Abstract
We identify Drosophila TACC (D–TACC) as a novel protein that is concentrated at centrosomes and interacts with microtubules. We show that D–TACC is essential for normal spindle function in the early embryo; if D–TACC function is perturbed by mutation or antibody injection, the microtubules emanating from centrosomes in embryos are short and chromosomes often fail to segregate properly. The C–terminal region of D–TACC interacts, possibly indirectly, with microtubules, and can target a heterologous fusion protein to centrosomes and microtubules in embryos. This C–terminal region is related to the mammalian transforming, acidic, coiled-coil-containing (TACC) family of proteins. The function of the TACC proteins is unknown, but the genes encoding the known TACC proteins are all associated with genomic regions that are rearranged in certain cancers. We show that at least one of the mammalian TACC proteins appears to be associated with centrosomes and microtubules in human cells. We propose that this conserved C–terminal ‘TACC domain’ defines a new family of microtubule-interacting proteins.
Keywords: cancer/centrosome/microtubules/mitosis/mitotic spindle
Introduction
Centrosomes, and the microtubules they nucleate, play a crucial role in organizing many processes in eukaryotic cells (Glover et al., 1993; Kellogg et al., 1994). The dynamic behaviour of microtubules is essential to their function, and drugs that interfere with microtubule dynamics invariably interfere with microtubule function (Jordan et al., 1992). The molecular mechanisms that regulate microtubule dynamics in the cell, however, are still poorly understood (Hyman and Karsenti, 1996; Desai and Mitchison, 1997; Andersen, 1999; Cassimeris, 1999).
A number of proteins have been shown to influence microtubule dynamics in vitro. These include various microtubule-associated proteins (MAPs) that bind directly to microtubules and appear to stabilize them (Gard and Kirschner, 1987; Andersen et al., 1994; Hirokawa, 1994; Vasquez et al., 1994; Andersen and Karsenti, 1997). More recently, factors that destabilize microtubules have also been identified (Belmont and Mitchison, 1996; Marklund et al., 1996; Walczak et al., 1996; Desai et al., 1999). Experiments in Xenopus extracts have provided strong evidence that several of these factors play important roles in regulating microtubule behaviour during the cell cycle (Gard and Kirschner, 1987; Andersen et al., 1994, 1997; Vasquez et al., 1994; Belmont and Mitchison, 1996; Walczak et al., 1996; Andersen and Karsenti, 1997; Tournebize et al., 1997; Andersen, 1998; Cha et al., 1998; Desai et al., 1999). The role of most of these proteins in regulating microtubule behaviour in vivo, however, is less clear.
Drosophila is a powerful, genetically tractable system in which a number of proteins have been identified that appear to regulate microtubule behaviour in vivo (see, for example, Gatti and Goldberg, 1991; Moritz et al., 1995; Sunkel et al, 1995; Saunders et al., 1997; Williams et al., 1997; Starr et al., 1998; Cullen et al., 1999; do Carmo Avides and Glover, 1999). By passing extracts of early Drosophila embryos over an affinity column of stabilized microtubules, Kellogg et al. (1989) isolated a large number of proteins that associate with microtubules in vitro. Antisera were raised against >20 of these proteins, and most of these antisera recognized proteins that were associated with microtubule structures in vivo. The distribution of the proteins varied: some were associated with centrosomes, either throughout the cell cycle or at specific stages of the cell cycle, while others were associated with spindle microtubules or kinetochores. The genes encoding several of these proteins have now been cloned and characterized, but, in most cases, their functions remain unknown (Kellogg et al., 1995; Whitfield et al., 1995; Kidd and Raff, 1997; Oegema et al., 1997). These studies demonstrate that the repertoire of proteins that interact with microtubules, either directly or indirectly, is likely to be large.
Here we report the cloning of a gene that encodes a Drosophila protein that co-purifies with microtubules in a microtubule spin-down experiment. We show that the protein, Drosophila TACC (D–TACC), is concentrated at centrosomes in vivo, and is essential for mitotic spindle function in the early fly embryo. In embryos where D–TACC function has been perturbed either by mutation or antibody injection, centrosomal microtubules are short. The C–terminal region of D–TACC is related to the mammalian TACC family of proteins. The function of these proteins is unknown, but it has been suggested that they are linked to cancer: the known TACC genes are all in genomic regions that are rearranged in certain cancer cells; TACC3 is overexpressed in some cancer cell lines; and overexpressing TACC1 transforms mouse cells (Still et al., 1999a,b). We show that this conserved C–terminal region targets D–TACC to centrosomes and microtubules in Drosophila, and that at least one mammalian TACC protein appears to be associated with centrosomes and microtubules in human cells. We propose that this conserved C–terminal ‘TACC domain’ defines a new family of microtubule-interacting proteins, and we discuss how this might explain the potential link between these genes and cancer.
Results
D–TACC is a centrosomal protein that interacts with microtubules in fly embryos
We isolated proteins from Drosophila embryo extracts that co-fractionated with the endogenous microtubules in a microtubule spin-down experiment, as described previously (Kellogg et al., 1989; Raff et al., 1993). We raised mouse antisera against several of these proteins, and one of them stained centrosomes strongly and microtubules weakly throughout the cell cycle in fly embryos. This serum recognized an ∼220 kDa protein in Western blotting experiments, and we used it to screen a cDNA expression library. A single positive clone was isolated and the cDNA was used to isolate a full-length cDNA that we call Drosophila tacc (d–tacc; see below).
We raised and affinity-purified antibodies against a recombinant D–TACC fusion protein. In embryos, these antibodies strongly stained centrosomes and weakly stained microtubules throughout the cell cycle (Figure 1A–C), just as the initial mouse sera did. D–TACC remained concentrated at centrosomes in embryos that were treated with colchicine to depolymerize their microtubules (Figure 1D), suggesting that it is an integral centrosomal protein (Kalt and Schliwa, 1993). To confirm the distribution of D–TACC, we expressed a full-length D–TACC–green fluorescent protein (GFP) fusion protein in early embryos. The fusion protein was fully functional as it rescued a mutation in the d–tacc gene (see below), and it was highly concentrated at centrosomes throughout the cell cycle in living embryos (Figure 2A). D–TACC–GFP also bound weakly to astral and spindle microtubules, often having a punctate distribution on the microtubules. During mitosis, D–TACC–GFP became slightly concentrated in the region of the spindle where the minus ends of the microtubules were clustered near to, but slightly detached from, the centrosomes (arrows, Figure 2A). In fixed embryos, the centrosomal protein γ–tubulin had a similar distribution during mitosis (Figure 2B). This suggests that D–TACC may interact preferentially with the minus ends of microtubules, as is widely believed to be the case for γ–tubulin.
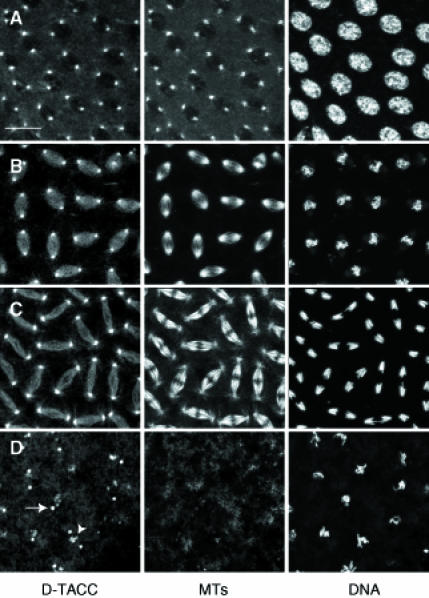
Fig. 1. Fixed wild-type embryos stained to reveal the distribution of D–TACC (left panels), microtubules (middle panels) and DNA (right panels). (A, B and C) Embryos in early prophase, metaphase and anaphase, respectively. (D) An embryo that was treated with colchicine prior to fixation to depolymerize the microtubules. D–TACC remains concentrated at the centrosomes (arrow); these bright dots were shown to be centrosomes in co-staining experiments with anti-γ–tubulin antibodies (not shown). D–TACC is also concentrated around some regions of the condensed chromatin in colchicine-treated embryos (arrowhead); the significance of this localization (if any) is not known. Scale bar, 20 μm.
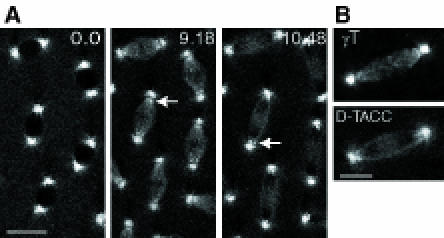
Fig. 2. (A) The distribution of D–TACC–GFP in a living embryo. Time is shown in minutes and seconds in the top right corner of each panel. In interphase (0.0), the protein is concentrated at centrosomes, but also spreads out in a slightly punctate fashion along the astral microtubules close to the centrosome. In metaphase (9.18), the protein remains concentrated at centrosomes but also associates with the mitotic spindle, where it becomes concentrated in the region of the spindle where the minus ends of the spindle microtubules are slightly separated from the centrosomes (arrow). In anaphase (10.48), the chromosomes (seen as dark shadows on the spindle) move to the poles of the spindle. The concentration of D–TACC–GFP in the region of the detached minus ends of the spindle microtubules is still apparent, and, in some spindles, small dots of D–TACC–GFP may be associating with the kinetochores of the chromosomes as they move towards the spindle poles (arrow). Scale bar, 20 μm. (B) A comparison of the distribution of γ–tubulin (top panel) in a methanol-fixed mitotic spindle and D–TACC–GFP (bottom panel) in the mitotic spindle of a living embryo (see text for details). Scale bar, 10 μm.
D–TACC appears to bind indirectly to microtubules via its C–terminal region
In Western blotting experiments, the anti-D–TACC antibodies recognized an ∼220 kDa protein (Figure 3A). The majority of this protein (60–80%) co-fractionated with the microtubules that were polymerized in embryo extracts and then pelleted through a sucrose cushion (Figure 3B, panel 1); for comparison, only 20–30% of the γ–tubulin co-pelleted with microtubules in this experiment (Figure 3B, panel 2).
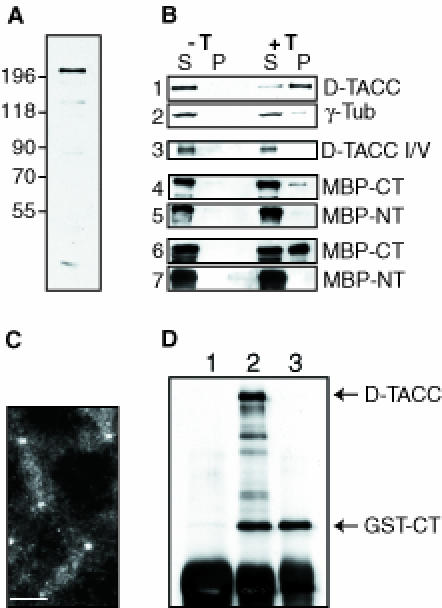
Fig. 3. An analysis of the microtubule-binding and centrosomal localization properties of D–TACC. (A) A Western blot of 0–4 h old embryos probed with affinity-purified anti-D–TACC antibodies. The antibodies recognize a major band of ∼220 kDa. They also recognize a number of smaller proteins that appear to be breakdown products of the ∼220 kDa protein as overexpressing the D–TACC protein from the full-length D–TACC cDNA increases the level of all of these bands, while a mutation in the D–TACC gene decreases the level of all of these bands (see, for example, Figure 4). (B) Western blots of various microtubule spin-down experiments. S, supernatant; P, pellet (equal fractions of which were loaded in each lane); –T is a control experiment where no taxol was added; and +T is an experiment where taxol was added to polymerize the microtubules. Panels 1 and 2, the endogenous D–TACC or γ–tubulin proteins in embryo extracts. Panel 3, in vitro translated D–TACC mixed with purified tubulin. (On longer exposures of this blot, a small amount of D–TACC was detectable in the pellet, not shown.) Panels 4 and 5, purified MBP–NT and MBP–CT fusion proteins mixed with purified tubulin. Panels 6 and 7, purified MBP–NT and MBP–CT fusion proteins mixed with embryo extracts. The MBP–Mid fusion protein behaved in a similar manner to the MBP–NT, while a GST–CT fusion protein behaved in a very similar manner to MBP–CT (not shown). (C) Immunofluorescence staining of a transgenic embryo expressing the GST–CT fusion protein with affinity-purified anti-GST antibodies. In wild-type embryos, these antibodies give only a weak background cytoplasmic staining. In the GST–CT-expressing embryos, the antibodies strongly stain centrosomes and weakly stain microtubules. Scale bar, 10 μm. (D) Immunoprecipitations were performed with extracts made from embryos expressing the GST–CT fusion protein. Anti-D–TACC antibodies (raised against the C–terminal region of D–TACC) precipitate both full-length D–TACC and the GST–CT fusion protein, as expected (lane 2). Anti-GST antibodies precipitate only the GST–CT protein (lane 3), demonstrating that, in embryo extracts, GST–CT does not oligomerize with the endogenous D–TACC protein. (This may explain why the GST–CT fusion protein does not appear to interfere dominantly with the function of the endogenous D–TACC). Random rabbit IgG precipitates neither of these proteins (lane 1). The IgG heavy chain from the antibodies used in the immunoprecipitation is recognized by the anti-rabbit secondary antibodies used to probe this blot, and it is shown here as a loading control (bottom band).
The interaction of D–TACC with stabilized microtubules appeared to be specific as the protein in embryo extracts did not co-pellet with actin filaments in microfilament spin-down experiments (not shown). Surprisingly, however, full-length D–TACC, translated in vitro, bound to microtubules only weakly, if at all, in spin-down experiments (Figure 3B, panel 3). To address further how D–TACC interacts with microtubules, we tested whether bacterially expressed and purified glutathione S-transferase (GST) or maltose-binding protein (MBP) fusion proteins that contained only the N–terminal (NT), middle (Mid) or C–terminal (CT) regions of D–TACC could associate with purified microtubules. None of these proteins bound strongly to microtubules, although a small fraction (∼5%) of the GST–CT and MBP–CT fusion proteins reproducibly co-pelleted with microtubules (Figure 3B, panels 4 and 5). If, however, these fusion proteins were mixed with embryo extracts, a significant fraction (∼50–60%) of the GST–CT or MBP–CT co-pelleted with microtubules, while none of the other fusion proteins interacted significantly with microtubules (Figure 3B, panels 6 and 7).
A GST–CT fusion protein also bound strongly to microtubules in extracts made from transgenic embryos that expressed the GST–CT fusion protein (not shown). In these transgenic embryos, the fusion protein was also localized in a manner very similar to the endogenous D–TACC protein, being strongly concentrated at centrosomes and more weakly concentrated on microtubules (Figure 3C). As this C–terminal region is predicted to form a coiled-coil structure (see below), it was possible that the GST–CT fusion protein was binding to centrosomes and microtubules in the transgenic embryos as an oligomer with the full-length endogenous D–TACC protein. Immunoprecipitation experiments, however, suggested that GST–CT did not form an oligomer with the endogenous D–TACC, at least in embryo extracts (Figure 3D). Thus, the C–terminal region of D–TACC appears to interact with microtubules and centrosomes in vivo, but a strong interaction with microtubules in vitro appears to require other factors present in the embryo extracts.
D–TACC is required for mitotic spindle function in the early embryo
To investigate the function of D–TACC, we isolated a mutation in the d–tacc gene, which we mapped to 82C within the deficiencies Df(3R)110 and Df(3R)ME15. None of the available mutations in this region appeared to be in the d–tacc gene, as none were rescued by the expression of the full-length D–TACC cDNA under the control of the polyubiquitin promoter (Pubq-D–TACC), which gives high levels of expression at all stages of development (Lee et al., 1988). We therefore performed an ethane methylsulfonate (EMS) mutagenesis screen to identify mutations that were either lethal or female sterile in combination with Df(3R)110. We screened ∼4000 chromosomes and found that the female sterility of one of the female sterile mutations isolated in this screen was fully rescued by the Pubq-D–TACC or Pubq-D–TACC–GFP transgenes. Western blotting revealed that this female sterile mutant produced an apparently full-length D–TACC protein, but at ∼10–fold lower levels than normal (Figure 4). We conclude that the female sterility of these flies is due to a mutation in the d-tacc gene and we call the mutation d-tacc1.
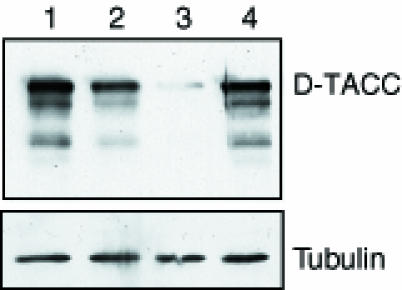
Fig. 4. Western blot showing the amount of D–TACC protein in 0–4 h embryos of the following genotypes: +/+ (lane 1); +/Df(3R)110 (lane 2); d-tacc1/Df(3R)110 (lane 3); and d-tacc1/Df(3R)110 embryos that also carry one copy of the PUbq-D–TACC transgene (lane 4). The same blot was also probed for α–tubulin as a loading control.
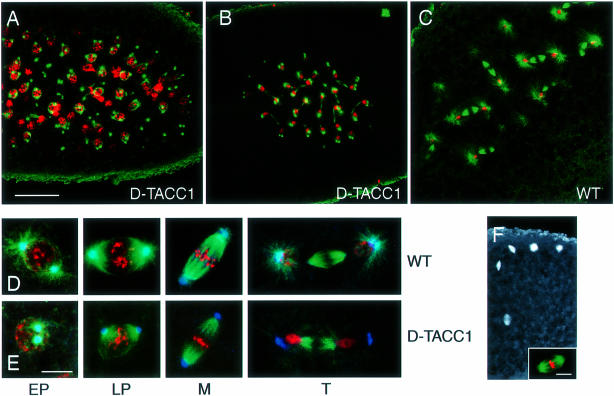
We fixed embryos laid by d–tacc1/Df(3R)110 females (hereafter referred to as D–TACC1 embryos) and examined the distribution of nuclei and microtubules. These embryos fell into two classes. About 30–50% of embryos initiated development but died during early embryogenesis from an accumulation of mitotic defects, with many free centrosomes and polyploid/aneuploid nuclei (Figure 5A). These mitotic defects looked similar in many respects to the defects seen in many other mitotic mutants, but these D–TACC1 embryos displayed one unusual feature: the nuclei often failed to migrate to the cortex and remained tightly clustered in the middle of the embryo (compare Figure 5B and C). Normal nuclear migration requires microtubules (Zalokar and Erk, 1976; Raff and Glover, 1989), and the microtubules emanating from the centrosomes in D–TACC1 embryos often appeared to be much shorter than normal at all stages of the cell cycle, even in embryos that had not yet developed mitotic defects (Figure 5B–E).
Fig. 5. The behaviour of chromosomes (red) and microtubules (green) in wild-type and D–TACC1 embryos. These images are projections that combine several cross-sections from each embryo. Thus, some spindles are not entirely within the sections shown, explaining why some of the spindles in (C) appear to have some nuclei missing. (A) A D–TACC1 embryo showing typical mitotic defects. (B) A D–TACC1 embryo and (C) a wild-type embryo that are both at nuclear cycle 6 (more nuclei are visible in the D–TACC1 embryo because they are tightly clustered in the middle of the embryo; in comparison, the nuclei in the wild-type embryo are well separated and are migrating towards the embryo cortex). From the degree of nuclear decondensation and centrosome separation, these embryos are at a very similar stage of late telophase. Nuclear migration has been postulated to depend on the long astral microtubules nucleated by the centrosomes during anaphase/telophase (Baker et al., 1993), and these microtubules are much shorter and weaker in the D–TACC1 embryo. (D and E) The behaviour of microtubules (green), chromosomes (red) and γ–tubulin (blue) at different stages of the cell cycle in wild-type (D) and D–TACC1 (E) embryos. The D–TACC1 images were all taken from embryos that had not yet developed significant mitotic defects. EP, early prophase; LP, late prophase; M, metaphase; T, telophase. The microtubules associated with the centrosomes in D–TACC1 embryos are abnormally short at all stages of the cell cycle, but γ–tubulin remains concentrated at the centrosomes. Note how the centrosomes in D–TACC1 embryos often fail to separate properly in interphase, perhaps because the microtubules associated with the centrosomes are short. (F) A D–TACC1 embryo that has failed in pronuclear fusion. The microtubules around the four female meiotic products are at the cortex, while those around the male pronucleus are in the middle of the embryo. The microtubules surrounding the female pronuclei in these embryos sometimes adopt a bipolar arrangement, as sometimes occurs in wild-type unfertilized eggs (J.W.Raff, unpublished observations). The inset shows the spindle surrounding the male pronucleus and the apparently haploid set of chromosomes typically found in these embryos arrested at the first nuclear cycle (this spindle is from a different embryo to that in the main figure). Centrosomes appear to be present at the poles of these spindles (not shown), as are astral microtubules (although these are usually hard to see as they are very short). Scale bar (A, B, C and F) = 30 μm; (D and E) = 10 μm; (F, inset = 5 μm).
The second class of D–TACC1 embryos (50–70%) failed to develop at all, and appeared to arrest at the first mitotic division with a single spindle surrounding an apparently haploid set of chromosomes (Figure 5F). In these embryos, the four female pronuclei seemed to form normally, but none of them migrated to fuse with the male pronucleus, a process dependent on the long astral microtubules nucleated by the sperm centrosome (Callaini and Riparbelli, 1996).
In agreement with the Western blotting results, when D–TACC was visualized by immunostaining in D–TACC1 embryos, it appeared to be present and localized correctly, but at much lower levels than normal (not shown). The centrosomal proteins γ–tubulin (Figure 5D and E), CP60 and CP190 (not shown) were also distributed normally, suggesting that centrosome structure was not grossly altered in D–TACC1 embryos. Similar defects to those shown in Figure 5 were seen in embryos laid by d–tacc1/Df(3R)ME15 females, and these defects were not seen in D–TACC1 embryos laid by mothers carrying a single copy of the PUbq-D–TACC or PUbq-D–TACC–GFP transgene (not shown). Recently, several other female sterile alleles of d–tacc have been identified on the basis of their failure in pronuclear fusion and their early mitotic phenotype (J.Kramer, B.Theurkauf and M.Goldberg, personal communication).
Spindle microtubules are short in embryos injected with anti-D–TACC antibodies
The defects seen in D–TACC1 embryos suggested that D–TACC might be involved normally in stabilizing or nucleating centrosomal microtubules, but the primary cause of the defects was difficult to determine from an analysis of a series of fixed embryos. We therefore injected syncytial blastoderm embryos with anti-D–TACC antibodies that had been labelled with Texas red; these embryos also expressed a tau–GFP fusion protein (Brand, 1995) so that we could follow the behaviour of the microtubules and antibodies at the same time in living embryos.
An injection of Texas red-labelled random IgG antibodies had no effect on the behaviour of microtubules, and the majority of injected embryos hatched as larvae. An injection of anti-D–TACC antibodies, in contrast, severely disrupted microtubule behaviour, and the injected embryos never hatched. The antibodies bound to the centrosomes around the injection site (note the increasingly orange/yellow colour of the centrosomes the closer they are to the injection site; Figure 6A, 1.00). The antibodies also started to form prominent clumps in the cytoplasm around the injection site, suggesting that they might be locally precipitating the D–TACC protein (Figure 6A, 2.20–10.54); this makes it difficult to tell whether the antibody is binding to centrosomes in this area at later time points. As the embryos entered mitosis, the spindles that formed closest to the injection site appeared to contain approximately normal numbers of microtubules, but the microtubules were shorter than normal (Figure 6A and B, 2.20–5.36). These spindles remained short throughout mitosis and they were delayed in exiting mitosis by ∼2 min (Figure 6A and B, 5.36–7.24). When the spindles eventually entered anaphase, the chromosomes moved towards the spindle poles (Figure 6A and B, 7.24; the position of the chromosomes can be inferred from their ‘shadows’ on the spindles, highlighted here with arrows), but they were poorly separated on the short spindles and often fused back together forming polyploid nuclei (Figure 6A and B, 10.54).
Fig. 6. The behaviour of microtubules (green in the colour panels, and also shown in black and white in the bottom panels) and injected anti-D–TACC antibodies (red in the colour panels) in living embryos. The time after injection (in minutes and seconds) is shown at the top right corner of each colour panel, and below the black and white panels. The site of injection is at the middle left of each colour panel. The black and white panels show a magnified view of a spindle that is close to (top panels) or far away from (bottom panels) the injection site. Both spindles are at approximately the same position on the anterior–posterior axis, and so would normally be going through the cell cycle synchronously. The spindles close to the injection site are delayed in exiting mitosis by ∼2 min in this embryo. Scale bar, 20 μm (colour panels) and 10 μm (black and white panels).
As a further control, we injected labelled antibodies against the centrosomal protein CP60. These antibodies also bound to centrosomes, but they did not induce these mitotic defects (not shown).
The C–terminal region of D–TACC is related to the mammalian TACC proteins
The sequence of the D–TACC cDNA revealed a single large open reading frame encoding a protein of 1190 amino acids. Analysis of this sequence revealed that the C–terminal ∼200 amino acids had a high probability of forming a coiled coil (Figure 7A). In BLAST searches of sequence databases (Altschul et al., 1990), this region of D–TACC identified a large number of coiled-coil proteins, but it was most homologous to the TACC family of proteins (a Blast E–value of between 5 × 10–17 and 2 × 10–18 compared with 7 × 10–6 for the next most closely related coiled-coil protein; Figure 7B). The functions of the TACC proteins are unknown, but the genes encoding the known TACC proteins are all associated with chromosomal rearrangements found in certain human cancers, and it has been proposed that these proteins might play a role in tumourigenesis (Still et al., 1999a,b). TACC1 and TACC3 have been fully sequenced (Still et al., 1999a,b; F.Gergely and J.W.Raff, unpublished results) and TACC2 partially sequenced (F.Gergely and J.W.Raff, unpublished results; I.Still and J.Cowell, personal communication). From the available sequences, the human proteins are highly conserved within the putative coiled-coil domain (60–75% identity), but are much less well conserved outside of this domain. Apart from this putative coiled-coil region, none of the TACC or D–TACC proteins show significant sequence homology to any other proteins in the databases.
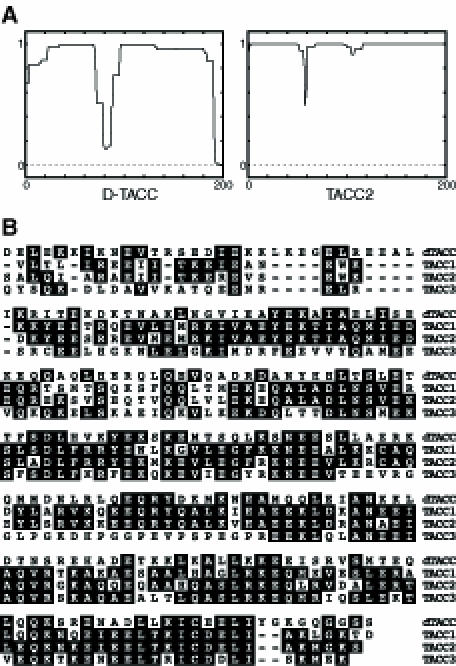
Fig. 7. (A) The C–terminal ∼200 amino acids of D–TACC are predicted to form a coiled-coil structure. The graph shows the predicted probability (from 0 to 1 on the y-axis) that the sequence will form a coiled-coil structure (Lupas et al., 1991). The C–terminal ∼200 amino acids of the TACC family of proteins are also predicted to form coiled coils (only TACC2 is shown here). (B) A sequence alignment of the C–terminal ∼200 amino acids of D–TACC and TACC1, 2 and 3.
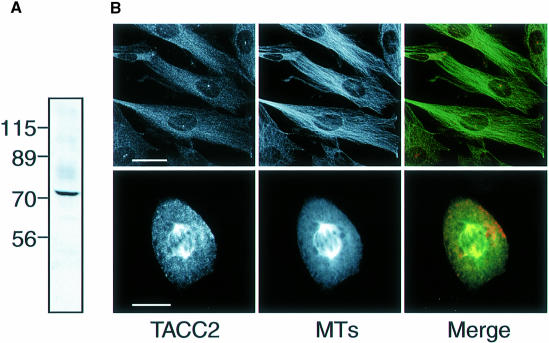
To test whether the human TACC proteins might also be associated with centrosomes and microtubules, we raised and affinity-purified antibodies against one of the TACC proteins, TACC2. The antibodies recognized a major band of ∼73 kDa in Western blots of HeLa cells (Figure 8A). In both HeLa cells and primary human fibroblasts, the antibodies stained centrosomes throughout the cell cycle as well as the microtubules of the mitotic spindle (Figure 8B), although some protein was also distributed throughout the cytoplasm. Thus, TACC2, like D–TACC, appears to be a centrosomal protein that can interact with microtubules.
Fig. 8. (A) Western blot of total HeLa cell extract probed with affinity-purified anti-TACC2 antibodies. (B) Primary fibroblast cells (derived from human foreskin) were fixed with methanol as described previously (Pines and Hunter, 1991). Primary fibroblast cells in interphase (top panels) and metaphase (bottom panels) stained with the affinity-purified anti-TACC2 antibodies (left panels) and with an anti–tubulin antibody (middle panels). The right panels show a merged image: TACC2 (red) and microtubules (green). Scale bar, 10 μm (top panels) and 4 μm (bottom panels).
Discussion
D–TACC is a novel centrosomal protein that can associate with microtubules. Although it binds strongly to microtubules in embryo extracts, full-length D–TACC, translated in vitro, binds only weakly, if at all, to purified microtubules. Purified fusion proteins containing the N–terminal, middle or C–terminal regions of D–TACC also do not bind strongly to purified microtubules, although a small fraction of the C–terminal fusion proteins reproducibly co-pellets with the microtubules. If, however, the C–terminal fusion proteins are mixed with embryo extract, then they strongly interact with microtubules, suggesting that D–TACC interacts with microtubules via its C–terminal region, and that this interaction requires other factors present in the embryo extracts. These factors in the extract may modify D–TACC to enable it to bind to microtubules, or they may bind to microtubules in a complex with D–TACC. We favour the latter possibility, as gel filtration and velocity sedimentation analysis of the endogenous D–TACC in embryo extracts suggest that D–TACC may be part of a larger complex (our unpublished observations).
Although D–TACC can interact with microtubules, its concentration at centrosomes may be independent of this interaction, as it remains concentrated at centrosomes in embryos where the microtubules have been depolymerized with colchicine. We cannot, however, exclude the possibility that some short microtubules remain associated with centrosomes under these conditions. Moreover, the same C–terminal region of D–TACC that interacts with microtubules in embryo extracts can also target a heterologous fusion protein to centrosomes. Thus, we remain cautious about whether D–TACC associates with centrosomes independently of microtubules.
D–TACC is essential for spindle function in the early embryo
To investigate the function of D–TACC, we have isolated a mutation in the D–TACC gene (d–tacc1). Flies homozygous for d–tacc1, or transheterozygous for d–tacc1 and a deficiency that uncovers d–tacc, are viable but female sterile. Centrosomal microtubules at all stages of the cell cycle are often shorter than normal in D–TACC1 embryos. Pronuclear fusion, nuclear migration and chromosome segregation are also often defective in these embryos; as all of these processes are thought to require centrosomal microtubules (Zalokar and Erk, 1976; Foe and Alberts, 1983; Raff and Glover, 1989; Baker et al., 1993; Foe et al., 1993; Callaini and Riparbelli, 1996), we suspect that they are defective as a secondary consequence of the abnormally short centrosomal microtubules.
As D–TACC1 embryos almost always develop severe mitotic defects prior to the migration of the nuclei to the cortex, it is not possible to observe microtubules directly at the cortex of living D–TACC1 embryos (Francis-Lang et al., 1999). We have therefore injected labelled anti-D–TACC antibodies into syncytial blastoderm embryos that expressed a tau–GFP fusion protein (Brand, 1995), allowing us to follow the behaviour of the antibodies and microtubules in real-time in living embryos. In these embryos, the injected antibodies bind to the centrosomes, and the spindles that form around the injection site are shorter than normal. Although chromosomes can move to the poles on these spindles, the chromosomes are often insufficiently separated on the short spindles and polyploid nuclei often form. These effects appear to be specific, as injecting labelled antibodies against CP60 (another centrosomal protein that binds to microtubules; Kellogg et al., 1995) does not cause similar defects, even though the antibodies bind strongly to centrosomes.
Taken together, our results suggest that D–TACC is normally required to organize or stabilize centrosomal microtubules. Although it is not clear how D–TACC functions, the interaction of the protein with microtubules and its concentration at centrosomes suggest that this effect is likely to be direct. One possibility is that D–TACC stabilizes microtubules that are nucleated from the centrosome. It is currently thought that microtubules are nucleated from γ–tubulin ring complexes (γ–TuRCs) in the centrosome (Moritz et al., 1995; Zheng et al., 1995). There is increasing evidence, however, that at least some of these microtubules can be released from their centrosomal nucleation sites, and, in many cell types, the released microtubules can be held in the vicinity of the centrosome by the action of microtubule motor proteins (Hyman and Karsenti, 1996; Keating et al., 1997; Compton, 1998). It is not clear how the minus ends of such free microtubules are stabilized in the cell (Rodionov et al., 1999). Perhaps D–TACC is involved in this stabilization. In support of this possibility, D–TACC–GFP becomes slightly concentrated in the area of the mitotic spindle where the minus ends of the microtubules are clustered near to, but slightly detached from, the centrosomes. γ–tubulin has a similar distribution in methanol-fixed embryos, raising the possibility that D–TACC might interact preferentially with the minus ends of microtubules, as is thought to be the case for γ–tubulin. We have no direct evidence, however, that D–TACC interacts preferentially with the minus ends of microtubules, and there are several other possible explanations for our results. D–TACC could, for example, be required to nucleate centrosomal microtubules, although D–TACC is unlikely to be a component of the γ–TuRC as there are apparently no proteins of the appropriate size in these complexes (Oegema et al., 1999). Alternatively, D–TACC could somehow serve to prevent the premature release of microtubules from the centrosome, perhaps by regulating the activity of centrosomal microtubule-severing activities (Karsenti, 1993).
Is D–TACC only required in the early embryo? The d–tacc1 mutation is viable but female sterile, and several other female sterile alleles of d–tacc have been isolated independently by other groups on the basis of their mitotic and pronuclear fusion defects (J.Kramer, B.Theurkauf and M.Goldberg, personal communication). These findings suggest that D–TACC may only be essential in the early embryo, perhaps because the rapid mitoses and large spindles of the early embryo impose a more stringent requirement for long spindle microtubules. In support of this possibility, the abnormally short spindles in the antibody-injected embryos are always delayed in exiting mitosis, suggesting that they transiently activate the spindle assembly checkpoint (Wells, 1996). Eventually, however, these spindles complete mitosis, although chromosome segregation often fails on the short spindles. Perhaps, at other stages of development, when mitosis is slower and the spindles no longer occupy a common cytoplasm, the activation of the spindle assembly checkpoint may allow the short spindles time to compensate for the loss of D–TACC function and complete mitosis successfully. On the other hand, our d–tacc1 mutation is unlikely to be a functional null as the mutants produce an apparently full-length protein but at only 10% of normal levels, and the protein is concentrated at centrosomes. The D–TACC protein is detectable at all stages of fly development (J.W.Raff, unpublished observations), and it is possible that a null mutation would be lethal.
D–TACC is related to the mammalian TACC family of proteins
The C–terminal ∼200 amino acids of D–TACC are predicted to form a coiled-coil structure, and this region is related to the mammalian transforming, acidic, coiled-coil-containing family of proteins. Although the sequence similarity between D–TACC and the three known mammalian TACC proteins is relatively weak (∼25–30% identity in the ∼200 amino acid C–terminal region), we believe it is functionally significant for three reasons. First, D–TACC is significantly more homologous to the known TACC proteins than to any other coiled-coil protein in the databases (a Blast E-value of ∼1 × 10–18 compared with 7 × 10–6 for the next most closely related coiled-coil protein). Secondly, although the mammalian TACC genes appear to have been generated by gene duplication events, the sequences of the known TACC proteins outside of this putative coiled-coil region are poorly conserved between family members (Still et al., 1999b; F.Gergely, J.W.Raff, I.Still and J.Cowell, unpublished data). Thus, it is perhaps not surprising that D–TACC is only related to the mammalian proteins in this region. Thirdly, TACC2 appears to have a very similar distribution in human cells to that of D–TACC in Drosophila cells, and the conserved C–terminal region of D–TACC appears to be important in determining this distribution in Drosophila cells. The simplest interpretation of these results is that this conserved region targets D–TACC and TACC2 to centrosomes and microtubules, and we speculate that the TACC proteins will all interact with microtubules via this conserved C–terminal domain. We are currently investigating whether this is the case.
Although the functions of the mammalian TACC proteins are unknown, the known TACC genes all map to regions of chromosomes that are rearranged in certain cancers, and it has been proposed that alterations in TACC gene function may contribute to tumorigenesis (Still et al., 1999a,b). All of the known TACC genes, however, are closely linked to fibroblast growth factor receptor (FGFR) genes (presumably the consequence of linked gene duplication events), and these genes are also attractive candidates for any gene(s) in these regions whose disruption could contribute to tumorigenesis. Thus, a direct link between the TACC genes and tumorigenesis remains to be established.
Recently, much attention has focused on the role of genetic instability in cancer progression (Li et al., 1997). As centrosomes organize the mitotic spindle, it has been suggested that defects in centrosome or microtubule function could contribute to the large-scale genetic instability that is usually associated with aggressive cancers (Brinkley and Goepfert, 1998; Doxsey, 1998). Many cancer cells have extra centrosomes, and some overexpress specific centrosomal or microtubule-associated proteins (Charrasse et al., 1998; Hanash et al., 1988; Lingle et al., 1998; Pihan et al., 1998; Zhou et al., 1998; Xu et al., 1999). While it is unclear whether alterations in TACC gene expression can contribute to cancer, our data demonstrate that D–TACC is involved in regulating microtubule behaviour in Drosophila, and a mutation in the d-tacc gene causes severe chromosome segregation defects in the early embryo. If the mammalian TACC proteins play similar roles in human cells, TACC defects could contribute to increased levels of genetic instability and thus to the development and progression of cancer. Our preliminary results suggest that the overexpression of TACC2 in human cells does perturb the microtubule cytoskeleton (F.Gergely, C.Karlsson, I.Still, J.Cowell, J.Kilmartin and J.W.Raff, unpublished observations).
Materials and methods
Cloning of tacc cDNAs
The original d–tacc clone was isolated by screening a 0–24 h embryo λ ZAPII cDNA expression library as described previously (Huynh et al., 1985; Kellogg and Alberts, 1992) with the mouse sera we call MA8. As the clone was not full length, we isolated a full-length clone by screening a plasmid cDNA library (Brown and Kafatos, 1988) using both conventional methods and nested PCR (full details available upon request). The sequence of the d–tacc cDNA has been submitted to the DDBJ/EMBL/GenBank databases under accession No. AF146700. Human TACC cDNAs were isolated from various commercially available cDNA libraries, using partial cDNAs that we identified in the expressed sequence tags (EST) databases as probes. During the course of this work, the full-length sequences of TACC1 and TACC3 were deposited in the databases by I.Still, J.Cowell and colleagues. At present, we do not have a full-length cDNA for TACC2 (although our longest clone encodes a predicted protein of ∼70 kDa, which is close to the size of the TACC2 protein we see in Western blotting experiments). Moreover, there appear to be several differentially spliced forms of TACC2 (F.Gergely, J.W.Raff, I.Still and J.Cowell, unpublished observations). A differentially spliced form of D–TACC has also been identified recently (J.Kramer and B.Theurkauf, personal communication; DDBJ/EMBL/GenBank accession No. AF146756). All sequencing was performed at the DNA sequencing facility at the Department of Biochemistry, Cambridge University.
Fusion protein production and purification
Bacterially expressed MBP (New England Biolabs) or GST (Smith and Johnson, 1988) fusion proteins were purified as described previously (Huang and Raff, 1999). If the fusion proteins were to be used in microtubule spin-down experiments, they were desalted on a Bio-Rad P6 column into BRB80 (80 mM PIPES pH 6.8, 1 mM MgCl2, 1 mM EGTA) with 50 mM KCl using a Bio-Rad Bio-Logic chromatography system. The proteins were stored at –20°C in this buffer with 50% glycerol. The following fusion proteins were used in this study: MBP–NT, containing amino acids 2–433 of D–TACC; MBP–Mid, containing amino acids 433–889 of D–TACC; MBP–CT and GST–CT, containing amino acids 853–1189 of D–TACC; and MBP–TACC2 containing the C–terminal 337 amino acids of TACC2.
Antibody production and purification
Several of the proteins that co-purified with microtubules from embryo extracts were separated by preparative SDS–PAGE. Individual bands were isolated from the gel as described previously (Kellogg et al., 1989), and antibodies were raised in Swiss Webster mice as described previously (Ou et al., 1993). Antibodies were raised in rabbits against bacterially expressed MBP (New England Biolabs) or GST (Smith and Johnson, 1988) fusion proteins containing the C–terminal 338 amino acids of D–TACC or the C–terminal 337 amino acids of TACC2. All injections and antisera production were performed by Eurogentec (Belgium). Antibodies were affinity purified from the rabbit sera using the appropriate fusion protein that had been coupled to either Affigel–10, Affigel–15 (Bio-Rad) or Amino-link (Pierce) resins as described previously (Huang and Raff, 1999).
Microtubule spin-down experiments
Microtubule spin-down experiments were performed essentially as described previously (Kellogg et al., 1989; Raff et al., 1993). Briefly, 0–4 h embryos were homogenized in an equal volume of C–buffer (50 mM HEPES pH 7.6, 1 mM MgCl2, 1 mM EGTA) with protease inhibitors. The extract was centrifuged for 1 h at 100 000 g. Dithiothreitol (DTT) and GTP were added to the supernatant (1 mM final concentration) and this was split into equal aliquots. To polymerize the microtubules, taxol was added to a final concentration of 10 μM. To polymerize actin filaments, KCl, ATP and phalloidin were added to the extract to 50 mM, 1 mM and 100 μM final concentrations, respectively. In control spin-downs, only buffer was added to the extracts. The supernatants were warmed to 25°C for 5 min to allow polymerization to initiate, and then shifted to 4°C for a further 15 min. The supernatants were layered onto a 2 vol. cushion of C-buffer with 50% sucrose, and this was centrifuged at 100 000 g for 10 min. The supernatant and cushion were aspirated, and the pellet was resuspended in protein sample buffer (Laemmli, 1970). Equal fractions of supernatant and pellet were loaded onto polyacrylamide gels, which were then blotted to nitrocellulose and probed with antibodies.
Microtubule spin-downs with in vitro translated D–TACC were performed by transcribing and translating the full-length D–TACC cDNA with the TNT-coupled reticulocyte lysate kit (Promega), following the manufacturer's instructions. In Western blotting experiments, the in vitro translated D–TACC co-migrated with the endogenous D–TACC in embryo extracts (not shown). The in vitro translation reaction mixture was centrifuged at 100 000 g for 15 min, and ∼40 μg of purified bovine tubulin was added to the supernatant (which we estimate to be an ∼50–fold molar excess relative to D–TACC). The microtubules were polymerized with taxol and pelleted through a sucrose cushion as described above. Microtubule spin-downs with bacterially expressed fusion proteins were performed by mixing 1–2 μg of purified fusion protein with ∼200 μg of purified tubulin. Microtubules were polymerized with taxol and pelleted through a sucrose cushion as described above. Alternatively, 1–2 μg of purified fusion protein was mixed with 100 μl of embryo extract; microtubules were then polymerized with taxol and the extracts treated as described above.
Immunoprecipitation
Immunoprecipitation experiments were performed using affinity-purified anti-D–TACC or anti-GST antibodies as described previously (Kidd and Raff, 1997).
SDS–PAGE and Western blotting
SDS–PAGE and Western blotting were performed as described previously (Laemmli, 1970; Towbin et al., 1979). Blots were incubated with primary antibodies at 1–2 μg/ml final concentration, and antibody detection was performed using either the ECL kit (Amersham), or the Supersignal kit (Pierce) according to the manufacturers' instructions. As these enhanced chemiluminescent detection methods are highly non-linear, all blots were quantitated by blotting serial dilutions of the appropriate fusion proteins or extracts on the same blots.
Fixation and antibody staining
The 0–3 h Drosophila embryos were fixed with methanol and processed for indirect immunofluorescence as described previously (Kellogg et al., 1989), except that RNase A was included with the primary antibodies, and propidium iodide was used to stain the DNA (Gonzalez and Glover, 1993). Embryos were treated with colchicine (500 μg/ml) prior to fixation as described previously (Raff et al., 1993). Human HeLa or primary fibroblast cells (MHF 181 primary foreskin fibroblasts) were cultured, fixed with methanol and stained with antibodies as described previously (Pines and Hunter, 1991). All affinity-purified primary antibodies were used at 1–2 μg/ml. The anti-CP60, anti-CP190 and anti-Drosophila γ–tubulin antibodies have all been described previously (Kellogg and Alberts, 1992; Raff et al., 1993; Kellogg et al., 1995); DM1a (Sigma) was used at 1/1000 to detect microtubules, while GTU–88 (Sigma) was used at 1/500 to detect γ–tubulin in some experiments. Cy5 or Cy3 (Jackson) anti-rabbit, and Cy3 (Jackson) or Alexa488 (Molecular Probes) anti-mouse secondary antibodies were used at 1/500. All imaging was performed using a Bio-Rad 1024 scanning confocal head attached to a Nikon microscope. Images were imported into Adobe Photoshop and were adjusted to use the full range of pixel intensities. In some cases, an unsharp mask filter was applied to the whole image.
Analysis of living embryos
Time-lapse video recordings of living embryos were made as described previously (Huang and Raff, 1999). For antibody injection, affinity-purified antibodies were desalted into 50 mM HEPES pH 8.6, 25 mM KCl, and labelled with Texas red–X succinimidyl ester (Molecular Probes) according to the supplier's instructions. The labelled antibodies were purified away from unincorporated label by desalting on a P6 (Bio-Rad) column into 25 mM HEPES pH 7.6, 10 mM KCl, and the antibodies were concentrated to 9–10 mg/ml in a Biomax concentrator (Millipore). The ratio of coupling was determined according to the supplier's instructions. Embryos were injected with antibody and observed on a confocal microscope as described above.
Screen for D–TACC mutations
Males of the genotype ru, st, e, ca were mutagenized with EMS as described previously (Ashburner, 1989). These males were mated to Pr, Dr/TM3 virgin females, and the resulting ru, st, e, ca/TM3 male progeny (F1) were selected and individually mated to two Df(3R)110/TM3 virgin females in a single vial. The progeny of these flies were screened to see if the mutated chromosome carried a lethal or female sterile mutation in the Df(3R)110 region using standard genetic methods (Roberts, 1986). All mutations used here are as described previously (Lindsley and Zimm, 1992).
Molecular methods
Standard molecular biology techniques were performed as described previously (Sambrook et al., 1989) or according to the supplier's instructions. The PUbq-D–TACC, PUbq-GST–CT and Pubq-D–TACC–GFP transformation vectors were derived from the full-length D–TACC cDNA either by isolating appropriate restriction fragments, or by amplifying appropriate fragments by PCR, and subcloning into the pWhite Rabbit-PUbq vector (N.Brown, personal communication; full details available on request). PUbq-D–TACC contained the full-length D–TACC cDNA; PUbq-GST–D–TACC contained the C–terminal 338 amino acids of D–TACC fused in-frame to the C–terminus of GST; and Pubq-D–TACC–GFP contained the full-length D–TACC cDNA fused in-frame at its C–terminus to mGFP6 (Schuldt et al., 1998). Flies were transformed with these constructs using standard techniques (Roberts, 1986).
Acknowledgments
Acknowledgements
We thank Ivan Still, John Cowell, Joe Kramer, Bill Theurkauf and Mike Goldberg for communicating results prior to publication, and Nick Brown, Jun-yong Huang and an anonymous reviewer for helpful comments on the manuscript. This work was supported by Wellcome Prize Studentships (F.G., D.K. and J.G.W.) and by a Wellcome Senior Fellowship in Basic Biomedical Research (K.J. and J.W.R.).
References
- Altschul S.F., Gish, W., Miller, W., Myers, E.W. and Lipman, D.J. (1990) Basic local alignment search tool. J. Mol. Biol., 215, 403–410. [DOI] [PubMed] [Google Scholar]
- Andersen S.S. (1998) Xenopus interphase and mitotic microtubule-associated proteins differentially suppress microtubule dynamics in vitro.Cell Motil. Cytoskeleton, 41, 202–213. [DOI] [PubMed] [Google Scholar]
- Andersen S.S. (1999) Balanced regulation of microtubule dynamics during the cell cycle: a contemporary view. BioEssays, 21, 53–60. [DOI] [PubMed] [Google Scholar]
- Andersen S.S. and Karsenti, E. (1997) XMAP310: a Xenopus rescue-promoting factor localized to the mitotic spindle. J. Cell Biol., 139, 975–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen S.S., Buendia, B., Dominguez, J.E., Sawyer, A. and Karsenti, E. (1994) Effect on microtubule dynamics of XMAP230, a microtubule-associated protein present in Xenopus laevis eggs and dividing cells [published erratum appears in J. Cell Biol., 1995, 128, following 988]. J. Cell Biol., 127, 1289–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen S.S., Ashford, A.J., Tournebize, R., Gavet, O., Sobel, A., Hyman, A.A. and Karsenti, E. (1997) Mitotic chromatin regulates phosphorylation of Stathmin/Op18. Nature, 389, 640–643. [DOI] [PubMed] [Google Scholar]
- Ashburner M. (1989) Drosophila, A Laboratory Handbook. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Baker J., Theurkauf, W.E. and Schubiger, G. (1993) Dynamic changes in microtubule configuration correlate with nuclear migration in the preblastoderm Drosophila embryo. J. Cell Biol., 122, 113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmont L.D. and Mitchison, T.J. (1996) Identification of a protein that interacts with tubulin dimers and increases the catastrophe rate of microtubules. Cell, 84, 623–631. [DOI] [PubMed] [Google Scholar]
- Brand A. (1995) GFP in Drosophila.Trends Genet., 11, 324–325. [DOI] [PubMed] [Google Scholar]
- Brinkley B.R. and Goepfert, T.M. (1998). Supernumerary centrosomes and cancer: Boveri's hypothesis resurrected. Cell Motil. Cytoskeleton, 41, 281–288. [DOI] [PubMed] [Google Scholar]
- Brown N.H. and Kafatos, F.C. (1988) Functional cDNA libraries from Drosophila embryos. J. Mol. Biol., 203, 425–437. [DOI] [PubMed] [Google Scholar]
- Callaini G. and Riparbelli, M.G. (1996) Fertilization in Drosophila melanogaster: centrosome inheritance and organisation of the first mitotic spindle. Dev. Biol., 176, 199–208. [DOI] [PubMed] [Google Scholar]
- Cassimeris L. (1999) Accessory protein regulation of microtubule dynamics throughout the cell cycle. Curr. Opin. Cell Biol., 11, 134–141. [DOI] [PubMed] [Google Scholar]
- Cha B.J., Error, B. and Gard, D.L. (1998) XMAP230 is required for the assembly and organization of acetylated microtubules and spindles in Xenopus oocytes and eggs. J. Cell Sci., 111, 2315–2327. [DOI] [PubMed] [Google Scholar]
- Charrasse S., Schroeder, M., Gauthier-Rouviere, C., Ango, F., Cassimeris, L., Gard, D.L. and Larroque, C. (1998) The TOGp protein is a new human microtubule-associated protein homologous to the Xenopus XMAP215. J. Cell Sci., 111, 1371–1383. [DOI] [PubMed] [Google Scholar]
- Compton D.A. (1998) Focusing on spindle poles. J. Cell Sci., 111, 1477–1481. [DOI] [PubMed] [Google Scholar]
- Cullen C.F., Deak,P., Glover,D.M. and Ohkura,H. (1999) mini spindles: a gene encoding a conserved microtubule-associated protein required for the integrity of the mitotic spindle in Drosophila. J. Cell Biol., 146, 1005–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai A. and Mitchison, T.J. (1997) Microtubule polymerization dynamics. Annu. Rev. Cell Dev. Biol., 13, 83–117. [DOI] [PubMed] [Google Scholar]
- Desai A., Verma, S., Mitchison, T.J. and Walczak, C.E. (1999) Kin I kinesins are microtubule-destabilizing enzymes. Cell, 96, 69–78. [DOI] [PubMed] [Google Scholar]
- do Carmo Avides M. and Glover, D.M. (1999) Abnormal spindle protein, Asp and the integrity of mitotic centrosomal microtubule organizing centers. Science, 283, 1733–1735. [DOI] [PubMed] [Google Scholar]
- Doxsey S. (1998) The centrosome—a tiny organelle with big potential. Nature Genet., 20, 104–106. [DOI] [PubMed] [Google Scholar]
- Foe V.E. and Alberts, B.M. (1983) Studies of nuclear and cytoplasmic behavior during the five mitotic cycles that precede gastrulation in Drosophila embryos. J. Cell Sci., 61, 31–70. [DOI] [PubMed] [Google Scholar]
- Foe V.E., Odell,G.M. and Edgar,B.A. (1993) Mitosis and morphogenesis in the Drosophila embryo: point and counterpoint. In Bate,M. and Martinez-Arias,A. (eds), The Development of Drosophila melanogaster. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp. 140–399. [Google Scholar]
- Francis-Lang H., Minden, J., Sullivan, W. and Oegema, K. (1999) Live confocal analysis with fluorescently labeled proteins. Methods Mol. Biol., 122, 223–239. [DOI] [PubMed] [Google Scholar]
- Gard D.L. and Kirschner, M.W. (1987) A microtubule-associated protein from Xenopus eggs that specifically promotes assembly at the plus-end. J. Cell Biol., 105, 2203–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatti M. and Goldberg, M.L. (1991) Mutations affecting cell division in Drosophila.Methods Cell Biol., 35, 543–586. [DOI] [PubMed] [Google Scholar]
- Glover D.M., Gonzalez, C. and Raff, J.W. (1993) The centrosome. Sci. Am., 268, 62–68. [DOI] [PubMed] [Google Scholar]
- Gonzalez C. and Glover,D.M. (1993) Techniques for studying mitosis in Drosophila. In Fantes,P. and Brooks,R. (eds), The Cell Cycle: A Practical Approach. Oxford University Press, Oxford, UK, pp. 143–174. [Google Scholar]
- Hanash S.M., Strahler, J.R., Kuick, R., Chu, E.H. and Nichols, D. (1988) Identification of a polypeptide associated with the malignant phenotype in acute leukemia. J. Biol. Chem., 263, 12813–12815. [PubMed] [Google Scholar]
- Hirokawa N. (1994) Microtubule organzation and dynamics dependent on microtubule-associated proteins. Curr. Opin. Cell Biol., 6, 74–81. [DOI] [PubMed] [Google Scholar]
- Huang J. and Raff, J.W. (1999) The disappearance of cyclin B at the end of mitosis is regulated spatially in Drosophila cells. EMBO J., 18, 2184–2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh T.V., Young,R.A. and Davis,R.W. (1985) Constructing and screening cDNA libraries in λ gt10 and λ gt11. In Glover,D.M. (ed.), DNA Cloning: A Practical Approach. IRL Press, Oxford, UK. Vol. 1, pp. 49–78. [Google Scholar]
- Hyman A.A. and Karsenti, E. (1996) Morphogenetic properties of microtubules and mitotic spindle assembly. Cell, 84, 401–410. [DOI] [PubMed] [Google Scholar]
- Jordan M.A., Thrower, D. and Wilson, L. (1992) Effects of vinblastine, podophyllotoxin and nocodazole on mitotic spindles. Implications for the role of microtubule dynamics in mitosis. J. Cell Sci., 102, 401–416. [DOI] [PubMed] [Google Scholar]
- Kalt A. and Schliwa, M. (1993) Molecular components of the centrosome. Trends Cell Biol., 3, 119–128. [DOI] [PubMed] [Google Scholar]
- Karsenti E. (1993) Severing microtubules in mitosis. Curr. Biol., 3, 208–210. [DOI] [PubMed] [Google Scholar]
- Keating T.J., Peloquin, J.G., Rodionov, V.I., Momcilovic, D. and Borisy, G.G. (1997) Microtubule release from the centrosome. Proc. Natl Acad. Sci. USA, 94, 5078–5083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg D.R. and Alberts, B.M. (1992) Purification of a multiprotein complex containing centrosomal proteins from the Drosophila embryo by chromatography with low-affinity polyclonal antibodies. Mol. Biol. Cell, 3, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg D.R., Field, C.M. and Alberts, B.M. (1989) Identification of microtubule-associated proteins in the centrosome, spindle and kinetochore of the early Drosophila embryo. J. Cell Biol., 109, 2977–2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg D.R., Moritz, M. and Alberts, B.M. (1994) The centrosome and cellular organization. Annu. Rev. Biochem., 63, 639–674. [DOI] [PubMed] [Google Scholar]
- Kellogg D.R., Oegema, K., Raff, J., Schneider, K. and Alberts, B.M. (1995) CP60: a microtubule associated protein that is localized to the centrosome in a cell cycle-specific manner. Mol. Biol. Cell, 6, 1673–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd D. and Raff, J.W. (1997) LK6, a short lived protein kinase in Drosophila that can associate with microtubules and centrosomes. J. Cell Sci., 110, 209–219. [DOI] [PubMed] [Google Scholar]
- Laemmli U.K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680–685. [DOI] [PubMed] [Google Scholar]
- Lee H.S., Simon, J.A. and Lis, J.T. (1988) Structure and expression of ubiquitin genes of Drosophila melanogaster.Mol. Cell. Biol., 8, 4727–4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Yerganian, G., Duesberg, P., Kraemer, A., Willer, A., Rausch, C. and Hehlmann, R. (1997) Aneuploidy correlated 100% with chemical transformation of Chinese Hamster cells. Proc. Natl Acad. Sci. USA, 94, 14506–14511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley D.L. and Zimm,G.G. (1992) The Genome of Drosophila melanogaster. Academic Press, New York, NY. [Google Scholar]
- Lingle W.L., Lutz, W.H., Ingle, J.N., Maihle, N.J. and Salisbury, J.L. (1998) Centrosome hypertrophy on human breast tumours: implications for genomic instability and cell polarity. Proc. Natl Acad. Sci. USA, 95, 2950–2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupas A., Van Dyke, M. and Stock, J. (1991) Predicting coiled coils from protein sequences. Science, 252, 1162–1164. [DOI] [PubMed] [Google Scholar]
- Marklund U., Larsson, N., Gradin, H.M., Brattsand, G. and Gullberg, M. (1996) Oncoprotein 18 is a phosphorylation-responsive regulator of microtubule dynamics. EMBO J., 15, 5290–5298. [PMC free article] [PubMed] [Google Scholar]
- Moritz M., Braunfeld, M.B., Sedat, J.W., Alberts, B. and Agard, D.A. (1995) Microtubule nucleation by γ tubulin containing rings in the centrosome. Nature, 378, 638–640. [DOI] [PubMed] [Google Scholar]
- Oegema K., Marshall, W.F., Sedat, J.W. and Alberts, B.M. (1997) Two proteins that cycle asynchronously between centrosomes and nuclear structures: Drosophila CP60 and CP190. J. Cell Sci., 110, 1573–1583. [DOI] [PubMed] [Google Scholar]
- Oegema K., Wiese, C., Martin, O.C., Milligan, R.A., Iwamatsu, A., Mitchison, T.J. and Zheng, Y. (1999) Characterization of two related Drosophila γ–tubulin complexes that differ in their ability to nucleate microtubules. J. Cell Biol., 144, 721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou S.K., Hwang, J.M. and Patterson, P.H. (1993) A modified method for obtaining large amounts of high titer polyclonal ascites fluid. J. Immunol. Methods, 165, 75–80. [DOI] [PubMed] [Google Scholar]
- Pihan G.A., Purohit, A., Wallace, J., Knecht, H., Woda, B., Queensberry, P. and Doxsey, S.J. (1998) Centrosome defects and genetic instability in malignant tumors. Cancer Res., 58, 3974–3985. [PubMed] [Google Scholar]
- Pines J. and Hunter, T. (1991) Human cyclins A and B1 are differentially located in the cell and undergo cell cycle-dependent nuclear transport. J. Cell Biol., 115, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff J.W. and Glover, D.M. (1989) Centrosomes and not nuclei, initiate pole cell formation in Drosophila embryos. Cell, 57, 611–619. [DOI] [PubMed] [Google Scholar]
- Raff J.W., Kellogg, D.R. and Alberts, B.M. (1993) Drosophila γ–tubulin is part of a complex containing two previously identified centrosomal MAPs. J. Cell Biol., 121, 823–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D. (1986) Drosophila, A Practical Approach. IRL Press, Oxford, UK. [Google Scholar]
- Rodionov V., Nadezhdina, E. and Borisy, G. (1999) Centrosomal control of microtubule dynamics. Proc. Natl Acad. Sci. USA, 96, 115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J., Fritsch,E.F. and Maniatis,T. (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Saunders R.D., Avides, M.C., Howard, T., Gonzalez, C. and Glover, D.M. (1997) The Drosophila gene abnormal spindle encodes a novel microtubule-associated protein that associates with the polar regions of the mitotic spindle. J. Cell Biol., 137, 881–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuldt A.J., Adams, J.H., Davidson, C.M., Micklem, D.R., Haseloff, J., Johnston, D.S. and Brand, A.H. (1998) Miranda mediates asymmetric protein and RNA localization in the developing nervous system. Genes Dev., 12, 1847–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D.B. and Johnson, K.S. (1988) Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene, 67, 31–40. [DOI] [PubMed] [Google Scholar]
- Starr D.A., Williams, B.C., Hays, T.S. and Goldberg, M.L. (1998) ZW10 helps recruit dynactin and dynein to the kinetochore. J. Cell Biol., 142, 763–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Still I.H., Hamilton, M., Vince, P., Wolfman, A. and Cowell, J. (1999a) Cloning of TACC1, an embryonically expressed, potentially transforming coiled coil containing gene, from the 8p11 breast cancer amplicon. Oncogene, 18, 4032–4038. [DOI] [PubMed] [Google Scholar]
- Still I.H., Vince, P. and Cowell, J.K. (1999b) The third member of the transforming acidic coiled coil-containing gene family, TACC3, maps in 4p16, close to translocation breakpoints in multiple myeloma and is upregulated in various cancer cell lines. Genomics, 58, 165–170. [DOI] [PubMed] [Google Scholar]
- Sunkel C.E., Gomes, R., Sampaio, P., Perdigao, J. and Gonzalez, C. (1995) γ tubulin is required for the structure and function of the microtubule organizing center in Drosophila neuroblasts. EMBO J., 14, 28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournebize R., Andersen, S.S., Verde, F., Doree, M., Karsenti, E. and Hyman, A.A. (1997) Distinct roles of PP1 and PP2A-like phosphatases in control of microtubule dynamics during mitosis. EMBO J., 16, 5537–5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin, T. and Gordon, J. (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellusose sheets: procedure and some applications. Proc. Natl Acad. Sci. USA, 76, 4350–4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasquez R.J., Gard, D.L. and Cassimeris, L. (1994) XMAP from Xenopus eggs promotes rapid plus end assembly of microtubules and rapid microtubule polymer turnover. J. Cell Biol., 127, 985–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walczak C.E., Mitchison, T.J. and Desai, A. (1996) XKCM1: a Xenopus kinesin-related protein that regulates microtubule dynamics during mitotic spindle assembly. Cell, 84, 37–47. [DOI] [PubMed] [Google Scholar]
- Wells W.A.E. (1996) The spindle assembly checkpoint: aiming for a perfect mitosis, every time. Trends Cell Biol., 6, 228–234. [DOI] [PubMed] [Google Scholar]
- Whitfield W.G., Chaplin, M.A., Oegema, K., Parry, H. and Glover, D.M. (1995) The 190 kDa centrosome-associated protein of Drosophila melanogaster contains four zinc finger motifs and binds to specific sites on polytene chromosomes. J. Cell Sci., 108, 3377–3387. [DOI] [PubMed] [Google Scholar]
- Williams B.C., Dernburg, A.F., Puro, J., Nokkala, S. and Goldberg, M.L. (1997) The Drosophila kinesin-like protein KLP3A is required for proper behavior of male and female pronuclei at fertilization. Development, 124, 2365–2376. [DOI] [PubMed] [Google Scholar]
- Xu X., Weaver, Z., Linke, S.P., Li, C., Gotay, J., Wang, X.W., Harris, C.C., Ried, T. and Deng, C.X. (1999) Centrosome amplification and a defective G2–M cell cycle checkpoint induce genetic instability in BRCA1 exon 11 isoform-deficient cells. Mol. Cell, 3, 389–395. [DOI] [PubMed] [Google Scholar]
- Zalokar M. and Erk, I. (1976) Division and migration of nuclei during early embryogenesis of Drosophila melanogaster.J. Microbiol. Cell, 25, 97–106. [Google Scholar]
- Zheng Y.X., Wong, M.L., Alberts, B. and Mitchison, T. (1995) Nucleation of microtubule assembly by a γ tubulin containing ring complex. Nature, 378, 578–583. [DOI] [PubMed] [Google Scholar]
- Zhou H., Kuang, J., Zhong, L., Kuo, W.L., Gray, J.W., Sahin, A., Brinkley, B.R. and Sen, S. (1998) Tumour amplified kinase STK15/BTAK induces centrosome amplification, aneuploidy and trans- formation. Nature Genet., 20, 189–193. [DOI] [PubMed] [Google Scholar]