Abstract
BACKGROUND AND PURPOSE
Pancreatitis represents a life-threatening inflammatory condition where leucocytes, cytokines and vascular endothelium contribute to the development of the inflammatory disease. The glucocorticoid-induced tumour necrosis factor (TNF) receptor family-related protein (GITR) is a costimulatory molecule for T lymphocytes, modulates innate and adaptive immune system and has been found to participate in a variety of immune responses and inflammatory processes. Our purpose was to verify whether inhibition of GITR triggering results in a better outcome in experimental pancreatitis.
EXPERIMENTAL APPROACH
In male GITR knock-out (GITR−/−) and GITR+/+ mice on Sv129 background, acute pancreatitis was induced after i.p. administration of cerulein. Other experimental groups of GITR+/+ mice were also treated with different doses of Fc-GITR fusion protein (up to 6.25 µg·mouse−1), given by implanted mini-osmotic pump. Clinical score and pro-inflammatory parameters were evaluated.
KEY RESULTS
A less acute pancreatitis was found in GITR−/− mice than in GITR+/+ mice, with marked differences in oedema, neutrophil infiltration, pancreatic dysfunction and injury. Co-treatment of GITR+/+ mice with cerulein and Fc-GITR fusion protein (6.25 µg·mouse−1) decreased the inflammatory response and tissue injury, compared with treatment with cerulein alone. Inhibition of GITR triggering was found to modulate activation of nuclear factor κB as well as the production of TNF-α, interleukin-1β, inducible nitric oxide synthase, nitrotyrosine, poly-ADP-ribose, intercellular adhesion molecule-1 and P-selectin.
CONCLUSIONS AND IMPLICATIONS
The GITR-GITR ligand system is crucial to the development of acute pancreatitis in mice. Our results also suggest that the Fc-GITR fusion protein could be useful in the treatment of acute pancreatitis.
Keywords: TNF receptor superfamily, fusion protein, pancreatitis, immunomodulation, anti-inflammatory treatment, costimulation, cytokines, endothelial function, GITR
Introduction
Acute pancreatitis is an inflammatory condition of the pancreas characterized by intra-acinar enzyme activation, leading to elevated levels of pancreatic enzymes in the blood, multiple organ dysfunction, activation of immune responses and inflammation (Malleo et al., 2008; Tamizhselvi et al., 2009). Interactions between leucocytes, soluble mediators (such as cytokines) and vascular endothelium contribute to the systemic progression of the inflammatory response, which determines disease severity and outcome. Notably, cytokines act as pro-inflammatory mediators in the pancreas, increasing vascular permeability and inducing thrombosis and haemorrhage, ultimately leading to tissue necrosis (Bhatia, 2002). Moreover, their increase correlates with life-threatening systemic manifestations which account for the majority of pancreatitis-associated mortality (Malleo et al., 2008).
An exaggerated systemic inflammatory response is a hallmark of severe acute pancreatitis with elevated circulating levels of tumour necrosis factor-alpha (TNF-α) and interleukin (IL)-1β (Dinarello, 1996). The primary role of TNF-α, produced by macrophages/monocytes, is to promote the inflammatory response and induce apoptotic cell death by binding to the TNF receptors, TNFR-1 and TNFR-2 (So et al., 2006). Both these receptors belong to the so-called TNF receptor superfamily (TNFRSF), which include other receptors such as Fas, CD40, CD27, and RANK and regulate diverse biological functions, including cell proliferation, differentiation and survival (Watts, 2005; So et al., 2006; Elgueta et al., 2009; Nolte et al., 2009; Strasser et al., 2009).
Classified as the 18th member of TNFRSF, the glucocorticoid-induced TNF receptor-related protein was cloned in a T-cell line treated with dexamethasone (Nocentini et al., 1997). The cytoplasm tail of this trans-membrane protein has similarities with that of other TNFRSF members, such as 4-1BB, OX40, CD27 and CD40 (Spinicelli et al., 2002). These molecules lack the death domain, which is required for the induction of apoptosis, and they mediate intracellular signalling by recruiting TNF receptor-associated factors (Watts, 2005).
Accumulating evidence suggests that T cell-mediated control of response against self contributes to the maintenance of natural immunological self-tolerance. Indeed, CD4+CD25+ regulatory T (Treg) cells are able to negatively modulate immune response. For instance, the depletion of CD4+CD25+ T cells leads to the spontaneous development of various autoimmune diseases in genetically susceptible animals. Glucocorticoid-induced TNF receptor family-related protein (GITR) is expressed in normal T lymphocytes, is up-regulated following T-cell activation and is constitutively expressed at high levels in CD4+CD25+ Treg cells (Nocentini et al., 1997; McHugh et al., 2002; Shimizu et al., 2002; Ronchetti et al., 2004; Gerli et al., 2009). GITR acts as a costimulatory molecule, contributing to activation of effector T lymphocytes and negatively controlling Treg cell regulatory activity (McHugh et al., 2002; Shimizu et al., 2002; Tone et al., 2003; Ronchetti et al., 2004; Nocentini et al., 2007) and is activated by its ligand (GITRL), which is expressed in antigen presenting cells, including macrophages and in endothelial cells (Kim et al., 2003; Yu et al., 2003; Krausz et al., 2007). Following GITR-GITRL interaction, GITRL also delivers signals to antigen-presenting cells (Lee et al., 2003; Agostini et al., 2005; Shin et al., 2005). In vivo studies suggest that GITR triggering exacerbates acute and chronic inflammatory responses not only due to T-cell control but also due to modulation of extravasation and innate immunity (Nocentini et al., 1997; 2007; Cuzzocrea et al., 2005; Suvas et al., 2005; Tamizhselvi et al., 2009). Moreover, recent evidence has shown that the treatment of wild type (GITR+/+) animals with Fc-GITR fusion protein ameliorates inflammation (Cuzzocrea et al., 2006; 2007;).
In the present study, we demonstrated that the genetic and pharmacological inhibition of GITR modified the development of acute pancreatitis.
Methods
Mice
Animal care was in compliance with regulations in Italy (D.M. 116192), Europe (O.J. of E.C. L 358/1 12/18/1986) and USA (Animal Welfare Assurance No A5594-01, Department of Health and Human Services, USA). The study was approved by the University of Messina Review Board for the care of animals. The animals were housed in a controlled environment and provided with standard rodent chow and water ad libitum. Male mice (4–5 weeks old, 20–22 g) with a targeted disruption of the glucocorticoid-induced TNFR-related gene (GITR-/-) and corresponding wild-type mice (GITR+/+) were obtained as previously described (Ronchetti et al., 2002).
Mini-osmotic pumps and implantation
Alzet pumps were used to deliver the fusion proteins at a constant rate. We used Alzet Model 2004 miniosmotic pumps (Charles River, Milan, Italy) implanted subcutaneously through a small incision in the skin between the scapulae, 3 h after the administration of cerulein. Using a hemostat, a small pocket was formed by spreading the subcutaneous connective tissues apart. The pump was inserted into the pocket with the flow moderator pointing away from the incision. The skin incision was closed with sutures. The pumping rate was 1 µL·h−1 (±0.15 µL·h−1) and the reservoir volume was 200 µL.
Induction of pancreatitis
Mice were randomly allocated into the following groups:
GITR+/++ cerulein group. Mice were treated hourly (×5) with cerulein (50 µg·kg−1, saline solution, i.p.) (n= 10).
GITR−/−+ cerulein group. Mice were treated hourly (×5) with cerulein (50 µg·kg−1, suspended in saline solution, i.p.) (n= 10).
GITR+/++ saline group. Sham-treated group in which identical treatments to the cerulein group were performed, except that the saline was administered instead of cerulein (n= 10).
GITR−/−+ saline group. Identical to GITR+/++ saline group, except for the use of mice with a targeted disruption of the GITR (GITR−/− mice) (n= 10).
GITR+/+ Cerulein + Fc-GITR protein group. Identical to the GITR+/+ cerulein group except for the administration of Fc-GITR protein (6.25, 3 or 1.5 µg·mouse−1) by mini-osmotic pump. Fc-GITR was purchased from Alexis (n= 10).
GITR+/+ Sham + Fc-GITR protein. Identical to GITR+/++ saline group except for the administration of Fc-GITR protein (6.25 µg·mouse−1) by mini-osmotic pump (n= 10).
Mice were killed by exsanguinations 24 h after the induction of pancreatitis. Blood samples were obtained by direct intracardiac puncture. The pancreas was removed immediately, frozen in liquid nitrogen and stored at −80°C until assayed. Portions of this organ were also fixed in formaldehyde for histological and immunohistochemical examination.
Morphological examination
Samples of pancreatic tissue were fixed for 24 h in 10% (w·v−1) phosphate-buffered saline (PBS)-buffered formaldehyde solution at room temperature, dehydrated using grade ethanol and embedded in Paraplast (Sherwood Medical, Mahwah, NJ, USA). The tissues were then embedded in paraffin and sectioned at 5 µm. Sections were then deparaffinized with xylene and stained with haematoxylin/eosin and silver impregnation for reticulum studies. The sections were then examined by an experienced histopathologist (without knowledge of the treatments) using light microscopy. Acinar-cell and pancreatic tissue injury/necrosis was quantified by morphometry as previously described (Dembinski et al., 2008). Ten randomly selected microscopic fields (×125) were chosen for each tissue sample and the extent of acinar cell injury/necrosis was expressed as the per cent of the total acinar tissue. The criteria for injury/necrosis were the following: (i) the presence of acinar cell ghosts or (ii) vacuolization and swelling of acinar cells and the destruction of the histoarchitecture of whole or parts of the acini, both of which had to be associated with an inflammatory reaction.
Determination of pancreatic oedema
The extent of pancreatic oedema was assayed by measuring tissue water content. For these latter measurements, a freshly obtained sample of pancreas was blotted dry, weighed and dried for 12 h at 95°C, and then weighed again. The difference between wet and dry tissue weight was calculated and expressed as a per cent of tissue wet weight.
Localization of nitro-tyrosine, poly ADP-ribose (PAR), P-selectin, intercellular adhesion molecule 1 (ICAM-1), inducible nitric oxide synthase (iNOS), Bax, Bcl-2, by immunohistochemistry
Pancreas tissues were fixed in 10% (w·v−1) PBS-buffered formaldehyde and 8 µm sections were prepared from paraffin-embedded tissues. After deparaffinization, endogenous peroxidase was quenched with 0.3% (v·v−1) hydrogen peroxide in 60% (v·v−1) methanol for 30 min. The sections were permeabilized with 0.1% (w·v−1) Triton X-100 in PBS for 20 min. Non-specific adsorption was minimized by incubating the section in 2% (v·v−1) normal goat serum in PBS for 20 min. Endogenous biotin- and avidin-binding sites were blocked by sequential incubation for 15 min with avidin and biotin respectively (DBA, Milan, Italy). Sections were incubated overnight with anti-GITR antibody (R&D Systems, Minneapolis, MN, USA; 1:100 in PBS, v·v−1), anti-nitrotyrosine antibody (Upstate Biotech, Saranac Lake, NY, USA) (1:500 in PBS, v·v−1), anti-PAR polyclonal antibody (1:500 in PBS, v·v−1) anti-P-selectin antibody (1:500 in PBS, v·v−1), mouse anti-rat ICAM-1 (CD54) antibody (1:500 in PBS, v·v−1) (DBA, Milan, Italy), anti-iNOS polyclonal antibody (1:500 in PBS, v·v−1), anti-Bax antibody (1:500 in PBS, v·v−1), anti-Bcl-2 antibody (1:500 in PBS, v·v−1). Specific labelling was detected with a biotin-conjugated goat anti-rabbit or goat anti-mouse IgG and avidin-biotin peroxidase complex (DBA, Milan, Italy). To confirm the binding specificity for nitrotyrosine, PAR, P-selectin, ICAM-1, iNOS, Bax and Bcl-2, some sections were also incubated with the only primary antibody or with the only secondary antibody. The counter stain was developed with diamino-benzidine (DAB; brown/black colour) and nuclear fast red (red background). A positive staining (brown/ black colour) was found in the sections, indicating that the immunoreactions were positive. To verify the binding specificity for PAR, P-selectin, ICAM-1, iNOS, Bax and Bcl-2, some sections were also incubated with primary antibody only (no secondary antibody) or with secondary antibody only (no primary antibody). In these situations, no positive staining was found in the sections indicating that the immunoreactions were positive in all the experiments carried out. To confirm the specificity of immunoreactions for nitrotyrosine, some sections were also incubated with the primary antibody (anti-nitrotyrosine) in the presence of excess nitrotyrosine (10 mM). Immunohistochemistry photographs (n= 5) were assessed by densitometry, using Optilab Graftek software (Milan, Italy).
Evaluation of GITR expression by real time PCR
To extract mRNA from pancreatic tissue, the pancreas was immediately placed in 200 µL of trizol LS reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) in 1.5 mL microfuge tube and homogenized using a Xenox Homogenizer (Karlsruhe, Germany). Then, 800 µL of trizol were added and total RNA was isolated according to the manufacturer's instructions. Conversion of total RNA (1 µg) to cDNA was performed with QuantiTect Reverse Transcription protocol (Qiagen, Valencia, CA, USA).
Real time PCR was performed with a Chromo 4 (MJ Research Bio-Rad, Milan, Italy) real time cycler using the specific FAM/MGB dye-labelled TaqMan probe GITR (Mm 00437136_m1). Gene expression was quantitated relative to the expression of endogenous control mouse GAPD (GAPDH) VIC/MGB probe amplified in the same tube of investigated genes. All probes were purchased from Applied Biosystem. All experiments were carried out in triplicate and the ΔΔCt method was used to determine expression of the genes of interest, as previously described (Livak and Schmittgen, 2001).
Subcellular fractionation, nuclear protein extraction and Western blot analysis for IκB-α, nuclear factor κB (NF-κB) p65, Bax and Bcl-2
Tissues were homogenized in cold lysis buffer A (HEPES 10 mM pH = 7.9, KCl 10 mM, EDTA 0.1 mM, EGTA 0.1 mM, dithiothreitol 1 mM, phenylmethyl suphonyl fluoride (PMSF) 0.5 mM, trypsin inhibitor 15 µg·mL−1, pepstatinA 3 µg·mL−1, leupeptin 2 µg·mL−1, benzamidine 40 µM). Homogenates were centrifuged at 13×g and the supernatant (cytosol + membrane extract) was collected to evaluate contents of IκB-α, Bax, Bcl-2 and β-actin. Buffer C (HEPES 20 mM, MgCl2 1.5 mM, NaCl 0.4 mM, EDTA 1 mM, EGTA 1 mM, dithiothreitol 1 mM, PMSF 0.5 µg·mL−1, leupeptin 2 µg·mL−1, benzamidine 40 µM, Nonidet P40 1%, glycerol 20%) was added to the cells; cells were then centrifuged at 16×g and the supernatant (nuclear extract) was collected to evaluate the content of NF-κB p65 and laminin B1 (Gharagozloo et al., 2010). Protein concentration in homogenate was determined by Bio-Rad Protein Assay (Bio-Rad, Richmond, CA, USA) and 50 µg of cytosol and nuclear extract from each sample were analysed. Proteins were separated by a 12% sodium dodecyl sulphate-polyacrylamide gel electrophoresis and transferred on PVDF membrane (Hybond-P Nitrocellulose, Amsherman Biosciences, UK). The membrane was blocked with 0.1% TBS-Tween containing 5% non-fat milk at room temperature for 1 h. Membranes were then incubated with the relative primary antibody overnight at 4°C: anti-IκB-α diluted 1:1000, anti-Bax diluted 1:500, anti-Bcl2 diluted 1:1000, anti-NF-κB p65 diluted 1:250, anti-β-actin 1:5000 (Santa Cruz Biotechnology, CA) and anti-Laminin B1. After the incubation, membranes were incubated for 1 h with peroxidase-conjugated anti-mouse or anti-rabbit secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) diluted 1:2000, and protein bands were detected with SuperSignal West Pico Chemioluminescent (PIERCE, Milan, Italy). Densitometric analysis was performed with a quantitative imaging system (ImageJ, Wayne Rasband., National Institute of Mental Health, USA).
Measurement of cytokines
Tumour necrosis factor-α and IL-1β levels were evaluated in plasma samples and samples of pancreas collected 24 h after cerulein injection. The tissues were homogenized in PBS containing 2 mmol·L−1 of PMSF (Sigma Chemical Co., Milan, Italy). The assay was carried out by using a commercial colorimetric kit (Diaclone Research Cell Science, Cedex, France), according to the manufacturer's instructions. All TNF-α and IL-1β determinations were performed in duplicate serial dilutions.
Biochemical assays
Serum amylase and lipase levels were measured 24 h after cerulein injection by a clinical laboratory. Results are expressed in international units per liter.
Myeloperoxidase activity
Myeloperoxidase activity, was determined as previously described (Genovese et al., 2006). Briefly, pancreas tissues were homogenized in a solution containing 0.5% hexa-decyl-trimethyl-ammonium bromide dissolved in 10 mM potassium phosphate buffer (pH 7) and centrifuged. An aliquot of the supernatant was then allowed to react with a solution of tetra-methyl-benzidine (1.6 mM) and 0.1 mM H2O2. The rate of change in absorbance was measured by a spectrophotometer at 650 nm. Myeloperoxidase activity was defined as the quantity of enzyme degrading 1 µmol of peroxide min−1 at 37°C and was expressed in units per gram weight of wet tissue.
Terminal deoxynucleotidyltransferase-mediated UTP nick-end labelling (TUNEL) assay
Terminal deoxynucleotidyltransferase-mediated UTP nick-end labelling assay was conducted by using a TUNEL detection kit according to the manufacturer's instructions (ApopTag, HRP kit DBA, Milan, Italy) as previously described (De Palma et al., 2009). Briefly, sections were incubated with 15 µg·mL−1 proteinase K for 15 min at room temperature and then washed with PBS. After endogenous peroxidase inactivation, sections were immersed in terminal deoxynucleotidyltransferase buffer containing deoxynucleotidyl transferase and biotinylated dUTP in terminal deoxynucleotidyltransferase buffer, incubated in a humid atmosphere at 37°C for 90 min, and then washed with PBS. After an incubation for 30 min with anti-horseradish peroxidase-conjugated antibody, signals were visualized with diaminobenzidine.
Data analysis
All the results were analysed by one-way anova followed by a Bonferroni post hoc test for multiple comparisons. A P-value of less than 0.05 was considered significant.
Materials
Unless otherwise stated, all compounds were obtained from Sigma-Aldrich Company (Milan, Italy). Primary monoclonal ICAM-1 antibodies (CD54) for immunohistochemistry were purchased by Pharmingen. Reagents and secondary and non-specific IgG antibody for immunohistochemical analysis were from Vector Laboratories Inc. (Milan, Italy). All other chemicals were of the highest commercial grade available. All stock solutions were prepared in non-pyrogenic saline (0.9% NaCl; Baxter Healthcare Ltd, Thetford, Norfolk, UK). The molecular target nomenclature conforms to Alexander et al. (2008).
Results
Reduced degree of acute pancreatitis in Fc-GITR-treated mice
Following intraperitoneal injection of the secretagogue cerulein in GITR+/+ mice, GITR staining was observed in pancreatic tissue sections, associated with infiltrating inflammatory cells (Figure 1), mostly neutrophils (Figure 1C2). In pancreatic sections from untreated GITR+/+ mice, GITR was not detectable by immunostaining (Figure 1A) and expressed at low levels, by real-time PCR (14.3 ± 3.8-fold less than activated T lymphocytes, used as positive control). Therefore, we determined if the inhibition of GITR triggering could decrease pancreatic inflammation.
Figure 1.
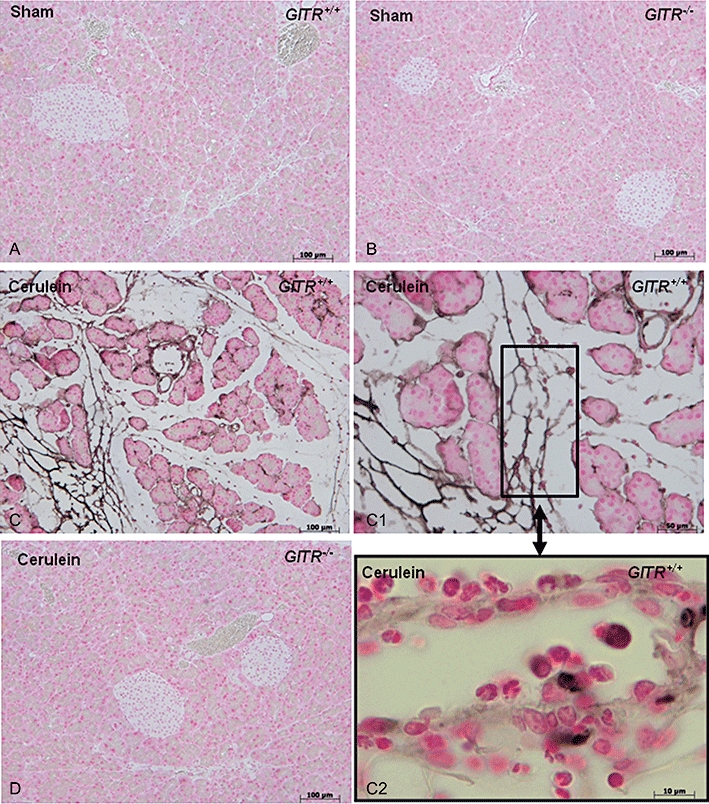
Expression of glucocorticoid-induced tumour necrosis factor receptor family-related protein (GITR) in the pancreas following cerulein treatment. Histological staining for GITR was absent in pancreatic sections from saline-treated (sham) GITR+/+ (A) and GITR−/− (B) or cerulein-treated GITR−/− mice (D). Only the sections from cerulein-treated GITR+/+ pancreas were positively stained for GITR (C, C1 and C2, at higher magnifications). Histological results shown are representative of two experiments performed in different experimental days.
In GITR+/+ mice, cerulein-induced pancreatitis was associated with a significant rise in serum levels of amylase (Figure 2A) and lipase (Figure 2B). These increases in amylase and lipase were markedly reduced in cerulein-treated GITR−/− mice.
Figure 2.
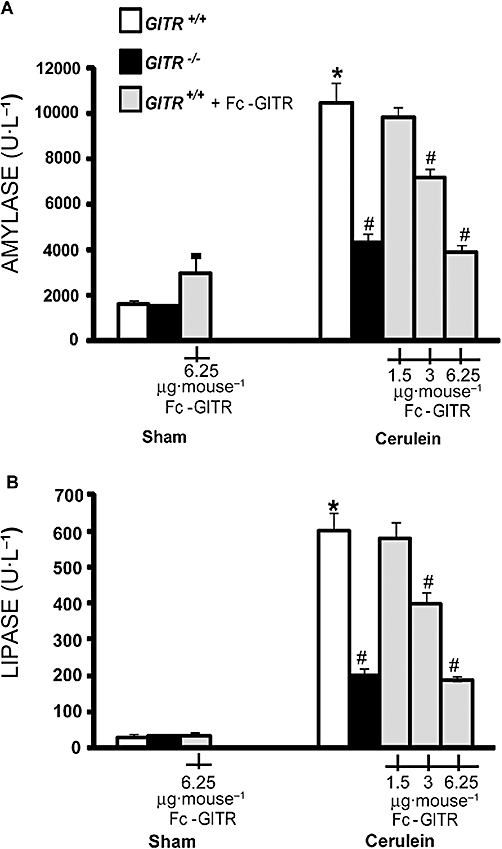
Effect of glucocorticoid-induced tumour necrosis factor receptor family-related protein (GITR) inhibition on cerulein-induced enzymic activity. Twenty-four hours after cerulein injection, GITR−/− mice and GITR+/+ mice, treated with Fc-GITR (at the different specified doses, by mini-osmotic pump), show lower levels of serum amylase (A) and lipase (B) in comparison with cerulein-treated GITR+/+ mice. Data from saline-treated (sham) mice are also reported. One representative experiment is shown. Data, are the mean ± SE of 10 mice each group. *P < 0.01 versus sham; #P < 0.01 versus cerulein-treated GITR+/+ mice.
Considering that the Fc-GITR. fusion protein ameliorates inflammation (Cuzzocrea et al., 2006; 2007;) by binding GITRL and inhibiting GITR activation (Tone et al., 2003; Ronchetti et al., 2007; O'Keeffe et al., 2008), we tested whether it would be useful also in controlling pancreatitis. To this aim, we administered a continuous infusion of Fc-GITR at 6.25 mg·mouse−1, as in previous studies (Cuzzocrea et al., 2006; 2007; Nocentini et al., 2008). In Fc-GITR/cerulein co-treated mice, the serum levels of amylase (Figure 2A) and lipase (Figure 2B) were similar to those observed in cerulein-treated GITR−/− mice. When lower doses of Fc-GITR were used, amylase (Figure 2A) and lipase (Figure 2B) were higher than those observed with the dose of 6.25 µg·mouse−1. The latter dose was used in the subsequent experiments. No elevation in the serum levels of amylase and lipase (Figure 2A and B, respectively) were observed in GITR+/+ or GITR−/− mice treated with vehicle (sham) and co-treated with Fc-GITR (6.25 µg·mouse−1).
Histological examination of pancreatic sections revealed tissue damage characterized by inflammatory cell infiltrates and acinar cell necrosis (Figure 3C) associated with reduction of collagen (Figure 3G). The extent and severity of the histological signs of pancreas injury were markedly reduced in cerulein-treated GITR−/− mice (Figure 3D and H) and in cerulein-/Fc-GITR-treated GITR+/+ mice (Figure 2E). No histological alterations (Figure 3A, B and F) were observed in GITR+/+ or GITR−/− mice treated with vehicle (sham). Histological scoring of acute pancreatitis (Table 1) and oedema (Figure 3I), further confirmed the role of GITR and the effect of Fc-GITR in the modulation of pancreatitis.
Figure 3.
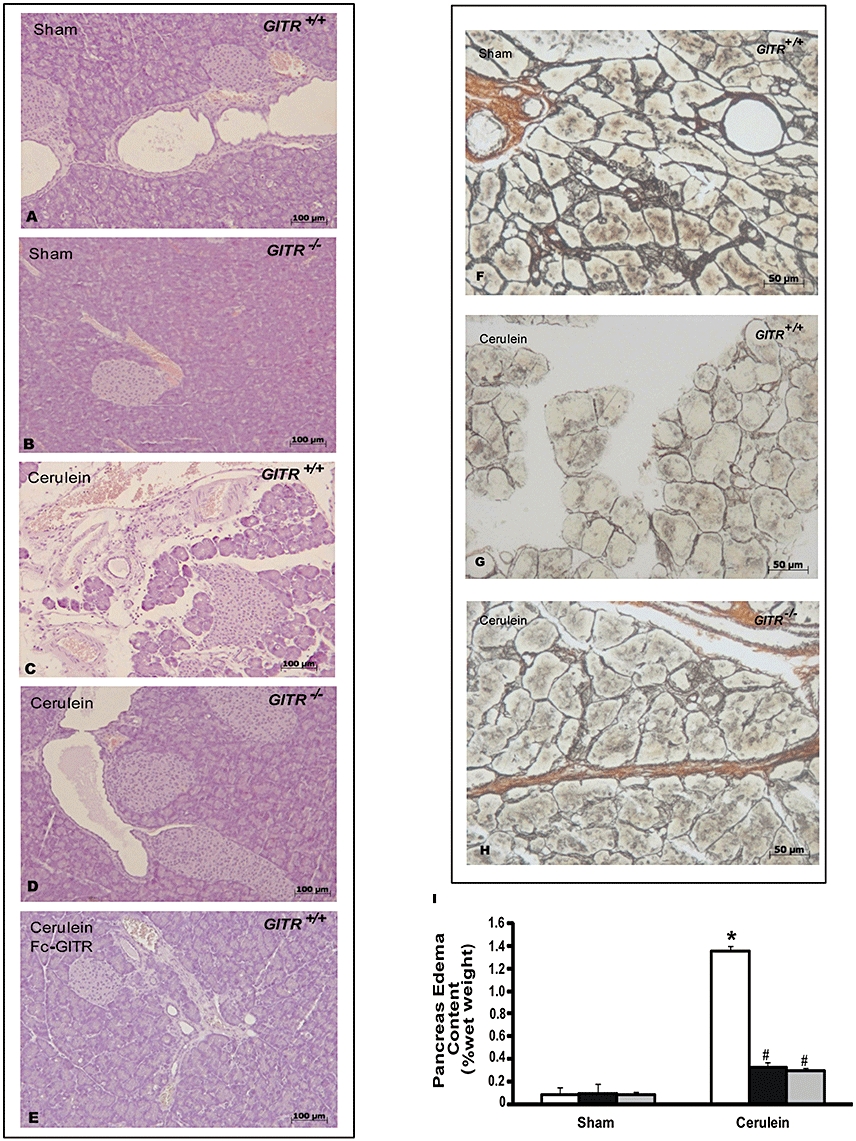
Effect of glucocorticoid-induced tumour necrosis factor receptor family-related protein (GITR) inhibition on cerulein-induced injury of the pancreas. Haemotoxylin and eosin stain (A–E panels): sections of pancreas from saline-treated (sham) GITR+/+ mice (A and B) show the normal architecture of the pancreas. On the contrary, sections of pancreas from GITR+/+ mice, show that 24 h after cerulein injection, marked inflammatory changes are present (C). Sections of pancreas from cerulein-treated GITR−/− and GITR+/+ mice co-treated with cerulein and Fc-GITR (6.25 µg·mouse−1, by mini-osmotic pump) show less pathological changes in the tissues (D and E respectively). Silver impregnation (F–H panels): in sham-treated mice (F) there was a normal presence of reticular and nervous fibres as well as connective tissues, while a significant alteration of the same tissues associated with a collagen reduction was observed in the pancreatic tissues of GITR+/+ mice (G). In the sections of pancreas from cerulein-injected GITR−/− mice, a significantly reduced alteration of reticular and nervous fibres as well as connective tissues was observed (H). Oedema following cerulein treatment is shown in panel I. Figure is representative of one out of three independent experiments.
Table 1.
Histological scoring of acute pancreatitis lesion
| Oedema | Inflammation | Necrosis | |
|---|---|---|---|
| GITR+/++ cerulein | 2.9 ± 0.1 | 3.4 ± 0.1 | 2.5 ± 0.1 |
| GITR−/−+ cerulein | 1.1 ± 0.1* | 1.0 ± 0.1* | 0.9 ± 0.1* |
| GITR+/++ cerulein + Fc-GITR | 1.2 ± 0.1* | 1.1 ± 0.2* | 1.0 ± 0.1* |
Data are means ± SE of 10 mice each group.
P < 0.01 versus cerulein-treated GITR+/+ mice.
GITR, glucocorticoid-induced tumour necrosis factor receptor family-related protein.
Absence of GITR reduces IκB-α degradation, NF-κB p65 activation and levels of TNF-α and IL-1β
To investigate the mechanisms by which the absence of GITR or its inactivation attenuated the development of cerulein-induced injury, we evaluated IκB-α degradation and nuclear NF-κB p65 translocation by Western blot analysis. A basal level of IκB-α was detected in the pancreatic tissues of sham-treated animals, whereas, in cerulein-treated GITR+/+ mice, IκB-α levels were substantially reduced (Figure 4A). This decrease in IκB-α levels induced by cerulein was prevented in cerulein-treated GITR−/−mice, with IκB-α levels similar to those of the sham group (Figure 4A). In addition, in the pancreatic tissues of cerulein-treated GITR+/+ mice, a significant increase of NF-κB p65 levels in the nuclear fraction was observed compared with the sham-treated mice (Figure 4B). Conversely, in GITR−/− mice, the level of nuclear NF-κB p65 was not increased following cerulein treatment (Figure 4B). To test whether GITR-GITRL interactions modulated the inflammatory process through the regulation of the secretion of cytokines, we analysed the plasma and pancreatic levels of the pro-inflammatory cytokines TNF-α and IL-1β after cerulein administration. In plasma and pancreatic samples collected from cerulein-treated GITR+/+ mice, both TNF-α (Figure 5A and B, respectively) and IL-1β formation (Figure 5C and D, respectively) were substantially increased, compared with sham-operated mice. Interestingly, in cerulein-treated GITR−/− mice as well as in cerulein/Fc-GITR co-treated GITR+/+ mice, levels of TNF-α (Figure 5A and B, respectively) and IL-1β (Figure 5C and D, respectively) were not significantly different from those of sham-treated mice.
Figure 4.
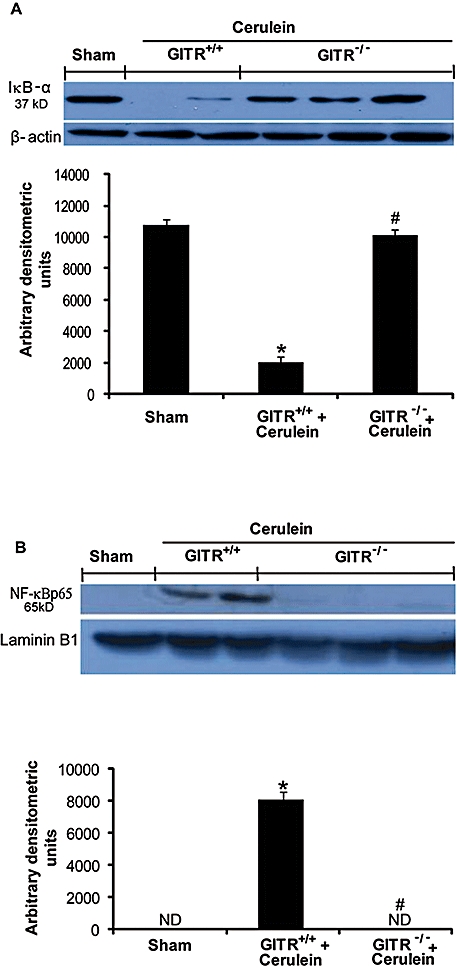
Effect of glucocorticoid-induced tumour necrosis factor receptor family-related protein (GITR) inhibition on cerulein-induced nuclear factor κB (NF-κB) activation in pancreas. Panels A: basal levels of inhibitor of kappa B-α (IκB-α) were detected by Western blot analysis in the homogenates of pancreatic tissues from saline-treated (sham) animals. IκB-α expression is low 24 h after cerulein administration, while it was not reduced in GITR−/− mice. β-actin was used as internal control. Panels B: levels of NF-κB p-65 subunit protein in the nuclear fractions of the pancreatic tissues were significantly increased by cerulein-administration compared to those in the sham-treated mice. The levels of NF-κB p-65 protein in the nuclear fractions of pancreatic tissues from GITR−/− mice were similar to that observed in sham-treated animals. Laminin B1 was used as internal control. Results shown in the lower half of panels A and B are expressed as mean ± SEM of the normalized densitometric analysis of five independent experiments. *P < 0.01 versus sham, #P < 0.01 versus cerulein-treated GITR+/+ mice.ND, not detectable.
Figure 5.
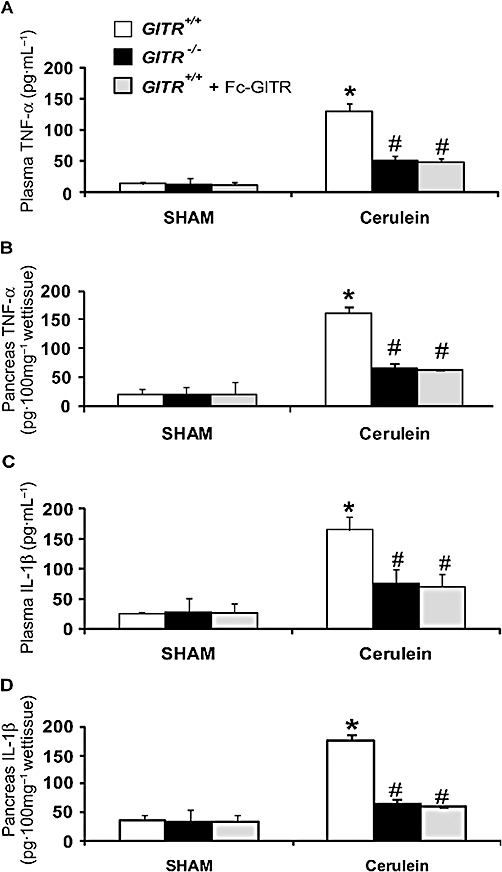
Effect of glucocorticoid-induced tumour necrosis factor (TNF) receptor family-related protein (GITR) inhibition on plasma and pancreatic tumour necrosis factor-α (TNF-α) and interleukin (IL)-1β levels. Twenty-four hours after cerulein injection an increase of plasma and pancreatic TNF-α (A and B, respectively) and IL-1β (C and D, respectively) levels was found in GITR+/+ mice when compared with GITR−/− and GITR+/+ mice that had been co-treated with Fc-GITR (6.25 µg/mouse of Fc-GITR, by mini-osmotic pump). No cytokine production was found in sham-operated mice. One representative experiment out of three is shown. Data are expressed as mean ± SE of 10 mice each group. *P < 0.01 versus sham; #P < 0.01 versus cerulein-treated GITR+/+ mice.
Reduced adhesion molecule expression and neutrophil infiltration in GITR−/− mice
A hallmark of acute pancreatitis is the accumulation of neutrophils in the pancreas, which augments tissue damage (Cuzzocrea et al., 2004). Therefore, we evaluated the expression of the adhesion molecules P-selectin and ICAM-1, which play a pivotal role in rolling and firm attachment of neutrophils to the endothelium. Assessment of neutrophil infiltration into the pancreas was also performed by measuring the activity of myeloperoxidase, an enzyme that is contained in (and specific for) neutrophil lysosomes.
Immunostaining for ICAM-1 (Figure 6A–D), P-selectin (Figure 6E–H) or myeloperoxidase activity (Figure 6I) were significantly enhanced in the pancreas samples collected from GITR+/+ mice and markedly reduced in pancreas from cerulein-treated GITR−/− mice after cerulein administration (Figure 6C, G and I respectively). Similarly, ICAM-1 and P-selectin staining as well as myeloperoxidase activity were significantly reduced in pancreatic tissues from cerulein-/Fc-GITR-treated GITR+/+ mice (Figure 6D, H and I respectively).
Figure 6.
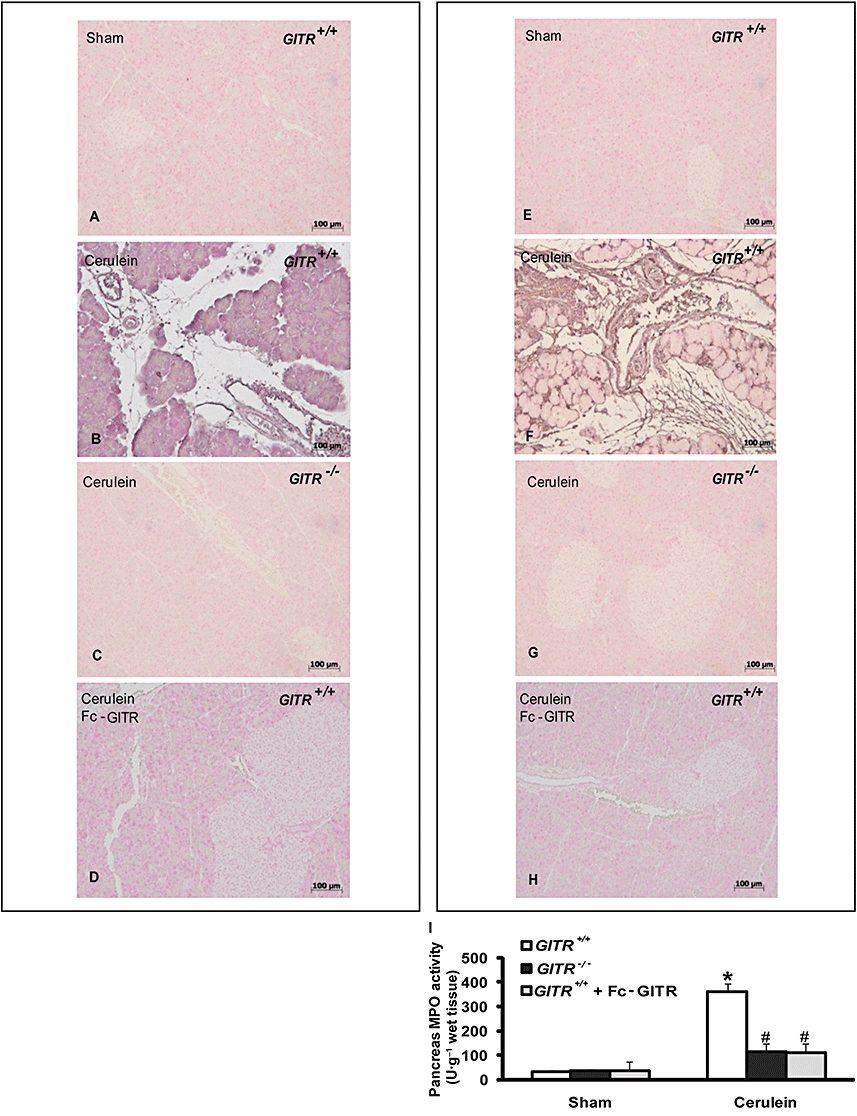
Effect of glucocorticoid-induced tumour necrosis factor receptor family-related protein (GITR) inhibition on expression of the adhesion molecules intercellular adhesion molecule 1 (ICAM-1) and P-selectin. Twenty-four hours after cerulein injection, immunohistochemical localization of ICAM-1 and P-selectin in pancreas of GITR+/+ mice showed a positive staining for both ICAM-1 (B) and P-selectin (F), when compared with sham-operated mice (A and E respectively). There was no detectable immunostaining for ICAM-1 and P-selectin in pancreas of cerulein-treated GITR−/− mice (C and G, respectively) and cerulein/Fc-GITR (6.25 µg/mouse of Fc-GITR, by mini-osmotic pump) co-treated GITR+/+ mice (D and H respectively). Photographs are representative of one out of three independent experiments (including five mice in each group). Panel I: myeloperoxidase activity in pancreatic samples of cerulein-treated GITR+/+ mice was significantly increased compared to sham-treated mice. Cerulein-treated GITR−/− mice and cerulein/Fc-GITR (6.25 µg·mouse−1 of Fc-GITR, by mini-osmotic pump) co-treated GITR+/+ mice showed a significantly decrease of MPO activity in pancreas (C). One representative experiment out of three is shown. Data are expressed as mean ± SE of 10 mice each group. *P < 0.01 versus sham; #P < 0.01 versus cerulein-treated GITR+/+ mice.
In samples of pancreas obtained from sham-treated GITR+/+ and GITR−/− mice, no myeloperoxidase activity (Figure 6I) and no positive staining for either P-selectin or ICAM-1 were observed (Figure 6A and E respectively).
Pancreatic tissue of GITR−/− mice shows a reduced iNOS expression, nitrosative stress and poly ADP-ribose polymerase (PARP) activation, measured by immunohistochemical methods
Inducible NOS expression was evaluated in sections of pancreas by immunohistochemical analysis. The samples obtained from sham-treated mice did not stain for iNOS (Figure 7A), whereas pancreatic tissue sections obtained from cerulein-treated GITR+/+ mice exhibited positive staining for iNOS (Figure 7B). Conversely, samples from GITR−/− (Figure 7C) and cerulein-/Fc-GITR-treated GITR+/+ mice (Figure 7D) showed a lower degree of iNOS staining.
Figure 7.
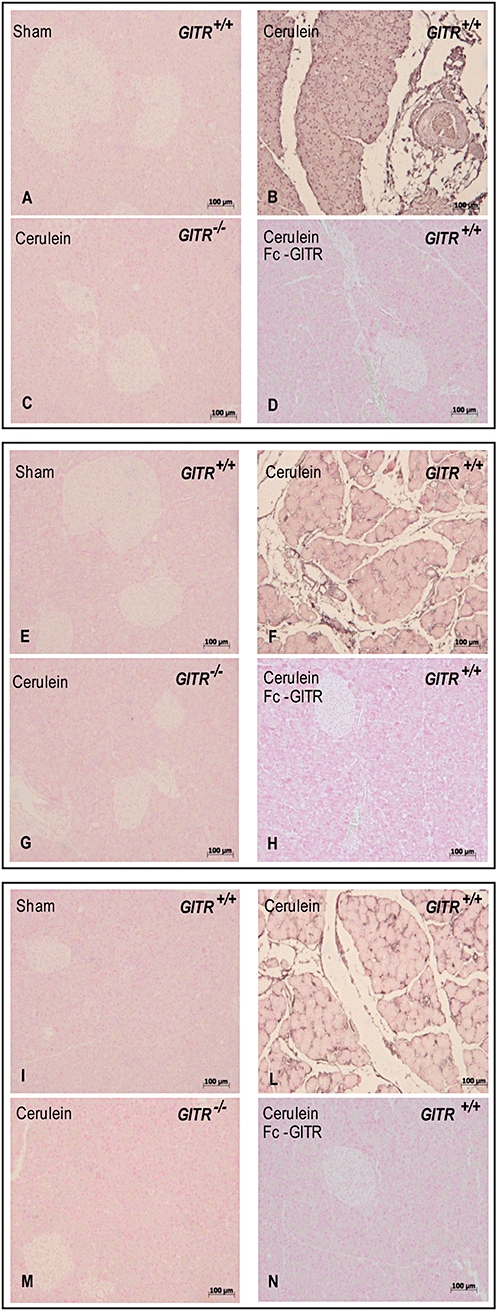
Effect of glucocorticoid-induced tumour necrosis factor receptor family-related protein (GITR) inhibition on inducible nitric oxide synthase (iNOS) expression, nitrosative stress and poly ADP-ribose polymerase activation. Twenty-four hours after cerulein injection, immunohistochemical localization of iNOS (A–D) nitrotyrosine (E–H) and poly ADP-ribose (PAR) (I–N) in the pancreatic tissue of GITR+/+ mice showed a positive iNOS (B) nitrotyrosine (F) and PAR (L) staining, when compared with sham operated mice (A, E and I respectively). After cerulein administration, in the pancreatic tissue of GITR−/− mice and Fc-GITR co-treated (6.25 µg·mouse−1, by mini-osmotic pump) GITR+/+ mice, there was no detectable immunostaining for iNOS (C and D, respectively), nitrotyrosine (G and H, respectively) and PAR (M and N respectively). One representative experiment out of three is shown.
To evaluate the amount of peroxynitrite formation and other nitrogen derivatives produced during cerulein-induced acute pancreatitis, nitrotyrosine, a specific marker of nitrosative stress, was measured by immunohistochemical analysis in sections of pancreas tissues, using a specific anti-nitrotyrosine antibody. The samples obtained from sham-treated mice showed no positive staining for nitrotyrosine (Figure 7E), while sections from cerulein-induced GITR+/+ mice exhibited positive staining for nitrotyrosine in pancreas (Figure 7F). Much less nitrotyrosine staining was found in the pancreas both of cerulein-treated GITR−/− mice (Figure 7G) and cerulein-/Fc-GITR-treated GITR+/+ mice (Figure 7H).
Sections of pancreas were also taken after cerulein administration to determine the activation of the nuclear enzyme PARP which has been implicated in the pathogenesis of acute pancreatitis. PARP activation was evaluated by assessing the presence of PAR, using an immunohistochemical approach. A positive staining for PAR was localized in sections of pancreas obtained from cerulein-treated GITR+/+ mice (Figure 7L). The samples from GITR−/− mice (Figure 7M) and cerulein-/Fc-GITR-treated GITR+/+ (Figure 7N) showed a reduction of the degree of positive staining for PAR in pancreas. No positive staining for PAR was found in tissues from sham-treated mice (Figure 7I).
Reduced apoptosis and modulation of apoptosis-related genes in pancreatic tissue of GITR−/− mice
The presence of apoptotic cells in pancreatic tissue is a measure of damage consequent to the inflammatory process (Gukovskaya and Pandol, 2004). TUNEL assay showed the presence of apoptotic cells in pancreatic sections obtained from cerulein-treated GITR+/+ mice (Figure 8A and E). By contrast, no positive TUNEL staining was found in tissue sections of cerulein-treated GITR−/− mice (Figure 8C and E) as well as in cerulein-/Fc-GITR-treated GITR+/+ (Figure 8D and E). As expected, no positive TUNEL staining was observed in pancreatic tissues of GITR−/− and GITR+/+ sham-treated mice (Figure 8A and E).
Figure 8.
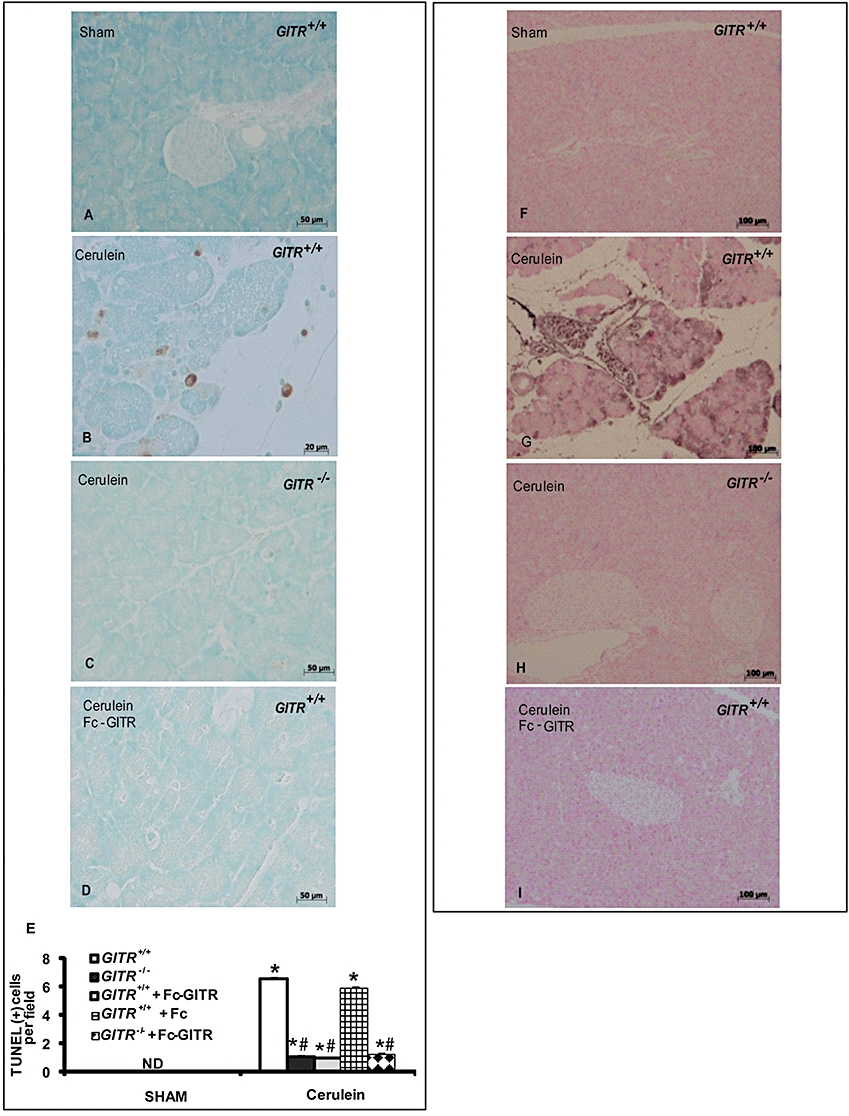
Effect of glucocorticoid-induced tumour necrosis factor receptor family-related protein (GITR) inhibition on apoptosis and S100 protein expression in pancreatic tissue. Twenty-four hours after cerulein administration, Terminal deoxynucleotidyltransferase-mediated UTP nick-end labelling (TUNEL) staining (A–E) showed a marked appearance of dark brown apoptotic cells and intercellular apoptotic fragments in pancreatic tissue of GITR+/+ mice (B, see cell count E), when compared with sham animals (A, see cell count E). In the pancreatic tissue of GITR−/− mice (C, see cell count E) and Fc-GITR co-treated (6.25 µg·mouse−1, by mini-osmotic pump) GITR+/+ mice (D, see cell count E), the number of dark brown cells was significantly reduced. One representative experiment out of three is shown. Data are expressed as number of cells per field (n= 10) and as mean ± SE. *P < 0.01 versus Sham; #P < 0.01 versus cerulein-group. ND, not detectable. Immunohistochemical localization of S100 protein (F–I) in the pancreatic tissue of GITR+/+ mice 24 h after cerulein injection showed a positive staining (G), when compared with sham operated mice (F). No detectable immunostaining in the pancreatic tissue of GITR−/− mice (H) and Fc-GITR-treated (6.25 µg·mouse−1, by mini-osmotic pump) GITR+/+ mice (I) was found. One representative experiment out of three is shown.
To confirm the apoptosis levels in our samples, we used immunohistochemical staining for S-100 protein, another marker of the apoptotic process, and revealed an increased level in cerulein-treated GITR+/+ mice (Figure 8G) as compared with cerulein-treated GITR−/− mice (Figure 8H) or cerulein-/Fc-GITR-treated GITR+/+ mice (Figure 8I). Sham-operated mice showed no staining for S-100 protein (Figure 8F).
We also investigated the expression of the pro-apoptotic molecule Bax and of the anti-apoptotic molecule Bcl-2, by Western blot of the pancreatic homogenates. The levels of Bax detected in pancreatic tissues from cerulein-treated GITR+/+ mice were much higher when compared with that detected GITR−/− mice (Figure 9A). Moreover, Bcl-2 was almost absent in samples from cerulein-treated GITR+/+ mice and present in GITR−/− mice (Figure 9F). Differences in Bax and Bcl-2 were also confirmed by immunohistochemical staining. Tissues from sham-treated mice did not stain for Bax (Figure 9B), whereas pancreatic sections obtained from cerulein-treated mice exhibited positive staining for Bax (Figure 9C). Deletion of GITR (Figure 9D) and treatment of GITR+/+ animals with Fc-GITR (Figure 9E) reduced the degree of positive staining for Bax in the pancreas of mice subjected to cerulein-induced pancreatitis. Moreover, pancreatic sections from sham-treated mice showed positive staining for Bcl-2 (Figure 9G), whereas in cerulein-treated mice Bcl-2 staining was significantly reduced (Figure 8H). GITR−/− (Figure 9I) and cerulein/Fc-GITR co-treated GITR+/+ mice (Figure 9L) showed a less pronounced loss of positive staining for Bcl-2 following cerulein-induced pancreatitis.
Figure 9.
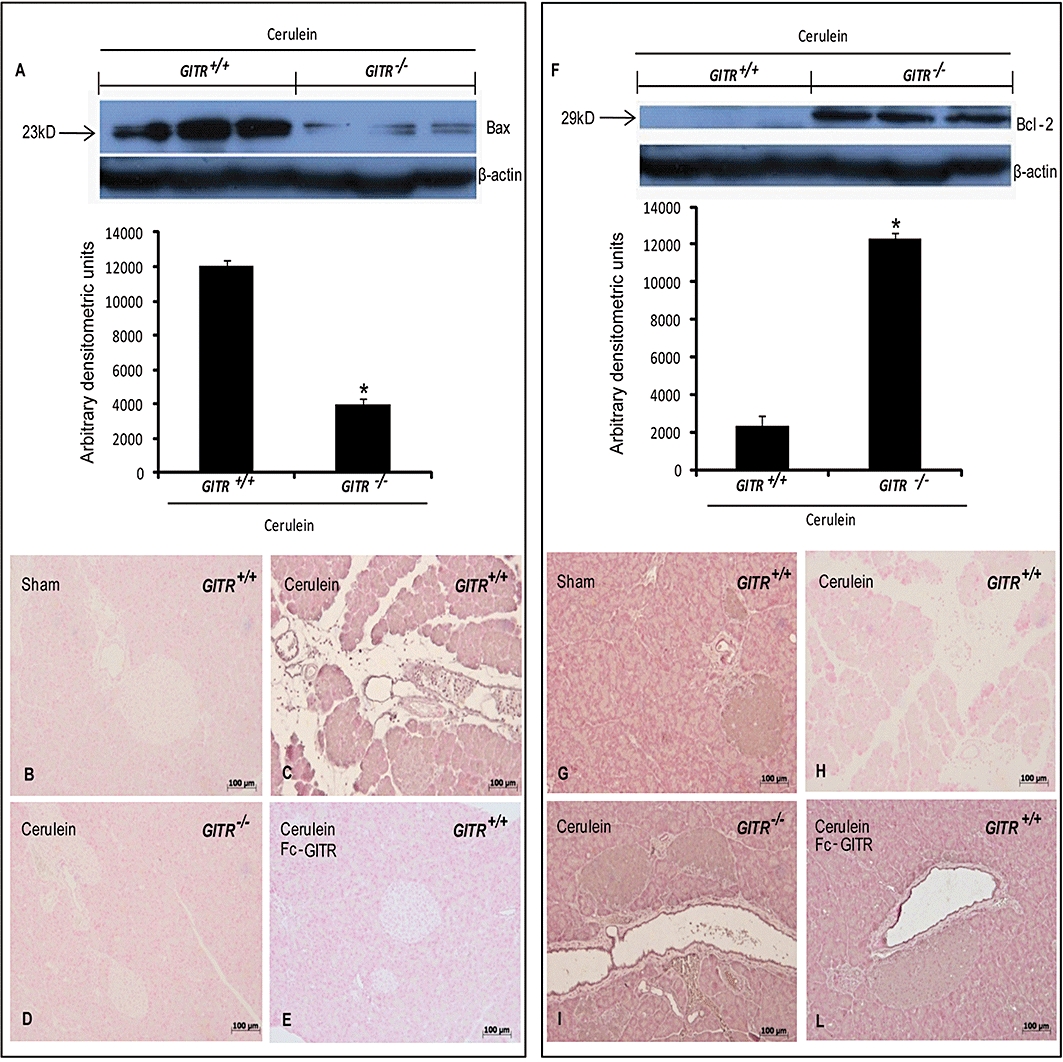
Effect of glucocorticoid-induced tumour necrosis factor receptor family-related protein (GITR) inhibition on pancreatic expression of Bcl-2 family members. Bax and Bcl-2 expression levels were detected in pancreatic samples 24 h after cerulein injection by Western blot. A significant increase of Bax expression was detected in GITR+/+ pancreatic tissue, whereas Bax levels were substantially reduced in GITR−/− mice (A). Bcl-2 expression was not observed in pancreas of GITR+/+ mice, while was more evident in the pancreatic tissue from cerulein-treated GITR−/− mice (F). Results shown in the lower half of panels A and F are expressed as mean ± SEM of the normalized densitometric analysis of five independent experiments. *P < 0.01 versus cerulein-treated GITR+/+ mice. Bax and Bcl-2 expression in pancreas was also evaluated by immunohistochemical analysis. Twenty-four hours after cerulein injection, positive Bax staining was found in pancreas of GITR+/+ mice (C). No positive staining for Bax was detected in pancreas of sham-treated GITR+/+ mice (B) cerulein-treated GITR−/− mice (D) and cerulein/Fc-GITR co-treated (6.25 µg·mouse−1 of Fc-GITR, by mini-osmotic pump) GITR+/+ mice (E). No positive staining for Bcl-2 was observed in pancreas of cerulein-treated GITR+/+ mice (G). A positive staining for Bcl-2 was observed in pancreas from sham-treated GITR+/+ mice (F), cerulein-treated GITR−/− mice (H) and cerulein/Fc-GITR (6.25 µg·mouse−1 of Fc-GITR, by mini-osmotic pump) co-treated GITR+/+ mice (I). One representative experiment out of three is shown.
Discussion
We and others have previously shown, in a number of experimental models of disease, that GITR plays an important role in regulating immune/inflammatory response (Ronchetti et al., 2004; Suvas et al., 2005; Cuzzocrea et al., 2006; 2007; Nocentini and Riccardi, 2009). We here show that the GITRL/GITR system modulates cerulein-induced acute pancreatitis.
Pancreatitis is a severe disease that rapidly leads to the activation of inflammatory processes, which entail the production of mediators and activation of mechanisms that cause an exacerbation of the inflammatory response and serious complications. The consequence of the acinar cell damage is the local activation of the immune/inflammatory components including dendritic cells, macrophages, fibroblasts and T cells as well as fibroblast and endothelial cells (Granger and Remick, 2005). To determine whether Fc-GITR could be useful to improve pancreatitis outcome, we treated mice with cerulein-induced acute pancreatitis with Fc-GITR. Moreover, to confirm the role of GITRL/GITR system and study the mechanism of action of Fc-GITR, we compared the clinical and histological outcome of pancreatitis in GITR−/− mice and control mice. Our results confirmed that Fc-GITR was useful in treating acute pancreatitis of the mice, at a dose of 6.25 µg·mouse−1.
To establish the main mechanism of action by which Fc-GITR exerts its activity, we should take into account that when the fusion protein Fc-GITR binds GITRL, it can induce at least two effects: inhibition of GITR triggering by its naturally expressed ligand and activation of GITRL. Inhibition of GITR triggering has critical effects to several cells of the immune system. In fact, GITR stimulates activation of CD4+ and CD8+ T lymphocytes and inhibits T regulatory suppressor activity (Tone et al., 2003; Ronchetti et al., 2004; 2007; Cho et al., 2009). Moreover, GITR modulates dendritic cell activity (Ronchetti et al., 2010) and the function of other cells of the innate immune system (Kim et al., 2006; Nocentini and Riccardi, 2009). The inhibition of each of these mechanisms may contribute to protection derived from Fc-GITR treatment, but CD4+ T-cell stimulation may be crucial in this case, as CD4+ T cells play a pivotal role in acute pancreatitis (Demols et al., 2000). The same mechanisms are operative in the protection of GITR−/− mice from cerulein-induced pancreatitis. Immunohistochemistry demonstrated that GITR was not expressed in the pancreatic tissue, while real time PCR showed that it was expressed at very low level. This discrepancy may be due to the lower sensitivity of the immunohistochemistry than real time PCR and also to the blood perfusing pancreas and including GITR positive cells. If GITR is expressed in the parenchyma, another mechanism of action of Fc-GITR might be the modulation of the response to inflammation by pancreatic cells.
GITRL triggering has been demonstrated to have tolerogenic effects in some models of inflammation through an action on dendritic cells (Cuzzocrea et al., 2005; 2006;). Therefore, the effect of Fc-GITR treatment might be attributed, at least in part, to this mechanism. However, two findings argue against this additional mechanism of action. Firstly, in GITR−/− mice, which are protected by cerulein-induced pancreatitis, GITRL triggering is not effective. Secondly, the development of pancreatitis is quite rapid and the involvement of dendritic cells may be marginal in the first hours. Furthermore, in macrophages, GITRL activation leads to the production of pro-inflammatory mediators such as IL-1α, IL-8, TNF-α, monocyte chemotactic protein 1, CCL2, iNOS and matrix metalloproteinase-9 (Shin et al., 2002; Bae et al., 2008). However, none of these effects was seen in Fc-GITR-treated mice. Therefore, it is reasonable that in this as well as other inflammatory models (Agostini et al., 2005), the pro-inflammatory effects of GITR triggering overcome the effects deriving from GITRL triggering (tolerogenic and/or pro-inflammatory). As a consequence, it seems that the protective effects of Fc-GITR fusion protein in cerulein-treated GITR+/+ mice are due to inhibition of GITR triggering more than to GITRL triggering. This Fc-GITR mechanism of action has been recently put forward by two other studies (Nocentini et al., 2008; Kim et al., 2010). Alternatively, it may be hypothesized that the Fc-GITR we have used inhibits GITR triggering but does not activate GITRL.
The transcription factor NF-κB, a key regulator of cytokine induction (Kim et al., 2000), plays a critical role in the pathogenesis of cerulein-induced acute pancreatitis by regulating the expression of many pro-inflammatory genes (Rakonczay et al., 2003). We have found here that after cerulein administration, inhibition of GITR activation reduced the levels of IκB-α degradation and NF-κB translocation. As NF-κB stimulates the release of proinflammatory mediators, we evaluated plasma and tissue levels of the production of such cytokines. The presence of IL-1β and TNF-α is characteristic of an ongoing inflammatory process and their serum and in situ levels correlate with the degree of inflammation. Our results revealed that mice with genetic and pharmacological inhibition of GITR triggering show a decreased production of pro-inflammatory mediators.
In agreement with the increased cytokine production during cerulein-induced pancreatitis, we also found, by immunohistochemistry, a greater expression of P-selectin and ICAM-1, endothelial adhesion molecules, that play a pivotal role in the rolling and firm attachment of neutrophils to endothelium. By contrast, we demonstrated significant reduction in the expression of P-selectin and ICAM-1 in cerulein-treated GITR−/− mice and cerulein/Fc-GITR co-treated GITR+/+ mice compared with cerulein-treated GITR+/+ mice. In the same samples, the consequently reduced neutrophilic infiltration was confirmed by lower levels of myeloperoxidase enzyme. Ongoing studies are further analysing the role of GITRL/GITR system on extravasation during the inflammatory response.
Peroxynitrite causes single-strand breaks of DNA (Inoue and Kawanishi, 1995) thus activating the nuclear enzyme poly(ADP-ribose) synthetase, the depletion of intracellular NAD and ATP pools and ultimately cell death (Zingarelli et al., 1996). Moreover, there is evidence that the activation of PARP may also play an important role in inflammation (Szabo and Dawson, 1998). Therefore, we analysed PAR expression, demonstrating that its presence was also reduced under genetic or pharmacological inhibition of GITR triggering, consistent with a lower degree of nuclear injury and cell death, suggesting that GITR-related modulation of the inflammatory process is due, at least in part, to inhibition of PARP activation. Notably, our results provide the first evidence that GITR regulates oxidative stress and the poly(ADP-ribose) synthetase pathway, which are activated during experimental pancreatitis.
Finally, acute pancreatitis causes the appearance of the macroscopic signs of injury, showing large areas of necrotic tissue, as a consequence of the activation of apoptotic pathways, activated by different receptors including TNFRSF members (Wallach et al., 1998). In particular, it has been demonstrated that GITR and CD27 bind and activate Siva (Spinicelli et al., 2002; Krausz et al., 2007), a proapoptotic protein that contains a death domain and interacts with TNF receptor-associated factor 2 and the anti-apoptotic protein Bcl-xL, leading to cell death (Xue et al., 2002; Barkinge et al., 2009; Gudi et al., 2009). We analysed the relation between GITR and apoptosis in this model by TUNEL assay and by evaluating the expression of S100 protein, Bax and Bcl-2. Results demonstrated that GITR activation was involved in modulation of apoptosis, possibly through Siva activation.
Blocking CD40–CD40 ligand or B7-CD28 costimulatory pathways has no effect on the severity of pancreatitis (Demols et al., 2000) and this is in contrast with the results here shown concerning the GITRL-GITR costimulatory pathway. These differing effects may have a variety of causes, including the fact that GITR triggering is more crucial for CD8+ T-cell activation than CD28 (Ronchetti et al., 2007), and the GITRL-GITR system is not only involved in T-cell costimulation, but also in the stimulation of other cells of the immune system (including macrophages and neutrophils) and extravasation (Krausz et al., 2007).
Our study indicates that GITR plays a pivotal role in the cellular mechanisms underlying experimental pancreatitis and resulting in severe inflammatory response, because deletion of GITR or its pharmacological inhibition by Fc-GITR ameliorates or even prevents the inflammatory process. For the first time, we show that the regulation of GITR-GITRL system might be a therapeutic target to reduce inflammatory defense mechanisms and the injury consequent to pancreatitis.
Acknowledgments
This work was supported by a research grant from the Italian Association for Cancer Research (AIRC) in Milan, Italy.
Glossary
Abbreviations
- GITR-/- mice
mice with a targeted disruption of the GITR
- GITRL
GITR ligand
- ICAM-1
intercellular adhesion molecule 1
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- NF-κB
nuclear factor κB
- PAR
poly ADP-ribose
- PARP
poly ADP-ribose polymerase
- TNF-α
tumour necrosis factor-α
- Treg cells
regulatory T cells
Conflicts of interest
The authors declare no conflicts of interests.
Supporting Information
Supporting Information: Teaching Materials; Figs 1–9 as PowerPoint slide.
References
- Agostini M, Cenci E, Pericolini E, Nocentini G, Bistoni G, Vecchiarelli A, et al. The glucocorticoid-induced tumor necrosis factor receptor-related gene modulates the response to Candida albicans infection. Infect Immun. 2005;73:7502–7508. doi: 10.1128/IAI.73.11.7502-7508.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC) Br J Pharmacol. (3rd edn) 2008;153(Suppl 2):S1–209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae EM, Kim WJ, Suk K, Kang YM, Park JE, Kim WY, et al. Reverse signaling initiated from GITRL induces NF-kappaB activation through ERK in the inflammatory activation of macrophages. Mol Immunol. 2008;45:523–533. doi: 10.1016/j.molimm.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Barkinge JL, Gudi R, Sarah H, Chu F, Borthakur A, Prabhakar BS, et al. The p53-induced Siva-1 plays a significant role in cisplatin-mediated apoptosis. J Carcinog. 2009;8:2. doi: 10.4103/1477-3163.45389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia M. Novel therapeutic targets for acute pancreatitis and associated multiple organ dysfunction syndrome. Curr Drug Targets Inflamm Allergy. 2002;1:343–351. doi: 10.2174/1568010023344517. [DOI] [PubMed] [Google Scholar]
- Cho JS, Hsu JV, Morrison SL. Localized expression of GITR-L in the tumor microenvironment promotes CD8+ T cell dependent anti-tumor immunity. Cancer Immunol Immunother. 2009;58:1057–1069. doi: 10.1007/s00262-008-0622-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzzocrea S, Genovese T, Mazzon E, Di Paola R, Muia C, Britti D, et al. Reduction in the development of cerulein-induced acute pancreatitis by treatment with M40401, a new selective superoxide dismutase mimetic. Shock. 2004;22:254–261. doi: 10.1097/01.shk.0000132490.79498.11. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Ayroldi E, Di Paola R, Agostini M, Mazzon E, Bruscoli S, et al. Role of glucocorticoid-induced TNF receptor family gene (GITR) in collagen-induced arthritis. FASEB J. 2005;19:1253–1265. doi: 10.1096/fj.04-3556com. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Nocentini G, Di Paola R, Agostini M, Mazzon E, Ronchetti S, et al. Proinflammatory role of glucocorticoid-induced TNF receptor-related gene in acute lung inflammation. J Immunol. 2006;177:631–641. doi: 10.4049/jimmunol.177.1.631. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Ronchetti S, Genovese T, Mazzon E, Agostini M, Di Paola R, et al. Genetic and pharmacological inhibition of GITR-GITRL interaction reduces chronic lung injury induced by bleomycin instillation. Faseb J. 2007;21:117–129. doi: 10.1096/fj.06-6611com. [DOI] [PubMed] [Google Scholar]
- De Palma C, Di Paola R, Perrotta C, Mazzon E, Cattaneo D, Trabucchi E, et al. Ibuprofen-arginine generates nitric oxide and has enhanced anti-inflammatory effects. Pharmacol Res. 2009;60:221–228. doi: 10.1016/j.phrs.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Dembinski A, Warzecha Z, Ceranowicz P, Warzecha AM, Pawlik WW, Dembinski M, et al. Dual, time-dependent deleterious and protective effect of anandamide on the course of cerulein-induced acute pancreatitis. Role of sensory nerves. Eur J Pharmacol. 2008;591:284–292. doi: 10.1016/j.ejphar.2008.06.059. [DOI] [PubMed] [Google Scholar]
- Demols A, Le Moine O, Desalle F, Quertinmont E, Van Laethem JL, Deviere J. CD4(+)T cells play an important role in acute experimental pancreatitis in mice. Gastroenterology. 2000;118:582–590. doi: 10.1016/s0016-5085(00)70265-4. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Cytokines as mediators in the pathogenesis of septic shock. Curr Top Microbiol Immunol. 1996;216:133–165. doi: 10.1007/978-3-642-80186-0_7. [DOI] [PubMed] [Google Scholar]
- Elgueta R, Benson MJ, de Vries VC, Wasiuk A, Guo Y, Noelle RJ. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev. 2009;229:152–172. doi: 10.1111/j.1600-065X.2009.00782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese T, Mazzon E, Di Paola R, Muia C, Crisafulli C, Menegazzi M, et al. Hypericum perforatum attenuates the development of cerulein-induced acute pancreatitis in mice. Shock. 2006;25:161–167. doi: 10.1097/01.shk.0000188326.82641.b7. [DOI] [PubMed] [Google Scholar]
- Gerli R, Nocentini G, Alunno A, Bocci EB, Bianchini R, Bistoni O, et al. Identification of regulatory T cells in systemic lupus erythematosus. Autoimmun Rev. 2009;8:426–430. doi: 10.1016/j.autrev.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Gharagozloo M, Velardi E, Bruscoli S, Agostini M, Di Sante M, Donato V, et al. Silymarin suppress CD4+ T cell activation and proliferation: effects on NF-kappaB activity and IL-2 production. Pharmacol Res. 2010;61:405–409. doi: 10.1016/j.phrs.2009.12.017. [DOI] [PubMed] [Google Scholar]
- Granger J, Remick D. Acute pancreatitis: models, markers, and mediators. Shock. 2005;24(Suppl 1):45–51. doi: 10.1097/01.shk.0000191413.94461.b0. [DOI] [PubMed] [Google Scholar]
- Gudi R, Barkinge J, Hawkins S, Prabhakar B, Kanteti P. Siva-1 promotes K-48 polyubiquitination of TRAF2 and inhibits TCR-mediated activation of NF-kappaB. J Environ Pathol Toxicol Oncol. 2009;28:25–38. doi: 10.1615/jenvironpatholtoxicoloncol.v28.i1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gukovskaya AS, Pandol SJ. Cell death pathways in pancreatitis and pancreatic cancer. Pancreatology. 2004;4:567–586. doi: 10.1159/000082182. [DOI] [PubMed] [Google Scholar]
- Inoue S, Kawanishi S. Oxidative DNA damage induced by simultaneous generation of nitric oxide and superoxide. FEBS Lett. 1995;371:86–88. doi: 10.1016/0014-5793(95)00873-8. [DOI] [PubMed] [Google Scholar]
- Kim H, Seo JY, Kim KH. NF-kappaB and cytokines in pancreatic acinar cells. J Korean Med Sci. 2000;15(Suppl):S53–S54. doi: 10.3346/jkms.2000.15.S.S53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JD, Choi BK, Bae JS, Lee UH, Han IS, Lee HW, et al. Cloning and characterization of GITR ligand. Genes Immun. 2003;4:564–569. doi: 10.1038/sj.gene.6364026. [DOI] [PubMed] [Google Scholar]
- Kim WJ, Bae EM, Kang YJ, Bae HU, Hong SH, Lee JY, et al. Glucocorticoid-induced tumour necrosis factor receptor family related protein (GITR) mediates inflammatory activation of macrophages that can destabilize atherosclerotic plaques. Immunology. 2006;119:421–429. doi: 10.1111/j.1365-2567.2006.02453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JI, Sonawane SB, Lee MK, Lee SH, Duff PE, Moore DJ, et al. Blockade of GITR-GITRL interaction maintains Treg function to prolong allograft survival. Eur J Immunol. 2010;40:1369–1374. doi: 10.1002/eji.200940046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krausz LT, Bianchini R, Ronchetti S, Fettucciari K, Nocentini G, Riccardi C. GITR-GITRL system, a novel player in shock and inflammation. Sci World J. 2007;7:533–566. doi: 10.1100/tsw.2007.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HS, Shin HH, Kwon BS, Choi HS. Soluble glucocorticoid-induced tumor necrosis factor receptor (sGITR) increased MMP-9 activity in murine macrophage. J Cell Biochem. 2003;88:1048–1056. doi: 10.1002/jcb.10456. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- McHugh RS, Whitters MJ, Piccirillo CA, Young DA, Shevach EM, Collins M, et al. CD4(+)CD25(+) immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 2002;16:311–323. doi: 10.1016/s1074-7613(02)00280-7. [DOI] [PubMed] [Google Scholar]
- Malleo G, Mazzon E, Genovese T, Di Paola R, Muia C, Crisafulli C, et al. Effects of thalidomide in a mouse model of cerulein-induced acute pancreatitis. Shock. 2008;29:89–97. doi: 10.1097/shk.0b013e318067df68. [DOI] [PubMed] [Google Scholar]
- Nocentini G, Riccardi C. GITR: a modulator of immune response and inflammation. In: Grewal I, editor. Therapeutic Targets of the Tnf Superfamily. Vol. 647. New York: Springer; 2009. pp. 156–173. [DOI] [PubMed] [Google Scholar]
- Nocentini G, Giunchi L, Ronchetti S, Krausz LT, Bartoli A, Moraca R, et al. A new member of the tumor necrosis factor/nerve growth factor receptor family inhibits T cell receptor-induced apoptosis. Proc Natl Acad Sci U S A. 1997;94:6216–6221. doi: 10.1073/pnas.94.12.6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nocentini G, Ronchetti S, Cuzzocrea S, Riccardi C. GITR/GITRL: more than an effector T cell co-stimulatory system. Eur J Immunol. 2007;37:1165–1169. doi: 10.1002/eji.200636933. [DOI] [PubMed] [Google Scholar]
- Nocentini G, Cuzzocrea S, Genovese T, Bianchini R, Mazzon E, Ronchetti S, et al. Glucocorticoid-induced tumor necrosis factor receptor-related (GITR)-Fc fusion protein inhibits GITR triggering and protects from the inflammatory response after spinal cord injury. Mol Pharmacol. 2008;73:1610–1621. doi: 10.1124/mol.107.044354. [DOI] [PubMed] [Google Scholar]
- Nolte MA, van Olffen RW, van Gisbergen KP, van Lier RA. Timing and tuning of CD27-CD70 interactions: the impact of signal strength in setting the balance between adaptive responses and immunopathology. Immunol Rev. 2009;229:216–231. doi: 10.1111/j.1600-065X.2009.00774.x. [DOI] [PubMed] [Google Scholar]
- O'Keeffe GW, Gutierrez H, Pandolfi PP, Riccardi C, Davies AM. NGF-promoted axon growth and target innervation requires GITRL-GITR signaling. Nat Neurosci. 2008;11:135–142. doi: 10.1038/nn2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakonczay Z, Jr, Jarmay K, Kaszaki J, Mandi Y, Duda E, Hegyi P, et al. NF-kappaB activation is detrimental in arginine-induced acute pancreatitis. Free Radic Biol Med. 2003;34:696–709. doi: 10.1016/s0891-5849(02)01373-4. [DOI] [PubMed] [Google Scholar]
- Ronchetti S, Nocentini G, Riccardi C, Pandolfi PP. Role of GITR in activation response of T lymphocytes. Blood. 2002;100:350–352. doi: 10.1182/blood-2001-12-0276. [DOI] [PubMed] [Google Scholar]
- Ronchetti S, Zollo O, Bruscoli S, Agostini M, Bianchini R, Nocentini G, et al. GITR, a member of the TNF receptor superfamily, is costimulatory to mouse T lymphocyte subpopulations. Eur J Immunol. 2004;34:613–622. doi: 10.1002/eji.200324804. [DOI] [PubMed] [Google Scholar]
- Ronchetti S, Nocentini G, Bianchini R, Krausz LT, Migliorati G, Riccardi C. GITR lowers the threshold of CD28 costimulation in CD8+ T cells. J Immunol. 2007;179:5916–5926. doi: 10.4049/jimmunol.179.9.5916. [DOI] [PubMed] [Google Scholar]
- Ronchetti S, Nocentini G, Petrillo MG, Bianchini R, Sportoletti P, Bastianelli A, et al. Glucocorticoid-Induced TNFR family Related gene (GITR) enhances dendritic cell activity. Immunol Lett. 2010 doi: 10.1016/j.imlet.2010.09.008. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3:135–142. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- Shin HH, Lee MH, Kim SG, Lee YH, Kwon BS, Choi HS. Recombinant glucocorticoid induced tumor necrosis factor receptor (rGITR) induces NOS in murine macrophage. FEBS Lett. 2002;514:275–280. doi: 10.1016/s0014-5793(02)02379-7. [DOI] [PubMed] [Google Scholar]
- Shin HH, Kim SJ, Lee DS, Choi HS. Soluble glucocorticoid-induced tumor necrosis factor receptor (sGITR) stimulates osteoclast differentiation in response to receptor activator of NF-kappaB ligand (RANKL) in osteoclast cells. Bone. 2005;36:832–839. doi: 10.1016/j.bone.2005.02.014. [DOI] [PubMed] [Google Scholar]
- So T, Lee SW, Croft M. Tumor necrosis factor/tumor necrosis factor receptor family members that positively regulate immunity. Int J Hematol. 2006;83:1–11. doi: 10.1532/IJH97.05120. [DOI] [PubMed] [Google Scholar]
- Spinicelli S, Nocentini G, Ronchetti S, Krausz LT, Bianchini R, Riccardi C. GITR interacts with the pro-apoptotic protein Siva and induces apoptosis. Cell Death Differ. 2002;9:1382–1384. doi: 10.1038/sj.cdd.4401140. [DOI] [PubMed] [Google Scholar]
- Strasser A, Jost PJ, Nagata S. The many roles of FAS receptor signaling in the immune system. Immunity. 2009;30:180–192. doi: 10.1016/j.immuni.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvas S, Kim B, Sarangi PP, Tone M, Waldmann H, Rouse BT. In vivo kinetics of GITR and GITR ligand expression and their functional significance in regulating viral immunopathology. J Virol. 2005;79:11935–11942. doi: 10.1128/JVI.79.18.11935-11942.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo C, Dawson VL. Role of poly(ADP-ribose) synthetase in inflammation and ischaemia-reperfusion. Trends Pharmacol Sci. 1998;19:287–298. doi: 10.1016/s0165-6147(98)01193-6. [DOI] [PubMed] [Google Scholar]
- Tamizhselvi R, Sun J, Koh YH, Bhatia M. Effect of hydrogen sulfide on PI3K-AKT pathway and on caerulein- induced cytokine production in isolated mouse pancreatic acinar cells. J Pharmacol Exp Ther. 2009;329:1166–1177. doi: 10.1124/jpet.109.150532. [DOI] [PubMed] [Google Scholar]
- Tone M, Tone Y, Adams E, Yates SF, Frewin MR, Cobbold SP, et al. Mouse glucocorticoid-induced tumor necrosis factor receptor ligand is costimulatory for T cells. Proc Natl Acad Sci U S A. 2003;100:15059–15064. doi: 10.1073/pnas.2334901100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallach D, Kovalenko AV, Varfolomeev EE, Boldin MP. Death-inducing functions of ligands of the tumor necrosis factor family: a Sanhedrin verdict. Curr Opin Immunol. 1998;10:279–288. doi: 10.1016/s0952-7915(98)80166-0. [DOI] [PubMed] [Google Scholar]
- Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- Xue L, Chu F, Cheng Y, Sun X, Borthakur A, Ramarao M, et al. Siva-1 binds to and inhibits BCL-X(L)-mediated protection against UV radiation-induced apoptosis. Proc Natl Acad Sci U S A. 2002;99:6925–6930. doi: 10.1073/pnas.102182299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu KY, Kim HS, Song SY, Min SS, Jeong JJ, Youn BS. Identification of a ligand for glucocorticoid-induced tumor necrosis factor receptor constitutively expressed in dendritic cells. Biochem Biophys Res Commun. 2003;310:433–438. doi: 10.1016/j.bbrc.2003.09.024. [DOI] [PubMed] [Google Scholar]
- Zingarelli B, O'Connor M, Wong H, Salzman AL, Szabo C. Peroxynitrite-mediated DNA strand breakage activates poly-adenosine diphosphate ribosyl synthetase and causes cellular energy depletion in macrophages stimulated with bacterial lipopolysaccharide. J Immunol. 1996;156:350–358. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


