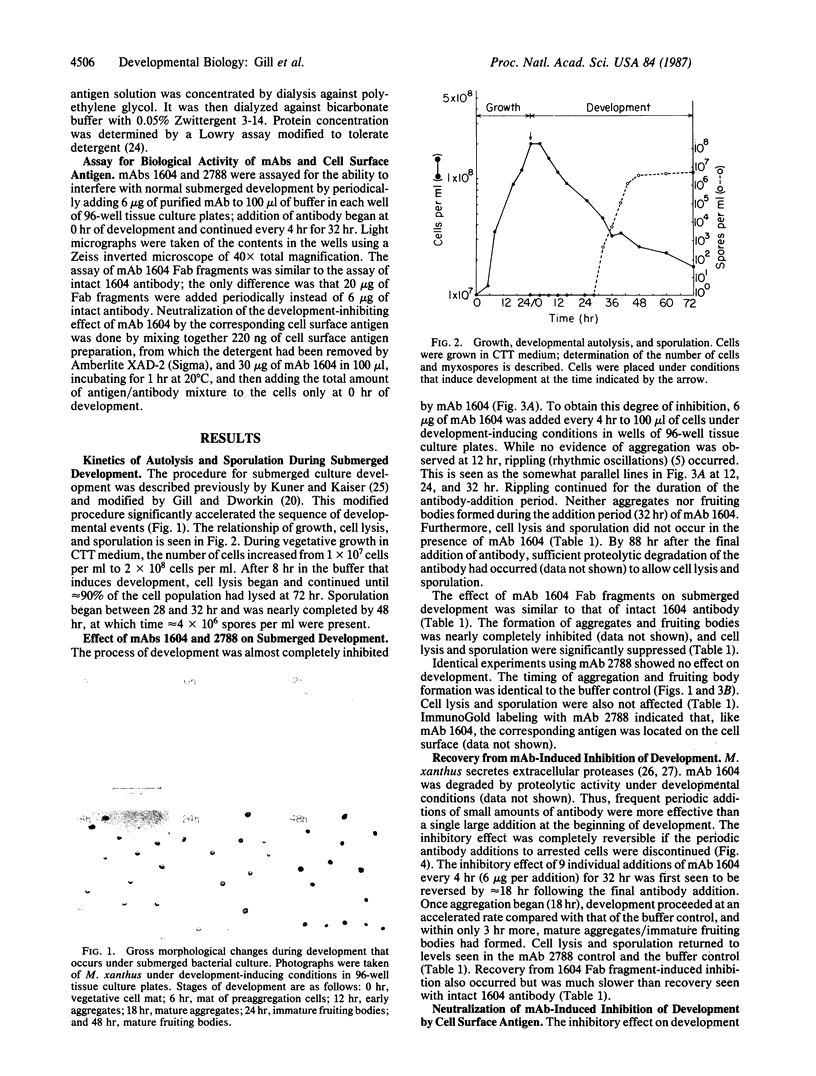
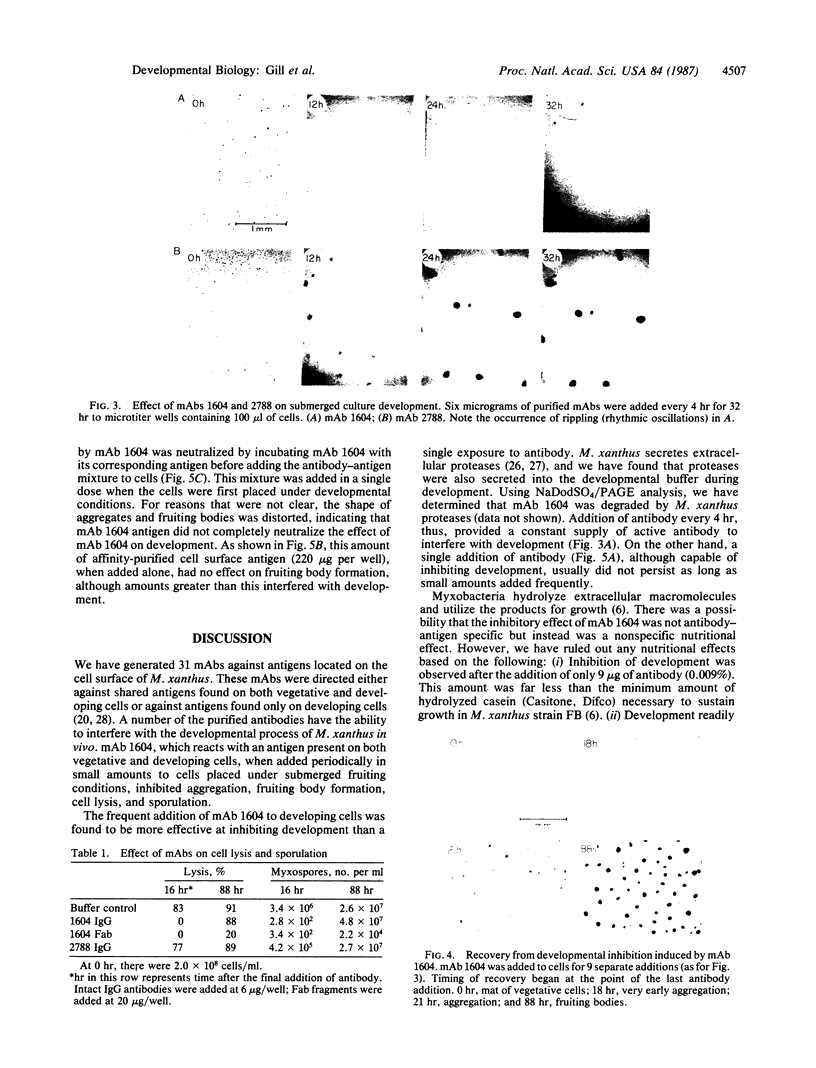
Monoclonal antibody (mAb) 1604 is directed against a cell surface antigen of Myxococcus xanthus. Purified antibody 1604 inhibited development of M. xanthus under conditions of submerged culture procedure otherwise leading to fruiting body formation. Intact molecules of mAb 1604, as well as its Fab fragments, inhibited developmental aggregation, autolysis, fruiting body formation, and sporulation. The addition of relatively small amounts of antibody every 4 hr was much more effective than a single large dose given at the onset of development. The inhibitory action of mAb 1604 on development was reversible after prolonged incubation of the antibody with cells; this was probably due to proteolytic degradation of the antibody. The effect of mAb 1604 on submerged bacterial development was neutralized by affinity-purified 1604 cell surface antigen. Another antibody, mAb 2788, directed against an M. xanthus cell surface antigen, did not block development. These data suggest that 1604 cell surface antigens is involved in contact-mediated cell interactions in M. xanthus.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brodie C., Klein C., Swierkosz J. Monoclonal antibodies: use to detect developmentally regulated antigens on D. discoideum amebae. Cell. 1983 Apr;32(4):1115–1123. doi: 10.1016/0092-8674(83)90295-7. [DOI] [PubMed] [Google Scholar]
- Decker C., Greggs R., Duggan K., Stubbs J., Horwitz A. Adhesive multiplicity in the interaction of embryonic fibroblasts and myoblasts with extracellular matrices. J Cell Biol. 1984 Oct;99(4 Pt 1):1398–1404. doi: 10.1083/jcb.99.4.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulley J. R., Grieve P. A. A simple technique for eliminating interference by detergents in the Lowry method of protein determination. Anal Biochem. 1975 Mar;64(1):136–141. doi: 10.1016/0003-2697(75)90415-7. [DOI] [PubMed] [Google Scholar]
- Dworkin M., Kaiser D. Cell interactions in myxobacterial growth and development. Science. 1985 Oct 4;230(4721):18–24. doi: 10.1126/science.3929384. [DOI] [PubMed] [Google Scholar]
- Edelman G. M. Cell adhesion molecules. Science. 1983 Feb 4;219(4584):450–457. doi: 10.1126/science.6823544. [DOI] [PubMed] [Google Scholar]
- Gill J. S., Dworkin M. Cell surface antigens during submerged development of Myxococcus xanthus examined with monoclonal antibodies. J Bacteriol. 1986 Nov;168(2):505–511. doi: 10.1128/jb.168.2.505-511.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill J., Stellwag E., Dworkin M. Monoclonal antibodies against cell-surface antigens of developing cells of Myxococcus xanthus. Ann Inst Pasteur Microbiol. 1985 Jan-Feb;136A(1):11–18. doi: 10.1016/s0769-2609(85)80015-6. [DOI] [PubMed] [Google Scholar]
- Hagen D. C., Bretscher A. P., Kaiser D. Synergism between morphogenetic mutants of Myxococcus xanthus. Dev Biol. 1978 Jun;64(2):284–296. doi: 10.1016/0012-1606(78)90079-9. [DOI] [PubMed] [Google Scholar]
- Hatta K., Okada T. S., Takeichi M. A monoclonal antibody disrupting calcium-dependent cell-cell adhesion of brain tissues: possible role of its target antigen in animal pattern formation. Proc Natl Acad Sci U S A. 1985 May;82(9):2789–2793. doi: 10.1073/pnas.82.9.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin J., Kaiser D. Cell-to-cell stimulation of movement in nonmotile mutants of Myxococcus. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2938–2942. doi: 10.1073/pnas.74.7.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen G. R., Dworkin M. Cell-cell interactions in developmental lysis of Myxococcus xanthus. Dev Biol. 1985 Nov;112(1):194–202. doi: 10.1016/0012-1606(85)90133-2. [DOI] [PubMed] [Google Scholar]
- Kaiser D., Manoil C., Dworkin M. Myxobacteria: cell interactions, genetics, and development. Annu Rev Microbiol. 1979;33:595–639. doi: 10.1146/annurev.mi.33.100179.003115. [DOI] [PubMed] [Google Scholar]
- Kaiser D. Social gliding is correlated with the presence of pili in Myxococcus xanthus. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5952–5956. doi: 10.1073/pnas.76.11.5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuner J. M., Kaiser D. Fruiting body morphogenesis in submerged cultures of Myxococcus xanthus. J Bacteriol. 1982 Jul;151(1):458–461. doi: 10.1128/jb.151.1.458-461.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRossa R., Kuner J., Hagen D., Manoil C., Kaiser D. Developmental cell interactions of Myxococcus xanthus: analysis of mutants. J Bacteriol. 1983 Mar;153(3):1394–1404. doi: 10.1128/jb.153.3.1394-1404.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochiai H., Schwarz H., Merkl R., Wagle G., Gerisch G. Stage-specific antigens reacting with monoclonal antibodies against contact site A, a cell-surface glycoprotein of Dictyostelium discoideum. Cell Differ. 1982 Jan;11(1):1–13. doi: 10.1016/0045-6039(82)90011-2. [DOI] [PubMed] [Google Scholar]
- Oi V. T., Herzenberg L. A. Localization of murine Ig-1b and Ig-1a (IgG 2a) allotypic determinants detected with monoclonal antibodies. Mol Immunol. 1979 Dec;16(12):1005–1017. doi: 10.1016/0161-5890(79)90034-8. [DOI] [PubMed] [Google Scholar]
- Rosenberg E., Keller K. H., Dworkin M. Cell density-dependent growth of Myxococcus xanthus on casein. J Bacteriol. 1977 Feb;129(2):770–777. doi: 10.1128/jb.129.2.770-777.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimkets L. J., Dworkin M. Excreted adenosine is a cell density signal for the initiation of fruiting body formation in Myxococcus xanthus. Dev Biol. 1981 May;84(1):51–60. doi: 10.1016/0012-1606(81)90369-9. [DOI] [PubMed] [Google Scholar]
- Springer W. R., Barondes S. H. Monoclonal antibodies block cell-cell adhesion in Dictyostelium discoideum. J Biol Chem. 1983 Apr 25;258(8):4698–4701. [PubMed] [Google Scholar]
- Toda K., Bozzaro S., Lottspeich F., Merkl R., Gerisch G. Monoclonal anti-glycoprotein antibody that blocks cell adhesion in Polysphondylium pallidum. Eur J Biochem. 1984 Apr 2;140(1):73–81. doi: 10.1111/j.1432-1033.1984.tb08068.x. [DOI] [PubMed] [Google Scholar]
- Toda K., Tharanathan R. N., Bozzaro S., Gerisch G. Monoclonal antibodies that block cell adhesion in Polysphondylium pallidum: reaction with L-fucose, a terminal sugar in cell-surface glycoproteins. Eur J Biochem. 1984 Sep 17;143(3):477–481. doi: 10.1111/j.1432-1033.1984.tb08395.x. [DOI] [PubMed] [Google Scholar]
- Wireman J. W., Dworkin M. Developmentally induced autolysis during fruiting body formation by Myxococcus xanthus. J Bacteriol. 1977 Feb;129(2):798–802. doi: 10.1128/jb.129.2.798-802.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]