Abstract
Environmental H2O2 creates several injuries in Escherichia coli, including the oxidative conversion of dehydratase [4Fe-4S] clusters to an inactive [3Fe-4S] form. To protect itself, H2O2-stressed E. coli activates the OxyR regulon. This regulon includes the suf operon, which encodes an alternative to the housekeeping Isc iron-sulfur-cluster assembly system. Previously studied [3Fe-4S] clusters are repaired by an Isc/Suf-independent pathway, so the rationale for Suf induction was not obvious. Using strains that cannot scavenge H2O2, we imposed chronic low-grade stress and found that suf mutants could not maintain the activity of isopropylmalate isomerase, a key iron-sulfur dehydratase. Experiments showed that its damaged cluster was degraded in vivo beyond the [3Fe-4S] state, presumably to an apoprotein form, and thus required a de novo assembly system for reactivation. Surprisingly, sub-micromolar H2O2 poisoned the Isc machinery, thereby creating a requirement for Suf both to repair the isomerase and to activate nascent Fe-S enzymes in general. The IscS and IscA components of the Isc system are H2O2-resistant, suggesting that oxidants disrupt Isc by oxidizing clusters as they are assembled on or transferred from the IscU scaffold. Consistent with these results, organisms that are routinely exposed to oxidants rely upon Suf rather than Isc for cluster assembly.
Keywords: Iron-sulfur clusters, the Suf system, the Isc system, and oxidative stress
Introduction
Contemporary organisms inherited their biochemical mechanisms and metabolic pathways from ancestors that evolved in an anaerobic world. Problems arise when this machinery is employed in aerobic habitats. Inside cells, molecular oxygen steals electrons from redox enzymes and continuously generates superoxide and hydrogen peroxide; these oxygen species are reactive and, therefore, potentially toxic. Organisms that live in aerobic habitats protect themselves from endogenous H2O2 by scavenging it with catalases and peroxidases. The titers of these enzymes are high enough to drive steady-state levels of H2O2 to levels as low as 20 nM (Seaver & Imlay, 2001b).
Nevertheless, because H2O2 can cross membranes, microbes are rapidly toxified if the extracellular concentration of H2O2 is high. This situation can result from abiotic processes, such as the oxidation of environmental sulfur or metals. It also occurs when organisms attempt to poison competitors by exploiting their vulnerability to reactive oxygen species. For example, lactic acid bacteria excrete H2O2 that inhibits the growth of other microbes; many plants and bacteria secrete redox-cycling antibiotics, which enter competitors and generate inhibitory oxidants; and mammalian macrophages deploy NADPH oxidase, which blasts captive bacteria with superoxide and H2O2 (Imlay, 2008a).
To understand oxidative stress we must identify the target biomolecules that are most vulnerable to it. Early work showed that millimolar doses of extracellular H2O2 are moderately bacteriocidal, while micromolar doses are bacteriostatic (Imlay & Linn, 1986). The identification of the damaged biomolecules was thwarted by the robust scavenging activities of cells, which quickly degrade the H2O2 that is added to lab media and thus interfere with the imposition of chronic micromolar H2O2 stress. To circumvent this problem, one can analyze the injuries that accumulate in mutant strains that are devoid of scavenging enzymes. A mutant strain of E. coli that lacks both its catalases and its NADH peroxidase (katE katG ahpCF, denoted Hpx−) does not degrade H2O2 at a significant rate (Seaver & Imlay, 2001a). When this strain is transferred from anaerobic to aerobic medium, endogenous H2O2 accumulates up to 1 micromolar. Since the H2O2 equilibrates between the intracellular and extracellular environments, external measurements allow intracellular concentrations to be tracked. Experiments with this strain have revealed several types of cell damage that occur rapidly. The Fenton reaction between H2O2 and unincorporated intracellular iron [reaction1] generates hydroxyl radicals, which oxidize a variety of biomolecules but exert their greatest toxicity by damaging DNA (Park et al., 2005, Henle et al., 1999, Imlay et al., 1988, Levin et al., 1982).
| (1) |
H2O2 also impedes the function of the Fur regulatory protein, apparently by oxidizing its iron cofactor; the consequence is a disruption of iron homeostatic mechanisms (Varghese et al., 2007). H2O2 creates covalent polypeptide damage by reacting with the iron cofactors of metalloproteins (Anjem et al., 2009). Finally, H2O2 oxidizes the solvent-exposed [4Fe-4S]2+ clusters of a family of dehydratases (Jang & Imlay, 2007, Flint et al., 1993).
| (2) |
The catalytic iron atom is released, thereby obviating enzyme activity. A residual [3Fe-4S]+ cluster remains, and it is subsequently reactivated to the [4Fe-4S]2+ form inside cells, through a process that has not been resolved. It is striking that all these mechanisms of cell damage derive from reactions between H2O2 and iron.
These are the targets that cells must protect when they enter H2O2-containing environments. To do so, a H2O2-responsive regulon is activated. In E. coli this regulon is controlled by the OxyR protein, which responds to as little as 0.2 micromolar intracellular H2O2 (Carmel-Harel & Storz, 2000, Zheng et al., 2001). This value appears to define a threshold concentration of H2O2 that is potentially toxic.
Genes that respond to OxyR should, therefore, comprise key defenses against H2O2; indeed, although Hpx− mutants can grow in aerobic medium, Hpx− oxyR mutants cannot (Park et al., 2005). Some of the OxyR-controlled defenses have been identified. Catalase (encoded by katG) and NADH peroxidase (ahpCF) are induced in order to drive down the level of H2O2 (Seaver & Imlay, 2001a). Dps, a ferritin-like protein, is induced to sequester loose iron; by doing so, it greatly suppresses Fenton-mediated damage to DNA and to proteins (Park et al., 2005, Grant et al., 1998, Ilari et al., 2002). Fur is induced in order to restore repression of iron-import proteins (Varghese et al., 2007). A manganese importer (mntH) protects simple metalloproteins by replacing their iron cofactors with an alternative metal that will not react with H2O2 (Anjem et al., 2009).
The suf operon is also induced (Zheng et al., 2001). The SufABCDSE proteins comprise an iron-sulfur-cluster assembly system. Under routine growth conditions, the Suf system is not synthesized in E. coli, as clusters are assembled by the housekeeping Isc system (Takahashi & Nakamura, 1999, Barras et al., 2005, Bandyopadhyay et al., 2008). Therefore, it seemed plausible that the Suf system is induced during H2O2 stress in order to repair damaged iron-sulfur clusters, in distinction to their de novo synthesis by the Isc apparatus. Consistent with this notion, experiments in some bacteria (but not others) (Tokumoto et al., 2004, Runyen-Janecky et al., 2008) indicated that Suf enhanced their resistance to a bolus of millimolar H2O2. Most strikingly, mutants of Erwinia chrysanthemi that lacked the suf operon exhibited hypersensitivity to paraquat, a generator of intracellular superoxide and H2O2 (Nachin et al., 2001). Subsequent work showed that the activities of oxidant-sensitive dehydratases were reduced (Nachin et al., 2003).
The availability of Hpx− mutants enabled us to test whether Suf is important in protecting E. coli from physiological, sub-micromolar levels of H2O2. Further, it allowed us to address the question of why Isc alone is inadequate. We considered several possibilities: that Suf is designed specifically to repair damaged clusters; that Suf is dedicated to cluster assembly for a protein(s) that is exclusively used during H2O2 stress; or that Isc is inoperative in H2O2-stressed cells. We determined that sub-micromolar H2O2 disables the Isc system and that Suf assumes the role of both de novo synthesis and cluster repair.
Results
The Suf system is critical during H2O2 stress
Previous studies have shown that when E. coli is grown in standard lab media the Isc system constitutes the major mechanism of [Fe-S] cluster assembly (Barras et al., 2005, Bandyopadhyay et al., 2008). In agreement with that conclusion, we observed that ΔhscA mutants grew substantially more slowly in a minimal glucose medium (tD = 89′) than did the wild-type parent strain (tD = 69′). Moreover, the enzymatic activities of two [Fe-S]-dependent enzymes, isopropylmalate isomerase and NADH dehydrogenase I, were reduced to 25% and 10% of their wild-type values (Fig. S1). (For this purpose ΔhscA mutants were studied because they lack Isc function but retain IscS-dependent activities that are unrelated to cluster assembly.) The residual activities of these enzymes were apparently due to the action of the Suf system, which is induced in Δisc mutants by the apo-IscR transcription factor. Double Δisc suf mutants cannot build clusters at all and are inviable (Takahashi & Tokumoto, 2002).
In contrast, deletions of the suf operon caused no growth defect (tD = 69′), nor were enzyme activities detectably diminished. Thus, as others have inferred (Outten et al., 2004, Djaman et al., 2004), the Suf system has no apparent role under standard growth conditions.
The observation that suf is controlled by the OxyR (Zheng et al., 2001, Lee et al., 2004) protein prompted us to examine whether the Suf system becomes important when cells are exposed to H2O2. Other workers have noted recovery defects when Δsuf mutants were challenged with a bolus of millimolar H2O2 (Nachin et al., 2001, Nachin et al., 2003, Tokumoto et al., 2004). Because the OxyR system is activated by sub-micromolar concentrations of intracellular H2O2 (Aslund et al., 1999), we sought to test the role of Suf when cells were chronically exposed to low, physiological doses. To do so we employed Δahp katG katE (Hpx−) mutants, which lack catalase and peroxidase activities and therefore cannot scavenge H2O2. When these mutants are cultured in aerobic medium, they accumulate 0.5–1.0 micromolar H2O2, which is generated by the spontaneous oxidation of intracellular redox enzymes (Seaver & Imlay, 2001b). This H2O2 comprises a constant oxidative stress. Hpx− mutants are able to grow under these conditions, but they display reduced activities of some [Fe-S] dehydratases because H2O2 oxidatively damages their solvent-exposed [Fe-S] clusters (Jang & Imlay, 2007).
When anaerobic cultures of Hpx− mutants were diluted into aerobic medium, the suf operon was expressed at a level 40-fold higher than in wild-type cells, consistent with its induction by the OxyR protein (Fig. S2). Induction was ten-fold lower when the OxyR binding site was deleted. Hpx−Δsuf mutants grew somewhat more slowly than did their suf+ parent. The addition of 6 μM H2O2 completely abolished their growth of the Hpx−Δsuf strain; under the same conditions the parent continued to grow well (Fig 1A). The growth defect of the Hpx−Δsuf mutant was eliminated when the suf operon was expressed from a plasmid (Fig. 1B). An Hpx− suf (non-inducible) mutant—which retained the intact suf operon but lacked the OxyR binding site upstream—exhibited a growth rate intermediate between that of the Hpx−Δsuf mutant and the Hpx− Suf+ strain (Fig. S3). Thus the Suf system is critical for growth during H2O2 stress, and OxyR must induce it to achieve sufficient levels.
Figure 1. The Suf system is important for growth during micromolar H2O2 stress.
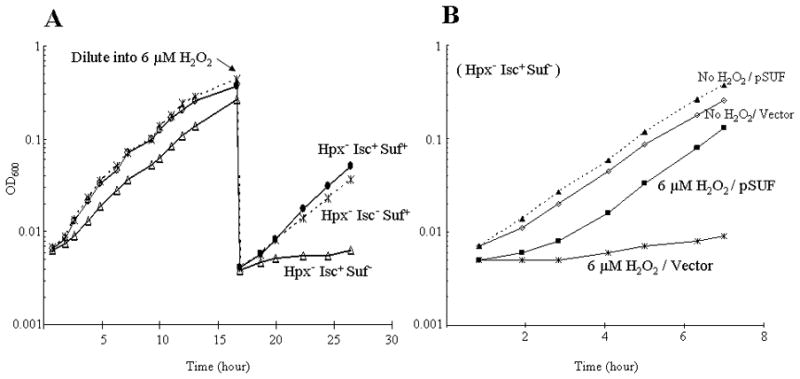
(A) Cells were cultured in aerobic glucose medium. At the arrow, cells were diluted into fresh medium containing 6 μM H2O2. (B) Anaerobic Hpx− Isc+ Suf− cells containing either pSUF (overexpressing the suf operon) or pWKS30 (an empty vector) were diluted into aerobic lactose minimal medium, with or without 6 μM H2O2.
Interestingly, Hpx− ΔhscA mutants were able to grow efficiently in aerobic medium, even when the exogenous H2O2 was added (Fig. 1A). This contrasts with the problems displayed by ΔhscA mutants in an Hpx+ background, and it suggests that the OxyR-driven induction of Suf might fully compensate for the absence of the Isc system.
Most [Fe-S] enzymes are dispensable for growth in glucose medium, but isopropylmalate isomerase (IPMI) is necessary for the synthesis of leucine. Indeed, leucine supplementation restored the growth of the Hpx−Δsuf mutant. IPMI is among the most sensitive of the dehydratases that can be inactivated by H2O2, and measurements showed that its activity diminished rapidly in both Hpx− Suf+ and Hpx−Δsuf mutants during their first hour of growth due to endogenous H2O2 (Fig. 2A). However, whereas the OxyR-mediated induction of suf ultimately enabled the IPMI activity in Hpx− Suf+ cells to regain about 10–15% of their original IPMI activity, the IPMI activity in Hpx−Δsuf mutants did not recover. After four hours of aeration, the latter strain lacked detectable activity. The absence of IPMI in the Δsuf background occurred despite a compensatory induction of leuCD transcription, which is responsive to the intracellular concentration of leucine (Fig. 2B).
Figure 2. Induction of the Suf system is necessary to ensure IPMI activity during H2O2 stress.
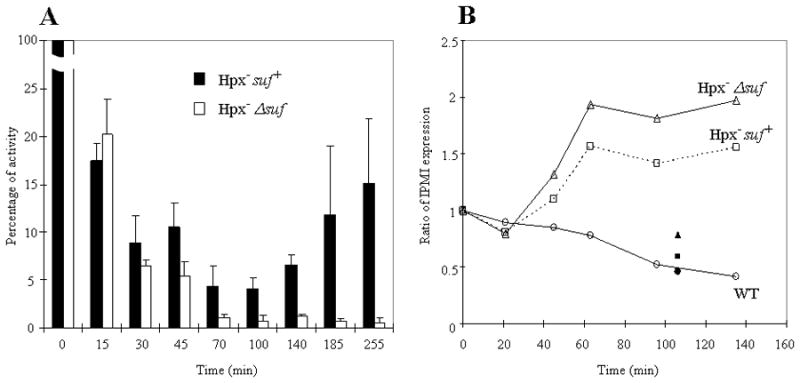
(A) Anaerobic cultures were aerated at time zero, and cellular IPMI activity was measured during subsequent aerobic growth. 100% activity was approximately 0.6 U/mg. (B) The expression of leuCD was monitored by transcriptional lacZ fusions. Expression was normalized to anaerobic levels (time zero). Expression was shut off when aerobic medium contained leucine (solid symbols).
Fumarase A and 6-phosphogluconate dehydratase are also [Fe-S] dehydratases whose activities are vulnerable to H2O2 (Jang & Imlay, 2007). The activities of these enzymes were similarly lower in Hpx−Δsuf mutants than in Hpx− Suf+ cells (Fig. 3). Therefore, we concluded that the Suf system is needed to preserve [Fe-S] dehydratase activities during low-grade H2O2 stress.
Figure 3. Suf was also necessary to support the activities of other labile [4Fe-4S] dehydratases.
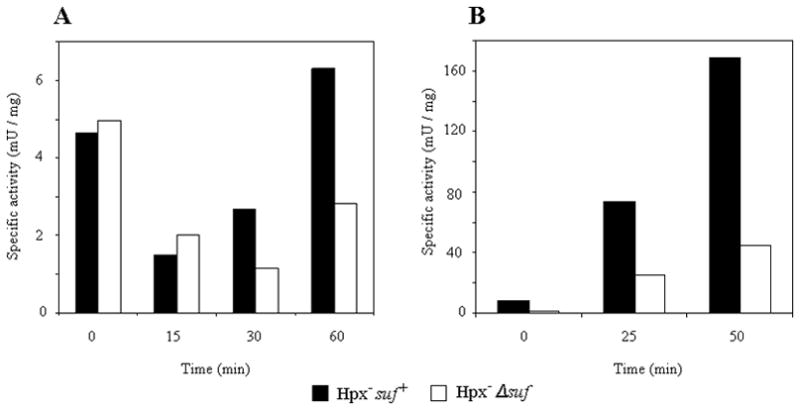
Anaerobic log-phase cells (time zero) were diluted into aerobic media, and enzyme activity was monitored during subsequent aerobic growth. (A) Fumarase A activity in aerobic glucose/casamino acids medium supplemented with 20 μM H2O2. (B) 6-phosphogluconate dehydratase activity. Anaerobic glucose-grown cells were washed and inoculated into aerobic gluconate medium at time zero, triggering the induction of 6-phosphogluconate dehydratase. The presence of casamino acids in both experiments ensured that cells continued to grow during the period of H2O2 stress. The data represent three independent trials.
The Suf system is needed to repair [Fe-S] clusters that have degraded beyond the [3Fe-4S]+ state
In principle Suf could enhance dehydratase activity in H2O2-stressed cells through either of two ways: by inserting clusters de novo into newly synthesized polypeptide, or by rebuilding clusters that had been oxidatively damaged by H2O2. The preceding experiments involved growing cells and therefore did not distinguish between these possibilities; however, given the circumstances, we suspected that the Suf system facilitated cluster repair. To test this idea, we monitored the activity of dehydratases under conditions in which de novo enzyme synthesis was blocked. An Hpx− Δsuf strain was constructed in which Suf was expressed from a plasmid under the control of the lac promoter; when this strain was cultured in lactose medium, the Suf system was synthesized, even in anaerobic conditions, when it is typically not expressed. Both this strain and its Hpx− Δsuf complement were grown to exponential phase in anaerobic lactose medium, and then chloramphenicol was added to block further protein synthesis. The cultures were then aerated, and the activity of IPMI was monitored during the subsequent period of H2O2 stress. The data in Fig. 4A show that the Suf system significantly decreased the rate at which H2O2 inactivated IPMI. Thus its protective effect was consistent with a repair activity that partially countervailed the damaging effects of H2O2.
Figure 4. The Suf system repairs damaged IPMI but not fumarase B.
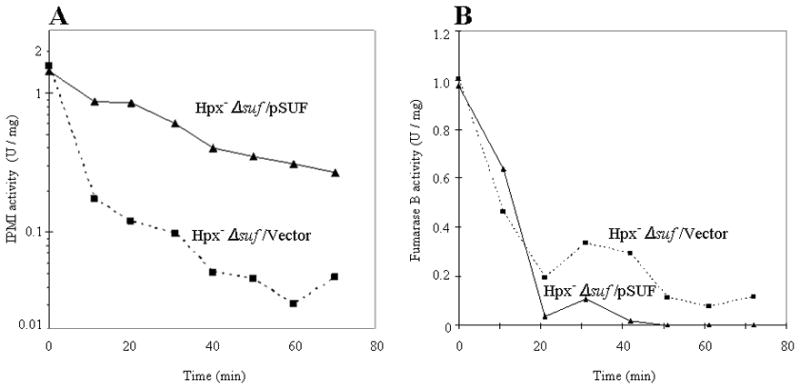
Hpx−Δsuf cells containing either pSUF or an empty vector were cultured anaerobically in lactose/18 amino acid medium that lacked leucine and cysteine. Chloramphenicol was added to block protein synthesis, and at time zero the cultures were aerated. IPMI (A) and fumarase B (B) activities were monitored. In the fumarase B experiment, 20 μM H2O2 was supplemented to the aerobic medium.
The experiment was repeated with fumarase B, the [4Fe-4S] isozyme that is expressed in anaerobic cells. Since this enzyme is less sensitive to H2O2, 20 μM exogenous H2O2 was added to the aerobic medium. In contrast to IPMI, fumarase B activity was unaffected by the presence or absence of the Suf system (Fig. 4B). This discrepancy led us to wonder whether the damaged clusters of oxidized IPMI and fumarase B might be in different forms.
When H2O2 initially oxidizes an enzymic [4Fe-4S]2+ cluster, the cluster quickly loses its catalytic iron atom and is converted to a [3Fe-4S]+ form. The cluster can be rapidly repaired to the original [4Fe-4S]2+ state by incubation in vitro with ferrous iron and dithiothreitol; full activity is restored. We have documented this behavior for both IPMI and fumarase (Jang & Imlay, 2007). However, the iron/dithiothreitol treatment is not expected to reactivate enzymes whose clusters have degraded beyond the [3Fe-4S]+ state—for example, to [2Fe-2S] or apo-protein forms—as sulfur atoms must be supplied to rebuild the cluster (Urbina et al., 2001, Smith et al., 2001). Based on this idea, we explored the status of the oxidized clusters in IPMI and fumarase B. Figure 5A shows that iron/dithiothreitol treatment completely restored the fumarase activity in extracts prepared from H2O2-exposed Hpx−Δsuf cells. Thus all the inactive fumarase enzymes were in the [3Fe-4S] form. In contrast, the same treatment restored less than 20% of the IPMI activity. Full restoration was achieved only when purified IscS and cysteine were provided as sulfur donors, indicating that the majority of clusters were degraded beyond the [3Fe-4S] form.
Figure 5. The oxidized Fe-S cluster of fumarase remains in a [3Fe-4S] form in vivo, while that in IPMI degrades further.
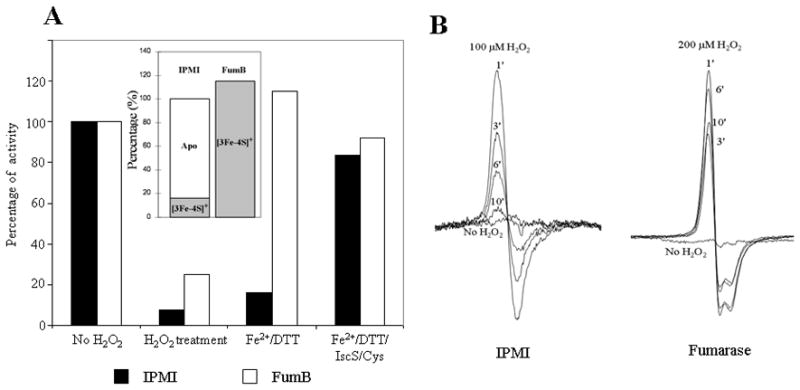
(A) Hpx−Δsuf cells were grown anaerobically to allow synthesis of active IPMI and fumarase B. Chloramphenicol was added to block further synthesis, and cultures were then aerated to create H2O2 stress. For fumarase B experiments, 20 μM H2O2 was supplemented to the aerobic medium. After 20 min, lysates were prepared and assayed without further treatment, after incubation with ferrous iron/dithiothreitiol, or after incubation with iron, DTT, and IscS/cysteine. Inset: Inferred status of the enzyme clusters upon harvesting. Initial activities were 1.6 U/mg (IPMI) and 1 U/mg (fumarase B). (B) Hpx−Δsuf cells that overproduce IPMI (left) or fumarase A (right) were harvested anaerobically, concentrated, and then exposed to 100 μM (IPMI) or 200 μM (fumarase) H2O2. The EPR signals represents the content of [3Fe-4S]+ clusters at subsequent time points. The signal is negligible in non-overproducing strains (not shown).
The observation that IPMI clusters degraded in vivo contrasted with our previous studies (Jang & Imlay, 2007), which showed that oxidation of enzyme in vitro creates a [3Fe-4S]+ form that is stable for at least 60 min. We reproduced that result, using EPR to confirm cluster status. Whole-cell EPR was then used to track the cluster status when IPMI-overproducing cells were exposed to H2O2. Initially, a strong [3Fe-4S]+ signal appeared; in contrast to the in vitro experiments, however, the signal decayed within ten minutes (Fig. 5B). During this period no activity was restored, suggesting that the disappearance of the signal was due to degradation of the cluster to a lesser form. EPR analysis failed to detect [2Fe-2S] clusters, raising the prospect that the cluster was fully removed. The mechanism of intracellular IPMI cluster degradation was probed but not solved (Discussion).
These data suggested the reason that IPMI, but not fumarase, required the Suf system for cluster repair. The Isc and Suf systems assemble full clusters on scaffold proteins, and they would therefore seem to be capable of transferring full clusters to client apoproteins; however, they would appear to be unsuitable for the reactivation of a protein that retains a partial cluster. In agreement with this idea, a recent study showed that the Isc system can reactivate apo-FNR (Mettert et al., 2008). We also observed that over-expression of the isc operon from a plasmid diminished the rate of IPMI inactivation by H2O2 (data not shown), suggesting that the Suf and Isc systems work in equivalent ways, albeit the Suf system might be more effective during H2O2 stress.
In contrast, the reactivation of enzymes that retain [3Fe-4S] clusters appears not to require a scaffold system. When purified fumarase A was exposed to H2O2 in vitro, the resultant [3Fe-4S]+ form of the enzyme was efficiently reactivated upon addition to cell extracts, whether or not they contained Suf proteins (data not shown). Fumarase was also repaired in vivo without any requirement for the Suf system (Fig. 6). It remains possible that some residual Isc function was sufficient to repair these mostly intact clusters, but earlier studies indicated that Isc was expendible for [3Fe-4S]+ repair under conditions in which Suf was poorly expressed (Djaman et al., 2004). The mechanism of reactivation presumably requires the consecutive donation of an electron donor and ferrous iron, but we have not identified any cell proteins that are essential. The di-iron protein YtfE has been suggested to be a possible partner in this process (Justino et al., 2006, Justino et al., 2007), but under our experimental conditions the rate of IPMI inactivation was not different in Hpx− and Hpx− ΔytfE mutants, and there was no difference in the rates at which [3Fe-4S]+ clusters of fumarase A were repaired in vivo after brief exposure to H2O2 (data not shown).
Figure 6. The repair of the [3Fe-4S]+ cluster of fumarase A does not depend upon Suf.
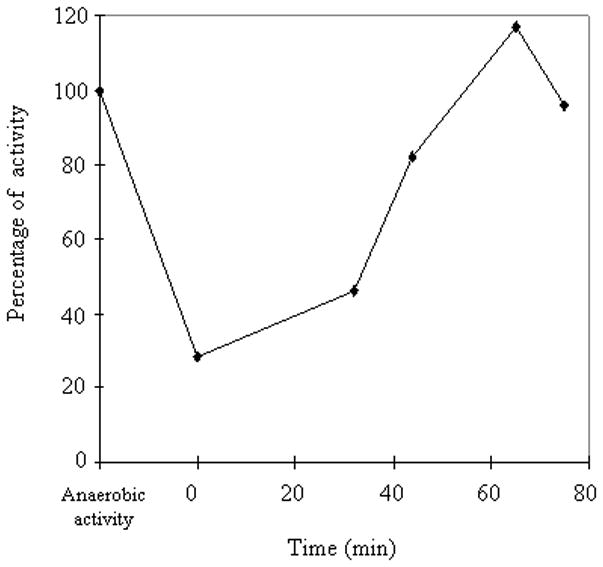
Chloramphenicol was added to anaerobic cultures of Hpx−Δsuf cells, and they were aerated in medium containing 20 μM H2O2 for 5 minutes. Catalase was then added, and the recovery of fumarase activity in the absence of H2O2 was monitored.
In summary, the Suf system is necessary for the repair of IPMI, because the oxidized IPMI cluster substantially degrades in vivo. In contrast, the [3Fe-4S]+ form of the oxidized fumarase cluster is stable and can be repaired without the involvement of Suf..
In H2O2-stressed cells the Suf system is also required for de novo cluster synthesis
Since the preceding data indicate that Suf is needed to build clusters in apo-IPMI, one might infer that the Isc system is poorly functional when E. coli is stressed by H2O2. To test this idea more directly, we examined the activation of enzymes which rely on [Fe-S] clusters but whose clusters are naturally shielded from H2O2 and therefore do not require repair. NADH dehydrogenase I and succinate dehydrogenase contain nine and three iron-sulfur clusters, respectively (Ohnishi, 1998, Condon et al., 1985). The clusters are buried in polypeptide, and so the enzymes do not lose activity when they are exposed to even 1 mM H2O2 in vivo or in vitro (data not shown). These respiratory enzymes are repressed in our anaerobic glucose medium, due to the action of ArcAB and Fnr (Gunsalus & Park, 1994, Crack et al., 2008), but they are induced when E. coli cells are aerated (data not shown). Figure 7 demonstrates that the enzyme activities quickly rose when Hpx− Suf+ cells were transferred to aerobic medium, but not when Hpx− Δsuf mutants were transferred, although there was no difference in the nuo transcription level between two strains. In addition, the defect in the latter cells was not due to a general problem with protein synthesis, since these enzymes are not requisite for growth in glucose medium and the Δsuf strain grew as well as its Suf+ partner. Instead, the result indicates that the submicromolar (0.5–1 μM) H2O2 that accumulated inside cells during these experiments was sufficient to prevent the Isc system from inserting new clusters into nascent polypeptides. In contrast, the progressive induction of the Suf system by OxyR circumvented this block.
Figure 7. The Suf system is needed for the de novo assembly of iron-sulfur clusters during H2O2 stress.
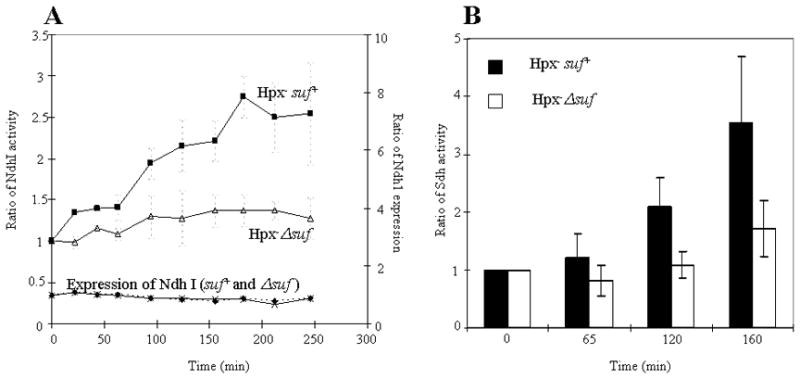
NADH dehydrogenase I (A) and succinate dehydrogenase (B) are induced when anaerobic cultures are aerated. Activities were measured at intervals after the aeration of Hpx− suf+ and Hpx−Δsuf cultures. Initial activities at time zero were approximately 30 mU/mg (NADH dehydrogenase I) and 40 mU/mg (succinate dehydrogenase). Panel A also indicates that nuo expression was independent of suf genotype.
To double-check this result, we indirectly monitored the presence of [2Fe-2S] clusters in the IscR protein. When cluster assembly is defective, the accumulation of apo-IscR protein allows it to serve as a transcription factor that activates expression of the suf operon (Giel et al., 2006, Yeo et al., 2006, Lee et al., 2008). A lacZ transcriptional fusion was constructed immediately downstream of the suf promoter, and the upstream OxyR binding site was removed, so that expression was primarily dictated by the status of IscR. This fusion was expressed at a two-fold higher level in an Hpx− strain relative to Hpx− ΔiscR mutants. A further two-fold induction was detected in Hpx− Δsuf mutants. These data confirm that H2O2 interferes with the activity of the Isc system (Fig. 8A), and they show that the Suf system substantially compensates.
Figure 8. The failure of the Isc system during H2O2 stress is not due to a lack of Isc synthesis.
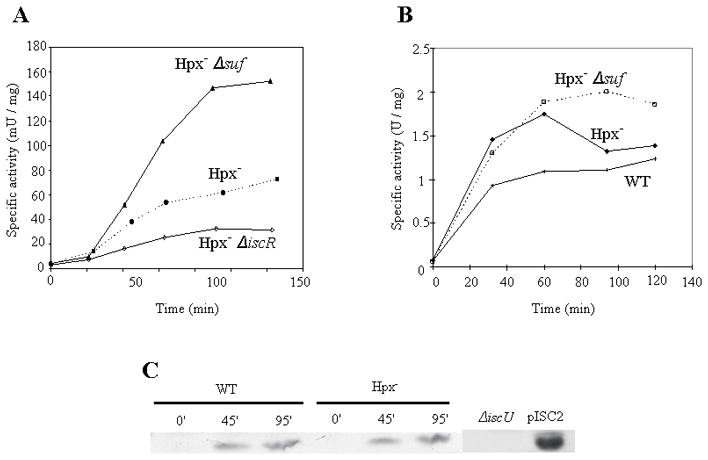
(A) Expression after aeration of a transcriptional lacZ fusion placed behind the sufNI promoter that lacks the OxyR binding site. Induction by apo-IscR is greatest in a suf mutant. (B) Expression after aeration of a transcriptional lacZ fusion placed behind the iscRSUA promoter. (C) IscU polypeptide was detected by western blot analysis using an anti-IscU antibody during aerobic growth. The experiments were performed using glucose/casamino acids medium.
The contribution of apo-IscR to suf induction during H2O2 stress was moderate (Fig. S2 and 8A) and may not be sufficient to ensure iron-sulfur assembly. Presumably the much larger impact of the OxyR system justifies the inclusion of suf in that regulon.
The Isc system is present but non-functional during H2O2 stress
Collectively, these data indicate that the Suf system is induced in H2O2-stressed cells because the Isc system is inactive. One explanation for its inactivity might be that its synthesis was repressed. A transcriptional fusion to the iscSUA promoter was constructed; the fusion retained the RyhB sRNA binding site to ensure that Fur-mediated control was active (Desnoyers et al., 2009). We observed that the expression of the Isc system was very low in anaerobic wild-type cells, and it rose rapidly when the cells were aerated. The same result was observed in Hpx− cells, indicating that the dysfunction of the Isc system was not due to a lack of expression (Fig. 8B). In fact isc expression was somewhat higher in the H2O2-stressed cells than in unstressed cells, presumably reflecting substantial conversion of [2Fe-2S]− IscR, a repressor of isc, to the apo-IscR form (Giel et al., 2006, Schwartz et al., 2001?). Western blot analysis using anti-IscU antibody verified the presence of this Isc protein in Hpx− cells (Fig. 8C). Thus H2O2 apparently inhibits the function of the extant Isc machinery.
The inactivity of the Isc system is reversible
We sought to identify the mechanism by which H2O2 disrupts the Isc system. As a first step, we tested whether the system would regain function when H2O2 was removed. To do so, we took advantage of the observation that rapidly synthesized [Fe-S] proteins transiently accumulate in an apo-protein form, due to the insufficient synthesis of [Fe-S] clusters. The fumA gene was strongly induced from a tac promoter in aerobically grown wild-type and Hpx− Δsuf strains. Under these conditions ca. 80% of the protein accumulated in the apo-protein form. Chloramphenicol was added to block further fumarase and Isc synthesis. When aerobic incubation continued, the wild-type cells progressively activated fumarase; however, no significant increase in fumarase activity occurred in the Hpx− Δsuf strain, indicating that the accumulated H2O2 continued to inhibit the Isc system. In contrast, when cells were moved to anaerobic medium, fumarase activity rebounded in the Hpx− Δsuf strain at a rate comparable to that of wild-type cells (Fig. 9). Thus once H2O2 stress ceases, the Isc system regains activity. This reversibility may explain why E. coli continues to synthesize the Isc proteins even when inhibitory levels of H2O2 are present. It also constrains the possible mechanisms by which H2O2 blocks Isc function.
Figure 9. The inactivation of the Isc system by H2O2 is reversible.
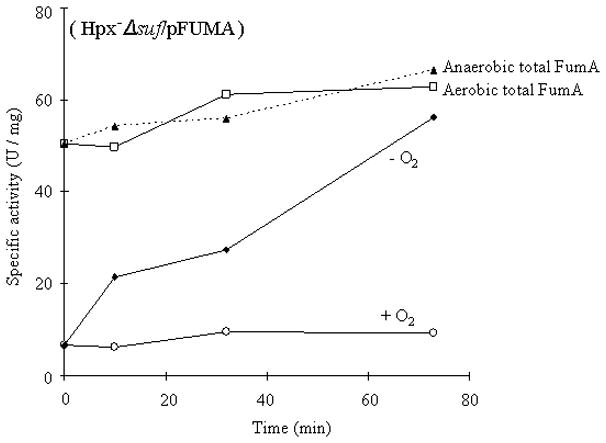
Fumarase A was strongly overexpressed in aerobic Hpx−Δsuf cells, forcing the accumulation of a large fraction of apoenzyme. Chloramphenicol was added to block protein synthesis. Cultures were then split and incubated either aerobically (+ O2) or anaerobically with catalase (−O2), and at intervals aliquots were harvested and assayed for fumarase activity. After cessation of H2O2 stress, Isc regained function and activated fumarase. Dashed lines: Total fumarase content was determined by treating the harvested extracts with ferrous iron, DTT, and IscS/cysteine.
How does H2O2 poison the Isc system?
The Suf system is induced during iron starvation (Outten et al., 2004), which led to the observation that the Isc system works poorly when iron levels are low. During H2O2 stress the OxyR system works to minimize Fenton chemistry by reducing the levels of free iron in the cell. This is accomplished by induction of the Dps ferritin-like protein, which sequesters unincorporated iron, and by induction of the Fur repressor, which diminishes synthesis of iron importers (Park et al., 2005, Varghese et al., 2007). Thus we explored the possibility that the effects of the OxyR-directed responses might starve the Isc system for iron.
Hpx− ΔoxyR mutants are not viable in aerobic medium (Park et al., 2005), which precluded the most direct test of this notion; however, the OxyR system is also induced in ahpCF mutants, due to modestly elevated levels of intracellular H2O2 (Seaver & Imlay, 2001b). Assays showed that NADH dehydrogenase I was synthesized at normal rates in a ahpCF suf strain (data not shown). Further, an oxyR2 expression plasmid that constitutively induces the OxyR response (Kullik et al., 1995) also did not create either growth defects or [Fe-S] enzyme deficiencies in a suf background (Fig. 10A). These data indicated that OxyR-controlled activities, including iron-depletion measures, are not sufficient to inactivate Isc. Indeed, EPR measurements, indicated that due to cluster damage the levels of unincorporated intracellular iron are actually higher in Hpx− mutants, in which Isc fails, than in wild-type cells, in which Isc is functional (Varghese et al., 2007). Finally, a cluster-synthesis defect could only be elicited in the ΔahpF suf strain when it was exposed to 20 μM exogenous H2O2; this defect was not diminished by the deletion of the dps gene (data not shown). Thus it appears to be H2O2 itself that poisons Isc function.
Figure 10. The inactivation of the Isc system is not due to OxyR induction or to the inactivation of IscS, IscA, or ferredoxin.
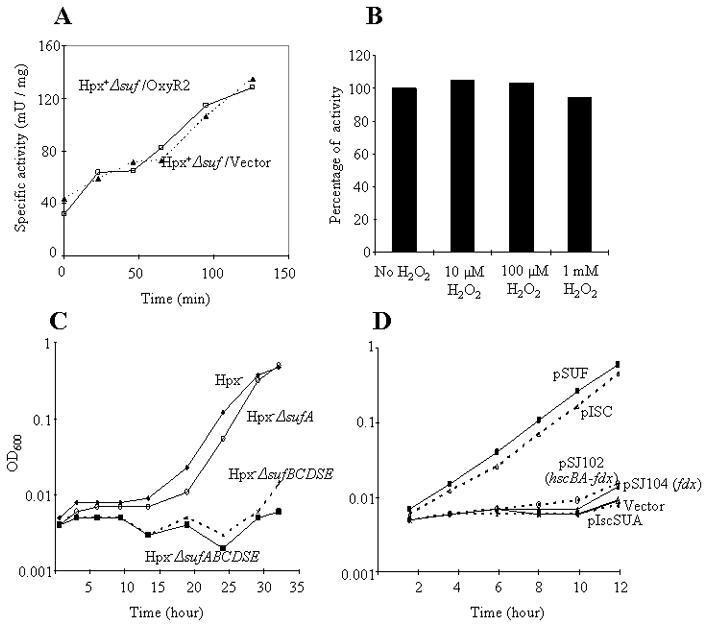
(A) Hpx+ cells (without H2O2 stress) were aerated at time zero. NADH dehydrogenase I activity was monitored in strains carrying a plasmid that strongly activates the OxyR regulon (pOxyR2) or an empty vector. (B) Purified IscS-(His)6 was treated with the indicated concentrations of H2O2 for 5 min. Catalase was then added, and activity was assayed. (C) Growing cultures in anaerobic glucose medium were diluted at time zero into aerobic medium containing 8 μM H2O2, and growth was monitored. The sufA gene was not essential for outgrowth, due to the persistent function of IscA, but other suf genes were required. (D) The full Isc system must be overproduced to compensate for damage by H2O2. Growing cultures in anaerobic medium were induced by IPTG in order to express the entire suf operon (pSUF), the full iscSUA hscAB-fdx gene set (pISC), or the hscBA-fdx, fdx, or iscSUA components of the Isc system. All genes were controlled by the lac promoter, and robust expression was confirmed by visualization of the EPR signals of ferredoxin and/or IscA (text).
The Isc system consists of the IscS desulfurase, the IscU scaffold protein on which nascent clusters are assembled, an IscA protein that serves either in cluster transfer or iron delivery, a ferredoxin that might adjust the cluster redox state, and the HscA/HscB chaperone proteins that apparently assist in cluster transfer to client apoproteins (Barras et al., 2005, Bandyopadhyay et al., 2008, Ayala-Castro et al., 2008). To date only a few chemical effects of low-micromolar H2O2 have been detected in vivo; these include the oxidation of reactive cysteinyl residues and of solvent-exposed iron-sulfur clusters (Imlay, 2008a). The Isc system involves both, and either type of reaction would be consistent with the reversibility of Isc inactivation. Thus the potential targets were examined in turn.
The IscS desulfurase features an exposed active-site cysteine (Cupp-Vickery et al., 2003) that seemed to be a plausible target for oxidation. Most protein cysteine residues react far too sluggishly to be affected by micromolar H2O2, but the OxyR, AhpC, and OhrR proteins feature active-site cysteines that are activated by local context so they are easily oxidized to sulfenic acid (Imlay, 2008a). However, we observed that purified IscS retained full activity when it was exposed to even 1 mM H2O2, whether the H2O2 was added prior to or during the desulfurase reaction (Fig. 10B).
Uniquely among Isc/Suf proteins, IscA and SufA can substitute for one another in vivo. Although ΔiscA sufA mutants are inviable, indicating that Fe/S biogenesis requires one or the other of these proteins, a ΔsufA mutant that lacks any of the Isc proteins other than IscA is viable, showing that the Suf system can function using IscA (Lu et al., 2008, Vinella et al., 2009). We observed that Hpx− mutants exhibited growth defects if they additionally lacked SufBCD, but Hpx− ΔsufA mutants were healthy (Fig. 10C). The implication is that IscA is functional in H2O2-stressed cells. Thus the Isc dysfunction must arise from damage to a different Isc protein.
The overexpression of the full iscSUA-hscAB-fdx gene set restored robust cluster synthesis in the Hpx− Δsuf mutants; however, overexpression of individual genes failed to do so (Fig. 10D). Ferredoxin may dissociate during the catalytic cycle; if so, the fact that ferredoxin overproduction did not restore Isc function strongly suggests that it is not the limiting component in the H2O2-stressed system. Although we observed that in vitro ferredoxin was rapidly oxidized by H2O2, the oxidized cluster was fully reducible, indicating that the apparent Fenton chemistry did not generate disabling damage to the protein (Fig. S4).
Aside from IscA and ferredoxin, the other isc gene products likely exist as part of a larger complex (Py & Barras, 2010) so that overproduction of a single member cannot easily compensate for damage. Our suspicion was that the nascent iron-sulfur clusters that are built on IscU might be vulnerable to oxidative degradation in vivo, since they are likely to be solvent-exposed when they are transferred to recipient proteins. Further, workers have reported that clusters in IscU are degraded when they are exposed to aerobic buffers in vitro (Agar et al., 2000, Shimomura et al., 2007). To test this idea in vivo, we attempted to overproduce the IscSU complex in order to use whole-cell EPR to monitor the status of IscU clusters during exposure to H2O2. In agreement with a previous report, this effort was unsuccessful(Raulfs et al., 2008); we were able to visualize clusters by EPR or visible spectroscopy only when the full iscSUA-hscAB-fdx operon was overexpressed. When these cells were exposed to 0.01–1 mM exogenous H2O2, no [3Fe-4S]+ signal appeared (Fig. S5A). Treatment of the unstressed cells with dithionite generated a signal that was consistent with [2Fe-2S]+ and/or [4Fe-4S]+ clusters; previous treatment with as much as 1 mM H2O2 did not diminish the dithionite-generated signal, indicating that H2O2 did not destroy the clusters (Fig. S5B). However, since the EPR analysis showed the total [Fe-S] cluster spectrum of the Isc system, oxidation or degradation of the cluster on IscU might be obscured by the dominant [2Fe-2S] signals of IscA and ferredoxin. Unfortunately, we were unable to make any definitive conclusion regarding the stability of IscU-bound clusters.
Discussion
When Barras and his colleagues exposed E. chrysanthemi suf mutants to phenazine methosulfate, a redox-cycling drug that generates reactive oxygen species, they discovered that dehydratase activities were very low (Nachin et al., 2003). Oxidants can damage dehydratase clusters (Flint et al., 1993, Jang & Imlay, 2007), and it seemed plausible that Suf was needed to repair them. But the observation was puzzling in two respects. First, why would a scaffold system be required to repair the [3Fe-4S] clusters that are produced by oxidation reactions? And second, in any case, why was the housekeeping Isc system inadequate for the job? The results from the present study begin to answer those questions. First, although damaged clusters are stable in vitro, some [3Fe-4S] clusters are further disassembled in vivo, thereby requiring the engagement of a de novo-style cluster-assembly system for their full repair. And second, the Isc system is poisoned by as little as 1 micromolar H2O2, leaving the apparently oxidant-resistant Suf system as the only viable Fe/S scaffold system. Thus during periods of oxidative stress bacteria rely upon the Suf system not only to maintain dehydratase activity but to support the activities of all Fe-S enzymes (Fig. 11). Still, these results raise some intriguing questions.
Figure 11. Iron-sulfur-cluster metabolism during H2O2 stress.
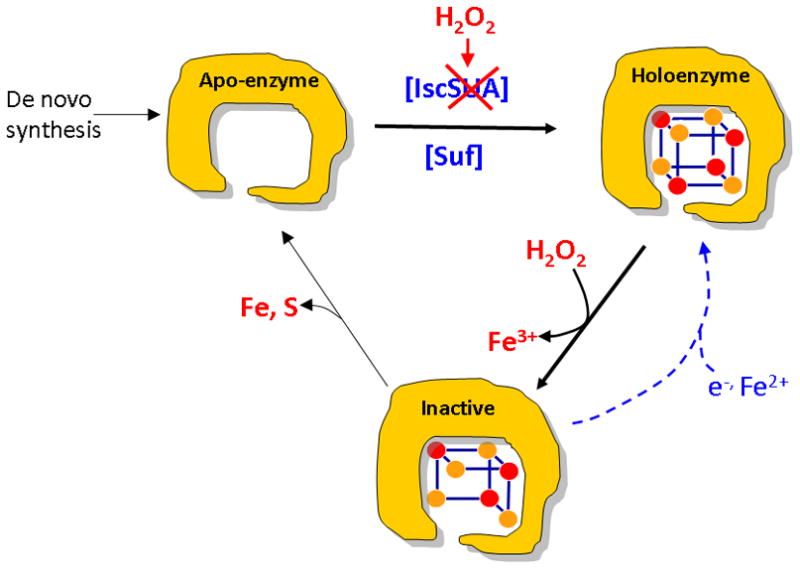
Iron-sulfur-cluster biogenesis relies on the Isc system under routine conditions, and the Suf system is not expressed. However, H2O2 both poisons the Isc system and damages dehydratase clusters. In some enzymes the [3Fe-4S] product is stable and is repaired by simple reduction/metallation, while in other enzymes the clusters are further degraded. Strong induction of the Suf system by OxyR compensates for Isc inactivity, restoring de novo assembly and repairing oxidized apoenzymes.
How are damaged clusters disassembled in vivo?
Repair of [3Fe-4S] clusters can easily be managed in vitro by dithiothreitol and ferrous iron, and we imagine that an analogous process occurs in vivo. A dithiol is required for reactivation, which suggests that the iron-sulfur bonds in the cluster can reorganize themselves internally and can interact with solutes. This fact led us to wonder whether the degradation of the [3Fe-4S] IPMI cluster inside cells might occur through sulfur-exchange reactions with metabolites. To test this notion, we attempted to replicate degradation by oxidizing IPMI in vitro and then incubating its [3Fe-4S] form with cysteine and with both oxidized and reduced glutathione; however, the [3Fe-4S]+ cluster remained intact. Thus it seems likely that degradation is catalyzed by enzymes. The Suf system is not requisite, since disassembly occurs in suf mutants. At present the basis of IPMI cluster decay remains uncertain.
Interestingly, the [4Fe-4S] cluster of IRP-1—which controls iron levels in mammalian cells—appears to behave much like that of IPMI, with potentially important consequences for metabolism. Workers found that in vitro H2O2 treatment converted the IRP-1 cluster to a [3Fe-4S] form, which lacks both the aconitase activity of the holo-protein and the RNA-binding activity of the apo-protein (Brazzolotto et al., 1999). However, upon oxidation in vivo the cluster was fully degraded, thereby exposing the RNA binding site that interacts with the transcripts of iron-import and –storage proteins (Castro et al., 1998, Cairo & Recalcati, 2007). Accumulation of the apo-protein form is normally a marker of iron starvation; thus, during oxidative stress the generation of the apo-protein form may inadvertently activate mechanisms that import more iron into the cell—thereby potentially aggravating the situation by stimulating Fenton chemistry. Aconitases have been proposed to serve similar iron-regulatory roles in B. subtilis and E. coli (Tang et al., 2005) and further experiments will be needed to determine whether oxidative stress affects them in a manner similar to IRP-1.
How does H2O2 poison the Isc system?
By refuting other possibilities, we inferred that H2O2 probably disrupts the Isc system by oxidizing the nascent clusters that are formed on the IscU scaffold protein. It seems particularly likely that the clusters are exposed to solvent—and to dissolved oxidants—when they are transferred to recipient apoproteins. It will be a challenge to test this idea biochemically, since H2O2 reacts rapidly with the ferrous iron that is used as an Isc substrate during turnover; in experimental systems this Fenton reaction will both scavenge the H2O2 and deplete the ferrous iron. Molecular oxygen does the same thing, forcing workers to use anaerobic conditions to study Isc function. Thus, it is not instructive to demonstrate that H2O2 disrupts continuous cluster assembly in vitro. Perhaps spectroscopic methods can reveal whether H2O2 interferes with single-turnover reactions.
Electron transfer from iron to H2O2 is an inner-sphere process; that is, in order to react with iron, H2O2 must bind it directly. For this reason dehydratase clusters can be fully protected from H2O2 by bound substrates, which provide fifth and sixth ligands to the erstwhile solvent-exposed iron atom. The iron atoms in IscU clusters are comparatively undercoordinated, as each derives three ligands from bridging sulfur atoms and another either from a protein aspartate or cysteine residue (Bandyopadhyay et al., 2008). Conceivably, they can be directly oxidized by species that would occupy a fifth coordination site. In fact, using UV-visible spectroscopy, other workers showed that H2O2 can directly oxidize [2Fe-2S] clusters even in resting IscU protein (Bitoun et al., 2008). (IscU-bound [4Fe-4S] clusters were not tested.) However, in those experiments approximately millimolar H2O2 concentrations were used, which is a thousand times the concentration of H2O2 that we found was sufficient to inhibit Isc in vivo. Thus the physiological relevance of this mechanism of cluster oxidation is uncertain.
If Isc inactivation were to derive from cluster oxidation, it would fall in line with the other molecular injuries produced by H2O2, all of which arise from iron-oxidation reactions. This includes DNA damage (Park et al., 2005), which is driven by a Fenton reaction between H2O2 and DNA-bound iron; dehydratase inactivation (Flint et al., 1993, Jang & Imlay, 2007); disruption of Fur protein, in which H2O2 apparently oxidizes the ferrous iron cofactor (Varghese et al., 2007); and protein carbonylation, which also arises through Fenton chemistry (Anjem et al., 2009). To date no non-iron-related injuries have been detected when bacteria are exposed to micromolar concentrations of H2O2. The rate constants for these reactions depend on the coordination environment of the iron atom but approximate 104 M−1 s−1 (Park et al., 2005, Jang & Imlay, 2007). The OxyR system is calibrated appropriately, responding when H2O2 levels exceed 0.2 micromolar (Carmel-Harel & Storz, 2000, Imlay, 2008a)—that is, at a dose above which the half-time of Fenton reactions approaches 1 min.
Since superoxide oxidizes dehydratase iron-sulfur clusters at a rate even higher than does H2O2, we wondered whether superoxide might also be able to inactivate Isc. We found that a suf::lacZ fusion is substantially induced in SOD− mutants (Fig. S6). Further, 6-phosphogluconate dehydratase and succinate dehydrogenase activities were substantially reduced when a suf mutation was introduced into the SOD− strain. The data indicate that superoxide, like H2O2, inhibits Isc function—which is consistent with an iron-sulfur target in the system. Indeed, the earlier observation that suf mutants are poisoned by phenazine methosulfate (Nachin et al., 2003) might owe more to superoxide, which is the direct product of PMS cycling, than to H2O2.
Does the distribution of the Suf and Isc systems correlate with environmental stress?
The fact that artificial Isc overproduction can suppress the growth defects of Hpx−Δsuf mutants could, in principle, suggest that Suf induction simply elevates the total cellular capacity for cluster assembly during a period of high demand—rather than that the Suf machinery is intrinsically more H2O2-resistant than is the Isc machinery. This notion is not excluded by any of the data in this study. However, if this were the case, one must wonder why the cell must switch from one assembly machine to the other. This consideration suggests that the Suf system somehow shields its nascent clusters from small oxidants. Similarly, the Suf system is more resistant to soft metals (Ranquet et al., 2007, Macomber & Imlay, 2009), which can disrupt solvent-exposed clusters, than is the Isc system. How Suf might shelter its clusters is entirely unclear. In any case, if the Suf system is more robust than the Isc system, one might guess that Isc is maintained in the biological world because it has its virtues, too, perhaps being catalytically more efficient than Suf. This notion has not been tested, but it fits the fact that E. coli employs Isc as its housekeeping system and Suf only as an emergency back-up.
More generally, one might expect that organisms that encounter oxidants routinely might dispense with Isc and exclusively express Suf, whereas ones that do not encounter oxidants might do the opposite. A phylogenetic survey of the two systems gives mixed results. Lactic acid bacteria, which generate millimolar doses of H2O2 through their pyruvate and lactate oxidases, contain Suf and lack Isc. So do eukaryote pathogens such as Mycobacterium tuberculosis, Enterococcus faecalis, and Xylella fastidiosa, which need to withstand the H2O2 that is generated by the host immune response (Robinson, 2009, Averyanov, 2009). Chloroplasts, which are substantial sources of reactive oxygen species, employ only the Suf system (Asada, 2006). These data are consistent with the hypothesis. However, most anaerobic bacteria also have dispensed with the Isc system, relying instead on Suf or on the Nif system. Perhaps the Isc system is ill-suited for environments that are occasionally iron-limited. An alternative is that these microbes must periodically contend with the H2O2 that is chemically generated when aerated waters mix with anaerobic solutions of reduced sulfur and metals. Obligate anaerobes all express catalases and/or peroxidases (Imlay, 2008b), presumably for such a circumstance.
In contrast, non-photosynthetic eukaryotes are distinguished among organisms in that they rely entirely on Isc for cluster assembly—they do not have Suf even as a back-up system. This unique arrangement may be viable only because Fe-S assembly in eukaryotes occurs in the mitochondrial matrix and thus is shielded from the external environment by two membranes. Because membranes are only semi-permeable to H2O2, efficient scavenging systems are able to reduce the cytoplasmic H2O2 concentration to a concentration that is an order of magnitude lower than that outside the cell. Mitochondria have additional scavengers (Park et al., 2000, Petrova et al., 2004), so that second gradient would exist between the mitochondrial matrix and the cytosol. The effects of these layers of scavenging should be multiplicative, and it should rigorously protect the Isc system from external H2O2—apparently making the Suf system unnecessary. We note, though, that these diffusion barriers would not protect Isc from drugs like antimycin, which produces H2O2 within mitochondria (Panduri et al., 2004). Such drugs potentially diminish the activities not only of oxidant-sensitive dehydratases but also of other Fe-S enzymes whose de novo activation would be impeded.
Materials and Methods
Reagents
Glycerol and D-glucose were obtained from Fisher Scientific; ethylenediaminetetraacetic acid disodium salt dehydrate (EDTA) and sodium dithionite, from Fluka; Amplex Red, from Invitrogen; His Gravitrap, from GE Healthcare; and ECL™ Western Blotting Detection Reagents, from Amersham. Sigma was the source of L-amino acids, bovine liver catalase, horse heart cytochrome c, rabbit muscle lactate dehydrogenase, chloramphenicol, ferric chloride, ferrous ammonium sulfate, 2,2′-dipyridyl, citraconate, disodium L(-) malic acid, D,L-dithiothreitol, hydrogen peroxide, isopropyl β-D-thiogalactopyranoside, β-lactose, NADH, deamino NADH (nicotinamide hypoxanthine dinucleotide, reduced form), trichloacetic acid, N, N-dimethyl-p-phenylenediamine, potassium D-gluconate, glutathione (reduced and oxidized), sodium sulfide, succinic acid, tri(cyclohexylammonium) 6-phosphogluconic acid, plumbagin, thiamine, o-nitrophenyl-b-D-galactopyraniside, DEAE-sepharose (DFF: Fast flow and DCL-6B), and horseradish peroxidase-conjugated anti-rabbit goat immunoglobulin G. Anti-A. vinelandii IscU antibody was a kind gift of Dr. Dennis R. Dean.
Bacterial strains
The strains and plasmids used in this study are listed in Table 1. Null mutations were created by the Red/Gam recombination method (Datsenko & Wanner, 2000) and were confirmed by either PCR analysis or enzyme assays. Mutations were introduced into new strains by P1 transduction and selected on media containing appropriate antibiotics. All strains in the Hpx− background were constructed anaerobically to avoid an outgrowth of suppressors, which may occur aerobically.
Table 1.
| Strains | Genotype and characteristics | Source or ref. |
|---|---|---|
| MG1655 | F− wild type E. coli | E. coli Genetic Stock Center |
| BW25113 | lacI rrnB ΔlacZ hsdK ΔaraBAD ΔrhaBAD | (Datsenko & Wanner, 2000) |
| OD114 | MG1655 ΔhscA2::cat zff-208::Tn10 | (Djaman et al., 2004) |
| OD510 | Δ(sufABCDSE)19::kan~zdi57::Tn10 sodA25::MudPR13 sodB1- Δ2::kan | (Djaman et al., 2004) |
| SJ89 | MG1655 Δ(sufABCDSE)19::kan~zdi57::Tn10 | P1(OD510) × MG1655 |
| LC106 | ΔahpF::kan Δ(katG17::Tn10)1 Δ(katE12::Tn10)1 | (Seaver & Imlay, 2001a) |
| SJ15 | LC106 Δ(sufABCDSE)19::kan~zdi57::Tn10 | P1(SJ89) × LC106 |
| SJ98 | MG1655 ΔlacZ1::cat | This work |
| SJ100 | LC106 ΔlacZ1::cat | P1(SJ98) × LC106 |
| SJ102 | SJ15 ΔlacZ1::cat | P1(SJ98) × SJ15 |
| SJ130 | MG1655 Δ(lacZ1::cat)1 | This work |
| SJ108 | LC106 Δ(lacZ1::cat)1 | This work |
| SJ160 | SJ15 Δ(lacZ1::cat)1 | This work |
| SJ172 | SJ130 attλ::[pSJ501::sufA′-lac+]~cat | This work |
| SJ186 | SJ108 attλ::[pSJ501::sufA′-lac+]~cat | P1(SJ172) × SJ108 |
| SJ57 | LC106 ΔhscA2::cat | P1(OD114) × LC106 |
| SJ283 | BW25113 Δ(OxyR binding site in PsufA)1::cat | This work |
| SJ288 | LC106 Δ(OxyR binding site in PsufA)1::cat | P1(SJ283) × LC106 |
| SJ440 | MG1655 Δ(lacZ1::cat)1 attλ::[pSJ501::leuL′-lac+]~cat | This work |
| SJ442 | LC106 Δ(lacZ1::cat)1 attλ::[pSJ501::leuL′-lac+]~cat | P1(SJ440) × SJ108 |
| SJ445 | SJ15 Δ(lacZ1::cat)1 attλ::[pSJ501::leuL′-lac+]~cat | P1(SJ440) × SJ160 |
| SJ40 | BW25113 Δ(fumC::cat)1 ΔfumB1::cat | This work |
| SJ53 | LC106 Δ(fumC::cat)1 Δ(fumB1::cat)1 | This work |
| SJ65 | SJ53 Δ(sufABCDSE)19::kan~zdi57::Tn10 | P1(SJ89) × SJ53 |
| SJ54 | LC106 Δ(fumCA::cat)1 Δ(fumB1::cat)1 | This work |
| SJ88 | SJ54 Δ(sufABCDSE)19::kan~zdi57::Tn10 | P1(SJ89) × SJ54 |
| SJ90 | BW25113 ΔytfE1::cat | This work |
| JI366 | ΔkatG17::Tn10 Δ(katE12::Tn10)1 | (Seaver & Imlay, 2001a) |
| SJ233 | JI366 ΔytfE1::cat | P1(SJ90) × JI366 |
| SJ91 | SJ54 ΔytfE1::cat | P1(SJ90) × SJ54 |
| SJ93 | SJ88 ΔytfE1::cat | P1(SJ90) × SJ88 |
| OD102 | hscA114::cat Φ(nuoAB-lacZ) | (Djaman et al., 2004) |
| SJ109 | SJ100 Φ(nuoAB-lacZ) | λ (OD102) × SJ100 |
| SJ110 | SJ102 Φ(nuoAB-lacZ) | λ (OD102) × SJ102 |
| λJS2 | Φ(sdhC-lacZ) lacY+ lacA+ | (Shen & Gunsalus, 1997) |
| SJ150 | MG1655 Δfrd1::cat | This work |
| SJ161 | SJ108 Δfrd1::cat | SJ108 P1(SJ150) |
| SJ163 | SJ160 Δfrd1::cat | P1(SJ150) × SJ160 |
| SJ179 | SJ161 Φ(sdhC-lacZ) | λ (λJS2)x SJ161 |
| SJ220 | SJ163 Φ(sdhC-lacZ) | λ (λJS2)x SJ163 |
| SJ263 | SJ130 attλ::[pSJ501::sufA′NI-OxyR-lac+]~cat | This work |
| SJ276 | SJ108 attλ::[pSJ501::sufA′NI-OxyR-lac+]~cat | P1(SJ263) × SJ108 |
| SJ334 | SJ160 attλ::[pSJ501::sufA′NI-OxyR-lac+]~cat | P1(SJ263) × SJ160 |
| SJ272 | BW25113 ΔiscR1::cat | This work |
| SJ291 | SJ130 attλ::[pSJ501::sufA′NI-OxyR-lac+] ΔiscR1::cat | P1(SJ272) × SJ263 |
| SJ293 | SJ108 attλ::[pSJ501::sufA′NI-OxyR-lac+] ΔiscR1::cat | P1(SJ272) × SJ276 |
| SJ332 | SJ263 Δ(sufABCDSE)19::kan~zdi57::Tn10 | P1(SJ89) × SJ263 |
| KI232 | Δ(sodB::kan)1 ΔsodA-1 | Laboratory stock |
| SJ259 | KI232 Δ(lacZ1::cat)1 | P1(SJ98) × SJ297 |
| SJ297 | SJ259 Δ(sufABCDSE)19::kan~zdi57::Tn10 | P1(SJ89) × SJ259 |
| SJ278 | SJ259 attλ::[pSJ501::sufA′NI-OxyR-lac+]~cat | P1(SJ263) × SJ59 |
| SJ295 | SJ259 attλ::[pSJ501::sufA′NI-OxyR-lac+] ΔiscR1::cat | P1(SJ272) × SJ278 |
| SJ336 | SJ297 attλ::[pSJ501::sufA′NI-OxyR-lac+]~cat | P1(SJ263) × SJ297 |
| SJ1010 | SJ130 attλ::[pSJ501::iscRS′-lac+]~cat | This work |
| SJ1019 | SJ108 attλ::[pSJ501::iscRS′-lac+]~cat | P1(SJ1010) × SJ108 |
| SJ1021 | SJ160 attλ::[pSJ501::iscRS′-lac+]~cat | P1(SJ1010) × SJ160 |
| JI370 | ΔahpF::kan | (Seaver & Imlay, 2001a) |
| SP65 | ΔmhpC281::Tn10 lacY1 Δdps-1::cat | (Park et al., 2005) |
| SJ181 | JI370 Δdps-1::cat | P1(SP65) × JI370 |
| SJ183 | SJ181 Δ(sufABCDSE)19::kan~zdi57::Tn10 | P1(SJ89) × SJ181 |
| SJ1001 | BW25113 ΔsufA1::cat | This work |
| SJ1004 | BW25113 Δ(sufBCDSE)1::cat | This work |
| SJ1023 | BW25113 Δ(sufSE)1::cat | This work |
| OD112 | ΔiscA112::cat | (Datsenko & Wanner, 2000) |
| SJ1013 | LC106 ΔiscA112::cat | P1(OD112) × LC106 |
| SJ1015 | LC106 ΔsufA1::cat | P1(SJ1001) × LC106 |
| SJ1017 | LC106 Δ(sufBCDSE)1::cat | P1(SJ1004) × LC106 |
| SJ1042 | LC106 Δ(sufSE)1::cat | P1(SJ1023) × LC106 |
| SJ1046 | LC106 Δ(sufA1::cat)1 ΔiscA112::cat | P1(OD112) × SJ1015 |
| SJ424 | BW25113 Δbfr-1::cat | This work |
| SJ448 | BW25113 Δ(bfd bfr)-1::cat | This work |
| KCI540 | LC106 ΔcyaY1::cat | Laboratory stock |
| JRG2953 | W3110 Δbfr::kan Δftn::spec | J.R. Guest |
| SJ428 | LC106 Δbfr-1::cat | P1(SJ424) × LC106 |
| SJ432 | SJ15 Δbfr-1::cat | P1(SJ424) × SJ15 |
| SJ459 | LC106 Δ(bfd bfr)-1::cat | P1(SJ448) × LC106 |
| SJ453 | LC106 Δftn::spec | P1(JRG2953) × LC106 |
| SJ463 | SJ15 ΔcyaY1::cat | P1(KCI540) × SJ15 |
| SJ461 | SJ453 Δ(bfd bfr)-1::cat | P1(SJ448) × SJ453 |
| SJ466 | SJ461 Δ(sufABCDSE)19::kan~zdi57::Tn10 | P1(SJ89) × SJ461 |
| SJ473 | SJ453 Δ((bfd bfr)-1::cat)1 ΔcyaY1::cat | P1(KCI540) × SJ461 |
| SJ487 | SJ473 Δ(sufABCDSE)19::kan~zdi57::Tn10 | P1(SJ89) × SJ473 |
| Plasmid | ||
| pCP20 | FLP expression plasmid; Ampr, temperature-sensitive replication and FLP synthesis | (Cherepanov & Wackernagel, 1995) |
| pKD3 | Template plasmid; amp, FRT-flanked cat | (Datsenko & Wanner, 2000) |
| pKD46 | λ Red recombinase (γ,β, and exo) expression plasmid; amp, ara- inducible expression, temperature-sensitive replication | (Datsenko & Wanner, 2000) |
| pCKR101 | Plac–lacI q Ptac polylinker Ampr (20–50 copies per cell) | Jeff Gardner |
| pWKS30 | Plac polylinker Ampr (6–8 copies per cell) | (Wang & Kushner, 1991) |
| pSUF1 | pWKS30 containing sufABCDSE | This work |
| pISC1 | pWKS30 containing iscSUA hsc BhscA fdx iscX | This work |
| pISC2 | pCKR101 containing iscSUA hscB hscA fdx iscX | This work |
| pIscSUA | pWKS30 containing iscSUA | This work |
| pSJ102 | pWKS30 containing hscB hscA fdx iscX | This work |
| pSJ104 | pWKS30 containing fdx iscX | This work |
| pET15b | PT7 polylinker Ampr | Lab stock |
| pIscS-(His)6 | pET15b containing iscS | This work |
| pTrcfdx | pTrc99 (Ptrc polylinker Ampr) containing fdx | (Ta & Vickery, 1992) |
| pFUMA | pCKR101 containing fumA | (Jang & Imlay, 2007) |
| pAH125 | CRIM reporter plasmid (Kmr) | (Haldimann & Wanner, 2001) |
| pSJ501 | pAH125 derivative, in which the Kam resistant marker has been replaced with a cat gene. | This work |
| pAH57 | CRIM helper plasmid | (Haldimann & Wanner, 2001) |
| pACYC184 | Tetr Cmr | (Kullik et al., 1995) |
| pGS058 | pACYC184 containing oxyR2 [A233V] | (Kullik et al., 1995) |
Growth conditions
Anaerobic cultures were grown in an anaerobic glove box (Coy Laboratory Products Inc.), and aerobic cultures were grown with vigorous shaking in a water bath at 37°C. Standard minimal medium contained minimal A salts, 0.2% glucose, 1 mM MgCl2, 5 mg/liter thiamine, and 0.5 mM histidine. Histidine was routinely added to the media because the parent strain, MG1655, is a histidine auxotroph under anaerobic conditions; to minimize the difference between anaerobic and aerobic cultures, histidine was also added to aerobic cultures. Casamino acids or specified L-amino acids were present at 0.2 % or 0.5 mM, respectively.
To ensure that Hpx− derivatives were growing exponentially before they were aerated, anaerobic overnight cultures were diluted to OD600 = 0.005 in appropriate fresh anaerobic media and grown to OD600 ~ 0.1 prior to dilution into aerobic media.
Enzyme assays
For assays of labile [4Fe-4S]-cluster-containing enzymes--isopropylmalate isomerase (IPMI), fumarase, and 6-phosphogluconate dehydratase--cell extracts were prepared by sonication in an anerobic chamber using anaerobic buffers. IPMI activity was measured by monitoring the decrease of citraconate, a pseudo-substrate, at 235 nm in 100 mM Tris-Cl (pH 7.6). The fumarase activity was determined in 50 mM sodium phosphate (pH 7.4) containing 50 mM of L-malate; production of fumarate was monitored at 250 nm (Jang & Imlay, 2007). To assay 6-phosphogluconate dehydratase, lysates were prepared from cultures grown in a minimal medium containing 0.2 % gluconate. The production of pyruvate by 6-phosphogluconate dehydratase (in 50 mM Tris-Cl, pH 7.65) was determined in the second reaction catalyzed by lactate dehydrogenase (Gardner & Fridovich, 1991).
Inverted membrane vesicles were prepared for NADH dehydrogenase I (Ndh1) and succinate dehydrogenase (Sdh) assays. The activities were measured in 50 mM aerobic potassium phosphate buffer (pH 7.8) by monitoring either deamino NADH (nicotinamide hypoxanthine dinucleotide, reduced form) oxidation by Ndh1 at 340 nm or cytochrome c reduction for Sdh at 550 nm in 50 mM aerobic potassium phosphate buffer (pH 7.8) (Djaman et al., 2004).
The desulfurase activity of IscS was assayed anaerobically by measuring sulfide production as described before with modifications (Siegel, 1965). Purified IscS was incubated with 2.5 mM cysteine and 5 mM dithiothreitol at 37°C for 5 min, and then the reaction was stopped by addition of 20 % trichloracetic acid. Precipitated IscS was removed by centrifugation, and the supernatant was incubated with 2 mM N, N-dimethyl-p-phenylenediamine (DPD) and 3 mM FeCl3 at ambient temperature for 30 min in the dark. The concentration of methylene blue that results from the reaction of DPD and sulfide was then measured at 670 nm.
As a reporter enzyme for transcriptional fusions, β-galactosidase was assayed using o-nitrophenyl-β-D-galactopyranoside (ONPG) as a substrate. The final product, ortho-nitrophenol, was detected at 420 nm (Miller, 1972).
H2O2 concentrations in culture media were determined by the Amplex Red/horseradish peroxidase method (Seaver & Imlay, 2001b). Because H2O2 rapidly equilibrates across the membranes of cells that lack scavenging activities, the extracellular H2O2 concentration is an excellent approximation of the intracellular concentration.
Plasmid constructions
The open reading frames of targeted genes were PCR-amplified from E. coli MG1655 by using the primers in Table 2. The PCR products were digested with XbaI and EcoRI and cloned into either pWKS30 or pCKR101 vectors. The plasmid constructions were confirmed by restriction/sequencing analyses. In addition, complementation experiments were performed in the Hpx+ backgrounds to verify that the plasmids were functional. The proteins encoded by pWKS30 or pCKR101 derivatives were expressed in lactose or glucose/1 mM IPTG media, respectively.
Table 2.
| Plasmid | Primer sequences |
|---|---|
| pSUF1 | Forward 5′-ATATCGAATTCTAAGTAAGAGGTAAATCGATGGACA-3′ |
| Reverse 5′-CATGGATCTAGATTAGCTAAGTGCAGCGGC-3′ | |
| pISC1 | Forward 5′-ATATCGAATTCTTTAATACGGAGTTTATAGAGCA-3′ |
| pISC2 | Reverse 5′-CATGGATCTAGA TTATTCGGCCTCGTCCAG-3′ |
| pIscS-(His)6 | Forward 5′-ATCGATCCATATGAAATTACCGATTTATCTCGAC-3′ |
| Reverse 5′-CATATAGGATCCTTAATGATGAGCCCATTCGATG-3′ | |
| pIscSUA | Forward 5′-ATATCGAATTCTTTAATACGGAGTTTATAGAGCA-3′ |
| Reverse 5′-CATGGATCTAGATCAAACGTGGAAGCTTTC-3′ | |
| pSJ104 | Forward 5′-ATATCGAATTCTTTAACGCAGCCCTGAGAATGTT-3′ |
| Reverse 5′-CATGGATCTAGA TTATTCGGCCTCGTCCAG-3′ | |
| pSJ107 | Forward 5′-ATATCGAATTCTTTAACCGTGGACGAGGTTTAAT-3′ |
| Reverse 5′-CATGGATCTAGA TTATTCGGCCTCGTCCAG-3′ | |
| pPsuf | Forward 5′-ATATGCCTGCAGCTTAAGGGTTTTCTTATTTC-3′ |
| Reverse 5′-TATACCGGTACCCATCGATTTACCTCACTTC-3′ | |
| pPsufNI | Forward 5′-ATATGCCTGCAGCTAACAATGAGATACCTAATTC-3′ |
| Reverse 5′-TATACCGGTACCCATCGATTTACCTCACTTC-3′ | |
| pPiscR | Forward 5′-ATATGCCTGCAGAGGTCGGATAAGGCGTTC-3′ |
| Reverse 5′-TATACCGGTACCCATGTCTTACTTCACCTC-3′ | |
| pPiscRS | Forward 5′-ATATGCCTGCAGAGGTCGGATAAGGCGTTC-3′ |
| Reverse 5′-TATACCGGATCCCATTGCTCTATAAACTCC-3′ | |
| pPleuL2 | Forward 5′-ATATGCCTGCAGTACTTAACTCCACTGTCA-3′ |
| Reverse 5′-TATACCGGTACCCTGTTCACCGTCGCGCAATG-3′ | |
Construction of transcriptional fusions
Single-copy chromosomal transcriptional fusions were constructed by the CRIM plasmid-host system (Haldimann & Wanner, 2001). Promoter regions of genes were PCR-amplified with primers in Table 2 and inserted into pSJ501 at KpnI and PstI restriction sites. The plasmids that contain transcriptional fusions were then integrated at the attB site (attλ) on chromosomal DNA by the integrase that is provided by pAH57. Single-copy integrants were verified by PCR. The transcriptional fusions in chromosomal DNA were introduced into desired strains by P1 transduction. Unless otherwise indicated, each fusion strain retained a working wild-type copy of the gene at its normal locus.
Inactivation and reactivation of enzymes
Cells were grown anaerobically for at least four generations to OD600 = 0.2. H2O2 was added to the cultures when they were aerated. At time points, aliquots were removed, catalase was added to 200 U/ml, and cells were returned to the anaerobic chamber for centrifugation, lysis, and assay.
In vivo inactivation of IPMI by endogenous H2O2 was initiated by aerating anaerobic Hpx− cultures without any addition of exogenous H2O2. Under these conditions the cells steadily generate H2O2, which was measured to be 0.5–1 μM. Cell extracts were then prepared and assayed in the anaerobic chamber.
The inactivation of enzymes in vitro was accomplished by the addition of H2O2 to lysates or to purified enzyme in anaerobic buffer. The H2O2 was subsequently removed by catalase prior to anaerobic assay. In some cases, damaged [3Fe-4S] clusters were chemically repaired by incubation with 100 μM Fe(NH4)2(SO4)2 and 2.5 mM dithiothreitol (DTT) for at least 10 min at room temperature. In vitro reconstitution of more extensively degraded iron-sulfur clusters was carried out by incubating enzymes in 50 mM Tris-Cl (pH 7.65) containing 500 μM Fe(NH4)2(SO4)2, 5 mM DTT, 2.5 mM cysteine, and purified IscS (0.16 mU) at room temperature for 20–60 min.
In vivo repair of fumarase was monitored in Hpx− cultures. Cells were incubated with 20 μM H2O2 in the presence of chloramphenicol for 5 min. Catalase was subsequently added to terminate H2O2 stress and fumarase activities were measured over time.
Purification of enzymes
Amplified iscS and fdx were inserted in pET15b at NdeI and BamHI restriction sites. E. coli BL21(DE3) cells containing pIsc-(His)6 were grown aerobically in LB to OD600 of approximately 0.1, and 1 mM IPTG was added, followed by another four-hour incubation at 37°C. The overexpressed IscS-(His)6 was purified using His Gravitrap (GE healthcare). The activity of the purified His-tagged enzyme was 22 mU/mg.
To purify ferredoxin, the Hpx− strain that contains pTrcfdx was grown anaerobically to OD600~ 0.1 at 37°C, and 1 mM IPTG was added. To achieve the optimum synthesis of ferredoxin, the cells were cultured for another 12 hours at room temperature. The cells were then harvested by centrifugation, and the cell pellets were resuspended in 15 ml of anaerobic 50 mM Tris-Cl/10 mM β–mercaptoethanol/0.1 mM EDTA (TBME, pH 7.4). Cell lysates were prepared by sonication, and cell debris was removed by centrifugation. The purification was performed as described before with modifications (Ta & Vickery, 1992). All steps of the purification were conducted in an anaerobic chamber at room temperature, and all buffers were anaerobic. The cell extract was loaded onto a DEAE-Sepharose column (DFF: Fast flow) and eluted by 0.2 M of KCl in TBME. The fractions that showed the visible spectrum of [2Fe-2S]2+ clusters were pooled and then loaded onto the second DEAE column (DCL-6B). The proteins were eluted by 0–0.4 M gradient of KCl in TBME. The purified ferredoxin was concentrated using Amicon ultra-15 centrifugal filter (3 kDa cutoff). The fractions that showed the spectrum of [2Fe-2S]2+ clusters were frozen in dry ice containing ethanol. Purified ferredoxin was more than 90% pure based on SDS-PAGE and was quantified by dye-binding assay using bovine serum albumin as a standard.
Fumarase A was overproduced and purified as described previously (Jang & Imlay, 2007).
Western blot analysis
Cells were grown for at least four generations anaerobically to an OD600 of approximately 0.1. Aerobic growth was then initiated by vigorous aeration of the cultures. At time points cells were harvested, and samples were prepared for sodium dodecyl sulfate-polyacrylamidegel electrophoresis (SDS-PAGE). Anaerobic cultures were also harvested as controls. Samples that contained 42 μg of proteins were loaded on 12 % SDS-PAGE gel. Gels were either stained with Coomassieblue dye or transferred to nitrocellulose. After blotting with a 5% skim milk solution, a 1:1,000 dilution of rabbit serum containing anti-A. vinelandii IscU was used as the primary antibody. Horseradish peroxidase-conjugated anti-rabbit goat immunoglobulin G was used as the secondary antiserum (1:20,000 dilution). The detection process was performed using chemiluminescent reagents (ECL™ Western Blotting Detection Reagents).
EPR analysis of Isc proteins
Hpx− cells expressing genes of the isc operon behind the tac promoter were grown in minimal glucose medium anaerobically, and 1 mM IPTG was added when cells were at an OD600 of 0.2. After another 4 hours of incubation, the cells were harvested by centrifugation at 7500 rpm for 10 min, and the cell pellets were resuspended in 1/500 of original culture volumes of 10 % glycerol. The resuspended cells were incubated with H2O2 for 5 min at room temperature, and then catalase was added to remove H2O2. In the attempts to detect [2Fe-2S] or [4Fe-4S] clusters, 10 mM sodium dithionite was added. The cell suspension (250 μl) then was transferred into an EPR tube and frozen in dry ice.
EPR spectra of [Fe-S]clusters were obtained with the following settings: microwave power, 1 milliwatt; microwave frequency, 9.05 GHz; modulation amplitude,8 Gauss at 100 KHz; time constant, 0.032; temperature, 15 K.
Intracellular free-iron measurements
Cells were grown in minimal glucose medium to an OD600 of 0.1–0.2 and harvested by centrifugation at 7000 g for 5 min at 4°C. A cell pellet was resuspended in 8 ml prewarmed fresh minimal medium that contain 10 mM DETAPAC (diethylentriaminepentaacetic acid, pH 7.0) and 20 mM desferrioxamine (pH 8.0). DETAPAC blocks further iron import, while desferrioxamine diffuses into cells and binds unincorporated iron in an EPR-visible ferric form. The concentrated cells were incubated at 37 °C for 15 min shaking in a water bath. The cells were washed with 5 ml of ice cold 20 mM Tris-Cl (pH 7.4) twice. Cells were then resuspended in 200 μl of ice cold 10% glycerol/20mM Tris-Cl (pH 7.4). The cell suspension (200 μl) then was transferred into an EPR tube and frozen in dry ice. Ferric sulfate standards were mixed with desferrioxamine and prepared in the same Tris buffer containing glycerol. The spectrometer settings were as follows: microwave power, 10 milliwatt; microwave frequency, 9.05 GHz; modulation amplitude,12.5 Gauss at 100 KHz; time constant, 0.032; temperature, 15 K.
Supplementary Material
Acknowledgments
We thank Mark Nilges of the Illinois EPR Research Center for assistance with EPR experiments, Gisela Storz and Larry E. Vickery for generously providing plasmids that were used in this study, and Dennis R. Dean for the kind donation of Azotobacter vinelandii IscU antibodies. This work was supported by grant GM49640 from the National Institutes of Health.
References
- Agar JN, Krebs C, Frazzon J, Huynh BH, Dean DR, Johnson MK. IscU as a Scaffold for Iron-Sulfur Cluster Biosynthesis: Sequential Assembly of [2Fe-2S] Clusters in IscU. Biochemistry. 2000;39:7856–7862. doi: 10.1021/bi000931n. [DOI] [PubMed] [Google Scholar]
- Anjem A, Varghese S, Imlay JA. Manganese import is a key element of the OxyR response to hydrogen peroxide in Escherichia coli. Mol Microbiol. 2009;72:844–858. doi: 10.1111/j.1365-2958.2009.06699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006;141:391–396. doi: 10.1104/pp.106.082040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslund F, Zheng M, Beckwith J, Storz G. Regulation of the OxyR transcriptional factor by hydrogen peroxide and the cellular thiol-disulfide status. Proc Natl Acad Sci USA. 1999;96:6161–6165. doi: 10.1073/pnas.96.11.6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averyanov A. Oxidative burst and plant disease resistance. Front Biosci (Elite Ed) 2009;1:142–152. doi: 10.2741/E14. [DOI] [PubMed] [Google Scholar]
- Ayala-Castro C, Saini A, Outten FW. Fe-S cluster assembly pathways in bacteria. Microbiol Mol Biol Rev. 2008;72:110–125. doi: 10.1128/MMBR.00034-07. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandyopadhyay S, Chandramouli K, Johnson MK. Iron-sulfur cluster biosynthesis. Biochem Soc Trans. 2008;36:1112–1119. doi: 10.1042/BST0361112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barras F, Loiseau L, Py B. How Escherichia coli and Saccharomyces cerevisiae build Fe/S proteins. Adv Microb Physiol. 2005;50:41–101. doi: 10.1016/S0065-2911(05)50002-X. [DOI] [PubMed] [Google Scholar]
- Bitoun JP, Wu G, Ding H. Escherichia coli FtnA acts as an iron buffer for re-assembly of iron-sulfur clusters in response to hydrogen peroxide stress. Biometals. 2008;21:693–703. doi: 10.1007/s10534-008-9154-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazzolotto X, Gaillard J, Pantopoulos K, Hentze MW, Moulis JM. Human cytoplasmic aconitase (Iron regulatory protein 1) is converted into its [3Fe-4S] form by hydrogen peroxide in vitro but is not activated for iron-responsive element binding. J Biol Chem. 1999;274:21625–21630. doi: 10.1074/jbc.274.31.21625. [DOI] [PubMed] [Google Scholar]
- Cairo G, Recalcati S. Iron-regulatory proteins: molecular biology and pathophysiological implications. Expert Rev Mol Med. 2007;9:1–13. doi: 10.1017/S1462399407000531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmel-Harel O, Storz G. Roles of the glutathione- and thioredoxin-dependent reduction systems in the Escherichia coli and Saccharomyces cerevisiae responses to oxidative stress. Annu Rev Microbiol. 2000;54:439–461. doi: 10.1146/annurev.micro.54.1.439. [DOI] [PubMed] [Google Scholar]
- Castro LA, Robalinho RL, Cayota A, Meneghini R, Radi R. Nitric oxide and peroxynitrite-dependent aconitase inactivation and iron-regulatory protein-1 activation in mammalian fibroblasts. Arch Biochem Biophys. 1998;359:215–224. doi: 10.1006/abbi.1998.0898. [DOI] [PubMed] [Google Scholar]
- Cherepanov PP, Wackernagel W. Gene disruption in Escherichia coli: TcR and KmR cassettes with the option of Flp-catalyzed excision of the antibiotic-resistance determinant. Gene. 1995;158:9–14. doi: 10.1016/0378-1119(95)00193-a. [DOI] [PubMed] [Google Scholar]
- Condon C, Cammack R, Patil DS, Owen P. The succinate dehydrogenase of Escherichia coli. Immunochemical resolution and biophysical characterization of a 4-subunit enzyme complex. J Biol Chem. 1985;260:9427–9434. [PubMed] [Google Scholar]
- Crack JC, Le Brun NE, Thomson AJ, Green J, Jervis AJ. Reactions of nitric oxide and oxygen with the regulator of fumarate and nitrate reduction, a global transcriptional regulator, during anaerobic growth of Escherichia coli. Methods Enzymol. 2008;437:191–209. doi: 10.1016/S0076-6879(07)37011-0. [DOI] [PubMed] [Google Scholar]
- Cupp-Vickery JR, Urbina H, Vickery LE. Crystal structure of IscS, a cysteine desulfurase from Escherichia coli. J Mol Biol. 2003;330:1049–1059. doi: 10.1016/s0022-2836(03)00690-9. [DOI] [PubMed] [Google Scholar]
- Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desnoyers G, Morissette A, Prevost K, Masse E. Small RNA-induced differential degradation of the polycistronic mRNA iscRSUA. EMBO J. 2009;28:1551–1561. doi: 10.1038/emboj.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djaman O, Outten FW, Imlay JA. Repair of oxidized iron-sulfur clusters in Escherichia coli. J Biol Chem. 2004;279:44590–44599. doi: 10.1074/jbc.M406487200. [DOI] [PubMed] [Google Scholar]
- Flint DH, Tuminello JF, Emptage MH. The inactivation of Fe-S cluster containing hydro-lyases by superoxide. J Biol Chem. 1993;268:22369–22376. [PubMed] [Google Scholar]
- Gardner PR, Fridovich I. Superoxide sensitivity of the Escherichia coli 6-phosphogluconate dehydratase. J Biol Chem. 1991;266:1478–1483. [PubMed] [Google Scholar]
- Giel JL, Rodionov D, Liu M, Blattner FR, Kiley PJ. IscR-dependent gene expression links iron-sulphur cluster assembly to the control of O2-regulated genes in Escherichia coli. Mol Microbiol. 2006;60:1058–1075. doi: 10.1111/j.1365-2958.2006.05160.x. [DOI] [PubMed] [Google Scholar]
- Grant RA, Filman DJ, Finkel SE, Kolter R, Hogle JM. The crystal structure of Dps, a ferritin homolog that binds and protects DNA. Nat Struct Biol. 1998;5:294–303. doi: 10.1038/nsb0498-294. [DOI] [PubMed] [Google Scholar]
- Gunsalus RP, Park SJ. Aerobic-anaerobic gene regulation in Escherichia coli: control by the ArcAB and Fnr regulons. Res Microbiol. 1994;145:437–450. doi: 10.1016/0923-2508(94)90092-2. [DOI] [PubMed] [Google Scholar]
- Haldimann A, Wanner BL. Conditional-replication, integration, excision, and retrieval plasmid-host systems for gene structure-function studies of bacteria. J Bacteriol. 2001;183:6384–6393. doi: 10.1128/JB.183.21.6384-6393.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle ES, Han Z, Tang N, Rai P, Luo Y, Linn S. Sequence-specific DNA cleavage by Fe2+-mediated Fenton reactions has possible biological implications. J Biol Chem. 1999;274:962–971. doi: 10.1074/jbc.274.2.962. [DOI] [PubMed] [Google Scholar]
- Ilari A, Ceci P, Ferrari D, Rossi G, Chiancone E. Iron incorporation into E. coli Dps gives rise to a ferritin-like microcrystalline core. J Biol Chem. 2002;277:37619–37623. doi: 10.1074/jbc.M206186200. [DOI] [PubMed] [Google Scholar]
- Imlay JA. Cellular defenses against superoxide and hydrogen peroxide. Annu Rev Biochem. 2008a;77:755–776. doi: 10.1146/annurev.biochem.77.061606.161055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imlay JA. How obligatory is anaerobiosis? Mol Microbiol. 2008b;68:801–804. doi: 10.1111/j.1365-2958.2008.06213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imlay JA, Chin SM, Linn S. Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science. 1988;240:640–642. doi: 10.1126/science.2834821. [DOI] [PubMed] [Google Scholar]
- Imlay JA, Linn S. Bimodal pattern of killing of DNA-repair-defective or anoxically grown Escherichia coli by hydrogen peroxide. J Bacteriol. 1986;166:519–527. doi: 10.1128/jb.166.2.519-527.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S, Imlay JA. Micromolar intracellular hydrogen peroxide disrupts metabolism by damaging iron-sulfur enzymes. J Biol Chem. 2007;282:929–937. doi: 10.1074/jbc.M607646200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justino MC, Almeida CC, Goncalves VL, Teixeira M, Saraiva LM. Escherichia coli YtfE is a di-iron protein with an important function in assembly of iron-sulphur clusters. FEMS Microbiol Lett. 2006;257:278–284. doi: 10.1111/j.1574-6968.2006.00179.x. [DOI] [PubMed] [Google Scholar]
- Justino MC, Almeida CC, Teixeira M, Saraiva LM. Escherichia coli di-iron YtfE protein is necessary for the repair of stress-damaged iron-sulfur clusters. J Biol Chem. 2007;282:10352–10359. doi: 10.1074/jbc.M610656200. [DOI] [PubMed] [Google Scholar]
- Kullik I, Toledano MB, Tartaglia LA, Storz G. Mutational analysis of the redox-sensitive transcriptional regulator OxyR: regions important for oxidation and transcriptional activation. J Bacteriol. 1995;177:1275–1284. doi: 10.1128/jb.177.5.1275-1284.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Yeo WS, Roe JH. Induction of the sufA operon encoding Fe-S assembly proteins by superoxide generators and hydrogen peroxide: involvement of OxyR, IHF and an unidentified oxidant-responsive factor. Mol Microbiol. 2004;51:1745–1755. doi: 10.1111/j.1365-2958.2003.03946.x. [DOI] [PubMed] [Google Scholar]
- Lee KC, Yeo WS, Roe JH. Oxidant-responsive induction of the suf operon, encoding a FeS assembly system, through Fur and IscR in Escherichia coli. J Bacteriol. 2008;190:8244–8247. doi: 10.1128/JB.01161-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin DE, Hollstein M, Christman MF, Schwiers EA, Ames BN. A new Salmonella tester strain (TA102) with A.T base pairs at the site of mutation detects oxidative mutagens. PNAS. 1982;79:7445–7449. doi: 10.1073/pnas.79.23.7445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Yang J, Tan G, Ding H. Complementary roles of SufA and IscA in the biogenesis of iron-sulfur clusters in Escherichia coli. Biochem J. 2008;409:535–543. doi: 10.1042/BJ20071166. [DOI] [PubMed] [Google Scholar]
- Macomber L, Imlay JA. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc Natl Acad Sci U S A. 2009;106:8344–8349. doi: 10.1073/pnas.0812808106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettert EL, Outten FW, Wanta B, Kiley PJ. The impact of O(2) on the Fe-S cluster biogenesis requirements of Escherichia coli FNR. J Mol Biol. 2008;384:798–811. doi: 10.1016/j.jmb.2008.09.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JH. Experiments in Molecular Genetics. Cold Spring Harbor Laboratory; Cold Spring Harbor, N.Y: 1972. [Google Scholar]
- Nachin L, El Hassouni M, Loiseau L, Expert D, Barras F. SoxR-dependent response to oxidative stress and virulence of Erwinia chrysanthemi: the key role of SufC, an orphan ABC ATPase. Mol Microbiol. 2001;39:960–972. doi: 10.1046/j.1365-2958.2001.02288.x. [DOI] [PubMed] [Google Scholar]
- Nachin L, Loiseau L, Expert D, Barras F. SufC: an unorthodox cytoplasmic ABC ATPase required for [Fe-S] biogenesis under oxidative stress. EMBO J. 2003;22:427–437. doi: 10.1093/emboj/cdg061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi T. Iron-sulfur clusters/semiquinones in Complex I. Biochim Biophys Acta. 1998;1364:186–206. doi: 10.1016/s0005-2728(98)00027-9. [DOI] [PubMed] [Google Scholar]
- Outten FW, Djaman O, Storz G. A suf operon requirement for Fe-S cluster assembly during iron starvation in Escherichia coli. Mol Microbiol. 2004;52:861–872. doi: 10.1111/j.1365-2958.2004.04025.x. [DOI] [PubMed] [Google Scholar]
- Panduri V, Weitzman SA, Chandel NS, Kamp DW. Mitochondrial-derived free radicals mediate asbestos-induced alveolar epithelial cell apoptosis. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1220–1227. doi: 10.1152/ajplung.00371.2003. [DOI] [PubMed] [Google Scholar]
- Park S, You X, Imlay JA. Substantial DNA damage from submicromolar intracellular hydrogen peroxide detected in Hpx- mutants of Escherichia coli. Proc Natl Acad Sci USA. 2005;102:9317–9322. doi: 10.1073/pnas.0502051102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SG, Cha MK, Jeong W, Kim IH. Distinct physiological functions of thiol peroxidase isoenzymes in Saccharomyces cerevisiae. J Biol Chem. 2000;275:5723–5732. doi: 10.1074/jbc.275.8.5723. [DOI] [PubMed] [Google Scholar]
- Petrova VY, Drescher D, Kujumdzieva AV, Schmitt MJ. Dual targeting of yeast catalase A to peroxisomes and mitochondria. Biochem J. 2004;380:393–400. doi: 10.1042/BJ20040042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Py B, Barras F. Building Fe-S proteins: bacterial strategies. Nat Rev Microbiol. 2010;8:436–446. doi: 10.1038/nrmicro2356. [DOI] [PubMed] [Google Scholar]
- Ranquet C, Ollagnier-de-Choudens S, Loiseau L, Barras F, Fontecave M. Cobalt stress in Escherichia coli. The effect on the iron-sulfur proteins. J Biol Chem. 2007;282:30442–30451. doi: 10.1074/jbc.M702519200. [DOI] [PubMed] [Google Scholar]
- Raulfs EC, O’Carroll IP, Dos Santos PC, Unciuleac MC, Dean DR. In vivo iron-sulfur cluster formation. Proc Natl Acad Sci U S A. 2008;105:8591–8596. doi: 10.1073/pnas.0803173105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JM. Phagocytic leukocytes and reactive oxygen species. Histochem Cell Biol. 2009;131:465–469. doi: 10.1007/s00418-009-0565-5. [DOI] [PubMed] [Google Scholar]
- Runyen-Janecky L, Daugherty A, Lloyd B, Wellington C, Eskandarian H, Sagransky M. Role and regulation of iron-sulfur cluster biosynthesis genes in Shigella flexneri virulence. Infect Immun. 2008;76:1083–1092. doi: 10.1128/IAI.01211-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz CJ, Giel JL, Patschkowski T, Luther C, Ruzicka FJ, Beinert H, Kiley PJ. IscR, an Fe-S cluster-containing transcription factor, represses expression of Escherichia coli genes encoding Fe-S cluster assembly proteins. Proc Natl Acad Sci U S A. 2001;98:14895–14900. doi: 10.1073/pnas.251550898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaver LC, Imlay JA. Alkyl hydroperoxide reductase is the primary scavenger of endogenous hydrogen peroxide in Escherichia coli. J Bacteriol. 2001a;183:7173–7181. doi: 10.1128/JB.183.24.7173-7181.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaver LC, Imlay JA. Hydrogen peroxide fluxes and compartmentalization inside growing Escherichia coli. J Bacteriol. 2001b;183:7182–7189. doi: 10.1128/JB.183.24.7182-7189.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Gunsalus RP. Role of multiple ArcA recognition sites in anaerobic regulation of succinate dehydrogenase (sdhCDAB) gene expression in Escherichia coli. Mol Microbiol. 1997;26:223–236. doi: 10.1046/j.1365-2958.1997.5561923.x. [DOI] [PubMed] [Google Scholar]
- Shimomura Y, Kamikubo H, Nishi Y, Masako T, Kataoka M, Kobayashi Y, Fukuyama K, Takahashi Y. Characterization and crystallization of an IscU-type scaffold protein with bound [2Fe-2S] cluster from the hyperthermophile, aquifex aeolicus. J Biochem. 2007;142:577–586. doi: 10.1093/jb/mvm163. [DOI] [PubMed] [Google Scholar]
- Siegel LM. A Direct Microdetermination for Sulfide. Anal Biochem. 1965;11:126–132. doi: 10.1016/0003-2697(65)90051-5. [DOI] [PubMed] [Google Scholar]
- Smith AD, Agar JN, Johnson KA, Frazzon J, Amster IJ, Dean DR, Johnson MK. Sulfur transfer from IscS to IscU: the first step in iron-sulfur cluster biosynthesis. J Am Chem Soc. 2001;123:11103–11104. doi: 10.1021/ja016757n. [DOI] [PubMed] [Google Scholar]
- Ta DT, Vickery LE. Cloning, sequencing, and overexpression of a [2Fe-2S] ferredoxin gene from Escherichia coli. J Biol Chem. 1992;267:11120–11125. [PubMed] [Google Scholar]
- Takahashi Y, Nakamura M. Functional assignment of the ORF2-iscS-iscU-iscA-hscB-hscA-fdx-ORF3 gene cluster involved in the assembly of Fe-S clusters in Escherichia coli. J Biochem. 1999;126:917–926. doi: 10.1093/oxfordjournals.jbchem.a022535. [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Tokumoto U. A third bacterial system for the assembly of iron-sulfur clusters with homologs in archaea and plastids. J Biol Chem. 2002;277:28380–28383. doi: 10.1074/jbc.C200365200. [DOI] [PubMed] [Google Scholar]
- Tang Y, Guest JR, Artymiuk PJ, Green J. Switching aconitase B between catalytic and regulatory modes involves iron-dependent dimer formation. Mol Microbiol. 2005;56:1149–1158. doi: 10.1111/j.1365-2958.2005.04610.x. [DOI] [PubMed] [Google Scholar]
- Tokumoto U, Kitamura S, Fukuyama K, Takahashi Y. Interchangeability and distinct properties of bacterial Fe-S cluster assembly systems: functional replacement of the isc and suf operons in Escherichia coli with the nifSU-like operon from Helicobacter pylori. J Biochem (Tokyo) 2004;136:199–209. doi: 10.1093/jb/mvh104. [DOI] [PubMed] [Google Scholar]
- Urbina HD, Silberg JJ, Hoff KG, Vickery LE. Transfer of sulfur from IscS to IscU during Fe/S cluster assembly. J Biol Chem. 2001;276:44521–44526. doi: 10.1074/jbc.M106907200. [DOI] [PubMed] [Google Scholar]
- Varghese S, Wu A, Park S, Imlay KRC, Imlay JA. Submicromolar hydrogen peroxide disrupts the ability of Fur protein to control free-iron levels in Escherichia coli. Mol Microbiol. 2007;64:822–830. doi: 10.1111/j.1365-2958.2007.05701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinella D, Brochier-Armanet C, Loiseau L, Talla E, Barras F. Iron-sulfur (Fe/S) protein biogenesis: phylogenomic and genetic studies of A-type carriers. PLoS Genet. 2009;5:e1000497. doi: 10.1371/journal.pgen.1000497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RF, Kushner SR. Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene. 1991;100:195–199. [PubMed] [Google Scholar]
- Yeo WS, Lee JH, Lee KC, Roe JH. IscR acts as an activator in response to oxidative stress for the suf operon encoding Fe-S assembly proteins. Mol Microbiol. 2006;61:206–218. doi: 10.1111/j.1365-2958.2006.05220.x. [DOI] [PubMed] [Google Scholar]
- Zheng M, Wang X, Templeton LJ, Smulski DR, LaRossa RA, Storz G. DNA microarray-mediated transcriptional profiling of the Escherichia coli response to hydrogen peroxide. J Bacteriol. 2001;183:4562–4570. doi: 10.1128/JB.183.15.4562-4570.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


