Abstract
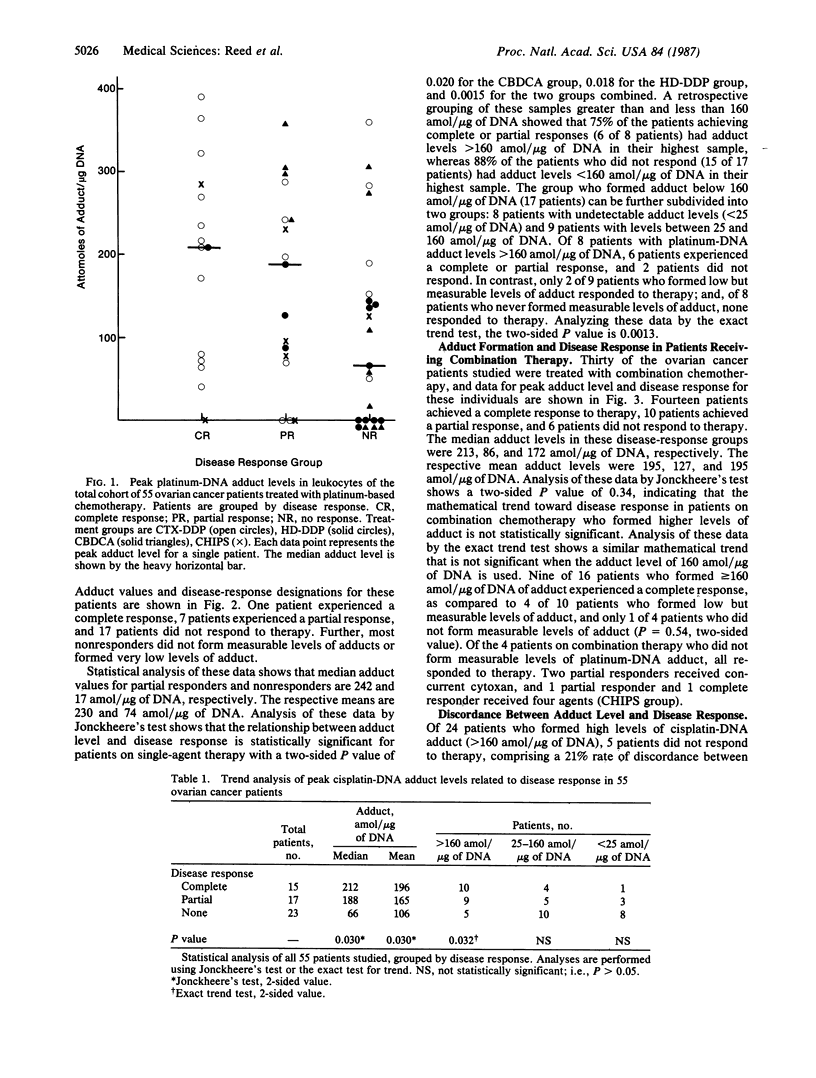
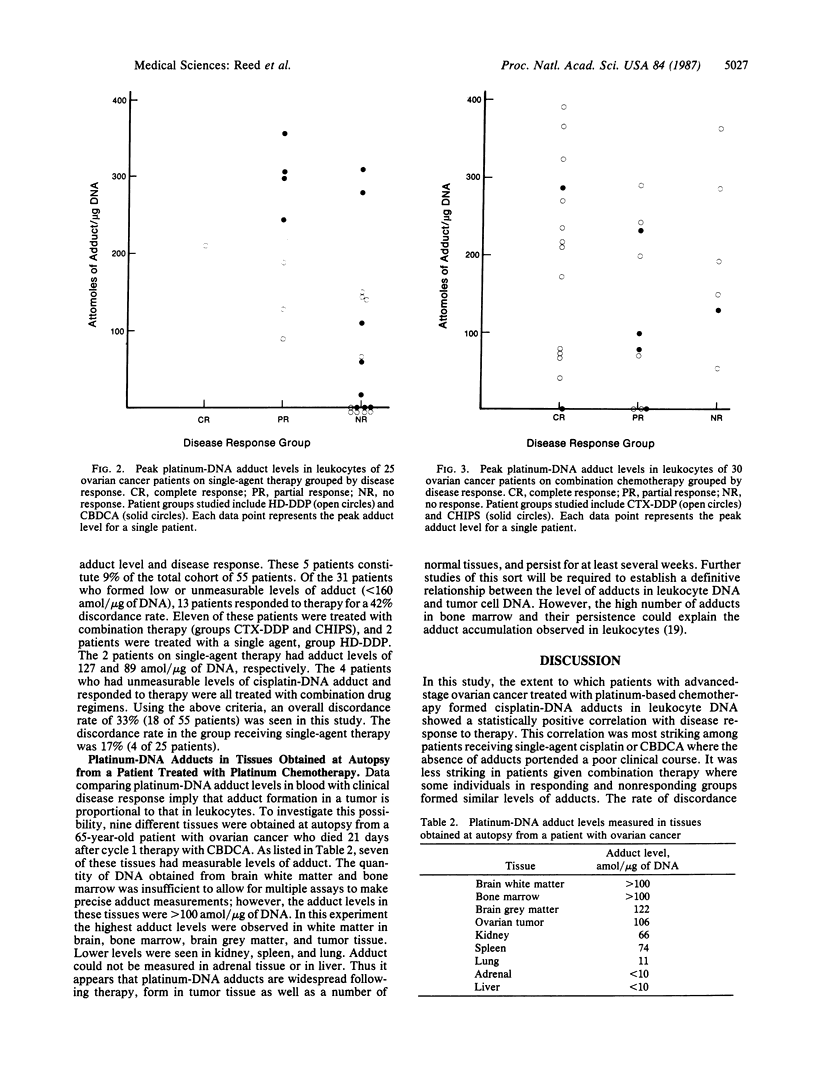
Fifty-five ovarian cancer patients receiving platinum drug-based chemotherapy have been studied prospectively to determine the extent of formation of the bidentate intrastrand adducts of diammineplatinum covalently attached to the N7 positions of adenosine and/or guanosine in leukocyte DNA. Data for clinical response, obtained from medical records, were then correlated with the adduct values. Patients were treated with platinum-based single-agent or combination chemotherapy containing cis-diamminedichloroplatinum (II) or diamminecyclobutane-dicarboxylatoplatinum on approved experimental protocols. Adduct measurements were performed by ELISA, and disease response to therapy was assessed by standard oncologic criteria. This study comprises a total of 101 blood samples obtained after intravenous cis-diamminedichloroplatinum (II) or diamminecyclobutane-dicarboxylatoplatinum infusion from 55 individuals, and in each case the highest (or "peak") adduct level for each patient was chosen for statistical analysis. Values for median adduct levels in patients grouped by complete response, partial response, and no response were 212, 193, and 62 amol of adduct per microgram of DNA, respectively. Analysis of these data by Jonckheere's test (an extension of the Mann-Whitney test) shows that higher levels of adduct formation correlates with disease response with a two-sided P value of 0.030. Of eight patients on single-agent therapy whose buffy-coat samples did not have measurable adduct levels, none responded to therapy. Analysis of these data using the exact test for trend shows that the formation of adduct at a level of 160 amol/micrograms of DNA or greater correlates with disease response with a two-sided P value of 0.032. Thus in ovarian cancer patients, the formation of the intrastrand diammineplatinum adducts in leukocyte DNA is associated with favorable disease response to cis-diamminedichloroplatinum (II) or diamminecyclobutane-dicarboxylatoplatinum chemotherapy.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnhart K. M., Bowden G. T. Cisplatin as an initiating agent in two-stage mouse skin carcinogenesis. Cancer Lett. 1985 Oct;29(1):101–105. doi: 10.1016/0304-3835(85)90129-6. [DOI] [PubMed] [Google Scholar]
- Bassett W. B., Weiss R. B. Acute leukemia following cisplatin for bladder cancer. J Clin Oncol. 1986 Apr;4(4):614–614. [PubMed] [Google Scholar]
- Endresen L., Schjerven L., Rugstad H. E. Tumours from a cell strain with a high content of metallothionein show enhanced resistance against cis-dichlorodiammineplatinum. Acta Pharmacol Toxicol (Copenh) 1984 Sep;55(3):183–187. doi: 10.1111/j.1600-0773.1984.tb02034.x. [DOI] [PubMed] [Google Scholar]
- Fichtinger-Schepman A. M., Lohman P. H., Reedijk J. Detection and quantification of adducts formed upon interaction of diamminedichloroplatinum (II) with DNA, by anion-exchange chromatography after enzymatic degradation. Nucleic Acids Res. 1982 Sep 11;10(17):5345–5356. doi: 10.1093/nar/10.17.5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fram R. J., Cusick P. S., Wilson J. M., Marinus M. G. Mismatch repair of cis-diamminedichloroplatinum(II)-induced DNA damage. Mol Pharmacol. 1985 Jul;28(1):51–55. [PubMed] [Google Scholar]
- Groopman J. D., Donahue P. R., Zhu J. Q., Chen J. S., Wogan G. N. Aflatoxin metabolism in humans: detection of metabolites and nucleic acid adducts in urine by affinity chromatography. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6492–6496. doi: 10.1073/pnas.82.19.6492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton T. C., Winker M. A., Louie K. G., Batist G., Behrens B. C., Tsuruo T., Grotzinger K. R., McKoy W. M., Young R. C., Ozols R. F. Augmentation of adriamycin, melphalan, and cisplatin cytotoxicity in drug-resistant and -sensitive human ovarian carcinoma cell lines by buthionine sulfoximine mediated glutathione depletion. Biochem Pharmacol. 1985 Jul 15;34(14):2583–2586. doi: 10.1016/0006-2952(85)90551-9. [DOI] [PubMed] [Google Scholar]
- Hanawalt P. C., Cooper P. K., Ganesan A. K., Smith C. A. DNA repair in bacteria and mammalian cells. Annu Rev Biochem. 1979;48:783–836. doi: 10.1146/annurev.bi.48.070179.004031. [DOI] [PubMed] [Google Scholar]
- Harris C. C., Vahakangas K., Newman M. J., Trivers G. E., Shamsuddin A., Sinopoli N., Mann D. L., Wright W. E. Detection of benzo[a]pyrene diol epoxide-DNA adducts in peripheral blood lymphocytes and antibodies to the adducts in serum from coke oven workers. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6672–6676. doi: 10.1073/pnas.82.19.6672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henne T., Schmähl D. Occurrence of second primary malignancies in man--a second look. Cancer Treat Rev. 1985 Jun;12(2):77–94. doi: 10.1016/0305-7372(85)90001-5. [DOI] [PubMed] [Google Scholar]
- Knox R. J., Friedlos F., Lydall D. A., Roberts J. J. Mechanism of cytotoxicity of anticancer platinum drugs: evidence that cis-diamminedichloroplatinum(II) and cis-diammine-(1,1-cyclobutanedicarboxylato)platinum(II) differ only in the kinetics of their interaction with DNA. Cancer Res. 1986 Apr;46(4 Pt 2):1972–1979. [PubMed] [Google Scholar]
- Knox R. J., Friedlos F., Lydall D. A., Roberts J. J. Mechanism of cytotoxicity of anticancer platinum drugs: evidence that cis-diamminedichloroplatinum(II) and cis-diammine-(1,1-cyclobutanedicarboxylato)platinum(II) differ only in the kinetics of their interaction with DNA. Cancer Res. 1986 Apr;46(4 Pt 2):1972–1979. [PubMed] [Google Scholar]
- Leopold W. R., Miller E. C., Miller J. A. Carcinogenicity of antitumor cis-platinum(II) coordination complexes in the mouse and rat. Cancer Res. 1979 Mar;39(3):913–918. [PubMed] [Google Scholar]
- Meister A. Selective modification of glutathione metabolism. Science. 1983 Apr 29;220(4596):472–477. doi: 10.1126/science.6836290. [DOI] [PubMed] [Google Scholar]
- Ozols R. F., Corden B. J., Jacob J., Wesley M. N., Ostchega Y., Young R. C. High-dose cisplatin in hypertonic saline. Ann Intern Med. 1984 Jan;100(1):19–24. doi: 10.7326/0003-4819-100-1-19. [DOI] [PubMed] [Google Scholar]
- Perera F. P., Poirier M. C., Yuspa S. H., Nakayama J., Jaretzki A., Curnen M. M., Knowles D. M., Weinstein I. B. A pilot project in molecular cancer epidemiology: determination of benzo[a]pyrene--DNA adducts in animal and human tissues by immunoassays. Carcinogenesis. 1982;3(12):1405–1410. doi: 10.1093/carcin/3.12.1405. [DOI] [PubMed] [Google Scholar]
- Plooy A. C., Fichtinger-Schepman A. M., Schutte H. H., van Dijk M., Lohman P. H. The quantitative detection of various Pt-DNA-adducts in Chinese hamster ovary cells treated with cisplatin: application of immunochemical techniques. Carcinogenesis. 1985 Apr;6(4):561–566. doi: 10.1093/carcin/6.4.561. [DOI] [PubMed] [Google Scholar]
- Poirier M. C., Lippard S. J., Zwelling L. A., Ushay H. M., Kerrigan D., Thill C. C., Santella R. M., Grunberger D., Yuspa S. H. Antibodies elicited against cis-diamminedichloroplatinum(II)-modified DNA are specific for cis-diamminedichloroplatinum(II)-DNA adducts formed in vivo and in vitro. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6443–6447. doi: 10.1073/pnas.79.21.6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier M. C. The use of carcinogen-DNA adduct antisera for quantitation and localization of genomic damage in animal models and the human population. Environ Mutagen. 1984;6(6):879–887. doi: 10.1002/em.2860060615. [DOI] [PubMed] [Google Scholar]
- Redman J. R., Vugrin D., Arlin Z. A., Gee T. S., Kempin S. J., Godbold J. H., Schottenfeld D., Clarkson B. D. Leukemia following treatment of germ cell tumors in men. J Clin Oncol. 1984 Oct;2(10):1080–1087. doi: 10.1200/JCO.1984.2.10.1080. [DOI] [PubMed] [Google Scholar]
- Reed E., Litterst C. L., Thill C. C., Yuspa S. H., Poirier M. C. cis-Diamminedichloroplatinum (II)-DNA adduct formation in renal, gonadal, and tumor tissues of male and female rats. Cancer Res. 1987 Feb 1;47(3):718–722. [PubMed] [Google Scholar]
- Reed E., Yuspa S. H., Zwelling L. A., Ozols R. F., Poirier M. C. Quantitation of cis-diamminedichloroplatinum II (cisplatin)-DNA-intrastrand adducts in testicular and ovarian cancer patients receiving cisplatin chemotherapy. J Clin Invest. 1986 Feb;77(2):545–550. doi: 10.1172/JCI112335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. G., Breslow N., Gart J. J. Trend and homogeneity analyses of proportions and life table data. Comput Biomed Res. 1977 Aug;10(4):373–381. doi: 10.1016/0010-4809(77)90006-4. [DOI] [PubMed] [Google Scholar]
- Zwelling L. A., Anderson T., Kohn K. W. DNA-protein and DNA interstrand cross-linking by cis- and trans-platinum(II) diamminedichloride in L1210 mouse leukemia cells and relation to cytotoxicity. Cancer Res. 1979 Feb;39(2 Pt 1):365–369. [PubMed] [Google Scholar]


