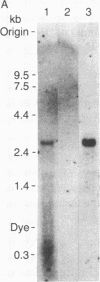
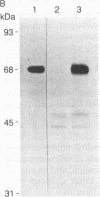
Abstract
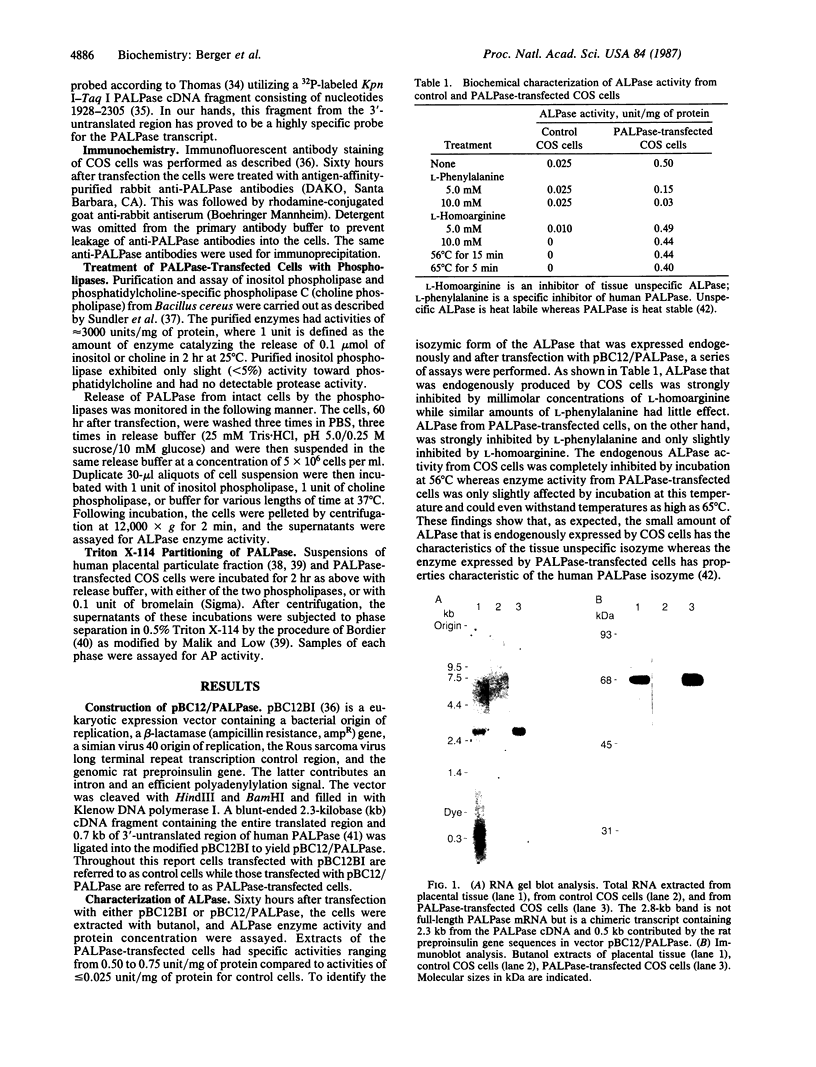
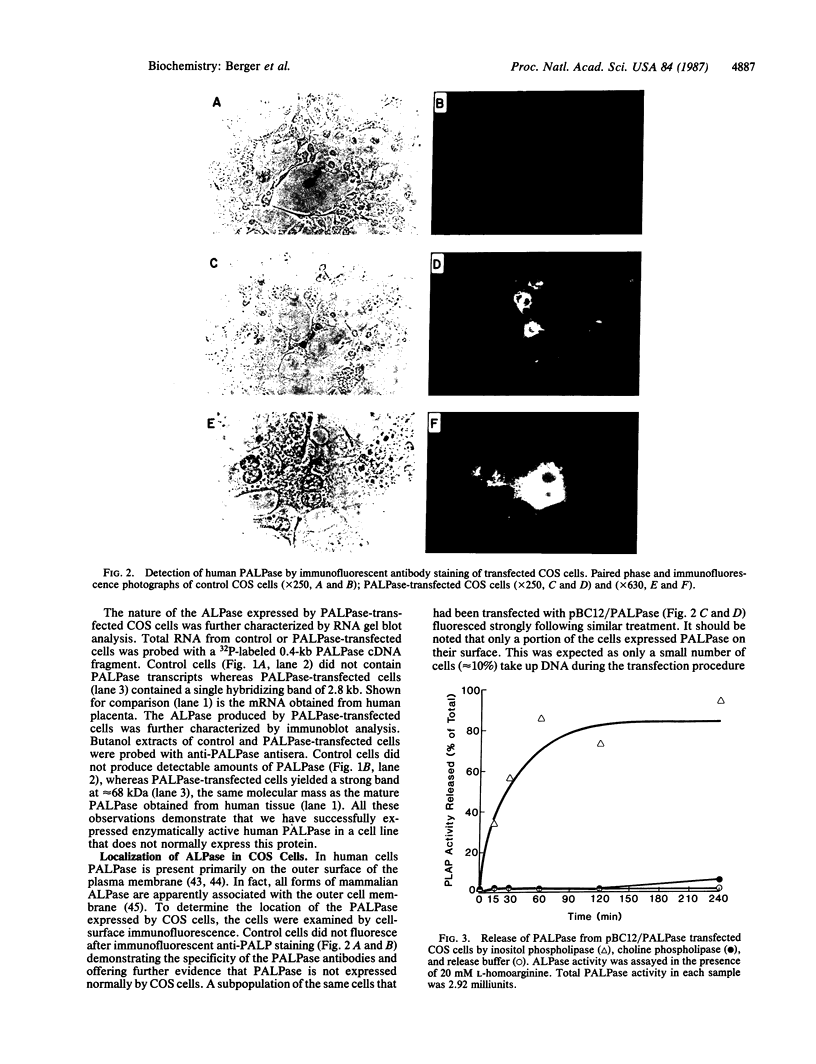
Human placental alkaline phosphatase (PALPase) has been transiently expressed in simian (COS) cells by transfection with a eukaryotic expression vector containing the corresponding cDNA. The level of expression of PALPase was high, and it was produced in an enzymatically active form. The bulk of PALPase was associated with the cell membrane as shown by immunocytochemistry and subcellular fractionation studies. The PALPase produced by transfected COS cells, like PALPase in human tissue, was specifically released from the intact cells in a hydrophilic form by phosphatidylinositol-specific phospholipase C and is, therefore, apparently attached to the outer membrane by means of a phosphatidylinositol-glycan. Transfected COS cells appear to be an excellent model for elucidating the mechanism of attachment of this phosphatidylinositol-glycan to a protein moiety.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abu-Hasan N. S., Sutcliffe R. G. Placental alkaline phosphatase integrates via its carboxy-terminus into the microvillous membrane: its allotypes differ in conformation. Placenta. 1985 Sep-Oct;6(5):391–404. doi: 10.1016/s0143-4004(85)80016-3. [DOI] [PubMed] [Google Scholar]
- Bangs J. D., Andrews N. W., Hart G. W., Englund P. T. Posttranslational modification and intracellular transport of a trypanosome variant surface glycoprotein. J Cell Biol. 1986 Jul;103(1):255–263. doi: 10.1083/jcb.103.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangs J. D., Hereld D., Krakow J. L., Hart G. W., Englund P. T. Rapid processing of the carboxyl terminus of a trypanosome variant surface glycoprotein. Proc Natl Acad Sci U S A. 1985 May;82(10):3207–3211. doi: 10.1073/pnas.82.10.3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger J., Garattini E., Hua J. C., Udenfriend S. Cloning and sequencing of human intestinal alkaline phosphatase cDNA. Proc Natl Acad Sci U S A. 1987 Feb;84(3):695–698. doi: 10.1073/pnas.84.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Borst P., Cross G. A. Molecular basis for trypanosome antigenic variation. Cell. 1982 Jun;29(2):291–303. doi: 10.1016/0092-8674(82)90146-5. [DOI] [PubMed] [Google Scholar]
- Butnick N. Z., Miyamoto C., Chizzonite R., Cullen B. R., Ju G., Skalka A. M. Regulation of the human c-myc gene: 5' noncoding sequences do not affect translation. Mol Cell Biol. 1985 Nov;5(11):3009–3016. doi: 10.1128/mcb.5.11.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conzelmann A., Spiazzi A., Hyman R., Bron C. Anchoring of membrane proteins via phosphatidylinositol is deficient in two classes of Thy-1 negative mutant lymphoma cells. EMBO J. 1986 Dec 1;5(12):3291–3296. doi: 10.1002/j.1460-2075.1986.tb04642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross G. A. Eukaryotic protein modification and membrane attachment via phosphatidylinositol. Cell. 1987 Jan 30;48(2):179–181. doi: 10.1016/0092-8674(87)90419-3. [DOI] [PubMed] [Google Scholar]
- Cullen B. R., Lomedico P. T., Ju G. Transcriptional interference in avian retroviruses--implications for the promoter insertion model of leukaemogenesis. Nature. 1984 Jan 19;307(5948):241–245. doi: 10.1038/307241a0. [DOI] [PubMed] [Google Scholar]
- Cullen B. R. Trans-activation of human immunodeficiency virus occurs via a bimodal mechanism. Cell. 1986 Sep 26;46(7):973–982. doi: 10.1016/0092-8674(86)90696-3. [DOI] [PubMed] [Google Scholar]
- Fatemi S. H., Tartakoff A. M. Hydrophilic anchor-deficient Thy-1 is secreted by a class E mutant T lymphoma. Cell. 1986 Aug 29;46(5):653–657. doi: 10.1016/0092-8674(86)90340-5. [DOI] [PubMed] [Google Scholar]
- Ferguson M. A., Cross G. A. Myristylation of the membrane form of a Trypanosoma brucei variant surface glycoprotein. J Biol Chem. 1984 Mar 10;259(5):3011–3015. [PubMed] [Google Scholar]
- Ferguson M. A., Duszenko M., Lamont G. S., Overath P., Cross G. A. Biosynthesis of Trypanosoma brucei variant surface glycoproteins. N-glycosylation and addition of a phosphatidylinositol membrane anchor. J Biol Chem. 1986 Jan 5;261(1):356–362. [PubMed] [Google Scholar]
- Ferguson M. A., Low M. G., Cross G. A. Glycosyl-sn-1,2-dimyristylphosphatidylinositol is covalently linked to Trypanosoma brucei variant surface glycoprotein. J Biol Chem. 1985 Nov 25;260(27):14547–14555. [PubMed] [Google Scholar]
- Futerman A. H., Fiorini R. M., Roth E., Low M. G., Silman I. Physicochemical behaviour and structural characteristics of membrane-bound acetylcholinesterase from Torpedo electric organ. Effect of phosphatidylinositol-specific phospholipase C. Biochem J. 1985 Mar 1;226(2):369–377. doi: 10.1042/bj2260369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futerman A. H., Low M. G., Ackermann K. E., Sherman W. R., Silman I. Identification of covalently bound inositol in the hydrophobic membrane-anchoring domain of Torpedo acetylcholinesterase. Biochem Biophys Res Commun. 1985 May 31;129(1):312–317. doi: 10.1016/0006-291x(85)91439-1. [DOI] [PubMed] [Google Scholar]
- Garattini E., Margolis J., Heimer E., Felix A., Udenfriend S. Human placental alkaline phosphatase in liver and intestine. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6080–6084. doi: 10.1073/pnas.82.18.6080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Haas R., Brandt P. T., Knight J., Rosenberry T. L. Identification of amine components in a glycolipid membrane-binding domain at the C-terminus of human erythrocyte acetylcholinesterase. Biochemistry. 1986 Jun 3;25(11):3098–3105. doi: 10.1021/bi00359a005. [DOI] [PubMed] [Google Scholar]
- Henthorn P. S., Knoll B. J., Raducha M., Rothblum K. N., Slaughter C., Weiss M., Lafferty M. A., Fischer T., Harris H. Products of two common alleles at the locus for human placental alkaline phosphatase differ by seven amino acids. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5597–5601. doi: 10.1073/pnas.83.15.5597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikezawa H., Yamanegi M., Taguchi R., Miyashita T., Ohyabu T. Studies on phosphatidylinositol phosphodiesterase (phospholipase C type) of Bacillus cereus. I. purification, properties and phosphatase-releasing activity. Biochim Biophys Acta. 1976 Nov 19;450(2):154–164. [PubMed] [Google Scholar]
- Jemmerson R., Klier F. G., Fishman W. H. Clustered distribution of human placental alkaline phosphatase on the surface of both placental and cancer cells. Electron microscopic observations using gold-labeled antibodies. J Histochem Cytochem. 1985 Dec;33(12):1227–1234. doi: 10.1177/33.12.4067276. [DOI] [PubMed] [Google Scholar]
- Jemmerson R., Shah N., Takeya M., Fishman W. H. Characterization of the placental alkaline phosphatase-like (Nagao) isozyme on the surface of A431 human epidermoid carcinoma cells. Cancer Res. 1985 Jan;45(1):282–287. [PubMed] [Google Scholar]
- Jemmerson R., Shah N., Takeya M., Fishman W. H. Functional organization of the placental alkaline phosphatase polypeptide chain. Prog Clin Biol Res. 1984;166:105–115. [PubMed] [Google Scholar]
- Krakow J. L., Hereld D., Bangs J. D., Hart G. W., Englund P. T. Identification of a glycolipid precursor of the Trypanosoma brucei variant surface glycoprotein. J Biol Chem. 1986 Sep 15;261(26):12147–12153. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Low M. G., Finean J. B. Release of alkaline phosphatase from membranes by a phosphatidylinositol-specific phospholipase C. Biochem J. 1977 Oct 1;167(1):281–284. doi: 10.1042/bj1670281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low M. G., Kincade P. W. Phosphatidylinositol is the membrane-anchoring domain of the Thy-1 glycoprotein. Nature. 1985 Nov 7;318(6041):62–64. doi: 10.1038/318062a0. [DOI] [PubMed] [Google Scholar]
- Malik A. S., Low M. G. Conversion of human placental alkaline phosphatase from a high Mr form to a low Mr form during butanol extraction. An investigation of the role of endogenous phosphoinositide-specific phospholipases. Biochem J. 1986 Dec 1;240(2):519–527. doi: 10.1042/bj2400519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W. L., Rosenberry T. L. Identification of covalently attached fatty acids in the hydrophobic membrane-binding domain of human erythrocyte acetylcholinesterase. Biochem Biophys Res Commun. 1985 Dec 17;133(2):621–627. doi: 10.1016/0006-291x(85)90950-7. [DOI] [PubMed] [Google Scholar]
- Roberts W. L., Rosenberry T. L. Selective radiolabeling and isolation of the hydrophobic membrane-binding domain of human erythrocyte acetylcholinesterase. Biochemistry. 1986 Jun 3;25(11):3091–3098. doi: 10.1021/bi00359a004. [DOI] [PubMed] [Google Scholar]
- Slein M. W., Logan G. F. Characterization of the Phospholipases of Bacillus cereus and Their Effects on Erythrocytes, Bone, and Kidney Cells. J Bacteriol. 1965 Jul;90(1):69–81. doi: 10.1128/jb.90.1.69-81.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stigbrand T. Present status and future trends of human alkaline phosphatases. Prog Clin Biol Res. 1984;166:3–14. [PubMed] [Google Scholar]
- Sundler R., Alberts A. W., Vagelos P. R. Enzymatic properties of phosphatidylinositol inositolphosphohydrolase from Bacillus cereus. Substrate dilution in detergent-phospholipid micelles and bilayer vesicles. J Biol Chem. 1978 Jun 25;253(12):4175–4179. [PubMed] [Google Scholar]
- Swerdlow P. S., Finley D., Varshavsky A. Enhancement of immunoblot sensitivity by heating of hydrated filters. Anal Biochem. 1986 Jul;156(1):147–153. doi: 10.1016/0003-2697(86)90166-1. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokumitsu S., Tokumitsu K., Fishman W. H. Immunocytochemical demonstration of intracytoplasmic alkaline phosphatase in HeLa TCRC-1 cells. J Histochem Cytochem. 1981 Sep;29(9):1080–1087. doi: 10.1177/29.9.7026668. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse A. G., Barclay A. N., Watts A., Williams A. F. A glycophospholipid tail at the carboxyl terminus of the Thy-1 glycoprotein of neurons and thymocytes. Science. 1985 Nov 29;230(4729):1003–1008. doi: 10.1126/science.2865810. [DOI] [PubMed] [Google Scholar]