Abstract
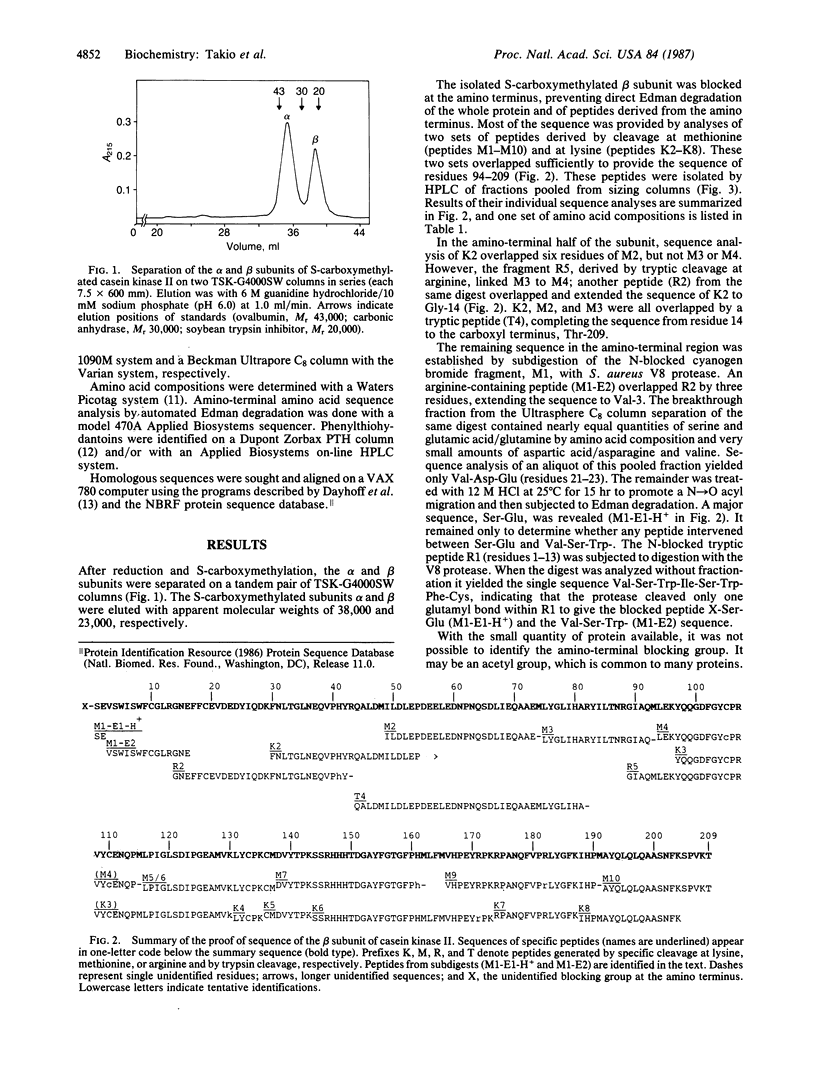
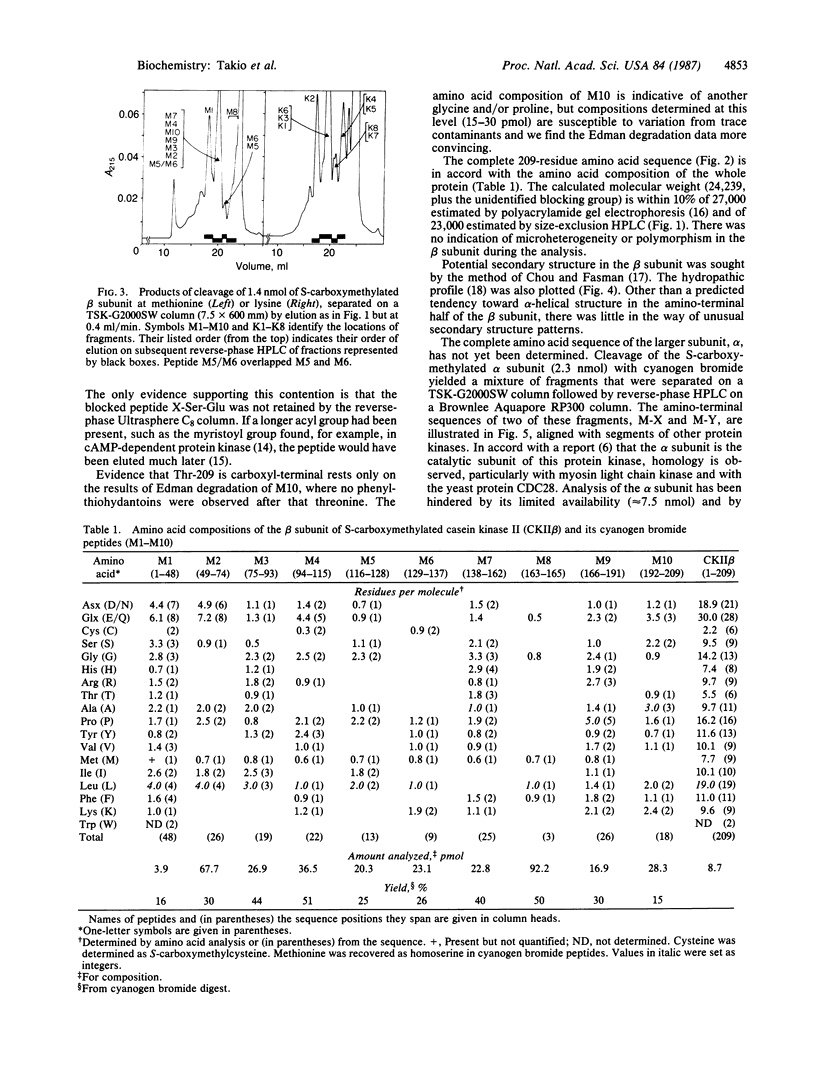
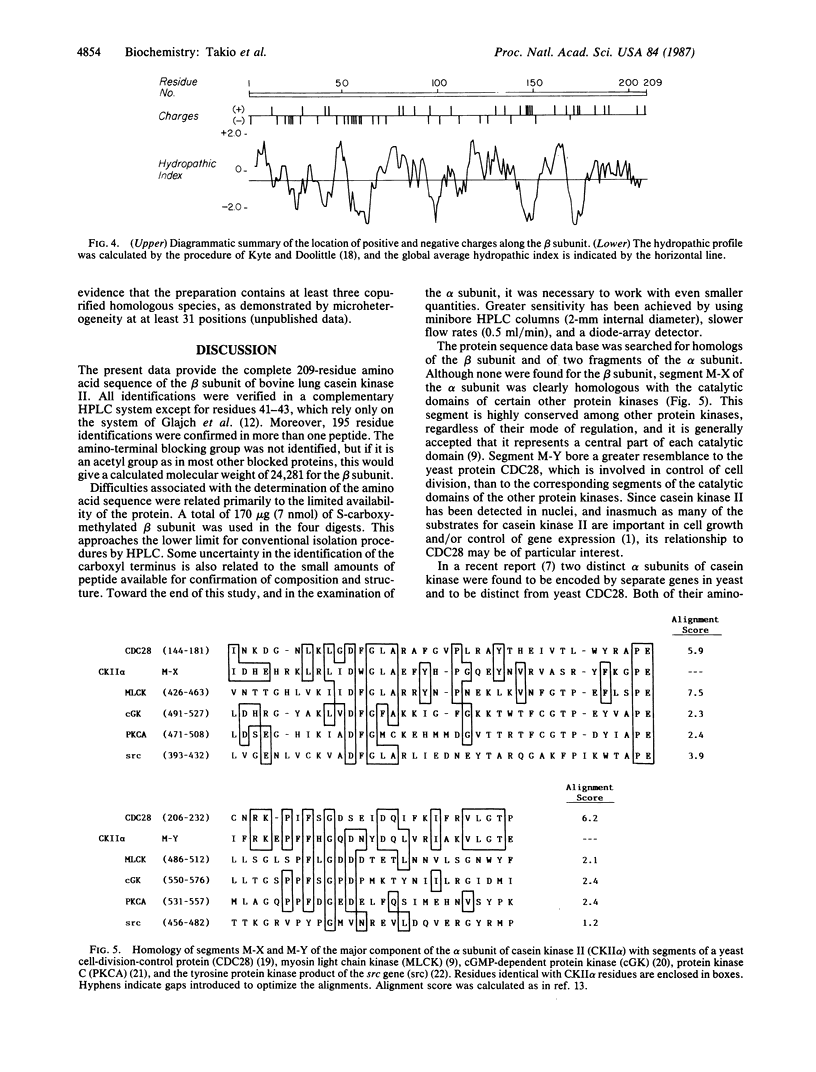
The amino acid sequence of the 209-residue beta subunit of bovine lung casein kinase II has been determined. Excluding the amino-terminal blocking group, which was not identified, the molecular weight of the polypeptide chain is 24,239. A marked polarity of the beta subunit is indicated by clusters of negative charges in the amino-terminal region and of positive charges in the carboxyl-terminal region. Whereas the beta subunit shows no homology with any known protein, a segment of the sequence of the larger and microheterogeneous alpha subunit exhibits homology with the catalytic domains of other protein kinases, particularly with the yeast cell-division-control protein CDC28.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bidlingmeyer B. A., Cohen S. A., Tarvin T. L. Rapid analysis of amino acids using pre-column derivatization. J Chromatogr. 1984 Dec 7;336(1):93–104. doi: 10.1016/s0378-4347(00)85133-6. [DOI] [PubMed] [Google Scholar]
- Carr S. A., Biemann K., Shoji S., Parmelee D. C., Titani K. n-Tetradecanoyl is the NH2-terminal blocking group of the catalytic subunit of cyclic AMP-dependent protein kinase from bovine cardiac muscle. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6128–6131. doi: 10.1073/pnas.79.20.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Dahmus M. E. Purification and properties of calf thymus casein kinases I and II. J Biol Chem. 1981 Apr 10;256(7):3319–3325. [PubMed] [Google Scholar]
- Dayhoff M. O., Barker W. C., Hunt L. T. Establishing homologies in protein sequences. Methods Enzymol. 1983;91:524–545. doi: 10.1016/s0076-6879(83)91049-2. [DOI] [PubMed] [Google Scholar]
- Feige J. J., Cochet C., Pirollet F., Chambaz E. M. Identification of the catalytic subunit of an oligomeric casein kinase (G type). Affinity labeling of the nucleotide site using 5'-[p-(fluorosulfonyl)benzoyl]adenosine. Biochemistry. 1983 Mar 15;22(6):1452–1459. doi: 10.1021/bi00275a020. [DOI] [PubMed] [Google Scholar]
- Feige J. J., Pirollet F., Cochet C., Chambaz E. M. Selective inhibition of a cyclic nucleotide-independent protein kinase (G-type casein kinase) by naturally occurring glycosaminoglycans. FEBS Lett. 1980 Nov 17;121(1):139–142. doi: 10.1016/0014-5793(80)81283-x. [DOI] [PubMed] [Google Scholar]
- Hathaway G. M., Lubben T. H., Traugh J. A. Inhibition of casein kinase II by heparin. J Biol Chem. 1980 Sep 10;255(17):8038–8041. [PubMed] [Google Scholar]
- Hathaway G. M., Traugh J. A. Casein kinases--multipotential protein kinases. Curr Top Cell Regul. 1982;21:101–127. [PubMed] [Google Scholar]
- Hathaway G. M., Traugh J. A. Cyclic nucleotide-independent protein kinases from rabbit reticulocytes. Purification of casein kinases. J Biol Chem. 1979 Feb 10;254(3):762–768. [PubMed] [Google Scholar]
- Hathaway G. M., Zoller M. J., Traugh J. A. Identification of the catalytic subunit of casein kinase II by affinity labeling with 5'-p-fluorosulfonylbenzoyl adenosine. J Biol Chem. 1981 Nov 25;256(22):11442–11446. [PubMed] [Google Scholar]
- Kuenzel E. A., Krebs E. G. A synthetic peptide substrate specific for casein kinase II. Proc Natl Acad Sci U S A. 1985 Feb;82(3):737–741. doi: 10.1073/pnas.82.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lörincz A. T., Reed S. I. Primary structure homology between the product of yeast cell division control gene CDC28 and vertebrate oncogenes. Nature. 1984 Jan 12;307(5947):183–185. doi: 10.1038/307183a0. [DOI] [PubMed] [Google Scholar]
- Masaki T., Tanabe M., Nakamura K., Soejima M. Studies on a new proteolytic enzyme from A chromobacter lyticus M497-1. I. Purification and some enzymatic properties. Biochim Biophys Acta. 1981 Jul 24;660(1):44–50. doi: 10.1016/0005-2744(81)90106-6. [DOI] [PubMed] [Google Scholar]
- Mäenpä P. H. Effects of polyamines and polyanions on a cyclic nucleotide-independent and a cyclic AMP-dependent protein kinase. Biochim Biophys Acta. 1977 Jul 21;498(1):294–305. doi: 10.1016/0304-4165(77)90267-7. [DOI] [PubMed] [Google Scholar]
- Padmanabha R., Glover C. V. Casein kinase II of yeast contains two distinct alpha polypeptides and an unusually large beta subunit. J Biol Chem. 1987 Feb 5;262(4):1829–1835. [PubMed] [Google Scholar]
- Parker P. J., Coussens L., Totty N., Rhee L., Young S., Chen E., Stabel S., Waterfield M. D., Ullrich A. The complete primary structure of protein kinase C--the major phorbol ester receptor. Science. 1986 Aug 22;233(4766):853–859. doi: 10.1126/science.3755547. [DOI] [PubMed] [Google Scholar]
- Schwartz D. E., Tizard R., Gilbert W. Nucleotide sequence of Rous sarcoma virus. Cell. 1983 Mar;32(3):853–869. doi: 10.1016/0092-8674(83)90071-5. [DOI] [PubMed] [Google Scholar]
- Takio K., Blumenthal D. K., Edelman A. M., Walsh K. A., Krebs E. G., Titani K. Amino acid sequence of an active fragment of rabbit skeletal muscle myosin light chain kinase. Biochemistry. 1985 Oct 22;24(22):6028–6037. doi: 10.1021/bi00343a002. [DOI] [PubMed] [Google Scholar]
- Takio K., Smith S. B., Walsh K. A., Krebs E. G., Titani K. Amino acid sequence around a "hinge" region and its "autophosphorylation" site in bovine Lung cGMP-dependent protein kinase. J Biol Chem. 1983 May 10;258(9):5531–5536. [PubMed] [Google Scholar]
- Takio K., Wade R. D., Smith S. B., Krebs E. G., Walsh K. A., Titani K. Guanosine cyclic 3',5'-phosphate dependent protein kinase, a chimeric protein homologous with two separate protein families. Biochemistry. 1984 Aug 28;23(18):4207–4218. doi: 10.1021/bi00313a030. [DOI] [PubMed] [Google Scholar]
- Thornburg W., Lindell T. J. Purification of rat liver nuclear protein kinase NII. J Biol Chem. 1977 Oct 10;252(19):6660–6665. [PubMed] [Google Scholar]
- Walsh K. A., Sasagawa T. High-performance liquid chromatography probes for posttranslationally modified amino acids. Methods Enzymol. 1984;106:22–29. doi: 10.1016/0076-6879(84)06005-5. [DOI] [PubMed] [Google Scholar]


