Abstract
Tesofensine is a novel monoamine reuptake inhibitor that inhibits both norepinephrine, 5-HT, and dopamine (DA) reuptake function. Tesofensine is currently in clinical development for the treatment of obesity, however, the pharmacological basis for its strong effect in obesity management is not clarified. Using a rat model of diet-induced obesity (DIO), we characterized the pharmacological mechanisms underlying the appetite suppressive effect of tesofensine. DIO rats treated with tesofensine (2.0 mg/kg, s.c.) for 16 days showed significantly lower body weights than vehicle-treated DIO rats, being reflected by a marked hypophagic response. Using an automatized food intake monitoring system during a 12 h nocturnal test period, tesofensine-induced hypophagia was investigated further by studying the acute interaction of a variety of monoamine receptor antagonists with tesofensine-induced hypophagia in the DIO rat. Tesofensine (0.5–3.0 mg/kg, s.c.) induced a dose-dependent and marked decline in food intake with an ED50 of 1.3 mg/kg. The hypophagic response of tesofensine (1.5 mg/kg, s.c.) was almost completely reversed by co-administration of prazosin (1.0 mg/kg, α1 adrenoceptor antagonist) and partially antagonized by co-administration of SCH23390 (0.03 mg/kg, DA D1 receptor antagonist). In contrast, tesofensine-induced hypophagia was not affected by RX821002 (0.3 mg/kg, α2 adrenoceptor antagonist), haloperidol (0.03 mg/kg, D2 receptor antagonist), NGB2904 (0.1 mg/kg, D3 receptor antagonist), or ritanserin (0.03 mg/kg, 5-HT2A/C receptor antagonist). Hence, the mechanism underlying the suppression of feeding by tesofensine in the obese rat is dependent on the drug's ability to indirectly stimulate α1 adrenoceptor and DA D1 receptor function.
Keywords: diet-induced obesity, monoamine reuptake inhibitor, food intake, anti-obesity effect, α1 adrenoceptor, dopamine D1 receptor
INTRODUCTION
Tesofensine is a novel centrally acting triple monoamine reuptake inhibitor (MRI) with intrinsic inhibitory activity on norepinephrine (NE), serotonin (5-HT), and dopamine (DA) transporter function (Lehr et al, 2008). Results from recent clinical trials show that tesofensine effectively produces a substantial weight loss in obese patients (Astrup et al, 2008a, 2008b). When corrected for placebo and diet effects, long-term tesofensine treatment produces a maximal weight loss of 10.6% in obese patients, which is twice that achieved by other anti-obesity agents. The robust weight loss produced by tesofensine is explained by a dose-dependent hypophagia because of stimulation of satiety (Astrup et al, 2008b), suggesting that tesofensine predominantly acts as an appetite suppressant to produce a negative energy balance. However, the pharmacological basis for the hypophagic effect is not resolved.
Monoaminergic neurotransmission is centrally involved in the homestatic control of appetite function, and it has become increasingly clear that there is a close association between dietary obesity and altered monoaminergic neurotransmission. For example, pharmacological manipulation studies have shown that stimulated central monoaminergic activity induces profound effects on feeding behavior and thus energy intake, and agents that enhance synaptic levels of NE, 5-HT, or DA by stimulating release or reducing reuptake can decrease feeding and weight gain (Nelson and Gehlert, 2006).
The hypothalamus is a key site of action for the anorexic effect of monoamine receptor agonists, as increased monoaminergic activity within the hypothalamus can markedly influence feeding behavior by triggering satiety signals (Meguid et al, 2000b; Wellman, 2000). Monoaminergic modulation of appetite function at the hypothalamic level is complex as several hypothalamic NE, DA, and 5-HT receptor subtypes are involved in the control of feeding activity. Notably, α1 adrenoceptor and 5-HT2C agonists inhibit food intake, and these monoaminergic signaling pathways are strongly implicated in the anorexic action NE and 5-HT (Clifton and Kennett, 2006). In addition, D1 and D2 receptors agonists can suppress feeding, and it is thought that these DA receptor subtypes can produce synergistic anorexic effects (Meguid et al, 2000b; Wellman, 2005).
There is a growing notion that mesolimbic dopaminergic neurotransmission contributes to the effect of DA on feeding behavior (Volkow and Wise, 2005). Food intake and food depriviation have opposing effects on extracellular DA levels in the nucleus accumbens, as feeding stimulates DA release and turnover whereas food deprivation causes the opposite effects (Nelson and Gehlert, 2006). Several DA receptors may be effectors of increased DA availability during feeding episodes, as both D1, D2, and D3 receptor agonists reduce food intake in animal models of obesity (Scislowski et al, 1999; McQuade et al, 2003; Davis et al, 2008).
Correspondingly, there are several indices of defective central monoaminergic neurotransmission in preclinical models of obesity, including reduced hypothalamic α1 adrenoceptor binding (Wilmot et al, 1988), enhanced electrophysiological responsiveness to intrahypothalamic NE application (Kraszewski and Cincotta, 2000), decreased basal hypothalamic 5-HT and DA levels (Meguid et al, 2000a), as well as reduced mesolimbic DA activity (Geiger et al, 2008).
Therefore, we reasoned that the anti-obesity effect of tesofensine treatment in obese individuals could be a consequence of combined modulation of several central monoaminergic pathways. In this study, the pharmacological mechanisms underlying the anti-obesity effect of tesofensine were investigated in a rat model of diet-induced obesity (DIO). Tesofensine induced a robust weight reduction in DIO rats during chronic tesofensine treatment, which was accompanied by a strong hypophagic response. To identify the principal monoamine receptor(s) being critically involved in hypophagic effect of tesofensine, we investigated whether tesofensine-induced hypophagia could be reversed by co-administration of various monoaminergic receptor antagonists.
MATERIALS AND METHODS
Animal Care and Housing
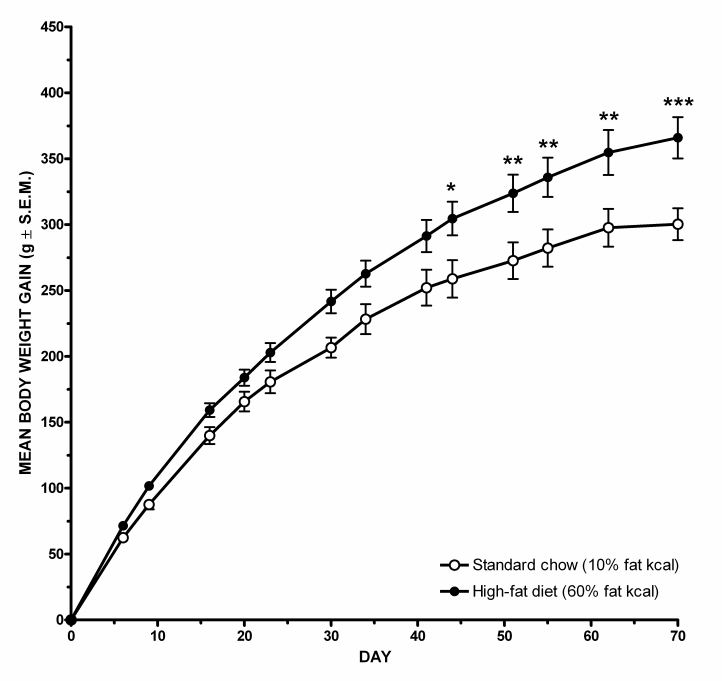
Five-weeks-old male Sprague-Dawley rats (150–180 g, Taconics, Ry, Denmark) were initially group housed (four rats per cage) in solid bottomed Plexiglas cages with dust-free wood chippings and a cardboard tube. Holding rooms were maintained under a 12-h light/dark cycle (lights off: 1500 h). Ambient temperature was 18.0–22.0°C and relative air humidity of 40–60%. A dim red light was the sole source of illumination during the dark period. The rats had ad libitum food and water, and they were made obese by switching to high-fat diet for a minimum of 10 weeks (#D12492; 60% kcal from fat, energy density 5.24 kcal/g, Research Diets, New Brunswick, NJ) in pellet form. After 3–4 weeks on high-fat diet, the rats were randomly matched (two rats per cage) with free access to animal diet throughout all experiments. Initially, an optimization study (see Figure 1) indicated that the rats obtained a significantly higher body weight gain, as compared with rats receiving standard chow (Altromin 1324, 10% kcal from fat, energy density 2.85 kcal/g, Altromin GmbH, Lage, Germany). Thus, after 10 weeks (day 70) on high-fat diet, the rats had a 22% higher net body weight gain (365±15 g, n=9) as compared with the net body weight gain (300±12 g, n=9) of rats on standard chow (repeated measure two-way ANOVA, p<0.001). The outbred rats in the present experiments were therefore after at least 10 weeks on high-fat diet considered to be DIO as a group, according to the term introduced by Levin and Keesey (1998). However, no discrimination was made between rats on high-fat diet with lowest or highest weight gain. Thus, rats were assigned randomly to experimental groups irrespectively of any individual differences in net weight gain.
Figure 1.
High-fat diet (Research Diets #D12492, 60% fat kcal., energy density 5.24 kcal/g) induces a significantly higher body weight gain over 10 weeks, as compared with rats receiving standard chow (Altromin 1324, 10% fat kcal, energy density 2.85 kcal/g). Data are shown as mean±SEM *p<0.05; **p<0.01; ***p<0.001.
All experiments were approved (permission no. 2007/561-1343) and conducted in accordance with the guidelines of the Animal Experimentation Inspectorate, Ministry of Justice, Denmark.
Drugs
Tesofensine (8-Azabicyclo[3.2.1]octane,3-[3,4-dichlorophenyl)-2-(ethoxymethyl)-8-methyl-[1R-(2-endo,3-exo)]-2-hydroxy-1,2,3-propanetricarboxylate) is a derivative of an azabicyclooctane citrate, synthesized at the Department of Medicinal Chemistry, NeuroSearch A/S. Prazosin, RX821002, SCH23390 and ritanserin were purchased from Sigma-Aldrich (St Louis, MI). Haloperidol and NGB2904 were from Janssen-Cilag (Beerse, Belgium) and Tocris (Ellisville, MO), respectively. All stock solutions were prepared daily and diluted to working concentration with the relevant vehicle. Tesofensine was dissolved in 0.9% saline solution, all other compounds were dissolved in 15% HP-β-cyclodextrine. All drug solutions were brought to pH 7.4 with 0.1 N NaOH.
Assessment of Feeding Kinetics Using Real-Time Feeding Monitoring
The DIO rats were re-housed after 9–10 weeks on high-fat diet (mean body weight 493±49 g, weight gain with high-fat diet 343±4.3 g) and transferred with its partnering rat to fully automated food intake monitoring cages (HM-2, MBRose Aps, Faaborg, Denmark; for further information, see http://www.mbrose.dk/products.htm). The HM-2 food intake monitoring cages were placed in a modified ventilated cabinet with lightproof doors and a light kit for cabinet-based control of light–dark cycle (Scanbur BK, Karslunde, Denmark) being similar to that in the holding rooms. Cabinet temperature was 24.0–26.0°C and relative air humidity of 40–60%. The animals were habituated to the HM-2 food intake monitoring system for at least 5 days before drug treatment. The feeding behavior of each animal was monitored on a constant basis during the habituation period to assure that the food intake of each animal returned to the pre-rehousing level. Food intake typically declined during the first day of habituation, but returned to the pre-rehousing level during the remainder of the habituation period (data not shown). On the day of re-housing to the fully automated food intake monitoring cages, the rats were subcutaneuosly injected with a microchip (#402575, eVet, Haderslev, Denmark). The tagging did not affect feeding behavior or body weight gain (data not shown) and allowed the HM-2 food intake monitoring system to simultaneously identify and in real-time mode to track feeding behavior of each individual animal throughout the entire experiment. Food intake was monitored on a constant basis after entry of the tagged animal into the tag-reading food channel. On exit of the animal from the food channel, the net food intake was calculated by the HM-2 control unit software (HMLab, MBRose, Faaborg, Denmark). Animal-allocated food intake was discriminated from spillage by defining a minimum bout size (⩾0.1 g) per entry. Food intake was measured on a constant basis and calculated as total food intake (gram) and cumulated food intake (gram per 2 h interval), respectively. In addition, microstructural analysis of nocturnal food intake was carried out by analyzing various meal consumption parameters, that is total number of meals, mean meal size, average meal duration, latency to first meal, and first meal size. Meals were defined using a minimum meal size criterion of 0.3 g, a minimum meal duration criterion of 30 s, and a minimum intermeal interval of 15 min, measured from the last measured intake of a meal (23). With the use of these criteria, all nocturnal feeding activity was resolved into meals.
All experimental compounds and vehicles were administered subcutaneously (1 ml/kg). The tesofensine dose–response and monoamine receptor antagonist interaction experiments were designed as between-subject studies with at least six (tesofensine dose–response study) or eight DIO rats (monamine receptor antagonist interaction study) per group. In all acute dosing experiments, food intake was measured on a constant basis throughout the 12 h period.
When performing drug interaction experiments, bilateral subcutaneous injections were given within 1 min between each injection. Drugs were administered 30–60 min before dark onset. The home cage was removed from the HM-2 food intake monitoring system during the drug administration procedure and returned immediately after completion of the drug injections, whereafter automated monitoration of the feeding behavior of each individual animal was started.
In addition, a time–response study of longer duration was conducted in DIO rats with a single dose of tesofensine (1.5 mg/kg, s.c., n=6) or saline vehicle (n=6) to assess the temporal dynamics on food intake over a 72 h measurement period. Data on food consumption were collected in 2 or 12 h intervals.
Body weight and microstructural food intake analysis were performed using a data reporting software (HMView, MBRose, Faaborg, Denmark). The data were imported into a standard graphic and statistical analysis programme (GraphPad Prism v.4.03 or SigmaStat v.3.5). All data were expressed as mean±SEM of n individual animals. The ED50 of tesofensine-induced hypophagia was calculated using a four parameter logistic equation for non-linear curvefitting of a sigmoidal dose–response curve. Statistical comparisons between groups were performed by a one-way ANOVA with Tukey's post hoc test (body weight gain, total food intake, and feeding kinetics) or a repeated measure two-way ANOVA with Bonferroni's multiple comparion test (cumulated food intake). A p-value <0.05 was considered statistically significant.
Chronic Treatment with Tesofensine
In a separate study, DIO rats (11 weeks on high-fat diet, mean body weight 556±12.1 g, weight gain with high-fat diet 406±4.8 g) were housed in the pair environment until 1 week before the study when they were singly housed in cages equipped with a food hopper allowing assessment of net food intake by manual weighing. The light–dark cycle was similar to that used in the acute drug treatment experiments. Tesofensine (2.0 mg/kg, n=5) or saline vehicle (n=5) was administered subcutaneously to the DIO rats once daily (∼1 h before dark onset) for 16 days. Body weight and food intake were monitored once daily before the daily drug treatment procedure. Body weight data were expressed as daily body weight gain relative to the first day of drug administration. The data were fed into a standard graphic and statistical analysis programme (GraphPad Prism v.4.03). Body weight gain and food intake were expressed as mean±SEM of n individual animals, and a repeated measure two-way ANOVA with Bonferroni's multiple comparion test was applied to perform statistical comparisons between treatment groups. A p-value <0.05 was considered statistically significant.
RESULTS
Acute Treatment with Tesofensine
Tesofensine dose dependently induces hypophagia in the DIO rat
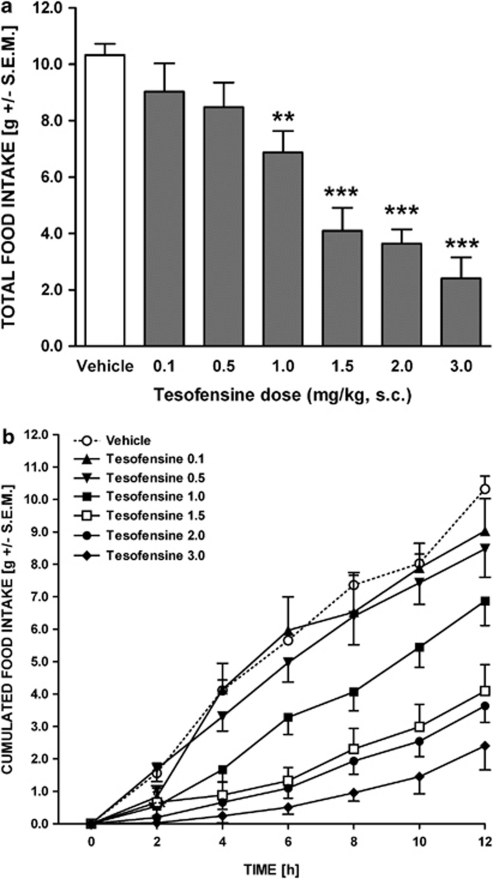
A single dose of tesofensine (0. 1–3 mg/kg, s.c.) robustly and dose dependently inhibited food intake in DIO rats over the 12 h nocturnal observation period (p<0.0001, Figure 2a). The threshold dose for inhibition of total food intake was 1.0 mg/kg (p<0.01). The ED50 for tesofensine-induced inhibition of total food intake in DIO rats was estimated to be 1.3 mg/kg.
Figure 2.
Tesofensine induces hypophagia in the DIO rat. (a) Dose–response effect of tesofensine (0.1–3.0 mg/kg, s.c., n⩾6 per group) on total food intake in the DIO rat during a 12 h nocturnal period. Total food intake over the entire observation period is shown in gram (left y-axis) as well as relative to vehicle-treated DIO rats (right y-axis). (b) Time–response effect of incrementing doses of tesofensine (0.1–3.0 mg/kg, s.c.) on cumulated nocturnal food intake (2 h intervals). Data are shown as mean±SEM **p<0.01; ***p<0.001.
During the 12 h-observation period, vehicle-treated rats consumed an amount of food corresponding to 54.1±2.1 kcal, whereas acute treatment with the highest dose of tesofensine (3.0 mg/kg) resulted in a total food intake amounting to 12.6±3.9 kcal, being equivalent to a 77% reduction of energy intake.
Analysis of the temporal effect of tesofensine indicated that doses of ⩾0.5 mg/kg significantly affected cumulated food intake (p<0.001, Figure 2b). The onset and duration of tesofensine-induced hypophagia was dose-dependent. For doses ⩾2.0 mg/kg, the hypophagic effect was introduced instantly after drug administration (p<0.05) and maintained throughout the nocturnal observation period. Lower doses (0.5–1.5 mg/kg) showed a progressive increase in the lag-time for onset of food intake suppression. Thus, though treatment with intermediate doses resulted in significant inhibitory effect on cumulative food intake at 2–12 h post injection (1.0 mg/kg, p<0.01; 1.5 mg/kg, p<0.001), the inhibitory effect of lower doses on food intake was further delayed, being only significant at 10–12 h post injection (0.5 mg/kg, p<0.05).
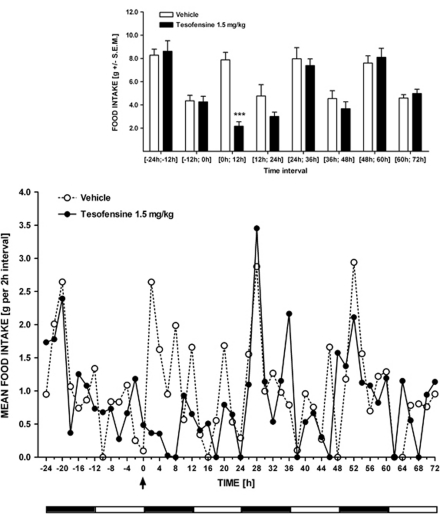
Although tesofensine treatment (1.5 mg/kg, s.c.) affected food intake during the initial 12 h post injection, feeding activity was re-established to vehicle control level in the remainder 12–72 h of light–dark cycle period (Figure 3), thus indicating that acute tesofensine treatment did not result in compensatory feeding activity.
Figure 3.
Temoral pharmacodynamics of acute tesofensine treatment in the DIO rat. Time–response effect of tesofensine (1.5 mg/kg, s.c., n=6) or saline vehicle (n=6) on food intake during a 72 h monitoration period. Upper panel, 12 h interval data points. Lower panel, 2 h interval data points with dark and white horizontal bars below the x-axis indicating dark and light phases, respectively. The arrow denotes when tesofensine or vehicle was administered. Data are shown as mean±SEM ***p<0.001 (compared with vehicle controls).
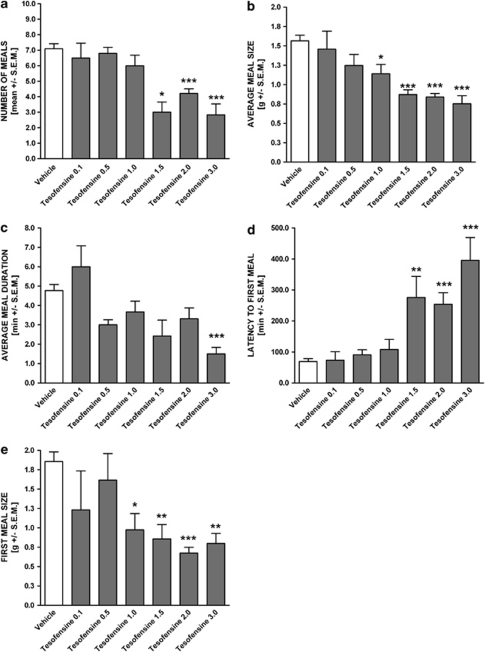
The effects of tesofensine on nocturnal food consumption were subjected to microstructural analysis (Figure 3). Tesofensine significantly affected several parameters of feeding kinetics, that is acute tesofensine treatment resulted in a decrease in the total number of meals (Figure 4a), average meal size (Figure 4b), and average meal duration (Figure 4c). In addition, tesofensine had a pronounced impact on first meal latency and size (Figure 4d, e).
Figure 4.
Feeding kinetics of tesofensine-induced hypophagia in the DIO rat. (0.1–3.0 mg/kg, s.c., n⩾6 per group, overall dose–response effects on food intake in the same animals are shown in Figure 1). (a) total number of meals, (b) average meal size, (c) average meal duration, (d) latency to first meal, and (e) first meal size during a 12 h nocturnal period. Data are shown as mean±SEM *p<0.05, **p<0.01, ***p<0.001 (compared with vehicle controls).
The lower food intake in tesofensine-exposed rats had an impact on body weight gain during the 12 h observation period. Vehicle-treated DIO rats gained +5.0±1.0 g (+1.0±0.2% of initial body weight) during the experiment. In contrast, tesofensine significantly reduced weight gain in the dose range of 1.0–3.0 mg/kg. Thus, treatment with doses of 1.0 and 3.0 mg/kg induced a weight loss of −2.0%±1.0 g (−0.4±0.3% of initial body weight, p<0.05) and −7.3±1.8 g (−1.5±0.4% of initial body weight, p<0.001), respectively.
To assess the effect of tesofensine on monoamine neurotransmission pathways, a dose of 1.5 mg/kg was selected for further analysis. This dose inhibited food intake corresponding to ∼50% of basal food consumption in the DIO rat, and was thus suitable for interaction studies with various monoamine receptor antagonists. Preliminary dose–response experiments were performed with each monoamine receptor antagonist in the DIO rat (data not shown), and the highest dose of each antagonist producing no effect on total food intake per se was selected.
Involvement of adrenoceptor function in tesofensine-induced hypophagia
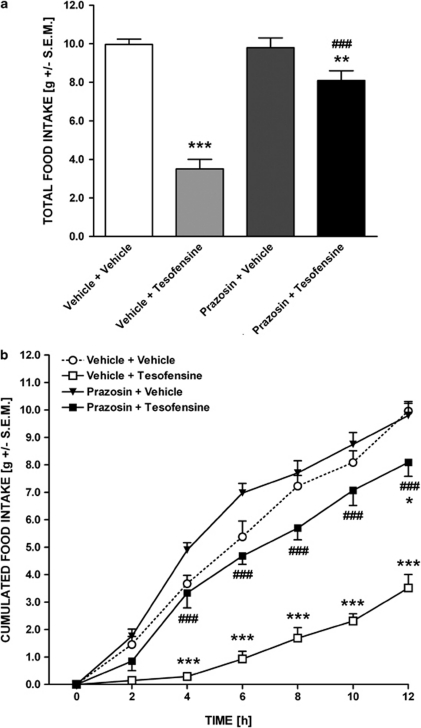
The reduction in food intake induced by tesofensine was almost completely abolished by co-administration of the α1 adrenoceptor antagonist, prazosin (1.0 mg/kg, s.c., Figure 5a). Although tesofensine significantly reduced total food intake (37±4.9% of vehicle control, p<0.001), combined prazosin treatment reversed the feeding level to 80±4.6% of the vehicle control level (p<0.01 and <0.001 as compared with vehicle and tesofensine alone, respectively). The antagonizing effect of prazosin had a fast onset (0–2 h, p<0.05) and was maintained throughout the 12 h nocturnal period, although the counteracting effect slightly wore off during the 6–12 h period (Figure 5b).
Figure 5.
Tesofensine-induced hypophagia is dependent on stimulated α1 adrenoceptor function. Effect of prazosin (1.0 mg/kg, s.c.) on tesofensine-induced (1.5 mg/kg, s.c.) hypophagia in the DIO rat. (a) Total food intake (gram, left y-axis; relative to vehicle controls, right y-axis) and (b) cumulated food intake (gram) during 12 h nocturnal period. Data are shown as mean±SEM *p<0.05, **p<0.01, ***p<0.001 (compared with vehicle controls); ###p<0.001 (compared with vehicle+tesofensine).
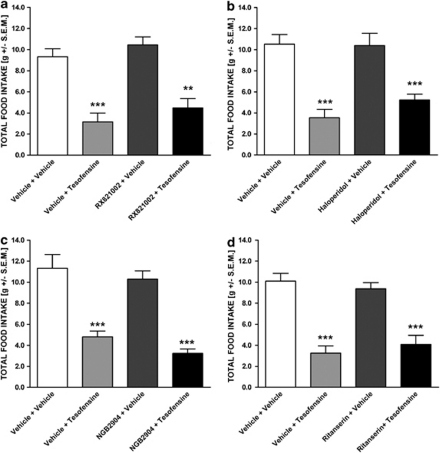
By contrast, co-administration of the α2 adrenoceptor antagonist, RX821002 (0.3 mg/kg, s.c.) did not affect the hypophagic effect of tesofensine (Figure 7a). Neither prazosin nor RX821002 administration alone influenced baseline total or cumulated food intake in the DIO rat.
Involvement of DA function in tesofensine-induced hypophagia
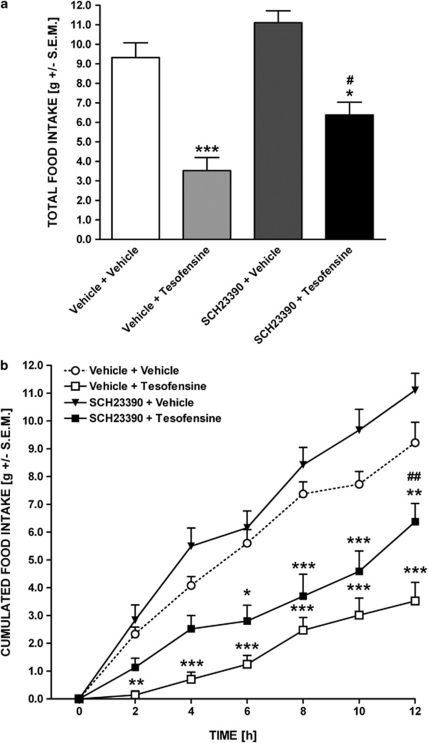
The D1 receptor antagonist SCH23390 (0.03 mg/kg, s.c.) partially antagonized the suppression of food intake with tesofensine (p=0.007). The level of total food intake after tesofensine alone or in combination with SCH23390 was 35±5.2% and 65±6.7%, respectively, of the vehicle control level (Figure 6a). Based on real-time analysis of cumulated food intake, onset of the antagonizing effect of SCH23390 was delayed, thus partially antagonizing the inhibitory effect of tesofensine on cumulative food intake at 10–12 h (p<0.01) post injection, also signifying that the efficacy of SCH23390 at intermediate time points was less robust (Figure 7b).
Figure 6.
Tesofensine-induced hypophagia is dependent on stimulated DA D1 receptor function. Effect of SCH23390 (0.03 mg/kg, s.c.) on tesofensine-induced (1.5 mg/kg, s.c.) hypophagia in the DIO rat. (a) Total food intake (gram, left y-axis; relative to vehicle controls, right y-axis) and (b) cumulated food intake (gram) during 12 h nocturnal period. Data are shown as mean±SEM *p<0.05, **p<0.01, ***p<0.001 (compared with vehicle controls); #p<0.05, ##p<0.01 (compared with vehicle+tesofensine).
Figure 7.
Tesofensine-induced hypophagia does not involve α2 adrenoceptor, DA D2, DA D3, or 5-HT2A/C receptor function. Effect of (a) RX821002 (0.3 mg/kg, s.c.), (b) haloperidol (0.03 mg/kg, s.c.), (c) NGB2904 (0.1 mg/kg, s.c.), or (d) ritanserin (0.03 mg/kg, s.c.) on tesofensine-induced (1.5 mg/kg, s.c.) hypophagia in the DIO rat. Data are shown total nocturnal food intake (gram) as mean±SEM **p<0.01, ***p<0.001 (compared with vehicle controls).
The D2 receptor antagonist haloperidol (0.03 mg/kg, s.c.) did not significantly (p=0.088) affect tesofensine's ability to inhibit food intake in the DIO rat (Figure 7b). In addition, the D3 receptor antagonist NGB2904 did not have any antagonizing effect on tesofensine-induced hypophagia (p=0.492, Figure 7c). SCH23390, haloperidol, and NGB2904 alone had no effect on baseline total or cumulated food intake in the DIO rat.
No involvement 5-HT2A/C receptor function in tesofensine-induced hypophagia
Ritanserin (0.03 mg/kg, s.c.), a 5-HT2A/C receptor antagonist, did not affect tesofensine-induced inhibition of food intake in the DIO rat (p=0.843, Figure 7d). Ritanserin treatment per se did not display a significant effect on total food consumption or cumulative food intake.
Chronic Treatment with Tesofensine
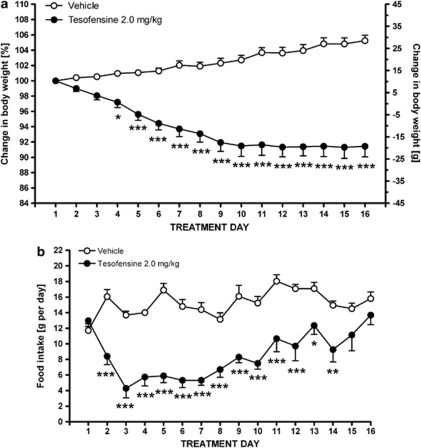
Daily administration of a moderate dose of tesofensine (2.0 mg/kg, s.c.) over 16 days triggered a significant reduction in body weight after 4 days of administration (p<0.05) relative to vehicle-treated controls (two-way ANOVA, overall p<0.0001), see Figure 8a. The weight lowering effect was sustained from day 4 throughout the remainder of the experiment, reaching a plateau from day 10 and onward. The average relative decrease in the body weight of tesofensine-treated DIO rats over the entire treatment period was 8.6±1.4%. When comparing to vehicle controls, the relative weight loss with tesofensine was 13.8±1.4%.
Figure 8.
Chronic tesofensine treatment causes a sustained body weight reduction with a temporal suppressive effect on food intake. Effect of a daily dose of tesofensine (2.0 mg/kg, s.c.) on body weight gain (a) and food intake (b) over a 16-day treatment period. Data are shown as mean±SEM *p<0.05, **p<0.01, ***p<0.001 (compared with vehicle controls).
Tesofensine also had a strong impact on food intake over the 16-day administration period. In accordance with the acute dosing experiments, tesofensine significantly reduced daily food intake on the first day of treatment, an effect that lasted to day 14 of treatment (two-way ANOVA, overall p<0.0001), see Figure 8b. The percentage of suppression of daily food intake induced by tesofensine over days 1–14 averaged 49.4±4.7% relative to the vehicle control group. The hypophagic response to tesofensine was thus transient, eventually returning to levels not significantly different from vehicle controls on treatment day 15.
DISCUSSION
Tesofensine dose-dependently suppressed food intake in DIO rats within 12 h of administration. The lowest dose that decreased the quantitity of food consumed during nocturnal feeding episodes was 1.0 mg/kg with a strong hypophagic effect being observed after administration of more than 2.0 mg/kg. The hypophagic effect of a single dose of tesofensine (⩾1.0 mg/kg) was sustained throughout the 12 h observation period. As a consequence of the strong hypophagic response, acute tesofensine administration resulted in a corresponding dose-dependent weight loss in the DIO rat.
Analysis of the time–response effect and microstructual feeding activity revealed that tesofensine had a pronounced effect on several behavioral aspects of food intake. Most strikingly, the highest dose of tesofensine (3.0 mg/kg, s.c.) strongly increased the latency time (571% increase) to the first meal and reduced the total number of meals and average meal size by 60 and 69%, respectively.
Given the strong acute effects of tesofensine on body weight and food consumption, the maintenance of tesofensine's activity after a longer duration of administration was investigated in the DIO model. The reduction in body mass of DIO rats produced by tesofensine could be sustained by daily dosing thoughout the 16-day treatment regimen. The decrease in body mass was accompanied by a significant suppression of daily food intake, although the hypophagic effect gradually returned to control levels after 14 days of treatment. In both normal (acute treatment) and DIO rats (acute or chronic treatment), tesofensine administration did not cause side effects, which could potentially influence feeding behavior, for example negative reinforcement or gastrointestinal upset (data not shown). We therefore conclude that tesofensine specifically reduces the drive to obtain food in DIO rats as a consequence of enhanced satiety signaling. This is also in close correspondence with observations from clinical settings, as the significant weight loss (10.6%) observed after 6 months with tesofensine treatment in obese patients is associated with suppression of hunger and increased satiety measures (Astrup et al, 2008a).
Considering that DIO rats gradually developed tolerance to the hypophagic effect of tesofensine during chronic dosing, this indicates that other factors than appetite regulation contributed to sustain the maximal weight loss. This notion is supported by the observation that stimulation of monoaminergic neurotransmission also impacts the metabolic rate by increasing energy expenditure, either directly by increasing thermogenesis through activation of peripheral β3 adrenoceptors in brown adipose tissue, or indirectly by increasing central DA receptor-dependent motor activity. These combined effects are reported for a number of MRIs, including the dual NE/5-HT and NE/DA reuptake inhibitors, sibutramine, and buproprion, respectively (Connoley et al, 1999; Liu et al, 2004; Golozoubova et al, 2006; Billes and Cowley, 2008). Although it should be noted that no tolerance to the appetite suppressing effect of tesofensine is observed in clinical settings (Astrup et al, 2008a), one possibility that warrants further investigation is whether sustained weight loss with tesofensine may involve increased energy expenditure.
As appetite suppression was an important factor for triggering and initially establishing the anti-obesity effect of tesofensine in the DIO rat, it was of particular interest to identify the principal monoaminergic system(s) underlying tesofensine-induced suppression of food intake in the DIO rat.
To this end, the interaction of acute tesofensine administration with a various monoamine receptor antagonists was investigated in the DIO rat. Although prazosin and SCH23390 were able to produce a significant reversal of tesofensine-induced hypophagia in the DIO rat, all other antagonists tested in this study with distinct monoamine receptor profiles had no effect. It should be noted that the individual doses of RX821002, haloperidol, NGB2904, and ritanserin, respectively, used in this study lie within ranges previously reported to be active in the rat in other in vivo settings (Pfeffer and Samson, 1988; Rittenhouse et al, 1994; Deupree et al, 2008; Spiller et al, 2008), thus excluding the possibility that the lack of an effect of these antagonists on tesofensine-induced hypophagia could be due to the use of physiologically inactive doses.
The hypophagic effect of tesofensine was strongly reversed by co-administration of prazosin. Correspondingly, prazosin also prevented the acute hypophagic effect of sibutramine (Jackson et al, 1997). In addition, selective NE reuptake inhibitors, such as nisoxetine, desmethylimipramine, and LY368975, produce anorexic effects in the rat (Durcan et al, 1988; Gehlert et al, 1998; Billes and Cowley, 2007). The triple MRI profile of tesofensine therefore suggests that its hypophagic effect to a major degree occurs after blockade of NE reuptake function, reflecting a subsequent activation of α1 adrenoceptors as a consequence of increased synaptic availability of NE. The relevance of stimulated α1 adrenoceptor function by tesofensine is supported by the consistent finding of anorexic effects after systemic administration of various α1 adrenoceptor agonists (Wellman and Davies, 1992; Morien et al, 1993; Racotta and Soto-Mora, 1993). In agreement, long-term α1 adrenoceptor antagonist treatment causes overeating and weight gain in clinical settings (Bray, 2000), indicating that positive modulation of α1 adrenoceptor activity is a clinically important target in obesity management.
The precise site of feeding modulatory action of adrenoceptor modulators in the CNS is not known. Both α1 and α2 adrenoceptors are localized within the hypothalamus (Young and Kuhar, 1980), and direct injections of prazosin into the hypothalamic paraventricular nucleus (PVN) can reverse the anorexic effect of a systemically administered α1 adrenoceptor agonist (Wellman and Davies, 1992). Moreover, there is evidence that NE efflux increases in the hypothalamus, including the PVN, during food consumption (Stanley et al, 1989; Morien et al, 1995). As extracellular NE levels in the rat PVN peak just before onset of dark being closely correlated to subsequent feeding activity (Morien et al, 1995), this region may therefore be important in providing satiety/hunger signals downstream of NE secretion. Interestingly, DIO rats exhibit reduced hypothalamic α1 adrenoceptor binding (Wilmot et al, 1988), which may thus reflect a reduced α1 adrenoceptor function. In addition, obese mice display enhanced electrophysiologic responsiveness to iontophoretically applied NE in the ventromedial hypothalamus as compared with lean mice, and high-fat feeding stimulates hypothalamic NE turnover in the obese rat (Shimizu et al, 1994; Kraszewski and Cincotta, 2000). This could possibly indicate that the hypothalamic NE system is primed in DIO rats, potentially facilitating an increased responsiveness to tesofensine-induced NE effects subsequent to NET blockade.
The lack of an effect of the selective α2 adrenoceptor antagonist RX821002 on tesofensine-induced hypophagia indicates that only α1 adrenoceptor function was affected, presumably at the hypothalamic level. Notably, the PVN is among the brain regions expressing the highest NE transporter (NET) binding level (Kung et al, 2004), which argues for tesofensine produced a blockade of NET function within the PVN, resulting in efficient accummulation of PVN synaptic NE favoring anorexic α1 adrenoceptor-induced signaling. Specificity for α1 adrenoceptor effects in the PVN after NET inhibition is further supported by the finding that sibutramine elevates synaptic NE levels in the PVN of lean rats, which is thought to be linked to its α1 adrenoceptor antagonist-sensitive hypophagic effect (Jackson et al, 1997; Wortley et al, 1999).
The selective D1 receptor antagonist SCH23390 partially blocked tesofensine-induced hypophagia. This suggests that tesofensine treatment promoted stimulated D1 receptor activity secondary to DA transporter (DAT) blockade. The finding that positive modulation of D1 receptor function contributed to the anorexic effect of tesofensine is in agreement with the role of D1 receptor receptors on feeding behavior. Accordingly, D1 receptor stimulation reduces food intake and weight gain in mouse models of obesity (Scislowski et al, 1999; Bina and Cincotta, 2000; Kuo, 2002). The inhibitory effect of D1 receptor activation on feeding is most likely linked to stimulated hypothalamic DA function, which can lead to suppression of hypothalamic orexigenic signaling (Kuo, 2002; Alberto et al, 2006). Moreover, changes in hypothalamic D1 receptor expression may contribute to the hyperphagic behavior of obese Zucker rats (Fetissov et al, 2002).
Hypothalamic DA neurotransmission has an important function in the control of the duration of feeding (Meguid et al, 1997). Interestingly, DA levels accumulate in the ventromedial hypothalamus during the initial period of food intake, reaching a peak, and declining toward baseline levels at the end of the feeding episode (Orosco and Nicolaidis, 1992; Orosco et al, 1995). In addition, intrahypothalamic DA infusions suppress feeding because of a reduction in the number of meal sizes (Yang et al, 1997). Interestingly, obese rats exhibit reduced basal hypothalamic DA levels levels (Meguid et al, 2000a). In this regard, it is noteworthy that tesofensine administration had a strong impact on the meal pattern of DIO rats, including a robust reduction in the average meal number and meal size. The close temporal association between hypothalamic DA kinetics and feeding suggests that high intrahypothalamic DA concentrations during feeding episodes are required to promote satiety. This may suggest that the effect of DA function in tesofensine-induced hypophagia is associated with an increase in hypothalamic DA tonus, which could enhance satiety responses in the DIO rat.
DIO rats have several indices of lowered striatal DA activity. These include reduced DA concentrations, impaired response to electrically evoked accumbal DA release, decreased basal tyrosine hydroxylase and DAT expression, as well as lower levels of D2 receptor binding (Pothos et al, 1998; Geiger et al, 2008). Correspondingly, striatal D2 receptor binding is inversely correlated to the degree of obesity in humans (Volkow and Wise, 2005), and D2 receptor agonists produce hypophagia and weight loss in animal models of obesity (Scislowski et al, 1999; Davis et al, 2008). In addition, mesolimbic D3 receptor function may also have a role in obesity, as suggested by findings of D3 receptor agonist-induced hypophagia in DIO mice and increased adiposity in D3 receptor knockout mice (McQuade et al, 2003; McQuade et al, 2004). Hence, the DAT inhibitory component of tesofensine would suggest that D2 and D3 receptor could have a role in tesofensine-induced hypophagia. However, the D2 and D3 receptor antagonists, haloperidol and NGB2904, did not influence tesofensine-induced attentuation of food intake. In the current experimental settings, these data therefore indicate that only the D1 receptor had a significant role in tesofensine-induced hypophagia.
The involvement of serotonin function in feeding behavior has been extensively studied, and there is strong evidence for anorexic effects of several 5-HT receptor subtypes (Halford et al, 2007). In particular, the 5-HT2C receptor has gained considerable interest as a potential anorexic target, and a number of 5-HT2C receptor agonists have anti-obesity effects in preclinical and clinical settings (Clifton and Kennett, 2006; Halford et al, 2007). However, the observation that ritanserin did not influence tesofensine's capacity to induce hypophagia indicates that 5-HT2A/C receptor function is not enhanced by tesofensine-induced 5-HT transporter inhibition.
In contrast to tesofensine, other MRIs are known to produce anorexic responses through stimulation of serotonergic activity, including fluoxetine, sertraline, and sibutramine (Clifton and Kennett, 2006; Halford et al, 2007). For sibutramine, suppression of food intake could be partially reversed by simultaneous ritanserin or SB206553 administration, thus implicating 5-HT2A/C or 5-HT2B/C receptor activation (Jackson et al, 1997; Grignaschi et al, 1999; Balcioglu and Wurtman, 2000). It is suggested that the serotonergic effects of sibutramine on feeding and body weight may likely be ascribed to its primary and secondary amine metabolites, which are triple MRIs, rather than sibutramine per se (Heal et al, 1998; Glick et al, 2000; Nelson and Gehlert, 2006). As tesofensine and the bioactive metabolites of sibutramine are almost equipotent 5-HTT inhibitors in vitro (Heal et al, 1998; Glick et al, 2000; Lehr et al, 2008), this suggests that methodological differences may account for this discrepancy between tesofensine and sibutramine on acute food intake. The observation that the hypophagic effect of tesofensine was not sensitive to ritanserin co-administration may be ascribed to the observation that higher doses (0.1–1.0 mg/kg) have been used in studies on sibutramine-induced hypophagia (Jackson et al, 1997; Grignaschi et al, 1999). However, the use of doses higher than 0.03 mg/kg was precluded because of an inhibitory effect of the ritanserin per se on food intake in the present DIO rat model (data not shown).
It is also noteworthy that all studies on the monoamine receptor-associated hypophagic effect of sibutramine have been conducted in lean rats, making it uncertain whether sibutramine displays similar effects on serotonergic receptor function in DIO rats. This is relevant, as obese rats have indices of altered hypothalamic 5-HT homeostasis, including reduced basal 5-HT levels, altered 5-HT turnover, and increased 5-HT responsiveness during high-fat feeding (Gardier et al, 1989; Mori et al, 1999; Meguid et al, 2000a).
In conclusion, tesofensine is an anorexic agent, which induces a strong acute hypophagic effect in a rat model of DIO. The mechanism underlying the robust and long-lasting suppression of acute feeding by tesofensine in the obese rats fed on a high-fat diet is dependent on the drug's ability to indirectly stimulate α1 adrenoceptor and DA D1 receptor function. Presumably, this reflects additive effects of increased NE and DA activity, which is consistent with tesofensine's ability to inhibit the reuptake of both NE and DA. Although it should be taken into account that the sustained weight loss obtained with long-term tesofensine treatment in DIO rats may in addition involve modulation of other monoaminergic receptor systems as well as stimulation of energy expenditure, the present data suggest that stimulated α1 adrenoceptor and D1 receptor function could contribute significantly to the robust weight loss observed with tesofensine treatment in obese patients (Astrup et al, 2008a, 2008b).
Acknowledgments
The authors thank Tine Østergaard Engelbrect and Peter Buhl for skillfull technical assistance.
This study was funded by NeuroSearch. The authors (AMDA, JDM, HHH) declare that, except for income received from our primary employer, no financial support or compensation has been received from any individual or corporate entity over the past 3 years for research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
References
- Alberto CO, Trask RB, Quinlan ME, Hirasawa M. Bidirectional dopaminergic modulation of excitatory synaptic transmission in orexin neurons. J Neurosci. 2006;26:10043–10050. doi: 10.1523/JNEUROSCI.1819-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Astrup A, Madsbad S, Breum L, Jensen TJ, Kroustrup JP, Larsen TM. Effect of tesofensine on bodyweight loss, body composition, and quality of life in obese patients: a randomised, double-blind, placebo-controlled trial. Lancet. 2008a;372:1906–1913. doi: 10.1016/S0140-6736(08)61525-1. [DOI] [PubMed] [Google Scholar]
- Astrup A, Meier DH, Mikkelsen BO, Villumsen JS, Larsen TM. Weight loss produced by tesofensine in patients with Parkinson′s or Alzheimer′s disease. Obesity (Silver Spring) 2008b;16:1363–1369. doi: 10.1038/oby.2008.56. [DOI] [PubMed] [Google Scholar]
- Balcioglu A, Wurtman RJ. Sibutramine, a serotonin uptake inhibitor, increases dopamine concentrations in rat striatal and hypothalamic extracellular fluid. Neuropharmacology. 2000;39:2352–2359. doi: 10.1016/s0028-3908(00)00083-6. [DOI] [PubMed] [Google Scholar]
- Billes SK, Cowley MA. Inhibition of dopamine and norepinephrine reuptake produces additive effects on energy balance in lean and obese mice. Neuropsychopharmacology. 2007;32:822–834. doi: 10.1038/sj.npp.1301155. [DOI] [PubMed] [Google Scholar]
- Billes SK, Cowley MA. Catecholamine reuptake inhibition causes weight loss by increasing locomotor activity and thermogenesis. Neuropsychopharmacology. 2008;33:1287–1297. doi: 10.1038/sj.npp.1301526. [DOI] [PubMed] [Google Scholar]
- Bina KG, Cincotta AH. Dopaminergic agonists normalize elevated hypothalamic neuropeptide Y and corticotropin-releasing hormone, body weight gain, and hyperglycemia in ob/ob mice. Neuroendocrinology. 2000;71:68–78. doi: 10.1159/000054522. [DOI] [PubMed] [Google Scholar]
- Bray GA. A concise review on the therapeutics of obesity. Nutrition. 2000;16:953–960. doi: 10.1016/s0899-9007(00)00424-x. [DOI] [PubMed] [Google Scholar]
- Clifton PG, Kennett GA. Monoamine receptors in the regulation of feeding behaviour and energy balance. CNS Neurol Disord Drug Targets. 2006;5:293–312. doi: 10.2174/187152706777452254. [DOI] [PubMed] [Google Scholar]
- Connoley IP, Liu YL, Frost I, Reckless IP, Heal DJ, Stock MJ. Thermogenic effects of sibutramine and its metabolites. Br J Pharmacol. 1999;126:1487–1495. doi: 10.1038/sj.bjp.0702446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis LM, Michaelides M, Cheskin LJ, Moran TH, Aja S, Watkins PA, et al. Bromocriptine administration reduces hyperphagia and adiposity and differentially affects Dopamine D2 receptor and transporter binding in leptin-receptor-deficient zucker rats and rats with diet-induced obesity. Neuroendocrinology. 2008;89:152–162. doi: 10.1159/000170586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deupree JD, Burke WJ, Bylund DB. Alpha-2 adrenergic-induced changes in rectal temperature in adult and 13-day old rats following acute and repeated desipramine administration. BMC Pharmacol. 2008;8:17. doi: 10.1186/1471-2210-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durcan MJ, McWilliam JR, Campbell IC, Neale MC, Dunn G. Chronic antidepressant drug regimes and food and water intake in rats. Pharmacol Biochem Behav. 1988;30:299–302. doi: 10.1016/0091-3057(88)90458-3. [DOI] [PubMed] [Google Scholar]
- Fetissov SO, Meguid MM, Sato T, Zhang LH. Expression of dopaminergic receptors in the hypothalamus of lean and obese Zucker rats and food intake. Am J Physiol Regul Integr Comp Physiol. 2002;283:R905–R910. doi: 10.1152/ajpregu.00092.2002. [DOI] [PubMed] [Google Scholar]
- Gardier AM, Trouvin JH, Orosco M, Nicolaidis S, Jacquot C. Effects of food intake and body weight on a serotonergic turnover index in rat hypothalamus. Brain Res Bull. 1989;22:531–535. doi: 10.1016/0361-9230(89)90106-8. [DOI] [PubMed] [Google Scholar]
- Gehlert DR, Dreshfield L, Tinsley F, Benvenga MJ, Gleason S, Fuller RW, et al. The selective norepinephrine reuptake inhibitor, LY368975, reduces food consumption in animal models of feeding. J Pharmacol Exp Ther. 1998;287:122–127. [PubMed] [Google Scholar]
- Geiger BM, Behr GG, Frank LE, Caldera-Siu AD, Beinfeld MC, Kokkotou EG, et al. Evidence for defective mesolimbic dopamine exocytosis in obesity-prone rats. FASEB J. 2008;22:2740–2746. doi: 10.1096/fj.08-110759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick SD, Haskew RE, Maisonneuve IM, Carlson JN, Jerussi TP. Enantioselective behavioral effects of sibutramine metabolites. Eur J Pharmacol. 2000;397:93–102. doi: 10.1016/s0014-2999(00)00216-8. [DOI] [PubMed] [Google Scholar]
- Golozoubova V, Strauss F, Malmlof K. Locomotion is the major determinant of sibutramine-induced increase in energy expenditure. Pharmacol Biochem Behav. 2006;83:517–527. doi: 10.1016/j.pbb.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Grignaschi G, Fanelli E, Scagnol I, Samanin R. Studies on the role of serotonin receptor subtypes in the effect of sibutramine in various feeding paradigms in rats. Br J Pharmacol. 1999;127:1190–1194. doi: 10.1038/sj.bjp.0702633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halford JC, Harrold JA, Boyland EJ, Lawton CL, Blundell JE. Serotonergic drugs : effects on appetite expression and use for the treatment of obesity. Drugs. 2007;67:27–55. doi: 10.2165/00003495-200767010-00004. [DOI] [PubMed] [Google Scholar]
- Heal DJ, Cheetham SC, Prow MR, Martin KF, Buckett WR. A comparison of the effects on central 5-HT function of sibutramine hydrochloride and other weight-modifying agents. Br J Pharmacol. 1998;125:301–308. doi: 10.1038/sj.bjp.0702067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson HC, Bearham MC, Hutchins LJ, Mazurkiewicz SE, Needham AM, Heal DJ. Investigation of the mechanisms underlying the hypophagic effects of the 5-HT and noradrenaline reuptake inhibitor, sibutramine, in the rat. Br J Pharmacol. 1997;121:1613–1618. doi: 10.1038/sj.bjp.0701311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraszewski KZ, Cincotta AH. Increased responsiveness of ventromedial hypothalamic neurons to norepinephrine in obese versus lean mice: relation to the metabolic syndrome. Int J Mol Med. 2000;5:349–355. doi: 10.3892/ijmm.5.4.349. [DOI] [PubMed] [Google Scholar]
- Kung MP, Choi SR, Hou C, Zhuang ZP, Foulon C, Kung HF. Selective binding of 2-[125I]iodo-nisoxetine to norepinephrine transporters in the brain. Nucl Med Biol. 2004;31:533–541. doi: 10.1016/j.nucmedbio.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Kuo DY. Co-administration of dopamine D1 and D2 agonists additively decreases daily food intake, body weight and hypothalamic neuropeptide Y level in rats. J Biomed Sci. 2002;9:126–132. doi: 10.1007/BF02256023. [DOI] [PubMed] [Google Scholar]
- Lehr T, Staab A, Tillmann C, Nielsen EO, Trommeshauser D, Schaefer HG, et al. Contribution of the active metabolite M1 to the pharmacological activity of tesofensine in vivo: a pharmacokinetic-pharmacodynamic modelling approach. Br J Pharmacol. 2008;153:164–174. doi: 10.1038/sj.bjp.0707539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin BE, Keesey RE. Defense of differing body weight set points in diet-induced obese and resistant rats. Am J Physiol. 1998;274:R412–R419. doi: 10.1152/ajpregu.1998.274.2.R412. [DOI] [PubMed] [Google Scholar]
- Liu YL, Connoley IP, Heal DJ, Stock MJ. Pharmacological characterisation of the thermogenic effect of bupropion. Eur J Pharmacol. 2004;498:219–225. doi: 10.1016/j.ejphar.2004.07.004. [DOI] [PubMed] [Google Scholar]
- McQuade JA, Benoit SC, Woods SC, Seeley RJ. 7-OH-DPAT selectively reduces intake of both chow and high fat diets in different food intake regimens. Pharmacol Biochem Behav. 2003;76:517–523. doi: 10.1016/j.pbb.2003.09.008. [DOI] [PubMed] [Google Scholar]
- McQuade JA, Benoit SC, Xu M, Woods SC, Seeley RJ. High-fat diet induced adiposity in mice with targeted disruption of the dopamine-3 receptor gene. Behav Brain Res. 2004;151:313–319. doi: 10.1016/j.bbr.2003.09.034. [DOI] [PubMed] [Google Scholar]
- Meguid MM, Fetissov SO, Blaha V, Yang ZJ. Dopamine and serotonin VMN release is related to feeding status in obese and lean Zucker rats. Neuroreport. 2000a;11:2069–2072. doi: 10.1097/00001756-200007140-00002. [DOI] [PubMed] [Google Scholar]
- Meguid MM, Fetissov SO, Varma M, Sato T, Zhang L, Laviano A, et al. Hypothalamic dopamine and serotonin in the regulation of food intake. Nutrition. 2000b;16:843–857. doi: 10.1016/s0899-9007(00)00449-4. [DOI] [PubMed] [Google Scholar]
- Meguid MM, Yang ZJ, Laviano A. Meal size and number: relationship to dopamine levels in the ventromedial hypothalamic nucleus. Am J Physiol. 1997;272:R1925–R1930. doi: 10.1152/ajpregu.1997.272.6.R1925. [DOI] [PubMed] [Google Scholar]
- Mori RC, Guimaraes RB, Nascimento CM, Ribeiro EB. Lateral hypothalamic serotonergic responsiveness to food intake in rat obesity as measured by microdialysis. Can J Physiol Pharmacol. 1999;77:286–292. [PubMed] [Google Scholar]
- Morien A, McMahon L, Wellman PJ. Effects on food and water intake of the alpha 1-adrenoceptor agonists amidephrine and SK&F-89748. Life Sci. 1993;53:169–174. doi: 10.1016/0024-3205(93)90664-o. [DOI] [PubMed] [Google Scholar]
- Morien A, Wellman PJ, Fojt J. Diurnal rhythms of paraventricular hypothalamic norepinephrine and food intake in rats. Pharmacol Biochem Behav. 1995;52:169–174. doi: 10.1016/0091-3057(95)00084-a. [DOI] [PubMed] [Google Scholar]
- Nelson DL, Gehlert DR. Central nervous system biogenic amine targets for control of appetite and energy expenditure. Endocrine. 2006;29:49–60. doi: 10.1385/endo:29:1:49. [DOI] [PubMed] [Google Scholar]
- Orosco M, Nicolaidis S. Spontaneous feeding-related monoaminergic changes in the rostromedial hypothalamus revealed by microdialysis. Physiol Behav. 1992;52:1015–1019. doi: 10.1016/0031-9384(92)90384-e. [DOI] [PubMed] [Google Scholar]
- Orosco M, Rouch C, Meile MJ, Nicolaidis S. Spontaneous feeding-related monoamine changes in rostromedial hypothalamus of the obese Zucker rat: a microdialysis study. Physiol Behav. 1995;57:1103–1106. doi: 10.1016/0031-9384(94)00383-g. [DOI] [PubMed] [Google Scholar]
- Pfeffer AO, Samson HH. Haloperidol and apomorphine effects on ethanol reinforcement in free feeding rats. Pharmacol Biochem Behav. 1988;29:343–350. doi: 10.1016/0091-3057(88)90167-0. [DOI] [PubMed] [Google Scholar]
- Pothos EN, Sulzer D, Hoebel BG. Plasticity of quantal size in ventral midbrain dopamine neurons: possible implications for the neurochemistry of feeding and reward. Appetite. 1998;31:405. doi: 10.1006/appe.1998.0210. [DOI] [PubMed] [Google Scholar]
- Racotta R, Soto-Mora LM. Specificity of alpha- and beta-adrenergic inhibition of water and food intake. Physiol Behav. 1993;53:361–365. doi: 10.1016/0031-9384(93)90218-5. [DOI] [PubMed] [Google Scholar]
- Rittenhouse PA, Bakkum EA, Levy AD, Li Q, Carnes M, van de Kar LD. Evidence that ACTH secretion is regulated by serotonin2A/2C (5-HT2A/2C) receptors. J Pharmacol Exp Ther. 1994;271:1647–1655. [PubMed] [Google Scholar]
- Scislowski PW, Tozzo E, Zhang Y, Phaneuf S, Prevelige R, Cincotta AH. Biochemical mechanisms responsible for the attenuation of diabetic and obese conditions in ob/ob mice treated with dopaminergic agonists. Int J Obes Relat Metab Disord. 1999;23:425–431. doi: 10.1038/sj.ijo.0800893. [DOI] [PubMed] [Google Scholar]
- Shimizu H, Fisler S, Bray GA. Extracellular hypothalamic monoamines measured by in vivo microdialysis in a rat model of dietary fat-induced obesity. Obes Res. 1994;2:100–109. doi: 10.1002/j.1550-8528.1994.tb00635.x. [DOI] [PubMed] [Google Scholar]
- Spiller K, Xi ZX, Peng XQ, Newman AH, Ashby CR, Jr, Heidbreder C, et al. The selective dopamine D3 receptor antagonists SB-277011A and NGB 2904 and the putative partial D3 receptor agonist BP-897 attenuate methamphetamine-enhanced brain stimulation reward in rats. Psychopharmacology (Berl) 2008;196:533–542. doi: 10.1007/s00213-007-0986-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley BG, Schwartz DH, Hernandez L, Hoebel BG, Leibowitz SF. Patterns of extracellular norepinephrine in the paraventricular hypothalamus: relationship to circadian rhythm and deprivation-induced eating behavior. Life Sci. 1989;45:275–282. doi: 10.1016/0024-3205(89)90136-7. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wise RA. How can drug addiction help us understand obesity. Nat Neurosci. 2005;8:555–560. doi: 10.1038/nn1452. [DOI] [PubMed] [Google Scholar]
- Wellman PJ. Norepinephrine and the control of food intake. Nutrition. 2000;16:837–842. doi: 10.1016/s0899-9007(00)00415-9. [DOI] [PubMed] [Google Scholar]
- Wellman PJ. Modulation of eating by central catecholamine systems. Curr Drug Targets. 2005;6:191–199. doi: 10.2174/1389450053174532. [DOI] [PubMed] [Google Scholar]
- Wellman PJ, Davies BT. Reversal of cirazoline- and phenylpropanolamine-induced anorexia by the alpha 1-receptor antagonist prazosin. Pharmacol Biochem Behav. 1992;42:97–100. doi: 10.1016/0091-3057(92)90452-l. [DOI] [PubMed] [Google Scholar]
- Wilmot CA, Sullivan AC, Levin BE. Effects of diet and obesity on brain alpha 1- and alpha 2-noradrenergic receptors in the rat. Brain Res. 1988;453:157–166. doi: 10.1016/0006-8993(88)90154-0. [DOI] [PubMed] [Google Scholar]
- Wortley KE, Hughes ZA, Heal DJ, Stanford SC. Comparison of changes in the extracellular concentration of noradrenaline in rat frontal cortex induced by sibutramine or d-amphetamine: modulation by alpha2-adrenoceptors. Br J Pharmacol. 1999;127:1860–1866. doi: 10.1038/sj.bjp.0702720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZJ, Meguid MM, Chai JK, Chen C, Oler A. Bilateral hypothalamic dopamine infusion in male Zucker rat suppresses feeding due to reduced meal size. Pharmacol Biochem Behav. 1997;58:631–635. doi: 10.1016/s0091-3057(97)00022-1. [DOI] [PubMed] [Google Scholar]
- Young WS, 3rd, Kuhar MJ. Noradrenergic alpha 1 and alpha 2 receptors: light microscopic autoradiographic localization. Proc Natl Acad Sci USA. 1980;77:1696–1700. doi: 10.1073/pnas.77.3.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]