Abstract
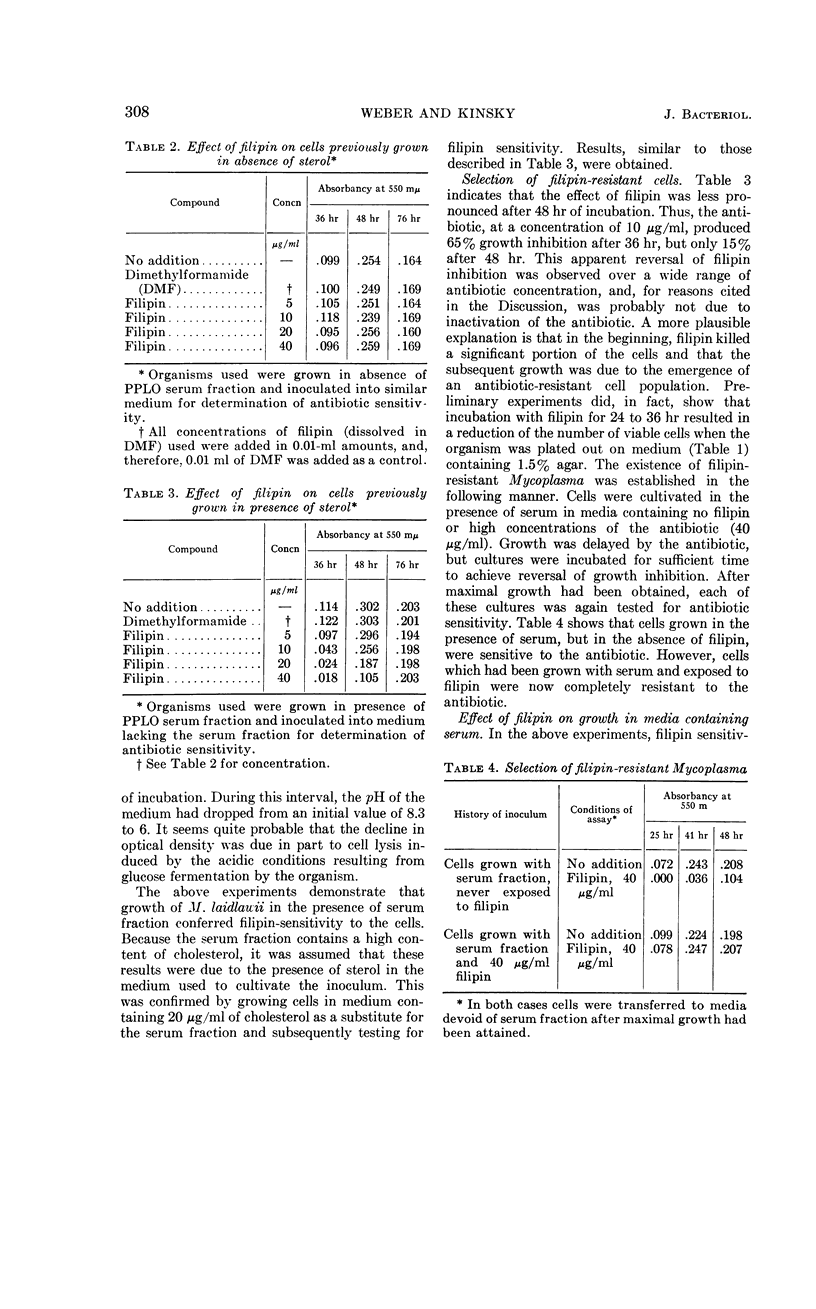
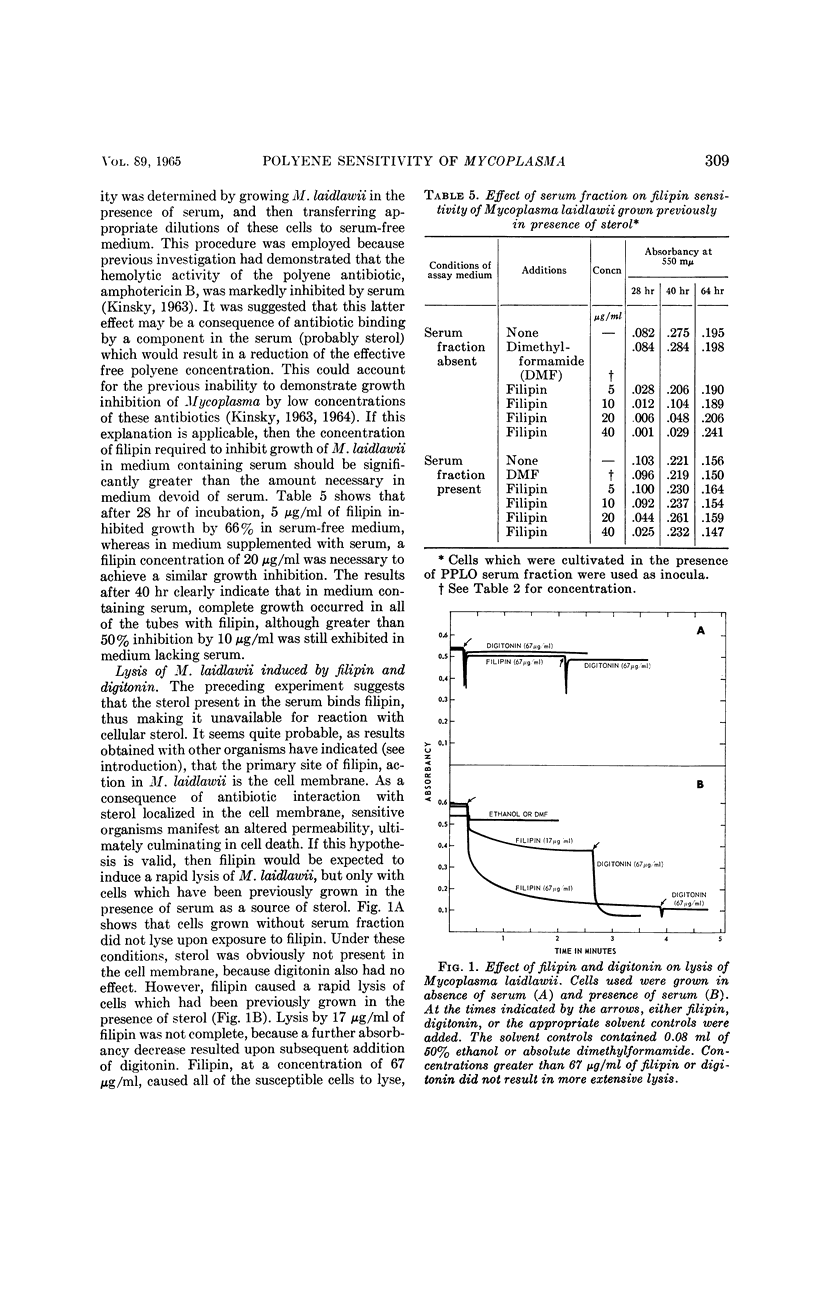
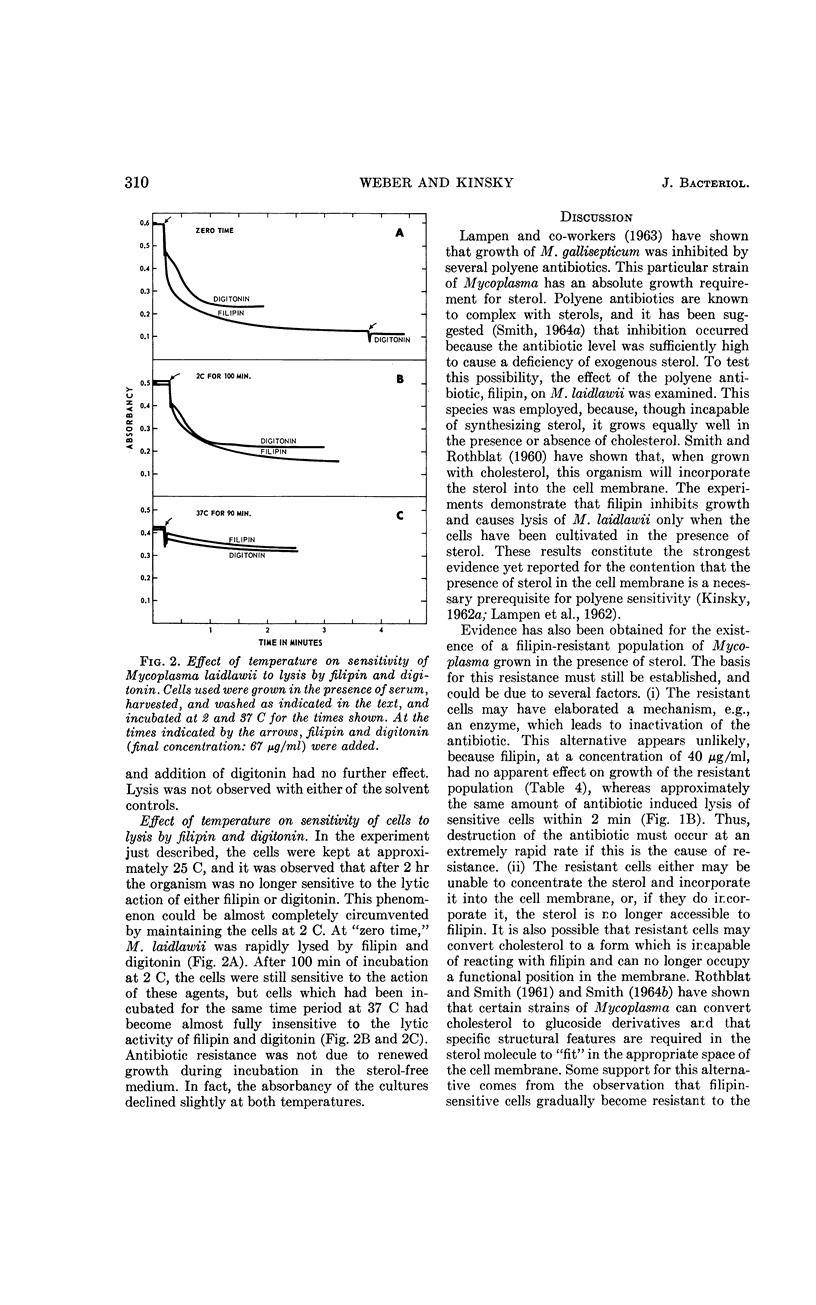
Weber, Morton M. (St. Louis University School of Medicine, St. Louis, Mo.), and Stephen C. Kinsky. Effect of cholesterol on the sensitivity of Mycoplasma laidlawii to the polyene antibiotic filipin. J. Bacteriol. 89:306–312. 1965.—The polyene antibiotic, filipin, inhibited growth and caused lysis of Mycoplasma laidlawii cells which had been cultured in the presence of cholesterol. The antibiotic did not inhibit growth and did not promote lysis of the organism when grown in the absence of cholesterol. These results constitute strong support for the contention that the presence of sterol in the cell membrane is a necessary prerequisite for polyene sensitivity. Higher concentrations of filipin were required to inhibit growth when serum was added to the assay medium than when it was absent. These results suggest binding of the antibiotic to some component in the serum and may partially account for the previous inability to demonstrate growth inhibition by low concentrations of the polyene antibiotics. The extent of growth inhibition due to filipin decreased upon prolonged incubation. Subculture in the presence of high concentrations of antibiotic indicated that the apparent reversal of inhibition was caused by emergence of a filipin-resistant cell population. It was also observed that cells, which originally were rapidly lysed by filipin and digitonin, were no longer responsive to the action of these agents upon incubation in sterol-free medium at 25 or 37 C for several hours. This effect could be prevented by keeping the cells at 2 C. These results may indicate that filipin-resistant cells carry out a metabolic conversion of membrane-localized sterol to a form which can no longer react with the antibiotic. Other possible causes of resistance, which cannot be excluded on the basis of the present data, are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- GHOSH B. K. Action of an antifungal antibiotic, nystatin, on the protozoa Leishmania donovani. IV. Studies on the cytological and cytochemical changes. Ann Biochem Exp Med. 1963 Jun;23:193–200. [PubMed] [Google Scholar]
- KINSKY S. C., AVRUCH J., PERMUTT M., ROGERS H. B. The lytic effect of polyene antifungal antibiotics on mammalian erythrocytes. Biochem Biophys Res Commun. 1962 Dec 19;9:503–507. doi: 10.1016/0006-291x(62)90116-x. [DOI] [PubMed] [Google Scholar]
- KINSKY S. C. Alterations in the permeability of Neurospora crassa due to polyene antibiotics. J Bacteriol. 1961 Dec;82:889–897. doi: 10.1128/jb.82.6.889-897.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KINSKY S. C. COMPARATIVE RESPONSES OF MAMMALIAN ERYTHROCYTES AND MICROBIAL PROTOPLASTS TO POLYENE ANTIBIOTICS AND VITAMIN A. Arch Biochem Biophys. 1963 Aug;102:180–188. doi: 10.1016/0003-9861(63)90169-3. [DOI] [PubMed] [Google Scholar]
- KINSKY S. C. Effect of polyene antibiotics on protoplasts of Neurospora crassa. J Bacteriol. 1962 Feb;83:351–358. doi: 10.1128/jb.83.2.351-358.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KINSKY S. C. MEMBRANE STEROLS AND THE SELECTIVE TOXICITY OF POLYENE ANTIFUNGAL ANTIBIOTICS. Antimicrob Agents Chemother (Bethesda) 1963;161:387–394. [PubMed] [Google Scholar]
- KINSKY S. C. Nystatin binding by protoplasts and a particulate fraction of Neurospora crassa, and a basis for the selective toxicity of polyene antifungal antibiotics. Proc Natl Acad Sci U S A. 1962 Jun 15;48:1049–1056. doi: 10.1073/pnas.48.6.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAMPEN J. O., ARNOW P. M., BOROWSKA Z., LASKIN A. I. Location and role of sterol at nystatin-binding sites. J Bacteriol. 1962 Dec;84:1152–1160. doi: 10.1128/jb.84.6.1152-1160.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAMPEN J. O., GILL J. W., ARNOW P. M., MAGANA-PLAZA I. INHIBITION OF THE PLEUROPNEUMONIA-LIKE ORGANISM MYCOPLASMA GALLISEPTICUM BY CERTAIN POLYENE ANTIFUNGAL ANTIBIOTICS. J Bacteriol. 1963 Nov;86:945–949. doi: 10.1128/jb.86.5.945-949.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVIN E. Y., BLOCH K. ABSENCE OF STEROLS IN BLUE-GREEN ALGAE. Nature. 1964 Apr 4;202:90–91. doi: 10.1038/202090a0. [DOI] [PubMed] [Google Scholar]
- RAZIN S. BINDING OF NYSTATIN BY MYCOPLASMA (PLEUROPNEUMONIA-LIKE ORGANISMS). Biochim Biophys Acta. 1963 Dec 13;78:771–773. doi: 10.1016/0006-3002(63)91056-4. [DOI] [PubMed] [Google Scholar]
- RAZIN S., KNIGHT B. C. A partially defined medium for the growth of Mycoplasma. J Gen Microbiol. 1960 Apr;22:492–503. doi: 10.1099/00221287-22-2-492. [DOI] [PubMed] [Google Scholar]
- ROTHBLAT G. H., SMITH P. F. Nonsaponifiable lipids of representative pleuropneumonia-like organisms. J Bacteriol. 1961 Oct;82:479–491. doi: 10.1128/jb.82.4.479-491.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH P. F. RELATION OF STEROL STRUCTURE TO UTILIZATION IN PLEUROPNEUMONIA-LIKE ORGANISMS. J Lipid Res. 1964 Jan;5:121–125. [PubMed] [Google Scholar]
- SMITH P. F. THE CAROTENOID PIGMENTS OF MYCOPLASMA. J Gen Microbiol. 1963 Sep;32:307–319. doi: 10.1099/00221287-32-3-307. [DOI] [PubMed] [Google Scholar]
- Smith P. F., Rothblat G. H. INCORPORATION OF CHOLESTEROL BY PLEUROPNEUMONIA-LIKE ORGANISMS. J Bacteriol. 1960 Dec;80(6):842–850. doi: 10.1128/jb.80.6.842-850.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]


