Abstract
BACKGROUND AND PURPOSE
Semaphorin 3A (Sema3A) is an important secreted repulsive guidance factor for many developing neurones. Sema3A continues to be expressed in adulthood, and expression of its receptor, neuropilin-1 (Nrp-1), can be altered by nerve injury. Autonomic neurones innervating the pelvic viscera are particularly susceptible to damage during pelvic surgical procedures, and failure to regenerate or aberrant growth of sympathetic and parasympathetic nerves lead to organ dysfunction. However, it is not known if adult pelvic neurones are potential targets for Sema3A.
EXPERIMENTAL APPROACH
The effects of Sema3A and activation or inhibition of cyclic nucleotide signalling were assessed in adult rat pelvic ganglion neurones in culture using a growth cone collapse assay.
KEY RESULTS
Sema3A caused growth cone collapse in both parasympathetic and sympathetic neurones expressing Nrp-1. However, the effect of Sema3A was mediated by distinct cyclic nucleotide signalling pathways in each neurone type. In parasympathetic neurones, cAMP and downstream activation of protein kinase A were required for growth cone collapse. In sympathetic neurones, cGMP was required for Sema3A-induced collapse; cAMP can also cause collapse but was not required. Sema3A-mediated, cGMP-dependent collapse in sympathetic neurones may require activation of cyclic nucleotide-gated ion channels (CNGCs).
CONCLUSIONS AND IMPLICATIONS
We propose that Sema3A is an important guidance factor for adult pelvic autonomic neurones, and that manipulation of their distinct signalling mechanisms could potentially promote functional selective regeneration or attenuate aberrant growth. To our knowledge, this is also the first study to implicate CNGCs in regulating growth cone dynamics of adult neurones.
Keywords: guidance, axotomy, regeneration, sprouting, autonomic, urogenital
Introduction
The guidance factor, semaphorin 3A (Sema3A), is a secreted protein that signals through a receptor complex of neuropilin-1 (Nrp-1) and plexin-A (He and Tessier-Lavigne, 1997; Takahashi et al., 1999). Sema3A has chemo-repulsive effects on developing axons to facilitate appropriate target innervation (Tran et al., 2007). For example, in the peripheral nervous system, Sema3A null mutant mice show severe abnormalities in morphology and projections of sympathetic ganglia (Behar et al., 1996; Taniguchi et al., 1997; Kawasaki et al., 2002) and premature entry of enteric neural precursors into the hindgut (Anderson et al., 2007).
Understanding the guidance factor signalling during development could reveal new strategies to promote regeneration of injured axons in the adult (Pasterkamp and Verhaagen, 2006; Pasterkamp and Giger, 2009). Direct evidence that Sema3A can inhibit regeneration in vivo is provided by the repulsion of established and reinnervating sensory axons by ectopic expression of Sema3A in rabbit cornea (Tanelian et al., 1997). A number of studies also indicate dynamic changes in Sema3A signalling after injury. For example, Nrp-1 is upregulated in dorsal root ganglion (DRG) neurones after damage to their peripheral processes and in regenerating olfactory axons (Pasterkamp et al., 1998; 2001; Gavazzi et al., 2000). Moreover, Sema3A may stabilize synaptic contacts and restrict sprouting at the neuromuscular junction (De Winter et al., 2006).
Damage to autonomic nerves regulating urogenital function is often unavoidable during pelvic surgery (e.g. prostatectomy and hysterectomy, Maas et al., 2003; Penson et al., 2005), having broad consequences for sexual and bladder function. The nerves most vulnerable to damage arise from the inferior hypogastric plexus, containing both sympathetic and parasympathetic neurones (Keast, 2006). This is of particular interest for pelvic parasympathetic neurones, which differ from other parasympathetic neurones in having unusually long axons and so are uniquely susceptible to damage. In rodents, functionally similar autonomic neurones are located in paired major pelvic ganglia (Keast, 2006). These neurones have some regenerative potential after axotomy, and restoration of function can be slow or incomplete; aberrant growth to inappropriate targets also occurs (Kepper and Keast, 1998; Palma and Keast, 2006; Nangle and Keast, 2007; 2009;). The potential role of guidance factors has not been examined in adult pelvic neurones, although Sema3A is expressed in some of their targets (e.g. uterus and lower bowel) and increased uterine Sema3A expression during pregnancy is associated with reduced sympathetic innervation (Marzioni et al., 2004; Anderson et al., 2007).
In this study, we aimed to determine if adult rat pelvic neurones are potential targets for Sema3A by performing growth cone collapse assays. This also provides a unique opportunity to investigate Sema3A actions on parasympathetic neurones, which in other locations are closely associated with their target organs. Cyclic nucleotides are heavily involved in mediating or modulating Sema3A signalling in other systems (Song et al., 1998; Huber et al., 2003; Nishiyama et al., 2008; Togashi et al., 2008; Pasterkamp and Giger, 2009), and we have found that both sympathetic and parasympathetic pelvic neurones respond to Sema3A but utilize completely distinct mechanisms. We propose that Sema3A is an important guidance factor for adult pelvic autonomic neurones, and that targeting of particular intracellular mechanisms could potentially be exploited to selectively promote appropriate growth.
Methods
All experiments were performed in accordance with the Code of Practice for the Care and Use of Animals for Experimental Purposes (National Health and Medical Research Council of Australia) and were approved by the Animal Care and Ethics Committees of the Royal North Shore Hospital and University of Sydney. Adult male outbred Wistar rats (7–11 weeks, n= 49) were anaesthetized with sodium pentobarbitone (60 mg·kg−1 i.p.), prior to decapitation. The major pelvic ganglia were removed and neurones were dissociated and cultured in the absence of serum or growth factors, as previously described (Wanigasekara and Keast, 2005). Briefly, pelvic ganglion neurones were incubated in 0.15% type I collagenase (Worthington, Lakewood, NJ, USA) and 0.25% trypsin in 1 mM ethylenediaminetetraacetic acid (Invitrogen Australia, Mulgrave, Australia) for 2 h before gentle mechanical trituration through a fire-polished glass pipette. Neurones were then dispersed onto glass cover slips pretreated with 500 µg·mL−1 poly-ornithine and 5 µg·mL−1 laminin (≈500 neurones per cover slip). Neurones were cultured in Neurobasal A medium containing 2% B27 supplement, L-alanyl-L-glutamine (200 µM GlutaMAX) and antibiotic/antifungal agents (all from Invitrogen Australia). Cultures were maintained for 2 days (44–48 h).
Immunocytochemistry
Cultures were fixed in pre-heated 4% phosphate-buffered formaldehyde (pH 7.4) containing 10% sucrose and incubated for 30 min at 37°C, washed, blocked and permeabilized in 10% horse serum and 0.1% triton X-100 in phosphate-buffered saline for 1 h, and incubated with primary antibodies for 2 h at room temperature. Antisera against neuronal nitric oxide synthase (NOS), raised in rabbit (1:100; Zymed Laboratories, San Francisco, CA, USA) or sheep (1:1000; gift from Dr Piers Emson, Babraham Institute, Cambridge, UK), or against vesicular acetylcholine transporter (VAChT), raised in goat (1:500; Chemicon, Temecula, CA, USA), were used to identify cholinergic neurones. We have referred to cholinergic/nitrergic neurones as ‘parasympathetic’, but recognize that a minority receives lumbar spinal inputs in vivo so are sympathetic (Dail et al., 1985; Keast, 1995). Antisera against tyrosine hydroxylase (TH), raised in rabbit (1:100; Chemicon) or mouse (1:100; Immunostar, Hudson, WI, USA), were used to identify sympathetic noradrenergic neurones. Technical details for the NOS, TH and VAChT antibodies, including specificity, have been previously published (Poulin et al., 2006; Yan and Keast, 2008). An antibody against Nrp-1 was also used, raised in goat (1:100; R&D Systems, Minneapolis, MN, USA). This antibody was produced using purified recombinant rat Nrp-1 extracellular domain derived from murine hybridoma cell lines, and its specificity has been demonstrated with a number of applications (see manufacturer's details). Furthermore, somatic and neurite staining has been identified in dissociated rat neonatal superior cervical ganglion neurones (Marko and Damon, 2008). Neurones were incubated for 1 h with Alexa Fluor 488-, Cy2- or Cy3-conjugated secondary antibodies (Jackson Immunoresearch Laboratories, West Grove, PA, USA) or with a biotin-labelled secondary antibody (Jackson Immunoresearch Laboratories) followed by AMCA avidin D (Vector Laboratories, Burlingame, CA, USA) to visualize sites of secondary antibody binding. Except for experiments using the Nrp-1 antibody, Alexa Fluor 488-conjugated phalloidin (1:50; Molecular Probes, Eugene, OR, USA) was used to identify F-actin in growth cones. 4′,6-diamidino-2-phenylindole (DAPI, 1 µg·mL−1; Sigma, Castle Hill, NSW, Australia) was used as a nuclear counterstain. We identified unhealthy or dead neurones as having irregular nuclei with condensed or peripheral chromatin. Such cells were rejected from analysis but were very rare. Cover slips were mounted onto slides with 0.5 M bicarbonate-buffered glycerol, and sealed with nail varnish.
Growth cone collapse assay
Collapsed growth cones were identified as ‘bullet shaped’ neurite endings lacking lamellipodia/filopodia, as previously described (Reza et al., 1999). We classified neurite-bearing neurones as ‘intact’ or ‘collapsed’ based on the morphology of their growth cone(s). A neurone was classed as ‘collapsed’ where a majority of its growth cones had a collapsed phenotype and otherwise referred to as ‘intact’. At least 50 neurite-bearing neurones were counted for any given treatment from each culture; approximately 20–25% of neurones did not have neurites and were not assessed. Growth cone collapse was expressed as a percentage of total neurones counted. Most neurite-bearing neurones had numerous neurite branches and growth cones, and in the vast majority of cases, the growth cones of each neurone were almost all collapsed or all intact. In our pilot experiments, we found that this method of classifying neurones gave similar growth cone collapse percentages as counting the total number of individual growth cones; however, the former is a more efficient means of achieving adequate sampling. To investigate the role of cyclic nucleotides in Sema3A-induced growth cone collapse, we treated cultures with relevant agonists (30 min), or added inhibitors of cyclic nucleotide signalling 1 h prior to Sema3A (100 ng·mL−1 for all experiments; R&D Systems). This concentration of Sema3A is optimal for causing growth cone collapse of embryonic and adult rat DRG neurones (Song et al., 1998; Wanigasekara and Keast, 2006).
Reagents
The following reagents were used: 2′,5′-dideoxyadenosine (DDA, 100 µM) and forskolin (FSK, 10 µM) to, respectively, inhibit and stimulate adenylyl cyclase; 1H-[1,2,4]oxadiazole[4,3-a]quinoxalin-1-one (ODQ, 1 µM) and sodium nitroprusside (SNP, 100 µM) to inhibit and stimulate, respectively, soluble guanylyl cyclase; rolipram (ROL, 10 µM) and zaprinast (ZAP, 10 µM) to inhibit cAMP- and cGMP-dependent phosphodiesterases, respectively; (9R,10S,12S)-2,3,9,10,11,12-hexahydro-10-hydroxy-9-methyl-1-oxo-9,12-epoxy-1H-diindolo[1,2,3-fg:3′,2′,1′-kl]pyrrolo[3,4-i][1,6]benzodiazocine-10-carboxylic acid, hexyl ester (KT-5720, 200 nM) and Rp-adenosine-3′,5′-cyclic monophosphorothioate (Rp-cAMPS, 20 µM) to inhibit protein kinase A (PKA); (9S,10R,12R)-2,3,9,10,11,12-hexahydro-10-methoxy-2,9-dimethyl-1-oxo-9,12-epoxy-1H-diindolo[1,2,3-fg:3′,2′,1′-kl]pyrrolo[3,4-i][1,6]benzodiazocine-10-carboxylic acid, methyl ester (KT-5823, 1 µM) and Rp-8-bromoguanosine-3′,5′-cyclic monophosphorothioate (Rp-8-Br-cGMPS, 20 µM) to inhibit protein kinase G (PKG); L-cis-diltiazem (LCD, 20 µM) and 2′,4′-dichlorobenzamil (DCB, 1 µM) to inhibit cyclic nucleotide-gated ion channels (CNGCs); the cAMP analogue, Sp-cAMPS (20 µM), and the cGMP analogue, 8-bromoguanosine 3′,5′-cyclic monophosphate (8-Br-cGMP, 100 µM). SNP was purchased from Fluka Chemika (Steinham, Germany), and 8-Br-cGMP and ZAP from Sigma. All other chemicals were purchased from BIOMOL International (Plymouth Meeting, PA, USA).
Imaging and figure production
Images were captured using an RT Spot camera (Diagnostic Instruments, Sterling Heights, MI, USA) mounted on an Olympus BX51 fluorescence microscope, or a fluorescence laser-scanning confocal microscope (Leica TCS SP5 system with Leica Application Suite software, Leica Microsystem, Wetzlar, Germany). Images were digitized using Image-Pro Plus 5.0 (Media Cybernetics, Bethesda, MD, USA). Small adjustments to brightness and contrast were made with Adobe Photoshop CS2 or Imaris x64 software (Bitplane AG, Zurich, Switzerland), in order to best represent staining as seen under the microscope. Figures were prepared using Adobe InDesign CS2.
Statistics
In every experiment, each replicate (n) corresponded to a measurement taken from isolated cultured neurones obtained from one rat. The neurones isolated from each rat were split across dishes cultured in parallel, with the control and each of the experimental conditions assigned one dish. Three or more replicates were analysed in each experiment. Statistical procedures were performed using Prism 5 for Mac OS X software. Data expressed as the percentage of neurones with collapsed growth cones were arcsine-root transformed prior to statistical analyses. To analyse the time course of the effects of Sema3A treatment, measurements at each of three time points were compared with the control condition using Dunnett's test. In all other experiments, multiple two-group comparisons were performed using Tukey's test procedure to control the experiment-wise Type I error rate (Quinn and Keough, 2002; Keppel and Wickens, 2004). All results are expressed as the mean ± standard error of the mean. P < 0.05 was regarded as statistically significant.
Results
Sema3A causes growth cone collapse in adult sympathetic and parasympathetic rat pelvic ganglion neurones expressing Nrp-1
We first wished to determine if Sema3A caused growth cone collapse in cultured adult pelvic autonomic neurones. By 48 h, most sympathetic and parasympathetic neurones (approximately 75%) had grown multiple neurites (Figure 1A,B). For both types of neurone, the majority had an ‘intact’ growth cone phenotype, but growth cones were, on average, larger and had more filopodia in sympathetic compared with parasympathetic neurones (Figure 1C,D). Approximately 25–35% of neurones had predominantly collapsed growth cones (Figure 1E,F). In Xenopus retinal ganglion neurones, Sema3A induces transient growth cone collapse that peaks at about 10 min and abates by 60 min (Campbell et al., 2001). In contrast, Sema3A-collapse is sustained in adult rat DRG neurones for at least 1 h after application (Wanigasekara and Keast, 2006). Therefore, we performed a time course experiment, adding Sema3A to cultures for 10, 30 or 60 min prior to fixation. Sema3A increased growth cone collapse at all time points in both sympathetic and parasympathetic neurones (Figure 1G,H). The response was sustained in sympathetic neurones, but was larger and transient (peaking at 30 min) in parasympathetic neurones.
Figure 1.
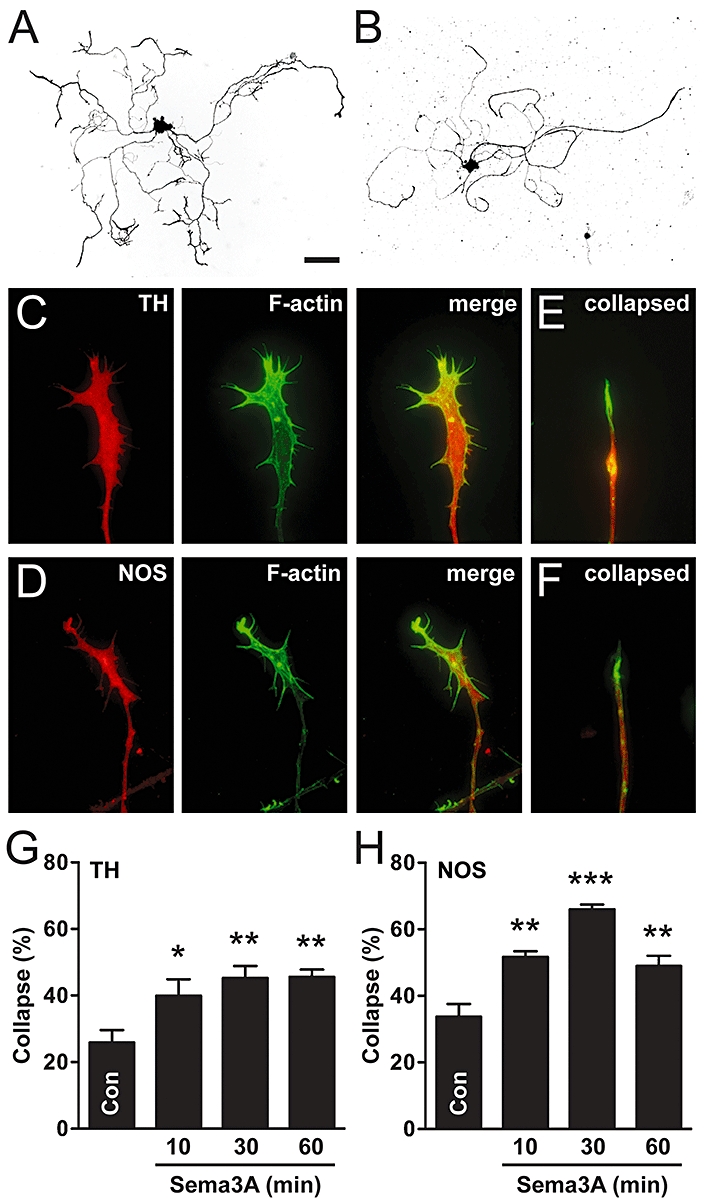
Sema3A causes growth cone collapse in adult sympathetic and parasympathetic neurones. (A,B) Inverted fluorescence images of cultured sympathetic (A; TH-positive) and parasympathetic (B; NOS-positive) pelvic ganglion neurones. (C, D) Intact growth cones of sympathetic (C) and parasympathetic (D) neurones. (E, F) Collapsed growth cones of sympathetic (E) and parasympathetic (F) neurones (merged images of TH or NOS with actin staining). Note the retraction of F-actin rich filopodia in collapsed growth cones. (G,H) Sema3A increased growth cone collapse in both sympathetic and parasympathetic neurones, at all time points tested (n= 4). Dunnett's test: *P < 0.05, **P < 0.01, ***P < 0.001 versus control. Scale bar = 100 µm in (A and B), 12 µm in (C–F). NOS, nitric oxide synthase; TH, tyrosine hydroxylase.
Next, we determined whether pelvic ganglion neurones express the Sema3A receptor, Nrp-1, and whether Sema3A-induced growth cone collapse only occurs in neurones expressing Nrp-1. We found that Nrp-1 was expressed in both sympathetic (32.3 ± 2.4%, n= 5) and parasympathetic neurones (53.9 ± 2.9%, n= 5). Nrp-1-immunoreactivity was observed in somata as well as neurites, including central and peripheral domains of growth cones and their filopodia (Figure 2A–D). Sema3A (30 min) caused growth cone collapse in approximately 80% of sympathetic and parasympathetic neurones expressing Nrp-1 (Figure 2E,F). However, in Nrp-1-negative neurones, Sema3A did not cause growth cone collapse (Figure 2E,F). These data demonstrate that specific populations of pelvic ganglion autonomic neurones are responsive to Sema3A, and that these neurones express Nrp-1.
Figure 2.
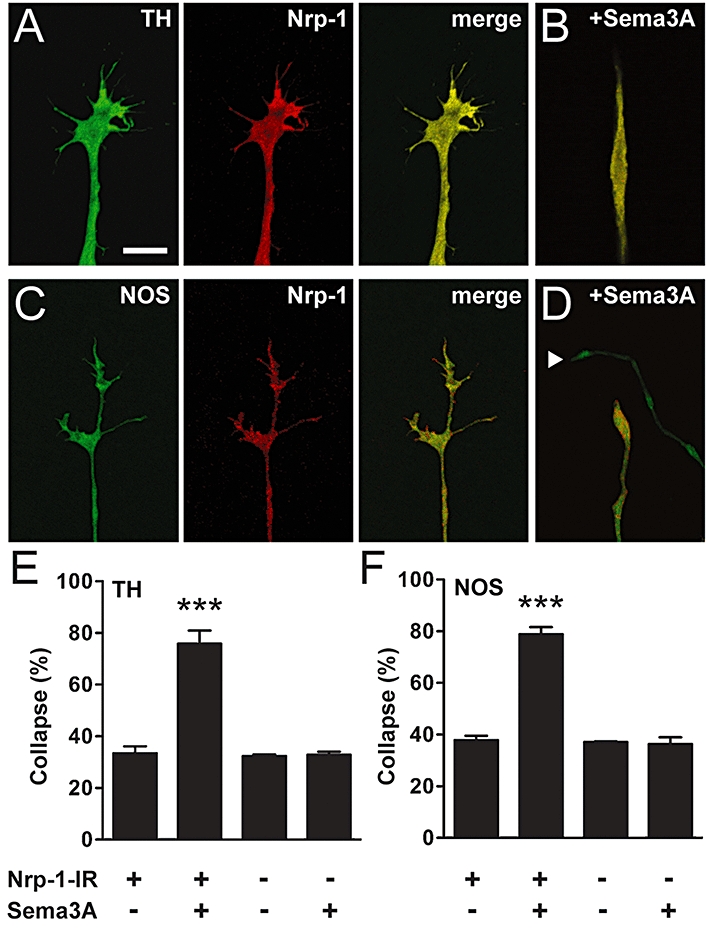
Sema3A-induced growth cone collapse occurs only in sympathetic and parasympathetic neurones expressing Nrp-1. (A–D) Confocal images of growth cones from sympathetic (TH-positive: A, intact; B, collapsed) and parasympathetic (NOS-positive: C, intact; D, collapsed) pelvic ganglion neurones expressing Nrp-1. Merged images of TH and NOS with Nrp-1 immunostaining are shown in (B) and (D) respectively. Arrowhead in (D) shows an Nrp-1-negative collapsed growth cone in close proximity to an Nrp-1-positive collapsed growth cone. (E, F) Sema3A caused growth cone collapse in sympathetic (E) and parasympathetic (F) neurones with Nrp-1-immunoreactivity (Nrp-1-IR), but did not cause collapse in Nrp-1-negative neurones (n= 3). Tukey's test: ***P < 0.001 versus Sema3A-negative control. Scale bar = 10 µm in (A–D). NOS, nitric oxide synthase; Nrp-1, neuropilin-1; Sema3A, semaphorin 3A; TH, tyrosine hydroxylase.
Cyclic AMP and PKA signalling mediate Sema3A-induced growth cone collapse in parasympathetic neurones
We used a pharmacological approach to determine whether cyclic nucleotide signalling is required for or modulates Sema3A-collapse in autonomic neurones, examining first the parasympathetic population. The adenylyl cyclase (AC) inhibitor, dideoxyadenosine (DDA, 100 µM), blocked Sema3A-induced collapse in parasympathetic neurones, but had no effect on its own (Figure 3A). In addition, increasing cAMP levels with FSK (10 µM), an activator of AC, caused growth cone collapse of similar magnitude to that observed with Sema3A (Figure 3A). ROL (10 µM), an inhibitor of cAMP-specific type 4 phosphodiesterase, the most abundant isoform in neural tissue (Nikulina et al., 2004), did not augment Sema3A-induced collapse in parasympathetic neurones (Figure 3B), implying a maximal effect for cAMP-mediated collapse was reached. However, by itself, ROL caused a small but significant increase in growth cone collapse (Figure 3B), suggesting that there is an appreciable basal production of cAMP. In contrast to the effect of AC inhibition, inhibition of soluble guanylyl cyclase (sGC) with ODQ (1 µM) did not block Sema3A-induced growth cone collapse in parasympathetic neurones (Figure 3C). Furthermore, elevating cGMP levels with the nitric oxide donor and sGC activator, SNP (100 µM), did not increase collapse.
Figure 3.
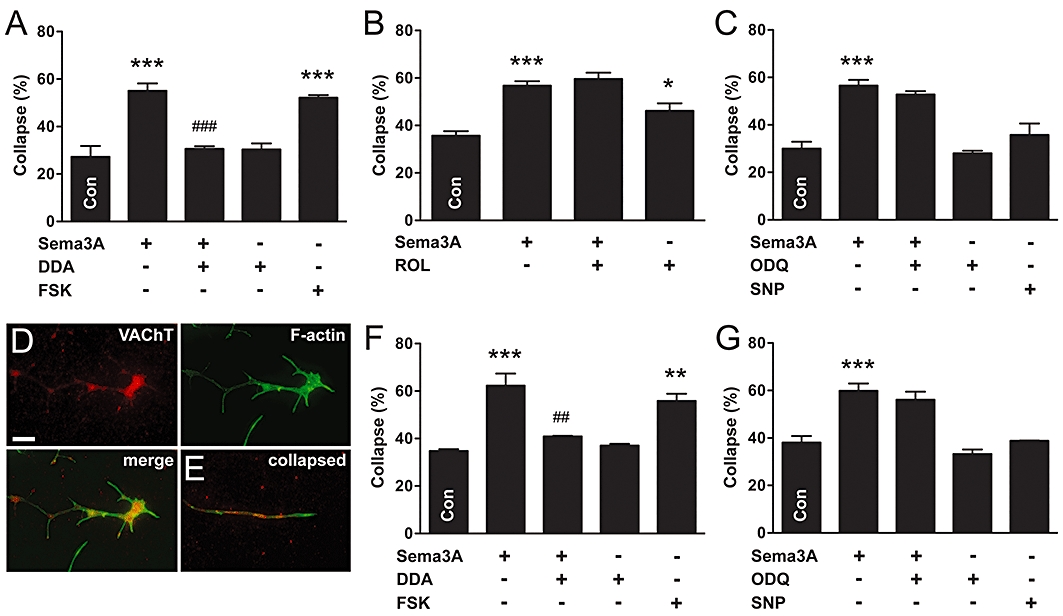
Adenylyl cyclase-cAMP signalling mediates Sema3A-induced growth cone collapse in parasympathetic neurones. (A) Inhibition of AC (DDA) prevented Sema3A-induced growth cone collapse, and stimulation of AC (FSK) caused growth cone collapse in NOS-positive parasympathetic neurones. (B) Cyclic AMP-dependent phosphodiesterase inhibition (ROL) did not augment Sema3A-induced collapse, but caused growth cone collapse by itself. (C) However, inhibition of sGC (ODQ) did not affect Sema3A-induced growth cone collapse, and stimulation of sGC (SNP) did not cause growth cone collapse. (D,E) Growth cones of parasympathetic neurones identified by VAChT-immunoreactivity (D, intact; E, collapsed). Merged images of VAChT and F-actin staining are shown in (E). Similar to NOS-positive neurones, inhibition of AC (F), but not sGC (G), prevented Sema3A-induced growth cone collapse in VAChT-positive/NOS-negative parasympathetic neurones. In addition, stimulation of AC (F), but not sGC (G), caused growth cone collapse. Tukey's test: *P < 0.05, **P < 0.01, ***P < 0.001 versus control; ##P < 0.01, ###P < 0.001 versus Sema3A (n= 3–6 for all experiments). Scale bar = 10 µm in (D) and (E). DDA, 2′,5′-dideoxyadenosine; FSK, forskolin; ODQ, 1H-[1,2,4]oxadiazole[4,3-a]quinoxalin-1-one; ROL, rolipram; Sema3A, semaphorin 3A; SNP, sodium nitroprusside; TH, tyrosine hydroxylase; VAChT, vesicular acetylcholine transporter.
Some parasympathetic pelvic ganglion neurones do not express NOS (Wanigasekara and Keast, 2005), so we assessed growth cone collapse in this population by counting VAChT-positive/NOS-negative neurones (examples of VAChT-immunoreactive neurites are shown in Figure 3D,E). In these parasympathetic neurones, Sema3A caused growth cone collapse of a similar magnitude to that observed in NOS-positive neurones (Figure 3F). Furthermore, DDA blocked Sema3A-induced collapse and FSK caused growth cone collapse (Figure 3F). Conversely, ODQ did not block Sema3A-induced collapse, and elevating cGMP levels with SNP did not cause collapse (Figure 3G). Together, these results suggest that activation of the AC-cAMP pathway mediates Sema3A-induced actin depolymerization in growth cones of nitrergic and non-nitrergic parasympathetic neurones, and that the sGC-cGMP pathway does not regulate repulsive growth cone dynamics.
To investigate Sema3A signalling downstream of cyclic nucleotides in nitrergic parasympathetic neurones, we used inhibitors of PKA and PKG, the respective prototypical mediators of cAMP and cGMP functions. The PKA inhibitors, KT-5720 (200 nM) and Rp-cAMPS (20 µM) prevented Sema3A-collapse without affecting growth cone collapse on their own (Figure 4A,B). These results suggest Sema3A-collapse is mediated by cAMP-dependent activation of PKA. In contrast to PKA inhibition, Sema3A-collapse was unaffected by PKG inhibition with either KT-5823 (1 µM, Figure 4C) or Rp-8-Br-cGMPS (20 µM, Figure 4D). However, both PKG inhibitors caused growth cone collapse on their own, implicating PKG signalling as a positive regulator of growth cone dynamics in parasympathetic neurones, independent of Sema3A signalling.
Figure 4.
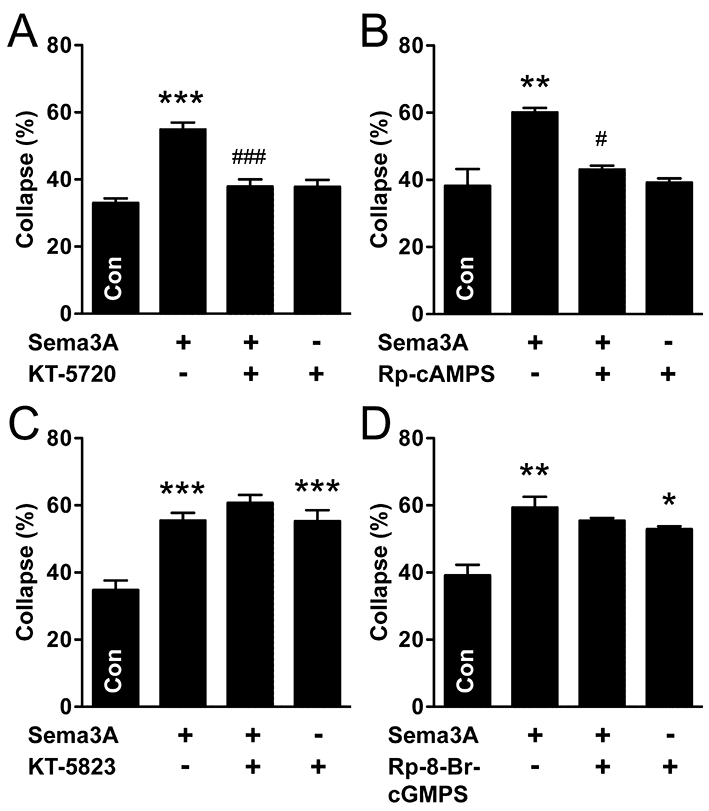
Protein kinase A signalling mediates Sema3A-induced growth cone collapse in nitrergic (NOS-positive) parasympathetic neurones. (A) The PKA inhibitor, KT-5720, prevented Sema3A-induced growth cone collapse in NOS-positive neurones. (B) Similar results were found with Rp-cAMPS. (C, D) In contrast, two separate inhibitors of PKG (C, KT-5823; D, Rp-8-Br-cGMPS) did not significantly affect Sema3A-collapse, but both caused growth cone collapse on their own. Tukey's test: *P < 0.05, **P < 0.01, ***P < 0.001 versus control; #P < 0.05, ###P < 0.001 versus Sema3A (n= 3–4 for all experiments). KT-5720, (9R,10S,12S)-2,3,9,10,11,12-hexahydro-10-hydroxy-9-methyl-1-oxo-9,12-epoxy-1H-diindolo[1,2,3-fg:3′,2′,1′-kl]pyrrolo[3,4-i][1,6]benzodiazocine-10-carboxylic acid, hexyl ester; KT-5823, (9S,10R,12R)-2,3,9,10,11,12-hexahydro-10-methoxy-2,9-dimethyl-1-oxo-9,12-epoxy-1H-diindolo[1,2,3-fg:3′,2′,1′-kl]pyrrolo[3,4-i][1,6]benzodiazocine-10-carboxylic acid, methyl ester; Rp-cAMPS, Rp-adenosine-3′,5′-cyclic monophosphorothioate; Rp-8-Br-cGMPS, Rp-8-bromoguanosine-3′,5′-cyclic monophosphorothioate; Sema3A, semaphorin 3A.
Cyclic GMP signalling mediates Sema3A-induced growth cone collapse in sympathetic neurones, but cAMP can also stimulate collapse
In sympathetic neurones, ODQ blocked Sema3A-collapse and caused a small decrease in growth cone collapse on its own (Figure 5A). Therefore, cyclic GMP signalling mediates Sema3A-induced growth cone collapse. Furthermore, both SNP (Figure 5A) and the cGMP analogue, 8-Br-cGMP (100 µM, data not shown), caused growth cone collapse. Consistent with this, DDA did not block Sema3A-induced collapse in sympathetic neurones (Figure 5B), suggesting that cAMP production is not required to mediate Sema3A-collapse. However, we found that elevating cAMP using either FSK (Figure 5B) or the cAMP analogue, Sp-cAMPS (20 µM, data not shown) caused growth cone collapse in sympathetic neurones. Together, these results suggest that in sympathetic neurones, Sema3A-induced growth cone collapse is mediated by the sGC-cGMP pathway, in contrast to parasympathetic neurones, where cAMP signalling is required. However, because cAMP can stimulate collapse, it is possible that chemo-repulsive guidance factor(s) other than Sema3A signal via this cyclic nucleotide.
Figure 5.
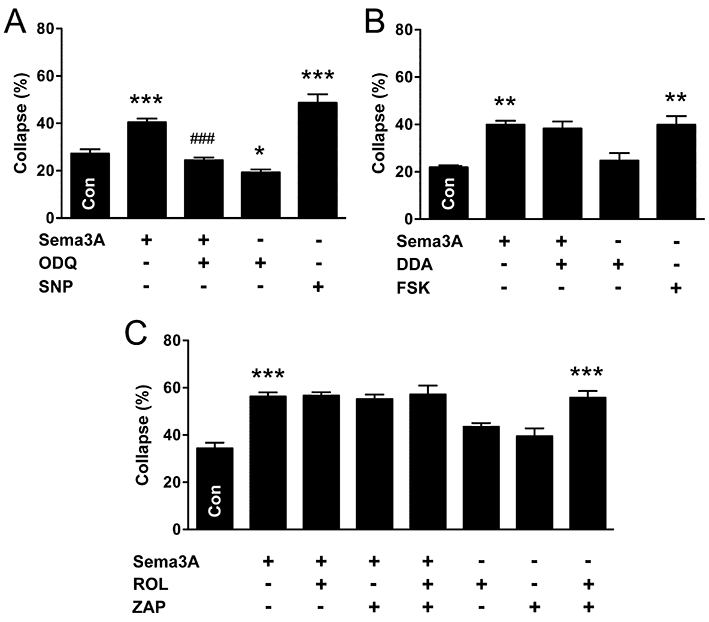
Soluble guanylyl cyclase-cGMP signalling mediates semaphorin 3A-induced growth cone collapse in sympathetic neurones. (A) Inhibition of sGC (ODQ) prevented Sema3A-induced growth cone collapse and stimulation of sGC (SNP) also caused collapse. (B) In contrast to parasympathetic neurones, inhibition of AC (DDA) did not prevent Sema3A-induced growth cone collapse in sympathetic neurones. However, stimulation of AC (FSK) did cause growth cone collapse. (C) Neither cAMP- nor cGMP-dependent phosphodiesterase inhibition (ROL and ZAP, respectively) augmented Sema3A-induced growth cone collapse, nor did these inhibitors significantly affect collapse on their own. However, when added together, ROL and ZAP caused growth cone collapse. Tukey's test: *P < 0.05, **P < 0.01, ***P < 0.001 versus control; ###P < 0.001 versus Sema3A (n= 4 for all experiments). DDA, 2′,5′-dideoxyadenosine; FSK, forskolin;; ROL, rolipram; Sema3A, semaphorin 3A; SNP, sodium nitroprusside; ZAP, zaprinast.
Because both cAMP and cGMP caused filopodial retraction in sympathetic neurones, we used ROL and the cGMP-specific type 5/6 phosphodiesterase inhibitor, ZAP (10 µM), to determine whether basal levels of either nucleotide affects growth cone dynamics (Figure 5C). Phosphodiesterase inhibition, separately or combined, did not augment Sema3A-induced collapse, suggesting that a maximal level of cyclic nucleotide-dependent collapse had been reached. Similarly, neither ROL nor ZAP caused statistically significant increases in collapse on their own. However, in combination, these inhibitors caused growth cone collapse to a similar degree as that caused by Sema3A, possibly via a synergistic effect (Figure 5C).
CNGCs mediate Sema3A-collapse in sympathetic, but not parasympathetic, neurones
In sympathetic neurones, we predicted from the dependence of Sema3A on sGC-cGMP signalling that PKG inhibitors would also inhibit the response to Sema3A, but both KT-5823 (Figure 6A) and Rp-8-Br-cGMPS (Figure 6B), failed to prevent Sema3A-collapse. Moreover, both agents caused growth cone collapse, implicating PKG signalling as a positive regulator of growth cone dynamics. This effect of the inhibitors alone may have impaired our ability to determine the role of PKG in the Sema3A response. As predicted by the lack of effect of AC inhibition on the Sema3A response, inhibition of PKA with either KT-5720 (Figure 6C) or Rp-cAMPS (Figure 6D) did not prevent Sema3A-collapse in sympathetic neurones. However, as seen for the PKG inhibitors in these neurones, each PKA inhibitor alone caused collapse, limiting the value of these reagents for assessing the downstream target of cAMP signalling. These data raise the possibility that neither PKA nor PKG signalling are required for growth cone collapse in response to the elevation of cyclic nucleotides, and that cGMP-dependent collapse in response to Sema3A may occur via a non-PKG downstream target.
Figure 6.
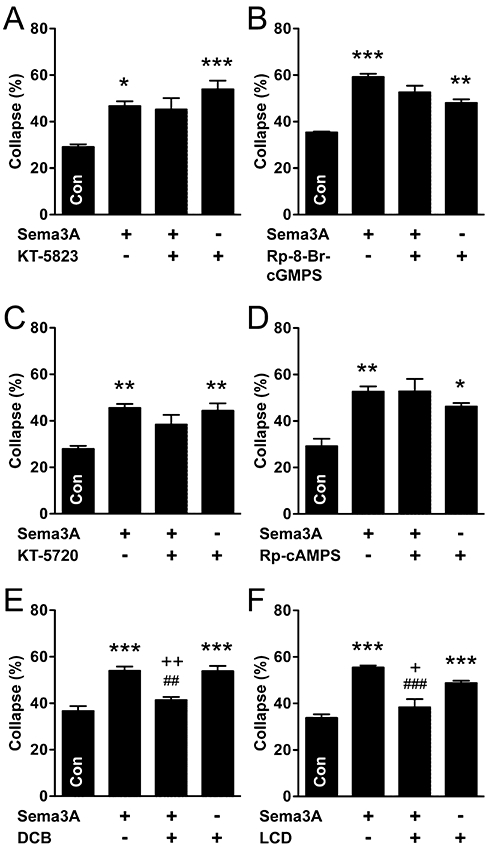
Cyclic nucleotide-gated ion channels, not PKA or PKG signalling, mediates Sema3A-induced growth cone collapse in sympathetic neurones. (A, B) Inhibition of PKG with KT-5823 (A) or Rp-8-Br-cGMPS (B) did not significantly alter Sema3A-induced growth cone collapse in sympathetic neurones, but both inhibitors caused growth cone collapse on their own. (C, D) Similarly, inhibition of PKA (C, KT-5720; D, Rp-cAMPS) did not alter Sema3A-collapse, but both of these inhibitors caused growth cone collapse. (E,F) However, inhibition of CNGCs (E, DCB; F, LCD) prevented Sema3A-collapse in sympathetic neurones. Furthermore, Sema3A prevented the collapse caused by DCB and LCD on their own. Tukey's test: *P < 0.05, **P < 0.01, ***P < 0.001 versus control (n= 3–5 for all experiments). **P < 0.01, ***P < 0.001 versus control; ##P < 0.01 versus Sema3A; +P < 0.05, ++P < 0.01 versus DCB or LCD. DCB, 2′,4′-dichlorobenzamil; KT-5720, (9R,10S,12S)-2,3,9,10,11,12-hexahydro-10-hydroxy-9-methyl-1-oxo-9,12-epoxy-1H-diindolo[1,2,3-fg:3′,2′,1′-kl]pyrrolo[3,4-i][1,6]benzodiazocine-10-carboxylic acid, hexyl ester; KT-5823, (9S,10R,12R)-2,3,9,10,11,12-hexahydro-10-methoxy-2,9-dimethyl-1-oxo-9,12-epoxy-1H-diindolo[1,2,3-fg:3′,2′,1′-kl]pyrrolo[3,4-i][1,6]benzodiazocine-10-carboxylic acid, methyl ester; LCD, L-cis-diltiazem; Rp-cAMPS, Rp-adenosine-3′,5′-cyclic monophosphorothioate; Rp-8-Br-cGMPS, Rp-8-bromoguanosine-3′,5′-cyclic monophosphorothioate; Sema3A, semaphorin 3A.
We next wished to investigate whether CNGCs function in cGMP-mediated growth cone collapse of sympathetic neurones. Recently, cGMP-specific (rod-type) CNGCs have been shown to mediate calcium-dependent chemo-repulsive effects of Sema3A in embryonic Xenopus spinal neurones (Togashi et al., 2008). CNGCs have not yet been implicated in guidance factor activity in adult neurones, although rod-type CNGCs have previously been identified in a neuroblastoma cell line derived from mouse sympathetic ganglia (Thompson, 1997). We found that treatment with the non-specific CNGC inhibitor, DCB (1 µM), or the CNGC-specific inhibitor, LCD (20 µM), prevented Sema3A-induced growth cone collapse in sympathetic neurons (Figure 6E,F). Therefore, CNGCs may mediate the cGMP-dependent effects of Sema3A. However, each inhibitor also caused collapse on its own (Figure 6E,F), raising the question of how collapsed growth cones caused by these agents could become less prevalent (i.e. comparable to controls) after exposure to Sema3A. One possibility is that collapsed growth cones are restored, or new growth cones formed during the period of Sema3A treatment, and that their stability is enhanced by Sema3A. Considering these observations together, we propose that under normal conditions, Sema3A-induced cGMP-dependent collapse is mediated by CNGCs, but that if these channels are inhibited, cGMP promotes growth cone stability, possibly via PKG. In parasympathetic neurones, LCD had no effect on Sema3A-induced growth cone collapse and did not significantly affect collapse on its own (data not shown).
Discussion and conclusions
We have shown that both sympathetic and parasympathetic adult pelvic autonomic neurones are targets for Sema3A. To our knowledge, this is the first time that Sema3A actions have been identified in parasympathetic neurones or pelvic neurones of any type, and suggest that Nrp-1 or its downstream effectors may be viable therapeutic targets for directing axonal growth after injury in this system. We also found that Sema3A caused growth cone collapse by completely different mechanisms in sympathetic and parasympathetic neurones. Our results suggest that Sema3A is an important guidance factor for adult pelvic autonomic neurones, and that separate pharmacological approaches could be used to promote appropriate, regenerative growth after injury. Moreover, we have revealed a role for CNG channels in sympathetic pelvic neurones, raising the possibility of their activity in other parts of the adult peripheral nervous system.
Growth cone collapse initiated by Sema3A in pelvic autonomic neurones was strongly correlated with Nrp-1 expression, occurring only in neurones expressing Nrp-1. However, a small proportion (∼20%) of Nrp-1 immunoreactive neurones did not display growth cone collapse in response to Sema3A. This observation raises the possibility that functionally distinct pelvic neurones (e.g. innervating different organs or tissues) may have different exposure or responses to Sema3A. The effects of Sema3A on pelvic autonomic neurones were unable to be predicted from previous studies in other parts of the nervous system because there is considerable diversity, depending on species, maturity and type of neurone. For example, elevating cGMP in embryonic rat DRG neurones prevents Sema3A-induced growth cone collapse, but causes collapse in embryonic chick DRG explants and mediates Sema3A-collapse in adult rat DRG neurones (Song et al., 1998; Dontchev and Letourneau, 2002; Wanigasekara and Keast, 2006).
We found that the properties of growth cones and Sema3A responses differed in a number of ways between sympathetic and parasympathetic pelvic neurones. They exhibited structural differences, with neurites of sympathetic neurones having larger growth cones and more filopodia than those of parasympathetic neurones. The physiological implications of these differences are not known, but may reflect distinct signalling or growth mechanisms. These may also be reflected by differing kinetics of the Sema3A response in the two groups, where sympathetic neurones showed a more sustained response.
The most important distinguishing feature of sympathetic and parasympathetic neurones was their responses to cyclic nucleotides and the mechanisms of Sema3A-induced growth cone collapse (Figure 7). Because the effects of agonists or antagonists of cyclic nucleotide signalling were examined concurrently in both sympathetic and parasympathetic neurones from the same animal, we are confident that the differential responses do not reflect any experiment-specific failure of our chosen reagents. In parasympathetic neurones of both types (nitrergic and non-nitrergic), the Sema3A response was mediated by cAMP, and cGMP did not cause collapse; in contrast, in sympathetic neurones, the Sema3A response was mediated by cGMP and mimicked by cAMP. Therefore, a broader range of mediators (e.g. neurotrophic factors, neurotransmitters) that affect cyclic nucleotide signalling may promote growth cone collapse in sympathetic neurones. For example, the potent activator of guanylyl cyclase, nitric oxide [released by many pelvic parasympathetic terminals (Keast, 2006; Nangle and Keast, 2007)], could potentially stimulate growth cone collapse in nearby sympathetic axons. Moreover, the ratio of cAMP and cGMP may determine the nature of the response of each class of pelvic neurones, as seen in turning assays conducted on other neurone types (Song et al., 1998; Huber et al., 2003; Pasterkamp and Giger, 2009).
Figure 7.
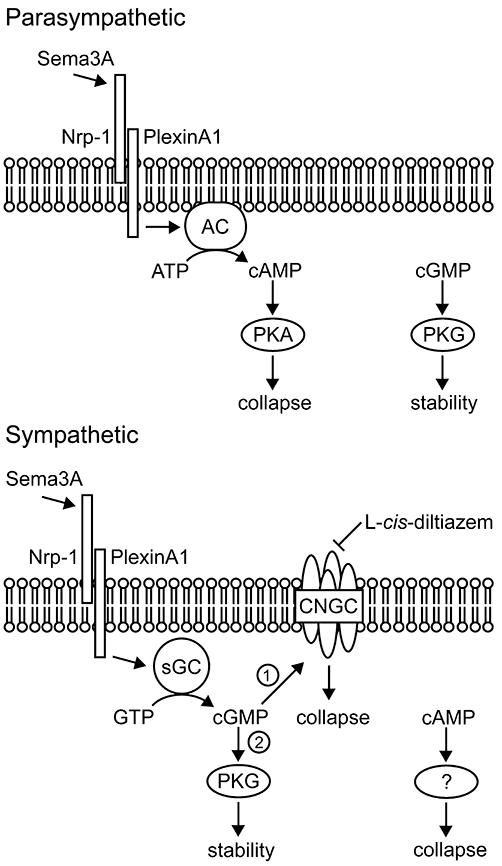
Proposed model for cyclic nucleotide-dependent signalling of growth cone collapse in adult autonomic neurones in response to Sema3A. In parasympathetic neurones, Sema3A binds to Nrp-1 and activates adenylyl cyclase, leading to the production of cAMP. In turn, cAMP activates PKA, which mediates growth cone collapse. However, cGMP may positively regulate growth cone dynamics independent of Sema3A signalling, since inhibition of PKG caused collapse. In sympathetic neurones, Sema3A binds Nrp-1 and stimulates cGMP production via soluble guanylyl cyclase. In turn, cGMP preferentially activates cyclic nucleotide-gated channels (CNGCs) (1), which mediate growth cone collapse. However, if CNGCs are inhibited, cGMP activates PKG (2), which does not cause collapse. Growth cone collapse is also caused by cAMP in sympathetic neurones, but this occurs independently of Sema3A and PKA signalling. AC, adenylyl cyclase; CNGCs, cyclic nucleotide-gated ion channel; Nrp-1, neuropilin-1; PKA, protein kinase A; PKG, protein kinase G; Sema3A, semaphorin 3A; sGC, soluble guanylyl cyclase.
Cyclic AMP mediated the Sema3A response in parasympathetic neurones but caused Sema3A-independent collapse in sympathetic neurones. Therefore, sympathetic neurones may be targeted by additional guidance factors. Moreover, it is possible that these two actions of cAMP differ in their downstream targets. In parasympathetic neurones, Sema3A actions were PKA-dependent, but this may not be the case in sympathetic neurones (although it is difficult to be certain of this because the PKA inhibitors alone caused collapse in these neurones). A number of recent studies have demonstrated that cAMP can signal via non-PKA targets in growth cones. For example, in embryonic rat commissural neurones, PKA is not required for cAMP-dependent netrin-1-induced growth cone attraction, although it regulates more subtle effects on turning behaviour (Moore and Kennedy, 2006). In sympathetic pelvic neurones, a PKA-independent response to cAMP could involve the exchange protein directly activated by cAMP (Epac), a guanine nucleotide exchange factor for the small G-protein, Rap1 (Kawasaki et al., 1998; de Rooij et al., 1998). In embryonic rat DRG neurones, Epac is responsible for PKA-independent attractive growth cone turning responses to netrin-1, but in early postnatal and adult neurones, cAMP production by netrin-1 causes PKA-dependent repulsive turning (Murray et al., 2009). Furthermore, in embryonic mouse olfactory neurones, cAMP is elevated in growth cones in response to stimulation of odorant receptors, and is coupled to non-selective (cAMP- and cGMP-responsive) ‘olfactory-type’ CNGCs (Maritan et al., 2009).
Cyclic GMP only caused growth cone collapse in sympathetic neurones, where it also mediated the response to Sema3A. Because PKG inhibitors caused growth cone collapse on their own, it is difficult to exclude PKG as a downstream target of cGMP. Irrespective, our studies provide evidence for CNGCs being required for the effects of Sema3A in this group of neurones. Although both CNGC inhibitors themselves promoted growth cone collapse, this treatment not only prevented further Sema3A-induced collapse but also restored growth cone collapse to control levels. We propose that in these neurones, Sema3A causes an increase in cGMP that activates CNGCs to cause collapse, and that if CNGCs are inhibited, cGMP promotes growth cone stability, possibly via PKG (Figure 7). A similar situation occurs in embryonic Xenopus spinal neurones, where cGMP-dependent (rod-type) CNGCs are responsible for the Ca2+ entry required to mediate repulsive growth cone turning in response to Sema3A (Togashi et al., 2008). In this study, Sema3A-mediated repulsion was switched to PKG-dependent attraction when CNGCs were inhibited (Togashi et al., 2008). Why Sema3A-induced elevation of cGMP predominantly affects CNGCs rather than PKG-dependent signalling in growth cones of Xenopus spinal or rat sympathetic neurones is not known, but different levels of CNGC expression, or cGMP production or compartmentalization, are plausible (Togashi et al., 2008).
Semaphorins can negatively influence axon regeneration, but could also limit inappropriate axon sprouting or redirect regenerating axons to their appropriate targets (Tang et al., 2007; Ziemba et al., 2008). For example, collaterals of injured DRG neurones selectively avoid Sema3A-secreting fibroblasts in spinal cord (Pasterkamp et al., 2001) and aberrant sprouting in the dorsal horn and hyperexcitability driven by nerve growth factor is attenuated by Sema3A (Cameron et al., 2006; Tang et al., 2007). Conversely, inhibition of Sema3A promotes regeneration after spinal cord injury (Kaneko et al., 2006). Very little is known about Sema3A in the adult autonomic nervous system, although cardiac overexpression of Sema3A is associated with reduced sympathetic innervation in mice (Ieda et al., 2007). Sema3A has also been strongly implicated as an inhibitory factor determining density of sympathetic innervation of blood vessels (Long et al., 2009), which may also be important in returning vascular supply after nerve damage or neuropathies. To our knowledge, there has been no previous study of semaphorins on parasympathetic autonomic neurones.
Pelvic ganglion neurones comprise both sympathetic and parasympathetic subtypes, and show diverse growth responses after injury (Keast, 2006) that could be modulated by guidance factors. For example, axotomy of the penile nerves in rats initiates growth of axon collaterals within the pelvic ganglion in parallel with slow regeneration of axons towards the target tissue (Palma and Keast, 2006; Nangle and Keast, 2007). Furthermore, after deafferentation, there is slow but restricted regeneration of spinal connections accompanied by growth of new axon collaterals within the denervated ganglion (Kepper and Keast, 1998; Keast, 2004). It is not known whether inhibitory guidance cues such as Sema3A impede regenerative efforts or exacerbate inappropriate plastic changes in the pelvic ganglia; however, modulation of Sema3A signalling pathways may provide an additional strategy to maximize return of function after injury. A better understanding of guidance factor expression and function in the adult may also benefit neurotrophic factor-driven therapeutic approaches to improve regeneration (Pasterkamp and Verhaagen, 2006; Ziemba et al., 2008), especially as Sema3A can inhibit neurotrophic factor effects in some neurones (Dontchev and Letourneau, 2002; Tang et al., 2004; Wanigasekara and Keast, 2006).
In conclusion, we propose that Sema3A is an important guidance factor for adult autonomic neurones, and that identification of differential Sema3A signalling between sympathetic and parasympathetic neurones may lead to selective therapeutic strategies to promote functional regeneration of pelvic organs or prevent aberrant reinnervation after injury. Our identification of parasympathetic neurones as a Sema3A target provides additional ways to modulate their function in development and adulthood.
Acknowledgments
We thank Dr Hui Yan for conducting pilot experiments and Prof Piers Emson (Babraham Institute, Babraham, UK) for NOS antibody raised in sheep. This work was supported by the National Health and Medical Research Council (Australia) Project Grant #570877 (to JK and MN) and Senior Research Fellowships #358709 and #632903 to JK.
Glossary
Abbreviations
- 8-Br-cGMP
8-bromoguanosine 3′,5′-cyclic monophosphate
- CNGC
cyclic nucleotide-gated ion channel
- DCB
2′,4′-dichlorobenzamil
- DDA
2′,5′-dideoxyadenosine
- FSK
forskolin
- KT-5720
(9R,10S,12S)-2,3,9,10,11,12-hexahydro-10-hydroxy-9-methyl-1-oxo-9,12-epoxy-1H-diindolo[1,2,3-fg:3′,2′,1′-kl]pyrrolo[3,4-i][1,6]benzodiazocine-10-carboxylic acid, hexyl ester
- KT-5823
(9S,10R,12R)-2,3,9,10,11,12-hexahydro-10-methoxy-2,9-dimethyl-1-oxo-9,12-epoxy-1H-diindolo[1,2,3-fg:3′,2′,1′-kl]pyrrolo[3,4-i][1,6]benzodiazocine-10-carboxylic acid, methyl ester
- LCD
L-cis-diltiazem
- NOS
nitric oxide synthase
- ODQ
1H-[1,2,4]oxadiazole[4,3-a]quinoxalin-1-one
- Nrp-1
neuropilin-1
- PKA
protein kinase A
- PKG
protein kinase G
- ROL (rolipram)
4-[3-(cyclopentyloxy)-4-methoxyphenyl]-2-pyrrolidinone
- Rp-8-Br-cGMPS
Rp-8-bromoguanosine-3′,5′-cyclic monophosphorothioate
- Rp-cAMPS
Rp-adenosine-3′,5′-cyclic monophosphorothioate
- Sema3A
semaphorin 3A
- SNP
sodium nitroprusside
- Sp-cAMPS
Sp-adenosine-3′,5′-cyclic monophosphorothioate
- TH
tyrosine hydroxylase
- VAChT
vesicular acetylcholine transporter
- ZAP (zaprinast)
1,4-dihydro-5-[2-propoxyphenyl]-7H-1,2,3-triazolo[4,5-d]pyrimidine-7-one
Conflicts of interest
None declared.
Supporting Information
Supporting Information: Teaching Materials; Figs 1–7 as PowerPoint slide.
References
- Anderson RB, Bergner AJ, Taniguchi M, Fujisawa H, Forrai A, Robb L, et al. Effects of different regions of the developing gut on the migration of enteric neural crest-derived cells: a role for Sema3A, but not Sema3F. Dev Biol. 2007;305:287–299. doi: 10.1016/j.ydbio.2007.02.020. [DOI] [PubMed] [Google Scholar]
- Behar O, Golden JA, Mashimo H, Schoen FJ, Fishman MC. Semaphorin III is needed for normal patterning and growth of nerves, bones and heart. Nature. 1996;383:525–528. doi: 10.1038/383525a0. [DOI] [PubMed] [Google Scholar]
- Cameron AA, Smith GM, Randall DC, Brown DR, Rabchevsky AG. Genetic manipulation of intraspinal plasticity after spinal cord injury alters the severity of autonomic dysreflexia. J Neurosci. 2006;26:2923–2932. doi: 10.1523/JNEUROSCI.4390-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DS, Regan AG, Lopez JS, Tannahill D, Harris WA, Holt CE. Semaphorin 3A elicits stage-dependent collapse, turning, and branching in Xenopus retinal growth cones. J Neurosci. 2001;21:8538–8547. doi: 10.1523/JNEUROSCI.21-21-08538.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dail WG, Manzanares K, Moll MA, Minorsky N. The hypogastric nerve innervates a population of penile neurons in the pelvic plexus. Neuroscience. 1985;16:1041–1046. doi: 10.1016/0306-4522(85)90114-9. [DOI] [PubMed] [Google Scholar]
- De Winter F, Vo T, Stam FJ, Wisman LA, Bar PR, Niclou SP, et al. The expression of the chemorepellent Semaphorin 3A is selectively induced in terminal Schwann cells of a subset of neuromuscular synapses that display limited anatomical plasticity and enhanced vulnerability in motor neuron disease. Mol Cell Neurosci. 2006;32:102–117. doi: 10.1016/j.mcn.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Dontchev VD, Letourneau PC. Nerve growth factor and semaphorin 3A signaling pathways interact in regulating sensory neuronal growth cone motility. J Neurosci. 2002;22:6659–6669. doi: 10.1523/JNEUROSCI.22-15-06659.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavazzi I, Stonehouse J, Sandvig A, Reza JN, Appiah-Kubi LS, Keynes R, et al. Peripheral, but not central, axotomy induces neuropilin-1 mRNA expression in adult large diameter primary sensory neurons. J Comp Neurol. 2000;423:492–499. [PubMed] [Google Scholar]
- He Z, Tessier-Lavigne M. Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell. 1997;90:739–751. doi: 10.1016/s0092-8674(00)80534-6. [DOI] [PubMed] [Google Scholar]
- Huber AB, Kolodkin AL, Ginty DD, Cloutier JF. Signaling at the growth cone: ligand-receptor complexes and the control of axon growth and guidance. Annu Rev Neurosci. 2003;26:509–563. doi: 10.1146/annurev.neuro.26.010302.081139. [DOI] [PubMed] [Google Scholar]
- Ieda M, Kanazawa H, Kimura K, Hattori F, Ieda Y, Taniguchi M, et al. Sema3a maintains normal heart rhythm through sympathetic innervation patterning. Nat Med. 2007;13:604–612. doi: 10.1038/nm1570. [DOI] [PubMed] [Google Scholar]
- Kaneko S, Iwanami A, Nakamura M, Kishino A, Kikuchi K, Shibata S, et al. A selective Sema3A inhibitor enhances regenerative responses and functional recovery of the injured spinal cord. Nat Med. 2006;12:1380–1389. doi: 10.1038/nm1505. [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Springett GM, Mochizuki N, Toki S, Nakaya M, Matsuda M, et al. A family of cAMP-binding proteins that directly activate Rap1. Science. 1998;282:2275–2279. doi: 10.1126/science.282.5397.2275. [DOI] [PubMed] [Google Scholar]
- Kawasaki T, Bekku Y, Suto F, Kitsukawa T, Taniguchi M, Nagatsu I, et al. Requirement of neuropilin 1-mediated Sema3A signals in patterning of the sympathetic nervous system. Development. 2002;129:671–680. doi: 10.1242/dev.129.3.671. [DOI] [PubMed] [Google Scholar]
- Keast JR. Visualization and immunohistochemical characterization of sympathetic and parasympathetic neurons in the male rat major pelvic ganglion. Neuroscience. 1995;66:655–662. doi: 10.1016/0306-4522(94)00595-v. [DOI] [PubMed] [Google Scholar]
- Keast JR. Remodelling of connections in pelvic ganglia after hypogastric nerve crush. Neuroscience. 2004;126:405–414. doi: 10.1016/j.neuroscience.2004.03.059. [DOI] [PubMed] [Google Scholar]
- Keast JR. Plasticity of pelvic autonomic ganglia and urogenital innervation. Int Rev Cytol. 2006;248:141–208. doi: 10.1016/S0074-7696(06)48003-7. [DOI] [PubMed] [Google Scholar]
- Keppel G, Wickens TD. Design and Analysis: A Researcher's Handbook. 4th edn. Upper Saddle River, NJ: Pearson Prentice Hall; 2004. [Google Scholar]
- Kepper ME, Keast JR. Specific targeting of ganglion cell sprouts provides an additional mechanism for restoring peripheral motor circuits in pelvic ganglia after spinal nerve damage. J Neurosci. 1998;18:7987–7995. doi: 10.1523/JNEUROSCI.18-19-07987.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JB, Jay SM, Segal SS, Madri JA. VEGF-A and Semaphorin3A: modulators of vascular sympathetic innervation. Dev Biol. 2009;334:119–132. doi: 10.1016/j.ydbio.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas CP, Trimbos JB, DeRuiter MC, van de Velde CJ, Kenter GG. Nerve sparing radical hysterectomy: latest developments and historical perspective. Crit Rev Oncol Hematol. 2003;48:271–279. doi: 10.1016/s1040-8428(03)00122-7. [DOI] [PubMed] [Google Scholar]
- Maritan M, Monaco G, Zamparo I, Zaccolo M, Pozzan T, Lodovichi C. Odorant receptors at the growth cone are coupled to localized cAMP and Ca2+ increases. Proc Natl Acad Sci USA. 2009;106:3537–3542. doi: 10.1073/pnas.0813224106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marko SB, Damon DH. VEGF promotes vascular sympathetic innervation. Am J Physiol Heart Circ Physiol. 2008;294:H2646–H2652. doi: 10.1152/ajpheart.00291.2008. [DOI] [PubMed] [Google Scholar]
- Marzioni D, Tamagnone L, Capparuccia L, Marchini C, Amici A, Todros T, et al. Restricted innervation of uterus and placenta during pregnancy: evidence for a role of the repelling signal Semaphorin 3A. Dev Dyn. 2004;231:839–848. doi: 10.1002/dvdy.20178. [DOI] [PubMed] [Google Scholar]
- Moore SW, Kennedy TE. Protein kinase A regulates the sensitivity of spinal commissural axon turning to netrin-1 but does not switch between chemoattraction and chemorepulsion. J Neurosci. 2006;26:2419–2423. doi: 10.1523/JNEUROSCI.5419-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray AJ, Tucker SJ, Shewan DA. cAMP-dependent axon guidance is distinctly regulated by Epac and protein kinase A. J Neurosci. 2009;29:15434–15444. doi: 10.1523/JNEUROSCI.3071-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nangle MR, Keast JR. Reduced efficacy of nitrergic neurotransmission exacerbates erectile dysfunction after penile nerve injury despite axonal regeneration. Exp Neurol. 2007;207:30–41. doi: 10.1016/j.expneurol.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Nangle MR, Keast JR. Deafferentation and axotomy each cause neurturin-independent upregulation of c-Jun in rodent pelvic ganglia. Exp Neurol. 2009;215:271–280. doi: 10.1016/j.expneurol.2008.10.012. [DOI] [PubMed] [Google Scholar]
- Nikulina E, Tidwell JL, Dai HN, Bregman BS, Filbin MT. The phosphodiesterase inhibitor rolipram delivered after a spinal cord lesion promotes axonal regeneration and functional recovery. Proc Natl Acad Sci USA. 2004;101:8786–8790. doi: 10.1073/pnas.0402595101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama M, von Schimmelmann MJ, Togashi K, Findley WM, Hong K. Membrane potential shifts caused by diffusible guidance signals direct growth-cone turning. Nat Neurosci. 2008;11:762–771. doi: 10.1038/nn.2130. [DOI] [PubMed] [Google Scholar]
- Palma CA, Keast JR. Structural effects and potential changes in growth factor signalling in penis-projecting autonomic neurons after axotomy. BMC Neurosci. 2006;7:41. doi: 10.1186/1471-2202-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasterkamp RJ, Giger RJ. Semaphorin function in neural plasticity and disease. Curr Opin Neurobiol. 2009;19:263–274. doi: 10.1016/j.conb.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasterkamp RJ, Verhaagen J. Semaphorins in axon regeneration: developmental guidance molecules gone wrong? Philos Trans R Soc Lond B Biol Sci. 2006;361:1499–1511. doi: 10.1098/rstb.2006.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasterkamp RJ, De Winter F, Holtmaat AJ, Verhaagen J. Evidence for a role of the chemorepellent semaphorin III and its receptor neuropilin-1 in the regeneration of primary olfactory axons. J Neurosci. 1998;18:9962–9976. doi: 10.1523/JNEUROSCI.18-23-09962.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasterkamp RJ, Anderson PN, Verhaagen J. Peripheral nerve injury fails to induce growth of lesioned ascending dorsal column axons into spinal cord scar tissue expressing the axon repellent Semaphorin3A. Eur J Neurosci. 2001;13:457–471. doi: 10.1046/j.0953-816x.2000.01398.x. [DOI] [PubMed] [Google Scholar]
- Penson DF, McLerran D, Feng Z, Li L, Albertsen PC, Gilliland FD, et al. 5-year urinary and sexual outcomes after radical prostatectomy: results from the prostate cancer outcomes study. J Urol. 2005;173:1701–1705. doi: 10.1097/01.ju.0000154637.38262.3a. [DOI] [PubMed] [Google Scholar]
- Poulin AN, Guerci A, Mestikawy SE, Semba K. Vesicular glutamate transporter 3 immunoreactivity is present in cholinergic basal forebrain neurons projecting to the basolateral amygdala in rat. J Comp Neurol. 2006;498:690–711. doi: 10.1002/cne.21081. [DOI] [PubMed] [Google Scholar]
- Quinn GP, Keough MJ. Experimental Design and Data Analysis for Biologists. Cambridge: Cambridge University Press; 2002. [Google Scholar]
- Reza JN, Gavazzi I, Cohen J. Neuropilin-1 is expressed on adult mammalian dorsal root ganglion neurons and mediates semaphorin3a/collapsin-1-induced growth cone collapse by small diameter sensory afferents. Mol Cell Neurosci. 1999;14:317–326. doi: 10.1006/mcne.1999.0786. [DOI] [PubMed] [Google Scholar]
- de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, et al. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature. 1998;396:474–477. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- Song H, Ming G, He Z, Lehmann M, McKerracher L, Tessier-Lavigne M, et al. Conversion of neuronal growth cone responses from repulsion to attraction by cyclic nucleotides. Science. 1998;281:1515–1518. doi: 10.1126/science.281.5382.1515. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Fournier A, Nakamura F, Wang LH, Murakami Y, Kalb RG, et al. Plexin-neuropilin-1 complexes form functional semaphorin-3A receptors. Cell. 1999;99:59–69. doi: 10.1016/s0092-8674(00)80062-8. [DOI] [PubMed] [Google Scholar]
- Tanelian DL, Barry MA, Johnston SA, Le T, Smith GM. Semaphorin III can repulse and inhibit adult sensory afferents in vivo. Nat Med. 1997;3:1398–1401. doi: 10.1038/nm1297-1398. [DOI] [PubMed] [Google Scholar]
- Tang XQ, Tanelian DL, Smith GM. Semaphorin3A inhibits nerve growth factor-induced sprouting of nociceptive afferents in adult rat spinal cord. J Neurosci. 2004;24:819–827. doi: 10.1523/JNEUROSCI.1263-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang XQ, Heron P, Mashburn C, Smith GM. Targeting sensory axon regeneration in adult spinal cord. J Neurosci. 2007;27:6068–6078. doi: 10.1523/JNEUROSCI.1442-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi M, Yuasa S, Fujisawa H, Naruse I, Saga S, Mishina M, et al. Disruption of semaphorin III/D gene causes severe abnormality in peripheral nerve projection. Neuron. 1997;19:519–530. doi: 10.1016/s0896-6273(00)80368-2. [DOI] [PubMed] [Google Scholar]
- Thompson SH. Cyclic GMP-gated channels in a sympathetic neuron cell line. J Gen Physiol. 1997;110:155–164. doi: 10.1085/jgp.110.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togashi K, von Schimmelmann MJ, Nishiyama M, Lim CS, Yoshida N, Yun B, et al. Cyclic GMP-gated CNG channels function in Sema3A-induced growth cone repulsion. Neuron. 2008;58:694–707. doi: 10.1016/j.neuron.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Tran TS, Kolodkin AL, Bharadwaj R. Semaphorin regulation of cellular morphology. Annu Rev Cell Dev Biol. 2007;23:263–292. doi: 10.1146/annurev.cellbio.22.010605.093554. [DOI] [PubMed] [Google Scholar]
- Wanigasekara Y, Keast JR. Neurturin has multiple neurotrophic effects on adult rat sacral parasympathetic ganglion neurons. Eur J Neurosci. 2005;22:595–604. doi: 10.1111/j.1460-9568.2005.04260.x. [DOI] [PubMed] [Google Scholar]
- Wanigasekara Y, Keast JR. Nerve growth factor, glial cell line-derived neurotrophic factor and neurturin prevent semaphorin 3A-mediated growth cone collapse in adult sensory neurons. Neuroscience. 2006;142:369–379. doi: 10.1016/j.neuroscience.2006.06.031. [DOI] [PubMed] [Google Scholar]
- Yan H, Keast JR. Neurturin regulates postnatal differentiation of parasympathetic pelvic ganglion neurons, initial axonal projections, and maintenance of terminal fields in male urogenital organs. J Comp Neurol. 2008;507:1169–1183. doi: 10.1002/cne.21593. [DOI] [PubMed] [Google Scholar]
- Ziemba KS, Chaudhry N, Rabchevsky AG, Jin Y, Smith GM. Targeting axon growth from neuronal transplants along preformed guidance pathways in the adult CNS. J Neurosci. 2008;28:340–348. doi: 10.1523/JNEUROSCI.3819-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


