Abstract
Pharmacological blockade of the anandamide-degrading enzyme, fatty acid amide hydrolase (FAAH), produces CB1 receptor (CB1R)-mediated analgesic, anxiolytic-like and antidepressant-like effects in murids. Using behavioral and electrophysiological approaches, we have characterized the emotional phenotype and serotonergic (5-HT) activity of mice lacking the FAAH gene in comparison to their wild type counterparts, and their response to a challenge of the CB1R antagonist, rimonabant. FAAH null-mutant (FAAH−/−) mice exhibited reduced immobility in the forced swim and tail suspension tests, predictive of antidepressant activity, which was attenuated by rimonabant. FAAH−/− mice showed an increase in the duration of open arm visits in the elevated plus maze, and a decrease in thigmotaxis and an increase in exploratory rearing displayed in the open field, indicating anxiolytic-like effects that were reversed by rimonabant. Rimonabant also prolonged the initiation of feeding in the novelty-suppressed feeding test. Electrophysiological recordings revealed a marked 34.68% increase in dorsal raphe 5-HT neural firing that was reversed by rimonabant in a subset of neurons exhibiting high firing rates (33.15% mean decrease). The response of the prefrontocortical pyramidal cells to the 5-HT2A/2C agonist (±)-1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane ((±)-DOI) revealed desensitized 5-HT2A/2C receptors, likely linked to the observed anxiolytic-like behaviors. The hippocampal pyramidal response to the 5-HT1A antagonist, WAY-100635, indicates enhanced tonus on the hippocampal 5-HT1A heteroreceptors, a hallmark of antidepressant-like action. Together, these results suggest that FAAH genetic deletion enhances anxiolytic-like and antidepressant-like effects, paralleled by altered 5-HT transmission and postsynaptic 5-HT1A and 5-HT2A/2C receptor function.
Keywords: cannabinoid CB1 receptor, depression/anxiety, electrophysiology, fatty acid amide hydrolase (FAAH) knockout, rimonabant, serotonin
INTRODUCTION
Major depression and anxiety disorders are prevalent, debilitating neuropsychiatric conditions with high comorbidity and treatment options that fall short of optimal efficacy. About a third of depressive patients are treatment-resistant, and many others experience relapsing symptoms (Bambico et al, 2009a; Zisook et al, 2008). Accordingly, some types of anxiety disorder, such as posttraumatic stress disorder, are both chronic and have low remission rates (Ohayon, 2006). Although the pathophysiology of mood disorders is not completely understood, it is widely accepted to be linked to impairments in the neurotransmission of 5-hydroxytryptamine (5-HT or serotonin) (Bambico et al, 2009a) produced by serotonergic neurons most densely found in the mesencephalic dorsal raphe (DR) nucleus (Dahlstrom and Fuxe, 1964; Descarries et al, 1982). Despite limited efficacies and a slow onset of action, drugs that augment 5-HT transmission, such as selective serotonin reuptake inhibitors (SSRIs), are the most preferred first-line treatments (Vaswani et al, 2003). Chronic antidepressant treatment facilitates exocytosis of 5-HT and/or increases its synaptic availability in corticolimbic regions extensively innervated by the DR, such as the prefrontal cortex, hippocampus, amygdala, and other forebrain structures implicated in mood regulation and stress adaptation (Steinbusch, 1981; Holmes, 2008). In contrast, 5-HT-depleting manipulations, such as through the 5-HT synthesis inhibitor, parachlorophenylalanine, or a diet deprived of the 5-HT precursor, tryptophan, precipitate anxiety and depression-like symptoms in animals (Blokland et al, 2002; Zhou et al, 2008; Roiser et al, 2008), exacerbate anxiogenic responses in human participants (Miller et al, 2000), and inflict a relapse in depressive patients (Smith et al, 1997). The delay in therapeutic onset of antidepressants has been attributed to gradual neuroplastic adaptations at the presynaptic and postsynaptic levels that result from the progressive augmentation in 5-HT activity. These modifications include desensitization of auto-inhibitory 5-HT1A receptors, sensitization or increased tonic activation of postsynaptic 5-HT1A receptors (Haddjeri et al, 1998; Besson et al, 2000; Szabo and Blier, 2001b), and downregulation of cortical 5-HT2A/2C receptors (Hollander et al, 1991a; Kennett et al, 1994a, 1994c; Quested et al, 1997). The receptor subtypes involved have been extensively explored for their roles in emotion and mood regulation, and for their modulatory impact on neurotrophic factor synthesis and neurogenesis, which are considered downstream biological signatures of antidepressant activity (Holmes, 2008; Bambico and Gobbi, 2008; Bambico et al, 2009a).
N-arachidonoylethanolamide (anandamide) and 2-arachidonoylglycerol, lipid endocannabinoid molecules that suppress excitatory and inhibitory transmission through retrograde signaling, are also present in the DR, along with CB1 receptors (CB1Rs)—their attendant Gi/o-protein-coupled receptor—and their catabolic enzyme, fatty acid amide hydrolase (FAAH) (Egertova et al, 1998; Haring et al, 2007; Moldrich and Wenger, 2000). Recent findings support a role for CB1R signaling in the modulation of the DR 5-HT system. Direct CB1R agonists modulate DR 5-HT neural firing in a biphasic manner in vivo (Bambico et al, 2007), inhibit 5-HT reuptake ex vivo (Johnson et al, 1976), and decrease 5-HT synthesis in vivo (Moranta et al, 2004). Inhibitors of FAAH also stimulate DR 5-HT neural firing by increasing anandamide-CB1R signaling (Gobbi et al, 2005). The spatio-temporal pattern of this 5-HT neural stimulation in the DR likely depends on the complementary expression of FAAH and CB1R and the on-demand characteristic of anandamide synthesis and release (Bambico and Gobbi, 2008; Bambico et al, 2009a). Consistent with the 5-HT-augmenting mechanism of antidepressants and anxiolytics, these drugs elicit identical effects detected in a wide range of behavioral tests and animal models for depression and anxiety (Bambico et al, 2007, 2009a; Bortolato et al, 2007; Gobbi et al, 2005; Hill and Gorzalka, 2005; Kathuria et al, 2003). The pharmacological properties of FAAH inhibitors relinquish much of the unwanted effects of direct CB1R agonists, including their reward properties, therefore offering favorability for these inhibitor drugs as potential alternative therapeutic agents (Hill et al, 2009a; Bambico et al, 2009a; Bambico and Gobbi, 2008).
Transgenic mice lacking FAAH (FAAH−/−) represent an invaluable tool in which to further evaluate the impact of increased endocannabinoid-CB1R signaling on emotional reactivity. As they are severely impaired in the capacity to degrade anandamide, they exhibit more than 10-fold higher levels of this endocannabinoid than wild types (WT) (Cravatt et al, 2001), while retaining normal brain CB1R density (Basavarajappa et al, 2006). Moreover, some of the inconsistent outcomes reported on the anxiolytic-like and antidepressant-like activity of FAAH inhibitors and FAAH genetic inactivation have been attributed to variable experimental conditions (Naidu et al, 2007; Moreira et al, 2008). We therefore subjected FAAH−/− mice to a battery of tests for antidepressant-like and anxiolytic-like activity. We then compared FAAH−/− and WT mice on the basis of their spontaneous DR 5-HT firing activity and function of prefrontocortical and hippocampal 5-HT1A and 5-HT2A/2C receptors. We also examined whether changes in behavioral and electrophysiological phenotypes were due to increased endocannabinoid–CB1R signaling by a challenge injection of the CB1R antagonist, rimonabant.
MATERIALS AND METHODS
Maintenance and Preparation of Animals
All experiments were carried out on male, adult FAAH knockout (FAAH−/−) mice and their WT counterparts (FAAH+/+). All mice used were from the F9 generation and weighed 25–40 g during the time of the experiments. FAAH−/− mice were generated as described earlier (Cravatt et al, 2001), and were backcrossed into the C57BL6/6J background. Mutant and WT mice were bred and maintained in the animal facilities of the University of California, Irvine, under standard conditions (12 : 12 light–dark cycle, lights on at 07:30; temperature at 20±2°C; 50–60% relative humidity, ad libitum access to food and water) until maturation. Thereafter, two batches from the colony were transported to the animal facilities of McGill University, where they were maintained under similar conditions, and caged in pairs or threes. The experimental procedures commenced about 2 months after arrival in the laboratory facility when the mice were approximately 5 months old. Additional experiments were performed on 8 to 9-month-old mice. Inspection of weight differences was carried out on 9- to 10-month old mice. All experiments on FAAH−/− mice were performed with the appropriate age-matched WT controls. All procedures were undertaken in compliance with the standards and ethical guidelines mandated by the local facility animal care committee of McGill University, the Canadian Institutes of Health Research, and the Canadian Council on Animal Care.
Drugs
The CB1R antagonist, rimonabant (SR141716A, provided by Dr Daniele Piomelli) and the carbamate inhibitor of FAAH, URB597 (3′-carbamoyl-biphenyl-3-yl-cyclohexylcarbamate, a kind gift from Dr Andrea Duranti and Dr Giorgio Tarzia, University of Urbino, Italy), were dissolved in a vehicle consisting of 5% Tween 80, 5% polyethylene glycol, and 90% saline (0.9% NaCl solution) to a final concentration of 300 and 90 μg/ml, respectively. All other drugs were obtained from Sigma-Aldrich Canada Ltd. The 5-HT1A receptor antagonist, N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-(2-pyridyl)cyclohexanecarboxamide (WAY-100635), was dissolved in saline (0.9% NaCl solution) to a final concentration of 150 μg/ml. The aforementioned drugs were administered intraperitoneally at a volume of 3.33 ml/kg body weight. For iontophoretic applications, the following drugs were used: the 5-HT1A receptor agonist 8-hydroxy-N,N-dipropyl-2-aminotetralin HBr (8-OH-DPAT, 50 mM in 200 mM NaCl, pH 4.0–4.5), the 5-HT2A/C receptor agonist (±)-DOI (10 mM in 200 mM NaCl, pH 4.0–4.5), serotonin creatinine sulfate (5-HT, 50 mM in 200 mM NaCl, pH 4.0–4.5) and quisqualic acid (1.5 mM in 400 mM NaCl, pH 8.0). Chloral hydrate was used as an anesthetic in all electrophysiological experiments.
Behavioral Assay
FAAH−/− and FAAH+/+ mice received a single injection of either the vehicle or rimonabant (1 mg/kg, intraperitoneal). Behavioral testing took place 30 min after drug/vehicle administration. We used a serial behavioral and electrophysiological testing procedure that has been validated and compared with single testing procedures in our laboratory, and by other investigators (Bambico et al, 2010; Walf and Frye, 2007). The novelty-suppressed feeding test (NSFT) and the open field test (OFT) were used to evaluate anxiety-like behaviors, whereas the forced swim test (FST) for antidepressant/depression-like coping behaviors. These were conducted on 5-month-old mice. To further validate behavioral data from these tests, we conducted additional ones, the elevated plus maze test (EPMT) for anxiety-like behavior and the tail suspension test (TST) for antidepressant-like behavior performed on 8- to 9-month-old mice. A 1- to 2-day interim period was interlaced between tests. On the day of testing, the mice were acclimated for about 60 min in the behavioral room before the procedures were initiated. The apparatus was cleaned with 70% alcohol and water after each run. The behaviors were recorded, stored, and analyzed as MPEG files using an automated behavioral tracking system (Videotrack, View Point Life Science, Montreal, Canada) equipped with infrared lighting-sensitive CCD cameras. The analog signals supplied by the camera were measurements of the luminosity of each point of the image scanned point-by-point and line-by-line at the rate of 25 images per second. These signals were transmitted to the Videotrack system and digitized on 8 bits by digital analog conversion. Before the experiments, animal/image background contrast detection thresholds were calibrated by visual inspection to distinguish diffferent behavioral patterns. Additional offline analyses were conducted by a rater who was blind to the experimental manipulations to further verify results.
Open field activity testing
Mice were each placed at the center of a white-painted open field arena (40 × 40 × 15 cm) and left to explore the whole field for 20 min of recording. The experiment took place under standard room lighting (350 lx); a white lamp (100 W) was suspended 2 m above the arena. Anxiety-like thigmotactic (‘wall-following') behavior was measured by the frequency and total duration of central zone (30 × 30 cm) visits. Anxiogenics decrease these behavioral endpoints whereas anxiolytics conversely increase them and attenuate freezing episodes (Archer, 1973; Choleris et al, 2001). Other ethological measures analyzed included grooming, rearing, turning, and locomotor activity; modified after Choleris et al (2001). This experiment was performed at 1400 hours.
Elevated plus maze test
The EPMT was used to assess anxiety-like reactivity as induced suppression of exploratory behavior. The maze was made of white Plexiglass and consisted of two open arms (16 × 5 cm) opposite each other and two walled arms (16 × 5 × 12 cm) opposite each other. The plus maze was raised 50 cm above ground and had a 5 × 5 cm central platform forming the intersection of the four arms. The mice were each placed on the central platform facing one of the open arms. EPMT behavior was recorded for 5 min under bright white light (100 W). Behavioral endpoints that were analyzed included time spent in the open vs closed arms, frequency of open arm vs closed arm entries, time spent (s) on the central platform, frequency of stretch-attend postures (SAP), and head dips into the open arms and beyond the borders of the open arms. Arm entries were defined as crossing of both forepaws into the predefined borders of each of the open and closed arms (modified after Rago et al (1988); Pellow and File (1986)). Anxiolytic agents are known to significantly increase open arm visits, head dips, and rearing behaviors while decreasing SAP and freezing behaviors. Anxiogenics increase closed arm visits, SAP, and freezing behaviors while decreasing head dips and rearing behaviors (Walf and Frye, 2007; Cole and Rodgers, 1995; Rodgers et al, 1995). To further enhance the procedure's sensitivity to anxiolytic agents and manipulations, the mice were singly caged for at least 3 days before the test, with the test itself conducted at 1400 hours (Hogg, 1996).
Forced swim test
The FST examines the dynamics of transition from an active (swimming) to a passive (immobility) mode of coping in an inescapable water-filled pool. Enhancement of immobility normally ensues after exposure; a phenomenon argued to reflect learned behavioral despair (Porsolt et al, 1977) and prevented by antidepressant treatment. The mice were placed individually into Plexiglas cylindrical bins (20 cm diameter, 50 cm high) filled with water (25–27°C water temperature) to a depth of 20 cm. This depth did not allow the tail and hindpaws to touch the floor of the bin. The test was conducted at 1900 hours when mice were allowed to swim for 6 min in a dimly lit environment and under minimal anxiogenic conditions (Kelliher et al, 2000). Custom-made plates arrayed with infrared light-emitting diodes were suspended above the bins. Infrared light-sensitive CCD cameras allowed for the capture and storage of images in the Videotrack system. After recording, the mice were rescued using a plastic grid and caged near a heat source (lamp). The behavioral tracking system was calibrated so that a mouse was considered immobile when making only those movements necessary to keep its head above water. The total duration of activity was determined during the last 4 min.
Tail suspension test
This procedure, which is also used to assess antidepressant-like activity, involved suspending the mouse by the tail from a lever in a 30 × 30 × 30 cm white-painted enclosure. The test was conducted at 1900 hours; movements were recorded and quantified for 6 min (the last 5 min were used for analysis) in a dimly lit environment and under minimal anxiogenic conditions. These experimental conditions were similar to those described by Gobbi et al (2005). Antidepressant treatment reduces the total time the mouse remains immobile (Steru et al, 1985).
Novelty-suppressed feeding test
This procedure was used to measure novelty-induced anxiety-like behaviours (neohypophagia, the inhibition of feeding upon exposure to an anxiety-provoking novel environment) and has been used to validate the acute effects of putative anxiolytics. It has also been used to predict the therapeutic effects of putative antidepressants upon long-term treatment (Bodnoff et al, 1988; Gross et al, 2000). The mice were food-deprived for 48 h, then each mouse was placed in a brightly illuminated (100 W, 350 lx) open arena (40 × 40 white-painted floor with walls 30 cm in height covered with blue textile) containing lab chow (12 pellets) evenly spread across the floor. The latency to initiate feeding (in seconds) was noted, and used as an index of anxiety/depression-like behavior. The cut-off time was 600 s (Bodnoff et al, 1988). Feeding latency was also observed in the home cage containing 12 pellets spread on the floor; the session was terminated immediately after the mice initiated feeding. The test was conducted at 1400 hours.
In Vivo Electrophysiology
Preparation for recording procedures
Three days after the last behavioral test, in vivo single-unit extracellular recordings of DR 5-HT neurons were performed as described earlier (Bambico et al, 2007; Gobbi et al, 2001, 2005). Another cohort of mice, naive to behavioral testing, was also used for these experiments. First, the mice were anesthetized with chloral hydrate (chloral hydrate, 400 mg/kg, 2% solution, intraperitoneal) then mounted in a stereotaxic frame (David Kopf Instruments, Tujunga, California) with the skull positioned horizontally (incisor bar at −3.3 mm). Anesthesia was confirmed by the absence of nociceptive reflex reaction to a tail or paw pinch and lack of an eye blink response to pressure. During the course of the experiments, anesthesia was maintained by a periodic intraperitoneal injection of supplemental doses of chloral hydrate (100 mg/kg) upon recovery of tail/paw nociceptive reflexes and eye blink response. Body temperature was maintained at 37±0.5°C throughout the experiment using a thermistor-controlled heating pad (Seabrook Medical Instrument, Inc.). Recordings were carried out using single-barreled (R&D Scientific Glass, Spencerville, MD) or microiontophoresis multi-barreled (Harvard/Applied Scientific Instrumentation, OR, USA) glass micropipettes pulled on a Narashige (Tokyo, Japan) PE-2 pipette puller. The micropipettes were preloaded with fiberglass strands to promote capillary filling with 2% Pontamine Sky Blue solution in 2 M NaCl and their tips were broken down to diameters of 1–3 μm for single-barreled and 10–15 μm for multi-barreled recordings. The impedances ranged from 2 to 6 MΩ. The stereotaxic brain coordinate system by Paxinos and Franklin (2001) was used in all electrophysiological experiments. Using a hydraulic micropositioner (model 650; David Kopf Instruments, Tujunga, California), the electrode was advanced slowly into the brain structure at approximately 0.15 mm/min to minimize the probability of missing slow-spiking neurons. To maximize sampling without introducing considerable tissue damage, three to five electrode descents were achieved. Single-unit activity was recorded as discriminated action potentials amplified by a Tennelec (Oakridge, TN) TB3 MDA3 amplifier, post-amplified and filtered by a Realistic 10 band frequency equalizer, digitized by a CED1401 interface system (Cambridge Electronic Design, Cambridge, UK), processed online, and analyzed off-line by Spike2 software version 5.20 for Windows PC (Microsoft, Seattle, WA). An Npi electronic Gmbh Microiontophoretic System (Tamm, Germany) was used for local (iontophoretic) drug applications. The spontaneous single-spike activity of neurons was recorded for at least 2 min; the first 30 s immediately after detecting the neuron was not considered to eliminate mechanical artifacts due to electrode displacement. For experiments requiring acute drug injection, a catheter was inserted intraperitoneally prior to electrophysiological recording to facilitate intraperitoneal administration. Drug response was considered inhibitory or excitatory if changes in neural activity exceeded 10% of the basal firing rate. At the end of each recording session, the recording site was marked by iontophoretic ejection (1–10 μA, negative current for 10 min) of Pontamine Sky Blue for later histological verification of recording sites. All recordings were carried out between 1400 and 2200 hours.
Extracellular recordings of spontaneous DR 5-HT single-spike and burst firing activity
The DR is the main source of 5-HT innervation in the brain. To record from single-unit 5-HT neurons in the DR, a burr hole was drilled on the cranial midline subtending regions above the entire rostrocaudal medial extent of the DR presumed to be richest in 5-HT neurons (Descarries et al, 1982). The electrode was lowered into the region of the DR (0.5–1.0 mm posterior to the interaural line on the midline, 2.5–3.5 mm from the dura mater, just beneath the Sylvian aqueduct). Under physiological conditions, spontaneously active 5-HT neurons exhibit characteristic electrophysiological properties distinguishable from non-5-HT neurons. These 5-HT neurons exhibit a slow (0.1–4 Hz) and a prominently regular firing rate (coefficient of variation (C.O.V.), ranges from 0.12 to 0.87), a broad biphasic (positive–negative) or triphasic waveforms (0.8–3.5 ms; 1.4 ms first positive and negative deflections) (Bambico et al, 2007; Allers and Sharp, 2003; Baraban and Aghajanian, 1980). Although these criteria may vary in response to pharmacological or ecological conditions (Bambico et al, 2009b), some spike features (waveform shape and spike duration) have been shown to be stable across conditions, and are therefore reliable indicators for 5-HT neurons (Aghajanian et al, 1978; Urbain et al, 2006; VanderMaelen and Aghajanian, 1983). When the regularity of firing was apparently altered by drug exposure/genetic manipulation (exceeding C.O.V. 0.87), the neurons were rigidly discriminated based on firing rates, spike shape, and duration. The regularity of neural firing activity was represented using inter-stimulus interval histograms or autocorrelograms. Burst firing activity was analyzed offline using a script designed for Spike 2 (Cambridge Electronic Design, Cambridge, UK). These analyses generated four parameters: mean ratio of the number of spikes within bursts to the sum total of recorded spikes (%), mean number of spikes contained in a burst, mean of inter-spike intervals (ISIs) in a burst, and mean burst length. The parameters used for analyzing the bursts were based on the criteria of Gobbi et al (2005), such that a train of at least two spikes with an onset defined by a maximum initial ISI of 20 ms within a regular low-frequency firing pattern was categorized as a burst. The longest ISI allowed within a burst was 40 ms.
Extracellular recordings and microiontophoresis from the CA3 dorsal hippocampus and the ventromedial prefrontal cortex
This procedure was modified after Gobbi et al (2001) and Labonte et al (2009). The multi-barreled micropipette was lowered into the CA3 regions of the dorsal hippocampus (2–3 mm lateral and 2.5–2.7 mm posterior to bregma) or the infralimbic (IL) region of the ventromedial prefrontal cortex (mPFCv) (1.5–2.0 mm anterior to bregma; 2.5–3.5 mm ventral to the dura mater; 0.25 mm from the midline, within layer 5 of the cortex). The side barrels had impedances ranging from 50 to 150 MΩ and contained 5-HT, 8-OH-DPAT, DOI, or quisqualic acid, and a NaCl solution (2 M) for automatic current balancing. As most of the pyramidal neurons are not spontaneously active under chloral hydrate anesthesia, prolonged low-current quisqualate ejections (from −2 to −5 nA) were introduced to activate them within their physiological firing rates (8–15 Hz in the hippocampus and 0.5–10 Hz in the mPFCv) (Ashby et al, 1990; ElMansari and Blier, 1997; Gobbi et al, 2001) and were retained with a current of +10 nA. It has been shown that there is no response difference between pharmacologically induced and spontaneously firing pyramidal neurons (Ashby et al, 1990). Neurons were also selected based on their steady response to standard short pulses of quisqualate and by large amplitudes, long durations, and single-action potential patterns alternating with complex spike discharges (Bartho et al, 2004; Gobbi and Janiri, 2006; Labonte et al, 2009). 5-HT was used to assess the function of the 5-HT transporter that is mainly responsible in the clearance of synaptic 5-HT through the tranmitter's reuptake. This was achieved by calculating the time required by neurons to recover 50% of the basal (initial) firing rate from the termination of the microiontophoretic application (designated as recovery time-50 (RT50)) (Gobbi et al, 2001; Pineyro et al, 1994). DOI was used to assess the sensitivity of 5-HT2A/2c receptors and was ejected as a cation (+5 to +50 nA 1–2 min-currents) and retained with a current of −11 nA. 8-OH-DPAT was used to assess the sensitivity of 5-HT1A receptors and was ejected as a cation (+2, +4, +6 and +8 nA in the hippocampus, +1, +2 and +3 nA in the mPFCv, 1–2 min currents) and retained with a current of −11 nA. Tonic hippocampal 5-HT1A receptor activity was assessed by a single injection of 0.5 mg/kg WAY-100635. This dose was fivefold greater than the maximal intravenous dose validated earlier and used in our laboratory (Besson et al, 2000) as the administration route used here was intraperitoneal. On this parameter, we also compared FAAH−/− mice response to that of WT that have received chronic (12 days; once-a-day) treatment with the FAAH inhibitor URB597 (0.3 mg/kg, intraperitoneal) with or without cotreatment with rimonabant (1 mg/kg, intraperitoneal, once daily during the last 7 days of treatment). Pyramidal activity was identified by large amplitudes (0.5–1.2 mV), long durations (0.8–1.2 ms), and as single action potentials alternating with complex spike discharges (Kandel and Spencer, 1961). Pyramidal neural response to systemic or microiontophoretic drug application was expressed as percentage increase/decrease from pre-drug (baseline) activity.
Euthanasia and histological verification
At the end of electrophysiological experiments, the animals were euthanized and the brains were harnessed and stored in paraformaldehyde. Coronal brain slices (20 μm) through regions of interests were prepared to inspect the location and extent of electrode lesions under a light microscope.
Data Analyses and Statistics
Data are presented as mean±standard error of the mean. All data were analyzed using SPSS version 17 (SPSS Inc., Chicago, Illinois) and Datasim version 1.2 (D.R. Bradley, Bates College, Lewiston, Maine). After testing for assumptions of normality of data distribution and of homogeneity of variance, the behavioral data were accordingly submitted to three-way or two-way analyses of variance (ANOVA) with genotype (FAAH−/− vs WT) and CB1R antagonist challenge (rimonabant vs vehicle) as between-group factors and time as a within-group factor, when appropriate. Electrophysiological data were submitted to Mann–Whitney U-tests for between-group comparisons or to two-way mixed design ANOVA (genotype × current) when accounting for repeated microiontophoretic current ejections. Tukey's honestly significant difference (HSD) test was used for multiple post hoc comparisons. Probability value of p⩽0.05 was considered to be statistically significant.
RESULTS
Behavioral Assay
Effect of genetic FAAH deletion on locomotor activity and spontaneous behaviors in the open field
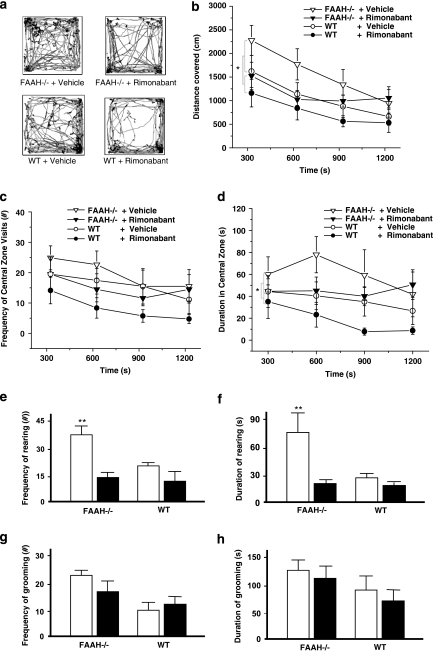
Figure 1 displays data obtained from the OFT. Only a main effect of genotype on distance covered was obtained after a three-way ANOVA with repeated measures across 5-min time bins (F(1, 28)=4.46, p=0.044) or a two-way ANOVA test on the area under the curve (AUC) (F(1, 28)=4.27, p=0.048); there was neither a significant main effect of drug (rimonabant) challenge nor genotype–rimonabant challenge interaction (Figure 1b). However, the administration of rimonabant resulted in an apparent decrease in locomotor activity regardless of genotype, attenuating distance covered by FAAH−/− mice to the level of vehicle-treated WT controls during the first 15 min. A significant main effect of time on distance covered was calculated (F(3, 84)=18.37, p<0.001). The interaction among factors (genotype, rimonabant challenge, and time) yielded no significance whereas rimonabant challenge-time interaction was marginally significant (F(3, 84)=2.34, p=0.079), indicating that the genetic and pharmacological interventions did not block habituation of locomotor activity. FAAH−/− mice, compared with the WT controls, displayed decreased thigmotactic behavior as determined by ANOVA on central zone duration (three-way ANOVA, main effect of genotype on central zone duration, F(1, 28)=4.91, p=0.035) or on AUC for central zone duration (two-way ANOVA, main effect of genotype, F(1, 28)=5.40, p=0.028) (Figure 1d). There was neither any significant main effect of time nor of rimonabant challenge, nor significant interactions among factors. No significant differences were calculated from the frequency of the central zone visits (Figure 1c). Ethological analyses of anxiety-related and exploratory behaviors revealed a significant main effect of genotype and rimonabant challenge, as well as significant interaction effect, on rearing frequency (genotype, F(1, 28)=9.14, p=0.005; rimonabant challenge, F(1, 28)=15.23, p<0.001; interaction, F(1, 28)=5.94, p=0.021; Figure 1e), and duration (genotype, F(1, 28)=15.01, p<0.001; rimonabant challenge, F(1, 28)=24.94, p<0.001; interaction, F(1, 28)=13.26, p=0.01; Figure 1f), but not on grooming (Figure 1g and h), turning, and immobility episodes (see Supplementary Table 1). Post hoc analyses on both frequency and duration of rearing indicated significantly lower values among drug-naive WT controls in comparison with drug-naive FAAH−/− mice (p<0.01); this difference was completely abolished by rimonabant challenge (Figure 1e and f). Rimonabant only attenuated the rearing behavior when administered in FAAH−/− mice (p<0.01).
Figure 1.
Effect of genetic deletion of FAAH on locomotor and exploratory activity in the open field test (OFT). (a) Horizontal movement traces of FAAH knockout mice (FAAH−/−) and wild type (WT) controls after intraperitoneal injection of vehicle or the CB1 receptor antagonist rimonabant (1 mg/kg) 30 min before the test. (b) Distance covered (cm) in horizontal locomotion. Rimonabant induced an apparent (nonsignificant) attenuation to the level of WT controls during the first 15 min of recording. Habituation of locomotor activity was not abolished by genetic FAAH deletion or by rimonabant. (c, d) FAAH−/− mice showed less thigmotaxis and longer times spent in the central zone; rimonabant induced an apparent nonsignificant attenuation to the level of WT controls. Reduced thigmotaxis in the open field indicated antianxiety effects. (e, f) Vertical exploratory activity (rearing) was less inhibited in FAAH−/− mice than in WT controls. This effect was reversed by rimonabant. (g, h) Genotype did not significantly influence grooming behaviour. Each trace in (a) is scaled from a 40 × 40 cm field dimension. Data points in (b–d) represent mean values±standard error of mean (SEM) drawn from FAAH−/− mice treated with vehicle (unshaded triangle) or rimonabant (shaded triangle) and from WT controls treated with vehicle (unshaded circle) or rimonabant (shaded circle). *p<0.05, main effect of genotype. Bar graphs in (e–h) represent mean values±SEM after treatment with vehicle (unshaded) or rimonabant (shaded); **p<0.01 vs rest of the groups, N=8 animals per group.
Effect of genetic FAAH deletion on anxiety-related behaviors in the elevated plus maze
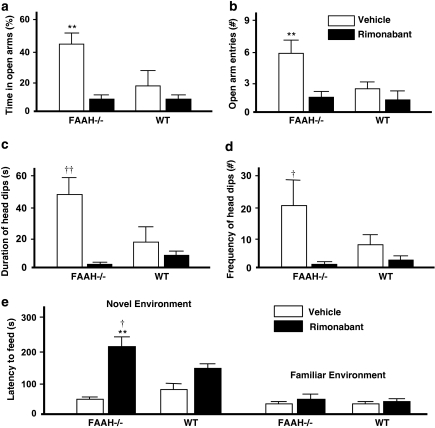
Data from the EPMT are presented in Figure 2a–d. Two-way ANOVA performed on the percentage of time spent in the open arm showed a significant main effect of genotype (F(1, 25)=4.36, p=0.047) and rimonabant challenge (F(1, 25)=11.81, p=0.002), and a marginally significant interaction between these two factors (F(1, 25)=3.62, p=0.069) (Figure 2a). Post hoc comparisons indicated significantly lesser time spent in the open arms among drug-naive WT controls in comparison with drug-naive FAAH−/− mice (p<0.01); this difference was completely abolished by rimonabant challenge. Rimonabant also exhibited attenuating effects on this measure when administered in FAAH−/− mice (p<0.01). Rimonabant failed to elicit significant effects in WT controls. A similar pattern of effects was observed from the frequency of open arm entries with significant main effects of genotype (F(1, 25)=7.12, p=0.013) and rimonabant challenge (F(1, 25)=8.04, p=0.008), and a significant interaction effect (F(1, 25)=5.45, p=0.027) (Figure 2b). Two-way ANOVA on head dips failed to reveal a significant effect of genotype (Figure 2c and d). A significant main effect of rimonabant challenge was calculated from head dip duration (F(1, 25)=8.46, p=0.008), accompanied by a significant genotype–rimonabant challenge interaction (F(1, 25)=5.76, p=0.024) (Figure 2c). Analysis of head dip frequency also yielded significant main effect of the rimonabant challenge (F(1, 25)=8.91, p=0.006), accompanied by a marginally significant genotype–rimonabant challenge interaction (F(1, 25)=3.5, p=0.073) (Figure 2d). Post hoc analyses indicated significantly lower head dip duration (p<0.01) and frequency (p<0.05) among drug-naive WT controls in comparison with drug-naive FAAH−/− mice. No difference was observed after rimonabant injection. Furthermore, when administered to FAAH−/− mice, rimonabant produced a robust attenuation in head dip duration and frequency falling below WT levels.
Figure 2.
Effect of genetic deletion of FAAH on anxiety-like behaviours in the elevated plus maze test (EAMT) (a–d) and novelty-suppressed feeding test (NFST) (e). (a, b) Vehicle-treated FAAH knockout (FAAH−/−) mice stayed significantly longer (a) and entered more frequently (b) in the open arms than did the vehicle-treated wild type (WT) controls. This resistance-like behaviour against the anxiogenic properties of the open arms were reversed by the intraperitoneal injection of the CB1 receptor antagonist rimonabant (1 mg/kg) 30 min before the test. (c, d) No significant effect of genotype was noted on head dipping behaviour. Rimonabant significantly attenuated duration (c) and frequency (d) of head dips only in vehicle-treated FAAH−/− mice. Bar graphs represent mean values±standard error of mean (SEM) after treatment with vehicle (unshaded) or rimonabant (shaded); **p<0.01 vehicle-treated FAAH−/− mice vs rest of the groups; †p<0.05, ††p<0.01 vehicle-treated FAAH−/− mice vs rimonabant-treated FAAH−/− mice. N=7–8 animals per group. (e) Impact of genetic deletion of FAAH on the suppressant effect of novelty on feeding behaviour. Left portion: latency to feed in a novel environment was slightly prolonged in vehicle-treated FAAH−/− mice as compared with vehicle-treated WT controls, indicating reduced anxiety in FAAH−/− mice. Intraperitoneal injection rimonabant (1 mg/kg) 30 min before the test slightly prolonged latency to feed in WT, suggesting that a constitutive activity of the CB1 receptor participates in the tonic inhibitory control of novelty-induced anxiety. The same dose of rimonabant led to an enormous and significant increase in the latency to feed in FAAH−/− mice, pointing to an increase in this CB1 receptor-mediated tonic inhibitory control on novelty-induced anxiety. Right portion: There were no noticeable differences in feeding behaviour among experimental groups when tested in a familiar environment. Bar graphs represent mean latency to feed in seconds±standard error of mean (SEM) after treatment with vehicle (unshaded) or rimonabant (shaded). **p<0.01 vs vehicle-treated FAAH−/−mice, †p<0.05 vs vehicle-treated and rimonabant-treated WT. N=8 animals per group.
Effect of genetic FAAH deletion on anxiety-related behaviors in the NSFT
Figure 2e shows the suppressant effect of novelty on feeding behavior. Two-way ANOVA on the latency to feed in the novel environment showed a significant main effect of rimonabant challenge (F(1, 60)=29.83, p<0.01), but not of genotype, and a significant genotype-rimonabant challenge interaction (F(1, 60)=8.29, p<0.006) (Figure 2e, left portion). The onset to feed was not any more augmented in vehicle-injected FAAH−/− mice than in vehicle-injected WT mice. However, cohorts injected with rimonabant showed longer delays in feeding onset regardless of the genotype, which were dramatically prolonged in FAAH−/− mice (74.27% longer delay relative to vehicle-injected FAAH−/− mice, p<0.001, 58.74% relative to vehicle-injected WT controls, p<0.001; and 35.74% relative to the rimonabant-injected WT controls, p=0.031; Tukey HSD test). Rimonabant administration in WT mice was already sufficient to prolong the onset to feed (35.79% longer delays relative to vehicle-injected WT controls) albeit failing to reach significance. When feeding behavior was examined in the familiar home cage environment, no observable differences were noted among the experimental groups (Figure 2e, right portion).
Effect of genetic FAAH deletion on stress-coping behavior in the FST
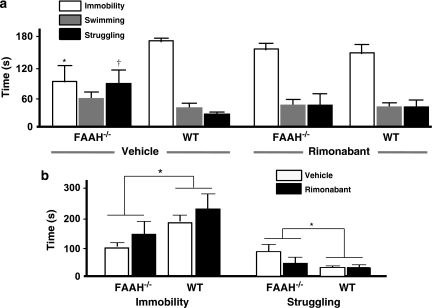
Figure 3a presents the behavioral profile in the FST, comparing differences in immobility, swimming, and struggling episodes predictive of antidepressant-like related activity. Two-way ANOVA on the duration of behaviors revealed that compared with their WT counterparts, FAAH−/− mice spent significantly less time immobile (main effect of genotype, F(1, 31)=5.02, p=0.032); the main effect of drug (rimonabant) challenge was not significant whereas the genotype–rimonabant challenge interaction was marginally significant (F(1, 31)=3.48, p=0.071). Post hoc comparisons showed that immobility duration was significantly lower (p=0.037) in vehicle-treated FAAH−/− mice than in vehicle-treated WT controls. The administration of rimonabant attenuated the decrease in immobility duration observed in FAAH−/− mice. Analysis of the duration of struggling revealed a significant main effect of genotype (F(1, 31)=4.67, p=0.039), a nonsignificant main effect of rimonabant challenge and a marginally significant genotype–rimonabant challenge interaction (F(1, 31)=3,69, p=0.064). Post hoc comparisons showed that the struggling duration was significantly higher (p=0.038) in vehicle-treated FAAH−/− mice than in vehicle-treated WT controls. The administration of rimonabant attenuated this increase in struggling duration observed in FAAH−/− mice. No significant effects were calculated from swimming duration. Two-way ANOVA on the frequency of behaviors revealed only a marginally significant main effect rimonabant challenge on the frequency of immobility episodes (F(1, 31)=3.37, p=0.076, data not shown); there were no other significant differences obtained from swimming or struggling frequencies.
Figure 3.
Antidepressant-like effects of genetic deletion of FAAH in the forced swim test (FST) and the tail suspension test (TST). (a) In the FST, vehicle-treated FAAH knockouts (FAAH−/− mice, left portion) displayed shorter and longer times in immobility and struggling, respectively, as compared with vehicle-treated wild type (WT) controls (right portion). As with treatment with standard antidepressants, this enhanced activity is predictive of antidepressant effects. Intraperitoneal injection of the CB1 antagonist rimonabant (1 mg/kg) 30 min before the test attenuated this antidepressant-like effect. Bar graphs represent mean total time spent immobile (white), swimming (gray) and struggling (black), expressed in seconds±standard error of mean (SEM) after treatment with vehicle or rimonabant as indicated. *p<0.05, immobility duration of vehicle-treated FAAH−/− mice vs vehicle-treated WT controls; †p<0.05, struggling duration of vehicle-treated FAAH−/− mice vs vehicle-treated WT controls. N=7–10 animals per group. (b) In the TST, FAAH−/− mice spent shorter times immobile as compared with WT controls, regardless of rimonabant administration. Rimonabant (1 mg/kg), intraperitoneally injected 30 min before the test slightly increased immobility. Bar graphs represent mean total time spent immobile expressed in seconds±SEM after treatment with vehicle (unshaded) or rimonabant (shaded). *p<0.05, immobility duration of FAAH−/− mice vs WT controls. N=7–12 animals per group.
Effect of genetic FAAH deletion on stress-coping behavior in the TST
Figure 3b presents the behavioral profile in the TST, comparing differences in immobility and struggling episodes predictive of antidepressant-related activity. Two-way ANOVA on the duration of behaviors showed that FAAH−/− mice, compared with their WT counterparts, exhibited significantly less time being immobile and reciprocally more time in struggling, regardless of rimonabant challenge (main effect of genotype, F(1, 33)=6.54, p=0.015) in the TST. The main effect of rimonabant challenge was marginally significant (F(1, 33)=3.17, p=0.084), although rimonabant, but not the vehicle, appeared to slightly increase the duration of immobility, regardless of genotype. No significant genotype–rimonabant challenge interaction was found. Two-way ANOVA on the frequency of behaviors showed no significant differences in immobility or struggling (data not shown).
Effect of genetic FAAH deletion on weight gain
There was no significant difference between the mean weight of FAAH−/− mice and WT controls (see Supplementary Table 1).
In Vivo Electrophysiology
Effect of genetic FAAH deletion on spontaneous single-spike and burst firing activity of DR 5-HT neurons
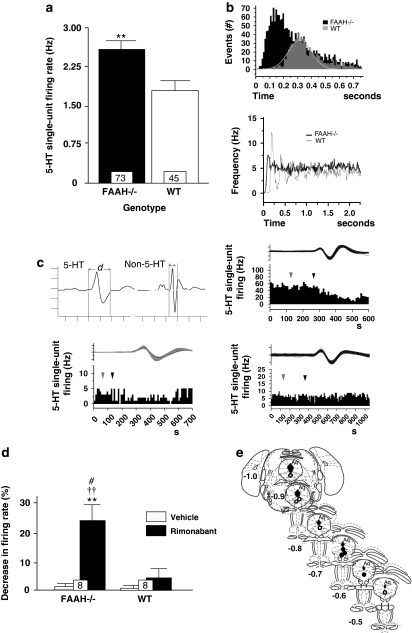
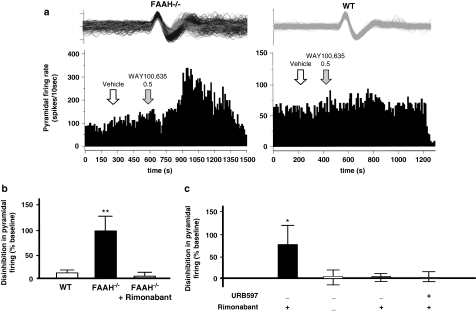
No significant differences between FAAH−/− and WT mice were observed in terms of the burst firing activity of 5-HT neurons in the DR (percentage of spikes in bursts, number of spikes within bursts, and ISI) except for an increase in mean burst length from 3.82±1.29 ms in WT controls to 7.20±0.68 ms in FAAH−/− mice (p=0.048, Mann–Whitney U-test). An inspection of ISI histograms generated from WT controls showed a Gausssian profile (Figure 4b top), and the autocorrelograms of WT single-spike activity indicated clear multiple peaks (Figure 4b bottom); these indicate a prominently regular neural firing among WT controls. By contrast, FAAH−/− mice exhibited positively skewed ISI histograms (Figure 4b, top) and flat autocorrelograms (Figure 4b, bottom), suggesting that the genetic FAAH deletion disrupts the regularity or rhythmicity of firing. In contrast, the mean spontaneous single-spike firing activity recorded from FAAH−/− mice was significantly greater than that of the WT controls (+35%, p=0.008, Mann–Whitney U-test; Figure 4a). k-means clustering aimed at grouping all 5-HT neurons recorded from FAAH−/− mice into two clusters segregated the neural population into fast-spiking neurons with a mean firing rate of 3.66 Hz (n=37) and normal-spiking neurons with a mean firing rate of 1.78 Hz (n=36) (F(1, 71)=138.12, p<0.001). The mean firing rate of WT controls (1.74±0.15 Hz) was significantly different from that of the high-spiking neurons (p<0.01) but not from that of the normal-spiking neurons of FAAH−/− mice. In an attempt to characterize the effect of acute rimonabant administration, the response of 5-HT neurons sampled across the rostrocaudal extent of the DR of FAAH−/− mice and WT controls was monitored. In WT mice, all eight neurons recorded were normal spiking, two of which were moderately inhibited by a challenge of 1 mg/kg rimonabant (22.15–33.33% representative, Figure 4c, lower left) whereas the rest were not responding. From among the responding neurons, higher rimonabant doses tested (>1.5 mg/kg, intraperitoneal) attenuated firing activity until complete inhibition. In FAAH−/− mice, six out of nine neurons recorded were fast spiking and exhibited more profound inhibitory responses (23.99–52.69% representative, Figure 4c, upper right) except for one non-responder, two of the nine were normal spiking and were non-responsive (representative, Figure 4c, lower right), and one of the nine was normal spiking and showed a slight excitatory response (inhibitory, excitatory, non-responders: FAAH−/− mice vs WT control, χ2(2)=6.0, p=0.049). Two-way ANOVA on rimonabant-induced decrease in firing activity from inhibited and non-responsive neurons showed a significant main effect of the rimonabant challenge (F(1, 14)=13.23, p=0.003) and a slight genotype effect (F(1, 13)=3.58, p=0.079), and interaction (F(1, 13)=3.31, p=0.090) (Figure 4d). Administration of vehicle did not elicit a response from both genotypes. In contrast, rimonabant, but not the vehicle, elicited a dramatic decrease in 5-HT neural firing in FAAH−/− mice (p<0.01), which was more profound than rimonabant's effect in WT controls (p<0.05) (Figure 4d, the systemic administration of WAY-100635 potently disinhibited pyramidal activity in FAAH−/− mice fivefold more efficiently than in WT controls (one-way ANOVA, F(2, 9)=72.28, p<0.001; Tukey's test, p<0.001). Systemic administration of the vehicle in either FAAH−/− mice or WT controls did not at all affect pyramidal firing activity. To examine locally possible changes in the sensitivity of hippocampal 5-HT1A and 5-HT2A/2C receptors, iontophoretic ejections of the 5-HT1A agonist 8-OH-DPAT and 5-HT2A/2C agonist DOI were performed while recording from pyramidal neurons. Both these agonists inhibited pyramidal firing, the degree of which was proportional to the amount of current ejected. However, between-genotype pyramidal response did not significantly differ (Figure 6b and c) indicating that the sensitivity of hippocampal 5-HT1A and 5-HT2A/2C receptors was no different in FAAH−/− mice than in WT controls. Figure 6a shows the stereotactic location of recording sites on one coronal section. Similarly, there was no significant difference between the RT50 of 5-HT-induced pyramidal inhibition in FAAH−/− mice and WT controls suggesting that the function of the 5-HT transporter system was not affected by the genetic deletion of FAAH (data not shown). We further ascertained whether the enhanced hippocampal 5-HT1A receptor tonic activity was truly because of increased anandamide-CB1R signaling and not merely to non-specific compensatory mechanisms resulting from a life-long condition of increased anandamide levels. First, we found that rimonabant (1.0 mg/kg, 7 days, once-daily, intraperitoneal) in FAAH−/− mice abrogated the disinhibitory response of pyramidal neurons to WAY-100635 (one-way ANOVA, F(2, 9)=72.28, p<0.001; FAAH−/− vs FAAH−/− plus rimonabant or vs WT, Tukey's test, p<0.001, Figure 5b). Interestingly, chronic (12 days, once daily) treatment with the FAAH inhibitor, URB597, at a dose (0.3 mg/kg, intraperitoneal) shown earlier by Bortolato et al (2007) to exhibit a potent antidepressant-like effect in the mouse chronic unpredictable mild stress model displayed enhanced disinhibitory response of pyramidal neurons to WAY-100635, achieving a significant difference from control (vehicle) levels in response to 1.0 mg/kg WAY-100635 (one-way ANOVA, F(4, 16)=4.44, p=0.013; URB597 vs control (vehicle), Tukey's test, p<0.031, Figure 5c); this effect was similar to that observed in FAAH−/− mice. We then found that similar to that observed in FAAH−/− mice, rimonabant (1.0 mg/kg, 7 days, once daily, intraperitoneal) co-applied with URB597 during the last 7 of the 12-day chronic URB597 regimen blocked the disinihbitory response of pyramidal neurons to WAY-100635 (URB597 vs URB597 plus rimonabant, Tukey's test, p<0.016, Figure 5c).
Figure 4.
Effect of genetic deletion of FAAH on 5-HT neural activity in the DR nucleus. (a) FAAH knockout (FAAH−/−) mice (shaded bar) exhibited significantly elevated mean spontaneous DR 5-HT neural firing activity relative to wild type (WT) control levels (unshaded bar). (b) Representative ISI histograms (top) and autocorrelograms (bottom) generated from a 5-HT neural activity of a FAAH−/− mouse (black bars/black trace) and a WT control (gray bars/gray trace) show profiles indicating irregular firing activity in FAAH−/− mice (skewed ISI profile, flat autocorrelogram) as opposed to the regular or rhythmic firing activity in WT controls (normally distributed ISI, multiple autocorrelogram peaks). (c) Representative integrated 5-HT neural firing rate histograms from a fast-spiking FAAH−/− mouse neuron (upper right), normal-spiking FAAH−/− mouse neuron (lower right) and from WT control neuron (lower left). Systemic injection of rimonabant (1 mg/kg, intraperitoneal, black arrows) was inhibitory to the fast-spiking FAAH−/− mouse neuron, was without effect on the normal-spiking FAAH−/− mouse neuron and slightly attenuative on the WT control neuron; vehicle injection (gray arrows) was without effect in all neurons. Upper left: typical spike characteristics of presumed 5-HT neurons (left) and non-5-HT neurons (right) encountered during electrophysiological recordings from the DR. Ordinate scale unit=1 mV, abscissa scale unit=1 ms, d=spike duration. (d) Intraperitoneal injection of rimonabant (shaded bars) more profoundly decreased mean DR 5-HT neural firing activity in FAAH−/− mice than in WT controls. (e) Postmortem histological verification of electrode descents against the recorded coordinates (based on the brain atlas of Paxinos and Franklin, 2001; numbers indicate millimeter distance of tissue section posterior to the interaural line, Aq=cerebral aqueduct) revelaed that in FAAH−/− mice, 5-HT neurons inhbited by rimonabant (black circles) were localized towards the rostral segment of the DR while the nonresponders (white circles) and slightly stimulated ones (gray circle) were found towards the caudal segment of the DR. Bar graphs in (a) and (d) represent mean values±SEM; the value at the bottom of each bar denotes the number of neurons recorded for that respective group. **p<0.01 vs WT control; ††p<0.01 vs vehicle-treated FAAH−/− mice or vehicle-treated WT control, #p<0.05 vs rimonabant-treated WT control.
Figure 5.
Effects of genetic and pharmacological deactivation of FAAH on postsynaptic 5-HT1A and 5-HT2A/2C receptors. Representative integrated hippocampal CA3 pyramidal firing rate histograms (a) showing that intraperitoneal administration of the 5-HT1A antagonist WAY-100635 (0.5 mg/kg) (shaded arrows in a) but not of vehicle (unshaded arrows in a) potently disinhibited hippocampal pyramidal neural activity in FAAH knockout (FAAH−/−) mice but not wild type (WT) controls. Bar graphs in (b) representing mean percentage of disinhibition (increase)±SEM in the firing of hippocampal CA3 pyramidal neurons in response to WAY-100635; this response in FAAH−/− mice (black bars in b) was significantly greater than in WT controls (white bars in b), indicating enhanced 5-HT tonus on hippocampal 5-HT1A receptors in FAAH−/− mice. This effect was abrogated by rimonabant treatment (1.0 mg/kg, 7 days, once-daily intraperitoneal administrations) (gray bars in b). N=4 neurons per group. **p<0.001 vs response of rimonabant-treated FAAH−/− mice or of WT control. (c) Bar graphs representing mean percentage of disinhibition (increase)±SEM in the firing of hippocampal CA3 pyramidal neurons in response to WAY-100635 (0.5–1.0 mg/kg, intraperitoneal) after chronic URB597 treatment (0.3 mg/kg, intraperitoneal, 12 dyas, once-daily, black bars in c) with or without cotreatment with rimonabant (1.0 mg/kg, intraperitoneal, during the last 7 days of URB597 treatment) in comparison with control (vehicle, white bars) or rimonabant alone (1.0 mg/kg, intraperitoneal, 7 days, once daily, gray bar). Chronic URB597 treatment produced a significant increase in tonic 5-HT1A activity that was prevented by rimonabant. Plus and minus signs below the bars indicate administration or nonadministration, respectively, of the drug indicated. N=4–5 neurons per group. *p<0.05 vs response of URB597 plus rimonabant, of rimonabant alone or of control (vehicle).
Effect of genetic FAAH deletion on prefrontocortical 5-HT1A and 5-HT2A/2C function
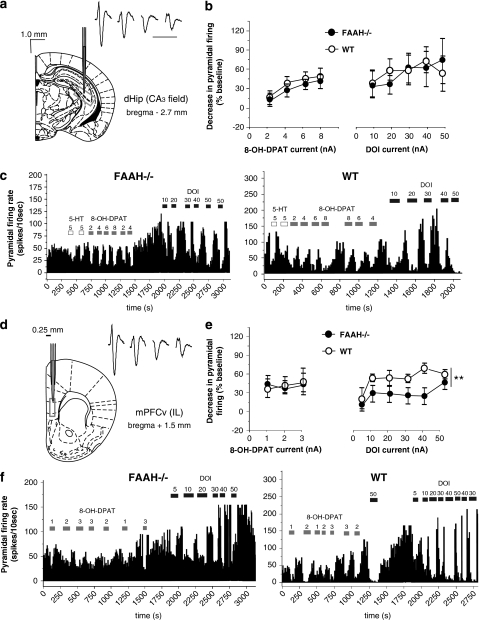
Microiontophoretic ejection of increasing 8-OH-DPAT currents failed to show significant differences in the response of pyramidal neurons in the IL prefrontal cortex (layer 5) of FAAH−/− mice and WT controls (Figure 6e and f). This indicates a lack of difference in the sensitivity of prefrontocortical 5-HT1A receptors of FAAH−/− and WT mice. In contrast, iontophoretic ejection of DOI showed a significant main effect of current (two-way ANOVA, F(5, 32)=4.12, p=0.005) and genotype (F(1, 32)=13.68, p<0.001), but without significant interaction between these two factors (Figure 6e and f). These observations indicate a current-dependent inhibition of prefrontocortical pyramidal neurons through 5-HT2A/2C receptor activation and that genetic FAAH deletion downregulates this 5-HT2A/2C receptor-mediated inhibition. Figure 6d shows the stereotactic location of recording sites on one coronal section.
Figure 6.
Effect of genetic deletion of FAAH on postsynaptic 5-HT1A and 5-HT2A/2C receptors. Stereotactic location of the recording sites in the CA3 field (boxed area, scale bar on the upper left of figure) of the dorsal hippocampus (dHip, a) or the ventromedial prefrontal cortex (mPFCv) (d), infralimbic (IL) region, layer 5 (0.25 mm lateral to midline, boxed area) and a representation of the complex spike waveform of pyramidal neurons (black bar=10 ms duration, scaled). (b) Line graphs showing the lack of difference in the mean decrease (±SEM) in hippocampal CA3 pyramidal neural activity (ordinate) between fatty acid amid hydrolase knockout (FAAH−/−) mouse (black circles) and a wild type (WT) control (white circles) in response to increasing doses of 8-OH-DPAT (abscissa, left panel) and DOI (abscissa, right panel). (c) Representative integrated firing rate histograms showing the activity of hippocampal CA3 pyramidal neurons (ordinate, spikes/10 s) plotted against time (abscissa). There is no difference between a FAAH−/− mouse (left panel) and a WT control (right panel) in the degree of response to microiontophoretic currents (values on top of horizontal bars) of 5-HT (white horizontal bars), the 5-HT1A agonist 8-OH-DPAT (gray horizontal bars) and the 5-HT2A/2C agonist DOI (black horizontal bars), meaning that both 5-HT1A and 5-HT2A/2C receptors remained normosensitive. (e) Line graphs showing between-genotype difference (FAAH−/− mouse: black circles; WT controls: white circles) in the response of mPFCv pyramidal neurons (ordinate, spike/10 s) to increasing microiontophoretic currents of DOI (abscissa, right panel) but not to those of 8-OH-DPAT (abscissa, left panel). **P<0.01. (f) Representative integrated firing rate histograms showing the activity of mPFCv pyramidal neurons (ordinate, spikes/10 s) plotted against time (abscissa). The response to microiontophoretic currents (values on top of horizontal bars) of DOI (back horizontal bars) but not of 8-OH-DPAT (gray hrizontal bars) is significantly blunted in FAAH−/− mouse (left panel) in comparison with a WT control (right panel).
DISCUSSION
This study provides evidence that genetic deletion of FAAH confers resistance to anxiety-like and depression-like behavioral responses, along with an enhancement in the spontaneous activity of DR 5-HT neurons. This phenotype is accompanied by the desensitization of prefrontocortcal 5-HT2A/2C receptors and increased tonic hippocamapal 5-HT1A activity, likely representing postsynaptic adaptations to increased impulse-dependent 5-HT release.
Compared with wild types (WT), FAAH knockout (FAAH−/−) mice were found to be less avoidant of the aversive open arms of the elevated plus maze and the central zone of the open field, and displayed significantly greater exploratory rearings—all indications of antianxiety. They were also more resistant to depression-like behavior in the forced swim and tail suspension tests, indicated by greater time spent in active coping. These observations are consistent with those already made by others in these FAAH−/− mice (Moreira et al, 2008; Naidu et al, 2007), and with results obtained from the pharmacological enhancement of anandamide levels through FAAH inhibitors (eg, URB597) and reuptake blockers (eg, AM404) (Gobbi et al, 2005; Moreira et al, 2008; Naidu et al, 2007; Patel and Hillard, 2006). Interestingly, similar to the effects of URB597 (Haller et al, 2009), the anxiolytic-like and antidepressant-like reactivity of FAAH−/− mice can be effectively evoked under certain laboratory and handling conditions (Moreira et al, 2008; Naidu et al, 2007). This is not surprising as other studies propose that optimization of experimental conditions, such as adjusting anxiogenic testing conditions in tests for anxiety-like reactivity (eg, the elevated plus maze) (Hogg, 1996) and behavioral despair tests (eg, the forced swim test) (Kelliher et al, 2000) more potently unmasks or enhances the sensitivity to even the conventionally used anxiolytics and antidepressants. Under highly anxiogenic conditions (eg, high-lighting) in tests for anxiety-related behavior and under less anxiogenic conditions (eg, dim-lighting) in behavioral despair tests, we were able to elicit potent genotype-dependent responses. All the anxiolytic-like responses elicited here were completely reversed by rimonabant, and are therefore mediated by an increased anandamide-CB1R signaling in FAAH−/− mice. Indeed, these mice have been reported to express 10-fold higher levels of intrinsic anandamide than those of WTs, without significant changes in the density of cerebral CB1R (Basavarajappa et al, 2006; Cravatt et al, 2001). Furthermore, CB1R appears to participate in a tonic inhibitory control of anxiety, evidenced by the rimonabant-induced reversal of responses of FAAH−/− mice below WT levels. In further support of this hypothesis, we also noted that rimonabant produced a slight anxiogenic-like response when administered to WTs, corroborating earlier reports of its anxiogenic-like effects in the defensive withdrawal test and elevated plus maze (Navarro et al, 1997). It is also possible that the anxiety-like response to rimonabant expressed by both genotypes observed here, which was particularly exaggerated in the novelty-suppressed feeding test is due to the interaction of the antagonism on tonic inhibition of anxiety and rimonabant's inverse agonistic property. This role on the tonic inhibitory control of anxiety is consistent with observations reported earlier that stress and anxiogenic stimuli lead to an increase in the hydrolytic activity of FAAH and a reduction in forebrain anandamide levels (Patel et al, 2005; Hill et al, 2009b). The attenuation of anandamide signaling would gate physiological responses associated with anxiety, including the activation of the hypothalamic-pituitary-adrenal axis and increased corticosterone production (for review, see Hill and McEwen (2009)). Under conditions of stable FAAH inactivity, as in FAAH−/− mice, the resultant elevation in anandamide tone would buffer stress-related responses; the behavioral impact of this buffering should be most evident under more aversive or anxiety-provoking conditions. The recruitment specifically of the CB1R by anandamide in this tonic regulation of anxiety-related processes is further supported by the fact that transgenic mice deficient in CB1R exhibit increased anxiety-like behavior in the light/dark test (Martin et al, 2002; Uriguen et al, 2004), elevated plus maze and social interaction test that persists after treatment with an otherwise effective dose of standard anxioytics (Uriguen et al, 2004). These CB1R knockout mice also show exaggerated increases in plasma corticosterone in response to restraint stress (Uriguen et al, 2004). In the forced swim and tail suspension tests, rimonabant also attenuated the antidepressant-like phenotype of FAAH−/− mice, convergent with that observed with URB597 in rats (Gobbi et al, 2005), implicating the involvement of the CB1R mediating the antidepressant-like response in FAAH−/− mice. However, caution must be taken in interpreting these data as under the testing conditions adopted here, FAAH−/− mice also exhibited moderate locomotor stimulation, possibly confounding results in these tests. Nevertheless, our electrophysiological experiments revealed a significantly elevated mean DR 5-HT neural activity in FAAH−/− mice that could provide the neurochemical substrate by which anxiolytic-like and antidepressant-like reactivity may be conveyed. Such an impact on 5-HT activity has been observed earlier with URB597 (Gobbi et al, 2005) and a low dose of the CB1R agonist, WIN55,212-2 (Bambico et al, 2007), and is consistent with the reported augmentation of the postsynaptic 5-HT content by cannabinoid agonists (Miczek and Dixit, 1980; Holtzman et al, 1969; Sagredo et al, 2006) or URB597 (Gobbi et al, 2005). This increase in 5-HT neural activity has been reliably replicated in other transgenic models of depression and antidepressant activity. For example, TREK-1 background potassium channel (Heurteaux et al, 2006) and NK1 receptor (Santarelli et al, 2001) null-mutant mice have been shown to exhibit a similar augmentation in spontaneous DR 5-HT neural firing associated with antidepressant-like behavior. Standard 5-HT-acting antidepressants, such as SSRI and monoaminoxidase inhibitors, initially suppress DR 5-HT neural firing but gradually enhance it thereafter, coinciding with the desensitization of 5-HT1A autoreceptors. This late 5-HT-increasing phase is linked to the onset of antidepressant effect (Bambico and Gobbi, 2008). Other new-generation antidepressants purportedly possessing faster onset of therapeutic action, such as reboxetine (Linner et al, 2004) and mirtazapine (Haddjeri et al, 1996), and other novel antidepressant treatments, such as NK-1 antagonists (Haddjeri and Blier, 2001) and vagus nerve stimulation (Dorr and Debonnel, 2006), also increase DR 5-HT neural firing. In contrast, 5-HT4 receptor (Conductier et al, 2006) and 5-HT transporter (Gobbi et al, 2001) null-mutant mice, and neonatal clomipramine treatment (Kinney et al, 1997), drug withdrawal (Pistis et al, 1997), and high-frequency subthalamic stimulation (Temel et al, 2007) have all been shown to be associated with decrements in spontaneous DR 5-HT firing and enhanced depression-like behaviors. Recently, genetic CB1R deletion and prolonged rimonabant treatment in mice have been shown to result in increased DR 5-HT neural firing ex vivo, instigated by impaired 5-HT1A autoreceptor negative feedback. As these mice simultaneously display enhanced depression-like behavior, this increase in 5-HT firing is interpreted to represent an adaptive response to depression-related neurobiological sequelae (Aso et al, 2009).
From our data, it is important to note the presence of fast-spiking and normal-spiking subsets of 5-HT neurons recorded along the midline axis of the DR in FAAH−/− mice. Interestingly, fast-spiking 5-HT neurons appear to be localized in the rostral DR subdivisions and are profoundly inhibited by a challenge of rimonabant, whereas normal-spiking neurons appear to be localized in the caudal regions and are unresponsive to rimonabant. This topographical pattern of reactivity to rimonabant is consistent with the presence of CB1Rs mainly on 5-HT neural terminals in the caudal DR, and on many non-5-HT, likely GABAergic interneurons in the rostral DR (Haring et al, 2007) that synapse with and impose tonic inhibition on neighboring 5-HT neurons. In these FAAH−/− mice, an increase in intrinsic anandamide leads to CB1R-mediated attenuations of GABAergic interneuronal activity and/or GABA efflux and a consequent disinhibition of 5-HT neurons. Rimonabant may conceivably reverse this disinhibition of 5-HT neurons by competing with anandamide on interneuronal CB1Rs. Alternatively, 5-HT-modulating CB1Rs may also be localized in the ventromedial prefrontal cortex (Bambico et al, 2007) or on glutamatergic terminals (Haj-Dahmane and Shen, 2009) that synapse with GABAergic interneurons or 5-HT neurons in the DR (Celada et al, 2001). The existence of this segregable 5-HT neural subpopulations may partly explain the region-selective alterations in postsynaptic 5-HT basal levels and evoked efflux we reported elsewhere (Bambico et al, 2009a).
Prefrontocortical pyramidal responses in FAAH−/− mice to microiontophoretically ejected agonists at 5-HT2A/2C and 5-HT1A receptors, DOI and 8-OH-DPAT respectively, showed normosensitive 5-HT1A receptors but subnormal 5-HT2A/2C receptor sensitivity. This 5-HT2A/2C receptor desensitization corroborates reported attenuations in 5-HT2A binding, 5-HT2A-associated signal transduction and associated behavioral responses (for review, see Hill et al (2009a)). Chronic treatment with antidepressants, such as SSRIs, that potentiate 5-HT efflux in the prefrontal cortex can similarly lead to a downregulation of 5-HT2A/2C receptors in this region (Hill et al, 2009a; Hollander et al, 1991b; Kennett et al, 1994b; Quested et al, 1997). This downregulation has been associated with the anxiolytic efficacy of these drugs, as the antagonism of these receptors possesses identical therapeutic effects (Deakin, 1988; Griebel et al, 1997; Adamec et al, 2004) or augments the effects of antidepressants (Marek et al, 2003; Hill et al, 2009a), whereas agonism produces panic and anxiety in otherwise healthy humans (Germine et al, 1994). Furthermore, prolonged corticosterone exposure results in an enhancement of frontocortical 5-HT2-related functions, an effect that was found to be reversed by chronic antidepressant treatment (Zahorodna and Hess, 2006). In contrast, hippocampal CA3 pyramidal neurons of FAAH−/− mice exhibited supranormal responses to the disinhibitory effects of systemically injected 5-HT1A antagonist WAY-100635, indicating an increase in the tonus on hippocampal 5-HT1A receptors in FAAH−/− mice, which were similarly observed after chronic URB597 treatment. The similarity of effects of genetic and pharmacological FAAH deactivation and the fact that both were blocked by prolonged co-application with rimonabant strengthen the notion that postsynaptic 5-HT receptor adaptations are due to the common mechanism of increased anandamide-CB1R signaling, and that the effects observed in FAAH−/− mice are not merely because of non-specific compensatory mechanisms resulting from a life-long condition of increased anandamide levels. These data are reminiscent of the hallmark enhancement in hippocampal 5-HT1A tonus that follows long-term administration of different classes of antidepressant treatments, including electroconvulsive shock (Haddjeri et al, 1998; Besson et al, 2000; Szabo and Blier, 2001b). This similar observation made in FAAH−/− mice may be explained by the increase in the depolarization-induced efflux of 5-HT in this structure, which has been shown in this transgenic mouse (Bambico et al, 2009a). We could also consider the possible involvement of an augmentation of noradrenergic neurotransmission, which has been reported to be associated with CB1R activation and FAAH inhibition (for review, see Bambico et al, 2009a). Furthermore, as enhanced hippocampal 5-HT1A function has been linked to the induction of cell proliferation and neurogenesis in the dentate gyrus (Jacobs et al, 2000) such as that shown with fluoxetine (Santarelli et al, 2003), this increase in tonic hippocampal 5-HT1A activity in FAAH−/− mice may contribute, at least in part, to the enhanced hippocampal neurogenic or proliferative processes observed in these mice (Aguado et al, 2005, 2006).
Although these data altogether highlight the role of increased anandamide-CB1R signaling in the anxiolytic-like and antidepressant-like phenotype of FAAH−/− mice, we cannot completely rule out the potential involvement of other neurobiological factors that are found to be similarly altered in these mice. For instance, FAAH inactivation has been documented to elicit hormonal changes and increased production of neurosteroids or other lipidic molecules, such as palmithoylethanolamide and oleoylethanolamide (Gobbi et al, 2005), whose behavioral functions are less explored, and whose interactions with the monoaminergic systems remain largely unknown. Nevertheless, the findings at hand strengthen the notion that FAAH is a potential pharmacological and genetic target for the development of treatment strategies for depression and anxiety. Through this approach, the interaction of the endocannabinoid and monoaminergic systems may be harnessed to modulate 5-HT neurotransmission. The resultant prefrontocortical 5-HT2A/2C desensitization and hippocampal 5-HT1A functional upregulation starkly contrast with 5-HT2A/2C upregulation and 5-HT1A downregulation observed after long-term treatment with direct CB1R agonists (Cheer et al, 1999; Hill et al, 2006), which are contrariwise asssociated with anxiogenic-like or depressogenic-like sequelae (Maes and Meltzer, 1995), further arguing for the favorability of indirect CB1R agonism through FAAH inactivation over direct CB1R agonism.
Acknowledgments
This work was supported by grants from the Fonds de la Recherche en Santé du Québec (GG), the Canadian Psychiatric Research Foundation (GG), National Institute on Drug Abuse (grant DA12413) (DP) and fellowships from the McGill University Health Center, McGill University Faculty of Medicine and FRSQ (FRB) and the Mexican Council for Science and Technology (SD). We would like to extend our gratitude to Jean-Philippe Garant for assistance in running a part of the data analyses and to Nhu-Tram Nguyen for assistance in running a part of the behavioural experiment. We also would like to acknowledge Mr Herculano Santos and Mr. Normand Champagne for their technical assistance in the handling and maintenance of the animals during the course of the experiments.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Adamec R, Creamer K, Bartoszyk GD, Burton P. Prophylactic and therapeutic effects of acute systemic injections of EMD 281014, a selective serotonin 2A receptor antagonist on anxiety induced by predator stress in rats. Eur J Pharmacol. 2004;504:79–96. doi: 10.1016/j.ejphar.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Aghajanian GK, Wang RY, Baraban J. Serotonergic and non-serotonergic neurons of dorsal raphe—reciprocal changes in firing induced by peripheral-nerve stimulation. Brain Res. 1978;153:169–175. doi: 10.1016/0006-8993(78)91140-x. [DOI] [PubMed] [Google Scholar]
- Aguado T, Monory K, Palazuelos J, Stella N, Cravatt B, Lutz B, et al. The endocannabinoid system drives neural progenitor proliferation. FASEB J. 2005;19:1704–1706. doi: 10.1096/fj.05-3995fje. [DOI] [PubMed] [Google Scholar]
- Aguado T, Palazuelos J, Monory K, Stella N, Cravatt B, Lutz B, et al. The endocannabinoid system promotes astroglial differentiation by acting on neural progenitor cells. J Neurosci. 2006;26:1551–1561. doi: 10.1523/JNEUROSCI.3101-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allers KA, Sharp T. Neurochemical and anatomical identification of fast- and slow-firing neurones in the rat dorsal raphe nucleus using juxtacellular labelling methods in vivo. Neuroscience. 2003;122:193–204. doi: 10.1016/s0306-4522(03)00518-9. [DOI] [PubMed] [Google Scholar]
- Archer J. Tests for emotionality in rats and mice—review. Anim Behav. 1973;21:205–235. doi: 10.1016/s0003-3472(73)80065-x. [DOI] [PubMed] [Google Scholar]
- Ashby CR, Jiang LH, Kasser RJ, Wang RY. Electrophysiological characterization of 5-hydroxytryptamine-2 receptors in the rat medial prefrontal cortex. J Pharmacol Exp Ther. 1990;252:171–178. [PubMed] [Google Scholar]
- Aso E, Renoir T, Mengod G, Ledent C, Hamon M, Maldonado R, et al. Lack of CB1 receptor activity impairs serotonergic negative feedback. J Neurochem. 2009;109:935–944. doi: 10.1111/j.1471-4159.2009.06025.x. [DOI] [PubMed] [Google Scholar]
- Bambico FR, Duranti A, Tontini A, Tarzia G, Gobbi G. Endocannabinoids in the treatment of mood disorders: evidence from animal models. Curr Pharm Des. 2009a;15:1623–1646. doi: 10.2174/138161209788168029. [DOI] [PubMed] [Google Scholar]
- Bambico FR, Gobbi G. The cannabinoid CB1 receptor and the endocannabinoid anandamide: possible antidepressant targets. Expert Opin Ther Targets. 2008;12:1347–1366. doi: 10.1517/14728222.12.11.1347. [DOI] [PubMed] [Google Scholar]
- Bambico FR, Katz N, Debonnel G, Gobbi G. Cannabinoids elicit antidepressant-like behavior and activate serotonergic neurons through the medial prefrontal cortex. J Neurosci. 2007;27:11700–11711. doi: 10.1523/JNEUROSCI.1636-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bambico FR, Nguyen NT, Gobbi G. Decline in serotonergic firing activity and desensitization of 5-HT1A autoreceptors after chronic unpredictable stress. Eur Neuropsychopharmacol. 2009b;19:215–228. doi: 10.1016/j.euroneuro.2008.11.005. [DOI] [PubMed] [Google Scholar]
- Bambico FR, Nguyen NT, Katz N, Gobbi G. Chronic exposure to cannabinoids during adolescence but not during adulthood impairs emotional behaviour and monoaminergic neurotransmission. Neurobiol Dis. 2010;37:641–655. doi: 10.1016/j.nbd.2009.11.020. [DOI] [PubMed] [Google Scholar]
- Baraban JM, Aghajanian GK. Suppression of serotonergic neuronal firing by alpha-adrenoceptor antagonists—evidence against GAHA mediation. Eur J Pharmacol. 1980;66:287–294. doi: 10.1016/0014-2999(80)90461-6. [DOI] [PubMed] [Google Scholar]
- Bartho P, Hirase H, Monconduit L, Zugaro M, Harris KD, Buzsaki G. Characterization of neocortical principal cells and Interneurons by network interactions and extracellular features. J Neurophysiol. 2004;92:600–608. doi: 10.1152/jn.01170.2003. [DOI] [PubMed] [Google Scholar]
- Basavarajappa BS, Yalamanchili R, Cravatt BF, Cooper TB, Hungund BL. Increased ethanol consumption and preference and decreased ethanol sensitivity in female FAAH knockout mice. Neuropharmacology. 2006;50:834–844. doi: 10.1016/j.neuropharm.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Besson A, Haddjeri N, Blier P, de Montigny C. Effects of the co-administration of mirtazapine and paroxetine on serotonergic neurotransmission in the rat brain. Eur Neuropsychopharmacol. 2000;10:177–188. doi: 10.1016/s0924-977x(00)00069-9. [DOI] [PubMed] [Google Scholar]
- Blokland A, Lieben C, Deutz NEP. Anxiogenic and depressive-like effects, but no cognitive deficits, after repeated moderate tryptophan depletion in the rat. J Psychopharmacol. 2002;16:39–49. doi: 10.1177/026988110201600112. [DOI] [PubMed] [Google Scholar]
- Bodnoff SR, Suranyicadotte B, Aitken DH, Quirion R, Meaney MJ. The effects of chronic antidepressant treatment in an animal-model of anxiety. Psychopharmacology. 1988;95:298–302. doi: 10.1007/BF00181937. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Mangieri RA, Fu J, Kim JH, Arguello O, Duranti A, et al. Antidepressant-like activity of the fatty acid amide hydrolase inhibitor URB597 in a rat model of chronic mild stress. Biol Psychiatry. 2007;62:1103–1110. doi: 10.1016/j.biopsych.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Celada P, Puig MV, Casanovas JM, Guillazo G, Artigas F. Control of dorsal raphe serotonergic neurons by the medial prefrontal cortex: involvement of serotonin-1A, GABA(A), and glutamate receptors. J Neurosci. 2001;21:9917–9929. doi: 10.1523/JNEUROSCI.21-24-09917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheer JF, Cadogan AK, Marsden CA, Fone KCF, Kendall DA. Modification of 5-HT2 receptor mediated behaviour in the rat by oleamide and the role of cannabinoid receptors. Neuropharmacology. 1999;38:533–541. doi: 10.1016/s0028-3908(98)00208-1. [DOI] [PubMed] [Google Scholar]
- Choleris E, Thomas AW, Kavaliers M, Prato FS. A detailed ethological analysis of the mouse open field test: effects of diazepam, chlordiazepoxide and an extremely low frequency pulsed magnetic field. Neurosci Biobehav Rev. 2001;25:235–260. doi: 10.1016/s0149-7634(01)00011-2. [DOI] [PubMed] [Google Scholar]
- Cole JC, Rodgers RJ. Ethological comparison of the effects of diazepam and acute chronic imipramine on the behavior of mice in the elevated plus-maze. Pharmacol Biochem Behav. 1995;52:473–478. doi: 10.1016/0091-3057(95)00163-q. [DOI] [PubMed] [Google Scholar]
- Conductier G, Dusticier N, Lucas G, Cote F, Debonnel G, Daszuta A, et al. Adaptive changes in serotonin neurons of the raphe nuclei in 5-HT4 receptor knock-out mouse. Eur J Neurosci. 2006;24:1053–1062. doi: 10.1111/j.1460-9568.2006.04943.x. [DOI] [PubMed] [Google Scholar]
- Cravatt BF, Demarest K, Patricelli MP, Bracey MH, Giang DK, Martin BR, et al. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc Natl Acad Sci USA. 2001;98:9371–9376. doi: 10.1073/pnas.161191698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlstrom A, Fuxe K. Evidence for existence of monoamine-containing neurons in central nervous system. I. Demonstration of monoamines in cell bodies of brain stem neurons. Acta Physiol Scand. 1964. p. 1.p. 55. [PubMed]
- Deakin JFW. 5HT2 receptors, depression and anxiety. Pharmacol Biochem Behav. 1988;29:819–820. doi: 10.1016/0091-3057(88)90215-8. [DOI] [PubMed] [Google Scholar]
- Descarries L, Watkins KC, Garcia S, Beaudet A. The serotonin neurons in nucleus raphe dorsalis of adult-rat—a light and electron-microscope autoradiographic study. J Comp Neurol. 1982;207:239–254. doi: 10.1002/cne.902070305. [DOI] [PubMed] [Google Scholar]
- Dorr AE, Debonnel G. Effect of vagus nerve stimulation on serotonergic and noradrenergic transmission. J Pharmacol Exp Ther. 2006;318:890–898. doi: 10.1124/jpet.106.104166. [DOI] [PubMed] [Google Scholar]
- Egertova M, Giang DK, Cravatt BF, Elphick MR. A new perspective on cannabinoid signalling: complementary localization of fatty acid amide hydrolase and the CB1 receptor in rat brain. Proc Roy Soc Lon B-Biol Sci. 1998;265:2081–2085. doi: 10.1098/rspb.1998.0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ElMansari M, Blier P. In vivo electrophysiological characterization of 5-HT receptors in the guinea pig head of caudate nucleus and orbitofrontal cortex. Neuropharmacology. 1997;36:577–588. doi: 10.1016/s0028-3908(97)00035-x. [DOI] [PubMed] [Google Scholar]
- Germine M, Goddard AW, Sholomskas DE, Woods SW, Charney DS, Heninger GR. Response to meta-chlorophenylpiperazine in panic disorder patients and healthy-subjects—influence of reduction in intravenous dosage. Psychiatry Res. 1994;54:115–133. doi: 10.1016/0165-1781(94)90001-9. [DOI] [PubMed] [Google Scholar]
- Gobbi G, Bambico FR, Mangieri R, Bortolato M, Campolongo P, Solinas M, et al. Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc Natl Acad Sci USA. 2005;102:18620–18625. doi: 10.1073/pnas.0509591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbi G, Janiri L. Sodium- and magnesium-valproate in vivo modulate glutamatergic and GABAergic synapses in the medial prefrontal cortex. Psychopharmacology. 2006;185:255–262. doi: 10.1007/s00213-006-0317-3. [DOI] [PubMed] [Google Scholar]
- Gobbi G, Murphy DL, Lesch KP, Blier P. Modifications of the serotonergic system in mice lacking serotonin transporters: an in vivo electrophysiological study. J Pharmacol Exp Ther. 2001;296:987–995. [PubMed] [Google Scholar]
- Griebel G, Rodgers RJ, Perrault G, Sanger DJ. Risk assessment behaviour: evaluation of utility in the study of 5-HT-related drugs in the rat elevated plus-maze test. Pharmacol Biochem Behav. 1997;57:817–827. doi: 10.1016/s0091-3057(96)00402-9. [DOI] [PubMed] [Google Scholar]
- Gross C, Santarelli L, Brunner D, Zhuang XX, Hen R. Altered fear circuits in 5-HT1A receptor KO mice. Biol Psychiatry. 2000;48:1157–1163. doi: 10.1016/s0006-3223(00)01041-6. [DOI] [PubMed] [Google Scholar]
- Haddjeri N, Blier P. Sustained blockade of neurokinin-1 receptors enhances serotonin neurotransmission. Biol Psychiatry. 2001;50:191–199. doi: 10.1016/s0006-3223(01)01162-3. [DOI] [PubMed] [Google Scholar]
- Haddjeri N, Blier P, de Montigny C. Long-term antidepressant treatments result in a tonic activation of forebrain 5-HT1A receptors. J Neurosci. 1998;18:10150–10156. doi: 10.1523/JNEUROSCI.18-23-10150.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddjeri N, Blier P, deMontigny C. Effect of the alpha-2 adrenoceptor antagonist mirtazapine on the 5-hydroxytryptamine system in the rat brain. J Pharmacol Exp Ther. 1996;277:861–871. [PubMed] [Google Scholar]
- Haj-Dahmane S, Shen RY. Endocannabinoids suppress excitatory synaptic transmission to Dorsal Raphe serotonin neurons through the activation of presynaptic CB1 receptors. J Pharmacol Exp Ther. 2009;331:186–196. doi: 10.1124/jpet.109.153858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller J, Barna I, Barsvari B, Pelczer KG, Yasar S, Panlilio LV, et al. Interactions between environmental aversiveness and the anxiolytic effects of enhanced cannabinoid signaling by FAAH inhibition in rats. Psychopharmacology. 2009;204:607–616. doi: 10.1007/s00213-009-1494-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haring M, Marsicano G, Lutz B, Monory K. Identification of the cannabinoid receptor type 1 in serotonergic cells of raphe nuclei in mice. Neuroscience. 2007;146:1212–1219. doi: 10.1016/j.neuroscience.2007.02.021. [DOI] [PubMed] [Google Scholar]
- Heurteaux C, Lucas G, Guy N, El Yacoubi M, Thummler S, Peng XD, et al. Deletion of the background potassium channel TREK-1 results in a depression-resistant phenotype. Nat Neurosci. 2006;9:1134–1141. doi: 10.1038/nn1749. [DOI] [PubMed] [Google Scholar]
- Hill MN, Gorzalka BB. Pharmacological enhancement of cannabinoid CB1 receptor activity elicits an antidepressant-like response in the rat forced swim test. Eur Neuropsychopharmacol. 2005;15:593–599. doi: 10.1016/j.euroneuro.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Hill MN, Hillard CJ, Bambico FR, Patel S, Gorzalka BB, Gobbi G. The therapeutic potential of the endocannabinoid system for the development of a novel class of antidepressants. Trends in Pharmacol Sci. 2009a;30:484–493. doi: 10.1016/j.tips.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Hill MN, McEwen BS.2009Involvement of the endocannabinoid system in the neurobehavioural effects of stress and glucocorticoids Prog Neuropsychopharmacol Biol PsychiatryE-pub ahead of print 10 November 2009). [DOI] [PMC free article] [PubMed]
- Hill MN, McLaughlin RJ, Morrish AC, Viau V, Floresco SB, Hillard CJ, et al. Suppression of amygdalar endocannabinoid signaling by stress contributes to activation of the hypothalamic-pituitary-adrenal axis. Neuropsychopharmacology. 2009b;34:2733–2745. doi: 10.1038/npp.2009.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Sun JC, Tse MTL, Gorzalka BB. Altered responsiveness of serotonin receptor subtypes following long-term cannabinoid treatment. Int J Neuropsychopharmacol. 2006;9:277–286. doi: 10.1017/S1461145705005651. [DOI] [PubMed] [Google Scholar]
- Hogg S. A review of the validity and variability of the elevated plus-maze as an animal model of anxiety. Pharmacol Bioch Behav. 1996;54:21–30. doi: 10.1016/0091-3057(95)02126-4. [DOI] [PubMed] [Google Scholar]
- Hollander E, Decaria C, Gully R, Nitescu A, Suckow RF, Gorman JM, et al. Effects of chronic fluoxetine treatment on behavioral and neuroendocrine responses to meta-chlorophenylpiperazine in obsessive-compulsive disorder. Psychiatry Res. 1991a;36:1–17. doi: 10.1016/0165-1781(91)90113-4. [DOI] [PubMed] [Google Scholar]
- Hollander E, Decaria C, Gully R, Nitescu A, Suckow RF, Gorman JM, et al. Effects of chronic fluoxetine treatment on behavioral and neuroendocrine responses to meta-chlorophenylpiperazine in obsessive-compulsive disorder. Psychiatry Res. 1991b;36:1–17. doi: 10.1016/0165-1781(91)90113-4. [DOI] [PubMed] [Google Scholar]
- Holmes A. Genetic variation in cortico-amygdala serotonin function and risk for stress-related disease. Neurosci Biobehav Rev. 2008;32:1293–1314. doi: 10.1016/j.neubiorev.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman D, Lovell RA, Jaffe JH, Freedman DX. 1-delta9-tetrahydrocannabinol—neurochemical and behavioral effects in mouse. Science. 1969;163:1464–1467. doi: 10.1126/science.163.3874.1464. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, van Praag H, Gage FH. Adult brain neurogenesis and psychiatry: a novel theory of depression. Mol Psychiatry. 2000;5:262–269. doi: 10.1038/sj.mp.4000712. [DOI] [PubMed] [Google Scholar]
- Johnson KM, Dewey WL, Harris LS. Delta-9-ThC induced elevations of mouse-brain tryptophan and consequent increased serotonin production. Pharmacologist. 1976;18:166. [Google Scholar]
- Kandel ER, Spencer WA. Electrophysiological properties of an archicortical neuron. Ann NY Acad Sci. 1961;94:570–603. doi: 10.1111/j.1749-6632.1961.tb35560.x. [DOI] [PubMed] [Google Scholar]
- Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A, et al. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- Kelliher P, Connor TJ, Harkin A, Sanchez C, Kelly JP, Leonard BE. Varying responses to the rat forced-swim test under diurnal and nocturnal conditions. Physiol Behav. 2000;69:531–539. doi: 10.1016/s0031-9384(00)00213-4. [DOI] [PubMed] [Google Scholar]
- Kennett GA, Lightowler S, Debiasi V, Stevens NC, Wood MD, Tulloch IF, et al. Effect of chronic administration of selective 5-hydroxytryptamine and noradrenaline uptake inhibitors on a putative index of 5-HT2C/2B receptor function. Neuropharmacology. 1994a;33:1581–1588. doi: 10.1016/0028-3908(94)90133-3. [DOI] [PubMed] [Google Scholar]
- Kennett GA, Lightowler S, Murphy O, Debiasi V, Stevens N, Tulloch LF, et al. Chronic treatment with paroxetine and fluoxetine, but not desipramine, desensitizes 5-HT2C receptor function. Br J Pharmacol. 1994b;112:U109. [Google Scholar]
- Kennett GA, Pittaway K, Blackburn TP. Evidence that 5-HT2C receptor antagonists are anxiolytic in the rat Geller-Seifter model of anxiety. Psychopharmacology. 1994c;114:90–96. doi: 10.1007/BF02245448. [DOI] [PubMed] [Google Scholar]
- Kinney GG, Vogel GW, Feng PF. Decreased dorsal raphe nucleus neuronal activity in adult chloral hydrate anesthetized rats following neonatal clomipramine treatment: Implications for endogenous depression. Brain Res. 1997;756:68–75. doi: 10.1016/s0006-8993(97)00119-4. [DOI] [PubMed] [Google Scholar]
- Labonte B, Bambico FR, Gobbi G. Potentiation of excitatory serotonergic responses by MK-801 in the medial prefrontal cortex. Naunyn-Schmiedebergs Arch Pharmacol. 2009;380:383–397. doi: 10.1007/s00210-009-0446-4. [DOI] [PubMed] [Google Scholar]
- Linner L, Wiker C, Arborelius L, Schalling M, Svensson TH. Selective noradrenaline reuptake inhibition enhances serotonergic neuronal activity and transmitter release in the rat forebrain. J Neural Transm. 2004;111:127–139. doi: 10.1007/s00702-003-0084-9. [DOI] [PubMed] [Google Scholar]
- Maes M, Meltzer HY.1995The serotonin hypothesis of major depressionIn: Bloom F, Kupfer D (eds). Psychopharmacology: the fourth generation of progress Raven Press: New York; 933–944. [Google Scholar]
- Marek GJ, Carpenter LL, McDougle CJ, Price H. Synergistic action of 5-HT2A antagonists and selective serotonin reuptake inhibitors in neuropsychiatric disorders. Neuropsychoparmacology. 2003;28:402–412. doi: 10.1038/sj.npp.1300057. [DOI] [PubMed] [Google Scholar]
- Martin M, Ledent C, Parmentier M, Maldonado R, Valverde O. Involvement of CB1 cannabinoid receptors in emotional behaviour. Psychopharmacology. 2002;159:379–387. doi: 10.1007/s00213-001-0946-5. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Dixit BN. Behavioral and biochemical effects of chronic delta-9-tetrahydrocannabinol in rats. Psychopharmacology. 1980;67:195–202. doi: 10.1007/BF00431977. [DOI] [PubMed] [Google Scholar]
- Miller HEJ, Deakin JFW, Anderson IM. Effect of acute tryptophan depletion on CO2-induced anxiety in patients with panic disorder and normal volunteers. Br J Psychiatry. 2000;176:182–188. doi: 10.1192/bjp.176.2.182. [DOI] [PubMed] [Google Scholar]
- Moldrich G, Wenger T. Localization of the CB1 cannabinoid receptor in the rat brain. An immunohistochemical study. Peptides. 2000;21:1735–1742. doi: 10.1016/s0196-9781(00)00324-7. [DOI] [PubMed] [Google Scholar]
- Moranta D, Esteban S, Garcia-Sevilla JA. Differential effects of acute cannabinoid drug treatment, mediated by CB1 receptors, on the in vivo activity of tyrosine and tryptophan hydroxylase in the rat brain. Naunyn-Schmiedebergs Arch Pharmacol. 2004;369:516–524. doi: 10.1007/s00210-004-0921-x. [DOI] [PubMed] [Google Scholar]
- Moreira FA, Kaiser N, Monory K, Lutz B. Reduced anxiety-like behaviour induced by genetic and pharmacological inhibition of the endocannabinoid-degrading enzyme fatty acid amide hydrolase (FAAH) is mediated by CB1 receptors. Neuropharmacology. 2008;54:141–150. doi: 10.1016/j.neuropharm.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Naidu PS, Varvel SA, Ahn K, Cravatt BF, Martin BR, Lichtman AH. Evaluation of fatty acid amide hydrolase inhibition in murine models of emotionality. Psychopharmacology. 2007;192:61–70. doi: 10.1007/s00213-006-0689-4. [DOI] [PubMed] [Google Scholar]
- Navarro M, Hernandez E, Munoz RM, delArco I, Villanua MA, Carrera MRA, et al. Acute administration of the CB1 cannabinoid receptor antagonist SR141716A induces anxiety-like responses in the rat. Neuroreport. 1997;8:491–496. doi: 10.1097/00001756-199701200-00023. [DOI] [PubMed] [Google Scholar]
- Ohayon MM. Anxiety disorders: prevalence, comorbidity and outcomes. J Psychiatric Res. 2006;40:475–476. doi: 10.1016/j.jpsychires.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Patel S, Hillard CJ. Pharmacological evaluation of cannabinoid receptor ligands in a mouse model of anxiety: further evidence for an anxiolytic role for endogenous cannabinoid signaling. J Pharmacol Exp Ther. 2006;318:304–311. doi: 10.1124/jpet.106.101287. [DOI] [PubMed] [Google Scholar]
- Patel S, Roelke CT, Rademacher DJ, Hillard CJ. Inhibition of restraint stress-induced neural and behavioural activation by endogenous cannabinoid signalling. Eur J Neurosci. 2005;21:1057–1069. doi: 10.1111/j.1460-9568.2005.03916.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates (Ed 2) Academic: London; 2001. [Google Scholar]
- Pellow S, File SE. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze—a novel test of anxiety in the rat. Pharmacol Biochem Beh. 1986;24:525–529. doi: 10.1016/0091-3057(86)90552-6. [DOI] [PubMed] [Google Scholar]
- Pineyro G, Blier P, Dennis T, deMontigny C. Desensitization of the neuronal 5-HT carrier following its long-term blockade. J Neurosci. 1994;14:3036–3047. doi: 10.1523/JNEUROSCI.14-05-03036.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistis M, Muntoni AL, Gessa G, Diana M. Effects of acute, chronic ethanol and withdrawal on dorsal raphe neurons: electrophysiological studies. Neuroscience. 1997;79:171–176. doi: 10.1016/s0306-4522(96)00643-4. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Lepichon M, Jalfre M. Depression—new animal-model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Quested DJ, Sargent PA, Cowen PJ. SSRI treatment decreases prolactin and hyperthermic responses to mCPP. Psychopharmacology. 1997;133:305–308. doi: 10.1007/s002130050406. [DOI] [PubMed] [Google Scholar]
- Rago L, Kiivet RA, Harro J, Pold M. Behavioral-differences in an elevated plus-maze—correlation between anxiety and decreased number of GABA and benzodiazepine receptors in mouse cerebral-cortex. Naunyn-Schmiedebergs Arch Pharmacol. 1988;337:675–678. doi: 10.1007/BF00175795. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Cole JC, Aboualfa K, Stephenson LH. Ethopharmacological analysis of the effects of putative anxiogenic agents in the mouse elevated plus-maze. Pharmacol Biochem Behav. 1995;52:805–813. doi: 10.1016/0091-3057(95)00190-8. [DOI] [PubMed] [Google Scholar]
- Roiser JP, Levy J, Fromm SJ, Wang HY, Hasler G, Sahakian BJ, et al. The effect of acute tryptophan depletion on the neural correlates of emotional processing in healthy volunteers. Neuropsychopharmacology. 2008;33:1992–2006. doi: 10.1038/sj.npp.1301581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagredo O, Ramos JA, Fernandez-Ruiz J, Rodriguez M, de Miguel R. Chronic delta(9)-tetrahydrocannabinol administration affects serotonin levels in the rat frontal cortex. Naunyn-Schmiedebergs Arch Pharmacol. 2006;372:313–317. doi: 10.1007/s00210-005-0026-1. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Gobbi G, Debs PC, Sibille EL, Blier P, Hen R, et al. Genetic and pharmacological disruption of neurokinin 1 receptor function decreases anxiety-related behaviors and increases serotonergi function. Proc Natl Acad Sci USAm. 2001;98:1912–1917. doi: 10.1073/pnas.041596398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Smith KA, Fairburn CG, Cowen PJ. Relapse of depression after rapid depletion of tryptophan. Lancet. 1997;349:915–919. doi: 10.1016/s0140-6736(96)07044-4. [DOI] [PubMed] [Google Scholar]
- Steinbusch HWM. Distribution of serotonin-immunoreactivity in the central nervous-system of the rat—cell-bodies and terminals. Neuroscience. 1981;6:557–618. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- Steru L, Chermat R, Thierry B, Simon P. The tail suspension test—a new method for screening antidepressants in mice. Psychopharmacology. 1985;85:367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- Szabo ST, Blier P. Effect of the selective noradrenergic reuptake inhibitor reboxetine on the firing activity of noradrenaline and serotonin neurons. E J Neurosci. 2001a;13:2077–2087. doi: 10.1046/j.0953-816x.2001.01583.x. [DOI] [PubMed] [Google Scholar]
- Szabo ST, Blier P. Effects of the selective norepinephrine reuptake inhibitor reboxetine on norepinephrine and serotonin transmission in the rat hippocampus. Neuropsychopharmacology. 2001b;25:845–857. doi: 10.1016/S0893-133X(01)00284-6. [DOI] [PubMed] [Google Scholar]
- Temel Y, Boothman LJ, Blokland A, Magill PJ, Steinbusch HWM, Visser-Vandewalle V, et al. Inhibition of 5-HT neuron activity and induction of depressive-like behavior by high-frequency stimulation of the subthalamic nucleus. Proc Natl Acad Sci USA. 2007;104:17087–17092. doi: 10.1073/pnas.0704144104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbain N, Creamer K, Debonnel G. Electrophysiological diversity of the dorsal raphe cells across the sleep-wake cycle of the rat. J Physiol London. 2006;573:679–695. doi: 10.1113/jphysiol.2006.108514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uriguen L, Perez-Rial S, Ledent C, Palomo T, Manzanares J. Impaired action of anxiolytic drugs in mice deficient in cannabinoid CB1 receptors. Neuropharmacology. 2004;46:966–973. doi: 10.1016/j.neuropharm.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Vandermaelen CP, Aghajanian GK. Electrophysiological and pharmacological characterization of serotonergic dorsal raphe neurons recorded extracellularly and intracellularly in rat-brain slices. Brain Res. 1983;289:109–119. doi: 10.1016/0006-8993(83)90011-2. [DOI] [PubMed] [Google Scholar]
- Vaswani M, Linda FK, Ramesh S. Role of selective serotonin reuptake inhibitors in psychiatric disorders: a comprehensive review. Prog Neuro-Psychopharmacol Biol Psychiatry. 2003;27:85–102. doi: 10.1016/s0278-5846(02)00338-x. [DOI] [PubMed] [Google Scholar]
- Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protocols. 2007;2:322–328. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahorodna A, Hess G. Imipramine and citalopram reverse corticosterone-induced alterations in the effects of the activation of 5-HT1A and 5-HT2 receptors in rat frontal cortex. J Physiol Pharmacol. 2006;57:389–399. [PubMed] [Google Scholar]
- Zhou JS, Li LJ, Tang SJ, Cao X, Li ZX, Li WH, et al. Effects of serotonin depletion on the hippocampal GR/MR and BDNF expression during the stress adaptation. Behav Brain Res. 2008;195:129–138. doi: 10.1016/j.bbr.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Zisook S, Ganadjian K, Moutier C, Prather R, Rao S. Sequenced treatment alternatives to relieve depression (STAR*D): lessons learned. J Clin Psychiatry. 2008;69:1184–1185. doi: 10.4088/jcp.v69n0719. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.