Abstract
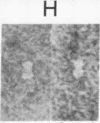
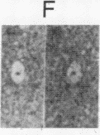
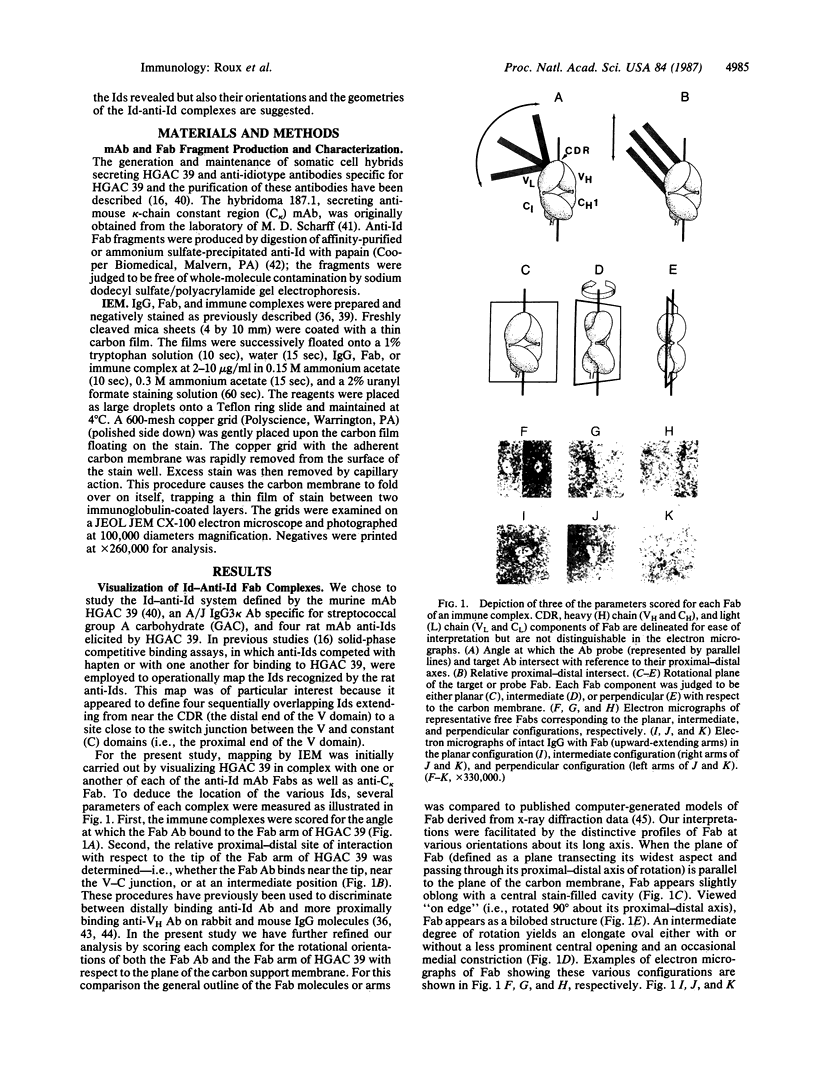
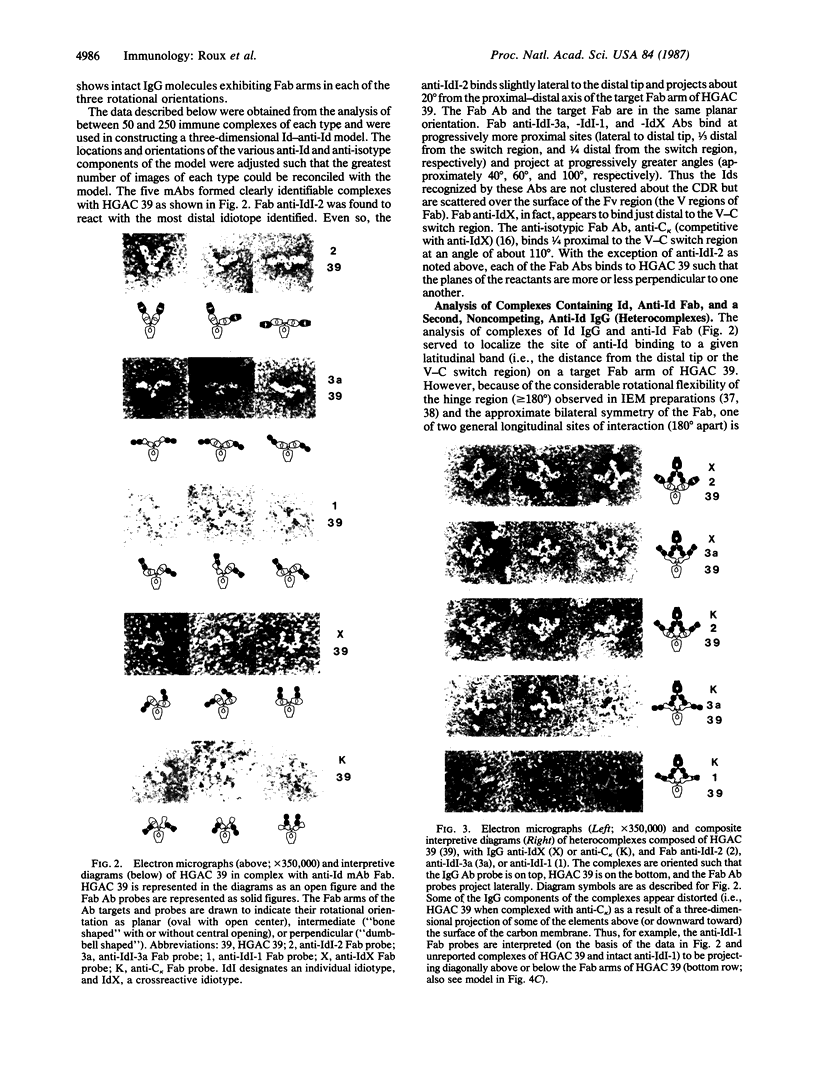
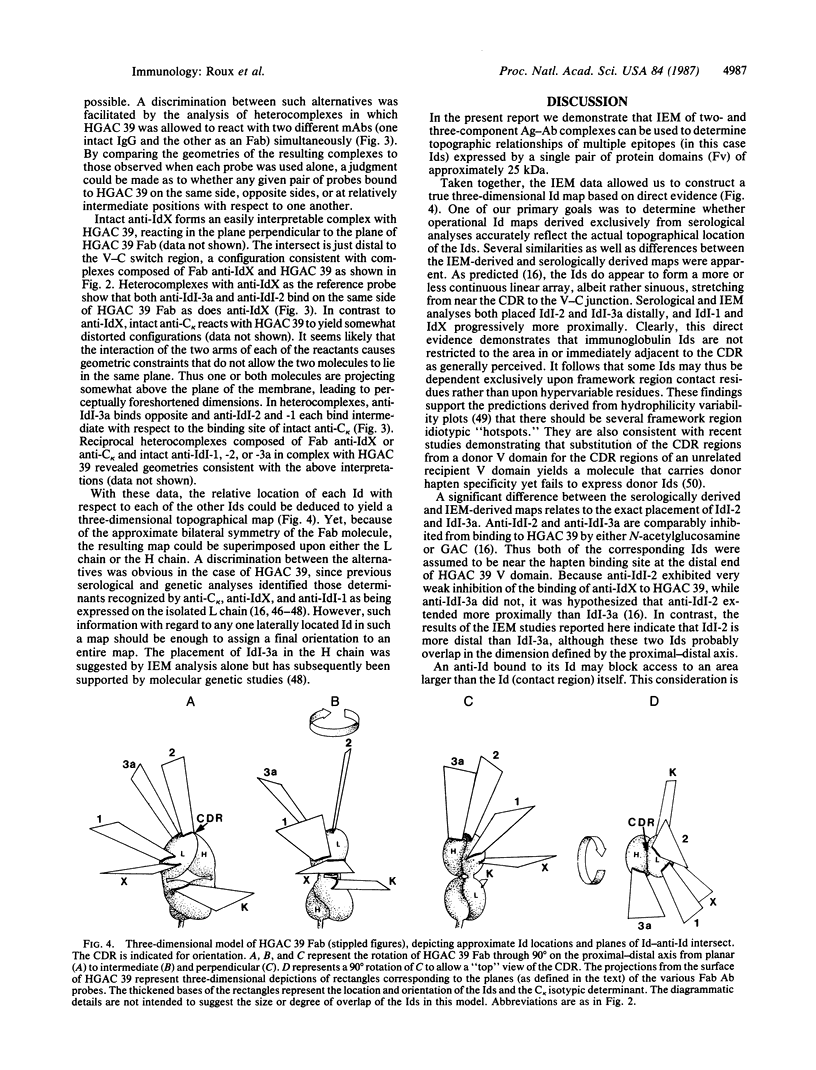
A three-dimensional map of the positions of four idiotypic determinants (idiotopes or Ids) and an isotypic determinant was derived by transmission electron microscopy of negatively stained immune complexes. Each complex was composed of a monoclonal Id-expressing IgG and one or two varieties of monoclonal anti-Id (or anti-isotype) Fab fragment or IgG. Data from the various combinations of Id and anti-Id (and anti-isotype) were used to construct a low-resolution three-dimensional model that revealed not only the approximate locations of Ids on the surface of the antibody variable domains but also details of the geometry of Id-anti-Id interactions not otherwise available. The Ids were shown to be dispersed over the variable domains, extending from the complementarity-determining region to near the variable-constant switch region. Thus, immunoelectron microscopy is a useful complement to serologic, biochemical, and genetic strategies for the topographical analysis of immunoglobulin Ids or other epitopes. This same approach should be of broader applicability in the study of epitopes and receptor sites on other macromolecules.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amit A. G., Mariuzza R. A., Phillips S. E., Poljak R. J. Three-dimensional structure of an antigen-antibody complex at 2.8 A resolution. Science. 1986 Aug 15;233(4765):747–753. doi: 10.1126/science.2426778. [DOI] [PubMed] [Google Scholar]
- Barlow D. J., Edwards M. S., Thornton J. M. Continuous and discontinuous protein antigenic determinants. Nature. 1986 Aug 21;322(6081):747–748. doi: 10.1038/322747a0. [DOI] [PubMed] [Google Scholar]
- Briles D. E., Davie J. M. Clonal nature of the immune response. II. The effect of immunization on clonal commitment. J Exp Med. 1980 Jul 1;152(1):151–160. doi: 10.1084/jem.152.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüggemann M., Müller H. J., Burger C., Rajewsky K. Idiotypic selection of an antibody mutant with changed hapten binding specificity, resulting from a point mutation in position 50 of the heavy chain. EMBO J. 1986 Jul;5(7):1561–1566. doi: 10.1002/j.1460-2075.1986.tb04397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüggemann M., Radbruch A., Rajewsky K. Immunoglobulin V region variants in hybridoma cells. I. Isolation of a variant with altered idiotypic and antigen binding specificity. EMBO J. 1982;1(5):629–634. doi: 10.1002/j.1460-2075.1982.tb01219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M., Bieber M., Levy R., Teng N. N. Influence of avidity and idiotope recognition on the modulation of surface immunoglobulin on malignant human B cells by rat monoclonal anti-idiotype antibodies. J Immunol. 1986 Apr 15;136(8):2983–2988. [PubMed] [Google Scholar]
- Cerny J., Wallich R., Hammerling G. J. Analysis of T15 idiotopes by monoclonal antibodies: variability of idiotopic expression on phosphorylcholine-specific lymphocytes from individual inbred mice. J Immunol. 1982 Apr;128(4):1885–1891. [PubMed] [Google Scholar]
- Chen P. P., Houghten R. A., Fong S., Rhodes G. H., Gilbertson T. A., Vaughan J. H., Lerner R. A., Carson D. A. Anti-hypervariable region antibody induced by a defined peptide: an approach for studying the structural correlates of idiotypes. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1784–1788. doi: 10.1073/pnas.81.6.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary M. L., Meeker T. C., Levy S., Lee E., Trela M., Sklar J., Levy R. Clustering of extensive somatic mutations in the variable region of an immunoglobulin heavy chain gene from a human B cell lymphoma. Cell. 1986 Jan 17;44(1):97–106. doi: 10.1016/0092-8674(86)90488-5. [DOI] [PubMed] [Google Scholar]
- Davie J. M., Seiden M. V., Greenspan N. S., Lutz C. T., Bartholow T. L., Clevinger B. L. Structural correlates of idiotopes. Annu Rev Immunol. 1986;4:147–165. doi: 10.1146/annurev.iy.04.040186.001051. [DOI] [PubMed] [Google Scholar]
- Furey W., Jr, Wang B. C., Yoo C. S., Sax M. Structure of a novel Bence-Jones protein (Rhe) fragment at 1.6 A resolution. J Mol Biol. 1983 Jul 5;167(3):661–692. doi: 10.1016/s0022-2836(83)80104-1. [DOI] [PubMed] [Google Scholar]
- Goldman M., Renversez J. C., Lambert P. H. Pathological expression of idiotypic interactions: immune complexes and cryoglobulins. Springer Semin Immunopathol. 1983;6(1):33–49. doi: 10.1007/BF01857365. [DOI] [PubMed] [Google Scholar]
- Greenspan N. S., Davie J. M. Serologic and topographic characterization of idiotopes on murine monoclonal anti-streptococcal group A carbohydrate antibodies. J Immunol. 1985 Feb;134(2):1065–1072. [PubMed] [Google Scholar]
- Greenspan N. S., Fulton R. J., Davie J. M. Analysis of anti-streptococcal group A carbohydrate idiotope levels in sera: correlation of magnitude of expression with idiotope position and VK haplotype. J Immunol. 1986 Jul 1;137(1):228–233. [PubMed] [Google Scholar]
- Greenspan N. S., Monafo W. J., Davie J. M. Interaction of IgG3 anti-streptococcal group A carbohydrate (GAC) antibody with streptococcal group A vaccine: enhancing and inhibiting effects of anti-GAC, anti-isotypic, and anti-idiotypic antibodies. J Immunol. 1987 Jan 1;138(1):285–292. [PubMed] [Google Scholar]
- Hansburg D., Briles D. E., Davie J. M. Analysis of the diversity of murine antibodies to dextran B1355. I. Generation of a larger, pauci-clonal response by a bacterial vaccine. J Immunol. 1976 Aug;117(2):569–575. [PubMed] [Google Scholar]
- Hansburg D., Briles D. E., Davie J. M. Analysis of the diversity of murine antibodies to dextran B1355. II. Demonstration of multiple idiotypes with variable expression in several strains. J Immunol. 1977 Oct;119(4):1406–1412. [PubMed] [Google Scholar]
- Jerne N. K. Towards a network theory of the immune system. Ann Immunol (Paris) 1974 Jan;125C(1-2):373–389. [PubMed] [Google Scholar]
- Jeske D., Milner E. C., Leo O., Moser M., Marvel J., Urbain J., Capra J. D. Molecular mapping of idiotopes of anti-arsonate antibodies. J Immunol. 1986 Apr 1;136(7):2568–2574. [PubMed] [Google Scholar]
- Jones P. T., Dear P. H., Foote J., Neuberger M. S., Winter G. Replacing the complementarity-determining regions in a human antibody with those from a mouse. 1986 May 29-Jun 4Nature. 321(6069):522–525. doi: 10.1038/321522a0. [DOI] [PubMed] [Google Scholar]
- Kaartinen M., Griffiths G. M., Markham A. F., Milstein C. mRNA sequences define an unusually restricted IgG response to 2-phenyloxazolone and its early diversification. 1983 Jul 28-Aug 3Nature. 304(5924):320–324. doi: 10.1038/304320a0. [DOI] [PubMed] [Google Scholar]
- Kelus A. S., Gell P. G. Immunological analysis of rabbit anti-antibody systems. J Exp Med. 1968 Jan 1;127(1):215–234. doi: 10.1084/jem.127.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieber-Emmons T., Kohler H. Towards a unified theory of immunoglobulin structure-function relations. Immunol Rev. 1986 Apr;90:29–48. doi: 10.1111/j.1600-065x.1986.tb01476.x. [DOI] [PubMed] [Google Scholar]
- Kim S., Davis M., Sinn E., Patten P., Hood L. Antibody diversity: somatic hypermutation of rearranged VH genes. Cell. 1981 Dec;27(3 Pt 2):573–581. doi: 10.1016/0092-8674(81)90399-8. [DOI] [PubMed] [Google Scholar]
- Kunkel H. G., Mannik M., Williams R. C. Individual Antigenic Specificity of Isolated Antibodies. Science. 1963 Jun 14;140(3572):1218–1219. doi: 10.1126/science.140.3572.1218. [DOI] [PubMed] [Google Scholar]
- Lutz C. T., Bartholow T. L., Greenspan N. S., Fulton R. J., Monafo W. J., Perlmutter R. M., Huang H. V., Davie J. M. Molecular dissection of the murine antibody response to streptococcal group A carbohydrate. J Exp Med. 1987 Feb 1;165(2):531–545. doi: 10.1084/jem.165.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannik M. Physicochemical and functional relationships of immune complexes. J Invest Dermatol. 1980 May;74(5):333–338. doi: 10.1111/1523-1747.ep12543582. [DOI] [PubMed] [Google Scholar]
- McKean D., Huppi K., Bell M., Staudt L., Gerhard W., Weigert M. Generation of antibody diversity in the immune response of BALB/c mice to influenza virus hemagglutinin. Proc Natl Acad Sci U S A. 1984 May;81(10):3180–3184. doi: 10.1073/pnas.81.10.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan S., Seiden M. V., Houghten R. A., Clevinger B., Davie J. M., Lerner R. A. Synthetic idiotypes: the third hypervariable region of murine anti-dextran antibodies. Cell. 1983 Dec;35(3 Pt 2):859–863. doi: 10.1016/0092-8674(83)90118-6. [DOI] [PubMed] [Google Scholar]
- Nahm M. H., Clevinger B. L., Davie J. M. Monoclonal antibodies to streptococcal group A carbohydrate. I. A dominant idiotypic determinant is located on Vk. J Immunol. 1982 Oct;129(4):1513–1518. [PubMed] [Google Scholar]
- OUDIN J., MICHEL M. [A new allotype form of rabbit serum gamma-globulins, apparently associated with antibody function and specificity]. C R Hebd Seances Acad Sci. 1963 Jul 17;257:805–808. [PubMed] [Google Scholar]
- Radbruch A., Zaiss S., Kappen C., Brüggemann M., Beyreuther K., Rajewsky K. Drastic change in idiotypic but not antigen-binding specificity of an antibody by a single amino-acid substitution. Nature. 1985 Jun 6;315(6019):506–508. doi: 10.1038/315506a0. [DOI] [PubMed] [Google Scholar]
- Rajewsky K., Takemori T. Genetics, expression, and function of idiotypes. Annu Rev Immunol. 1983;1:569–607. doi: 10.1146/annurev.iy.01.040183.003033. [DOI] [PubMed] [Google Scholar]
- Rousseaux J., Rousseaux-Prévost R., Bazin H. Optimal conditions for the preparation of Fab and F(ab')2 fragments from monoclonal IgG of different rat IgG subclasses. J Immunol Methods. 1983 Nov 11;64(1-2):141–146. doi: 10.1016/0022-1759(83)90392-7. [DOI] [PubMed] [Google Scholar]
- Roux K. H. Direct demonstration of multiple VH allotopes on rabbit Ig molecules: allotope characteristics and Fab arm rotational flexibility revealed by immunoelectron microscopy. Eur J Immunol. 1984 May;14(5):459–464. doi: 10.1002/eji.1830140514. [DOI] [PubMed] [Google Scholar]
- Roux K. H., Metzger D. W. Immunoelectron microscopic localization of idiotypes and allotypes on immunoglobulin molecules. J Immunol. 1982 Dec;129(6):2548–2553. [PubMed] [Google Scholar]
- Roux K. H., Metzger D. W., Kazdin D. S., Horng W. J. Induced latent allotypes within rabbit anti-cross-reactive idiotype reagents. Direct immunoelectron microscopic evidence. Eur J Immunol. 1984 Oct;14(10):910–915. doi: 10.1002/eji.1830141010. [DOI] [PubMed] [Google Scholar]
- Rudikoff S. Immunoglobulin structure--function correlates: antigen binding and idiotypes. Contemp Top Mol Immunol. 1983;9:169–209. doi: 10.1007/978-1-4684-4517-6_6. [DOI] [PubMed] [Google Scholar]
- Seegan G. W., Smith C. A., Schumaker V. N. Changes in quaternary structure of IgG upon reduction of the interheavy-chain disulfide bond. Proc Natl Acad Sci U S A. 1979 Feb;76(2):907–911. doi: 10.1073/pnas.76.2.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverton E. W., Navia M. A., Davies D. R. Three-dimensional structure of an intact human immunoglobulin. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5140–5144. doi: 10.1073/pnas.74.11.5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens F. J., Jwo J., Carperos W., Köhler H., Schiffer M. Relationships between liquid- and solid-phase antibody association characteristics: implications for the use of competitive ELISA techniques to map the spatial location of idiotopes. J Immunol. 1986 Sep 15;137(6):1937–1944. [PubMed] [Google Scholar]
- Streicher H. Z., Cuttitta F., Buckenmeyer G. K., Kawamura H., Minna J., Berzofsky J. A. Mapping the idiotopes of a monoclonal anti-myoglobin antibody with syngeneic monoclonal anti-idiotypic antibodies: detection of a common idiotope. J Immunol. 1986 Feb 1;136(3):1007–1014. [PubMed] [Google Scholar]
- Teillaud J. L., Desaymard C., Giusti A. M., Haseltine B., Pollock R. R., Yelton D. E., Zack D. J., Scharff M. D. Monoclonal antibodies reveal the structural basis of antibody diversity. Science. 1983 Nov 18;222(4625):721–726. doi: 10.1126/science.6356353. [DOI] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Valentine R. C., Green N. M. Electron microscopy of an antibody-hapten complex. J Mol Biol. 1967 Aug 14;27(3):615–617. doi: 10.1016/0022-2836(67)90063-0. [DOI] [PubMed] [Google Scholar]
- Van Cleave V. H., Murti K. G., Metzger D. W. Mouse monoclonal antibodies induced by anti-allotype antibody display internal images of the rabbit VHa1 allotype: direct visualization by immunoelectron microscopy. Eur J Immunol. 1986 Jun;16(6):701–707. doi: 10.1002/eji.1830160619. [DOI] [PubMed] [Google Scholar]
- Wrigley N. G., Brown E. B., Skehel J. J. Electron microscopic evidence for the axial rotation and inter-domain flexibility of the Fab regions of immunoglobulin G. J Mol Biol. 1983 Sep 25;169(3):771–774. doi: 10.1016/s0022-2836(83)80170-3. [DOI] [PubMed] [Google Scholar]
- Yelton D. E., Desaymard C., Scharff M. D. Use of monoclonal anti-mouse immunoglobulin to detect mouse antibodies. Hybridoma. 1981;1(1):5–11. doi: 10.1089/hyb.1.1981.1.5. [DOI] [PubMed] [Google Scholar]
- Zenke G., Eichmann K., Emmrich F. Idiotope mapping on the variable region of an antibody clonotype produced by normal (nonmalignant) human B cells. J Immunol. 1985 Dec;135(6):4066–4072. [PubMed] [Google Scholar]
- de la Paz P., Sutton B. J., Darsley M. J., Rees A. R. Modelling of the combining sites of three anti-lysozyme monoclonal antibodies and of the complex between one of the antibodies and its epitope. EMBO J. 1986 Feb;5(2):415–425. doi: 10.1002/j.1460-2075.1986.tb04227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]