Abstract
BACKGROUND AND PURPOSE
The extent to which behavioural effects vary as a function of CB1 receptor agonist efficacy is not clear. These studies tested the hypothesis that cannabinoid tolerance and cross-tolerance depend upon the CB1 agonist efficacy of drugs to which tolerance/cross-tolerance develops.
EXPERIMENTAL APPROACH
Sensitivity to cannabinoids, including the cannabinoid antagonist rimonabant, low efficacy agonist Δ9-tetrahydrocannabinol (Δ9-THC), and high efficacy agonists CP 55940 and WIN 55212-2, was determined before and after chronic Δ9-THC treatment in rhesus monkeys. Two measures of behavioural effect were assessed: effects of drugs to decrease fixed ratio responding for food presentation and stimulus-shock termination and discriminative stimulus effects in monkeys discriminating Δ9-THC (0.1 mg·kg−1, i.v.).
KEY RESULTS
Δ9-THC decreased responding for both food presentation and stimulus-shock termination; these effects were antagonized by the CB1 antagonist rimonabant. Chronic Δ9-THC (1 mg·kg−1 per 12 h, s.c.) resulted in tolerance to the rate-decreasing effects of Δ9-THC and cross-tolerance to CP 55940 and WIN 55212-2; however, cross-tolerance was less than tolerance. Chronic Δ9-THC increased sensitivity to rimonabant without changing sensitivity to the non-cannabinoids midazolam and ketamine. In monkeys discriminating Δ9-THC (0.1 mg·kg−1, i.v.), both CP 55940 and WIN 55212-2 produced high levels of drug-lever responding. Chronic Δ9-THC (1 mg·kg−1 per day, s.c.) decreased sensitivity to Δ9-THC without producing cross-tolerance to CP 55940 or WIN 55212-2.
CONCLUSIONS AND IMPLICATIONS
In Δ9-THC-treated monkeys, the magnitude of tolerance and cross-tolerance to other CB1 receptor agonists varied inversely with agonist efficacy, suggesting that CB1 agonist efficacy is an important determinant of behavioural effects.
Keywords: cannabinoid, cross-tolerance, drug discrimination, efficacy, fixed ratio responding, rhesus monkey, tolerance
Introduction
Pharmacological efficacy has been defined as the relationship between the magnitude of physiological response produced by a drug or ligand at a certain amount of receptor occupancy (Ariens, 1954; Stephenson, 1956; Kenakin, 2002). Agonists acting at a common receptor subtype can vary in efficacy; for example, an agonist producing the maximum response at full or less-than-full receptor occupancy has higher efficacy than agonists producing less than the maximum at full receptor occupancy. When a low efficacy agonist is able to produce the same maximum response as that obtained with a high efficacy agonist, the low efficacy agonist occupies a greater number of receptors than a higher efficacy agonist at the smallest concentrations or doses producing the maximum response. Agonists not only can differ from each other in efficacy, but the same agonist can vary in its potency and maximum response obtained, depending on receptor density and the efficiency of receptor coupling to second messenger pathways (e.g. G-protein-coupled receptors) which, in turn, can vary among different organ systems or responses (Kenakin, 1981). Therefore, when receptor density and coupling efficiency are high, an agonist can be relatively more potent and produce a higher level of response than when receptor density and coupling efficiency are low.
For some drug classes (e.g. µ opioid agonists), in vivo effects critically depend on agonist efficacy (Bergman et al., 2000 for review), with increasing levels of µ agonist efficacy associated with increasing levels of effect (e.g. analgesia and abuse liability). However, for other drugs (e.g. cannabinoids), relationships between agonist efficacy and behavioural effects are less clear. CB1 receptors are coupled to inhibitory G-proteins and CB1 agonists typically inhibit adenylyl cyclase (Howlett, 2004). G-protein activation assessed with [35S]-guanosine 5′-3′thio-triphosphate binding has been used to demonstrate marked differences in CB1 receptor agonist efficacy among ligands; for example, rank order efficacy was WIN 55212-2 > CP 55940 > Δ9-tetrahydrocannabinol (Δ9-THC; Breivogel and Childers, 2000). However, despite having low efficacy, Δ9-THC often produces the same maximum effect in vivo as that obtained with high efficacy cannabinoid agonists. For example, Δ9-THC, CP 55940 and WIN 55212-2 produced similar maximum effects in drug discrimination procedures (e.g. McMahon, 2006) and assays concurrently assessing hypothermia, antinociception, locomotor activity and immobility (Fan et al., 1994). The ability of Δ9-THC to achieve the same level of response as high efficacy agonists across a broad range of conditions likely reflects the large number of CB1 receptors (i.e. spare receptors) in the nervous system (Gifford et al., 1999) and high coupling efficiency to G-proteins.
One strategy for assessing relative efficacy among agonists is to decrease receptor function through chronic agonist treatment, and studies with µ opioids provide a compelling example of this approach. Chronic morphine treatment decreases sensitivity to morphine (i.e. tolerance) and other µ agonists (i.e. cross-tolerance) by decreasing downstream signalling through µ receptors (He et al., 2002; Martini and Whistler, 2007). In morphine-treated animals, the magnitude of decrease in sensitivity to some behavioural effects varies with agonist efficacy such that greater loss of sensitivity occurs for low efficacy as compared with high efficacy agonists (Walker et al., 1997), as would be predicted by receptor theory. Chronic Δ9-THC treatment can decrease CB1 receptor number and function (Sim et al., 1996; Breivogel et al., 1999) and can produce tolerance and cross-tolerance (Fan et al., 1994); however, relationships between tolerance/cross-tolerance and CB1 agonist efficacy have not been firmly established. Such a relationship would suggest that the potency and maximum effect of various cannabinoid agonists, including endogenous ligands such as anandamide, vary significantly as a function of different levels of marijuana smoking or other cannabinoid use (oral Δ9-THC) and corresponding CB1 receptor desensitization.
In the current study, rhesus monkeys received chronic Δ9-THC treatment. Changes in sensitivity to cannabinoids were assessed with two measures of behavioural effect: decreases in the rate of fixed ratio responding and discriminative stimulus effects. One group of monkeys responded under fixed ratio schedules of food presentation and stimulus-shock termination (SST) and sensitivity to the effects of cannabinoids (rimonabant, Δ9-THC, CP 55940 and WIN 55212-2) and non-cannabinoids (midazolam and ketamine) to decrease rate of responding was assessed before, during and after discontinuation of chronic Δ9-THC treatment (1 mg·kg−1 per 12 h, s.c. for 71 days). Another group of monkeys discriminated Δ9-THC (0.1 mg·kg−1, i.v.) and sensitivity to the discriminative stimulus effects of Δ9-THC and to the Δ9-THC-like discriminative stimulus effects of WIN 55212-2 and CP 55940 was assessed before and after daily Δ9-THC (1 mg·kg−1, s.c.) treatment; discrimination training was suspended during daily Δ9-THC treatment. Overall, these studies tested the hypothesis that the magnitude of tolerance and cross-tolerance to cannabinoid agonists in Δ9-THC-treated animals varies inversely as a function of cannabinoid agonist efficacy.
Methods
Animals
One group of adult rhesus monkeys (Macaca mulatta), consisting of two females and four males, responded for food presentation and SST. Another group of adult rhesus monkeys, consisting of one female and four males, discriminated Δ9-THC. Monkeys were housed individually on a 14 h light/10 h dark schedule, were maintained at 95% free-feeding weight (range 4–9 kg) with a diet consisting of primate chow (High Protein Monkey Diet, Harlan Teklad, Madison, WI, USA), fresh fruit and peanuts, and were provided with water in the home cage. Monkeys responding for food presentation and SST were pharmacologically and behaviourally naïve prior to this study; monkeys discriminating Δ9-THC were trained previously and had received cannabinoids and non-cannabinoids (e.g. McMahon, 2006, 2009). All monkeys were maintained in accordance with the Institutional Animal Care and Use Committee, The University of Texas Health Science Center at San Antonio, and with the ‘Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research’ (National Research Council, 1996).
Catheter insertion
Monkeys were anaesthetized with ketamine (10 mg·kg−1, i.m.) and isoflurane (1.5–3.0% inhalation). Using sterile techniques, chronic indwelling catheters (heparin-coated polyurethane, outer diameter = 1.68 mm, inner diameter = 1.02 mm; Instech Solomon, Plymouth Meeting, PA) were inserted into a femoral or subclavian vein. Suture silk (coated vicryl, Ethicon Inc., Somerville, NJ, USA) was used to anchor the catheter to the vessel and to ligate the section of the vessel proximal to the catheter insertion. The distal end of the catheter was attached to a vascular access port (Mida-cbas-c50, Instech Solomon) positioned s.c. at the mid-scapular region of the back.
Apparatus
Monkeys were seated in chairs (Model R001, Primate Products, Miami, FL, USA) and were placed in ventilated, sound-proof chambers equipped with levers and lights. Feet were placed in shoes containing brass electrodes to which a brief electric stimulus (3 mA, 250 ms) could be delivered from an a/c generator. An interface (MedAssociates, St. Albans, VT, USA) connected the chambers to a computer, which controlled and recorded experimental events with Medical-PC software.
Schedules of food presentation and SST: acute effects of Δ9-THC and rimonabant
In the presence of a green light, two female and two male monkeys responded under a schedule of continuous reinforcement to obtain 300-mg banana-flavoured food pellets (BioServ, Frenchtown, NJ, USA); the response requirement was systematically increased to an FR5 over days. Thereafter, sessions were divided into multiple cycles, each beginning with a 15-min timeout; the chamber was dark and responses had no programmed consequence. The timeout was followed by a 5-min response period; the green light was illuminated and a food pellet could be obtained upon completion of the response requirement up to delivery of 10 food pellets in a cycle. If 10 food pellets were obtained in less than 5 min, then the green light was extinguished and the remainder of the 5-min period was a timeout.
Sessions consisted of two to six cycles and were conducted 7 days per week. Vehicle was administered i.v. in the first minute of the first cycle, followed by vehicle or sham (dull pressure applied to the skin overlying the vascular access port) non-systematically in the first minute of subsequent cycles. Sessions were conducted until the response rate for individual monkeys stabilized, defined as five consecutive days with response rate within ±20% of the mean rate for those days. Thereafter, tests were conducted by administering vehicle or a dose (0.32–3.2 mg·kg−1, i.v.) of rimonabant, 15 min before sessions in which vehicle was administered in the first cycle, followed by Δ9-THC i.v. in cumulative doses increasing by 0.25 or 0.5 log unit per cycle. The dose-effect function included ineffective doses (i.e. doses not modifying response rate) up to doses that markedly decreased response rate (i.e. doses resulting in delivery of no food pellets in a cycle). The order of testing with different doses of rimonabant was non-systematic, and two control dose-effect tests with Δ9-THC were determined, one before and the other after tests with rimonabant in combination with Δ9-THC were completed. Test sessions were conducted twice weekly so long as response rate in the preceding training session was within ±20% of the mean rate for the five previous vehicle-sessions; otherwise, testing was postponed until this criterion was satisfied.
Upon completion of tests under the schedule of food presentation, monkeys were trained to respond for SST under a schedule of continuous reinforcement. A red light was illuminated and an electric stimulus was scheduled for delivery every 40 s until completion of the response requirement or until four electric stimuli were delivered, whichever occurred first. Completion of the response requirement extinguished the light and prevented delivery of the electric stimulus for 30 s; thereafter, the light was illuminated and the schedule was repeated. The response requirement was systematically increased to an FR5 and sessions were divided into multiple cycles consisting of a 15-min timeout followed by a 5-min period of SST. Tests under the schedule of SST ended when four electric stimuli were delivered in a cycle. The criterion for testing and order of Δ9-THC and rimonabant administration in monkeys responding for SST were identical to that described for food-maintained responding; test sessions ended when electric stimuli were delivered.
Multiple schedule of food presentation and SST: sensitivity of drugs before and during chronic treatment with Δ9-THC
Chronic Δ9-THC was studied in one female monkey that participated in the experiment described above and two other naïve male monkeys that were trained to respond for food presentation and SST as described above. In order to study food presentation and SST simultaneously during chronic treatment, monkeys responded for the reinforcers under a multiple FR5/FR5 schedule. Sessions consisted of two to six, 20-min cycles, each comprising a 12-min timeout period during which the chamber was dark and responses had no programmed consequence. This was followed by an 8-min period consisting of food presentation followed by SST. Monkeys responded for food under an FR5 schedule as described above; the schedule of food presentation ended after 2 min or the delivery of 10 food pellets, in which case the remainder of the 2-min food component was a timeout. The food component was followed by a 1-min timeout and then a 5-min period of SST with parameters identical to that described above. Vehicle i.v. or sham was administered at the beginning of cycles. Each cycle ended after 8 min of schedule presentations (including the 2-min food component, 1-min timeout and 5-min SST component).
Training was conducted until stable rates of responding were established during both components, defined as five consecutive days with response rates for both components within ±20% of the mean rate for those days. Thereafter, monkeys received cumulative i.v. doses of Δ9-THC, CP 55940, WIN 55212-2, rimonabant, midazolam and ketamine. Δ9-THC was tested first followed by the other drugs in non-systematic order. Doses were increased by 0.25 or 0.5 log unit each cycle and the largest dose resulted in delivery of electric stimuli. Monkeys were then treated daily with Δ9-THC (1 mg·kg−1 per 12 h, s.c. at 0600 h and 1800 h) for 71 days; sessions were conducted daily at noon. During chronic Δ9-THC treatment, monkeys received vehicle or sham during sessions until all three monkeys satisfied the criterion above. Beginning on day 24 of treatment, monkeys were tested with cumulative i.v. doses of Δ9-THC, CP 55940, WIN 55212-2, rimonabant, midazolam and ketamine; order of testing was non-systematic. The effects of Δ9-THC, CP 55940, WIN 55212-2 and rimonabant were determined twice on separate days; midazolam and ketamine were studied once. After Δ9-THC was administered at 0600 h on day 71 of treatment, Δ9-THC treatment was discontinued and sessions were conducted by administering vehicle or sham. Twenty-eight days after the discontinuation of chronic Δ9-THC treatment, cumulative doses of Δ9-THC and rimonabant were administered in separate test sessions. Tests with drugs before, during and after discontinuation of chronic Δ9-THC treatment were separated by at least two vehicle-sessions and were only conducted when responding for both food presentation and SST in the immediately preceding vehicle-session was ±20% of the mean rate for the five previous vehicle-sessions; otherwise, testing was postponed until the criterion was satisfied.
Drug discrimination procedure
Five monkeys discriminated Δ9-THC (0.1 mg·kg−1, i.v.) from vehicle (1 part absolute ethanol, 1 part Emulphor-620 and 18 parts saline) while responding under a fixed ratio 5 (FR5) schedule of SST in a multiple-cycle procedure. Each cycle began with a 15-min timeout; responses during the timeout had no programmed consequence. The timeout was followed by a 5-min schedule of SST, which was signalled by illumination of red lights (one positioned above each lever). Five consecutive responses on the correct lever extinguished the red lights, prevented delivery of an electric stimulus and initiated a 30-s timeout. Otherwise, an electric stimulus was delivered every 40 s. Responding on the incorrect lever reset the response requirement on the correct lever. Determination of correct levers varied among monkeys (i.e. left lever associated with drug; right lever associated with vehicle) and remained the same for that monkey for the duration of the study.
Training sessions were conducted by administering Δ9-THC (0.1 mg·kg−1) or vehicle within the first minute of a cycle, followed by vehicle or sham within the first minute of subsequent cycles. Δ9-THC training consisted of two cycles and was preceded by zero to four vehicle-training cycles; vehicle training consisted of two to six cycles. Completion of the FR on the correct lever was required for a reinforcer during each training cycle. Monkeys had previously satisfied the criteria for testing, that is, at least 80% of the total responses occurred on the correct lever and fewer than five responses occurred on the incorrect lever before completion of the FR on the correct lever for all cycles during five consecutive or six of seven training sessions. During test sessions, five consecutive responses on either lever postponed the shock schedule. Δ9-THC, CP 55940 and WIN 55212-2 were studied by administering vehicle in the first cycle, followed by cumulative i.v. doses increasing by 0.5 log unit in subsequent cycles. The dose-effect function included ineffective doses (i.e. doses producing responses predominantly on the vehicle lever) up to doses that produced greater than 80% of responses on the Δ9-THC lever.
The day after a Δ9-THC dose-effect determination, vehicle test sessions were conducted on consecutive days for 1, 3 or 6 days by administering vehicle in the first cycle followed by sham in a second cycle; monkeys received Δ9-THC (1 mg·kg−1, s.c.) after each of these sessions. On the day after 1, 3 or 6 days of Δ9-THC treatment, a test session was conducted by administering vehicle in the first cycle followed by cumulative doses of Δ9-THC in subsequent cycles. After the 6-day treatment regimen, cumulative Δ9-THC dose-effect tests were conducted every other day until sensitivity was not different from the initial dose-effect findings, as determined for individual monkeys; vehicle-test sessions consisting of two cycles were conducted on intervening days. After sensitivity to Δ9-THC was determined before and after once daily Δ9-THC (1 mg·kg−1) treatment for 1, 3, or 6 days, sensitivity to CP 55940 and WIN 55212-2 was determined in non-systematic order among monkeys before and after 3 days of Δ9-THC treatment, in the same manner as that described for Δ9-THC. Following tests with CP 55940 and WIN 55212-2, sensitivity to Δ9-THC was determined a second time before and after 3 days of Δ9-THC treatment. Successive episodes of daily Δ9-THC treatment were separated by at least 30 days. Before the initial dose-effect tests prior to Δ9-THC treatment, performance for consecutive training sessions, including both vehicle and drug training sessions, satisfied the test criteria. The type of training sessions preceding these test sessions varied non-systematically.
Drugs
Drug/molecular target nomenclature conforms to the British Journal of Pharmacology Guide to Receptors and Channels (Alexander et al., 2009). Rimonabant, Δ9-THC (100 mg·mL−1 in absolute ethanol; The Research Technology Branch, National Institute on Drug Abuse, Rockville, MD, USA), CP 55940 ((1R,3R,4R)-3-[2-hydroxy-4-(1,1-dimethylheptyl) phenyl]-4-(3-hydroxypropyl)cyclohexan-1-ol; Tocris, Ellisville, MO, USA) and WIN 55212-2 ((R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo-[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone mesylate; Sigma, St. Louis, MO, USA) were dissolved in 1 part absolute ethanol, 1 part Emulphor-620 (Rhodia Inc., Cranbury, NJ, USA) and 18 parts physiological saline. Midazolam hydrochloride (5 mg·mL−1 in physiological saline; Bedford Laboratories, Bedford, OH, USA) and ketamine hydrochloride (100 mg·mL−1 in physiological saline; Bioniche, Athens, GA, USA) were diluted in physiological saline as needed. All drugs were administered i.v., and dose was expressed as the weight of the forms listed above.
Data analyses
Control rate of responding (responses·s−1) was calculated as the average rate for all cycles in the five non-drug (i.e. vehicle) sessions immediately preceding a drug session, excluding vehicle sessions conducted the day after a drug session. The control response rate for data collected during chronic Δ9-THC treatment was calculated as the average rate of the five non-drug (i.e. vehicle) sessions immediately preceding Δ9-THC treatment. Response rate data were expressed as a percentage of the control response rate and data were analysed separately for each reinforcer (food presentation and SST). Discrimination data were expressed as a percentage of responses on the Δ9-THC lever out of total responses on both the Δ9-THC and vehicle levers. When response rate was decreased to less than 50% of the control or responding on the Δ9-THC lever was increased to greater than 50%, potency was calculated by simultaneously fitting straight lines to individual dose-effect data by means of GraphPad Prism version 5.0 for Windows (San Diego, CA, USA) with linear regression. Straight lines were fitted to the linear portion of dose-effect curves. The slopes of dose-effect curves were compared with an F-ratio test using GraphPad; if the slopes were not significantly different; then, a common, best-fitting slope was used for further analyses (Kenakin, 1997). Doses corresponding to the 50% level of the effect (ED50 values), potency ratios and their 95% confidence limits were calculated with parallel line analyses of data from individual subjects (Tallarida, 2000). The potencies were considered significantly different when the 95% confidence limits of the potency ratio did not include 1.
Rimonabant alone (i.e. without Δ9-THC treatment) did not decrease responding to below 50% of control in all monkeys (see Results); differences in potency to modify rate of responding were examined by determining whether slopes of rimonabant dose–response curves were significantly different from 0. Rimonabant tended to decrease response rate when administered in single bolus doses before cumulative doses of Δ9-THC; therefore, antagonism of the rate-decreasing effects of Δ9-THC by rimonabant was analysed by expressing data as a percentage of the response rate following each respective dose of rimonabant alone.
Results
The effects of Δ9-THC to decrease response rate: antagonism by rimonabant
For 10 non-drug sessions, average (SEM) rates of lever-pressing for food presentation in four monkeys were 0.90 (0.05), 1.18 (0.06), 1.34 (0.10) and 1.58 (0.07) responses·s−1. For the schedule of SST, average (SEM) rates of responding were 0.84 (0.06), 0.56 (0.05), 0.94 (0.06) and 1.84 (0.14) responses·s−1 for the same monkeys respectively. Response rate did not significantly differ as a function of reinforcer (P > 0.05).
When administered as a single bolus dose, rimonabant (3.2 mg·kg−1) decreased responding to 54% and 70% of control for food presentation and SST, respectively [Figure 1, top, diamonds above vehicle (VEH)]; the slope of the rimonabant dose–response curve was significantly different from 0 when responding was maintained by SST (P < 0.05) but not food presentation (P > 0.05). Δ9-THC produced a marked, dose-dependent decrease in responding for food and SST (Figure 1, circles, left and right, respectively). The ED50 values determined from the two control Δ9-THC dose–response curves (i.e. one determined before and the other after tests with rimonabant in combination with Δ9-THC) were not significantly different from each other; these data were averaged for graphic presentation (Figure 1) and further analyses. The slopes of the Δ9-THC dose–response curves alone and in combination with various doses of rimonabant were not significantly different. The ED50 values (95% confidence limits) for Δ9-THC to decrease responding for food presentation and SST were 0.17 and 0.058 mg·kg−1 respectively (Table 1). Rimonabant dose-dependently antagonized the effects of Δ9-THC to decrease responding. For food presentation, rimonabant (3.2 mg·kg−1) significantly decreased the potency of Δ9-THC 14-fold; smaller doses of rimonabant did not produce significant antagonism (Table 1, Food). For SST, doses of 1 and 3.2 mg·kg−1 of rimonabant significantly decreased the potency of Δ9-THC 6.7- and 14-fold, respectively, whereas the smallest dose (0.32 mg·kg−1) of rimonabant did not produce significant antagonism (Table 1, SST).
Figure 1.
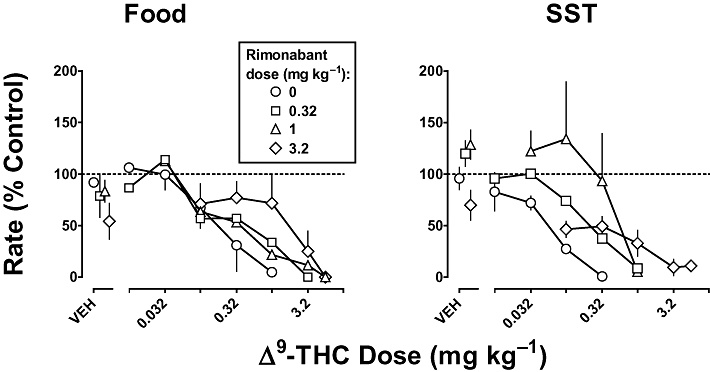
Effects of rimonabant and Δ9-tetrahydrocannabinol (Δ9-THC), alone and in combination, on responding for food presentation (left) and stimulus-shock termination (SST; right). Abscissae: vehicle (VEH) or i.v. dose of Δ9-THC in mg·kg−1 body weight. Ordinates: mean (±SEM) rate of responding expressed as a percentage of control. Points above VEH represent the average response rate after vehicle or a dose (0.32-3.2 mg·kg−1, i.v.) of rimonabant alone. Rimonabant (0.32-3.2 mg·kg−1) was administered prior to cumulative doses of Δ9-THC.
Table 1.
ED50 values and 95% confidence limits for Δ9-tetrahydrocannabinol (Δ9-THC) alone and in combination with various doses (0.32–3.2 mg·kg−1) of rimonabant in monkeys responding for food presentation (Food) and stimulus-shock termination (SST)
| ED50 in mg·kg−1 (95% confidence limits) | Potency ratio† (95% confidence limits) | |
|---|---|---|
| Food | ||
| Δ9-THC alone | 0.17 (0.055–0.54) | |
| +0.32 Rimonabant | 0.22 (0.097–0.48) | 1.3 (0.4–4.0) |
| +1 Rimonabant | 0.60 (0.19–1.9) | 3.5 (1.0–13) |
| +3.2 Rimonabant | 2.3* (0.60–8.8) | 14 (3.6–50) |
| SST | ||
| Δ9-THC alone | 0.058 (0.024–0.14) | |
| +0.32 Rimonabant | 0.13 (0.058–0.38) | 2.2 (0.6–8.6) |
| +1 Rimonabant | 0.39* (0.06–2.6) | 6.7 (1.1–41) |
| +3.2 Rimonabant | 0.79* (0.31–2.0) | 14 (4.0–46) |
Potency ratios and 95% confidence limits are equal to the ED50 values of Δ9-THC in combination with a dose of rimonabant divided by the ED50 value of Δ9-THC alone.
Significantly different from Δ9-THC alone.
The effects of drugs on response rate before and during chronic Δ9-THC treatment
In three monkeys responding for food presentation and SST under the multiple schedule, average (SEM) rates of lever-pressing for 10 non-drug sessions conducted before chronic Δ9-THC treatment were 0.46 (0.02), 0.51 (0.04) and 0.64 (0.03) responses·s−1 for food presentation and 1.75 (0.12), 2.35 (0.28) and 1.70 (0.09) responses·s−1 for SST for each respective monkey. Response rate was significantly lower for food presentation as compared with SST under the multiple schedule (P < 0.05).
Before and during chronic Δ9-THC treatment, Δ9-THC, CP 55940 and WIN 55212-2 markedly decreased responding for food presentation and SST (Figure 2). Analysis of dose–response curves separately for each drug and reinforcer showed that the slopes did not significantly differ as a function of Δ9-THC treatment (P > 0.05). Before chronic Δ9-THC treatment, the ED50 values of Δ9-THC were 0.094 and 0.047 mg·kg−1 in the food and SST components respectively (Table 2). The ED50 values of CP 55940 were 0.0085 and 0.0095 mg·kg−1 in the food and SST components respectively (Table 2); CP 55940 was 11- and 5-fold more potent than Δ9-THC in the respective schedule components. WIN 55212-2 was slightly less potent than Δ9-THC, that is, the ED50 values of WIN 55212-2 were 0.24 and 0.21 mg·kg−1 in the food and SST components respectively.
Figure 2.
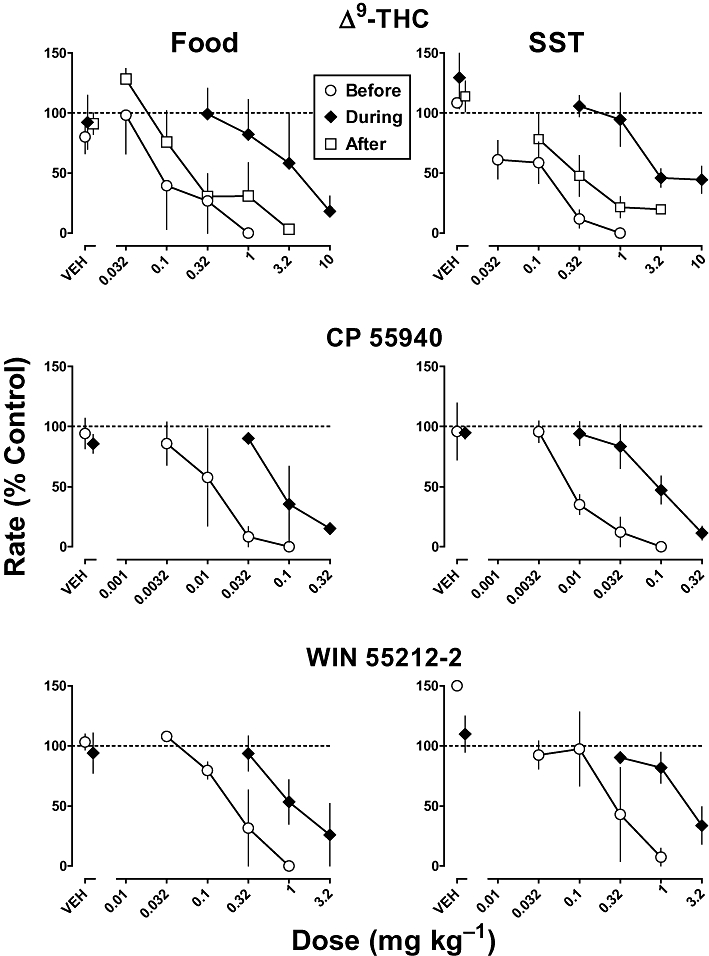
Effect of Δ9-tetrahydrocannabinol (Δ9-THC) (top), CP 55940 (middle) and WIN 55212-2 (bottom) on responding for food presentation (left) and stimulus-shock termination (SST; right) before and during chronic Δ9-THC (1 mg·kg−1 per 12 h, s.c.). Abscissae: vehicle (VEH) or dose administered i.v. in mg·kg−1 body weight. Ordinates: mean (±SEM) rate of responding expressed as a percentage of control. Squares (top) show the effects Δ9-THC determined after discontinuation of Δ9-THC (1 mg·kg−1 per 12 h, s.c.) treatment.
Table 2.
ED50 values and 95% confidence limits of drugs determined before and during daily Δ9-tetrahydrocannabinol (Δ9-THC) treatment (1 mg·kg−1 per 12 h, s.c.) in monkeys responding for food presentation (Food) and stimulus-shock termination (SST)
| ED50 in mg·kg−1 (95% confidence limits) | Potency ratio† (95% confidence limits) | |
|---|---|---|
| Δ9-THC food | ||
| Before | 0.094 (0.013–0.65) | |
| During | 2.2* (0.27–17) | 23 (1.8–290) |
| After†† | 0.31 (0.028–3.5) | 3.3 (0.2–49) |
| Δ9-THC SST | ||
| Before | 0.047 (0.016–0.14) | |
| During | 7.6* (1.2–46) | 160 (27–930) |
| After†† | 0.24* (0.066–0.90) | 5.1 (1.2–22) |
| CP 55940 food | ||
| Before | 0.0085 (0.0024–0.029) | |
| During | 0.082* (0.019–0.34) | 9.6 (1.9–49) |
| CP 55940 SST | ||
| Before | 0.0095 (0.0052–0.017) | |
| During | 0.085* (0.047–0.15) | 8.9 (4.2–19) |
| WIN 55212-2 food | ||
| Before | 0.24 (0.037–1.6) | |
| During | 1.2 (0.32–4.4) | 4.9 (0.8–32) |
| WIN 55212-2 SST | ||
| Before | 0.21 (0.047–0.93) | |
| During | 2.4* (0.85–6.7) | 11 (2.4–54) |
| Rimonabant food | ||
| Before | 3.2 | |
| During | 0.87 (0.17–4.5) | 0.27^ |
| Rimonabant SST | ||
| Before | 3.2 | |
| During | 0.50 (0.21–1.2) | 0.16^ |
| Midazolam food | ||
| Before | 0.17 (0.05–0.59) | |
| During | 0.24 (0.08–0.73) | 1.4 (0.4–4.9) |
| Midazolam SST | ||
| Before | 0.057 (0.029–0.12) | |
| During | 0.073 (0.04–0.13) | 1.3 (0.6–2.9) |
| Ketamine food | ||
| Before | 1.2 (0.81–1.7) | |
| During | 0.61 (0.33–1.1) | 0.51 (0.2–1.1) |
| Ketamine SST | ||
| Before | 1.2 (0.59–2.6) | |
| During | 0.84 (0.45–1.6) | 0.70 (0.3–1.7) |
Potency ratios and 95% confidence limits are equal to the ED50 values determined during or after discontinuation of daily Δ9-THC treatment divided by the ED50 value determined before daily Δ9-THC treatment.
ED50 values and 95% confidence limits for Δ9-THC determined after discontinuation of Δ9-THC treatment also are shown.
Significantly different from Before.
ED50 value Before equal to largest dose studied; 95% confidence limits could not be calculated.
Upon chronic Δ9-THC (1 mg·kg−1 per 12 h, s.c.) treatment, responding in both schedule components was decreased as compared with before treatment (Figure 3, compare Days 1–24, during chronic Δ9-THC to before). Beginning on day 24 of chronic Δ9-THC treatment, sensitivity to Δ9-THC and other drugs was assessed. Sensitivity to the rate-decreasing effects of Δ9-THC was decreased during chronic Δ9-THC treatment (Figure 2, top, compare diamonds to circles). Before chronic treatment, 0.32 mg·kg−1 of Δ9-THC decreased responding in the food and SST components to 27% and 12% of control respectively. During chronic Δ9-THC treatment, larger doses (3.2 and 10 mg·kg−1) were required to produce comparable decreases in responding. Chronic Δ9-THC treatment significantly increased the ED50 value of Δ9-THC by 23- and 160-fold in the food and SST components respectively (Table 2). Sensitivity to the rate-decreasing effects of CP 55940 also was decreased during chronic Δ9-THC treatment (Figure 2, middle); the ED50 values of CP 55940 were increased 9.6- and 8.9-fold in the food and SST components respectively (Table 2). During chronic Δ9-THC treatment, the potency of WIN 55212-2 to decrease responding in the SST component decreased significantly by 11-fold (Table 2). Although the potency of WIN 55212-2 to decrease responding for food was less during chronic Δ9-THC as compared with before treatment, the difference was not statistically significant. Overall, during chronic Δ9-THC treatment, sensitivity to Δ9-THC decreased more than sensitivity to CP 55490 and WIN 55212-2 (Figure 2, compare top panel to middle and bottom panels).
Figure 3.
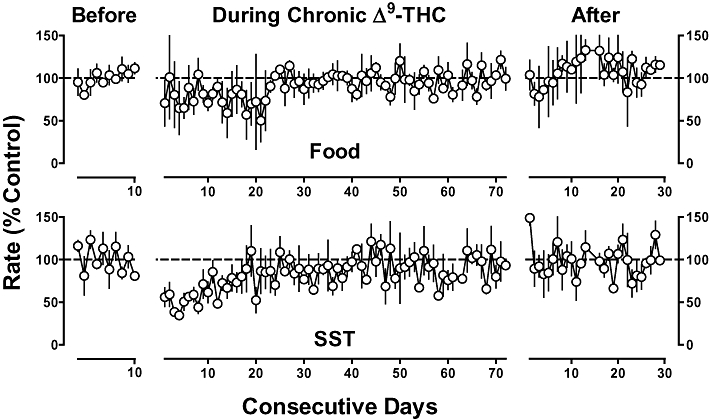
Vehicle performance before, during and after discontinuation of chronic Δ9-tetrahydrocannabinol (Δ9-THC) treatment in monkeys responding for food presentation (top) and stimulus-shock termination (SST; bottom). Abscissae: consecutive sessions before, during and after discontinuation of chronic Δ9-THC (1 mg·kg−1 per 12 h, s.c.) treatment. Ordinates: mean (±SEM) rate of responding expressed as a percentage of control.
Rimonabant, when administered up to a cumulative dose of 3.2 mg·kg−1 before chronic Δ9-THC treatment, decreased responding in the food and SST components to a maximum of 68% and 85% of control respectively (Figure 4; circles). The effects of rimonabant before chronic Δ9-THC did not significantly vary as a function of dose, that is, the slope of the dose–response curve was not significantly different from 0 (P > 0.05). During chronic Δ9-THC treatment, rimonabant (1 mg·kg−1) decreased responding in the food and SST components to 39% and 35%, respectively; the dose–response curve for rimonabant during chronic Δ9-THC treatment was significantly different from 0 in the food component (F1,13= 4.82; P < 0.05) and approached significance in the SST component (F1,13= 3.20; P= 0.09). The ED50 values for rimonabant during chronic Δ9-THC were 0.87 and 0.50 mg·kg−1 for the food and SST components respectively (Table 2). Before chronic Δ9-THC treatment, a conservative estimate of the ED50 was the largest dose (3.2 mg·kg−1) tested. Although sensitivity to rimonabant was increased 3.7- and 6.4-fold for the respective schedule components, the data were not amenable to statistical analysis (i.e. 95% confidence limits of the potency ratios could not be calculated).
Figure 4.
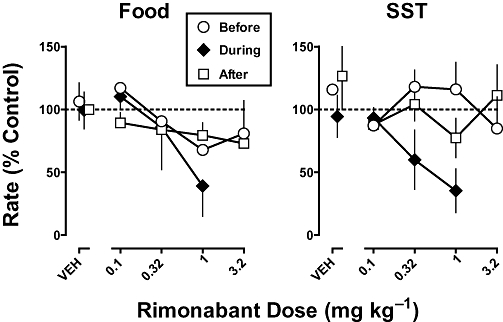
Effects of rimonabant on responding for food presentation (left) and stimulus-shock termination (SST; right) before, during and after discontinuation of chronic Δ9-tetrahydrocannabinol (Δ9-THC); (1 mg·kg−1 per 12 h, s.c.). Abscissae: vehicle (VEH) or dose administered i.v. in mg·kg−1 body weight. Ordinates: mean (±S.E.M.) rate of responding expressed as a percentage of control.
Before chronic Δ9-THC treatment, the non-cannabinoids midazolam and ketamine dose-dependently decreased responding (Figure 5, circles). In the food and SST components, the ED50 values for midazolam were 0.17 and 0.057 mg·kg−1, respectively; the ED50 values for ketamine were 1.2 mg·kg−1 in both schedule components (Table 2). In contrast to the cannabinoids, sensitivity to the effects of midazolam and ketamine were not significantly modified by chronic Δ9-THC treatment; the ED50 values did not differ by more than 2-fold before as compared with during chronic Δ9-THC treatment (Table 2).
Figure 5.

Effects of midazolam (top) and ketamine (bottom) on responding for food presentation (left) and stimulus-shock termination (SST) before and during chronic Δ9-tetrahydrocannabinol (Δ9-THC) (1 mg·kg−1 per 12 h, s.c.). Abscissae: vehicle (VEH) or dose administered i.v. in mg·kg−1 body weight. Ordinates: mean (±SEM) rate of responding expressed as a percentage of control.
When chronic Δ9-THC (1 mg·kg−1 per 12 h, s.c.) treatment was discontinued, there was a trend for food-maintained responding to decrease and then increase over days (Figure 3, top), whereas SST-maintained responding was elevated during the first session conducted during the discontinuation period and, thereafter, not substantially modified relative to control (Figure 3, bottom). In the food component, sensitivity to Δ9-THC determined after discontinuation of chronic Δ9-THC treatment was no longer significantly different from sensitivity determined before chronic Δ9-THC treatment (Figure 2, top left, compare squares and circles; Table 2). In contrast, in the SST component, sensitivity to Δ9-THC remained significantly decreased (5.1-fold) after discontinuation of chronic treatment as compared with before chronic treatment, albeit the ED50 value of Δ9-THC determined after discontinuation of Δ9-THC treatment was markedly (32-fold) less than the ED50 value of Δ9-THC determined during chronic treatment (Figure 2, top right, compare square and diamonds; Table 2). After discontinuation of Δ9-THC treatment, sensitivity to the rate-decreasing effects of rimonabant was less than that determined during chronic Δ9-THC treatment (Figure 4); up to a dose of 3.2 mg·kg−1, the slope of the dose–response functions in each schedule component were not significantly different from 0 (P > 0.05).
Discriminative stimulus effects of drugs before and immediately after Δ9-THC treatment
In monkeys discriminating Δ9-THC, the training drug dose-dependently increased the percentage of responses on the Δ9-THC lever, that is, at the training dose (0.1 mg·kg−1, i.v.), percentage responding on the Δ9-THC lever was 100% (Figure 6, top, circles). In contrast, vehicle produced 0% of the responses on the Δ9-THC lever (Figure 6, top, circles above VEH). During the test conducted 1 day after a single injection of 1 mg·kg−1 of Δ9-THC s.c., % Δ9-THC lever responding was 25% when monkeys received vehicle during that test (Figure 6, top left, diamond above VEH). In subsequent cycles, Δ9-THC dose-dependently increased Δ9-THC appropriate responding (Figure 6, top left, diamonds). The slopes and ED50 values (Table 3) of the Δ9-THC dose–response curves determined before and after 1 day of Δ9-THC treatment (1 mg·kg−1, s.c.) were not significantly different (P > 0.05). In contrast, both 3 and 6 days of treatment with Δ9-THC (1 mg·kg−1, s.c.) produced a significant decrease in the potency of Δ9-THC to produce discriminative stimulus effects (Figure 6, top, three rightmost panels). Sensitivity to Δ9-THC decreased comparably (2.7- to 3.0-fold; Table 3) for 6 days of treatment and both determinations of the 3-day Δ9-THC treatment, that is, one occurring before and the other after studies with CP 55490 and WIN 55212-2. Sensitivity to Δ9-THC was assessed periodically after the 6-day treatment without intervening training sessions; the ED50 value was no longer significantly different from control 7 days after treatment was discontinued (Figure 6, top right, compare triangle and circles; Table 3). For 10 non-drug sessions, average (SEM) rates of lever-pressing in five monkeys were 0.84 (0.07), 0.85 (0.04), 0.90 (0.03), 0.92 (0.10) and 1.63 (0.03) responses·s−1. Up to a dose of 0.32 mg·kg−1, Δ9-THC did not significantly modify response rate during any of the tests conducted before and after 1, 3 or 6 days of Δ9-THC treatment (P > 0.05).
Figure 6.
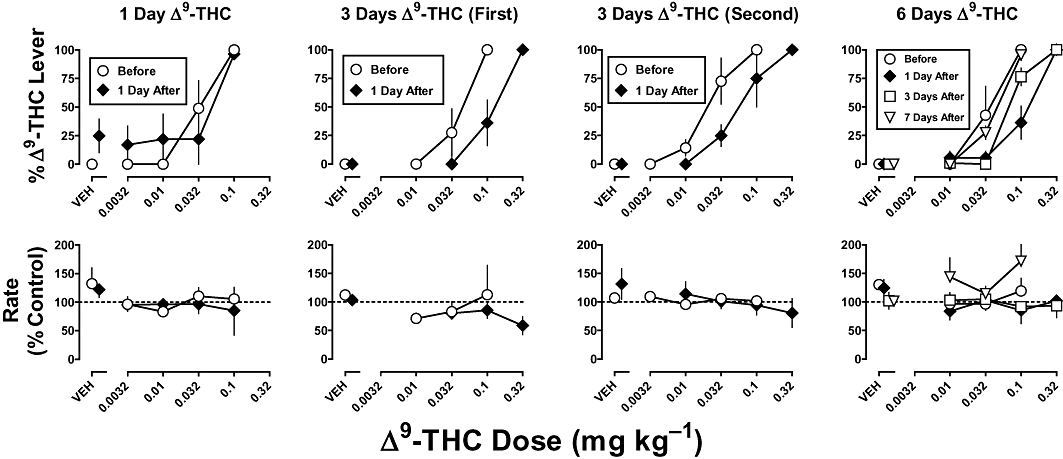
Discriminative stimulus effects of Δ9-tetrahydrocannabinol (Δ9-THC) in rhesus monkeys before and after 1, 3 or 6 days of Δ9-THC (1 mg·kg−1 per day, s.c.). Abscissae: vehicle (VEH) or dose administered i.v. of Δ9-THC in mg·kg−1 body weight. Ordinates: mean (± SEM) percentage of responding on the Δ9-THC lever (top) and rate of responding expressed as a percentage of control (bottom). The effects of the 3-day treatment were determined twice (First and Second; see Methods for details). The squares and triangles (rightmost panel) show sensitivity to Δ9-THC determined 3 and 7 days after discontinuation of the 6-day treatment regimen respectively.
Table 3.
ED50 values and 95% confidence limits determined before and 1 day after Δ9-tetrahydrocannabinol (Δ9-THC) treatment (1 mg·kg−1 per day, s.c. for up to 6 days) in monkeys discriminating Δ9-THC
| ED50 in mg·kg−1 (95% confidence limits) | Potency ratio† (95% confidence limits) | |
|---|---|---|
| Δ9-THC | ||
| 1 day Δ9-THC | ||
| Before | 0.041 (0.013–0.13) | |
| 1 day after | 0.030 (0.0097–0.093) | 0.7 (0.2–2.9) |
| 3 days Δ9-THC (First) | ||
| Before | 0.046 (0.021–0.097) | |
| 1 day after | 0.13* (0.068–0.24) | 2.8 (1.3–6.4) |
| 3 days Δ9-THC (Second) | ||
| Before | 0.021 (0.012–0.037) | |
| 1 day after | 0.057* (0.030–0.11) | 2.7 (1.3–5.7) |
| 6 days Δ9-THC | ||
| Before | 0.037 (0.022–0.069) | |
| 1 day after | 0.11* (0.080–0.16) | 3.0 (1.6–5.4) |
| 7 days after | 0.042 (0.025–0.077) | 1.1 (0.5–2.7) |
| CP 55940 | ||
| 3 days Δ9-THC | ||
| Before | 0.0045 (0.0020–0.010) | |
| 1 day after | 0.0047 (0.0025–0.0090) | 1.0 (0.4–2.4) |
| WIN 55212-2 | ||
| 3 days Δ9-THC | ||
| Before | 0.12 (0.032–0.44) | |
| 1 day after | 0.083 (0.029–0.24) | 0.7 (0.2–2.7) |
Potency ratios and 95% confidence limits are equal to the ED50 values determined 1 day after Δ9-THC treatment divided by the ED50 value determined before daily Δ9-THC treatment.
Significantly different from before.
CP 55940 and WIN 55212-2 both dose-dependently increased responding on the Δ9-THC lever (Figure 7, top left and right, respectively, circles); the ED50 values were 0.0045 and 0.12 mg·kg−1 respectively (Table 3). When the dose–response curves were re-determined after 3 days of Δ9-THC (1 mg·kg−1) treatment (Figure 7, top, diamonds), the potencies of CP 55940 and WIN 55212-2 were not significantly different from their respective controls (P > 0.05; Table 3). There was no significant relationship between dose (up to 0.032 mg·kg−1) of CP 55940 and rate of responding (Figure 7, bottom left). WIN 55212-2, up to a dose of 0.32 mg·kg−1, tended to decrease response rate, that is, the slope of the WIN 55212-2 dose–response curve determined before Δ9-THC treatment was significantly different from 0 (P < 0.05; Figure 7, bottom right, circles).
Figure 7.

Discriminative stimulus effects of CP 55940 (left) and WIN 55212-2 (right) in rhesus monkeys before and after 3 days of Δ9-tetrahydrocannabinol (Δ9-THC); (1 mg·kg−1 per day, s.c.). Abscissae: vehicle (VEH) or dose administered i.v. in mg·kg−1 body weight. Ordinates: mean (± SEM) percentage of responding on the Δ9-THC lever (top) and rate of responding expressed as a percentage of control (bottom).
Discussion
Chronic Δ9-THC treatment resulted in tolerance when measuring the effects of Δ9-THC to decrease rates of operant responding and to produce discriminative stimulus effects; the development of tolerance was time-dependent and reversible (i.e. tolerance diminished after treatment was discontinued). Tolerance to Δ9-THC was accompanied by a smaller loss of sensitivity to the rate-decreasing effects of CP 55940 and WIN 55212-2 (i.e. cross-tolerance), whereas sensitivity to the rate-decreasing effects of the cannabinoid antagonist rimonabant increased. Chronic Δ9-THC treatment had pharmacologically selective effects inasmuch as there was no change in sensitivity to the rate-decreasing effects of non-cannabinoids (midazolam and ketamine). The potency of drugs to decrease responding and the pattern of tolerance/cross-tolerance did not vary with the type of reinforcer (food presentation versus SST). For discriminative stimulus effects, tolerance to Δ9-THC developed without cross-tolerance to CP 55940 and WIN 55212-2. The inverse relationship between magnitude of tolerance/cross-tolerance in the current study and cannabinoid agonist efficacy of the drugs to which tolerance/cross-tolerance developed, as established elsewhere in vitro (Breivogel and Childers, 2000), suggests that CB1 agonist efficacy is an important determinant of behavioural effects.
According to receptor theory (Kenakin, 1997), low efficacy agonists occupy more receptors than high efficacy agonists at equal levels of effect. When CB1 receptor number and function are decreased (e.g. by chronic Δ9-THC treatment; Sim et al., 1996; Breivogel et al., 1999), fractional occupancy and dose need to increase in order to retain the effect (i.e. tolerance and cross-tolerance develop). Because low efficacy agonists occupy a relatively large number of receptors, receptor down-regulation produces a disproportionate change and greater increase in the fractional occupancy needed to retain effect for a low as compared with a high efficacy agonist which, in turn, can result in greater tolerance/cross-tolerance to the low efficacy agonist as compared with the high efficacy agonist. With sufficient receptor down-regulation, the maximum effect can also decrease for the low efficacy agonist. Chronic Δ9-THC treatment produced a greater decrease in the potency of the low efficacy agonist Δ9-THC than the higher efficacy agonists CP 55940 and WIN 55212-2. These results strongly suggest that, for some behavioural effects, Δ9-THC needs to occupy a greater number of cannabinoid receptors than CP 55940 and WIN 55212-2 for all three to achieve the same level of effect. However, the maximum effect was unchanged, suggesting the presence of unoccupied or spare CB1 receptors.
An alternative explanation for these data is that Δ9-THC, CP 55940 and WIN 55212-2 vary in selectivity for different receptor subtypes including CB1, CB2 and perhaps other receptors (Palmer et al., 2002). However, in rhesus monkeys discriminating 0.1 mg·kg−1 of Δ9-THC i.v. (i.e. the discrimination assay used in the current study), the results of a previous study strongly suggested that Δ9-THC, CP 55940 and WIN 55212-2 produced their discriminative stimulus effects through a common receptor type (McMahon, 2006). In that study, Schild analysis of rimonabant in combination with Δ9-THC, CP 55940 and WIN 55212-2 was consistent with simple, competitive and reversible antagonism; moreover, the potency (i.e. apparent affinity or pA2) of rimonabant was similar in the presence of all three agonists. Rimonabant is more selective for CB1 than CB2 receptors (Rinaldi-Carmona et al., 1994); moreover, a selective CB2 agonist did not substitute for the Δ9-THC discriminative stimulus, and the Δ9-THC discriminative stimulus was not blocked by a selective CB2 antagonist (McMahon, 2006). Collectively, these results suggest that differences in the magnitude of tolerance and cross-tolerance to discriminative stimulus effects is not due to differences in receptor-subtype selectivity, but rather to differences in agonist efficacy at a common site (i.e. CB1 receptors).
The effects of drugs to decrease fixed ratio responding have been used to assess tolerance and cross-tolerance to a variety of drugs including γ-aminobutyric acidA modulators (McMahon and France, 2002) and opioids (Brandt and France, 2000). However, relatively large doses of drug are often required to decrease operant responding and the mechanisms underlying rate-decreasing effects sometimes differ from the mechanisms responsible for other behavioural effects (e.g. discriminative stimulus effects) of the same drug. For example, low efficacy µ agonists (e.g. nalbuphine) shared discriminative stimulus effects with other µ opioids and those effects were blocked by the µ opioid antagonist naltrexone; in contrast, the rate-decreasing effects of nalbuphine were not blocked by naltrexone (Walker and Young, 1993; Walker et al., 1994). However, in the current study, rimonabant antagonized the rate-decreasing effects of Δ9-THC, implicating a CB1 receptor mechanism. Antagonism occurred at doses of rimonabant that also decreased responding, consistent with previous studies (Järbe et al., 2003; De Vry and Jentzsch, 2004). The marked tolerance to Δ9-THC (23- and 160-fold for food presentation and SST, respectively) and the lesser cross-tolerance to CP 55940 (10- and 9-fold, respectively) and WIN 55212-2 (5- and 11-fold, respectively) are consistent with a rank order efficacy in vivo of WIN 55212-2 ≥ CP 55940 > Δ9-THC, similar to what has been reported at CB1 receptors in vitro (Breivogel and Childers, 2000). However, the current study cannot reject the possibility that differential tolerance/cross-tolerance is due, in part, to differential binding to multiple receptor subtypes that mediate rate-decreasing effects. Moreover, drug metabolism and disposition were not assessed in the current study to determine whether chronic treatment selectively increases metabolism of Δ9-THC. However, previous studies suggested that Δ9-THC tolerance was not accompanied by significant changes in metabolism in birds (Dewey et al., 1973; McMillan et al., 1973), rats (Siemens and Kalant, 1974), dogs (Martin et al., 1976) and humans (Hunt and Jones, 1980; but see Lemberger et al., 1971).
Tolerance to Δ9-THC has been previously demonstrated for many effects, including discriminative stimulus effects and effects on rates of operant responding, rectal temperature, locomotor activity, nociception, ingestion and learning (McMillan et al., 1970; Miczek, 1979; Wiley et al., 1993; Fan et al., 1994; Delatte et al., 2002; McKinney et al., 2008). When treatment was constant, tolerance to Δ9-THC did not develop equally for all effects (e.g. Miczek, 1979; Fan et al., 1994), perhaps reflecting differences in cannabinoid receptor number and G-protein-coupling efficiency in brain regions that differentially mediated the various effects of Δ9-THC. In the current study, tolerance to Δ9-THC for producing rate-decreasing and discriminative stimulus effects was generated with different treatment parameters, thereby precluding assessment of whether tolerance develops equally for the two behavioural measures.
Chronic agonist treatment with opioids (Goldberg and Schuster, 1967) and benzodiazepines (McMahon and France, 2002) can increase the sensitivity of a pharmacologically-related antagonist to decrease rates of operant responding. This can reflect agonist dependence inasmuch as the effects of the antagonist are due to withdrawal. Δ9-THC treatment increased the sensitivity of monkeys to the rate-decreasing effects of rimonabant, consistent with rimonabant inducing Δ9-THC withdrawal. However, in the absence of Δ9-THC treatment, rimonabant tended to decrease responding at a dose (3.2 mg·kg−1) 0.5–1 log unit larger than the smallest doses decreasing response rate during chronic Δ9-THC treatment. The mechanism(s) responsible for the effects of rimonabant alone have not been established. Δ9-THC treatment (e.g. 1 mg·kg−1 per 12 h, s.c.) was shown in another study to increase sensitivity to other effects of rimonabant (e.g. head shaking) in rhesus monkeys (e.g. Stewart and McMahon, 2010). Such enhancement might reflect CB1 receptor inverse agonism (Bouaboula et al., 1997) in as much as chronic treatment with other types of agonist can increase sensitivity to inverse agonists at the same receptor (e.g. benzodiazepine receptor ligands; Sannerud et al., 1991).
In summary, in Δ9-THC-treated monkeys, there was a predictable inverse relationship between the magnitude of tolerance and cross-tolerance and the efficacy of the agonists to which tolerance and cross-tolerance developed, suggesting that cannabinoid agonist efficacy is an important determinant of behavioural effects. These results have significant implications for marijuana and other cannabinoid use inasmuch as the effectiveness of low efficacy agonists will be impacted to a greater degree than high efficacy agonists. Overall, these studies underscore the broad utility of using receptor theory to predict the effects of G-protein-coupled receptor agonists in animals, and suggest that the potential psychotherapeutic and other behavioural effects of cannabinoids depend on the magnitude to which they stimulate CB1 receptors and downstream effector mechanisms.
Acknowledgments
The author thanks Dr Lisa R. Gerak for helpful editorial comments, Dr Wouter Koek for assistance with statistical analysis, Drs J. Elliott and M. Leland for assistance with surgical procedures and D. Aguirre, C. Rock, D. Schulze and W. Holbein for technical assistance. This research was supported by grants from the U.S. Public Health Service, National Institutes of Health, National Institute on Drug Abuse (R01-DA19222 and R01-DA26781).
Glossary
Abbreviations
- CB
cannabinoid
- CP 55940
(1R,3R,4R)-3-[2-hydroxy-4-(1,1-dimethylheptyl) phenyl]-4-(3-hydroxypropyl)cyclohexan-1-ol
- Δ9-THC
Δ9-tetrahydrocannabinol
- FR
fixed ratio
- WIN 55212-2
(R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo-[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone mesylate
Conflicts of interest
None.
Supporting Information
Supporting Information: Teaching Materials; Figs 1–7 as PowerPoint slide.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC) Br J Pharmacol. (4th edn) 2009;158:S1–S254. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariens EJ. Affinity and intrinsic activity in the theory of competitive inhibition. Arch Int Pharmacodyn Ther. 1954;99:32–49. [PubMed] [Google Scholar]
- Bergman J, France CP, Holtzman SG, Katz JL, Koek W, Stephens DN. Agonist efficacy, drug dependence, and medications development: preclinical evaluation of opioid, dopaminergic, and GABAA-ergic ligands. Psychopharmacology. 2000;153:67–84. doi: 10.1007/s002130000567. [DOI] [PubMed] [Google Scholar]
- Bouaboula M, Perrachon S, Milligan L, Canat X, Rinaldi-Carmona M, Portier M, et al. A selective inverse agonist for central cannabinoid receptor inhibits mitogen-activated protein kinase activation stimulated by insulin or insulin-like growth factor 1. Evidence for a new model of receptor/ligand interactions. J Biol Chem. 1997;272:22330–22339. doi: 10.1074/jbc.272.35.22330. [DOI] [PubMed] [Google Scholar]
- Brandt MR, France CP. Chronic l-alpha-acetylmethadol (LAAM) in rhesus monkeys: tolerance and cross-tolerance to the antinociceptive, ventilatory, and rate-decreasing effects of opioids. J Pharmacol Exp Ther. 2000;294:168–178. [PubMed] [Google Scholar]
- Breivogel CS, Childers SR. Cannabinoid agonist signal transduction in rat brain: comparison of cannabinoid agonists in receptor binding, G-protein activation, and adenylyl cyclase inhibition. J Pharmacol Exp Ther. 2000;295:328–336. [PubMed] [Google Scholar]
- Breivogel CS, Childers SR, Deadwyler SA, Hampson RE, Vogt LJ, Sim-Selley LJ. Chronic Δ9-tetrahydrocannabinol treatment produces a time-dependent loss of cannabinoid receptors and cannabinoid receptor-activated G proteins in rat brain. J Neurochem. 1999;73:2447–2459. doi: 10.1046/j.1471-4159.1999.0732447.x. [DOI] [PubMed] [Google Scholar]
- De Vry J, Jentzsch KR. Partial agonist-like profile of the cannabinoid receptor antagonist SR141716A in a food-reinforced operant paradigm. Behav Pharmacol. 2004;15:13–20. doi: 10.1097/00008877-200402000-00002. [DOI] [PubMed] [Google Scholar]
- Delatte MS, Winsauer PJ, Moerschbaecher JM. Tolerance to the disruptive effects of Δ9-THC on learning in rats. Pharmacol Biochem Behav. 2002;74:129–140. doi: 10.1016/s0091-3057(02)00966-8. [DOI] [PubMed] [Google Scholar]
- Dewey WL, McMillan DE, Harris LS, Turk RF. Distribution of radioactivity in brain of tolerant and nontolerant pigeons treated with 3H-Δ9-tetrahydrocannabinol. Biochem Pharmacol. 1973;22:399–405. doi: 10.1016/0006-2952(73)90420-6. [DOI] [PubMed] [Google Scholar]
- Fan F, Compton DR, Ward S, Melvin L, Martin BR. Development of cross-tolerance between delta Δ9-tetrahydrocannabinol, CP 55,940 and WIN 55,212. J Pharmacol Exp Ther. 1994;271:1383–1390. [PubMed] [Google Scholar]
- Gifford AN, Bruneus M, Gatley SJ, Lan R, Makriyannis A, Volkow ND. Large receptor reserve for cannabinoid actions in the central nervous system. J Pharmacol Exp Ther. 1999;288:478–483. [PubMed] [Google Scholar]
- Goldberg SR, Schuster CR. Conditioned suppression by a stimulus associated with nalorphine in morphine-dependent monkeys. J Exp Anal Behav. 1967;10:235–242. doi: 10.1901/jeab.1967.10-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Fong J, von Zastrow M, Whistler JL. Regulation of opioid receptor trafficking and morphine tolerance by receptor oligomerization. Cell. 2002;108:271–282. doi: 10.1016/s0092-8674(02)00613-x. [DOI] [PubMed] [Google Scholar]
- Howlett AC. Efficacy in CB1 receptor-mediated signal transduction. Br J Pharmacol. 2004;142:1209–1218. doi: 10.1038/sj.bjp.0705881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt CA, Jones RT. Tolerance and disposition of tetrahydrocannabinol in man. J Pharmacol Exp Ther. 1980;215:35–44. [PubMed] [Google Scholar]
- Järbe TU, Lamb RJ, Liu Q, Makriyannis A. R)-Methanandamide and Δ9-tetrahydrocannabinol-induced operant rate decreases in rats are not readily antagonized by SR-141716A. Eur J Pharmacol. 2003;466:121–127. doi: 10.1016/s0014-2999(03)01491-2. [DOI] [PubMed] [Google Scholar]
- Kenakin TP. An in vitro quantitative analysis of the alpha adrenoceptor partial agonist activity of dobutamine and its relevance to inotropic selectivity. J Pharmacol Exp Ther. 1981;216:210–219. [PubMed] [Google Scholar]
- Kenakin T. Pharmacologic Analysis of Drug-Receptor Interaction. Philadelphia: Lippincott-Raven Publishers; 1997. [Google Scholar]
- Kenakin T. Drug efficacy at G protein-coupled receptors. Annu Rev Pharmacol Toxicol. 2002;42:349–379. doi: 10.1146/annurev.pharmtox.42.091401.113012. [DOI] [PubMed] [Google Scholar]
- Lemberger L, Silberstein SD, Axelrod J, Kopin IJ. Marihuana: studies on the disposition and metabolism of Δ9-tetrahydrocannabinol in man. Science. 1971;170:1320–1322. doi: 10.1126/science.170.3964.1320. [DOI] [PubMed] [Google Scholar]
- Martin BR, Dewey WL, Harris LS, Beckner JS. 3H-Δ9-tetrahydrocannabinol tissue and subcellular distribution in the central nervous system and tissue distribution in peripheral organs of tolerant and nontolerant dogs. J Pharmacol Exp Ther. 1976;196:128–144. [PubMed] [Google Scholar]
- Martini L, Whistler JL. The role of mu opioid receptor desensitization and endocytosis in morphine tolerance and dependence. Curr Opin Neurobiol. 2007;17:556–564. doi: 10.1016/j.conb.2007.10.004. [DOI] [PubMed] [Google Scholar]
- McKinney DL, Cassidy MP, Collier LM, Martin BR, Wiley JL, Selley DE, et al. Dose-related differences in the regional pattern of cannabinoid receptor adaptation and in vivo tolerance development to Δ9-tetrahydrocannabinol. J Pharmacol Exp Ther. 2008;324:664–673. doi: 10.1124/jpet.107.130328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon LR. Characterization of cannabinoid agonists and apparent pA2 analysis of cannabinoid antagonists in rhesus monkeys discriminating Δ9-tetrahydrocannabinol. J Pharmacol Exp Ther. 2006;319:1211–1218. doi: 10.1124/jpet.106.107110. [DOI] [PubMed] [Google Scholar]
- McMahon LR. Apparent affinity estimates of rimonabant in combination with anandamide and chemical analogs of anandamide in rhesus monkeys discriminating Δ9-tetrahydrocannabinol. Psychopharmacology. 2009;203:219–228. doi: 10.1007/s00213-008-1230-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon LR, France CP. Daily treatment with diazepam differentially modifies sensitivity to the effects of gamma-aminobutyric acidA modulators on schedule-controlled responding in rhesus monkeys. J Pharmacol Exp Ther. 2002;300:1017–10125. doi: 10.1124/jpet.300.3.1017. [DOI] [PubMed] [Google Scholar]
- McMillan DE, Harris LS, Frankenheim JM, Kennedy JS. l-Δ9-trans-tetrahydrocannabinol in pigeons: tolerance to the behavioral effects. Science. 1970;169:501–503. doi: 10.1126/science.169.3944.501. [DOI] [PubMed] [Google Scholar]
- McMillan DE, Dewey WL, Turk RF, Harris LS, McNeil JH. Blood levels of 3H-Δ9-tetrahydrocannabinol and its metabolites in tolerant and nontolerant pigeons. Biochem Pharmacol. 1973;22:383–397. doi: 10.1016/0006-2952(73)90419-x. [DOI] [PubMed] [Google Scholar]
- Miczek KA. Chronic Δ9-tetrahydrocannabinol in rats: effect on social interactions, mouse killing, motor activity, consummatory behavior, and body temperature. Psychopharmacology. 1979;60:137–146. doi: 10.1007/BF00432284. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academy Press; 1996. [Google Scholar]
- Palmer SL, Thakur GA, Makriyannis A. Cannabinergic ligands. Chem Phys Lipids. 2002;121:3–19. doi: 10.1016/s0009-3084(02)00143-3. [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Héaulme M, Shire D, Calandra B, Congy C, et al. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350:240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- Sannerud CA, Allen M, Cook JM, Griffiths RR. Behavioral effects of benzodiazepine ligands in non-dependent, diazepam-dependent, and diazepam-withdrawn baboons. Eur J Pharmacol. 1991;202:159–169. doi: 10.1016/0014-2999(91)90290-7. [DOI] [PubMed] [Google Scholar]
- Siemens AJ, Kalant H. Metabolism of delta1-tetrahydrocannabinol by rats tolerant to cannabis. Can J Physiol Pharmacol. 1974;52:1154–11566. doi: 10.1139/y74-151. [DOI] [PubMed] [Google Scholar]
- Sim LJ, Hampson RE, Deadwyler SA, Childers SR. Effects of chronic treatment with Δ9-tetrahydrocannabinol on cannabinoid-stimulated [35S]GTPgammaS autoradiography in rat brain. J Neurosci. 1996;16:8057–8066. doi: 10.1523/JNEUROSCI.16-24-08057.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson RP. A modification of receptor theory. Br J Pharmacol. 1956;11:379–393. doi: 10.1111/j.1476-5381.1956.tb00006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart JL, McMahon LR. Rimonabant-induced Δ9-THC withdrawal in rhesus monkeys: discriminative stimulus effects and other withdrawal signs. J Pharmacol Exp Ther. 2010;334:347–356. doi: 10.1124/jpet.110.168435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallarida RJ. Drug Synergism and Dose-Effect Data Analysis. Boca Raton, FL: Chapman and Hall/CRC; 2000. [Google Scholar]
- Walker EA, Young AM. Discriminative-stimulus effects of the low efficacy mu agonist nalbuphine. J Pharmacol Exp Ther. 1993;267:322–330. [PubMed] [Google Scholar]
- Walker EA, Makhay MM, House JD, Young AM. In vivo apparent pA2 analysis for naltrexone antagonism of discriminative stimulus and analgesic effects of opiate agonists in rats. J Pharmacol Exp Ther. 1994;271:959–968. [PubMed] [Google Scholar]
- Walker EA, Richardson TM, Young AM. Tolerance and cross-tolerance to morphine-like stimulus effects of mu opioids in rats. Psychopharmacology. 1997;133:17–28. doi: 10.1007/s002130050366. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Barrett RL, Balster RL, Martin BR. Tolerance to the discriminative stimulus effects of Δ9-tetrahydrocannabinol. Behav Pharmacol. 1993;4:581–585. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


