Abstract
Recent studies of several key developmental transitions have brought into question the long held view of the basal transcriptional apparatus as ubiquitous and invariant. In an effort to better understand the role of core promoter recognition and coactivator complex switching in cellular differentiation, we have examined changes in transcription factor IID (TFIID) and cofactor required for Sp1 activation/Mediator during mouse liver development. Here we show that the differentiation of fetal liver progenitors to adult hepatocytes involves a wholesale depletion of canonical cofactor required for Sp1 activation/Mediator and TFIID complexes at both the RNA and protein level, and that this alteration likely involves silencing of transcription factor promoters as well as protein degradation. It will be intriguing for future studies to determine if a novel and as yet unknown core promoter recognition complex takes the place of TFIID in adult hepatocytes and to uncover the mechanisms that down-regulate TFIID during this critical developmental transition.
Keywords: TATA box-binding protein-associated factor, hepatoblast, hepatogenesis
The precise and orderly differentiation of embryonic progenitors to committed adult cell types requires exquisite spatial and temporal control of gene expression. Until recently, the dynamic changes in transcriptional output that accompany developmental transitions were assumed to depend exclusively on regulatory DNA and its associated sequence-specific activators and repressors, whereas core promoter recognition, coactivator, and chromatin modifier complexes were generally taken to be ubiquitous and invariant from one cell type to the next. Indeed, these so-called core factors are highly conserved from yeast to man (1). However, recent evidence suggests that several key developmental transitions are accompanied by, and actually require, dramatic changes in components of this general machinery including transcription factor IID (TFIID) composed of the TATA box-binding protein (TBP) and its associated factors (TAFs), the cofactor required for Sp1 activation/Mediator (CRSP/Med) complex, and the Brg1/Brm-associated factor (BAF) complex (for review, see 2).
The first evidence that some cell types may contain alterations of the general machinery came with the identification of germ-cell-specific TFIID subcomplexes in which key subunits are replaced by paralogous components. Initially oogenesis and spermatogenesis were found to require an altered TFIID in which paralogous TAF4b replaces the canonical TAF4 (3, 4). Later spermatocytes were found to also express paralogous TAF7L that may cooperate with TBP and TAF1 in the regulation of spermatogenesis genes (5, 6). Although unique paralogs of other canonical TAFs have been identified, their tissue-specific expression and functional significance remain to be elucidated (7). The finding that TAF8 is significantly up-regulated and may be required during adipogenesis is one of the few examples to date of a canonical TAF that is normally associated with TFIID but which may also have an independent developmental function (8). The striking finding that some developmental programs such as muscle differentiation can proceed normally in the near absence of intact TFIID provided an additional clue that core promoter complex switching may be more widespread than initially appreciated (9, 10). In myotubes, an alternative core component, TAF3, is retained and required for myogenic differentiation. An additional recent example of the relative dispensability of TFIID was the finding that inactivation of TAF10 has little or no affect on adult hepatic gene expression (11). Thus, the notion of switching or “remodeling” of the core promoter machinery may be more widespread than initially appreciated.
Almost simultaneously with the discovery of “atypical” TAFs such as TAF4b, TBP-related factors (TRFs) or TBP-like proteins (TBPLs) were identified, further expanding potential core promoter recognition complex diversity. The first of these subunits, TRF1, is restricted to Drosophila where it was shown to direct transcription by RNA polymerase III (12). The evolutionarily conserved TRF2 has been primarily examined during spermatogenesis, although its expression appears to be widespread (13–15). Most recently, the vertebrate-specific TRF3 has been examined in oogenesis, hematopoiesis, and myogenesis. The earliest reports suggested that TRF3 may be widely expressed, albeit at a low level, but significantly enriched in the developing oocyte (16, 17). More detailed and simultaneous studies found that a unique TAF3-TRF3 complex may in some instances largely replace canonical TFIID in the regulation of hematopoietic and myogenic genes, and that this change in core promoter recognition complexes was essential for both the myogenic transition in mice and hematopoietic differentiation in zebrafish and mouse cells (10, 18, 19). Importantly, the extensive down-regulation of canonical TFIID that accompanies myogenic development is accompanied by the finding of a wholesale depletion of the CRSP/Med coactivator complex (9). Most recently, ablation of the TRF3 locus has revealed its essential role in germ cell development and the transcription of oogenic genes (20). As developmental changes in the core promoter recognition machinery continue to be uncovered, yet other studies have revealed the developmental dynamics of the chromatin remodeling machinery (for review, see ref. 21).
Collectively these recent examples suggest that the long held view of TFIID, BAF, and CRSP/Med complexes as “general” transcription factors that function at a majority of promoters in every cell type needs to be reconsidered. Historically these factors have been studied in transformed or highly proliferative cell types such as HeLa, and recent advancements in the purification of more relevant primary cells have improved our ability to study the dynamics of such factors in a physiologically and developmentally relevant context. To further broaden our understanding of how core machinery switching contributes to developmental transitions and to establish the role of TFIID and CRSP/Med changes during the development of multiple cell types, we have examined the expression of TFIID and CRSP/Med subunits in additional primary cells and their associated developmental programs. Previous reports suggested that the adult mammalian liver might contain unusually low levels of both TBP and TAF4, critical components of canonical TFIID (3, 17). Here we report dramatic changes in the cellular levels of both TFIID and CRSP/Med during mouse liver development.
Results and Discussion
Differential Expression of TFIID in Hepatoblasts and Hepatocytes.
As a first step toward identifying additional developmental programs that may involve down-regulation of TFIID, we generated whole cell extracts from adult mouse tissues and compared the TBP, TAF1, and TAF4 levels to those in myoblasts by immunoblotting. Consistent with previous results, we find that the adult liver contains significantly lower levels of these canonical TFIID subunits than either myoblasts or the whole embryo, and that fully differentiated tissues in general have low levels of these three proteins (3, 17).
Based on these initial experiments, we determined that significant differences in both extracellular protein concentration and cell type heterogeneity between related but developmentally discrete tissues substantially complicates meaningful comparisons of TFIID levels. This observation, in combination with the previous finding that efficient purification of myoblasts and myotubes was required to uncover the extent of TFIID changes in the myogenic program, prompted us to employ highly purified single cell types in our analysis (10). Hepatoblasts are the definitive fetal liver progenitors that give rise to the parenchymal cell types of the adult liver, including hepatocytes, which account for a majority of the liver mass and are the most critical components of liver physiology (22). Bipotential hepatoblasts comprise approximately 20% of the cells in the fetal liver, whereas hepatocytes make up approximately 60% of the adult liver mass. To investigate whether differences in the core machinery existed between the distinct stages of hepatogenesis represented by these two cell populations, we established a protocol for the purification of hepatoblasts from E13.5 mouse embryos based on enzymatic digestion of individually dissected fetal livers followed by magnetic-bead-based depletion of TER119+ erythrocytes and CD45+ leukocytes. Both fluorescence-activated cell sorting and immunofluorescence staining of CD45, TER119, E-Cadherin, and DLK1 showed these preparations to contain greater than 90% pure hepatoblasts, consistent with previous results (23). Adult hepatocytes from 8 to 10-wk-old mice were purified using established in situ collagenase perfusion protocols (24).
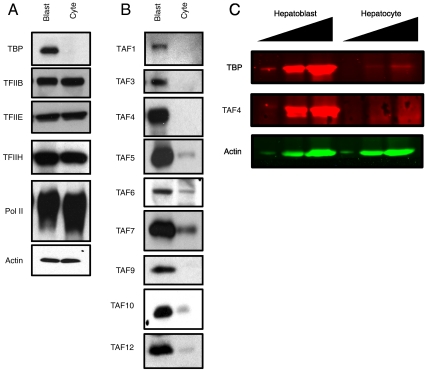
We compared the levels of TBP, multiple TAFs, and other general transcription factors in these highly purified hepatoblasts to those in hepatocytes by immunoblot (Fig. 1 A and B). Strikingly, protein levels of TBP and every TAF subunit examined are dramatically lower in adult hepatocytes compared to fetal hepatoblasts. Quantification of these changes by fluorescence-based immunoblotting shows that for at least two subunits, TBP and TAF4, this change spans two orders of magnitude or more (Fig. 1C). This apparently near-wholesale disruption of canonical TFIID includes key structural subunits such as TAF4 and TAF5, subunits which are known to make critical DNA contacts such as TAF1, and subunits which are understood to contact activators such as TAF4 (25–29). Additionally this down-regulation includes subunits that are known components of alternative TAF-containing complexes such as the TBP-free TAF-containing (TFTC) and SPT3/TAF9/GCN5 acetyltransferase (STAGA) complexes, an observation which was previously made in the myogenic program (30). In contrast to the myogenic program, TAF3 appears to be down-regulated in hepatocytes, suggesting that the TAF3–TRF3 complex is likely not required for hepatogenesis; similarly, although we find TRF3 and TRF2 levels to be largely unchanged during the hepatogenic program, levels of these two proteins are low and near the limit of detection. We also observe that the extent of protein down-regulation varies between subunits (compare TAF4 and TAF6) and in subsequent experiments noticed that the extent of down-regulation is much greater between purified hepatoblasts and hepatocytes than between whole fetal and adult livers. Based on this last observation, we speculate that resident liver cells other than hepatocytes may contain abundant intact TFIID and that previous studies may not have noticed major changes in TFIID levels because they employed whole liver tissue as opposed to highly purified single-cell-type populations. Importantly, as in the myogenic program, other general transcription factors including, but not limited to, pol II, TFIIB, TFIIE, and TFIIH appear to be largely unchanged, suggesting that the observed reductions in transcription factors are specific to TAF-containing complexes and do not reflect a general down-regulation of the complete transcription machinery (Fig. 1A).
Fig. 1.
Analysis of core transcription machinery in hepatoblasts and hepatocytes. (A) Immunoblot of purified hepatoblasts (Blast) and hepatocytes (Cyte) for TBP, TFIIB, TFIIE, TFIIH, RNA polymerase II, and actin. (B) Immunoblot for canonical TAFs. (C) Fluorescence-based quantitative immunoblot of hepatoblasts and hepatocytes for TBP, TAF4, and actin spanning two orders of magnitude.
Down-Regulation of CRSP/Med in Adult Hepatocytes.
Much like TFIID, the CRSP/Med complex was until recently widely assumed to act universally on a majority of promoters in nearly all cell types. Genetic evidence previously suggested that several key CRSP/Med subunits may be required for some critical and well-studied developmental transitions, including hematopoiesis and adipogenesis (31, 32). However, recent findings identify smaller CRSP/Med subcomplexes with promoter-selective properties, suggesting that a ubiquitous and invariant holo-CRSP/Med complex may not participate in the regulation of all genomic promoters as previously thought (33). In the case of myogenesis, severe down-regulation of six critical CRSP/Med subunits was found to accompany the extensive decrease in TFIID, suggesting that the myogenic program may be independent of CRSP/Med activity (9).
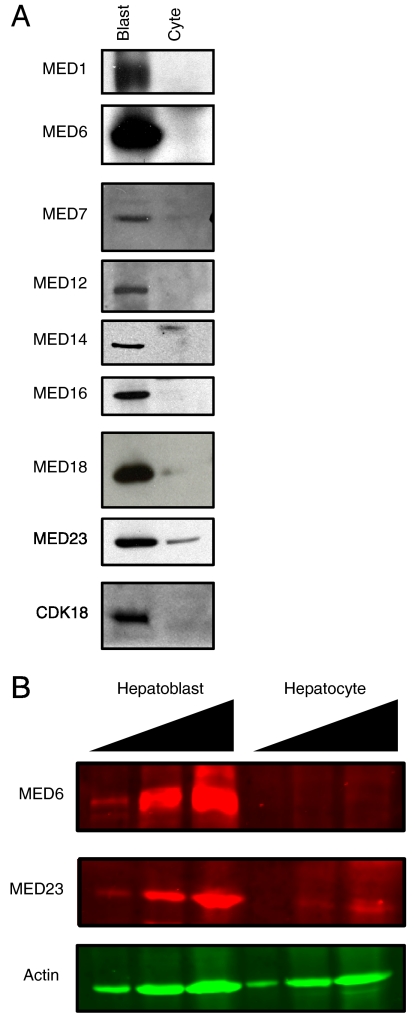
Our analysis shows that the transition from hepatoblasts to hepatocytes is also accompanied by significant decreases in CRSP/Med protein levels (Fig. 2A). As with TFIID, fluorescence-based quantitative immunoblots show at least several CRSP/Med subunits to be decreased by two orders of magnitude or more in adult hepatocytes versus hepatoblasts at the protein level (Fig. 2B). The decrease in CRSP/Med abundance includes multiple subunits from each of the four discrete regions within CRSP/Med including the head (MED6 and 18), middle (MED1 and 7), tail (MED14 and 16), and CDK8 submodule (CDK8 and MED12) (34). Although the abundance of CRSP/Med subunits and the availability of appropriate antibodies precludes analysis of every subunit, it seems likely that if some subunits did remain in hepatocytes they would contribute to a complex which is substantially different from the previously studied canonical CRSP/Med complex.
Fig. 2.
Analysis of CRSP/Med subunits in hepatoblasts and hepatocytes. (A) Immunoblot of purified hepatoblasts (Blast) and hepatocytes (Cyte) for various CRSP/Med (MED) subunits. (B) Fluorescence-based quantitative immunoblot of hepatoblasts and hepatocytes for MED6, MED23, and actin spanning two orders of magnitude.
Transcriptional Silencing of TFIID and CRSP/Med Promoters.
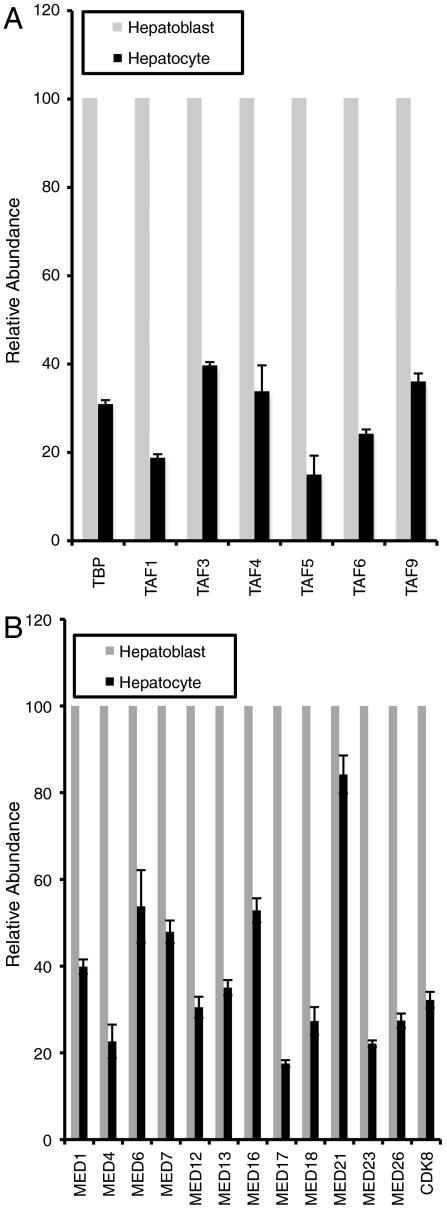
Having established that hepatogenesis is accompanied by significant changes in the level of TFIID and CRSP/Med polypeptides, we sought to understand if transcription of the individual subunits that make up these complexes is also altered. To this end, we performed quantitative reverse transcription-PCR (qRT-PCR) using RNA purified from hepatoblasts or hepatocytes, and gene-specific primers. When compared to GAPDH, mRNA levels of TBP and multiple TAFs were clearly lower in adult hepatocytes relative to hepatoblasts (Fig. 3A). Similarly, mRNA levels of multiple CRSP/Med subunits including components of the head, middle, tail, and CDK8 domains were also reduced in hepatocytes when compared to hepatoblasts (Fig. 3B). Thus it appears that changes in the active transcription of individual TFIID and CRSP/Med subunits and/or changes in mRNA stability contribute at least in part to the decreased protein abundance of these complexes in adult liver. Intriguingly, the changes observed at the mRNA level are less severe than at the protein level, suggesting that an active mechanism of protein turnover or translational repression may contribute to the down-regulation of these complexes in hepatocytes. Although cell-cycle-dependent modification and inactivation of TFIID has been demonstrated, the role of cell-type-specific modification and proteasome-dependent TFIID turnover represents an important area for future analysis (35). The recent finding that micro-RNAs regulate significant changes in BAF complex composition during neuronal development suggests that multiple mechanisms may synergize to alter core promoter recognition complex diversity and trigger the formation of an alternative preinitiation apparatus (36)
Fig. 3.
Analysis of core machinery mRNA abundance in purified hepatoblasts and hepatocytes. (A) Relative abundance of various TAF transcripts in purified hepatoblasts and hepatocytes normalized to GAPDH. (B) Relative abundance of various MED transcripts in purified hepatoblasts and hepatocytes normalized to GAPDH.
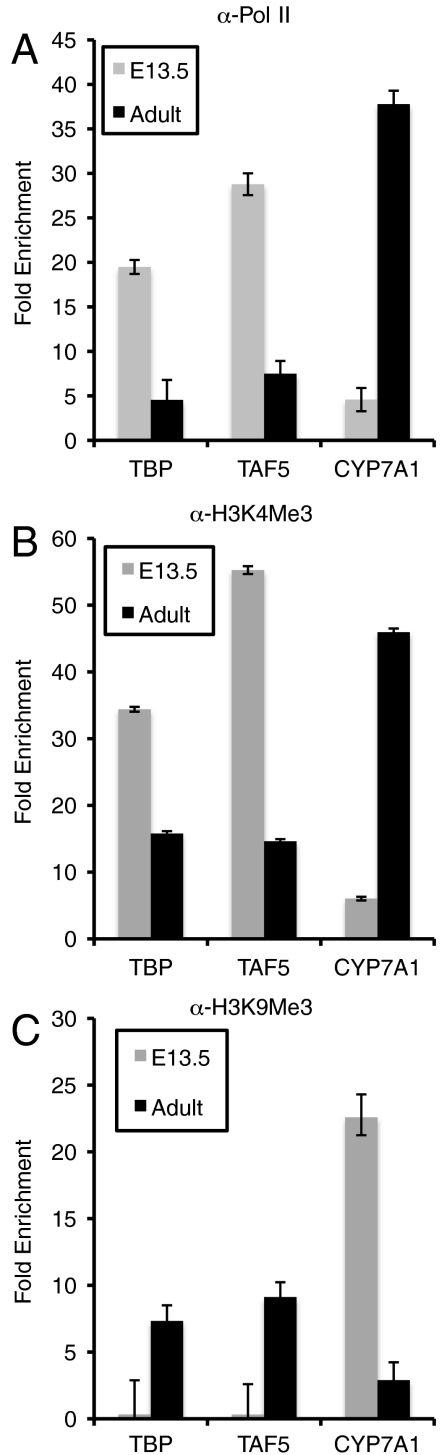
To further investigate the transcriptional repression of TFIID subunit genes, we performed chromatin immunoprecipitation with antibodies against pol II and established histone marks followed by quantitative PCR with primers directed against TBP and TAF proximal promoters (Fig. 4 A–C) (37). This analysis reveals that serine 5 phosphorylated pol II and K4 trimethylated histone H3, two marks for active transcription, were significantly enriched at the TBP and TAF5 promoters in hepatoblasts relative to hepatocytes. Conversely, K9 trimethylated histone H3, a mark of transcriptional repression, was enriched on these promoters in hepatocytes but relatively absent from them in hepatoblasts. As a control, we used the cytochrome P450 7A1 gene that is active exclusively in fully differentiated hepatocytes (38). As expected, this promoter is enriched for active histone marks in hepatocytes but shows enhanced repressive marks in hepatoblasts (Fig. 4 A–C). Hence, it appears that TBP and TAF promoters may be actively repressed by modifications of nearby histone tails and possibly by the recruitment of as yet unidentified transcriptional repressors upon differentiation. Uncovering the signaling events and mechanisms that produce this transcriptional repression in the developing liver warrants a more extensive analysis beyond the scope of this study.
Fig. 4.
Enrichment of RNA polymerase II and histone marks on TFIID subunit promoters. Purified hepatoblasts and hepatocytes were cross-linked and chromatin was subjected to immunoprecipitation with antibodies against (A) pol II, (B) triemethylated H3K4, or (C) trimethylated H3K9. DNA was purified and the abundance of TBP, TAF5, or CYP7A1 proximal promoter DNA was compared to an IgG control by qPCR.
TFIID Changes in an in Vivo Model of Hepatogenesis.
Primary tissue derived cell lines that can mimic a specific differentiation program under defined conditions have proven invaluable in the study of transcription; the C2C12 system was critical in the initial finding of TFIID changes during myogenesis (10). To explore hepatogenesis ex vivo, we employed the hepatoblast derived hepatic progenitors proliferating on laminin (HPPL) cell line (39). These cells proliferate and maintain a progenitor-like state displaying the expression of many bipotential markers when maintained on laminin in the presence of epidermal and hepatocyte growth factor. When grown in medium containing Oncostatin-M, an essential in vivo regulator of hepatic differentiation, and Matrigel, HPPLs efficiently differentiate into hepatocyte-like cells that express adult hepatocyte markers at levels similar to primary isolates.
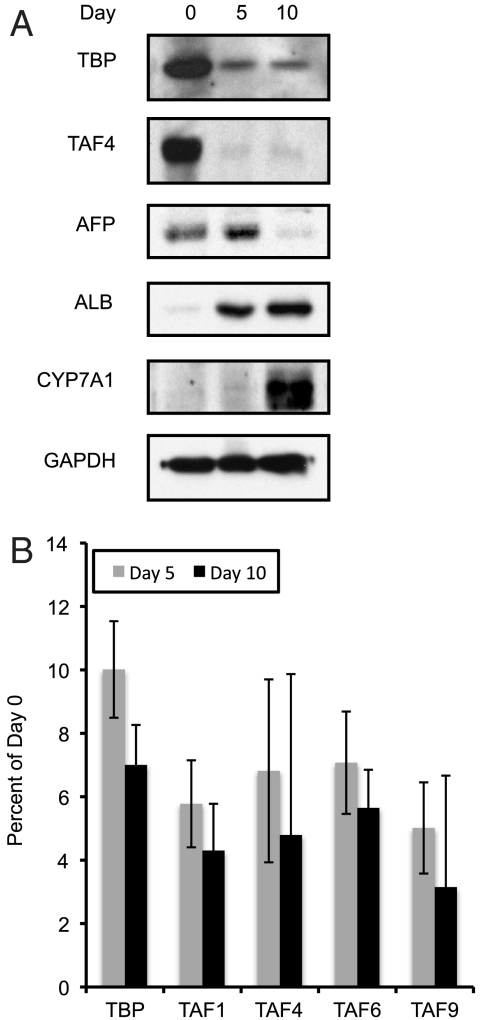
We have differentiated HPPLs as reported and find the down-regulation of bipotential markers and induction of hepatocyte-specific genes at both the protein and mRNA levels is consistent with previous studies (Fig. 5A) (39). Comparison of proliferative hepatoblast-like HPPLs to those that have undergone an extensive differentiation protocol reveals a decrease in TFIID levels that closely mimics that observed in purified primary hepatoblasts versus hepatocytes (Fig. 5A). Importantly, these changes are observed at both the mRNA and protein levels, suggesting that the same mechanism of down-regulation seen in primary cells may also take place in this ex vivo model system (Fig. 5B). It is also interesting to note that the majority of TFIID seems to be severely depleted just halfway into the differentiation process and before the final step of hepatogenic differentiation that is characterized by expression of mature hepatocyte markers such as CYP7A1 (Fig. 5A). This finding suggests that down-regulation of TFIID occurs relatively early in the hepatogenic program, an observation also made during myogenesis.
Fig. 5.
Analysis of core machinery changes in a cell culture model of hepatogenesis. HPPLs were allowed to proliferate (0), differentiated to 5 d in the presence of Oncostatin-M (5), or further matured for an additional 5 d in the presence of Matrigel (10). (A) Immunoblots of TBP, TAF 4, a hepatoblast marker (AFP), or hepatocyte markers (ALB, CYP7A1) from proliferative and differentiated HPPLs. (B) Quantitative RT-PCR of TBP and TAF transcripts from proliferating and differentiated HPPLs normalized to GAPDH.
Concluding Remarks
Here we show that the differentiation of bipotential fetal liver progenitors into lineage-committed hepatocytes is accompanied by a near-wholesale depletion of canonical TFIID and CRSP/Med. These results extend the recent finding of core promoter recognition complex switching in myogenesis to yet another differentiation program and suggest that this mechanism may be a general approach to cell-type-specific transcriptional control (9, 10). Importantly, these changes are observed at both the protein and mRNA level, and initial experiments suggest that an active mechanism of transcriptional silencing may contribute to decreased transcription factor levels. An ex vivo model of hepatogenesis recapitulates these results and may prove useful in future mechanistic studies.
Although the developmental down-regulation of TFIID we observe in liver development closely mirrors that observed in the myogenic program, one striking difference is the apparent absence of a TAF3–TRF3 complex in the adult hepatocyte. It therefore seems possible that an as yet unidentified liver-specific core promoter recognition complex partially replaces canonical TFIID during or following differentiation. Although it has been suggested that fetal or postnatal induction of transcription by TFIID would be sufficient to establish a gene’s expression throughout the organism’s life, and that future rounds of transcription may be core promoter recognition complex independent, such a model has the clear disadvantage of excluding alterations in expression following physiological or environmental change (11). Future identification of a hepatocyte-specific complex or complexes would provide a more parsimonious mechanism of transcriptional change during liver development, regeneration, and homeostasis. The role of TFIID and CRSP/Med in the less abundant cell types of the adult liver, such as cholangiocytes, is also unknown and warrants further study to determine how they compare to what we observe in the adult hepatocyte. In the myogenic program, core promoter recognition complex changes were found to coordinate with the actions of developmentally regulated sequence-specific activators (9). Hence, investigating the differential interaction of TFIID and potentially new core promoter recognition factors with the well-established repertoire of liver-specific activators would further refine our mechanistic understanding of liver-specific transcription.
As the critical role of core promoter recognition complex switching to developmental dynamics becomes increasingly clear, many questions remain. For example, although we identify changes in the transcription of TFIID subunit genes, others report ubiquitin-dependent proteolysis of TAF4 during neuronal differentiation, suggesting that the developmental timing and relative contribution of both transcriptional and posttranscriptional regulation to TFIID abundance is likely complex (40). One obvious similarity between the changes we observe in both the myogenic and hepatogenic programs is that those progenitors which contain high levels of TFIID and CRSP/Med are also highly proliferative, whereas the differentiated cells that lack these complexes are essentially quiescent; similarly, the transformed cell lines traditionally used to study these complexes such as HeLa are also rapidly dividing. Hence, one major function of TFIID and CRSP/Med in the progenitor, and even in transformed cells, may be to promote transcription of positive cell cycle regulators, and, more importantly, their near complete loss in differentiated cells may be necessary to ensure that these genes are turned off, allowing committed cells to withdraw from the cell cycle. Furthermore, transcription by pol I and pol III requires TBP, and we have yet to understand how differentiated cells with reduced TBP concentrations maintain steady-state transcription by these essential RNA polymerases (41, 42). Finally, the extracellular events and signaling cascades which induce developmental changes in TFIID and CRSP/Med must be uncovered if we are to fully appreciate the dynamics of these factors during a given developmental transition. Thus it appears that core promoter recognition and coactivator complex switching in cell-type-specific transcription will require much further study and should yield many seminal findings in the future.
Materials and Methods
Tissue Extracts and Primary Cell Purification.
Mouse tissues were hand-dissected from 8–10-wk-old CD-1 mice and used to make whole cell extract in 0.15 mM NaCl/0.05 mM Tris•HCl, pH 7.2/1% Triton X-100/1% sodium deoxycholate/0.1% SDS (RIPA buffer). E13–13.5 embryos were harvested from timed pregnant CD-1 females and fetal livers individually dissected in PBS at 4 °C. Livers from three to six females were dissociated for 10 min at 37 °C with 1.0 mg/mL Liberase 3 and 0.1% DNAse 1 (Roche). Erythrocytes were lysed with PharmaLyse (BD Bioscience) and single-cell suspensions achieved by passage through a 70 μm filter. Hepatoblasts were isolated by depletion with CD45 and TER119 microbeads using an LD column and MidMacs magnet (Miltenyi Biotec). Hepatocytes were isolated by intraportal perfusion of 8–10-wk-old CD-1 mice with 0.1 mg/mL Liberase 3 (Roche) for 10–15 min followed by passage through a 70-μm filter and gravity sedimentation.
HPPL Cell Culture.
Proliferative HPPL cells were maintained in DMEM/F12 (Invitrogen), 10% heat inactivated FBS, 1x insulin/transferrin/selenium (Invitrogen), 10 mM nicotinamide (Sigma), 0.1 μm, dexamethasone (Novagen), 5 mM l-glutamine (Invitrogen), 20 ng/mL hepatocyte growth factor (HGF) (Peprotech), and 20 ng/mL epidermal growth factor (EGF) (Peprotech) on laminin-coated plates (BD Bioscience). Confluent cultures were differentiated by incubation for 5 d in the same media minus HGF and EGF and containing 20 ng/mL Oncostatin M (OSM) (Peprotech). For complete differentiation, OSM media was removed and cells were overlaid with fresh media containing 0.350 mg/mL growth factor reduced Matrigel (BD Bioscience) for an additional 5 d as reported (39).
Antibodies and Immunoblotting.
Whole cell lysates were prepared by sonication in RIPA buffer, separated on denaturing polyacrylamide gels, and transferred to 0.22 µm nitrocellulose membranes. The following antibodies were used: rabbit anti-MED1 (Bethyl), rabbit anti-MED6 (Bethyl), rabbit anti-MED12 (Novus), rabbit anti-MED18 (Novus), rabbit anti-MED23 (Bethyl), goat anti-CDK8 (Abcam), mouse anti-TBP (Biodesign), rabbit anti-TRF2 (Abcam), rabbit anti-TRF3 (Deato and Tjian 2007), goat anti-TAF1 (Santa Cruz), rabbit anti-TAF3 (Deato and Tjian 2007), mouse anti-TAF4 (BD Bioscience), mouse anti-TAF5 (Eurogentec), rabbit anti-TAF6 (Abcam), rabbit anti-TAF7 (Abnova), goat anti-TAF9 (Santa Cruz), mouse anti-TAF10 (Chemicon), rabbit anti-TAF12. Quantitative Western blotting was performed with ECL-Plex reagents and a Typhoon scanner (GE Bioscience).
Quantitative RT-PCR.
RNA was prepared using the RNeasy Plus Mini Kit (Qiagen) and reverse transcribed with high-capacity cDNA reagents (Applied Biosystems). Quantitative PCR of total cDNA was performed on an ABI 7300 with Power SYBR Green Master Mix (Applied Biosystems) gene-specific primers. All experiments were normalized to GAPDH in triplicate and represent at least two biological replicates.
ChIP.
Purified cells were cross-linked for 10 min with 1% formaldehyde and quenched by addition of glycine to 125 mM. Cells were collected at 1,000 × g for 10 min and washed twice for 20 min in hypotonic lysis buffer [25 mM Hepes pH 7.9, 10 mM KCl, 0.250 M sucrose, 2 mM EDTA, 1.5 mM MgCl2, 0.1% NP-40, 1 mM DTT, 0.5 mM spermidine, Protease Inhibitor Cocktail (Roche)]. Nuclei were collected at 1,000 × g and resuspended in an equal volume of nuclear lysis buffer [50 mM Hepes pH 7.9, 140 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.1% sodium deoxycholate, 0.1% SDS, Protease Inhibitor Cocktail (Roche)]. Chromatin was sheared to 300–600 bp fragments by sonication and cleared by centrifugation at 15,000 × g. Equal amounts of cleared chromatin were precipitated by overnight incubation with rabbit IgG, rabbit anti-pol II, rabbit anti-H3K4Me3, or rabbit anti-H3K9Me3 (Active Motif). Immunocomplexes were incubated for 2 h with preblocked protein A and protein G magnetic beads (Invitrogen). Samples were washed, chromatin eluted, and cross-linking reversed before purification of DNA using QIAquick spin columns (Qiagen). Quantitative PCR was performed with primers surrounding the relevant proximal promoter and compared to the IgG control.
Footnotes
The authors declare no conflict of interest.
References
- 1.Thomas MC, Chiang CM. The general transcription machinery and general cofactors. Crit Rev Biochem Mol Biol. 2006;41:105–178. doi: 10.1080/10409230600648736. [DOI] [PubMed] [Google Scholar]
- 2.D’Alessio JA, Wright KJ, Tjian R. Shifting players and paradigms in cell-specific transcription. Mol Cell. 2009;36:924–931. doi: 10.1016/j.molcel.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freiman RN, et al. Requirement of tissue-selective TBP-associated factor TAFII105 in ovarian development. Science. 2001;293:2084–2087. doi: 10.1126/science.1061935. [DOI] [PubMed] [Google Scholar]
- 4.Falender AE, et al. Maintenance of spermatogenesis requires TAF4b, a gonad-specific subunit of TFIID. Genes Dev. 2005;19:794–803. doi: 10.1101/gad.1290105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng Y, et al. Abnormal sperm in mice lacking the Taf7l gene. Mol Cell Biol. 2007;27:2582–2589. doi: 10.1128/MCB.01722-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pointud JC, et al. The intracellular localisation of TAF7L, a paralogue of transcription factor TFIID subunit TAF7, is developmentally regulated during male germ-cell differentiation. J Cell Sci. 2003;116:1847–1858. doi: 10.1242/jcs.00391. [DOI] [PubMed] [Google Scholar]
- 7.Frontini M, et al. TAF9b (formerly TAF9L) is a bona fide TAF that has unique and overlapping roles with TAF9. Mol Cell Biol. 2005;25:4638–4649. doi: 10.1128/MCB.25.11.4638-4649.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guermah M, Ge K, Chiang CM, Roeder RG. The TBN protein, which is essential for early embryonic mouse development, is an inducible TAFII implicated in adipogenesis. Mol Cell. 2003;12:991–1001. doi: 10.1016/s1097-2765(03)00396-4. [DOI] [PubMed] [Google Scholar]
- 9.Deato MD, et al. MyoD targets TAF3/TRF3 to activate myogenin transcription. Mol Cell. 2008;32:96–105. doi: 10.1016/j.molcel.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deato MD, Tjian R. Switching of the core transcription machinery during myogenesis. Genes Dev. 2007;21:2137–2149. doi: 10.1101/gad.1583407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tatarakis A, et al. Dominant and redundant functions of TFIID involved in the regulation of hepatic genes. Mol Cell. 2008;31:531–543. doi: 10.1016/j.molcel.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 12.Hansen SK, Takada S, Jacobson RH, Lis JT, Tjian R. Transcription properties of a cell type-specific TATA-binding protein, TRF. Cell. 1997;91:71–83. doi: 10.1016/s0092-8674(01)80010-6. [DOI] [PubMed] [Google Scholar]
- 13.Zhang D, Penttila TL, Morris PL, Teichmann M, Roeder RG. Spermiogenesis deficiency in mice lacking the Trf2 gene. Science. 2001;292:1153–1155. doi: 10.1126/science.1059188. [DOI] [PubMed] [Google Scholar]
- 14.Ohbayashi T, Makino Y, Tamura TA. Identification of a mouse TBP-like protein (TLP) distantly related to the drosophila TBP-related factor. Nucleic Acids Res. 1999;27:750–755. doi: 10.1093/nar/27.3.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rabenstein MD, Zhou S, Lis JT, Tjian R. TATA box-binding protein (TBP)-related factor 2 (TRF2), a third member of the TBP family. Proc Natl Acad Sci USA. 1999;96:4791–4796. doi: 10.1073/pnas.96.9.4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gazdag E, Rajkovic A, Torres-Padilla ME, Tora L. Analysis of TATA-binding protein 2 (TBP2) and TBP expression suggests different roles for the two proteins in regulation of gene expression during oogenesis and early mouse development. Reproduction. 2007;134:51–62. doi: 10.1530/REP-06-0337. [DOI] [PubMed] [Google Scholar]
- 17.Persengiev SP, et al. TRF3, a TATA-box-binding protein-related factor, is vertebrate-specific and widely expressed. Proc Natl Acad Sci USA. 2003;100:14887–14891. doi: 10.1073/pnas.2036440100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hart DO, Raha T, Lawson ND, Green MR. Initiation of zebrafish haematopoiesis by the TATA-box-binding protein-related factor Trf3. Nature. 2007;450:1082–1085. doi: 10.1038/nature06349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hart DO, Santra MK, Raha T, Green MR. Selective interaction between Trf3 and Taf3 required for early development and hematopoiesis. Dev Dyn. 2009;238:2540–2549. doi: 10.1002/dvdy.22083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gazdag E, et al. TBP2 is essential for germ cell development by regulating transcription and chromatin condensation in the oocyte. Genes Dev. 2009;23:2210–2223. doi: 10.1101/gad.535209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ho L, Crabtree GR. Chromatin remodelling during development. Nature. 2010;463:474–484. doi: 10.1038/nature08911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zaret KS, Grompe M. Generation and regeneration of cells of the liver and pancreas. Science. 2008;322:1490–1494. doi: 10.1126/science.1161431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taniguchi H, et al. Clonogenic colony-forming ability of flow cytometrically isolated hepatic progenitor cells in the murine fetal liver. Cell Transplant. 2000;9:697–700. doi: 10.1177/096368970000900517. [DOI] [PubMed] [Google Scholar]
- 24.Wang X, et al. The origin and liver repopulating capacity of murine oval cells. Proc Natl Acad Sci USA. 2003;100(Suppl 1):11881–11888. doi: 10.1073/pnas.1734199100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wright KJ, Marr MT, 2nd, Tjian R. TAF4 nucleates a core subcomplex of TFIID and mediates activated transcription from a TATA-less promoter. Proc Natl Acad Sci USA. 2006;103:12347–12352. doi: 10.1073/pnas.0605499103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verrijzer CP, Chen JL, Yokomori K, Tjian R. Binding of TAFs to core elements directs promoter selectivity by RNA polymerase II. Cell. 1995;81:1115–1125. doi: 10.1016/s0092-8674(05)80016-9. [DOI] [PubMed] [Google Scholar]
- 27.Tanese N, Saluja D, Vassallo MF, Chen JL, Admon A. Molecular cloning and analysis of two subunits of the human TFIID complex: hTAFII130 and hTAFII100. Proc Natl Acad Sci USA. 1996;93:13611–13616. doi: 10.1073/pnas.93.24.13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goodrich JA, Hoey T, Thut CJ, Admon A, Tjian R. Drosophila TAFII40 interacts with both a VP16 activation domain and the basal transcription factor TFIIB. Cell. 1993;75:519–530. doi: 10.1016/0092-8674(93)90386-5. [DOI] [PubMed] [Google Scholar]
- 29.Wright KJ, Tjian R. Wnt signaling targets ETO coactivation domain of TAF4/TFIID in vivo. Proc Natl Acad Sci USA. 2008;106:55–60. doi: 10.1073/pnas.0811914106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wieczorek E, Brand M, Jacq X, Tora L. Function of TAF(II)-containing complex without TBP in transcription by RNA polymerase II. Nature. 1998;393:187–191. doi: 10.1038/30283. [DOI] [PubMed] [Google Scholar]
- 31.Urahama N, et al. The role of transcriptional coactivator TRAP220 in myelomonocytic differentiation. Genes Cells. 2005;10:1127–1137. doi: 10.1111/j.1365-2443.2005.00906.x. [DOI] [PubMed] [Google Scholar]
- 32.Ge K, et al. Transcription coactivator TRAP220 is required for PPAR gamma 2-stimulated adipogenesis. Nature. 2002;417:563–567. doi: 10.1038/417563a. [DOI] [PubMed] [Google Scholar]
- 33.Taatjes DJ, Tjian R. Structure and function of CRSP/Med2; a promoter-selective transcriptional coactivator complex. Mol Cell. 2004;14:675–683. doi: 10.1016/j.molcel.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 34.Guglielmi B, et al. A high resolution protein interaction map of the yeast Mediator complex. Nucleic Acids Res. 2004;32:5379–5391. doi: 10.1093/nar/gkh878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Segil N, Guermah M, Hoffmann A, Roeder RG, Heintz N. Mitotic regulation of TFIID: Inhibition of activator-dependent transcription and changes in subcellular localization. Genes Dev. 1996;10:2389–2400. doi: 10.1101/gad.10.19.2389. [DOI] [PubMed] [Google Scholar]
- 36.Yoo AS, Staahl BT, Chen L, Crabtree GR. MicroRNA-mediated switching of chromatin-remodelling complexes in neural development. Nature. 2009;460:642–646. doi: 10.1038/nature08139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldberg AD, Allis CD, Bernstein E. Epigenetics: A landscape takes shape. Cell. 2007;128:635–638. doi: 10.1016/j.cell.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 38.Cohen JC, et al. Cloning of the human cholesterol 7 alpha-hydroxylase gene (CYP7) and localization to chromosome 8q11-q12. Genomics. 1992;14:153–161. doi: 10.1016/s0888-7543(05)80298-8. [DOI] [PubMed] [Google Scholar]
- 39.Tanimizu N, Saito H, Mostov K, Miyajima A. Long-term culture of hepatic progenitors derived from mouse Dlk+ hepatoblasts. J Cell Sci. 2004;117:6425–6434. doi: 10.1242/jcs.01572. [DOI] [PubMed] [Google Scholar]
- 40.Perletti L, Kopf E, Carre L, Davidson I. Coordinate regulation of RARgamma2, TBP, and TAFII135 by targeted proteolysis during retinoic acid-induced differentiation of F9 embryonal carcinoma cells. BMC Mol Biol. 2001;2:4. doi: 10.1186/1471-2199-2-4. 10.1186/1471-2199-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Russell J, Zomerdijk JC. The RNA polymerase I transcription machinery. Biochem Soc Symp. 2006;73:203–216. doi: 10.1042/bss0730203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kassavetis GA, Geiduschek EP. Transcription factor TFIIIB and transcription by RNA polymerase III. Biochem Soc Trans. 2006;34:1082–1087. doi: 10.1042/BST0341082. [DOI] [PubMed] [Google Scholar]