Matrix metalloproteinases (MMPs) and their tissue inhibitors (TIMPs) are important mediators of host-induced tissue destruction and are implicated in the pathogenesis of neuroinflammatory diseases.1 MMPs are secreted by all cell types in the CNS and degrade constituents of the blood–brain barrier (BBB) basement membrane, such as type IV collagen, laminin, aggrin, and fibronectin.1 Models of CNS infections have shown infiltrating leukocytes use MMPs to traverse the glia limitans. Mmp knockout mice showed less immune pathology, BBB breakdown, and cellular infiltration,2 and MMP inhibitors gave better outcomes in experimental pneumococcal meningitis.3 However, the role of MMPs/TIMPs in the pathogenesis of CNS infection in humans is not well-defined.
Methods.
We measured pretreatment CSF MMP-1 to -10 and TIMP-1, -2, and -4 concentrations by ELISA (R&D, Abingdon, UK) in 96 HIV-negative, randomly chosen Vietnamese adults admitted to the Hospital for Tropical Diseases, Ho Chi Minh City, Vietnam, with bacterial (n = 31), tuberculous (n = 11), fungal (n = 13), parasitic (n = 11), or viral (n = 10) meningitis. Ten patients with cerebral malaria and 10 patients without infections served as controls. All were recruited between November 2006 and November 2008. We followed a predefined plan to explore relationships between CSF MMP/TIMP expression and prospectively recorded clinical features and outcome.
Standard protocol approvals, registrations, and patient consent.
The study was approved by the hospital's ethical and scientific committees and written informed consent was obtained from patients or relatives.
Results.
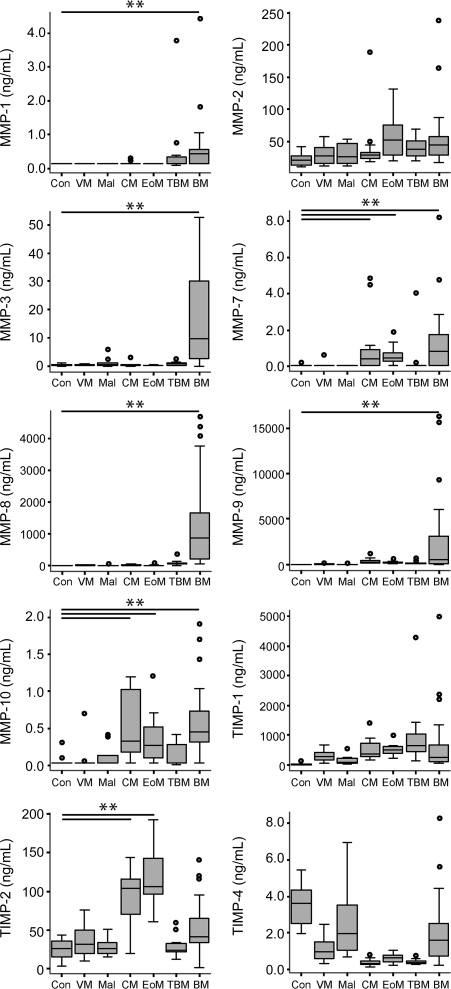
The baseline clinical and laboratory features of the patients are summarized in table e-1 on the Neurology® Web site at www.neurology.org. CSF MMP and TIMP concentrations varied according to the etiology of meningitis (figure 1). Patients with pyogenic bacterial meningitis had elevated MMP-1, -3, -7, -8, -9, and -10 concentrations (p < 0.01). Cryptococcal and eosinophilic meningitis were associated with increased MMP-7, -10, and TIMP-2 concentrations (p < 0.01), and tuberculous meningitis was characterized by a trend toward elevated MMP-1 concentrations. MMP-9 and CSF white cell count (WCC) were strongly correlated (R2 = 0.73, p < 0.01, figure e-1), regardless of the etiology. CSF MMP-3, -8, and -9 concentrations were significantly correlated with one another: MMP-8/-9 (R2 = 0.52, p < 0.01), MMP-3/-8 (R2 = 0.47, p < 0.01), and MMP-3/-9 (R2 = 0.13, p < 0.01). TIMP-1 concentrations were correlated with CSF WCC (R2 = 0.35, p < 0.01) and MMP-9 (R2 = 0.37, p < 0.01). MMP-8 and -9 were also correlated with CSF lactate concentration (R2 = 0.62, p < 0.01, and R2 = 0.33, p < 0.01, respectively). We did not find correlations between CSF lymphocyte count and CSF MMP/TIMP concentrations or CSF total protein and MMP/TIMP concentrations.
Figure 1. Matrix metalloproteinases (MMP) and their tissue inhibitors (TIMP) concentrations in the CSF of HIV-negative patients with CNS infections vary by etiology.
CSF samples taken within 24 hours of admission prior to antimicrobial treatment, immediately stored at −70°C, were analyzed by MMP/TIMP ELISA as annotated. The black line represents the median value and the box the interquartile range. Whiskers represent 1.5 times the interquartile range away from the box. Outliers are represented by open circles. BM = bacterial meningitis; CM = cryptococcal meningitis; Con = control; EoM = eosinophilic meningitis; Mal = malaria; TBM = tuberculous meningitis; VM = viral meningitis. Patients with cryptococcal, tuberculous, and pyogenic bacterial meningitis were all confirmed by culture of the causative agent from CSF. Patients with cerebral malaria were comatose with a positive peripheral blood smear for Plasmodium falciparum (alternative causes of coma were excluded by performing routine laboratory tests, as well as confirming negative blood and CSF cultures). Patients with eosinophilic meningitis were diagnosed on typical presenting features and CSF white cells >20% eosinophils. Patients with viral meningitis were diagnosed on the basis of clinical features and complete recovery without any antimicrobial treatment (including prior to admission). Normal control CSF was defined as having no cells and normal protein concentration, confirmed with normal clinical follow-up. *p < 0.05, **p < 0.01.
We examined the clinical features associated with severe coma (which we defined by Glasgow Coma Score ≤11) at presentation. On univariate analysis, low peripheral blood WCC, high CSF lactate concentration, and high CSF MMP-3 and -7 and TIMP-2 were associated with severe coma (p < 0.1; table e-2). On multivariable logistic regression analysis, high CSF TIMP-2 concentration was associated with Glasgow Coma Score >11 (odds ratio 0.2, 95% confidence interval 0.04–0.97, p < 0.05) and high CSF lactate concentration remained predictive of severe coma (odds ratio 6.4, 95% confidence interval 1.02–39.8, p < 0.05). In tuberculous, cryptococcal, and eosinophilic meningitis, coma severity was independently associated with high CSF MMP-8 concentration. In-hospital deaths occurred in those with cerebral malaria (5/10), cryptococcal (4/13), eosinophilic (3/11), tuberculous (3/11), and bacterial meningitis (1/31), but we did not find any significant relationships between death and CSF MMP/TIMP concentrations.
Discussion.
Our work demonstrates that CSF MMP/TIMP expression in CNS infections is determined by etiologic agent. CSF MMP-1 was highest in bacterial meningitis and elevated in patients with tuberculous meningitis. The BBB is rich in collagen and MMP-1 is a potent collagenase, which may explain the extensive BBB breakdown observed in these meningitides. CSF MMP-3, -8, and -9 concentrations correlated with CSF WCC; neutrophil granules are rich in MMP-8 and -9, but do not contain MMP-3, suggesting other cellular sources of MMP-3.4 High CSF MMP-3 concentrations were associated with severe coma, suggesting this molecule might contribute to disease severity, for example by its known role of activating MMP-1. Experimental models implicate MMP-8 and -9 in the pathogenesis of bacterial meningitis and the strong correlation between MMP-8 and -9 with CSF WCC suggests neutrophils may be the source of these molecules.5,6 TIMP-2 is a potent MMP inhibitor expressed by neurons, and low CSF concentrations were an independent predictor of severe coma,7 suggesting a neuroprotective effect of TIMP-2 since uninhibited MMP activity is linked to disease severity and cerebral injury. There was no association between MMP/TIMP concentration and death, but our study was insufficiently powered to detect such an effect. MMP-1/-3/-8/-9 and TIMP-2 are potentially implicated in the pathogenesis of human CNS infections.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the doctors and nurses at The Hospital for Tropical Diseases who cared for the patients and allowed collection of samples for this study to occur; and Catherine Ong for additional laboratory work and commenting on the manuscript.
Footnotes
Supplemental data at www.neurology.org
Study funding: J.A.G. was a Medical Research Council (UK) Clinical Training Fellow. This work was supported by grants from the Wellcome Trust (G.E.T., J.J.F.), the Hammersmith Hospital Special Trustees (J.A.G.), and the Oxford Tropical Network (J.A.G.). J.S.F. is supported by the NIHR Biomedical Research Centre funding scheme. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure: Dr. Green is an employee of (since 10/2010) and holds stock in GlaxoSmithKline; and has received research support from Medical Research Council (UK), the Oxford Tropical Network, and Hammersmith Hospitals Special Trustees. Dr. Chau and Dr. Farrar report no disclosures. Dr. Friedland has received research support from UKCRC, NIHR Biomedical Research Centre, Medical Research Council UK, the NHS, MRC (Singapore), and the Wellcome Trust. Dr. Thwaites serves as Associate Editor of BMC Infectious Diseases; and receives research support from the Wellcome Trust.
AUTHOR CONTRIBUTIONS
J.A.G., G.E.T., and J.J.F. designed the study. T.T.H.C. collected the clinical data and samples. J.A.G. and T.T.H.C. performed the experiments. J.A.G. and G.E.T. analyzed the data. J.A.G. wrote the first draft of the paper. J.A.G., G.E.T., T.T.H.C., J.S.F., and J.J.F. all contributed to the review of data and writing of the paper.
References
- 1. Yong VW. Metalloproteinases: mediators of pathology and regeneration in the CNS. Nat Rev Neurosci 2005;6:931–944 [DOI] [PubMed] [Google Scholar]
- 2. Asahi M, Wang X, Mori T, et al. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J Neurosci 2001;21:7724–7732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Leib SL, Leppert D, Clements J, Tauber MG. Matrix metalloproteinases contribute to brain damage in experimental pneumococcal meningitis. Infect Immun 2000;68:615–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cuadrado E, Ortega L, Hernandez-Guillamon M, et al. Tissue plasminogen activator (t-PA) promotes neutrophil degranulation and MMP-9 release. J Leukoc Biol 2008;84:207–214 [DOI] [PubMed] [Google Scholar]
- 5. Williams PL, Leib SL, Kamberi P, et al. Levels of matrix metalloproteinase-9 within cerebrospinal fluid in a rabbit model of coccidioidal meningitis and vasculitis. J Infect Dis 2002;186:1692–1695 [DOI] [PubMed] [Google Scholar]
- 6. Leppert D, Leib SL, Grygar C, Miller KM, Schaad UB, Hollander GA. Matrix metalloproteinase (MMP)-8 and MMP-9 in cerebrospinal fluid during bacterial meningitis: association with blood-brain barrier damage and neurological sequelae. Clin Infect Dis 2000;31:80–84 [DOI] [PubMed] [Google Scholar]
- 7. Pagenstecher A, Stalder AK, Kincaid CL, Shapiro SD, Campbell IL. Differential expression of matrix metalloproteinase and tissue inhibitor of matrix metalloproteinase genes in the mouse central nervous system in normal and inflammatory states. Am J Pathol 1998;152:729–741 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.