Abstract
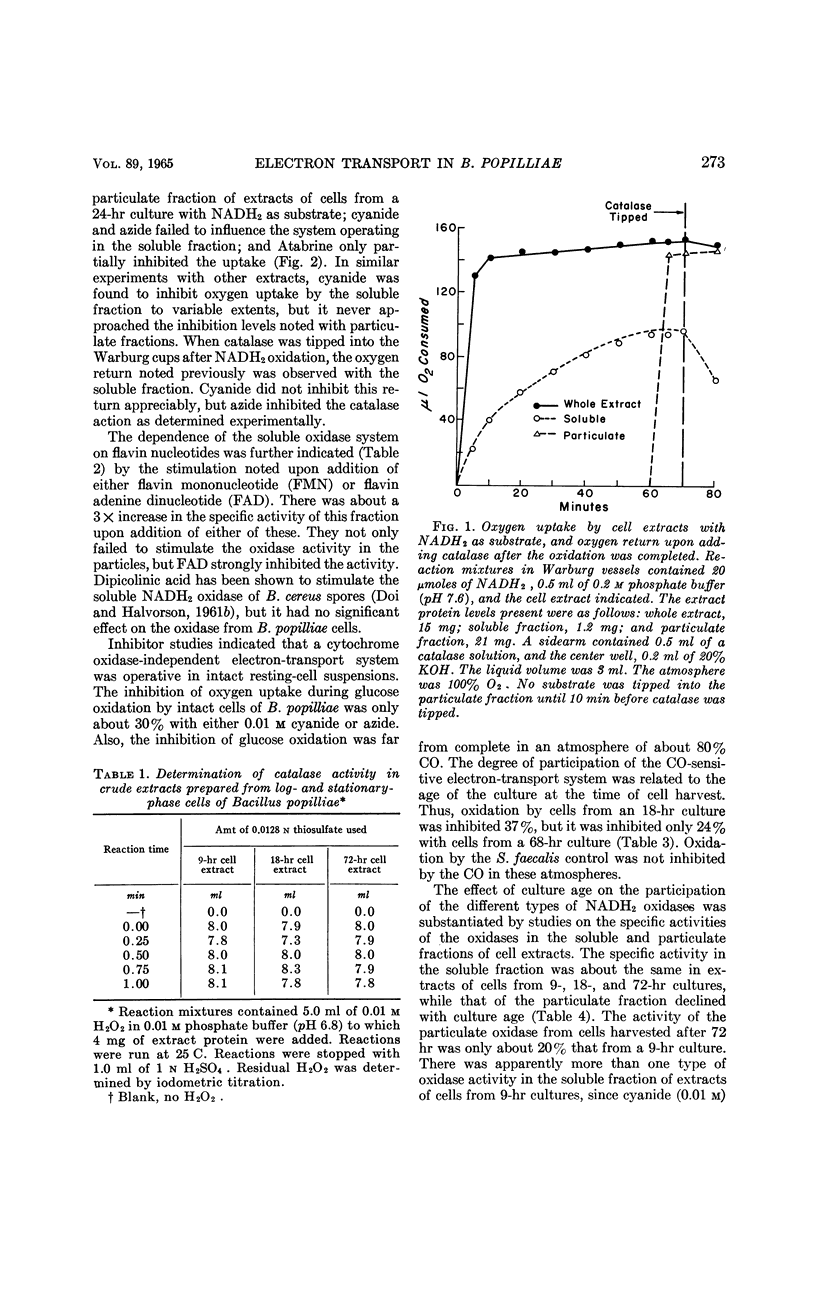
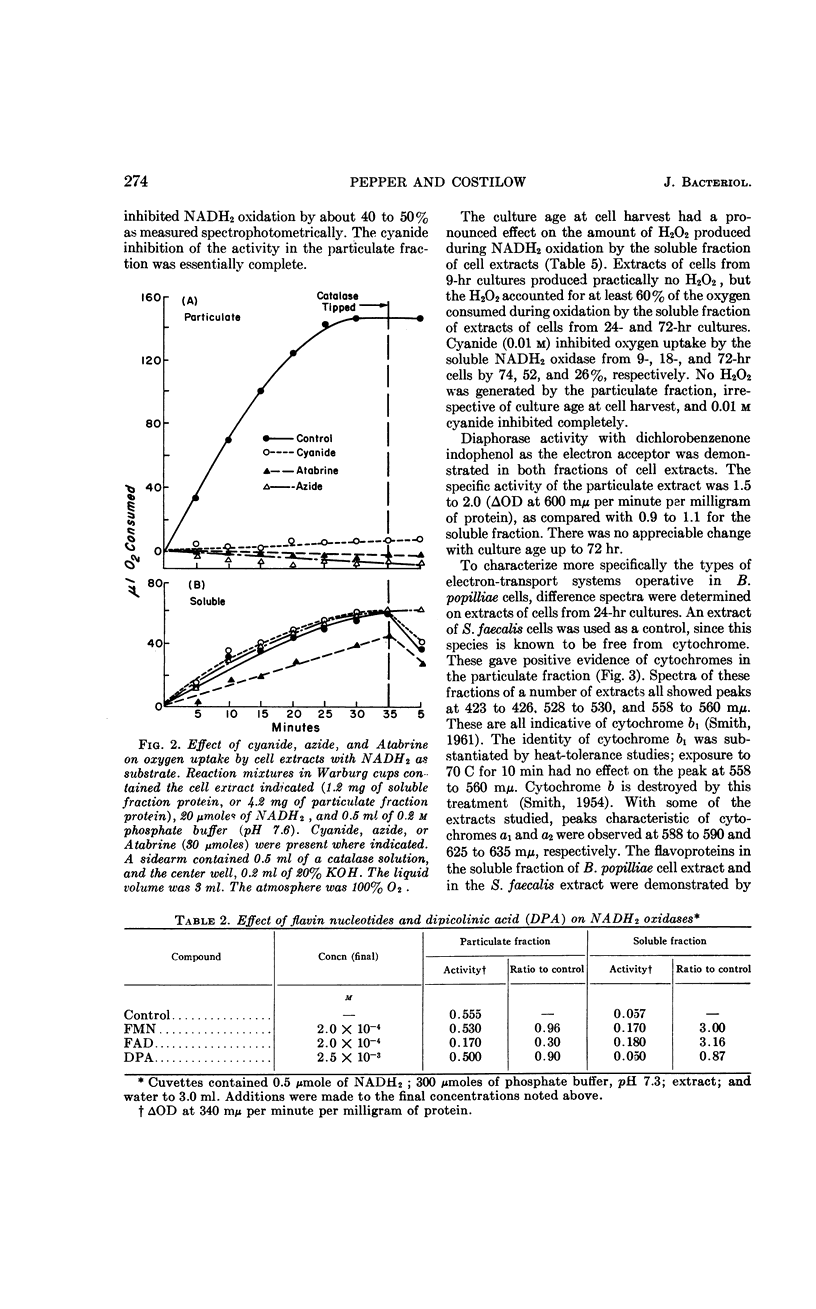
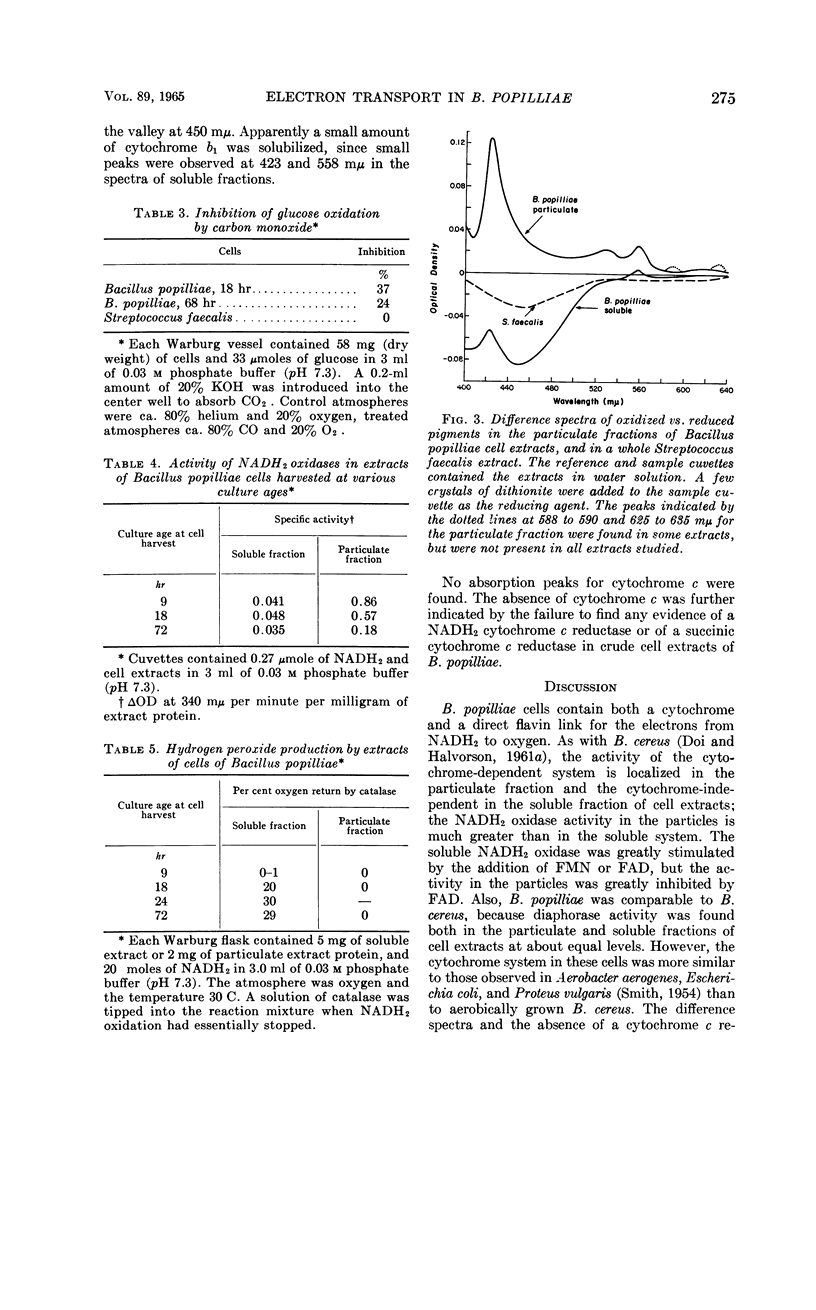
Pepper, Rollin E. (Michigan State University, East Lansing), and Ralph N. Costilow. Electron transport in Bacillus popilliae. J. Bacteriol. 89:271–276. 1965.—Bacillus popilliae was found to be unique among aerobic microorganisms in that it was deficient in a hydrogen peroxide-scavenging system. Neither catalase nor peroxidase was found. At the same time, a system for producing hydrogen peroxide during oxidation of reduced nicotinamide adenine dinucleotide (NADH2) was consistently present in the soluble fraction of extracts of cells from older cultures. Cells harvested from 9-hr cultures did not produce a significant amount of peroxide. The soluble NADH2 oxidase was apparently a flavoprotein, since it was stimulated by flavin nucleotides, insensitive to cyanide and azide, and inhibited by Atabrine. Also, difference spectra demonstrated the presence of a reducible flavin in the soluble fraction of cell extracts. The particulate fraction of cell extracts was shown by difference spectra to contain cytochrome b1; the strong inhibition of NADH2 oxidation by cyanide, azide, and carbon monoxide indicated that a terminal cytochrome oxidase was also present. This system was also flavin-dependent, since it was strongly inhibited by Atabrine. The specific activity of the NADH2 oxidase in the particulate fraction was lower in extracts of cells from older cultures than in those from exponentially growing cultures. Cytochrome c was not found in extracts of these cells. It is believed that the increased participation of the hydrogen peroxide-generating NADH2 oxidase in cells of older cultures may be responsible for the rapid loss in cell viability noted in stationary-phase cultures.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DOI R. H., HALVORSON H. Mechanism of dipicolinic acid stimulation of the soluble reduced diphosphopyridine nucleotide oxidase of spores. J Bacteriol. 1961 Apr;81:642–648. doi: 10.1128/jb.81.4.642-648.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOLIN M. I. The Streptococcus faecalis oxidases for reduced diphosphopyridine nucleotide. III. Isolation and properties of a flavin peroxidase for reduced diphosphopyridine nucleotide. J Biol Chem. 1957 Mar;225(1):557–573. [PubMed] [Google Scholar]
- DOWLER W. M., SHAW P. D., GOTTLIEB D. TERMINAL OXIDATION IN CELL-FREE EXTRACTS OF FUNGII. J Bacteriol. 1963 Jul;86:9–17. doi: 10.1128/jb.86.1.9-17.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- PEPPER R. E., COSTILOW R. N. GLUCOSE CATABOLISM BY BACILLUS POPILLIAE AND BACILLUS LENTIMORBUS. J Bacteriol. 1964 Feb;87:303–310. doi: 10.1128/jb.87.2.303-310.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAEFFER P. Recherches sur le métabolisme bactérien des cytochromes et des porphyrines. I. Disparition partielle des cytochromes par culture anaérobie chez certaines bactéries aérobies facultatives. Biochim Biophys Acta. 1952 Sep;9(3):261–270. doi: 10.1016/0006-3002(52)90160-1. [DOI] [PubMed] [Google Scholar]
- SMITH L. Bacterial cytochromes; difference spectra. Arch Biochem Biophys. 1954 Jun;50(2):299–314. doi: 10.1016/0003-9861(54)90045-4. [DOI] [PubMed] [Google Scholar]


