Abstract
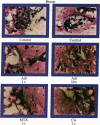
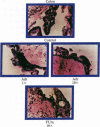
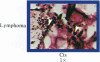
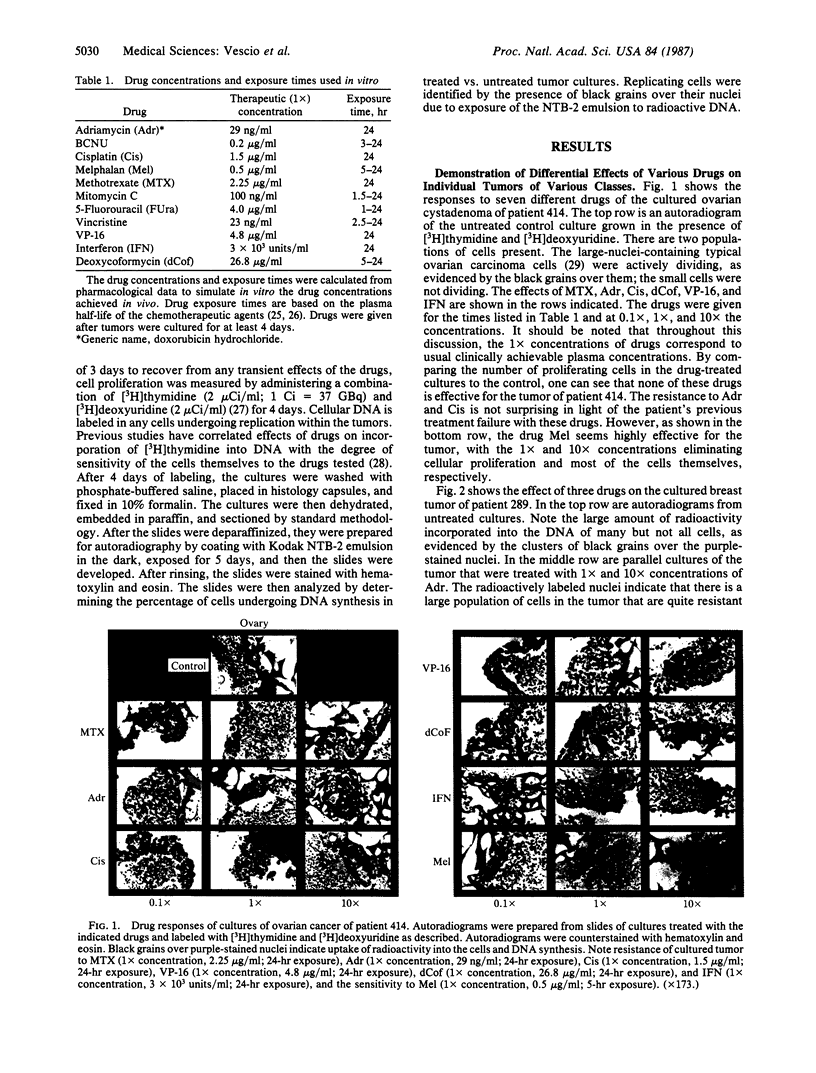
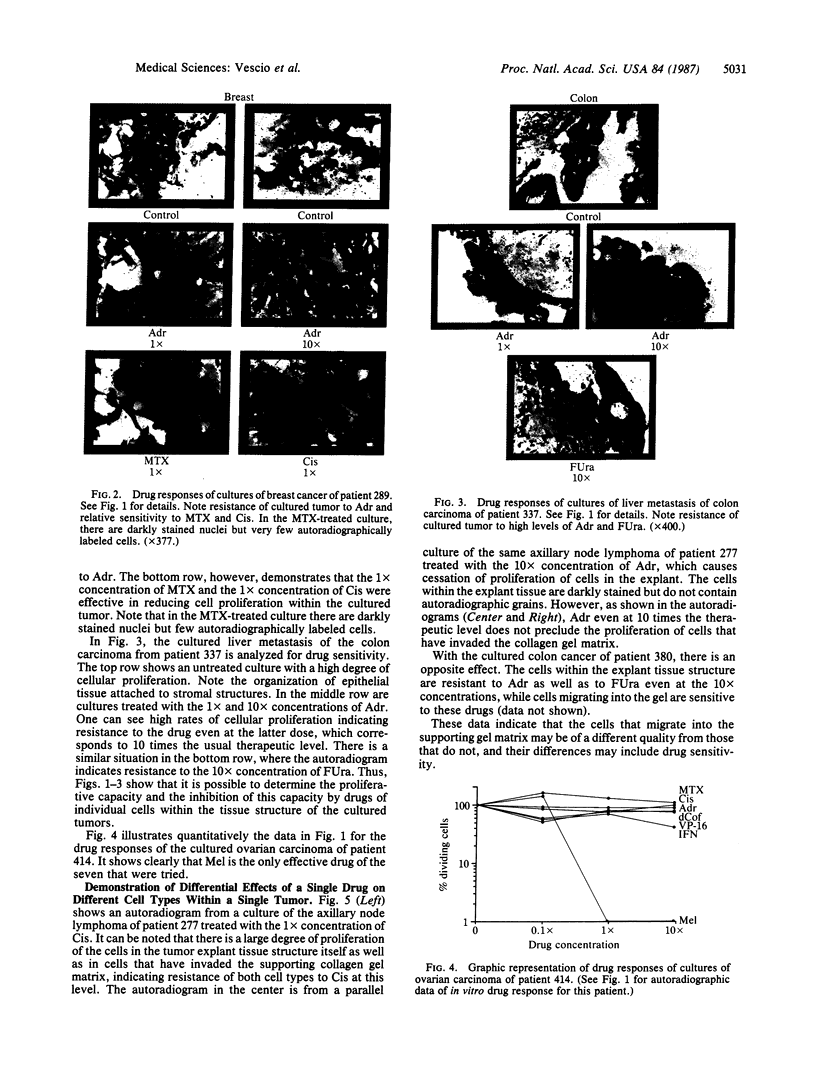
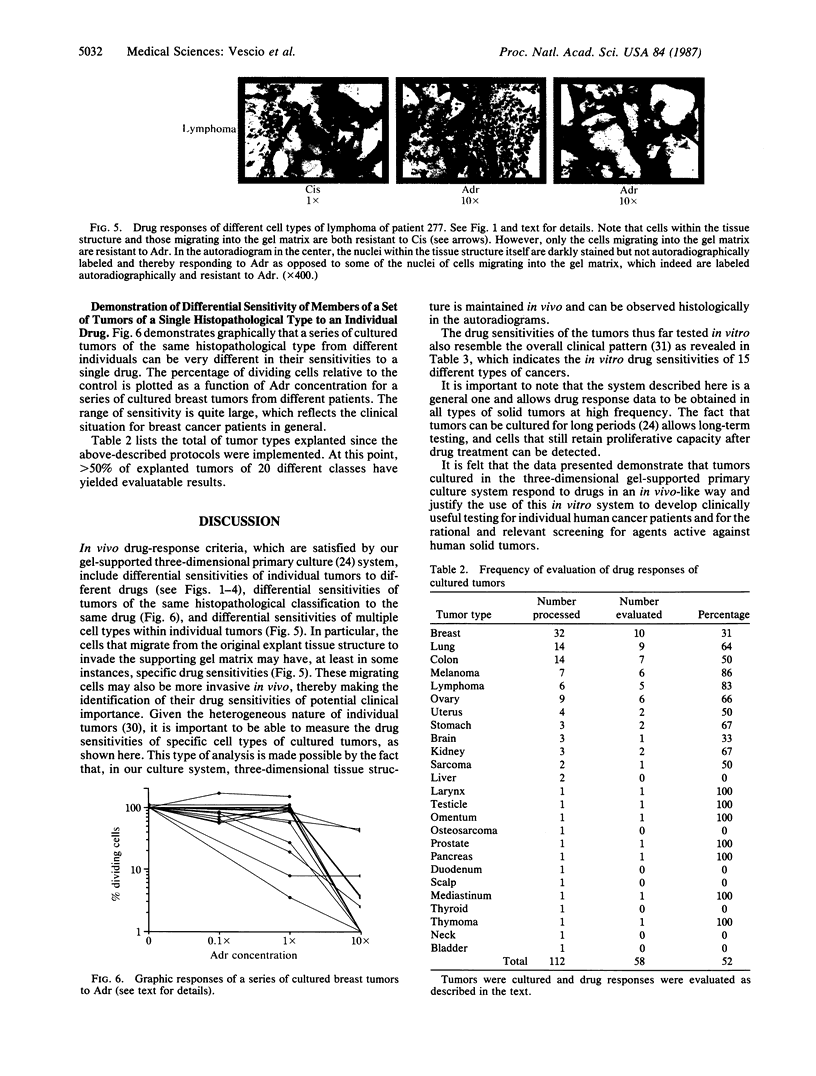
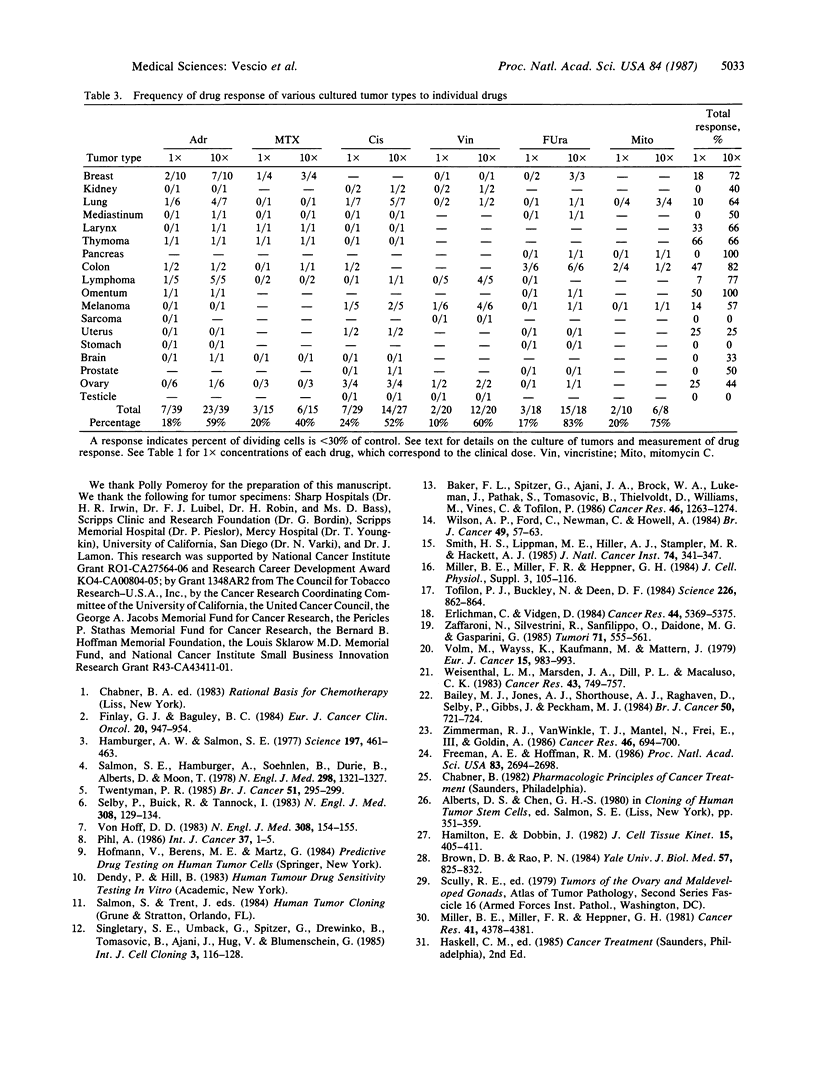
An in vitro test of cell sensitivity to drugs that indicates in vivo response is an important need in cancer therapy and cancer drug development. Toward this end, we previously developed a collagen gel-supported culture system for growth of human tumors. This three-dimensional culture system is general and grows tumors at high frequency directly from surgery or biopsy that maintain important in vivo properties in vitro, including tissue architecture. We report here that with autoradiographic techniques measuring cellular DNA synthesis the drug responses of individual cells within the tissue structure of in vitro-grown tumors can be determined. Twenty tumor classes, including all the major ones, have been measured in toto at greater than 50% frequency. Quantitative and qualitative results show increasing cell kill with rising cytotoxic drug concentration, differential drug sensitivities of multiple cell types within individual cultured tumors, differential sensitivities of a series of tumors of the same histopathological classification to a single drug, differential sensitivities of individual tumors to a series of drugs, and sensitivity patterns of various tumor types similar to the sensitivities found in vivo. Therefore, the results indicate that potentially important therapeutic data can be obtained from tumor specimens growing in vitro for the individual cancer patient as well as for rational and relevant screening for new agents active against human solid tumors.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey M. J., Jones A. J., Shorthouse A. J., Raghaven D., Selby P., Gibbs J., Peckham M. J. Limitations of the human tumour xenograft system in individual patient drug sensitivity testing. Br J Cancer. 1984 Nov;50(5):721–724. doi: 10.1038/bjc.1984.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker F. L., Spitzer G., Ajani J. A., Brock W. A., Lukeman J., Pathak S., Tomasovic B., Thielvoldt D., Williams M., Vines C. Drug and radiation sensitivity measurements of successful primary monolayer culturing of human tumor cells using cell-adhesive matrix and supplemented medium. Cancer Res. 1986 Mar;46(3):1263–1274. [PubMed] [Google Scholar]
- Brown D. B., Rao P. N. In vitro drug sensitivity of tumor cells is correlated with drug-induced inhibition of DNA synthesis. Yale J Biol Med. 1984 Nov-Dec;57(6):825–832. [PMC free article] [PubMed] [Google Scholar]
- Erlichman C., Vidgen D. Cytotoxicity of adriamycin in MGH-U1 cells grown as monolayer cultures, spheroids, and xenografts in immune-deprived mice. Cancer Res. 1984 Nov;44(11):5369–5375. [PubMed] [Google Scholar]
- Finlay G. J., Baguley B. C. The use of human cancer cell lines as a primary screening system for antineoplastic compounds. Eur J Cancer Clin Oncol. 1984 Jul;20(7):947–954. doi: 10.1016/0277-5379(84)90169-x. [DOI] [PubMed] [Google Scholar]
- Freeman A. E., Hoffman R. M. In vivo-like growth of human tumors in vitro. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2694–2698. doi: 10.1073/pnas.83.8.2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger A. W., Salmon S. E. Primary bioassay of human tumor stem cells. Science. 1977 Jul 29;197(4302):461–463. doi: 10.1126/science.560061. [DOI] [PubMed] [Google Scholar]
- Hamilton E., Dobbin J. [3H]thymidine labels less than half of the DNA-synthesizing cells in the mouse tumour, carcinoma NT. Cell Tissue Kinet. 1982 Jul;15(4):405–411. doi: 10.1111/j.1365-2184.1982.tb01058.x. [DOI] [PubMed] [Google Scholar]
- Miller B. E., Miller F. R., Heppner G. H. Assessing tumor drug sensitivity by a new in vitro assay which preserves tumor heterogeneity and subpopulation interactions. J Cell Physiol Suppl. 1984;3:105–116. doi: 10.1002/jcp.1041210413. [DOI] [PubMed] [Google Scholar]
- Miller B. E., Miller F. R., Heppner G. H. Interactions between tumor subpopulations affecting their sensitivity to the antineoplastic agents cyclophosphamide and methotrexate. Cancer Res. 1981 Nov;41(11 Pt 1):4378–4381. [PubMed] [Google Scholar]
- Pihl A. UICC Study Group on chemosensitivity testing of human tumors. Problems--applications--future prospects. Int J Cancer. 1986 Jan 15;37(1):1–5. doi: 10.1002/ijc.2910370102. [DOI] [PubMed] [Google Scholar]
- Salmon S. E., Hamburger A. W., Soehnlen B., Durie B. G., Alberts D. S., Moon T. E. Quantitation of differential sensitivity of human-tumor stem cells to anticancer drugs. N Engl J Med. 1978 Jun 15;298(24):1321–1327. doi: 10.1056/NEJM197806152982401. [DOI] [PubMed] [Google Scholar]
- Selby P., Buick R. N., Tannock I. A critical appraisal of the "human tumor stem-cell assay". N Engl J Med. 1983 Jan 20;308(3):129–134. doi: 10.1056/NEJM198301203080304. [DOI] [PubMed] [Google Scholar]
- Singletary S. E., Umbach G. E., Spitzer G., Drewinko B., Tomasovic B., Ajani J., Hug V., Blumenschein G. The human tumor stem cell assay revisited. Int J Cell Cloning. 1985 Mar;3(2):116–128. doi: 10.1002/stem.5530030205. [DOI] [PubMed] [Google Scholar]
- Smith H. S., Lippman M. E., Hiller A. J., Stampfer M. R., Hackett A. J. Response to doxorubicin of cultured normal and cancerous human mammary epithelial cells. J Natl Cancer Inst. 1985 Feb;74(2):341–347. [PubMed] [Google Scholar]
- Tofilon P. J., Buckley N., Deen D. F. Effect of cell-cell interactions on drug sensitivity and growth of drug-sensitive and -resistant tumor cells in spheroids. Science. 1984 Nov 16;226(4676):862–864. doi: 10.1126/science.6494917. [DOI] [PubMed] [Google Scholar]
- Twentyman P. R. Predictive chemosensitivity testing. Br J Cancer. 1985 Mar;51(3):295–299. doi: 10.1038/bjc.1985.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volm M., Wayss K., Kaufmann M., Mattern J. Pretherapeutic detection of tumour resistance and the results of tumour chemotherapy. Eur J Cancer. 1979 Jul;15(7):983–993. doi: 10.1016/0014-2964(79)90282-2. [DOI] [PubMed] [Google Scholar]
- Von Hoff D. D. "Send this patient's tumor for culture and sensitivity". N Engl J Med. 1983 Jan 20;308(3):154–155. doi: 10.1056/NEJM198301203080310. [DOI] [PubMed] [Google Scholar]
- Weisenthal L. M., Marsden J. A., Dill P. L., Macaluso C. K. A novel dye exclusion method for testing in vitro chemosensitivity of human tumors. Cancer Res. 1983 Feb;43(2):749–757. [PubMed] [Google Scholar]
- Wilson A. P., Ford C. H., Newman C. E., Howell A. A comparison of three assays used for the in vitro chemosensitivity testing of human tumours. Br J Cancer. 1984 Jan;49(1):57–63. doi: 10.1038/bjc.1984.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaffaroni N., Silvestrini R., Sanfilippo O., Daidone M. G., Gasparini G. In vitro activity of alkylating agents on human tumors as measured by a short-term antimetabolic assay. Tumori. 1985 Dec 31;71(6):555–561. doi: 10.1177/030089168507100607. [DOI] [PubMed] [Google Scholar]
- Zimmerman R. J., VanWinkle T. J., Mantel N., Frei E., 3rd, Goldin A. 5-Fluorouracil treatment of a human colon adenocarcinoma implanted in the subrenal capsule site of athymic mice. Cancer Res. 1986 Feb;46(2):694–700. [PubMed] [Google Scholar]