Abstract
The migratory locust, Locusta migratoria, shows a striking phenotypic plasticity. It transitions between solitary and gregarious phases in response to population density changes. However, the molecular mechanism underlying the phase-dependent behavior changes remains elusive. Here we report a genome-wide gene expression profiling of gregarious and solitary nymphs at each stadium of the migratory locust, and we identified the most differentially expressed genes in the fourth stadium of the two phases. Bioinformatics analysis indicated that the catecholamine metabolic pathway was the most significant pathway up-regulated in the gregarious phase. We found pale, henna, and vat1, involved in dopamine biosynthesis and synaptic release, were critical target genes related to behavioral phase changes in the locusts. The roles of these genes in mediating behavioral changes in the gregarious individuals were confirmed by RNAi and pharmacological intervention. A single injection of dopamine or its agonist initiated gregarious behavior. Moreover, continuous and multiple injections of a dopamine agonist coupled with crowding resulted in more pronounced gregarious behavior. Our study thus provides insights into the relationships between genes and behavior in phase transition of this important pest species.
Keywords: microarray, RNA interference, polyphenism
Phenotypic plasticity associated with locust phase polyphenism arises when extrinsic factors induce alternative phenotypes in individuals with the same genetic background (1). Solitary locusts usually live in an isolated state, with their cryptic body color blending well into the surroundings (2). Population density is a substantial source of extrinsic factors that leads to the reversible transformation between solitary and gregarious phases. Under high population density, migratory locusts (Locusta migratoria) form large, fast-flying swarms that wreak havoc on local vegetation (3, 4). Gregarious locusts have a distinctive orange body color with dark patterns as nymphs. In addition, they are attracted to one another and exhibit collective social behavior (2).
Behavior is one of the most obvious traits to examine to evaluate the phase state of the migratory locust (1–6). Although the neuronal circuitry responsible for processing and integrating phase-shifting cues is not clear, several neurochemicals have been implicated (1). For instance, the biogenic amine serotonin was found to be necessary and sufficient for inducing gregarious behavior in the desert locust, Schistocerca gregaria (7).
The first step in identifying the mechanisms underlying phenotypic plasticity in locusts is to understand how behavior is regulated. A void in our understanding of the mechanism of the phenomena is the interaction of gene expression and environment in regulating phase change of the migratory locust (1). Because of the inherent complexity associated with phase transition, the full complement of behavioral and physiological changes is underwritten by genetic and epigenetic factors (8, 9). In previous studies, we have found significant differences in the expression of coding and noncoding RNA genes between the two phases, indicating the importance of genetic and epigenetic factors in determining or differentiating alternative phenotypes of the migratory locust (8–10). Although the behavioral characteristics can obviously change during the phase transition (1, 2), the underlying molecular and genetic mechanism leading to the manifestation of phase-dependent characteristics in migratory locusts remain elusive. To systematically examine the functions and roles of pathways and genes that regulate phase-specific phenotypes of the migratory locust, we used a high-density locust oligonucleotide microarray to monitor genome-wide transcriptional expression profiles and detect the differentially expressed genes (DEGs) and their regulation between the two phases.
Results and Discussion
Solitary and Gregarious Nymphs Exhibit Phase-Specific Phenotypes.
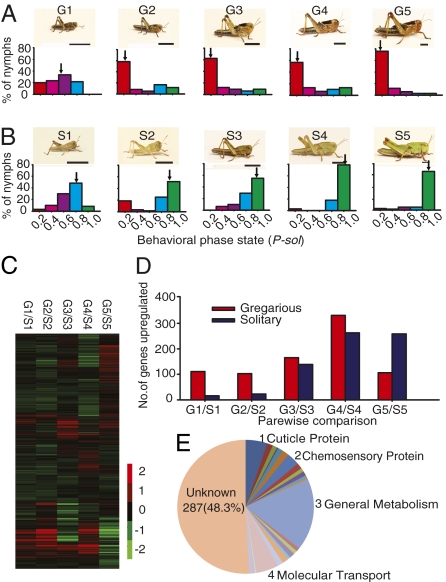
The solitary and gregarious nymphs were reared either in crowded condition or in solitude as previous described (8), and they displayed phase-specific phenotypes of body color from the first to fifth stadium (Fig. 1 A and B). To quantify behavior traits at each nymphal stadium, we used a modified test arena with an EthoVision system (11). This setup allowed us to simultaneously monitor and record 11 behavioral parameters that were fit into a binary logistic regression model for a single probabilistic value, P-solitary (P-sol) (SI Appendix, Table S1) (6). A P-sol of 0 would indicate fully gregarious behavior and a P-sol of 1.0 would indicate fully solitary behavior. As shown by Fig. 1 A and B, solitary and gregarious individuals showed partial phase-specific behavior in the first two stadia, whereas the nymphs of the two phases displayed phase-specific behavior from the third to the fifth stadium.
Fig. 1.
Phenotype-associated pattern and genome-wide expression profile. (A and B) Typical gregarious (A) and solitary (B) nymphs and the distribution of P-sol values in each population are shown. Arrows indicate median P-sol values. (Scale bars = 5 mm.) (C) Heat map of all genes in the five comparisons between the two phases. (D) The number of DEGs identified in five stadia. (E) Gene classification of 594 DEGs from the fourth-stadium comparison. S, solitary phase; G, gregarious phase; 1–5, nymph stadia 1–5.
Genome-Wide Expression Identified the Catecholamine Metabolic Pathway Involved in Phase Change.
To systematically delineate gene activity changes potentially associated with the regulation of phase-dependent traits, we performed genome-wide expression profiling of gregarious and solitary nymphs at each stadium by using a high-density oligonucleotide array with 9,154 locust unigenes (8, 9). After performing the fixed-model ANOVA, we generated hierarchical clusters of DEGs (Fig. 1C). As the nymphs aged from the first to the fourth stadium, the number of DEGs between the two phases increased drastically, with more genes being expressed at higher levels in the gregarious phase (Fig. 1D). However, at the fifth stadium, the number of DEGs decreased significantly, and more genes were expressed at higher levels in the solitary phase (Fig. 1D and SI Appendix, Table S2). The shift in the number of DEGs between the fourth and fifth stadium suggests that the fourth stadium is a key developmental stage, when the largest number of DEGs between the two phases is observed. After the fourth stadium, the phase difference tends to be stable (12).
All of the DEGs in the fourth stadium are classified into 21 functional categories, among which the top four regulated ones were those involved in general metabolism, molecular transport, the production of cuticle protein, and chemosensory transduction (Fig. 1E). As the most highly differential group, the general metabolism category contained more genes expressed at higher levels in the gregarious phase in the first four stadia, while the number of up-regulated DEGs in this group decreased in the fifth stadium of this phase. When comparing the gene profiling in the two phases, the genes in the general metabolism category displayed similar trends as the global expression pattern, whereas genes in the classes of chemosensory protein, cuticle protein, and molecular transport exhibited distinguished expression patterns, which were different from the global expression profiling through the whole developmental stage (SI Appendix, Fig. S1). The general metabolism group contained 33 genes that are involved in biosynthesis of carbohydrates, L-3,4-dihydroxyphenylalanine(L-DOPA), lipids, chitin, and other metabolic intermediates (SI Appendix, Table S3). More importantly, gene enrichment analysis ranked the pathway involved in catecholamine biosynthesis and metabolism as the top pathway affected in the fourth-stadium gregarious nymphs (SI Appendix, Tables S4 and S5), implying the potential significance of this pathway in controlling phase-dependent traits.
Temporal and Spatial Expression of Pivotal Genes in the Catecholamine Metabolic Pathway Is Phase-Specific.
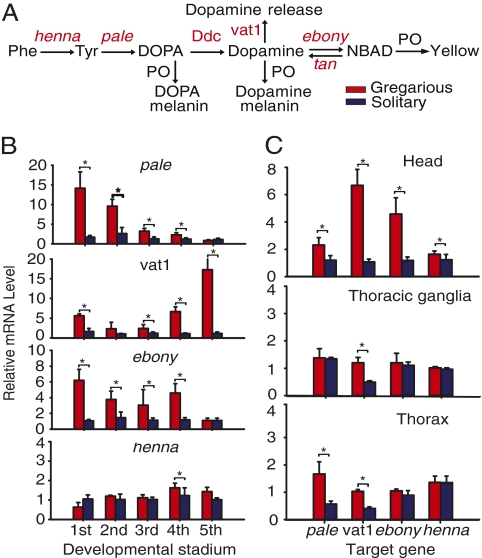
In insects, catecholamine biosynthesis is closely linked to dopamine production (13), in which tyrosine is hydroxylated to generate L-DOPA and then L-DOPA is decarboxylated to become dopamine (Fig. 2A). This metabolic pathway is controlled by the rate-limiting enzyme tyrosine hydroxylase, encoded by the pale gene (13). Intermediates L-DOPA and dopamine can be further converted into melanin pigment derivatives to generate the black coloration. Alternatively, dopamine can be converted into N-β-alanyldopamine by the ebony-encoded enzyme to produce a yellow color (14, 15). Quantitative PCR analyses of head tissues revealed a descending trend for temporal expression of pale in gregarious nymphs, starting with a strong first-stadium expression and gradually weakening to background levels in the fifth stadium (Fig. 2B). In contrast, expression of vesicular amine transporter 1 (vat1), which controls the synaptic release of dopamine (16), began at a moderate level in the first stadium and then rapidly increased to peak level in the fifth stadium (Fig. 2B). Elevated expression of pale and vat1 appeared to be a unique feature in head tissues of gregarious nymphs because peripheral expression was relatively low in thoracic ganglia and nonneuronal thoracic tissues at the fourth stadium (Fig. 2C). This temporal expression of pale and vat1 may reflect an early-stage requirement for a high level of dopamine production and a late-stage surge in dopamine release. The gene ebony, which is responsible for controlling the production of a yellow color (15), was also expressed at a higher level in gregarious head tissues in the first four stadia (Fig. 2B). In contrast, the low expression of pale, vat1, and ebony in solitary nymphs probably reduced synthesis and transportation of dopamine, which potentially leads to the quiescent behavior of solitary nymphs (1). The gene henna in this pathway encodes phenylalanine hydroxylase for tyrosine synthesis and tryptophan hydroxylase for the synthesis of 5-hydrotryptophan (5-HTP), the precursor of serotonin (17). Except for a small but significant increase in the gregarious head tissue at the fourth stadium, little difference in henna expression was observed between the two phases in investigated tissues or stadia (Fig. 2B). Other non-rate-limiting genes tan, ddc, and PO did not show changes related to phase transition (SI Appendix, Fig. S2). Genes in this metabolic pathway were expressed temporally and spatially in a phase-specific manner, which indicates their possible involvement in regulating phase-dependent traits of the migratory locust.
Fig. 2.
Temporal and spatial expression of genes related to dopamine metabolism show phase-specific expression patterns. (A) Schematic representation of dopamine metabolic pathway. NBAD, N-β-alanyldopamine. (B and C) Quantitative PCR analyses of pale, ebony, vat1, and henna expression in gregarious and solitary phases were performed at different intervals throughout the stadium (B) or in different tissues (C). Levels of gene expression in solitary nymphs are shown in blue, and levels of gene expression in gregarious nymphs are shown in red. Asterisks denotes statistically significant change (mean ± SE; P < 0.05, Student t test).
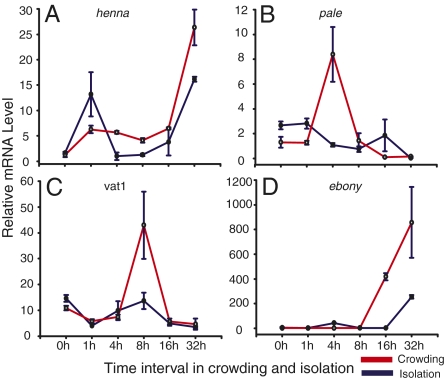
To validate the relationship of the critical genes in this pathway with phase transition, we further examined the dynamic expression of these genes during the time courses of isolation and crowding in the fourth-stadium nymphs. After 4 h of crowding, the expression of henna was significantly increased and maintained at higher levels from 4 to 32 h than its expression in isolated locusts (Fig. 3A). The expression of pale quickly increased after crowding for 1 h, and vat1 expression showed transient increase after crowding for 4 h (Fig. 3 B and C). The expression of ebony also began to increase in crowding from 8 to 32 h (Fig. 3D). Thus, the expression changes of genes in this metabolic pathway are closely associated with the time course of phase transition in the migratory locust.
Fig. 3.
The expression tendency of genes in the catecholamine metabolic pathway in the crowding and isolation process of the fourth-stadium nymphs. Genes henna (A), pale (B), vat1 (C), and ebony (D) expressed differentially in the crowding and isolating of the migratory locust (one-way ANOVA, P < 0.01).
Pivotal Genes in the Catecholamine Metabolic Pathway Mediate Behavior Changes Associated with Phase Transition.
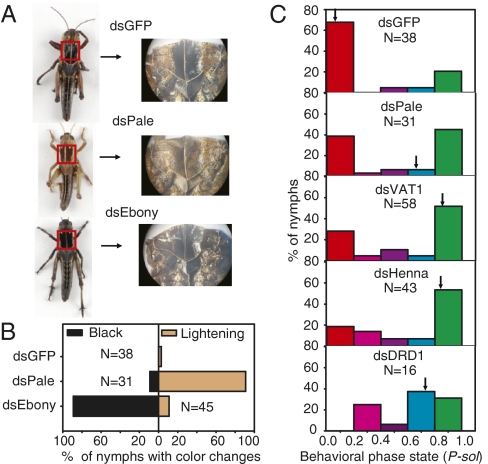
The high expression of genes encoding enzymes that control catecholamine metabolism in gregarious nymphs or in the crowding of solitary nymphs suggests that blocking dopamine synthesis, synaptic release, or its signal transduction may interfere with melanin deposition and induce solitary behavior. Expression patterns revealed by microarray and RNA-seq (12) implied that the fourth stadium is the key developmental stage. Thus, the fourth-stadium nymphs were chosen to determine the short-term effects of pharmacological intervention on the behavioral changes of the two phases. The long-term effects of RNAi and pharmacological treatments were investigated with the third-stadium nymphs, and the behavior was assayed at the fourth stadium. To confirm the physiological significance of the genes involved in catecholamine metabolism in phase transition, we injected dsRNAs against these genes into thoracic hemocoels of gregarious nymphs at the third stadium, and body color pattern was assayed. After molting, >90% of dsPale-injected nymphs showed remarkable lightening of the pronotum compared with <5% in the dsGFP-injected group. dsRNA silencing of ebony caused a darkening of the pronotum in 89% of injected gregarious nymphs (Fig. 4 A and B and SI Appendix, Fig. S3). These results are consistent with the previously reported mechanism of dopamine melanization (14). When subjected to behavioral tests in the fourth stadium, the dsPale-injected nymphs shifted toward the solitary state, with ∼45% falling into the P-sol interval of 0.8–1.0 compared with 21% in the dsGFP-injected group (Fig. 4C). Reducing vat1 expression also rendered a solitary shift, with 51% of the dsRNA-injected nymphs falling into the P-sol interval of 0.8–1.0 (Fig. 4C), which further supports the importance of dopamine release in phase transition. RNAi of dopamine receptor D1 led to a significant shift toward the solitary phase (Fig. 4C), suggesting that the dopamine receptor pathway is responsible for maintaining gregarious behavior. Silencing of henna expression caused 53% of the injected nymphs to shift into the P-sol interval of 0.8–1.0 (Fig. 4C), but no decrease in pigment deposition was detected. In these experiments, injection of dsRNAs efficiently reduced 70% of the targeted gene expression (SI Appendix, Fig. S4). Thus, the dopamine metabolic pathway evidently impacts behavior changes associated with phase transition for the migratory locust.
Fig. 4.
RNAi-mediated silencing of dopamine metabolic genes alters pigmentation and induces solitary behavioral change in gregarious nymphs. (A and B) Representative (A) and statistical (B) body color changes induced by RNAi-mediated silencing are shown. Third-stadium nymphs were injected with dsRNA three times at one stadium interval. (C) Behavioral changes induced by RNAi (Mann–Whitney U test, P < 0.01, relative to dsGFP-injected controls). Arrows indicate median P-sol values of the population. DRD1, dopamine receptor D1.
To further demonstrate the role of dopamine metabolism in phase transition, we used HPLC-MS to measure the levels of dopamine and serotonin, two main biogenic amines, in the brain of the migratory locust at the fourth stadium and to manipulate dopamine production through pharmacological intervention. The results showed that the level of dopamine, but not serotonin, was significantly higher in gregarious nymphs than in their solitary counterparts (SI Appendix, Fig. S5). Gregarious nymphs at the third stadium were injected with α-methyl-dl-tyrosine methyl ester hydrochloride (AMPT), an antagonist of tyrosine hydroxylase (18), at consecutive 48-h intervals, and the resulting behavioral state was assayed. After molting, solitary behavior was observed at the fourth stadium, with 48% of AMPT-injected nymphs segregating into the P-sol interval of 0.8–1.0, in comparison with only 10.2% for buffer-injected control nymphs (Fig. 5A). Similarly, injection of the fourth-stadium gregarious nymphs with reserpine, a competitive inhibitor of vat1 (19), induced solitary behavior in 47% of nymphs compared with 32% in DMSO-injected controls (Fig. 5B). To rule out the “off-target” effect of reserpine on the release of other monoamines (20), we blocked the function of the dopamine receptor by injecting SCH23390 and other antagonists into thoracic hemocoels of gregarious nymphs at the fourth stadium (Fig. 5C and SI Appendix, Fig. S6). The results revealed evident solitary behavioral traits, indicating that dopamine production, synaptic release, and its signaling pathway control the gregarious behavior phase state.
Fig. 5.
Behavioral responses of gregarious and solitary nymphs to dopamine biosynthesis, synaptic release, and receptor signaling. (A–C) Three consecutive injections of the tyrosine hydroxylase inhibitor AMPT (80 μg/μL) at 48-h intervals (A), one injection of the dopamine receptor antagonist SCH23390 (80 μg/μL) at 1-h intervals (B), or one injection of the Vat1 inhibitor reserpine (80 μg/μL) at 48-h intervals (C) were sufficient to induce solitarization of gregarious nymphs. (D and E) Direct injection of dopamine (80 μg/μL; D) or its agonist apomorphine (10 μg/μL; E) partially induced gregarization of the fourth-stadium solitary nymphs. (F) The fourth-stadium solitary nymphs injected with apomorphine (10 μg/μL) and exposed to 1 h of crowding showed a behavioral shift toward the gregarious phase. (G) The third-stadium solitary nymphs injected with apomorphine (10 μg/μL) and exposed to one stadium of crowding showed a behavioral shift toward the gregarious phase. Arrows indicate median P-sol values of the population. All statistical analyses were conducted with Mann–Whitney U test relative to control groups (P < 0.05).
Considering the link between locomotion and dopamine (20), the requirement of dopamine biosynthesis, synaptic release, and signaling in locust phase transition suggests that dopamine is potentially a key neurotransmitter in the induction of gregarious behavior. This hypothesis was confirmed by the direct injection of dopamine or its agonist apomorphine into thoracic hemocoels of solitary nymphs, which caused a significant but incomplete shift toward gregarious behavior for 1 h after the injection (Fig. 5 D and E), indicating that dopamine can significantly induce gregarious-like behavior in migratory locusts.
Involvement of dopamine metabolism and transportation in mediating gregarious behavior raised the possibility that increased dopamine synthesis and activation of its receptors could amplify the effects of outer stimuli in crowding. Solitary nymphs that received apomorphine and were exposed to 1 h of crowding showed a similar pattern of gregarious behavior as observed in the apomorphine-injected solitary group. Saline-injected solitary nymphs exposed to 1 h of crowding still remained solitary (Fig. 5F). These results indicate that the agonist induction coupled with 1 h of crowding is not sufficient to fully induce the behavioral transformation toward the gregarious phase. When apomorphine was injected into third-stadium solitary nymphs at 48-h intervals three consecutive times, these solitary nymphs exposed to crowding for one stadium showed evident gregarious behavior compared to undrugged and crowded controls (Fig. 5G). Therefore, we propose that the dopamine pathway can induce gregarious behavior of the migratory locust.
Serotonin Potentiates the Behavior Changes Associated with Phase Transition.
The significant but incomplete shift toward gregarious behavior in the migratory locust induced by dopamine agonists indicates that one or more neurotransmitters other than dopamine are still required to elicit full completeness of gregarization. Indeed, serotonin and its agonists showed similar partial inductive effects (SI Appendix, Fig. S7 C and D); injection of antagonists for tryptophan hydroxylase and serotonin receptor also induced solitary behavior of gregarious nymphs, confirming the role of serotonin in regulating phase-specific behavior of the migratory locust (SI Appendix, Fig. S7 A and B) (7). Moreover, after 5-HTP injection and exposure to brief crowding, solitary nymphs became highly gregarious compared with undrugged and crowded controls (SI Appendix, Fig. S7E). However, upon injection of 5-HTP at 48-h intervals three consecutive times and crowding, we found that solitary nymphs retained their solitary behavior (SI Appendix, Fig. S7F). Thus, serotonin can initiate gregarious behavior, but it is not able to induce and control gregarious behavior during long-term crowding of the migratory locust.
In this study, we identified the catecholamine metabolic pathway as critical for controlling biosynthesis and synaptic release of biogenic amines that affect behavior and body color of the migratory locust. The hyperactivity of the dopamine metabolic pathway in gregarious nymphs suggests its significance in integrating social environmental clues leading to their aggregation. The silencing of henna, pale, vat1, and DRD1 in the catecholamine metabolic pathway can initiate solitary behavior of gregarious nymphs, whereas dopamine receptor agonists stimulate gregarious behavior in solitary nymphs, indicating that the gregarious behavior is induced and maintained by dopaminergic modulation. Silencing of vat1 resulted in a bigger impact on the gregarious behavior in comparison with silencing of pale, suggesting that the release of prestored dopamine potentially plays a prominent role in controlling phase-specific behavior. Unlike in the desert locust, S. gregaria, behavioral gregarization of fourth-stadium solitary nymphs in the migratory locust occurred slowly, but the rate of solitarization of gregarious nymphs was much faster. This slow transition from solitary to gregarious phase in the migratory locust was confirmed as being a species-specific trait (21). Another possible reason for the asymmetric kinetics associated with the bidirectional phase transition in the migratory locust is that there is the difference in the accumulation and removal of dopamine. Therefore, the relationship of the gene expression with catalysis activities of pale- and ebony-encoded enzymes in isolation and crowding should be further investigated in the migratory locust. In addition, the role of melanin deposition by pale and ebony in the catecholamine metabolic pathway is different from that of the neuropeptide corazonin. The genes pale and ebony mediate the melanin deposition by regulating the metabolism of L-DOPA, dopamine, and their intermediates, whereas the neuropeptide corazonin induces dark pigmentation at the neuroendocrine level (22–24). Therefore, investigating the underlying mechanism for body color change related to phase transition is also a challenge in future.
Previous studies on the desert locust S. gregaria showed that dopamine also exhibited a significant increase during crowding of solitary nymphs for 4 and 24 h, but serotonin levels in the thoracic ganglia increased in crowding for 4 h (25). In our study, brain dopamine levels in the fourth-stadium gregarious nymphs were higher than in solitary nymphs, but there was no significant difference in serotonin levels between the two phases. Dopaminergic and serotonergic systems probably play a synergistic role in regulating body physiologies and behaviors (26, 27). The incomplete phase changes in the locusts induced by dopamine and serotonin signaling indicate the potential existence of other inducing or inhibitory factors in the phase changes, but the impacts of locust species specificity also need to be considered (21). Another possibility is that the functions of dopamine, serotonin, or other neurochemicals interact. Therefore, it is necessary to depict the detailed interactions of these neurotransmitters in regulating phase transition. The potential regulation of phase-related features by the identified candidate genes (8, 12) requires further investigations of the synergistic activities mediated by dopamine and other chemical messengers (28).
In summary, we demonstrated that the catecholamine metabolic pathway is involved in initiation or maintenance of phase transition of the migratory locust. Our study then reveals an insight into the molecular mechanisms of phase change in this worldwide pest species.
Materials and Methods
Animals and Husbandry.
Animals used in experiments were from gregarious and solitary locust colonies maintained in the Institute of Zoology, Chinese Academy of Sciences. Gregarious nymphs were cultured in large boxes (40 × 40× 40cm) at a density of 500–1,000 insects per container for eight generations. Solitary nymphs were cultured alone in white metal boxes (10 ×10 × 25 cm) supplied with charcoal-filtered compressed air for five generations before experiment action. This colony was maintained under a 14-h:10-h light:dark photocycle regime at 30 ± 2 °C and fed on fresh wheat seedlings and bran (8).
Microarray Design and Hybridization.
Total RNA was extracted according to the protocol of the RNeasy Mini Kit (Qiagen). OligoWiz was used to design oligos (29), and 9,154 probes were synthesized. Probes were dissolved in 3× SSC at 40 μM and spotted on poly-l-lysine–coated slides with a SpotArray Enterprise Microarrayer (PerkinElmer Life Sciences). The locust microarray contained 19,200 spots with 4 × 12 blocks of 400 spots, duplicated for each gene. Empty, positive, and negative controls were included. Total RNA (40 μg) was used to prepare cDNA probes, which were labeled with monofunctional Cy3 and Cy5 and then purified, mixed, and hybridized to arrays. Three to five independent hybridizations with biological replicates were performed with dye-reversal strategy, and we chose the direct-comparison method for microarray hybridization. Washed microarrays were scanned and quantified with a GenePix 4000B Microarray Scanner and GenePix Pro 5.0 (Axon Instruments).
Pharmacological Intervention.
We used the third-stadium nymphs to determine the long-term effects of drugs and the fourth-stadium nymphs to determine short-term effects. The behavior assay was performed at the fourth stadium for all of the experiments. The tyrosine hydroxylase inhibitor AMPT was injected into the third-stadium gregarious nymphs, and reserpine (the inhibitor of vat1) was injected into the fourth-stadium gregarious nymphs. Moreover, the fourth-stadium gregarious nymphs were injected with four dopamine receptor antagonists, including chlorpromazine, SCH23390, raclopride, and apokyn hydrate, and were reared for 1 h. After injection of dopamine or its agonist apomorphine, the fourth-stadium solitary nymphs stayed alone or lived in crowding for 1 h. After injection of apomorphine, the third-stadium solitary animals lived in crowding for one stadium before the behavior assay.
We injected α-methyltryptophan (AMTP) into the third-stadium gregarious nymphs. After injection of a serotonin receptor antagonist solution containing ketanserin and methiothepin, the fourth-stadium gregarious nymphs were then reared for 1 h. In addition, serotonin, its receptor agonist α-methylserotonin, and 5-carboxamidotryptamine were injected into the fourth-stadium solitary nymphs, and behavior was assayed 1 h later. After injection of 5-HTP, the fourth-stadium solitary nymphs were exposed to crowding for 1 h. Moreover, the third-stadium solitary nymphs injected with 5-HTP lived in crowding for one stadium. See SI Appendix for more details.
RNAi.
dsRNA of target genes in the catecholamine metabolic pathway was prepared by using the T7 RiboMAX system (Promega) and annealed by incubation at 70 °C for 10 min. Then, 2 μL of dsRNA (6 μg/μL) from target genes or GFP control was injected into the thoracic hemocoels of third-stadium gregarious nymphs three times at 48-h intervals. See SI Appendix for more details.
Quantitative PCR.
Quantitative PCR was performed by using the SYBR Green I RNA Amplification Kit (Tiangen) according to the manufacturer's instructions. RNA samples were prepared independently from those used for microarray detection. Four independent biological replicates were prepared for expression analysis by real-time quantitative PCR. β-Actin was used as the positive control, and quantification was based on the requirement of PCR cycle number (Ct) to cross or exceed the fluorescence intensity level; the 2−ΔΔCt method was used to analyze gene expression levels (30).
Behavioral Assay.
Behavioral assays of locust nymphs from the first to the third nymphal stadia were performed in a rectangular arena (20 ×15 ×10 cm). The behavior of the fourth and fifth stadia of the two phases was analyzed in another rectangular arena (40 × 30 × 10 cm). The walls of these arenas were opaque plastic. A stimulus chamber was separated from the main observation area by a clear Perspex partition, and the other chamber remained empty. Twenty gregarious nymphs of the fourth stadium were used as the stimulus group, and the other end was left empty. Both ends were equally illuminated to prevent a mirror image formation in the end partitions. The floor of the arena was covered with sheets of filter paper during the behavioral assay. Nymphs were gently placed into the assay arena, and every nymph was examined only once. The EthoVision system (Noldus) was used for video recording and behavioral data extraction (11). Eleven different behavioral parameters were recorded and expressed as a mixture of behavioral or categorical markers.
To measure and quantify the behavioral phenotype of all five stadia of the two phases, a binary logistic regression model of phase state was constructed in SPSS version 15.0 for every nymph stadium of the migratory locust (31). Eleven behavioral variables were acquired as follows: (i) entry frequency in stimulus area (EFISA; stimulus area was defined as 25% of the arena closest to the stimulus group), (ii) latency of first occurrence in stimulus area (LFOISA), (iii) total duration in area close to the wall (TDCW), (iv) entry frequency in area close to the wall (EFCW), (v) entry frequency in the region opposite the stimulus area (EFIOSA; opposite of stimulus area was defined as 25% of the arena at the opposite end of the stimulus group), (vi) latency of first occurrence opposite the stimulus area (LFOIOSA), (vii) mean distance to the stimulus group (MDTSG), (viii) attraction index [AI; attraction index stands for the extent of tested animals attracted by the stimulus group (total duration in the stimulus area, in the opposite of the stimulus area, and in the middle area were weighted by 1, −1, and 0, respectively): AI = 1 × total duration in stimulus area + (−1) × total duration in the opposite of stimulus area + 0 × total duration in middle area], (ix) total distance moved (TDM), (x) total duration of movement (TDMV), and (xi) frequency of movement (FOM). The untransformed data of these 11 behavioral parameters were chosen for model building by a forward stepwise approach. The building process continued until no further improvement was obtained by adding further behavior variables. The inclusion or elimination of independent variables was closely correlated with the significant level of their regression coefficient β in the Wald test. The behavioral parameters of this model were adjusted until the regression model could discriminate the two phases at an optimal level, according to the following equation: P-sol = eη/(1 + eη), where η = β0 + β1·X1 + β2·X2 + … + βk·Xk. P-sol is the probability that the nymphs will be regarded as a member of the solitary phase population, and values ranged from 1 to 0, where 1 means that individuals displayed solitary behavior and 0 means that individuals displayed gregarious behavior. The most robust indicators of phase state were retained in the model, and all individual model parameters are given in SI Appendix, Table S1. The regression model of the fourth stadium of the migratory locust was used to discriminate the behavior state of locusts treated with dsRNA or drugs.
Microarray Data Analysis.
The Limma package was used for raw data background correction with the “minimum” function in R (2.6.0) (32). Intensity signals were normalized for bias elimination by using the “rlowess” function, and then data were fit into a fixed-effect ANOVA model. All statistical analyses were conducted in R using the R/MAANOVA software package with array as random effect (33). An FS test P value was calculated for each gene. Within the stringent permissive cutoff of P < 0.001, we considered the smallest fold change of 1.5 as statistically significant. Hierarchical clustering (average linkage clustering) was performed with Cluster software, and dendrograms and expression maps were generated by using Java TreeView (34). (Cluster and TreeView are available from the Eisen laboratory, http://rana.lbl.gov/EisenSoftware.htm.) DEGs were mapped to the Gene Ontology database by Blast2GO and enriched by using Fisher's test (35); pathways with P < 0.05 were considered differentially regulated.
Behavioral Data Analysis.
Statistical analyses of behavior studies, as indicated in figure legends, were performed by using SPSS version 15.0 software.
Supplementary Material
Acknowledgments
We thank Drs. S. J. Simpson and S. Y. Cheng for valuable feedback and suggestions on an early version of this manuscript. BGI (formerly known as the Beijing Genome Institute) made the microarray. This research was supported by National Natural Science Foundation of China Grant 30830022, Chinese Academy of Sciences Grant KSCX2-YW-N-087, and National Basic Research Program of China Grant 2006CB102000.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1015098108/-/DCSupplemental.
References
- 1.Pener MP, Simpson SJ. Locust phase polyphenism: An update. Adv Insect Physiol. 2009;36:1–272. [Google Scholar]
- 2.Uvarov B. Grasshopper and Locusts. Vol 1. 5 Cambridge: Cambridge Univ. Press; 1966. [Google Scholar]
- 3.Enserink M. Can the war on locusts be won? Science. 2004;306:1880–1882. doi: 10.1126/science.306.5703.1880. [DOI] [PubMed] [Google Scholar]
- 4.Pener MP, Yerushalmi Y. The physiology of locust phase polymorphism: An update. J Insect Physiol. 1998;44:365–377. doi: 10.1016/s0022-1910(97)00169-8. [DOI] [PubMed] [Google Scholar]
- 5.Simpson SJ, Despland E, Hägele BF, Dodgson T. Gregarious behavior in desert locusts is evoked by touching their back legs. Proc Natl Acad Sci USA. 2001;98:3895–3897. doi: 10.1073/pnas.071527998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simpson SJ, McCaffery AR, Hagele BF. A behavioural analysis of phase change in the desert locust. Biol Rev Camb Philos Soc. 1999;74:461–480. [Google Scholar]
- 7.Anstey ML, Rogers SM, Ott SR, Burrows M, Simpson SJ. Serotonin mediates behavioral gregarization underlying swarm formation in desert locusts. Science. 2009;323:627–630. doi: 10.1126/science.1165939. [DOI] [PubMed] [Google Scholar]
- 8.Kang L, et al. The analysis of large-scale gene expression correlated to the phase changes of the migratory locust. Proc Natl Acad Sci USA. 2004;101:17611–17615. doi: 10.1073/pnas.0407753101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma ZY, Yu J, Kang L. LocustDB: A relational database for the transcriptome and biology of the migratory locust (Locusta migratoria) BMC Genomics. 2006;7:11. doi: 10.1186/1471-2164-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei YY, Chen S, Yang PC, Ma ZY, Kang L. Characterization and comparative profiling of the small RNA transcriptomes in two phases of locust. Genome Biol. 2009;10:R6. doi: 10.1186/gb-2009-10-1-r6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noldus LPJJ, Spink AJ, Tegelenbosch RAJ. Etho Vision: A versatile video tracking system for automation of behavioral experiments. Behav Res Methods Instrum Comput. 2001;33:398–414. doi: 10.3758/bf03195394. [DOI] [PubMed] [Google Scholar]
- 12.Chen S, et al. De novo analysis of transcriptome dynamics in the migratory locust during the development of phase traits. PLoS ONE. 2010;5:e15633. doi: 10.1371/journal.pone.0015633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walter MF, et al. Catecholamine metabolism and in vitro induction of premature cuticle melanization in wild type and pigmentation mutants of Drosophila melanogaster. Arch Insect Biochem Physiol. 1996;31:219–233. doi: 10.1002/(SICI)1520-6327(1996)31:2<219::AID-ARCH9>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 14.Wittkopp PJ, True JR, Carroll SB. Reciprocal functions of the Drosophila yellow and ebony proteins in the development and evolution of pigment patterns. Development. 2002;129:1849–1858. doi: 10.1242/dev.129.8.1849. [DOI] [PubMed] [Google Scholar]
- 15.Wittkopp PJ, Carrol SB, Kopp A. Reciprocal functions of the Drosophila Yellow and Ebony proteins in the development and evolution of pigment patterns. Trends Genet. 2003;19:495–504. [Google Scholar]
- 16.Eiden LE. Evolution in black and white: Genetic control of pigment patterns in Drosophila. FASEB J. 2000;14:2396–2400. [Google Scholar]
- 17.Coleman CM, Neckameyer WS. Substrate regulation of serotonin and dopamine synthesis in Drosophila. Invert Neurosci. 2004;5:85–96. doi: 10.1007/s10158-004-0031-y. [DOI] [PubMed] [Google Scholar]
- 18.Pendleton RG, Rasheed A, Hillman R. Effects of adrenergic agents on locomotor behavior and reproductive development in Drosophila. Drug Dev Res. 2000;50:142–146. [Google Scholar]
- 19.Liu Y, Edwards RH. The role of vesicular transport proteins in synaptic transmission and neural degeneration. Annu Rev Neurosci. 1997;20:125–156. doi: 10.1146/annurev.neuro.20.1.125. [DOI] [PubMed] [Google Scholar]
- 20.Andretic R, van Swinderen B, Greenspan RJ. Dopaminergic modulation of arousal in Drosophila. Curr Biol. 2005;15:1165–1175. doi: 10.1016/j.cub.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 21.Guo W, et al. CSP and Takeout genes modulate the switch between attraction and repulsion during behavioral phase change in the migratory locust. PLoS Genet. 2011;7(2):e1001291. doi: 10.1371/journal.pgen.1001291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanaka S, Pener MP. A neuropeptide controlling the dark pigmentation in color polymorphism of the migratory locust, Locusta migratoria. J Insect Physiol. 1994;40:997–1005. [Google Scholar]
- 23.Tawfik AI, et al. Identification of the gregarization-associated dark-pigmentotropin in locusts through an albino mutant. Proc Natl Acad Sci USA. 1999;96:7083–7087. doi: 10.1073/pnas.96.12.7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verlinden H, Badisco L, Marchal E, Van Wielendaele P, Vanden Broeck J. Endocrinology of reproduction and phase transition in locusts. Gen Comp Endocrinol. 2009;162:79–92. doi: 10.1016/j.ygcen.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 25.Rogers SM, et al. Substantial changes in central nervous system neurotransmitters and neuromodulators accompany phase change in the locust. J Exp Biol. 2004;207:3603–3617. doi: 10.1242/jeb.01183. [DOI] [PubMed] [Google Scholar]
- 26.Boureau YL, Dayan P. Opponency revisited: Competition and cooperation between dopamine and serotonin. Neuropsychopharmacology. 2011;36:74–97. doi: 10.1038/npp.2010.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Condron B, Zinn K. Dopaminergic control of serotonergic neuron development in the grasshopper central nervous system. Adv Pharmacol. 1998;42:949–951. doi: 10.1016/s1054-3589(08)60904-7. [DOI] [PubMed] [Google Scholar]
- 28.Stevenson PA. The key to Pandora's box. Science. 2009;323:594–595. doi: 10.1126/science.1169280. [DOI] [PubMed] [Google Scholar]
- 29.Nielsen HB, Wernersson R, Knudsen S. Design of oligonucleotides for microarrays and perspectives for design of multi-transcriptome arrays. Nucleic Acids Res. 2003;31:3491–3496. doi: 10.1093/nar/gkg622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−Δ Δ CT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 31.Roessingh P, Simpson SJ, James S. Analysis of phase-related changes in behaviour of desert locust nymphs. Proc R Soc Lond B Biol Sci. 1993;252:43–49. [Google Scholar]
- 32.Smyth GK, Speed TP. Normalization of cDNA microarray data. Methods. 2003;31:265–273. doi: 10.1016/s1046-2023(03)00155-5. [DOI] [PubMed] [Google Scholar]
- 33.Cui X, Hwang JT, Qiu J, Blades NJ, Churchill GA. Improved statistical tests for differential gene expression by shrinking variance components estimates. Biostatistics. 2005;6:59–75. doi: 10.1093/biostatistics/kxh018. [DOI] [PubMed] [Google Scholar]
- 34.Eisen MB, Spellman PT, Brown PO, Botstein D. Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Conesa A, et al. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics. 2005;21:3674–3676. doi: 10.1093/bioinformatics/bti610. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.