Abstract
Lüderitz, O. (Max-Planck-Institut für Immunbiologie, Frieburg, Germany), H. J. Risse, H. Schulte-Holthausen, J. L. Strominger, I. W. Sutherland, and O. Westphal. Biochemical studies of the smooth-rough mutation in Salmonella minnesota. J. Bacteriol. 89:343–354. 1965.—A comparative study of the O antigen from the smooth strain of Salmonella minnesota and of the two R antigens derived from two rough forms of S. minnesota (strains R 60 and R 345) has been carried out. The O-specific polysaccharide of the smooth form is composed of heptose, galactose, glucose, glucosamine, galactosamine, and ketodeoxyoctanoate (KDO). R 60 polysaccharide contains KDO, heptose, galactose, glucose, and glucosamine, whereas the R 345 polysaccharide contains only KDO, heptose, galactose, and glucose. Serologically, R 345 and R 60 polysaccharides belong to serogroups R I and R II, respectively. Enzymatic studies revealed that the acetylgalactosamine-synthesizing enzyme, uridine diphosphate-N-acetylglucosamine-4-epimerase, is present in wild-type and R 345 cells but is absent from R 60 cells. Two distinct polysaccharides were obtained from the R 345 cells: a polysaccharide derived from the R antigen (lipopolysaccharide) containing no galactosamine and exerting R specificity, and a soluble polysaccharide containing galactosamine and exerting O specificity. The structure of O and R antigens is discussed, together with the general significance of the results for the biosynthesis of the O antigens of the genus Salmonella.
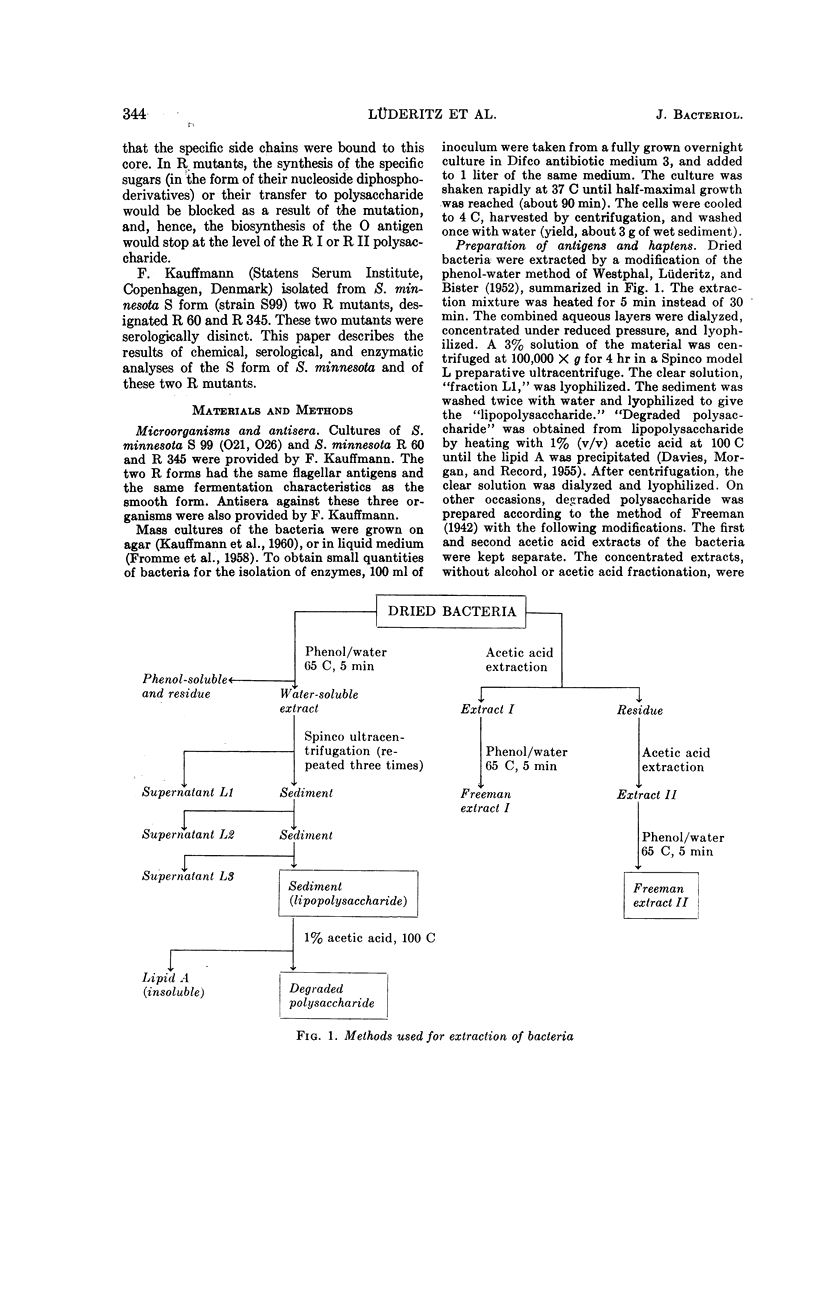
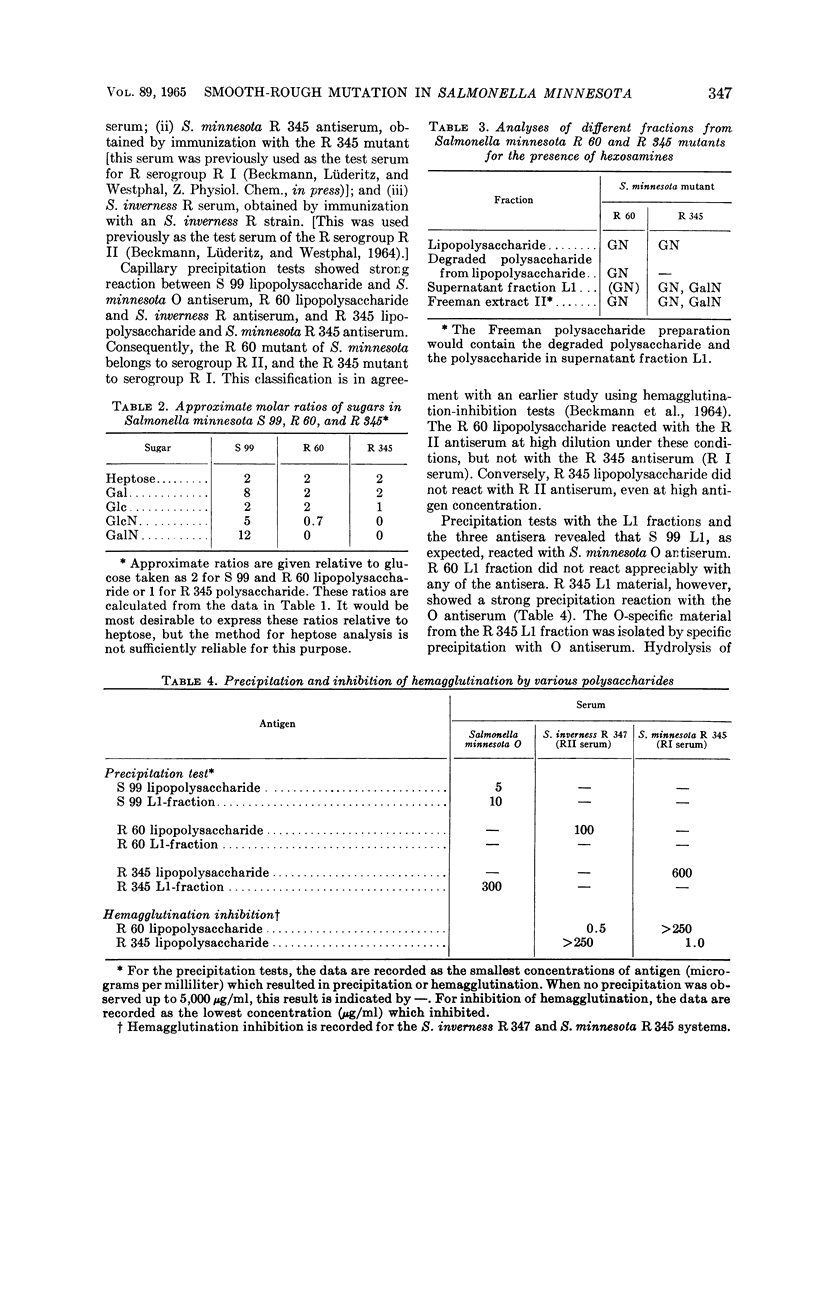
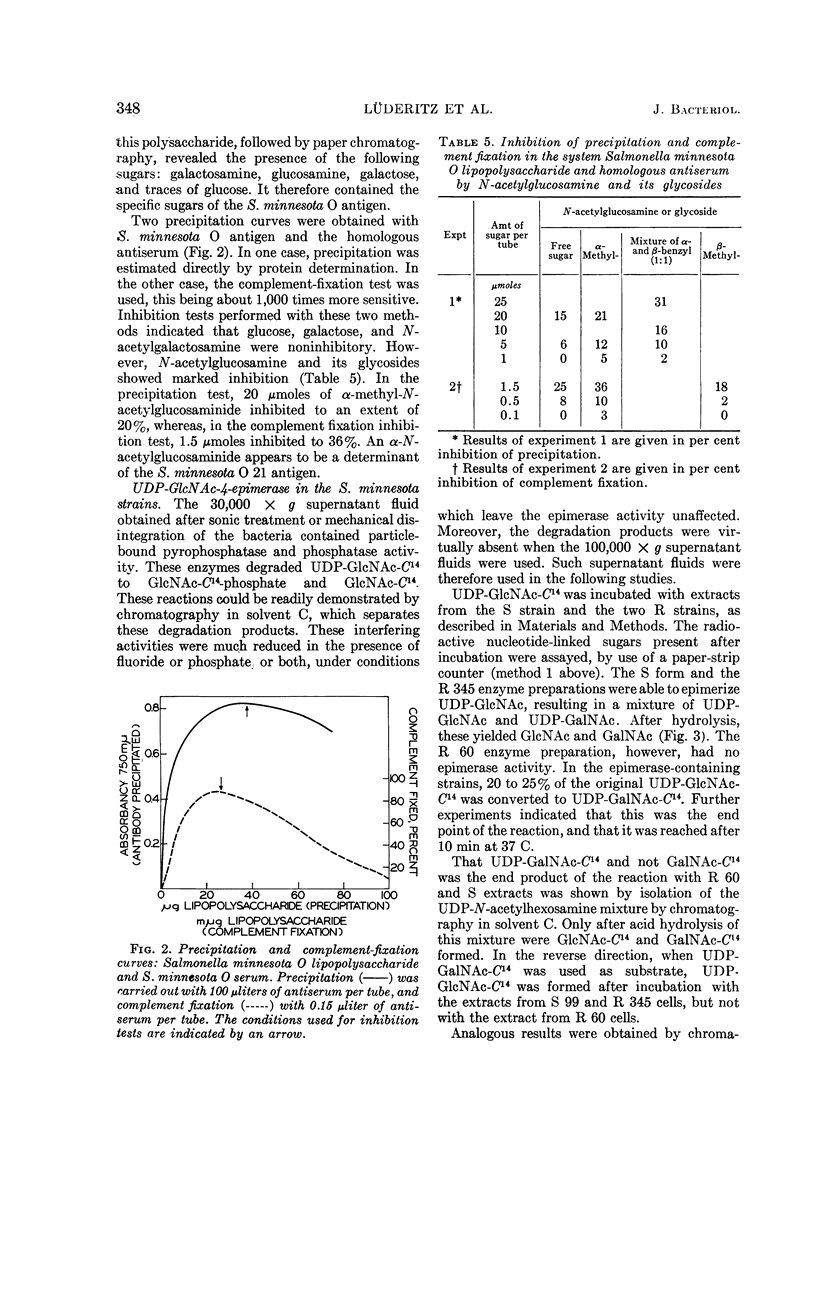
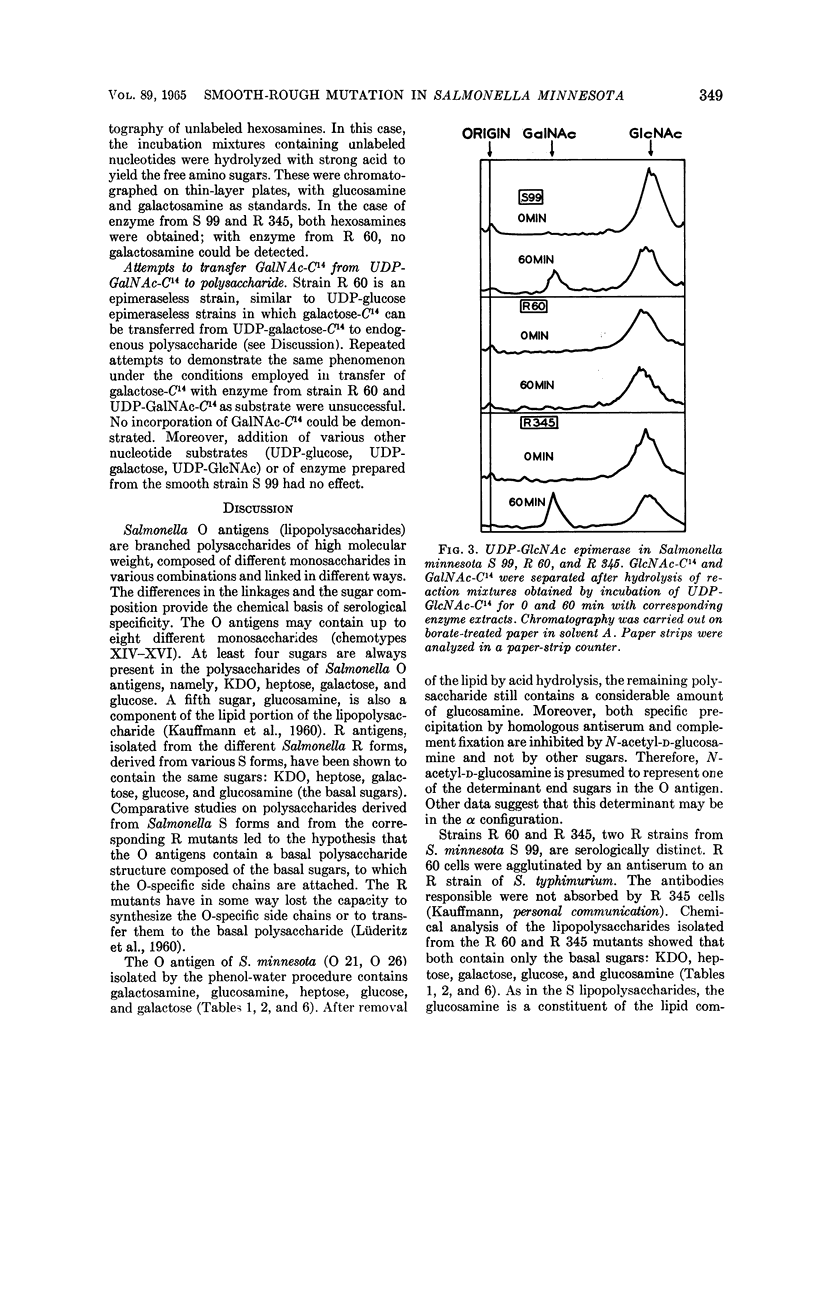
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BECKMANN I., LUEDERITZ O., WESTPHAL O. ZUR IMMUNCHEMIE DER SOMATISCHEN ANTIGENE VON ENTEROBACTERIACEAE. IX. SEROLOGISCHE TYPISIERUNG VON SALMONELLA-R-ANTIGENEN. Biochem Z. 1964 May 22;339:401–415. [PubMed] [Google Scholar]
- BECKMANN I., SUBBAIAH T. V., STOCKER B. A. ROUGH MUTANTS OF SALMONELLA TYPHIMURIUM. II. SEROLOGICAL AND CHEMICAL INVESTIGATIONS. Nature. 1964 Mar 28;201:1299–1301. doi: 10.1038/2011299a0. [DOI] [PubMed] [Google Scholar]
- CARDINI C. E., LELOIR L. F. Enzymatic formation of acetylgalactosamine. J Biol Chem. 1957 Mar;225(1):317–324. [PubMed] [Google Scholar]
- DAVIES D. A., MORGAN W. T., RECORD B. R. Studies in immunochemistry. 15. The specific polysaccharide of the dominant 'O' somatic antigen of Shigella dysenteriae. Biochem J. 1955 Jun;60(2):290–303. doi: 10.1042/bj0600290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIES D. A. Polysaccharides of gram-negative bacteria. Adv Carbohydr Chem. 1960;15:271–340. doi: 10.1016/s0096-5332(08)60190-3. [DOI] [PubMed] [Google Scholar]
- Edstrom R. D., Heath E. C. Sugar nucleotide transferases in Escherichia coli lipopolysaccharide biosynthesis. Biochem Biophys Res Commun. 1964 Aug 11;16(6):576–581. doi: 10.1016/0006-291x(64)90195-0. [DOI] [PubMed] [Google Scholar]
- FISCHER F. G., DORFEL H. Die papierchromatographische Trennung und Bestimmung der Uronsäuren. Hoppe Seylers Z Physiol Chem. 1955 Sep 2;301(4-6):224–234. [PubMed] [Google Scholar]
- FROMME I., LUDERITZ O., NOWOTNY A., WESTPHAL O. Chemische Analyse des Lipopolysaccharids aus Salmonella abortus equi. Pharm Acta Helv. 1958 Aug-Oct;33(8-10):391–400. [PubMed] [Google Scholar]
- FUKASAWA T., JOKURA K., KURAHASHI K. A new enzymic defect of galactose metabolism in Escherichia coli K-12 mutants. Biochem Biophys Res Commun. 1962 Apr 3;7:121–125. doi: 10.1016/0006-291x(62)90158-4. [DOI] [PubMed] [Google Scholar]
- Freeman G. G. The preparation and properties of a specific polysaccharide from Bact. typhosum Ty(2): With an addendum by J. St L. Philpot, From the Department of Biochemistry, Oxford. Biochem J. 1942 Apr;36(3-4):340–356. doi: 10.1042/bj0360340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLASER L. The biosynthesis of N-acetylgalactosamine. J Biol Chem. 1959 Nov;234:2801–2805. [PubMed] [Google Scholar]
- KAUFFMANN F., KRUEGER L., LUEDERITZ O., WESTPHAL O. [On the immunochemistry of the O-antigen of Enterobacteriaceae. VI. Comparison of the sugar components of polysaccharides from S and R forms of Salmonella]. Zentralbl Bakteriol. 1961 May;182:57–66. [PubMed] [Google Scholar]
- KAUFFMANN F., LUEDERITZ O., STIERLIN H., WESTPHAL O. [On the immunochemistry of O antigens of Enterobacteriaceae. I. Analysis of the sugar component of Salmonella O antigens]. Zentralbl Bakteriol. 1960 May;178:442–458. [PubMed] [Google Scholar]
- LOWRY O. H., ROBERTS N. R., LEINER K. Y., WU M. L., FARR A. L. The quantitative histochemistry of brain. I. Chemical methods. J Biol Chem. 1954 Mar;207(1):1–17. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LUEDERITZ O., BECKMANN I., WESTPHAL O. ZUR IMMUNCHEMIE DER SOMATISCHEN ANTIGENE VON ENTEROBACTERIACEAE. X. R-SPEZIFISCHE STRUKTUREN IN SALMONELLA-O-ANTIGENEN. Biochem Z. 1964 May 22;339:416–435. [PubMed] [Google Scholar]
- LUEDERITZ O., KAUFFMANN F., STIERLIN H., WESTPHAL O. [On the immunochemistry of O antigens of enterobacteriaceae. II. Comparison of the sugar componet of the S. R and T forms of Salmonella]. Zentralbl Bakteriol. 1960 Jun;179:180–186. [PubMed] [Google Scholar]
- LUEDERITZ O., SIMMONS D. A., WESTPHAL O., STROMINGER J. L. A SPECIFIC MICRODETERMINATION OF GLUCOSAMINE AND THE ANALYSIS OF OTHER HEXOSAMINES IN THE PRESENCE OF GLUCOSAMINE. Anal Biochem. 1964 Nov;9:263–271. doi: 10.1016/0003-2697(64)90184-8. [DOI] [PubMed] [Google Scholar]
- NATHENSON S. G., STROMINGER J. L. ENZYMATIC SYNTHESIS OF N-ACETYLGLUCOSAMINYLRIBITOL LINKAGES IN TEICHOIC ACID FROM STAPHYLOCOCCUS AUREUS, STRAIN COPENHAGEN. J Biol Chem. 1963 Oct;238:3161–3169. [PubMed] [Google Scholar]
- NIKAIDO H. Galactose-sensitive mutants of Salmonella. I. Metabolism of galactose. Biochim Biophys Acta. 1961 Apr 15;48:460–469. doi: 10.1016/0006-3002(61)90044-0. [DOI] [PubMed] [Google Scholar]
- NIKAIDO H., MIKAIDO K., SUBBAIAH T. V., STOCKER B. A. ROUGH MUTANTS OF SALMONELLA TYPHIMURIUM. III. ENZYMATIC SYNTHESIS OF NUCLEOTIDE-SUGAR COMPOUNDS. Nature. 1964 Mar 28;201:1301–1302. doi: 10.1038/2011301a0. [DOI] [PubMed] [Google Scholar]
- NIKAIDO H. Studies on the biosynthesis of cell-wall polysaccharide in mutant strains of Salmonella. I. Proc Natl Acad Sci U S A. 1962 Aug;48:1337–1341. doi: 10.1073/pnas.48.8.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSBORN M. J., ROSEN S. M., ROTHFIELD L., HORECKER B. L. Biosynthesis of bacterial lipopolysaccharide. I. Enzymatic incorporation of galactose in a mutant strain of Salmonella. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1831–1838. doi: 10.1073/pnas.48.10.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSBORN M. J. STUDIES ON THE GRAM-NEGATIVE CELL WALL. I. EVIDENCE FOR THE ROLE OF 2-KETO- 3-DEOXYOCTONATE IN THE LIPOPOLYSACCHARIDE OF SALMONELLA TYPHIMURIUM. Proc Natl Acad Sci U S A. 1963 Sep;50:499–506. doi: 10.1073/pnas.50.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J., D'Ari L. Enzymatic incorporation of N-acetylglucosamine into cell wall lipopolysaccharide in a mutant strain of Salmonella typhimurium. Biochem Biophys Res Commun. 1964 Aug 11;16(6):568–575. doi: 10.1016/0006-291x(64)90194-9. [DOI] [PubMed] [Google Scholar]
- SCHLOSSHARDT J. UNTERSUCHUNGEN UEBER DIE ENTSTEHUNG VON MUTAGENEN IM ZELLSTOFFWECHSEL UND IHRE ROLLE IM S-R-FORMENWECHSEL BEI SALMONELLEN. Zentralbl Bakteriol Orig. 1964 Feb;192:54–66. [PubMed] [Google Scholar]
- STROMINGER J. L., PARK J. T., THOMPSON R. E. Composition of the cell wall of Staphylococcus aureus: its relation to the mechanism of action of penicillin. J Biol Chem. 1959 Dec;234:3263–3268. [PubMed] [Google Scholar]
- STROMINGER J. L., SMITH M. S. The preparation of uridine diphosphoacetyl-galactosamine. J Biol Chem. 1959 Jul;234(7):1828–1829. [PubMed] [Google Scholar]
- SUBBAIAH T. V., STOCKER B. A. ROUGH MUTANTS OF SALMONELLA TYPHIMURIUM. I. GENETICS. Nature. 1964 Mar 28;201:1298–1299. doi: 10.1038/2011298a0. [DOI] [PubMed] [Google Scholar]
- SUNDARARAJAN T. A., RAPIN A. M., KALCKAR H. M. Biochemical observations on E. coli mutants defective in uridine diphosphoglucose. Proc Natl Acad Sci U S A. 1962 Dec 15;48:2187–2193. doi: 10.1073/pnas.48.12.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARAVDEKAR V. S., SASLAW L. D. A sensitive colorimetric method for the estimation of 2-deoxy sugars with the use of the malonaldehyde-thiobarbituric acid reaction. J Biol Chem. 1959 Aug;234(8):1945–1950. [PubMed] [Google Scholar]
- WASSERMAN E., LEVINE L. Quantitative micro-complement fixation and its use in the study of antigenic structure by specific antigen-antibody inhibition. J Immunol. 1961 Sep;87:290–295. [PubMed] [Google Scholar]
- WESTPHAL O., NOWOTNY A., LUDERITZ O., HURNI H., EICHENBERGER E., SCHONHOLZER G. Die Bedeutung der Lipoid-Komponente (Lipoid A) für die biologischen Wirkungen bakterieller Endotoxine (Lipopolysaccharide). Pharm Acta Helv. 1958 Aug-Oct;33(8-10):401–411. [PubMed] [Google Scholar]


