Summary
Relatively few transcription factors that govern the virulence of Aspergillus fumigatus are known. We constructed 11 A. fumigatus transcription factor mutants and screened them for altered virulence in Galleria mellonella larvae. We discovered that the zinc cluster transcription factor, AcuM, is essential for maximal virulence in this model, as well as in murine models of hematogenously disseminated and invasive pulmonary aspergillosis. Transcriptional profiling experiments suggested that AcuM suppresses sreA and induces hapX to stimulate expression of genes involved in both reductive iron assimilation and siderophore-mediated iron uptake. Consistent with these results, a ΔacuM mutant had reduced iron incorporation, decreased extracellular siderophore production, and impaired capacity to grow under iron-limited conditions. Interestingly, an Aspergillus nidulans ΔacuM mutant had normal extracellular siderophore production and growth under iron-limited conditions, indicating that AcuM does not govern iron acquisition in this organism. A. fumigatus AcuM also regulated genes involved in gluconeogenesis, and the ΔacuM mutant had impaired growth on gluconeogenic carbon sources. Deletion of sreA in the ΔacuM mutant restored iron uptake, extracellular siderophore production, and virulence, but not the defect in gluconeogenesis. Thus, AcuM represses SreA and thereby induces iron acquisition, a process that is essential for the maximal virulence of A. fumigatus.
Introduction
Aspergillus fumigatus is a ubiquitous saprophytic mold that causes the majority of cases of invasive aspergillosis (Maschmeyer et al., 2007; Patterson et al., 2005). The incidence of invasive aspergillosis has risen substantially due to the increasing number of immunosuppressed patients (Marr et al., 2002). Even with current antifungal therapy, the mortality associated with invasive aspergillosis is approximately 50%, and it approaches 100% when hematogenously disseminated disease is present (Patterson et al., 2000; Patterson et al., 2005; Pegues et al., 2001). The mechanisms by which A. fumigatus causes invasive disease are incompletely understood. Identifying virulence factors of this fungus is important because this information holds promise for developing new approaches for diagnosing and treating invasive aspergillosis.
One approach to identifying virulence factors is to determine the signaling pathways that regulate them. Transcription factors are highly useful in this regard, because they frequently govern the expression of multiple target genes. Thus, deletion of a single transcription factor gene has a higher probability of influencing virulence compared to deletion of a gene encoding a putative virulence factor. Among the 9900 genes annotated in A. fumigatus genome, approximately 322 specify transcription factors (Nierman et al., 2005). To date, the list of transcription factors and transcriptional regulators that have been found to govern A. fumigatus virulence is relatively short. This list includes Ace2, SrbA, Hac1, CrzA, LaeA, ZafA, CpcA, MedA, and DvrA (Bok et al., 2005; Cramer et al., 2008; Ejzykowicz et al., 2009; Ejzykowicz et al., 2010; Gravelat et al., 2009; Krappmann et al., 2004; Moreno et al., 2007a; Soriani et al., 2008; Sugui et al., 2007; Willger et al., 2008).
To identify additional transcription factors that govern the virulence of A. fumigatus, we selected 12 genes that were predicted to specify A. fumigatus transcription factors that might influence pathogenicity, based on their homology to transcription factors in other organisms, expression in response to environmental stress, and uniqueness to A. fumigatus and other fungi. From this list, we constructed 11 transcription factor deletion strains and screened them for alterations in virulence. We discovered that the zinc cluster transcription factor, AcuM is required for maximal virulence during both hematogenously disseminated and invasive pulmonary aspergillosis in mice.
In other fungi such as Saccharomyces cerevisiae and Aspergillus nidulans, orthologs of A. fumigatus AcuM govern gluconeogenesis (Hynes et al., 2007; Soontorngun et al., 2007). We found that in A. fumigatus, AcuM not only regulates gluconeogenesis, but it also governs both reductive iron assimilation and siderophore-mediated iron uptake. In Aspergillus spp. iron acquisition under iron-depleted conditions is governed by SreA and HapX (Hortschansky et al., 2007; Schrettl et al., 2008). Under low iron conditions, expression of the GATA factor SreA is reduced, which results in derepression of the bZip transcription factor, HapX, as well as genes involved in both reductive iron assimilation and siderophore activity (Schrettl et al., 2008). HapX binds to the CCAAT-binding complex and also induces the expression of iron assimilation and siderophore genes (Hortschansky et al., 2007). We found that AcuM regulates iron acquisition under iron-depleted conditions mainly by repressing SreA and possibly by stimulating HapX.
Results
Screening of A. fumigatus transcription factor deletion mutants suggests that AcuM may be required for virulence
We selected 12 putative A. fumigatus transcription factors, based on their homology to transcription factors in other organisms, expression in response to environmental stress, and uniqueness to A. fumigatus and other fungi (Table 1). For 11 of these transcription factors, we were able to construct corresponding deletion mutants in the wild-type strain, Af293. However, despite multiple attempts, we were unable to delete gene Afu2g10770, which specifies an ortholog of Con7 in Magnaporthe grisea. Thus, this gene may either be essential in A. fumigatus, or required for growth under the selection conditions.
Table 1.
List of A. fumigatus genes that are predicted to specify transcription factors, and for which null mutants were constructed.
| Strain | A. fumigatus Gene | Ortholog | Function of ortholog | Virulence of null mutant (Median survival time in days)a |
|---|---|---|---|---|
| Af293 (wild-type) | 8.5 | |||
| ΔAfu1g02860 | Afu1g02860 | Fusarium graminearum TRI6 | Regulator of mycotoxin production (Pinson-Gadais et al., 2008; Proctor et al., 1995; Seong et al., 2009) | 7.5 |
| ΔAfu1g04140 | Afu1g04140 | A. fumigatus specific transcription factor | 7.5 | |
| ΔAfu2g04600 | Afu2g04600 | Aspergillus. parasticus SugR | Regulator of sugar utilization (Yu et al., 2000) | 7.5 |
| ΔacuM | Afu2g12330 (acuM) | Saccharomyces cerevisiae Rds2; Candida albicans Cwt1; Aspergillus nidulans AcuM | Regulator of cell wall integrity and gluconeogenesis (Moreno et al., 2003; Moreno et al., 2007a; Moreno et al., 2008; Soontorngun et al., 2007) | 12.0b |
| ΔacuM ΔsreA | Afu2g12330 (acuM) Afu5g11260 (sreA) | A. nidulans SreA | Negative regulator of iron homeostasis | NDc |
| ΔAfu2g16310 | Afu2g16310 | Fungal specific transcription factor | 8.0 | |
| ΔAfu4g09710 | Afu4g09710 | A. nidulans NosA | Regulator of sexual development (Vienken and Fischer, 2006) | 8.0 |
| ΔsteA | Afu5g06190 (steA) | S. cerevisiae Ste12; C. albicans Cph1 | Regulator of filamentation and mating (Braun and Johnson, 2000; Lewis et al., 2002; Liu et al., 1994; Ramer and Davis, 1993; Vallim et al., 2000) | 9.0 |
| ΔAfu5g10130 | Afu5g10130 | A. fumigatus specific transcription factor | 7.0 | |
| ΔAfu6g07170 | Afu6g07170 | A. nidulans RosA | Regulator of sexual development, repressor of NosA (Vienken et al., 2005) | 10.0 |
| Δyap1 | Afu6g09930 (yap1) | S. cerevisiae Yap1 | Regulator of oxidative stress tolerance (Delaunay et al., 2000) | 9.0 |
| ΔAfu8g05460 | Afu8g05460 | Up-regulated in heat shock (Do et al., 2009) | 7.0 | |
| Afu2g10770 | Magnaporthe. grisea Con7 | Essential for appressorium formation and disease-related morphogenesis (Odenbach et al., 2007) | ---d |
Survival of G. mellonella larvae after infection with 106 conidia of the indicated null mutant Data are the combined results two experiments, each using 20–30 larvae per strain
P < 0.001 compared to larvae infected with Af293
Not determined
Deletion mutant for this gene was unable to be constructed
Next, we screened these deletion mutants for alterations in virulence using the G. mellonella larva model of invasive aspergillosis. Of the 11 mutants tested, only the Afu2g12330 deletion mutant had significantly reduced virulence (Table 1). Afu2g12330 was initially selected for deletion analysis because its gene product has 26% identity to Candida albicans Cwt1 (orf19.5849) and 25% identity to Saccharomyces cerevisiae Rds2 (YPL133C). Both of these zinc cluster transcription factors govern cell wall composition and integrity (Moreno et al., 2003; Moreno et al., 2008). Rds2 also regulates gluconeogenesis in S. cerevisiae (Soontorngun et al., 2007); whether Cwt1 governs this process in C. albicans is currently unknown. A close ortholog of Afu2g12330 in Aspergillus nidulans is acuM (ANIA_06293; 54% amino acid identity), which also governs gluconeogenesis (Hynes et al., 2007). Based on the extensive homology between A. nidulans AcuM and the A. fumigatus Afu2g12330 gene product, we named Afu2g12330 acuM. Orthologs of acuM are present in many filamentous ascomycetes, including other species of Aspergillus, Coccidioides spp., Paracoccidioides brasiliensis, and Penicillium spp (Hynes et al., 2007).
As annotated in the A. fumigatus genome sequencing project, the protein specified by acuM contains only 4 conserved cysteine residues, whereas most zinc cluster transcription factors have 6 (Naar and Thakur, 2009). Therefore, we amplified acuM cDNA by high-fidelity PCR and sequenced the resulting product to determine if there had been an error during genome sequencing. We found that there had been an error in intron prediction and that acuM actually specifies a 627 amino acid protein that contains the expected 6 cysteines. This corrected sequence has been deposited in Genbank (GU290314).
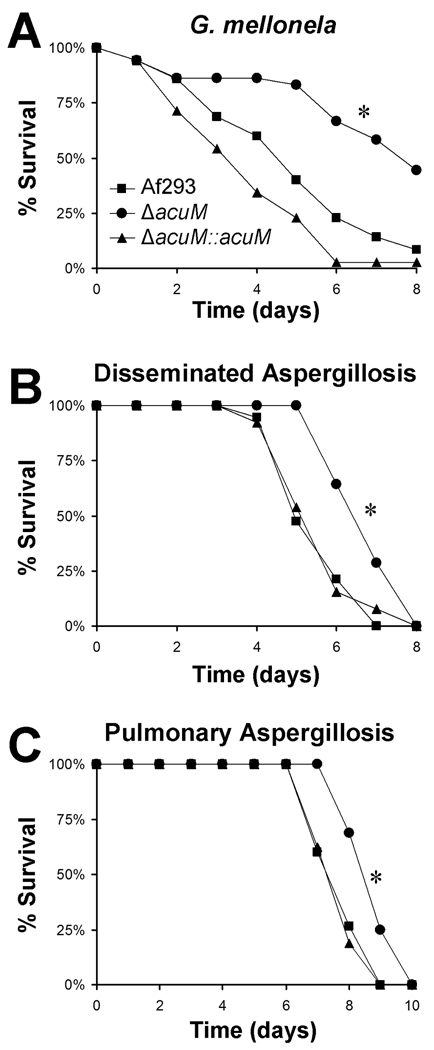
To confirm that the attenuated virulence of the ΔacuM mutant was due to the absence of AcuM, we constructed a ΔacuM∷acuM complemented strain. The acuM mRNA expression in this strain was similar to that of the wild type strain, as determined by real-time PCR (data not shown). Next, we compared the virulence of the wild-type strain, ΔacuM mutant, and the ΔacuM∷acuM complemented strain in G. mellonella. As predicted, complementation of the ΔacuM mutant with a wild-type copy of acuM restored virulence to wild-type levels (Fig. 1A), thus verifying that acuM is essential for maximal virulence in this model.
Fig. 1.
Deletion of acuM results in reduced virulence in G. mellonella and murine models of invasive aspergillosis. (A) Survival of G. mellonella larvae after injection with conidia of the indicated strains. Representative results of one of three experiments, each with 35 larvae per strain. (B) Survival of neutropenic mice with hematogenously disseminated aspergillosis. Mice were immunosuppressed with cyclophosphamide and cortisone acetate, inoculated intravenously with germlings of the indicated strain, and followed for survival. Results are the combined data from two experiments for a total of 13–19 mice per strain. (C) Survival of non-neutropenic mice with invasive pulmonary aspergillosis. Mice were immunosuppressed with cortisone acetate and then inoculated by placing them in an acrylic chamber filled with an aerosol of conidia from the indicated strains of A. fumigatus. Results are the combined data from two experiments for a total of 15–16 mice per strain. *P ≤ 0.005 compared to Af293 and the ΔacuM∷acuM complemented strains.
The ΔacuM mutant has attenuated virulence in mouse models of hematogenously disseminated and invasive pulmonary aspergillosis
To determine whether AcuM governs A. fumigatus virulence in mammals, we tested the ΔacuM mutant in two different murine models of invasive aspergillosis. The first was a neutropenic model of hematogenously disseminated disease. In this model, the mice were immunosuppressed with cyclophosphamide and cortisone acetate, and then inoculated intravenously with A. fumigatus germlings. Mice infected with the ΔacuM mutant survived significantly longer than those infected with the wild-type and ΔacuM∷acuM complemented strains (p ≤ 0.005) (Fig. 1B). In the second model, the mice were immunosuppressed with high-dose cortisone acetate, and then invasive pulmonary disease was induced by placing them in an acrylic chamber filled with an aerosol of A. fumigatus conidia. Infection with the ΔacuM mutant also resulted in significantly delayed mortality in this model (p < 0.005 compared to the wild-type and ΔacuM∷acuM complemented strains) (Fig. 1C). Collectively, these results indicate that acuM is necessary for the normal virulence of A. fumigatus during both disseminated and invasive pulmonary aspergillosis.
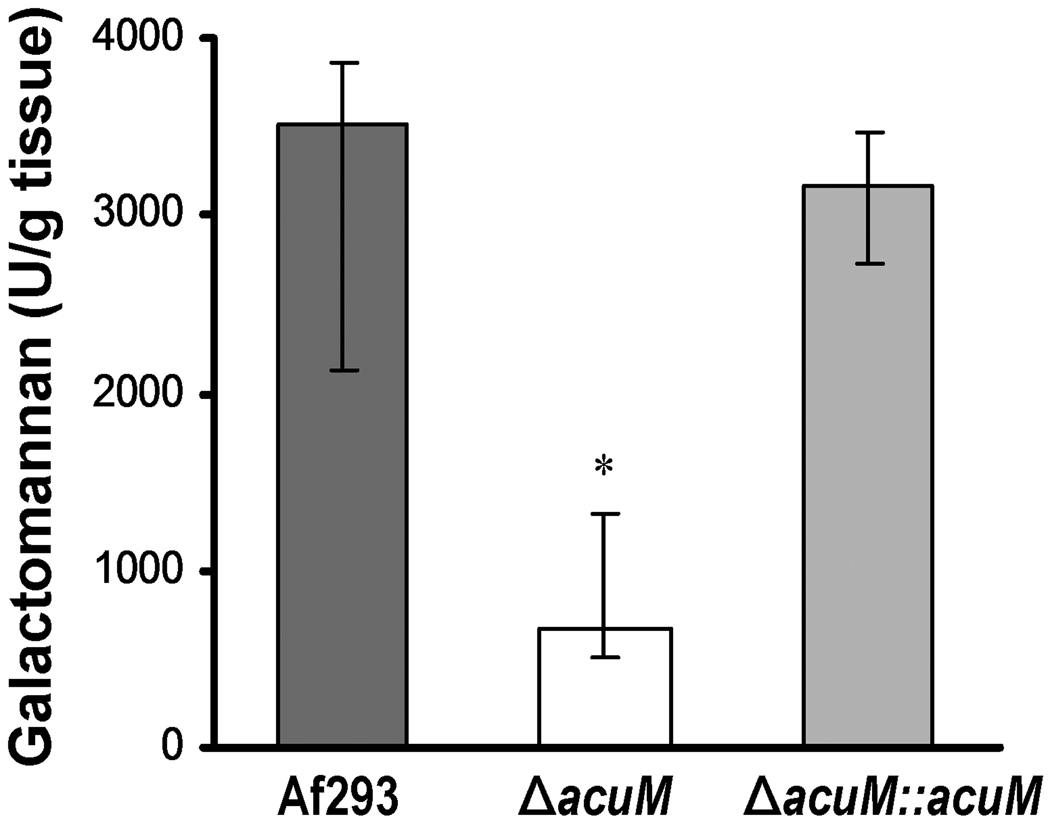
To verify the reduced virulence of the ΔacuM mutant, we investigated the pulmonary fungal burden of mice infected in the aerosol chamber by measuring the level of galactomannan in their lungs after 4 days infection (Sheppard et al., 2006). Preliminary in vitro studies demonstrated that the ΔacuM mutant released a similar amount of galactomannan into the medium as did the wild-type strain (data not shown), thus confirming that galactomannan can be used as a measure of organ fungal burden with this strain. As anticipated, the pulmonary galactomannan content of mice infected with the ΔacuM mutant was significantly lower than that of mice infected with either the wild-type or ΔacuM∷acuM complemented strains (p < 0.001) (Fig. 2). Therefore, acuM is required for maximal growth of A. fumigatus in the lungs. Furthermore, the delayed mortality of mice with invasive pulmonary aspergillosis caused by the ΔacuM mutant was likely due in part to their lower pulmonary fungal burden.
Fig. 2.
Mice infected with the ΔacuM mutant had a lower pulmonary fungal burden. Non-neutropenic mice were infected with the indicated strains in the aerosol chamber and then sacrificed after 4 days for determination of pulmonary galactomannan content. Results are median ± interquartile range of 7 to 9 mice per strain. *P < 0.001 compared to Af293 and the ΔacuM∷acuM complemented strain.
Analysis of the time course of acuM mRNA expression
To investigate the function of AcuM in A. fumigatus, we first used real-time PCR to analyze the time course of acuM mRNA expression in the wild-type strain when it was grown in Aspergillus minimal medium (AMM) with iron at 37°C. acuM appeared to be expressed constitutively as acuM mRNA levels were similar in swollen conidia, germlings and hyphae (data not shown).
Transcription profiling suggests that AcuM governs genes involved in reductive and siderophore mediated iron acquisition, and carbon metabolism
Next, we performed microarray analysis of the ΔacuM mutant to identify genes that require AcuM for normal expression. To simulate the relatively nutrient-poor conditions within the host, the organisms were grown in liquid RPMI 1640 medium at 37°C as described in Experimental Procedures. A total of 251 genes were down-regulated and 119 genes were up-regulated in the ΔacuM mutant compared to the wild-type strain and the ΔacuM∷acuM complemented strain at the 18 h and/or 24 h time points. A complete list of these genes is contained in Supplemental Table S2. Gene ontology (GO) term analysis of the genes that were down-regulated in the ΔacuM mutant indicated that they were significantly (p < 0.001) enriched in terms related to iron acquisition and homeostasis, carbon metabolism, and synthesis of methionine and glutamate (Table 2). The genes contained in each of the categories are listed in Supplemental Table 3. The genes that were up-regulated in the ΔacuM mutant were not significantly enriched in any GO term. Although the AcuM orthologs, Rds2 and Cwt1 govern cell wall structure in S. cerevisiae and C. albicans, respectively (Moreno et al., 2003; Moreno et al., 2008), very few genes involved in cell wall synthesis showed AcuM-dependent expression, suggesting that AcuM does not play a significant role in governing cell wall structure in A. fumigatus.
Table 2.
Overrepresented functional categories of A. fumigatus genes that were down-regulated in the ΔacuM mutant compared to the wild-type and ΔacuM∷acuM complemented strain.
| GO Term | P value | |
|---|---|---|
| 18 h | 24 h | |
| Siderophore-iron transport | 0.17 | 0.0000015 |
| Iron ion homeostasis | 0.45 | 0.00028 |
| Gluconeogenesis | 0.00035 | 0.00095 |
| Glyoxylate cycle | 1.0 | 0.00092 |
| One-carbon compound metabolism | 0.00036 | 1.0 |
| Methionine metabolism | 0.0000088 | 1.0 |
| Glutamate biosynthesis | 0.0012 | 0.0000045 |
A. fumigatus can obtain iron from the environment by two mechanisms, reductive iron assimilation and production of siderophores (Schrettl et al., 2004). We found that two genes in the reductive iron assimilation pathway (ftrA and fre2) were significantly down-regulated in the ΔacuM mutant, as shown by the microarray data and verified by real-time PCR (Table 3). Furthermore, multiple genes involved in siderophore synthesis (sidA, sidC, sidF, sidG, and Afu3g03390) and siderophore transport (mirB, sit1, and Afu7g04730) were also down-regulated in this mutant (Table 3). Thus, the transcriptional profiling data suggested that AcuM controls both reductive iron assimilation and siderophore-mediated iron acquisition.
Table 3.
List of genes involved in siderophore synthesis and transport, and iron uptake that were down-regulated in the ΔacuM mutant compared to Af293.
| A. fumigatus gene | Fold-change in mRNA level in the ΔacuM mutant | Putative function | ||
|---|---|---|---|---|
| Microarray (18 h)a | Microarray (24 h)a | Real-time PCR (24 h)b | ||
| Afu1g17270 (fre2) | −3.2 | −2.4 | −2.3 | Ferric-chelate reductase |
| Afu5g03800 (ftrA) | −2.8 | −1.6 | −1.6 | High-affinity iron permease |
| Afu2g07680 (sidA) | −4.0 | −1.4 | −4.2 | L-ornithine N5-oxygenase |
| Afu1g17200 (sidC) | −1.7 | −1.2 | −1.7 | Nonribosomal peptide synthase |
| Afu3g03420 (sidD) | −3.3 | −2.0 | −3.1 | Fusarinine C nonribosomal peptide synthetase |
| Afu3g03650 (sidG) | −3.0 | −1.9 | −11.4 | Fusarinine C acetyltransferase |
| Afu3g03400 (sidF) | −3.4 | −2.1 | −7.2 | Siderophore biosynthesis acetylase |
| Afu3g03390 | −2.3 | −1.7 | −5.0 | Siderophore biosynthesis lipase/esterase |
| Afu3g03640 (mirB) | −4.0 | −2.7 | −4.1 | Siderophore transporter |
| Afu7g06060 (sit1) | −2.2 | −2.1 | −2.1 | Siderophore transporter |
| Afu7g04730 | −2.4 | −1.8 | −2.1 | Siderophore transporter |
| Afu6g07720 (acuF) | −9.4 | −6.4 | −16.2 | Phosphoenolpyruvate carboxykinase |
| Afu4g11310 (fbp1) | −2.9 | −1.7 | −7.2 | Fructose-1,6-bisphosphatase |
Mean of 4 biological replicates in RPMI 1640 medium
Mean of 3 biological replicates in AMM without iron and with 300 µM ferrozine
Deletion of acuM results in increased expression of sreA and reduced expression of hapX
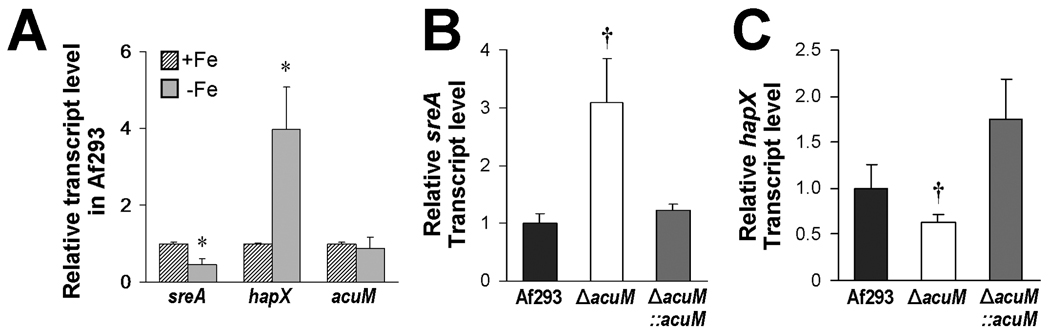
In Aspergillus spp. iron acquisition under iron-depleted conditions is governed by SreA and HapX (Hortschansky et al., 2007; Schrettl et al., 2008). Because AcuM also governs the expression of genes involved in these processes, we investigated the expression of sreA and hapX in the ΔacuM mutant. First, we verified that growth of wild-type A. fumigatus under iron-depleted conditions results in reduced sreA expression and increased hapX expression (Fig. 3A). Interestingly, acuM transcript levels were unaffected by the iron content of the medium.
Fig. 3.
Effects of extracellular iron and acuM deletion on sreA and hapX mRNA levels. (A) A. fumigatus Af293 was grown in Aspergillus minimal medium (AMM) containing ferrozine and FeSO4 (+Fe) or AMM containing ferrozine without FeSO4 (−Fe) for 24 h, after which the relative transcript levels of the indicated genes were determined by real-time PCR. (B and C) The indicated strains of A. fumigatus were grown for 24 h in AMM containing ferrozine without FeSO4, and then the relative transcript levels of sreA (B) and hapX (C) were determined by real-time PCR. Results are the mean ± SD of three biological replicates, each tested in triplicate. *P < 0.05 compared to organisms grown in the presence of FeSO4; †p < 0.05 compared to Af293 or the ΔacuM∷acuM complemented strain.
When the ΔacuM mutant was grown under iron-limited conditions, sreA mRNA levels were 3-fold higher in this strain than in the wild-type strain (Fig. 3B). Also, hapX expression was reduced by 1.6-fold compared to the wild-type strain (Fig. 3C). These alterations in sreA and hapX transcript levels were due to the absence of acuM because they were restored to wild-type levels in the ΔacuM∷acuM complemented strains (Figs. 3B and C). Collectively, these results suggest that acuM either directly or indirectly regulates the expression of sreA and hapX.
Deletion of SreA is known to result in the increased expression of 49 genes, most of which are involved in iron acquisition, siderophore production and siderophore transport (Schrettl et al., 2008). The finding that sreA had increased expression in the ΔacuM mutant (Fig. 3B) predicted that many SreA-responsive genes would be down-regulated in this strain. Indeed, we found that 26 of 49 SreA-responsive genes had significantly reduced expression in the ΔacuM mutant (Fig. 4). It is known that many SreA-responsive genes are located in clusters along the chromosomes (Schrettl et al., 2008). We determined that 5 of the 8 SreA-responsive gene clusters were down-regulated in the ΔacuM mutant (Fig. 4). These gene clusters were located on chromosomes 1, 3, 5 and 8, and contained genes involved in siderophore biosynthesis and transport. Collectively, these results suggest that AcuM governs siderophore-mediate iron acquisition at least in part by repressing SreA.
Fig. 4.
Cluster analysis of SreA-responsive gene expression in the ΔacuM mutant. Microarray data comparing the response of the ΔacuM mutant with Af293 (WT) and the ΔacuM∷acuM complemented strain (Comp) after 18 and 24 h incubation in RPMI 1640 medium at 37°C are shown. The bar at the top indicates the colors that correspond to the observed expression ratios. The genes are displayed in the order of their chromosomal location and the vertical colored boxes indicate gene clusters. The numbers in these boxes correspond to the SreA-responsive gene clusters in (Schrettl et al., 2008). The gene names in blue font denote genes that had significantly reduced transcript levels in the ΔacuM mutant compared to the control strains.
AcuM is required for normal iron incorporation, extracellular siderophore production, and growth in iron deficient media
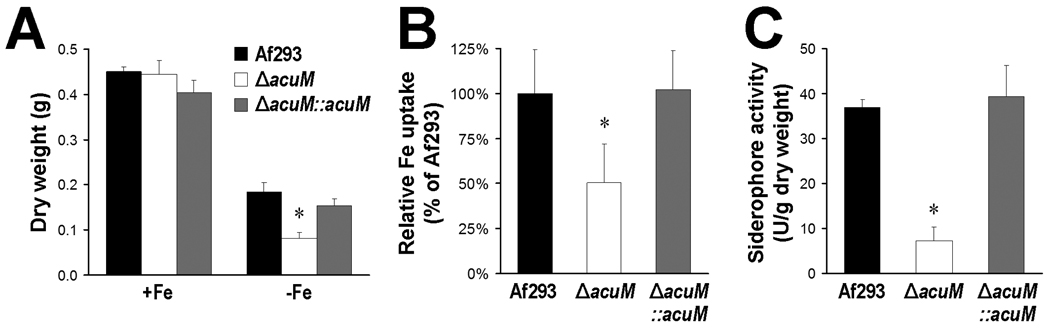
To test the predictions that AcuM governs both reductive iron assimilation and siderophore-mediated iron uptake, we first tested the capacity of the ΔacuM mutant to grow under iron limited conditions. These experiments were performed using organisms grown in Sabouraud broth containing the iron chelator, phenanthroline because the ΔacuM mutant grew more slowly than the wild-type strain in AMM, even with iron supplementation. Similar results have been reported with an A. fumigatus ΔsidA mutant (Hissen et al., 2005). As predicted by the transcriptional profiling data, the ΔacuM mutant grew significantly more slowly than the wild-type or ΔacuM∷acuM complemented strains under iron-limited conditions (Fig. 5A). This growth defect was rescued by supplementing the medium with iron. Next, we measured the capacity of washed germlings of the ΔacuM mutant to incorporate 55FeCl3 from the medium. We found that the ΔacuM mutant incorporated approximately 50% less iron did than the wild-type and ΔacuM∷acuM complemented strains (p < 0.001) (Fig. 5B). Because the germlings were washed prior to being tested in this assay, it is probable that the defective iron uptake of the ΔacuM mutant was due to impaired reductive iron assimilation rather than reduced extracellular siderophore production. Finally, we measured the accumulation of extracellular siderophore activity in the culture medium using the chrome Azurol S (CAS) assay (Schwyn and Neilands, 1987). When grown in AMM without iron, the extracellular siderophore activity of the ΔacuM mutant was reduced by at least 80% compared to the wild-type and ΔacuM∷acuM complemented strains (p < 0.001) (Fig. 5C). Collectively, these results indicate that the impaired growth of the ΔacuM mutant under iron-limited conditions was due to defects in both reductive iron assimilation and extracellular siderophore production.
Fig. 5.
Deletion of acuM resulted in decreased growth in a low iron medium, impaired iron incorporation, and reduced extracellular siderophore production. (A) Dry weight of the indicated strains after 40 h of incubation in Sabouraud broth containing phenanthroline with (+Fe) or without FeSO4 (−Fe). (B) Relative incorporation of 55FeCl3 after 1 h by washed germlings of the indicated strains. (C) Extracellular siderophore activity of the indicated strains after 30 h of growth. Results are mean ± SD of three (A and B) or four (C) experiments, each performed in triplicate. *P < 0.001 compared to Af293 and the ΔacuM∷acuM complemented strain.
AcuM is dispensable for siderophore production and growth under iron-limited conditions of A. nidulans
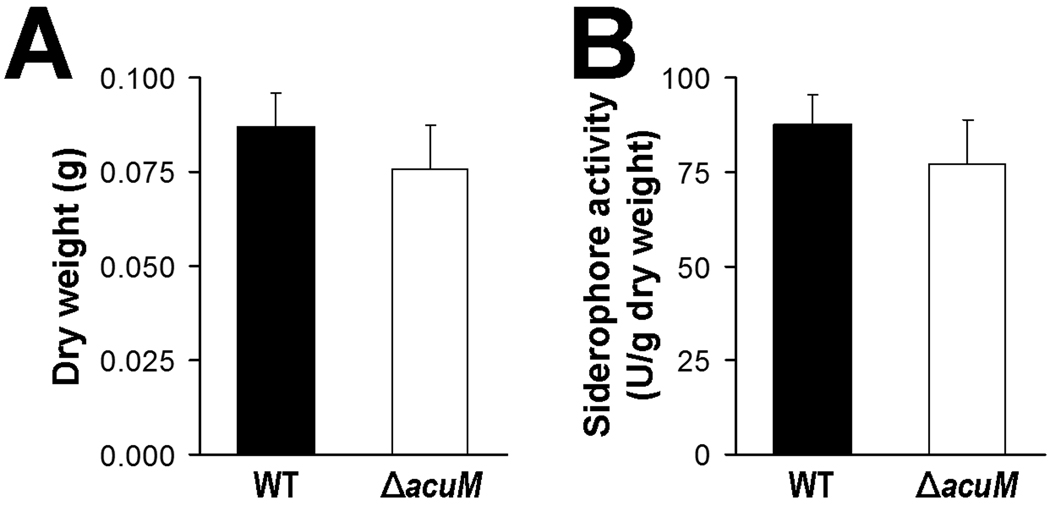
A. fumigatus AcuM shares significant homology with A. nidulans AcuM (Hynes et al., 2007). Therefore, we investigated whether AcuM also governs iron homeostasis in A. nidulans. We found that an A. nidulans ΔacuM mutant did not have any significant defects in extracellular siderophore activity or growth under iron-limited conditions (Fig. 6). Therefore, AcuM does not appear to play a significant role in regulating siderophore-mediated iron assimilation or growth under iron-limited conditions in A. nidulans.
Fig. 6.
Role of A. nidulans AcuM in extracellular siderophore production and growth under iron limited conditions. (A) Dry weight of the indicated strains after 24 h growth in AMM without iron. (B) The indicated strains of A. nidulans were grown in AMM without iron for 24 h, after which the total extracellular siderophore activity was determined. Results are mean ± SD of three independent experiments.
The ΔacuM mutant is defective in gluconeogenesis
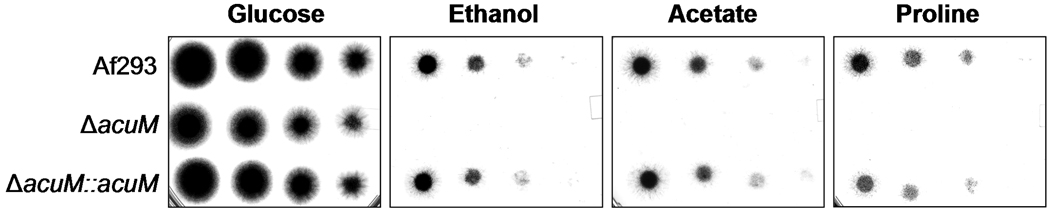
In A. nidulans, AcuM governs gluconeogenesis (Hynes et al., 2007), and our transcriptional profiling results indicated that AcuM also controls gluconeogenesis in A. fumigatus. For example, acuF and fbp1 were significantly down-regulated in the ΔacuM mutant (Table 3). These genes specify phosphoenolpyruvate carboxykinase and fructose-1,6,bisphosphatase, respectively, important enzymes that are specific for gluconeogenesis (Hynes et al., 2007). To verify that the ΔacuM mutant was defective in gluconeogenesis, we examined its growth in media containing carbon sources other than glucose. The ΔacuM mutant had a minor growth defect on AMM agar containing supplemental iron when glucose was the carbon source (Fig. 7). However, it was virtually unable to grow when the carbon source was ethanol, acetate, or proline. Taken together, these data indicate that AcuM is required for normal gluconeogenesis in A. fumigatus.
Fig. 7.
AcuM is required for normal growth on gluconeogenic carbon sources. Serial 10-fold dilutions of the indicated strains were grown on Aspergillus minimal medium supplemented with FeCl3 and containing the indicated carbon sources. The plates were imaged after incubation at 37°C for 40 h.
AcuM is not required for resistance to environmental stress and antifungal agents
The AcuM orthologs, Cwt1 and Rds2, govern cell wall structure in C. albicans and S. cerevisiae, respectively (Moreno et al., 2003; Moreno et al., 2008). Also, sreA, whose expression is governed in part by AcuM, is required for normal resistance to oxidative stress and amphotericin B (Schrettl et al., 2008). Therefore, we investigated the susceptibility of the ΔacuM mutant to various stressors and antifungal agents in iron replete conditions. This mutant was similar to the wild-type strain in its susceptibility to Congo red, calcofluor white, hydrogen peroxide, SDS, caspofungin, and amphotericin B (data not shown). Therefore, under the conditions tested, AcuM does not appear to influence cell wall integrity, or susceptibility to oxidative stress or antifungal agents.
Absence of AcuM results in increased susceptibility to killing by neutrophil-like and macrophage-like HL-60 cells
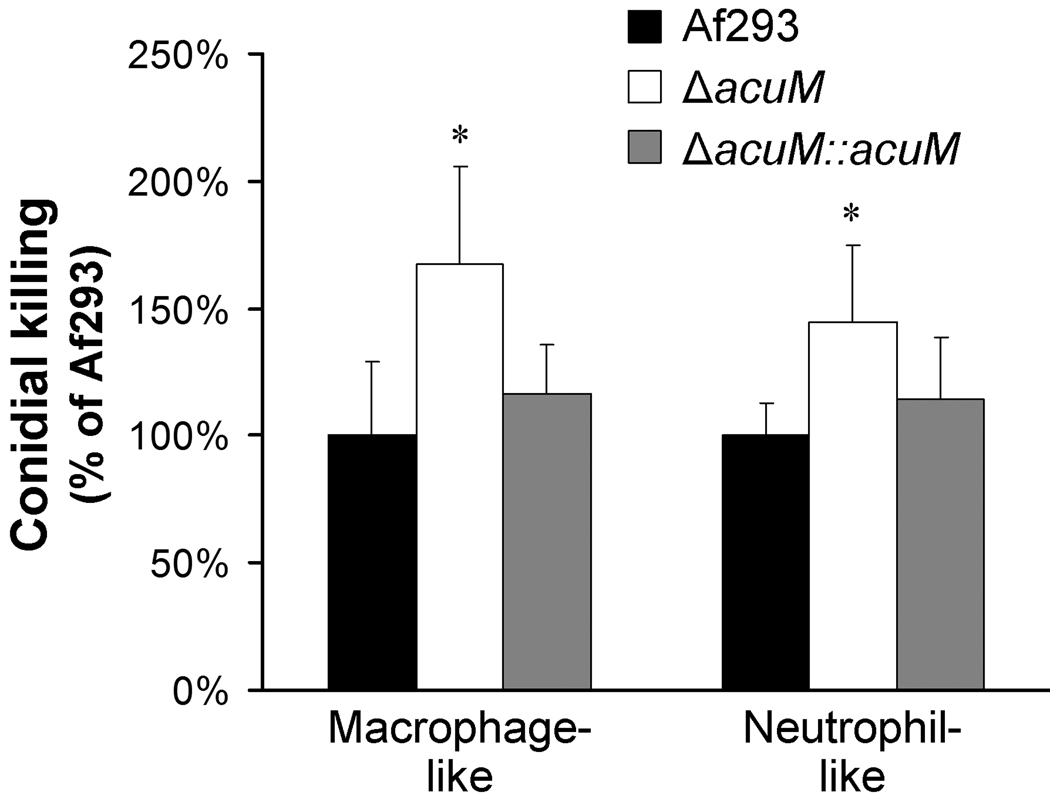
Macrophages and neutrophils sequester iron and make it unavailable to microbial pathogens (Seifert et al., 2008; Zarember et al., 2007). In addition, C. albicans cells that have been phagocytosed by either macrophages or neutrophils have increased gluconeogenesis, suggesting that the phagocytic vacuole is a low-glucose environment (Barelle et al., 2006). These data led us to test whether deletion of acuM influenced the susceptibility of A. fumigatus to leukocyte killing. We found that swollen conidia of the ΔacuM mutant were significantly more susceptible to killing by HL-60 cells that had been differentiated into either macrophage-like or neutrophil-like cells (Fig. 8). These results demonstrate that AcuM is required for A. fumigatus to resist killing by phagocytic leukocytes. They further suggest that increased susceptibility to phagocyte killing contributes to the attenuated virulence of the ΔacuM mutant.
Fig. 8.
Increased susceptibility of the ΔacuM mutant to phagocyte killing. Swollen conidia of the indicated strains were incubated for 3 h with HL-60 cells that had been differentiated into macrophage-like or neutrophil-like cells. The number of organisms killed was determined by quantitative culture. Results are mean ± SD of three independent experiments each performed in triplicate. *P < 0.01 compared to Af293 or the ΔacuM∷acuM complemented strain.
Deletion of sreA rescues the defects in iron acquisition and virulence of the ΔacuM mutant
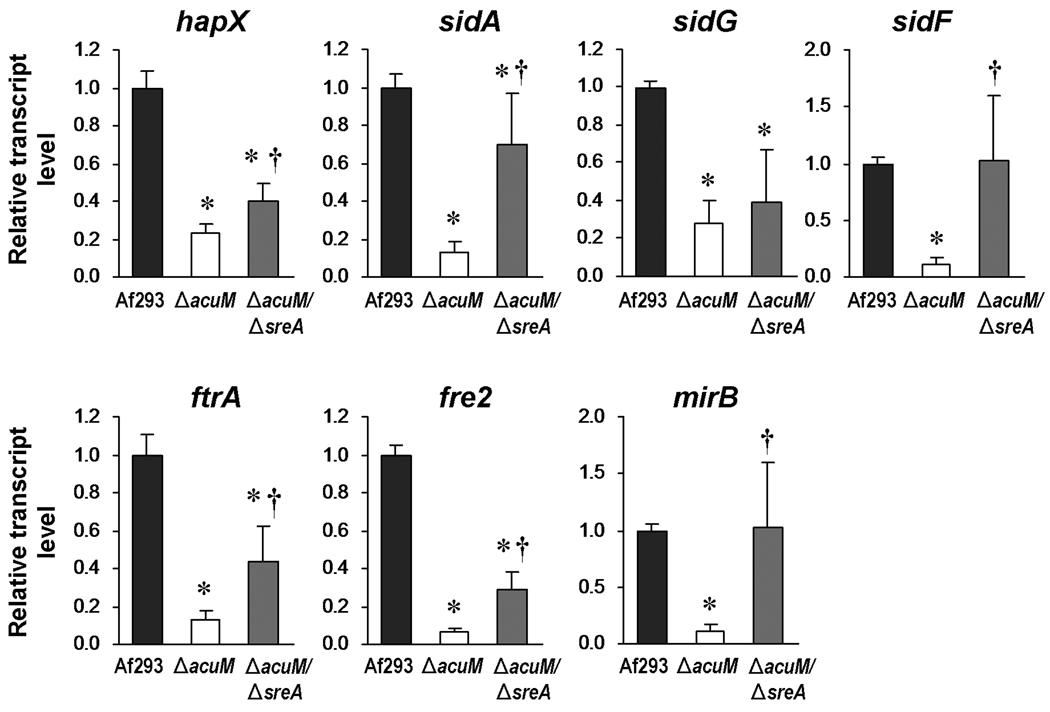
SreA functions as a negative regulator repressor of iron acquisition (Schrettl et al.), and sreA transcript levels were increased by 3-fold in the ΔacuM mutant (Fig. 3B). This finding suggested that the reduced iron acquisition of the ΔacuM mutant was due in part to the elevated expression of sreA. To test this hypothesis, we constructed a ΔacuM ΔsreA double mutant. Deletion of sreA in the ΔacuM mutant resulted in higher transcript levels of the regulatory gene, hapX, as well as genes involved in siderophore biosynthesis and transport (sidA, sigF, and mirB), and high-affinity iron uptake (ftrA and fre2) (Fig. 9). However, mRNA levels of sidG were not significantly increased in the ΔacuM ΔsreA double mutant, suggesting that the expression of this gene may not be repressed by SreA in the absence of AcuM.
Fig. 9.
Deletion of sreA in the ΔacuM mutant results in enhanced expression of genes involved in iron acquisition. The indicated strains of A. fumigatus were incubated for 24 h at 37°C in AMM without iron containing 300 µM ferrozine, after which the relative transcript levels of the indicated genes were determined by real-time PCR. Results are the mean ± SD of 3 biological replicates, each tested in triplicate. *P < 0.05 compared to Af293, †p < 0.05 compared to the ΔacuM single mutant.
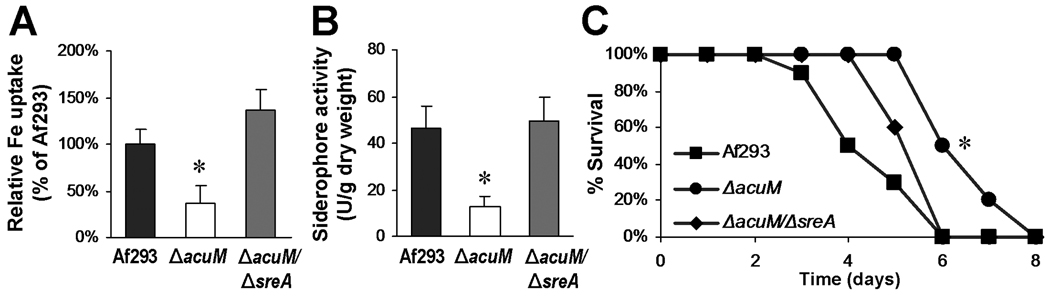
Next, we investigated the phenotype of the ΔacuM ΔsreA double mutant. Consistent with transcriptional profiling results, deletion of sreA the ΔacuM background restored incorporation of 55FeCl3 and extracellular siderophore production to wild-type levels (Figs. 10A and B). In addition, when tested in the neutropenic mouse model of disseminated aspergillosis, the ΔacuM ΔsreA double mutant was significantly more virulent than the ΔacuM mutant (p = 0.003). Mice infected with the ΔacuM ΔsreA double mutant survived slightly longer than those infected with the wild-type strain, but this difference was not significant (p = 0.062). Collectively, these results indicate that the reduced iron uptake, decreased siderophore production, and attenuated virulence of the ΔacuM mutant are largely the result of increased expression of sreA.
Fig. 10.
The ΔacuM ΔsreA double mutant has wild-type iron uptake, extracellular siderophore production, and virulence. (A) Relative incorporation of 55FeCl3 after 1 h by washed germlings of the indicated strains. Results are mean ± SD of three experiments, each performed in triplicate. (B) Extracellular siderophore activity of the indicated strains after 30 h of growth. Results are mean ± SD of three experiments, each performed in triplicate. (C) Survival of neutropenic mice after intravenous inoculation with conidia of the indicated strains. Each strain was inoculated into 10 mice. *P < 0.005 compared to Af293 and the ΔacuM ΔsreA double mutant.
Discussion
AcuM was selected for inclusion in our screen for transcription factors that govern virulence because AcuM orthologs participate in governing cell wall integrity in C. albicans and S. cerevisiae, as well as gluconeogenesis in S. cerevisiae and A. nidulans (Hynes et al., 2007; Moreno et al., 2003; Moreno et al., 2008; Soontorngun et al., 2007). The extent of homology between A. fumigatus AcuM and A. nidulans AcuM predicted our findings that the ΔacuM mutant had reduced expression of genes involved in gluconeogenesis and defective ability to grow on gluconeogenic carbon sources. However, a novel discovery was that, in A. fumigatus, AcuM plays a central role in iron homeostasis, governing both high-affinity iron assimilation and siderophore-mediated iron uptake. This conclusion is supported by the transcriptional profiling results, as well as phenotypic data which showed that the ΔacuM mutant had reduced iron incorporation, decreased siderophore production, and impaired growth under iron-limited conditions.
In Aspergillus species, iron homeostasis is governed by the SreA and HapX transcription factors. We found that when the ΔacuM mutant was grown under iron-limited conditions, sreA mRNA levels were inappropriately elevated whereas hapX mRNA levels were abnormally low. Because deletion of acuM increased the transcript levels of sreA by 3-fold, but only reduced hapX transcript levels by 1.6-fold, it is likely that AcuM governs iron homeostasis predominantly via its effects on SreA. In support of this hypothesis, 26 of 49 of previously identified SreA-responsive genes (Schrettl et al., 2008) had reduced expression in the ΔacuM mutant. Also, most of these AcuM-dependent genes were located in the same chromosomal clusters as the SreA-responsive genes. This overlap between AcuM and SreA is even more impressive when one considers that the transcriptional profiling experiments of the ΔacuM and ΔsreA mutants used organisms grown under significantly different conditions. In experiments with the ΔacuM mutant, the organisms were grown under iron-limited conditions, whereas the transcriptional profiling experiments of the ΔsreA mutants used organisms that had been initially iron starved and then switched to iron-replete conditions (Schrettl et al., 2008). Our finding that deletion of sreA in the ΔacuM mutant restored iron uptake and extracellular siderophore production provides additional evidence that AcuM controls iron homeostasis mainly via SreA. At present, it is unknown whether AcuM directly binds to the promoter of sreA or whether it represses sreA transcript levels by an indirect mechanism.
Schrettl et al. (Schrettl et al., 2008) also identified 9 iron repressed genes that were not under the control of SreA. Interestingly, 3 of these genes (Afu5g02330 [ribotoxin AspF1], Afu5g00720 [GCN5-related N-acetyltransferase], and Afu1g03150 [C-14 sterol reductase]) had reduced expression in the ΔacuM mutant. These results suggest that AcuM may also govern some responses to iron independently of SreA. In A. nidulans, HapX and SreA exhibit mutual transcriptional control, and each influences iron homeostasis (Hortschansky et al., 2007; Schrettl et al., 2008). The transcriptional profile of an A. fumigatus ΔhapX mutant has not been reported. However, some proteins whose levels are reduced in an A. nidulans ΔhapX mutant (Hortschansky et al., 2007) correspond to genes that are down-regulated in the A. fumigatus ΔacuM mutant. This overlap includes 3-hydroxy-3-methylglutaryl CoA synthase (Afu3g10660), hydantoinase/oxoprolinase (Afu4g11460), and GMC oxidoreductase (Afu3g01580). We predict that the transcriptional profile of an A. fumigatus ΔhapX mutant would be even more similar to that of the ΔacuM mutant.
It is also notable that the transcriptional profiling studies of the A. fumigatus ΔsreA mutant indicated that SreA governs the expression of some genes involved in carbon metabolism (Schrettl et al., 2008). At least one of these genes, Afu6g04920 (NAD-dependent formate dehydrogenase) (Kennedy et al., 2009) was down-regulated in the ΔacuM mutant. This result provides an additional link between regulation of iron homeostasis and carbon metabolism in A. fumigatus. It further suggests that in A. fumigatus, AcuM may govern carbon metabolism partly by repressing SreA. However, deletion of sreA in the ΔacuM mutant did not restore its capacity to grow on gluconeogenic carbon sources (data not shown). Thus, gluconeogenesis is likely governed predominantly by AcuM, independently of SreA.
We found that the ΔacuM mutant had attenuated virulence in G. mellonela larvae. This virulence defect was subsequently verified in murine models of disseminated and invasive pulmonary aspergillosis. Our finding that deletion of sreA in the ΔacuM mutant restored both iron acquisition and virulence suggests that virulence defect of the ΔacuM mutant was largely due to impaired growth within the iron-limited environment of the host. Moreover, the near wild-type virulence of the ΔacuM ΔsreA double mutant, which remained defective in growth on gluconeogenic substrates, indicates that there are adequate glyoclytic substrates within the host and that gluoconeogenesis is dispensable for A. fumigatus virulence. Although the ΔacuM mutant had attenuated virulence in murine models of invasive aspergillosis, mice infected with this mutant still died. In contrast, the A. fumigatus siderophore mutants, ΔsidA, ΔsidC, ΔsidD, and ΔsidF, appear to have a much greater attenuation in virulence (Hissen et al., 2005; Schrettl et al., 2004; Schrettl et al., 2007). The probable explanation for this result is that the expression of most of the genes involved in siderophore-mediated iron uptake was reduced, but not completely abrogated in the ΔacuM mutant. It is possible that these genes may be under the control of hapX, the expression of which was only partially governed by AcuM.
A surprising finding was that the A. nidulans ΔacuM mutant grew similarly to the wild-type strain under iron-limited conditions and had normal extracellular siderophore production. These results indicate that there has been significant transcriptional rewiring between A. fumigatus and A. nidulans. Although AcuM regulates gluconeogenesis in both species, it has evolved the additional function of controlling iron homeostasis in A. fumigatus. In A. nidulans, a second zinc cluster transcription factor, AcuK, functions with AcuM to control gluconeogenesis (Hynes et al., 2007). An ortholog of AcuK is present in the A. fumigatus genome (Hynes et al., 2007). Thus, it is possible that AcuK may also regulate iron homeostasis as well as gluconeogenesis in this organism. Transcriptional rewiring of metabolic regulatory pathways has been reported to occur between yeast such as S. cerevisiae and C. albicans (Martchenko et al., 2007), which separated from each other 150 to 300 million years ago (Pesole et al., 1995). A. nidulans and A. fumigatus diverged from each other over a similar time span, approximately 200 million years ago (Galagan et al., 2005). During this time, AcuM and probably other genes that contribute to the enhanced pathogenicity of A. fumigatus developed a new function.
In summary, this work demonstrates that A. fumigatus AcuM governs both iron acquisition and gluconeogenesis. Its effects on iron acquisition are mediated mainly by repression of SreA. AcuM-regulated iron acquisition, but not gluconeogenesis is essential for maximal virulence during both hematogenously disseminated and invasive pulmonary aspergillosis. Although AcuM regulates iron homeostasis in A. fumigatus it does not do so in A. nidulans, indicating a significant divergence in transcriptional regulation between these two organisms has occurred.
Experimental procedures
Strains and growth conditions
A. fumigatus strain Af293 was used as the wild-type strain in all experiments with this organism. The A. fumigatus mutant strains used in the experiments are listed in Table 1. They were routinely grown on Sabouraud agar (Difco, Detroit, MI) at 37°C for 1 week prior to use. The Aspergillus nidulans control strain and ΔacuM mutant strain were a generous gift from Dr. Michael Hynes (University of Melbourne, Victoria, Australia) and grown as described (Hynes et al., 2007). The conidia of all organisms were harvested by gently rinsing the agar plates with PBS containing 0.1% Tween 80 (Sigma-Aldrich, St. Louis, MO) and enumerated using a hemacytometer.
For iron-depleted conditions, the organisms were grown in either Sabouraud dextrose broth (Difco, Detroit, MI) containing 30 µM phenanthroline (Sigma-Aldrich) or liquid AMM containing 1 % glucose (wt/vol) and 10 mM ammonium tartrate, without supplemental iron (Hortschansky et al., 2007). Iron–replete medium was prepared by supplementing Sabouraud dextrose broth plus phenanthroline with 1 mM FeSO4, and the AMM with 50 µM FeSO4. In each experiment, 2 × 108 conidia of the various strains were inoculated into 100 ml of culture medium and incubated at 37°C in a shaking incubator for 40 h.
Construction of A. fumigatus mutant strains
Each of the A. fumigatus mutant strains in Table 1 was generated using split-marker approach with a minor modification of our previously described method (Sheppard et al., 2005). To delete acuM (Afu2g12330), a DNA fragment encompassing 1416 bp upstream of the acuM protein coding region was amplified from A.fumigatus Af293 genomic DNA by high-fidelity PCR using primers AcuM-F4 and AcuM-F3 (Supplemental Table S1). This fragment was cloned into plasmid pNLC106 (Catlett et al., 2002), yielding (pAcuM-HY), which contained the 5’ portion of the hph hygromycin resistance cassette. Next, a DNA fragment containing the upstream acuM flanking region and the 5’ portion of the hygromycin resistance cassette was amplified by high-fidelity PCR from pAcuM-HY using primers AcuM-F4 and HY. To generate the second half of the split marker, 1516 bp of the acuM downstream flanking sequence was PCR amplified using primers AcuM-F2 and AcuM-F1. Next, the 3’ region of the hygromycin resistance cassette was amplified from plasmid pAN7-1 (Punt et al., 1987) using primers HYG-F and YG. The downstream sequence was combined with the 3’ resistance cassette by fusion PCR using primers AcuM-F1 and YG. A. fumigatus Af293 was then transformed by protoplasting with the two fragments containing the upstream and downstream flanking sequences of acuM (Weidner et al., 1998). Hygromycin-resistant clones were screened for disruption of acuM by colony PCR using the primers AcuM-VER-UP and AcuM-VER-R (Table S1). The other mutant strains of A. fumigatus were generated in a similar manner using the primers listed in Supplemental Table S1.
To complement the ΔacuM mutant, the acuM open reading frame along with 2968 bp of upstream sequence and 918 bp downstream sequence was amplified from genomic DNA of strain Af293 by high-fidelity PCR using primes AcuM-REV-F and AcuM-REV-R (Supplemental Table S1). The resulting 6180 bp DNA fragment was ligated into pGEM-T Easy (Promega Corp., Madison, WI) and sequence verified. This fragment was excised from pGEM-T Easy by NotI digestion and cloned into plasmid p402, which contains the phleomycin resistance gene (Richie et al., 2007). The resulting plasmid was linearized with AfeI and transformed into the ΔacuM mutant. Phleomycin-resistant clones were screened by whole cell PCR using primers AcuM-REV-VER-F and AcuM-REV-VER-R (Supplemental Table S1) to select clones with the proper integration.
Disruption of acuM and correct integration of a single copy of the acuM complementation plasmid were verified by Southern blotting with a 569 bp fragment encompassing the hygromycin resistance cassette and the 5’ acuM flanking sequence.
To delete sreA (Afu5g11260) in the ΔacuM background, the Gateway® cloning system (Invitrogen, Carlsbad, CA) was used to fuse sreA flanking sequences with the ble phleomycin resistance cassette as follows. A 1402 bp sequence of the sreA upstream flanking sequence was amplified from A. fumigatus Af293 genomic DNA by high-fidelity PCR using the primers SreA-gate1 and SreA-gate2 (Table S1). This fragment was first cloned into the pENTR-D-TOPO plasmid, and then transferred to the pBL plasmid so that the 3’ end of the fragment was positioned next to the 5’ end of the ble cassette. Using PCR with the SreA-gate1 and BL primers, a 2285 bp product containing the upstream sreA flanking region and the 5’ portion of the phleomycin resistance cassette was amplified from this plasmid. Similarly, a 1242 bp fragment of the sreA downstream flanking sequence was generated by PCR, cloned in pENTR-D-TOPO, and then transferred to pLE immediately downstream of the ble cassettee. A 2015 bp product containing the 3’ portion of the phleomycin resistance cassette followed by the downstream sreA flanking region was amplified from this plasmid by PCR with the primers LE and SreA-gate4. Finally, the A. fumigatus ΔacuM mutant was transformed with the two fragments containing the upstream and downstream flanking sequences of sreA. Phleomycin-resistant clones were screened for disruption of sreA by colony PCR using the primers SreA-ext1 and SreA-ext4 (Table S1). Positive clones were then screened for the absence of sreA mRNA by real-time PCR.
Galleria mellonella model of invasive aspergillosis
The various transcription factor mutants were screened for virulence in the Galleria mellonella larva model of disseminated aspergillosis (Fan et al., 2007; Gravelat et al., 2009; Jackson et al., 2009; Mylonakis, 2008; Reeves et al., 2004; Renwick et al., 2006). G. mellonella larvae in the final instar stage were obtained from Vanderhorst Wholesale (St. Mary’s, OH). Larvae were stored at room temperature in wood shavings in the dark prior to use and were used within 1 week of delivery. The larvae were injected with 5 × 106 conidia in 5 µl of phosphate buffered saline (PBS) in the last pro-leg with a Hamilton syringe. Each strain of A. fumigatus was used to infect 30 to 35 larvae, and the experiments were repeated 2 or 3 times. The infected larvae were maintained in Petri dishes in a dark humidified incubator at 37°C. Mortality was assessed based on lack of movement in response to tactile stimulation.
Murine model of hematogenously disseminated aspergillosis
Male BALB/c mice (Taconic Farms Inc, Hudson, NY) were immunosuppressed with cortisone acetate (Sigma-Aldrich) administered subcutaneously at 250 mg/kg on days −2 and +3 relative to infection, and with cyclophosphamide (Western Medical Supply, Arcadia, CA) administered intraperitoneally at 250 mg/kg on days −2 and +3. The mice received daily intraperitoneal injections of 5 mg of ceftazidime while they were immunosuppressed to prevent bacterial infections. Germlings of the three strains were prepared by incubating conidia in Petri dishes containing Sabouraud dextrose broth (5 × 105 conidia/ml) at 37°C for 3 h, then storing them at 4°C overnight, and incubating them at 37°C for an additional 4 h the next day. After gently rinsing the resulting germlings twice with PBS, the organisms were harvested with a cell scraper, suspended in PBS, and enumerated with a hemacytometer. The immunosuppressed mice were inoculated with 3 ×103 germlings via the lateral tail vein, and the inocula were verified by quantitative culture. Control mice were immunosuppressed, but not infected. In each experiment, 5 to 10 mice were infected with each strain.
Murine model of invasive pulmonary aspergillosis
To induce invasive pulmonary aspergillosis, male BALB/c mice were immunosuppressed with 5 doses of 10 mg cortisone acetate administered subcutaneously every other day, starting on day −4 relative to infection and finishing on day +4 (Ejzykowicz et al., 2009; Spikes et al., 2008). The mice received daily intraperitoneal injections of 5 mg of ceftazidime while they were immunosuppressed. They were infected by placing them for 1 h in an acrylic chamber into which 109 conidia/ML were aerosolized (Sheppard et al., 2004). Control mice were immunosuppressed, but not infected. In the survival studies, 11 mice were infected with each strain of A. fumigatus. Shortly after inoculation, 3 mice from each group were sacrificed, after which their lungs were homogenized and quantitatively cultured to verify conidial delivery to the lungs. The remaining mice were monitored for survival. The survival experiments were repeated twice and the results were combined.
The pulmonary fungal burden was assessed by measuring the pulmonary galactomannan content using the Platelia Aspergillus kit (Bio-Rad, Hercules, CA) as previously described (Ejzykowicz et al., 2009; Gravelat et al., 2009). Briefly, 10 to 12 mice per strain were infected as above. Three mice were sacrificed after inoculation with each strain to verify pulmonary conidial delivery by quantitative culture. The remaining mice were sacrificed after 4 days of infection. Their lungs were harvested, weighed, and then homogenized in ice cold PBS containing protease inhibitor cocktail (Sigma-Aldrich). The homogenates were clarified by centrifugation and aliquots of the resulting supernatants were stored at −80°C for later analysis. To determine the galactomannan content, each pulmonary homogenate was diluted 1:10 in ultra-pure water and processed according to the manufacturer's instructions. The resulting optical densities were compared with a standard curve, which was made using serial dilutions of a pool of lung homogenates from 5 heavily infected immunosuppressed mice (7 days after intranasal infection with strain Af293). All animal studies were approved by the Institutional Animal Use and Care Committee, and performed according to the National Institutes of Health guidelines for animal housing and care.
Microarray analysis
To obtain RNA for microarray analysis, conidia of the various strains were used to inoculate RPMI 1640 medium (Cat. No. R6504; Sigma-Aldrich) buffered with 4-morpholinepropanesulfonicacid to pH 7.0 (34.5 g/l; Sigma-Aldrich). This medium contains 0.2% glucose as the carbon source and 0.03% glutamine as the nitrogen source. For the 8 h time point, a one liter flask containing 500 ml of medium was used. For the 18 and 24 h time point, 250 ml flasks containing 100 ml of medium were used. The concentration of conidia in these flasks was 5 × 105 cells per ml. After incubating the organisms in a shaking incubator at 37°C for 8, 18, and 24h, the resulting hyphae were harvested by filtration and snap frozen in liquid nitrogen. Next the RNA was extracted using the RNeasy Plant Mini Kit (Qiagen, Germantown, MD) following the manufacturer instructions.
An A. fumigatus Af293 DNA amplicon microarray containing triplicate probes for 9516 genes was used in this study (Nierman et al., 2005). The labeling reactions with RNA and hybridizations were performed as described in the J. Craig Venter Institute standard operating procedure (Http://pfgrc.jcvi.org/index.php/microarray/protocols.html). cDNA from the ΔacuM mutant was hybridized against cDNA from the wild-type strain in two biological replicates and against cDNA from the ΔacuM∷acuM complemented strain in two biological replicates. Dye swaps were performed. Because hybridization against the wild-type and ΔacuM∷acuM complemented strains gave similar results, the data were combined for the final analysis. The gene expression ratios were log2-transformed and imported into JCVI MultiExperiment Viewer software (http://www.tm4.org/mev.html) (Saeed et al., 2006). The Significance Analysis for Microarrays (Tusher et al., 2001) method was used (false discovery rate of 0.1%) to identify genes subject to differential transcriptional regulation between the control strains and the ΔacuM mutant after 18h and/or 24h of growth. Genes down-regulated in the ΔacuM mutant were further analyzed by the Expression Analysis Systematic Explorer (Hosack et al., 2003) within JCVI MultiExperiment Viewer to identify overrepresented Gene Ontology (GO) terms and Pfam domains. Using Fisher’s exact test and stepdown Bonferroni correction, a cutoff value of adjusted P < 0.05 was set to assess statistical significance.
Selected microarray results were verified by real-time PCR using the primers listed in Supplemental Table S1. Relative gene expression levels were quantified by the 2−ΔΔCTmethod using TEF1 as the reference gene (Ejzykowicz et al., 2009; Gravelat et al., 2008; Sheppard et al., 2005). The real-time PCR experiments were performed using 3 biological replicates, each tested in triplicate.
Quantification of iron incorporation
The capacity of the different strains to incorporate ferric iron from the medium was determined by a minor modification of our previously described method (Fu et al., 2004). Briefly, 5×106 conidia/ml of the various A. fumigatus strains were incubated in AMM without iron containing 1 mM ferrozine (Sigma-Aldrich) and 1 mM ascorbic acid (Sigma) in a shaking incubator at 37 °C for 8 hours. Next, the swollen conidia were harvested, washed twice with assay buffer (AMM without iron, 10 mM MOPS pH 6.1, and 2% glucose), and enumerated. After adjusting the concentration of conidia to 108 cells per ml, 200 µl of this suspension was added to 400 µl of assay buffer containing 1.5 µM 55FeCl3 and the organisms were incubated at 37°C for 60 minutes. The suspension was then chilled on ice, after which the organisms were washed three times with washing buffer (1 mM EDTA, 20 mM Na3 citrate pH 4.2, 1 mM KH2PO4, 1 mM CaCl2, 5 mM MgSO4, 1 mM NaCl, 0.05% Tween 80). The fungal associated 55Fe was measured by liquid scintillation counting. Each experiment was performed in triplicate on 3 different occasions.
Measurement of extracellular siderophore production
The CAS assay was used to measure the total extracellular siderophore activity of the different strains (Schwyn and Neilands, 1987). This assay is based on competition for iron by the indicator dye (CAS) and the siderophore produced by the microorganism. Removal of iron from CAS by the siderophore causes it to change color (from blue to orange), which is measured by absorbance at 630Å. Siderophores were generated by adding 3 × 108 conidia to AMM without iron, and incubating them at 37°C in a shaking incubator. For the A. fumigatus studies, the incubation time was 30 h. Because A. nidulans had greater extracellular siderophore activity than A. fumigatus, a 24 h incubation time was used. Next, the conditioned medium was collected by filtration, and an aliquot was added to the CAS assay solution. After adding shuttle solution to the mixture, the OD630 was determined using a spectrophotometer. A standard curve was constructed using different dilutions of ferrichrome. The extracellular siderophore activity of each strain was measured in 3 independent experiments.
Effects of different carbon sources on growth
To test the effects of different carbon sources on growth, AMM plus iron without glucose was supplemented with 0.5% ethanol, 50 mM acetate, 50 mM L-proline, or 1% glucose (Hynes et al., 2007). Serial 10-fold dilutions of conidia ranging from 105 to 102 cells in a volume of 5 µl were spotted onto AMM agar plates. The plates were incubated at 37 °C for 40 h and then imaged to determine the relative sizes of the colonies. Each experiment was repeated 3 times.
Susceptibility to environmental stress and antifungal agents
To determine susceptibility to environmental stress, 103 conidia or germlings of the various A. fumigatus strains were added to individual wells of 96-well plates containing serial 2-fold dilutions of the various stressors in Sabouraud broth. These stressors included Congo red (5 to 250 µg/ml), calcofluor white (5 to 250 µg/ml), hydrogen peroxide (0.1 to 5 mM), SDS (0.0002 to 0.01%), caspofungin (0.001 to 0.2 µg/ml), amphotericin B (0.25 to 2.2 µg/ml). The plates were incubated at 37°C for 48 h, after which the growth in the wells was scored visually. The minimal inhibitory concentration was defined as the concentration of that stressor that inhibited growth by at least 80% compared to organisms grown in the absence of that stressor.
A. fumigatus killing by macrophage-like and neutrophil-like HL-60 cells
HL-60 cells (American Type Culture Collection, Manassas, VA) were cultured in RPMI 1640 medium, supplemented with glutamine (Irvine Scientific, Santa Ana, CA), 10% fetal bovine serum (Gemini BioProducts, Woodland, CA), 1% penicillin, streptomycin, and 50 µM β-mercaptoethanol (Sigma-Aldrich, St. Louis, MO) at 37°C in 5% CO2. To induce differentiation into macrophage-like cells, the HL-60 cells were stimulated with 50 nM 12-o-tetradecanoyl-phorbol-13-acetate for 2 days (Collins, 1987). To induce differentiation into neutrophil-like cells, the HL-60 cells incubated in 1.3% (v/v) DMSO and 2.5 µM retinoic acid for 3 days (Spellberg et al., 2005). Conidia from the 3 strains A. fumigatus were incubated in RPMI 1640 medium at 37°C for 4 h to obtain swollen conidia. Next, 103 swollen conidia were added to polypropylene tubes containing differentiated HL-60 cells in 1 ml RPMI 1640 medium supplemented with 5% pooled human serum (Sigma-Aldrich) to achieve a target to effector cell ratio of 1:50. After incubation at 37°C in 5% CO2 for 3 h, the tubes were vortexed vigorously, and the number of surviving organisms was determined by quantitative culture. Swollen conidia incubated in the absence of HL-60 cells were processed in parallel as a negative control. Each experiment was performed in triplicate and repeated 3 times.
Statistical analyses
The survival data were analyzed using the Log-Rank test, and pulmonary fungal burden results were analyzed with the Wilcoxon Rank Sum test. The data from the in vitro experiments were analyzed by Analysis of Variance. A p value of ≤ 0.05 was considered to be significant.
Supplementary Material
Supplemental Table S1. List of PCR primers used in the studies.
Supplemental Table S2. List of A. fumigatus genes that were significantly up- or down-regulated in the ΔacuM mutant at 18 and/or 24 h compared to Af293 and the ΔacuM∷acuM complemented strain. Data represent microarray results as well as real-time PCR verification of selected genes.
Supplemental Table S3. List of genes in the GO term categories that were significantly down-regulated in the ΔacuM mutant at 18 and/or 24 h compared to Af293 and the ΔacuM∷acuM complemented strain.
ACKNOWLEDGEMENTS
We thank Marie-Claude Ouimet for technical assistance. This work was supported in part by grants R21AI064511 and R01AI073829, as well as contract no. N01-AI-30041 from the National Institutes of Health, U.S.A. D.C.S was supported in part by a Clinician-Scientist award and Operating Grant from the Canadian Institutes of Health Research.
REFERENCES
- Barelle CJ, Priest CL, Maccallum DM, Gow NA, Odds FC, Brown AJ. Niche-specific regulation of central metabolic pathways in a fungal pathogen. Cell Microbiol. 2006;Vol. 8:961–971. doi: 10.1111/j.1462-5822.2005.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bok JW, Balajee SA, Marr KA, Andes D, Nielsen KF, Frisvad JC, Keller NP. LaeA, a regulator of morphogenetic fungal virulence factors. Eukaryot Cell. 2005;4:1574–1582. doi: 10.1128/EC.4.9.1574-1582.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun BR, Johnson AD. TUP1, CPH1 and EFG1 make independent contributions to filamentation in candida albicans. Genetics. 2000;155:57–67. doi: 10.1093/genetics/155.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlett NL, Lee B, Yoder OC, Turgeon BG. Split-Marker Recombination for Efficient Targeted Deletion of Fungal Genes. Fungal Genet. 2002;Vol. News.:9–11. [Google Scholar]
- Collins SJ. The HL-60 promyelocytic leukemia cell line: proliferation, differentiation, and cellular oncogene expression. Blood. 1987;70:1233–1244. [PubMed] [Google Scholar]
- Cramer RA, Jr, Perfect BZ, Pinchai N, Park S, Perlin DS, Asfaw YG, Heitman J, Perfect JR, Steinbach WJ. Calcineurin target CrzA regulates conidial germination, hyphal growth, and pathogenesis of Aspergillus fumigatus. Eukaryot Cell. 2008;7:1085–1097. doi: 10.1128/EC.00086-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaunay A, Isnard AD, Toledano MB. H2O2 sensing through oxidation of the Yap1 transcription factor. Embo J. 2000;19:5157–5166. doi: 10.1093/emboj/19.19.5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do JH, Yamaguchi R, Miyano S. Exploring temporal transcription regulation structure of Aspergillus fumigatus in heat shock by state space model. BMC Genomics. 2009;10:306. doi: 10.1186/1471-2164-10-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejzykowicz DE, Cunha MM, Rozental S, Solis NV, Gravelat FN, Sheppard DC, Filler SG. The Aspergillus fumigatus transcription factor Ace2 governs pigment production, conidiation and virulence. Mol Microbiol. 2009;72:155–169. doi: 10.1111/j.1365-2958.2009.06631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejzykowicz DE, Solis NV, Gravelat FN, Chabot J, Li X, Sheppard DC, Filler SG. Role of Aspergillus fumigatus DvrA in Host Cell Interactions and Virulence. Eukaryot Cell. 2010 doi: 10.1128/EC.00055-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W, Idnurm A, Breger J, Mylonakis E, Heitman J. Eca1, a sarcoplasmic/endoplasmic reticulum Ca2+-ATPase, is involved in stress tolerance and virulence in Cryptococcus neoformans. Infect Immun. 2007;75:3394–3405. doi: 10.1128/IAI.01977-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Lee H, Collins M, Tsai HF, Spellberg B, Edwards JE, Jr, Kwon-Chung KJ, Ibrahim AS. Cloning and functional characterization of the Rhizopus oryzae high affinity iron permease (rFTR1) gene. FEMS Microbiol Lett. 2004;235:169–176. doi: 10.1016/j.femsle.2004.04.031. [DOI] [PubMed] [Google Scholar]
- Galagan JE, Calvo SE, Cuomo C, Ma LJ, Wortman JR, Batzoglou S, Lee SI, Basturkmen M, Spevak CC, Clutterbuck J, Kapitonov V, Jurka J, Scazzocchio C, Farman M, Butler J, Purcell S, Harris S, Braus GH, Draht O, Busch S, D'Enfert C, Bouchier C, Goldman GH, Bell-Pedersen D, Griffiths-Jones S, Doonan JH, Yu J, Vienken K, Pain A, Freitag M, Selker EU, Archer DB, Penalva MA, Oakley BR, Momany M, Tanaka T, Kumagai T, Asai K, Machida M, Nierman WC, Denning DW, Caddick M, Hynes M, Paoletti M, Fischer R, Miller B, Dyer P, Sachs MS, Osmani SA, Birren BW. Sequencing of Aspergillus nidulans and comparative analysis with A. fumigatus and A. oryzae. Nature. 2005;438:1105–1115. doi: 10.1038/nature04341. [DOI] [PubMed] [Google Scholar]
- Gravelat FN, Doedt T, Chiang LY, Liu H, Filler SG, Patterson TF, Sheppard DC. In vivo analysis of Aspergillus fumigatus developmental gene expression determined by real-time reverse transcription-PCR. Infect Immun. 2008;76:3632–3639. doi: 10.1128/IAI.01483-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravelat FN, Ejzykowicz DE, Chiang LY, Chabot JC, Urb M, Macdonald KD, Al-Bader N, Filler SG, Sheppard DC. Aspergillus fumigatus MedA governs adherence, host cell interactions and virulence. Cell Microbiol. 2009 doi: 10.1111/j.1462-5822.2009.01408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hissen AH, Wan AN, Warwas ML, Pinto LJ, Moore MM. The Aspergillus fumigatus siderophore biosynthetic gene sidA, encoding L-ornithine N5-oxygenase, is required for virulence. Infect Immun. 2005;73:5493–5503. doi: 10.1128/IAI.73.9.5493-5503.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hortschansky P, Eisendle M, Al-Abdallah Q, Schmidt AD, Bergmann S, Thon M, Kniemeyer O, Abt B, Seeber B, Werner ER, Kato M, Brakhage AA, Haas H. Interaction of HapX with the CCAAT-binding complex--a novel mechanism of gene regulation by iron. Embo J. 2007;26:3157–3168. doi: 10.1038/sj.emboj.7601752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosack DA, Dennis G, Jr, Sherman BT, Lane HC, Lempicki RA. Identifying biological themes within lists of genes with EASE. Genome Biol. 2003;4:R70. doi: 10.1186/gb-2003-4-10-r70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes MJ, Szewczyk E, Murray SL, Suzuki Y, Davis MA, Sealy-Lewis HM. Transcriptional control of gluconeogenesis in Aspergillus nidulans. Genetics. 2007;176:139–150. doi: 10.1534/genetics.107.070904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson JC, Higgins LA, Lin X. Conidiation color mutants of Aspergillus fumigatus are highly pathogenic to the heterologous insect host Galleria mellonella. PLoS One. 2009;4:e4224. doi: 10.1371/journal.pone.0004224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy CJ, Boyle PM, Waks Z, Silver PA. Systems-level engineering of nonfermentative metabolism in yeast. Genetics. 2009;183:385–397. doi: 10.1534/genetics.109.105254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krappmann S, Bignell EM, Reichard U, Rogers T, Haynes K, Braus GH. The Aspergillus fumigatus transcriptional activator CpcA contributes significantly to the virulence of this fungal pathogen. Mol Microbiol. 2004;52:785–799. doi: 10.1111/j.1365-2958.2004.04015.x. [DOI] [PubMed] [Google Scholar]
- Lewis RE, Lo HJ, Raad II, Kontoyiannis DP. Lack of catheter infection by the efg1/efg1 cph1/cph1 double-null mutant, a Candida albicans strain that is defective in filamentous growth. Antimicrob Agents Chemother. 2002;46:1153–1155. doi: 10.1128/AAC.46.4.1153-1155.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Kohler J, Fink GR. Suppression of hyphal formation in Candida albicans by mutation of a STE12 homolog. Science. 1994;266:1723–1726. doi: 10.1126/science.7992058. [DOI] [PubMed] [Google Scholar]
- Marr KA, Carter RA, Crippa F, Wald A, Corey L. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2002;34:909–917. doi: 10.1086/339202. [DOI] [PubMed] [Google Scholar]
- Martchenko M, Levitin A, Hogues H, Nantel A, Whiteway M. Transcriptional rewiring of fungal galactose-metabolism circuitry. Curr Biol. 2007;17:1007–1013. doi: 10.1016/j.cub.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maschmeyer G, Haas A, Cornely OA. Invasive aspergillosis: epidemiology, diagnosis and management in immunocompromised patients. Drugs. 2007;67:1567–1601. doi: 10.2165/00003495-200767110-00004. [DOI] [PubMed] [Google Scholar]
- Moreno I, Pedreno Y, Maicas S, Sentandreu R, Herrero E, Valentin E. Characterization of a Candida albicans gene encoding a putative transcriptional factor required for cell wall integrity. FEMS Microbiol Lett. 2003;226:159–167. doi: 10.1016/S0378-1097(03)00588-3. [DOI] [PubMed] [Google Scholar]
- Moreno I, Castillo L, Sentandreu R, Valentin E. Global transcriptional profiling of Candida albicans cwt1 null mutant. Yeast. 2007a;24:357–370. doi: 10.1002/yea.1444. [DOI] [PubMed] [Google Scholar]
- Moreno I, Tutrone N, Sentandreu R, Valentin E. Saccharomyces cerevisiae Rds2 transcription factor involvement in cell wall composition and architecture. Int Microbiol. 2008;11:57–63. [PubMed] [Google Scholar]
- Mylonakis E. Galleria mellonella and the study of fungal pathogenesis: making the case for another genetically tractable model host. Mycopathologia. 2008;165:1–3. doi: 10.1007/s11046-007-9082-z. [DOI] [PubMed] [Google Scholar]
- Naar AM, Thakur JK. Nuclear receptor-like transcription factors in fungi. Genes Dev. 2009;23:419–432. doi: 10.1101/gad.1743009. [DOI] [PubMed] [Google Scholar]
- Nierman WC, Pain A, Anderson MJ, Wortman JR, Kim HS, Arroyo J, Berriman M, Abe K, Archer DB, Bermejo C, Bennett J, Bowyer P, Chen D, Collins M, Coulsen R, Davies R, Dyer PS, Farman M, Fedorova N, Feldblyum TV, Fischer R, Fosker N, Fraser A, Garcia JL, Garcia MJ, Goble A, Goldman GH, Gomi K, Griffith-Jones S, Gwilliam R, Haas B, Haas H, Harris D, Horiuchi H, Huang J, Humphray S, Jimenez J, Keller N, Khouri H, Kitamoto K, Kobayashi T, Konzack S, Kulkarni R, Kumagai T, Lafon A, Latge JP, Li W, Lord A, Lu C, Majoros WH, May GS, Miller BL, Mohamoud Y, Molina M, Monod M, Mouyna I, Mulligan S, Murphy L, O'Neil S, Paulsen I, Penalva MA, Pertea M, Price C, Pritchard BL, Quail MA, Rabbinowitsch E, Rawlins N, Rajandream MA, Reichard U, Renauld H, Robson GD, Rodriguez de Cordoba S, Rodriguez-Pena JM, Ronning CM, Rutter S, Salzberg SL, Sanchez M, Sanchez-Ferrero JC, Saunders D, Seeger K, Squares R, Squares S, Takeuchi M, Tekaia F, Turner G, Vazquez de Aldana CR, Weidman J, White O, Woodward J, Yu JH, Fraser C, Galagan JE, Asai K, Machida M, Hall N, Barrell B, Denning DW. Genomic sequence of the pathogenic and allergenic filamentous fungus Aspergillus fumigatus. Nature. 2005;438:1151–1156. doi: 10.1038/nature04332. [DOI] [PubMed] [Google Scholar]
- Odenbach D, Breth B, Thines E, Weber RW, Anke H, Foster AJ. The transcription factor Con7p is a central regulator of infection-related morphogenesis in the rice blast fungus Magnaporthe grisea. Mol Microbiol. 2007;64:293–307. doi: 10.1111/j.1365-2958.2007.05643.x. [DOI] [PubMed] [Google Scholar]
- Patterson TF, Kirkpatrick WR, White M, Hiemenz JW, Wingard JR, Dupont B, Rinaldi MG, Stevens DA, Graybill JR. Invasive aspergillosis. Disease spectrum, treatment practices, and outcomes. I3 Aspergillus Study Group. Medicine (Baltimore) 2000;79:250–260. doi: 10.1097/00005792-200007000-00006. [DOI] [PubMed] [Google Scholar]
- Patterson TF, Boucher HW, Herbrecht R, Denning DW, Lortholary O, Ribaud P, Rubin RH, Wingard JR, DePauw B, Schlamm HT, Troke P, Bennett JE. Strategy of following voriconazole versus amphotericin B therapy with other licensed antifungal therapy for primary treatment of invasive aspergillosis: impact of other therapies on outcome. Clin Infect Dis. 2005;41:1448–1452. doi: 10.1086/497126. [DOI] [PubMed] [Google Scholar]
- Pegues CF, Daar ES, Murthy AR. The epidemiology of invasive pulmonary aspergillosis at a large teaching hospital. Infect Control Hosp Epidemiol. 2001;22:370–374. doi: 10.1086/501915. [DOI] [PubMed] [Google Scholar]
- Pesole G, Lotti M, Alberghina L, Saccone C. Evolutionary origin of nonuniversal CUGSer codon in some Candida species as inferred from a molecular phylogeny. Genetics. 1995;141:903–907. doi: 10.1093/genetics/141.3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinson-Gadais L, Richard-Forget F, Frasse P, Barreau C, Cahagnier B, Richard-Molard D, Bakan B. Magnesium represses trichothecene biosynthesis and modulates Tri5, Tri6, and Tri12 genes expression in Fusarium graminearum. Mycopathologia. 2008;165:51–59. doi: 10.1007/s11046-007-9076-x. [DOI] [PubMed] [Google Scholar]
- Proctor RH, Hohn TM, McCormick SP, Desjardins AE. Tri6 encodes an unusual zinc finger protein involved in regulation of trichothecene biosynthesis in Fusarium sporotrichioides. Appl Environ Microbiol. 1995;61:1923–1930. doi: 10.1128/aem.61.5.1923-1930.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punt PJ, Oliver RP, Dingemanse MA, Pouwels PH, van den Hondel CA. Transformation of Aspergillus based on the hygromycin B resistance marker from Escherichia coli. Gene. 1987;56:117–124. doi: 10.1016/0378-1119(87)90164-8. [DOI] [PubMed] [Google Scholar]
- Ramer SW, Davis RW. A dominant truncation allele identifies a gene, STE20, that encodes a putative protein kinase necessary for mating in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1993;90:452–456. doi: 10.1073/pnas.90.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves EP, Messina CG, Doyle S, Kavanagh K. Correlation between gliotoxin production and virulence of Aspergillus fumigatus in Galleria mellonella. Mycopathologia. 2004;158:73–79. doi: 10.1023/b:myco.0000038434.55764.16. [DOI] [PubMed] [Google Scholar]
- Renwick J, Daly P, Reeves EP, Kavanagh K. Susceptibility of larvae of Galleria mellonella to infection by Aspergillus fumigatus is dependent upon stage of conidial germination. Mycopathologia. 2006;161:377–384. doi: 10.1007/s11046-006-0021-1. [DOI] [PubMed] [Google Scholar]
- Richie DL, Fuller KK, Fortwendel J, Miley MD, McCarthy JW, Feldmesser M, Rhodes JC, Askew DS. Unexpected link between metal ion deficiency and autophagy in Aspergillus fumigatus. Eukaryot Cell. 2007;6:2437–2447. doi: 10.1128/EC.00224-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed AI, Bhagabati NK, Braisted JC, Liang W, Sharov V, Howe EA, Li J, Thiagarajan M, White JA, Quackenbush J. TM4 microarray software suite. Methods Enzymol. 2006;411:134–193. doi: 10.1016/S0076-6879(06)11009-5. [DOI] [PubMed] [Google Scholar]
- Schrettl M, Bignell E, Kragl C, Joechl C, Rogers T, Arst HN, Jr, Haynes K, Haas H. Siderophore biosynthesis but not reductive iron assimilation is essential for Aspergillus fumigatus virulence. J Exp Med. 2004;200:1213–1219. doi: 10.1084/jem.20041242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrettl M, Bignell E, Kragl C, Sabiha Y, Loss O, Eisendle M, Wallner A, Arst HN, Jr, Haynes K, Haas H. Distinct roles for intra- and extracellular siderophores during Aspergillus fumigatus infection. PLoS Pathog. 2007;3:1195–1207. doi: 10.1371/journal.ppat.0030128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrettl M, Kim HS, Eisendle M, Kragl C, Nierman WC, Heinekamp T, Werner ER, Jacobsen I, Illmer P, Yi H, Brakhage AA, Haas H. SreA-mediated iron regulation in Aspergillus fumigatus. Mol Microbiol. 2008;70:27–43. doi: 10.1111/j.1365-2958.2008.06376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwyn B, Neilands JB. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987;160:47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- Seifert M, Nairz M, Schroll A, Schrettl M, Haas H, Weiss G. Effects of the Aspergillus fumigatus siderophore systems on the regulation of macrophage immune effector pathways and iron homeostasis. Immunobiology. 2008;213:767–778. doi: 10.1016/j.imbio.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Seong KY, Pasquali M, Zhou X, Song J, Hilburn K, McCormick S, Dong Y, Xu JR, Kistler HC. Global gene regulation by Fusarium transcription factors Tri6 and Tri10 reveals adaptations for toxin biosynthesis. Mol Microbiol. 2009;72:354–367. doi: 10.1111/j.1365-2958.2009.06649.x. [DOI] [PubMed] [Google Scholar]
- Sheppard DC, Rieg G, Chiang LY, Filler SG, Edwards JE, Jr, Ibrahim AS. Novel inhalational murine model of invasive pulmonary aspergillosis. Antimicrob Agents Chemother. 2004;48:1908–1911. doi: 10.1128/AAC.48.5.1908-1911.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard DC, Doedt T, Chiang LY, Kim HS, Chen D, Nierman WC, Filler SG. The Aspergillus fumigatus StuA protein governs the up-regulation of a discrete transcriptional program during the acquisition of developmental competence. Mol Biol Cell. 2005;16:5866–5879. doi: 10.1091/mbc.E05-07-0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard DC, Marr KA, Fredricks DN, Chiang LY, Doedt T, Filler SG. Comparison of three methodologies for the determination of pulmonary fungal burden in experimental murine aspergillosis. Clin Microbiol Infect. 2006;12:376–380. doi: 10.1111/j.1469-0691.2005.01349.x. [DOI] [PubMed] [Google Scholar]
- Soontorngun N, Larochelle M, Drouin S, Robert F, Turcotte B. Regulation of gluconeogenesis in Saccharomyces cerevisiae is mediated by activator and repressor functions of Rds2. Mol Cell Biol. 2007;27:7895–7905. doi: 10.1128/MCB.01055-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriani FM, Malavazi I, da Silva Ferreira ME, Savoldi M, Von Zeska Kress MR, de Souza Goldman MH, Loss O, Bignell E, Goldman GH. Functional characterization of the Aspergillus fumigatus CRZ1 homologue, CrzA. Mol Microbiol. 2008;67:1274–1291. doi: 10.1111/j.1365-2958.2008.06122.x. [DOI] [PubMed] [Google Scholar]
- Spellberg BJ, Collins M, French SW, Edwards JE, Jr, Fu Y, Ibrahim AS. A phagocytic cell line markedly improves survival of infected neutropenic mice. J Leukoc Biol. 2005;78:338–344. doi: 10.1189/jlb.0205072. [DOI] [PubMed] [Google Scholar]
- Spikes S, Xu R, Nguyen CK, Chamilos G, Kontoyiannis DP, Jacobson RH, Ejzykowicz DE, Chiang LY, Filler SG, May GS. Gliotoxin production in Aspergillus fumigatus contributes to host-specific differences in virulence. J Infect Dis. 2008;197:479–486. doi: 10.1086/525044. [DOI] [PubMed] [Google Scholar]
- Sugui JA, Pardo J, Chang YC, Mullbacher A, Zarember KA, Galvez EM, Brinster L, Zerfas P, Gallin JI, Simon MM, Kwon-Chung KJ. Role of laeA in the Regulation of alb1, gliP, Conidial Morphology, and Virulence in Aspergillus fumigatus. Eukaryot Cell. 2007;6:1552–1561. doi: 10.1128/EC.00140-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A. 2001;98:5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallim MA, Miller KY, Miller BL. Aspergillus SteA (sterile12-like) is a homeodomain-C2/H2-Zn+2 finger transcription factor required for sexual reproduction. Mol Microbiol. 2000;36:290–301. doi: 10.1046/j.1365-2958.2000.01874.x. [DOI] [PubMed] [Google Scholar]
- Vienken K, Scherer M, Fischer R. The Zn(II)2Cys6 putative Aspergillus nidulans transcription factor repressor of sexual development inhibits sexual development under low-carbon conditions and in submersed culture. Genetics. 2005;169:619–630. doi: 10.1534/genetics.104.030767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vienken K, Fischer R. The Zn(II)2Cys6 putative transcription factor NosA controls fruiting body formation in Aspergillus nidulans. Mol Microbiol. 2006;61:544–554. doi: 10.1111/j.1365-2958.2006.05257.x. [DOI] [PubMed] [Google Scholar]
- Weidner G, d'Enfert C, Koch A, Mol PC, Brakhage AA. Development of a homologous transformation system for the human pathogenic fungus Aspergillus fumigatus based on the pyrG gene encoding orotidine 5'-monophosphate decarboxylase. Curr Genet. 1998;33:378–385. doi: 10.1007/s002940050350. [DOI] [PubMed] [Google Scholar]
- Willger SD, Puttikamonkul S, Kim KH, Burritt JB, Grahl N, Metzler LJ, Barbuch R, Bard M, Lawrence CB, Cramer RA., Jr A sterol-regulatory element binding protein is required for cell polarity, hypoxia adaptation, azole drug resistance, and virulence in Aspergillus fumigatus. PLoS Pathog. 2008;4:e1000200. doi: 10.1371/journal.ppat.1000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Chang P, Bhatnagar D, Cleveland TE. Cloning of a sugar utilization gene cluster in Aspergillus parasiticus. Biochim Biophys Acta. 2000;1493:211–214. doi: 10.1016/s0167-4781(00)00148-2. [DOI] [PubMed] [Google Scholar]
- Zarember KA, Sugui JA, Chang YC, Kwon-Chung KJ, Gallin JI. Human polymorphonuclear leukocytes inhibit Aspergillus fumigatus conidial growth by lactoferrin-mediated iron depletion. J Immunol. 2007;178:6367–6373. doi: 10.4049/jimmunol.178.10.6367. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table S1. List of PCR primers used in the studies.
Supplemental Table S2. List of A. fumigatus genes that were significantly up- or down-regulated in the ΔacuM mutant at 18 and/or 24 h compared to Af293 and the ΔacuM∷acuM complemented strain. Data represent microarray results as well as real-time PCR verification of selected genes.
Supplemental Table S3. List of genes in the GO term categories that were significantly down-regulated in the ΔacuM mutant at 18 and/or 24 h compared to Af293 and the ΔacuM∷acuM complemented strain.