Abstract
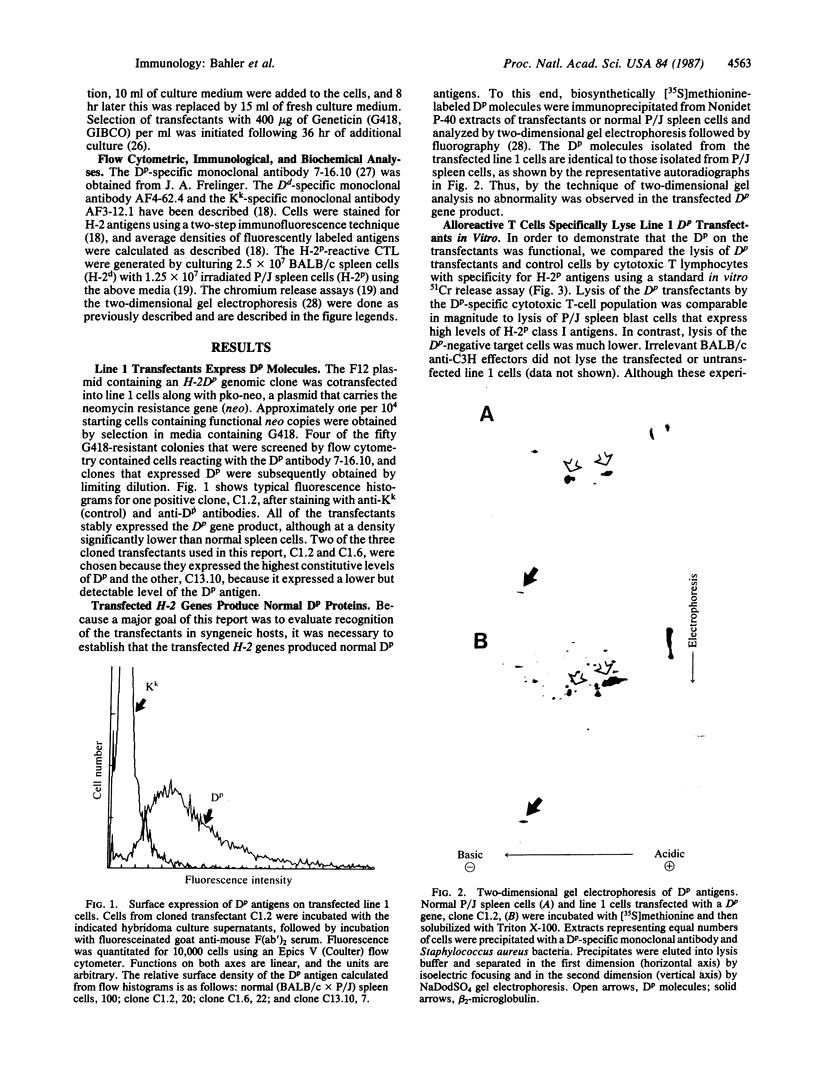
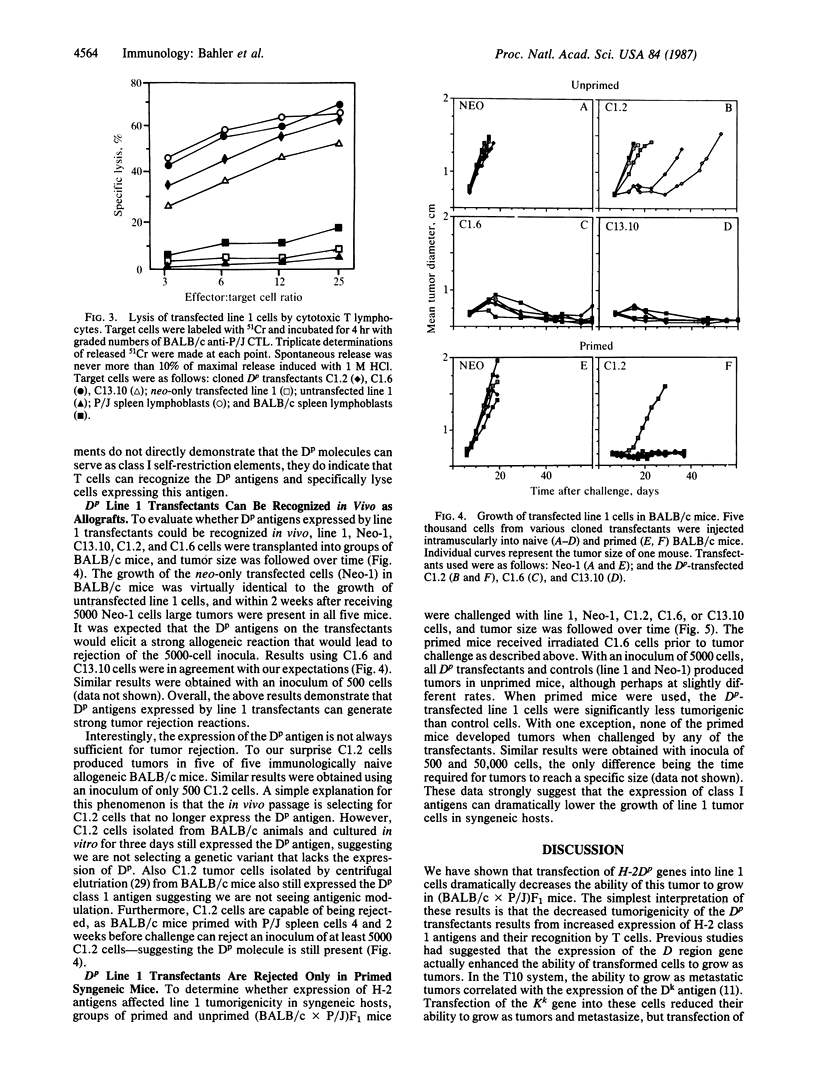
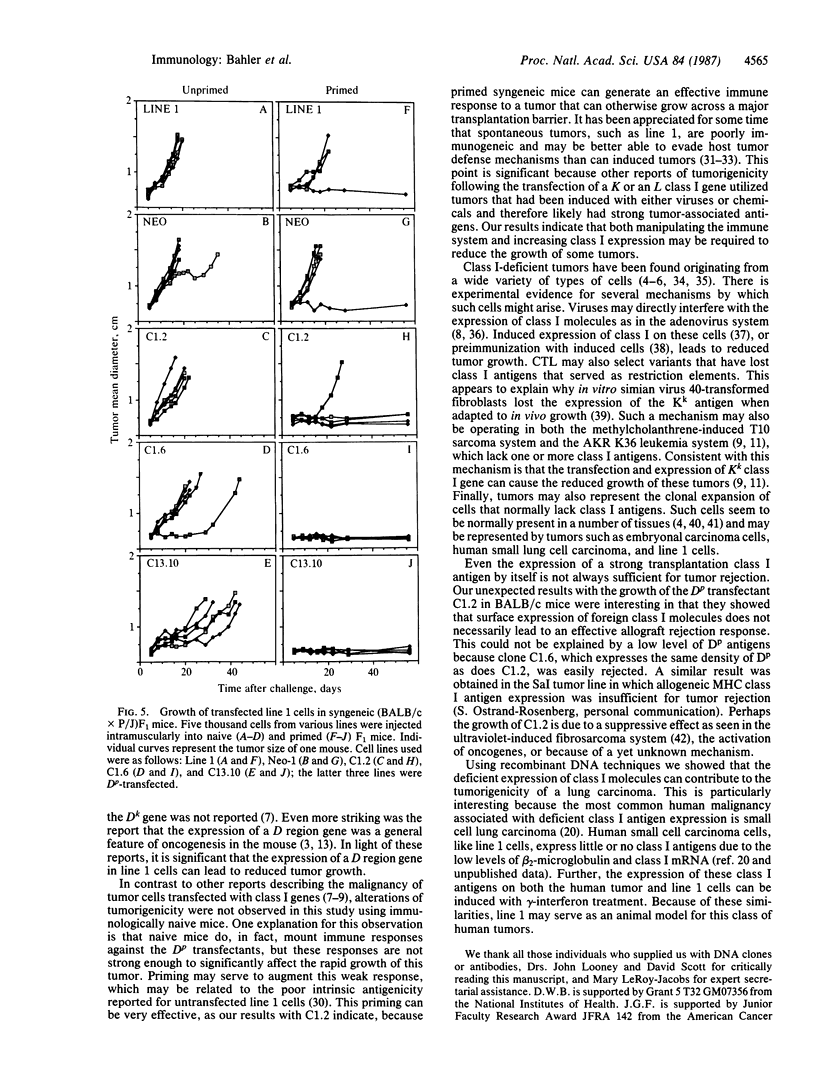
Cultured cells of the murine lung carcinoma called line 1 express very low levels of H-2 class I antigens and are resistant to lysis mediated by alloreactive T cells. In order to investigate how the expression of class I antigens affects the in vivo growth of this spontaneous tumor, H-2Dp genes were transferred into line 1 cells. Cloned transfectants that displayed H-2Dp surface antigens were identified using flow cytometry. The transfected H-2Dp antigens appeared normal by two-dimensional gel electrophoresis and could also function as excellent targets for T-cell-mediated lysis in vitro. Marked differences in tumorigenicity (defined as tumor growth in immunologically competent hosts) were observed between the Dp transfected cells and untransfected or control transfected line 1 cells in syngeneic mice only if the animals had previously received injections of irradiated Dp transfectants. Expression of Dp antigens did not appreciably affect the growth of line 1 tumors in immunologically naive syngeneic mice or necessarily cause rejection in allogeneic mice. Our in vivo results show that increased expression of class I antigens can reduce the growth of tumors like line 1 that lack all class I antigens. Our results also suggest that increasing class I antigens alone on some spontaneous tumors deficient in expression will not by itself be sufficient for tumor rejection.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bahler D. W., Lord E. M. Dimethyl sulfoxide induces expression of H-2 antigens on mouse lung carcinoma cells. J Immunol. 1985 Apr;134(4):2790–2798. [PubMed] [Google Scholar]
- Bahler D. W., Lord E. M., Kennel S. J., Horan P. K. Heterogeneity and clonal variation related to cell surface expression of a mouse lung tumor-associated antigen quantified using flow cytometry. Cancer Res. 1984 Aug;44(8):3317–3323. [PubMed] [Google Scholar]
- Bernards R., Schrier P. I., Houweling A., Bos J. L., van der Eb A. J., Zijlstra M., Melief C. J. Tumorigenicity of cells transformed by adenovirus type 12 by evasion of T-cell immunity. 1983 Oct 27-Nov 2Nature. 305(5937):776–779. doi: 10.1038/305776a0. [DOI] [PubMed] [Google Scholar]
- Brickell P. M., Latchman D. S., Murphy D., Willison K., Rigby P. W. Activation of a Qa/Tla class I major histocompatibility antigen gene is a general feature of oncogenesis in the mouse. Nature. 1983 Dec 22;306(5945):756–760. doi: 10.1038/306756a0. [DOI] [PubMed] [Google Scholar]
- Brickell P. M., Latchman D. S., Murphy D., Willison K., Rigby P. W. The class I major histocompatibility antigen gene activated in a line of SV40-transformed mouse cells is H-2Dd, not Qa/Tla. Nature. 1985 Jul 11;316(6024):162–163. doi: 10.1038/316162a0. [DOI] [PubMed] [Google Scholar]
- Darsley M. J., Takahashi H., Macchi M. J., Frelinger J. A., Ozato K., Appella E. New family of exon-shuffled recombinant genes reveals extensive interdomain interactions in class I histocompatibility antigens and identifies residues involved. J Exp Med. 1987 Jan 1;165(1):211–222. doi: 10.1084/jem.165.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle A., Martin W. J., Funa K., Gazdar A., Carney D., Martin S. E., Linnoila I., Cuttitta F., Mulshine J., Bunn P. Markedly decreased expression of class I histocompatibility antigens, protein, and mRNA in human small-cell lung cancer. J Exp Med. 1985 May 1;161(5):1135–1151. doi: 10.1084/jem.161.5.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming K. A., McMichael A., Morton J. A., Woods J., McGee J. O. Distribution of HLA class 1 antigens in normal human tissue and in mammary cancer. J Clin Pathol. 1981 Jul;34(7):779–784. doi: 10.1136/jcp.34.7.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frelinger J. G., Shigeta M., Infante A. J., Nelson P. A., Pierres M., Fathman C. G. Multiple functional sites on a single Ia molecule defined using T cell clones and antibodies with chain-determined specificity. J Exp Med. 1984 Mar 1;159(3):704–715. doi: 10.1084/jem.159.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenow R. S., McMillan M., Nicolson M., Sher B. T., Eakle K., Davidson N., Hood L. Identification of the class I genes of the mouse major histocompatibility complex by DNA-mediated gene transfer. Nature. 1982 Nov 18;300(5889):231–237. doi: 10.1038/300231a0. [DOI] [PubMed] [Google Scholar]
- Goodenow R. S., Vogel J. M., Linsk R. L. Histocompatibility antigens on murine tumors. Science. 1985 Nov 15;230(4727):777–783. doi: 10.1126/science.2997918. [DOI] [PubMed] [Google Scholar]
- Gooding L. R. Characterization of a progressive tumor from C3H fibroblasts transformed in vitro with SV40 virus. Immunoresistance in vivo correlates with phenotypic loss of H-2Kk. J Immunol. 1982 Sep;129(3):1306–1312. [PubMed] [Google Scholar]
- Harmon R. C., Stein N., Frelinger J. A. Monoclonal antibodies reactive with H-2 determinants. Immunogenetics. 1983;18(5):541–545. doi: 10.1007/BF00364395. [DOI] [PubMed] [Google Scholar]
- Hayashi H., Tanaka K., Jay F., Khoury G., Jay G. Modulation of the tumorigenicity of human adenovirus-12-transformed cells by interferon. Cell. 1985 Nov;43(1):263–267. doi: 10.1016/0092-8674(85)90031-5. [DOI] [PubMed] [Google Scholar]
- Hewitt H. B., Blake E. R., Walder A. S. A critique of the evidence for active host defence against cancer, based on personal studies of 27 murine tumours of spontaneous origin. Br J Cancer. 1976 Mar;33(3):241–259. doi: 10.1038/bjc.1976.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui K., Grosveld F., Festenstein H. Rejection of transplantable AKR leukaemia cells following MHC DNA-mediated cell transformation. Nature. 1984 Oct 25;311(5988):750–752. doi: 10.1038/311750a0. [DOI] [PubMed] [Google Scholar]
- Katzav S., Segal S., Feldman M. Immuno-selection in vivo of H-2D phenotypic variants from a metastatic clone of sarcoma cells results in cell lines of altered metastatic competence. Int J Cancer. 1984 Mar 15;33(3):407–415. doi: 10.1002/ijc.2910330320. [DOI] [PubMed] [Google Scholar]
- Klein G., Klein E. Immune surveillance against virus-induced tumors and nonrejectability of spontaneous tumors: contrasting consequences of host versus tumor evolution. Proc Natl Acad Sci U S A. 1977 May;74(5):2121–2125. doi: 10.1073/pnas.74.5.2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppers R. C., Ballas Z. K., Green W. R., Henney C. S. Quantitative appraisal of H-2 products in T cell-mediated lysis by allogeneic and syngeneic effector cells. J Immunol. 1981 Aug;127(2):500–504. [PubMed] [Google Scholar]
- Kärre K., Ljunggren H. G., Piontek G., Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature. 1986 Feb 20;319(6055):675–678. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- Lampson L. A., Fisher C. A., Whelan J. P. Striking paucity of HLA-A, B, C and beta 2-microglobulin on human neuroblastoma cell lines. J Immunol. 1983 May;130(5):2471–2478. [PubMed] [Google Scholar]
- Lord E. M., Keng P. C. Methods for using centrifugal elutriation to separate malignant and lymphoid cell populations. J Immunol Methods. 1984 Mar 30;68(1-2):147–155. doi: 10.1016/0022-1759(84)90145-5. [DOI] [PubMed] [Google Scholar]
- McLaughlin-Taylor E., Woodward J. G., Macchi M. J., McMillan M., Frelinger J. A. Functional characterization of the H-2Dp gene product. Immunogenetics. 1984;19(3):205–216. doi: 10.1007/BF00364764. [DOI] [PubMed] [Google Scholar]
- Morello D., Daniel F., Baldacci P., Cayre Y., Gachelin G., Kourilsky P. Absence of significant H-2 and beta 2-microglobulin mRNA expression by mouse embryonal carcinoma cells. Nature. 1982 Mar 18;296(5854):260–262. doi: 10.1038/296260a0. [DOI] [PubMed] [Google Scholar]
- Natali P. G., Cavaliere R., Bigotti A., Nicotra M. R., Russo C., Ng A. K., Giacomini P., Ferrone S. Antigenic heterogeneity of surgically removed primary and autologous metastatic human melanoma lesions. J Immunol. 1983 Mar;130(3):1462–1466. [PubMed] [Google Scholar]
- PREHN R. T., MAIN J. M. Immunity to methylcholanthrene-induced sarcomas. J Natl Cancer Inst. 1957 Jun;18(6):769–778. [PubMed] [Google Scholar]
- Philipps C., McMillan M., Flood P. M., Murphy D. B., Forman J., Lancki D., Womack J. E., Goodenow R. S., Schreiber H. Identification of a unique tumor-specific antigen as a novel class I major histocompatibility molecule. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5140–5144. doi: 10.1073/pnas.82.15.5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponder B. A., Wilkinson M. M., Wood M., Westwood J. H. Immunohistochemical demonstration of H2 antigens in mouse tissue sections. J Histochem Cytochem. 1983 Jul;31(7):911–919. doi: 10.1177/31.7.6343482. [DOI] [PubMed] [Google Scholar]
- Rosloniec E. F., Kuhn M. H., Genyea C. A., Reed A. H., Jennings J. J., Giraldo A. A., Beisel K. W., Lerman S. P. Aggressiveness of SJL/J lymphomas correlates with absence of H-2Ds antigens. J Immunol. 1984 Feb;132(2):945–952. [PubMed] [Google Scholar]
- Sher B. T., Nairn R., Coligan J. E., Hood L. E. DNA sequence of the mouse H-2Dd transplantation antigen gene. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1175–1179. doi: 10.1073/pnas.82.4.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Tanaka K., Hayashi H., Hamada C., Khoury G., Jay G. Expression of major histocompatibility complex class I antigens as a strategy for the potentiation of immune recognition of tumor cells. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8723–8727. doi: 10.1073/pnas.83.22.8723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Isselbacher K. J., Khoury G., Jay G. Reversal of oncogenesis by the expression of a major histocompatibility complex class I gene. Science. 1985 Apr 5;228(4695):26–30. doi: 10.1126/science.3975631. [DOI] [PubMed] [Google Scholar]
- Van Doren K., Hanahan D., Gluzman Y. Infection of eucaryotic cells by helper-independent recombinant adenoviruses: early region 1 is not obligatory for integration of viral DNA. J Virol. 1984 May;50(2):606–614. doi: 10.1128/jvi.50.2.606-614.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallich R., Bulbuc N., Hämmerling G. J., Katzav S., Segal S., Feldman M. Abrogation of metastatic properties of tumour cells by de novo expression of H-2K antigens following H-2 gene transfection. Nature. 1985 May 23;315(6017):301–305. doi: 10.1038/315301a0. [DOI] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R., Urlaub G., Chasin L. DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1373–1376. doi: 10.1073/pnas.76.3.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuhas J. M., Pazmino N. H., Proctor J. O., Toya R. E. A direct relationship between immune competence and the subcutaneous growth rate of a malignant murine lung tumor. Cancer Res. 1974 Apr;34(4):722–728. [PubMed] [Google Scholar]
- Yuhas J. M., Toya R. E., Wagner E. Specific and nonspecific stimulation of resistance to the growth and metastasis of the line 1 lung carcinoma. Cancer Res. 1975 Jan;35(1):242–244. [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]