Abstract
Reactive oxygen species (ROS) and reactive nitrogen species (RNS) are constantly produced and are tightly regulated to maintain a redox balance (or homeostasis) together with antioxidants (e.g. superoxide dismutase and glutathione) under normal physiological circumstances. These ROS/RNS have been shown to be critical for various biological events including signal transduction, aging, apoptosis, and development. Despite the known beneficial effects, an overproduction of ROS/RNS in the cases of receptor-mediated stimulation and disease-induced oxidative stress can inflict severe tissue damage. In particular, these ROS/RNS are capable of degrading macromolecules including proteins, lipids and nucleic acids as well as polysaccharides, and presumably lead to their dysfunction. The purpose of this review is to highlight (1) chemical mechanisms related to cell-free and cell-based depolymerization of polysaccharides initiated by individual oxidative species; (2) the effect of ROS/RNS-mediated depolymerization on the successive cleavage of the glycosidic linkage of polysaccharides by glycoside hydrolases; and (3) the potential biological outcome of ROS/RNS-mediated depolymerization of polysaccharides.
Keywords: degradation, nitric oxide, polysaccharide, superoxide
Introduction
Reactive oxygen species (ROS) is defined as chemically active products generated by the partial reduction in oxygen, whereas reactive nitrogen species (RNS) refers to reactive products derived from reactions with nitric oxide (NO). Because an extensive overlap and cross-talk exist in the production, function, and decomposition of ROS and RNS, these species are often referred to interchangeably (Winterbourn 2008), and in this minireview collectively termed ROS/RNS. Both reactive species can be generated from either exogenous (γ- or UV-irradiation and ionization, etc.) or endogenous (mitochondrial respiration, ROS/RNS-producing enzymes, etc.) sources (Kohen and Nyska 2002).
In living organisms, the outcome of the ROS/RNS action may be either beneficial or deleterious. On one hand, ROS/RNS function as intra- and extracellular messengers playing an important role in various signal transduction pathways and in transcriptional regulation (Fialkow et al. 2007; Yao et al. 2007). On the other hand, prolonged elevated levels of ROS/RNS can cause intense oxidative stress, a deleterious process that can be an important mediator of damage to various cellular structures, including lipids and membranes, proteins, and DNA (Valko et al. 2007).
Polysaccharides are biopolymers possessing repeating units (mono- or oligosaccharide) joined together by glycosidic bonds with an enormous diversity of structure. Abundant polysaccharides are biosynthesized by animals, fungi, algae, microorganisms, and plants. These polysaccharides, in addition to being storage polymers or structure forming macromolecules, are increasingly recognized as key substances with versatile bioactivities and broad applications (Heinze et al. 2006). Studies have established that the functional roles of a given polysaccharide from both prokaryotic and eukaryotic cells are linked to its degree of polymerization (molecular size; Paoletti et al. 1992; Kasper et al. 1996; Wessels et al. 1998). In the past several decades, accumulating evidence has shown that ROS/RNS attack can result in the fragmentation of polysaccharides, and thus presumably alter the functions of these molecules. This paper will focus on the oxidative cleavage of polysaccharides by ROS/RNS and its potential outcome.
Chemical depolymerization of polysaccharides by ROS/RNS
ROS/RNS can be classified as either free radicals, which contain one unpaired electron or nonradicals. The former generally includes superoxide ion radical (O2−), hydroxyl radical (OH·), peroxyl (ROO·), alkoxyl radicals (RO·), and NO radical (NO·). The latter contains hydrogen peroxide (H2O2), organic peroxide (ROOR′), ozone (O3), hypochlorous acid (HClO), singlet oxygen (1O2), aldehydes (HCOR), and peroxynitrite (ONOOH), etc. (Kohen and Nyska 2002). Several ROS/RNS can individually cause the backbone scission of polysaccharides, generating smaller fragments.
Ozone
Ozone can directly oxidize organic polymers possessing olefinic or double bonds. In addition, ozone breakdown to dioxygen gives rise to oxygen-free radicals, which are highly reactive and capable of damaging many organic molecules (Rao and Davis 2001). Furthermore, ozone was reported to react with acetal functions in a specific manner to give esters in which one of the alkoxy residues in the acetal was retained in the acyloxy group (Deslongchamps and Moreau 1971; Wang et al. 1998). The polymerization of polysaccharides by ozone in aqueous solution involves three mechanisms (Wang et al. 1999): (1) selective ozonolytic oxidation of β-d-aldosidic linkages, (2) nonselective oxidative degradation by radical species, and (3) nonselective acid hydrolysis. Among them, the first is the predominant reaction in which aldonic acid esters are formed and are spontaneously saponified to yield smaller fragments (Scheme 1). The ozonolytic oxidation of aldosides proceeds under a strong stereoelectronic control and prefers the aglycone conformation, in which each oxygen has one of its lone-pair orbitals antiperiplanar to the alkylidene C–H bond. Therefore, glycosidic linkages with different conformations can have different reaction rates with ozone, allowing for selectivity in cleaving β-d-linkages of polysaccharides (Wang et al. 1999).
Scheme 1.
The ozonolytic cleavage of β-d-linked glycoside (adapted from Wang et al. 1998, 1999).
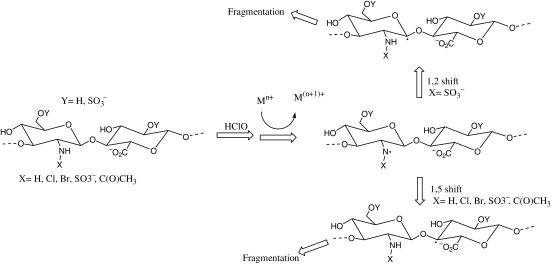
Hypochlorite
N-acetyl, NH2, N-SO3H residues on glycosaminoglycans (GAGs) can react with hypoclorite to generate polymer-derived N-chloro derivatives (chloramines, dichloramines, chlorosulfonamides) (Rees et al. 2003, 2004; Rees and Davies 2006; Scheme 2). In the presence of transition-metal ions and/or superoxide, the decomposition of these derivatives gives rise to nitrogen-centered radicals. These radicals undergo rapid intramolecular abstraction reactions to give carbon-centered radicals at C-2 on the amino sugar rings (via a 1,2-hydrogen atom shift; Rees and Davies 2006) and/or at C-4 on the neighboring glycosidic residues (via 1,5-hydrogen atom shifts; Rees et al. 2004). These products finally cause the cleavage of glycosidic bonds through β-scission (Rees et al. 2004; Rees and Davies 2006).
Scheme 2.
The cleavage of glycosidic bonds in GAGs through β-scission induced by hyperchlorite (adapted from Rees et al. 2004; Rees and Davies 2006).
OH radical and peroxynitrite
The hydroxyl radical is the most reactive ROS. This radical can abstract hydrogen atoms at all ring C–H bonds of aldoses, uronic acids, and other sites on carbohydrates except C-2 of N-acetyl hexosamine (Gilbert et al. 1981; Hawkins and Davies 1996). The abstraction of hydrogen atom will generate carbon-center radicals. The radicals at carbons which form glycosidic bonds will undergo a β-scission reaction resulting in the breakdown of polysaccharide chains (Gilbert et al. 1981; Hawkins and Davies 1996; Rees et al. 2008). Notably, sulfated polysaccharides are shown more resistant to hydroxyl radical attack (Moseley et al. 1995).
Peroxynitrite has been shown to degrade hyaluronic acid (HA) and chondroitin sulfate via a hydroxyl radical-like mechanism (Li et al. 1997; Al-Assaf et al. 2003; Kennett and Davies 2007). The susceptibility of the various GAGs to degradation by peroxynitrite appears to depend on their degree of sulfation, for example, hyaluronan (lacking sulfate) was the most susceptible and heparin (highly sulfated) was the least susceptible to peroxynitrite-mediated degradation (Kennett and Davies 2007).
Singlet oxygen
Singlet oxygen was considered to be involved in the initiation step of the photodegradation of polymers (Jan et al. 1974). In a model system, singlet oxygen was produced by xanthine oxidase, and as a result, there was a dramatic decline in the solution viscosity (30–50%) of plant polysaccharides, such as methylcellulose, starch, pectin, and guar gum (Kon and Schwimmer 1977). The decreased viscosity suggested that the degradation of the polysaccharide chains had taken place. Further experiments of ROS quenchers and scavengers proved that this process was dependent on hydroxyl radicals and singlet oxygen (Kon and Schwimmer 1977). In another study, singlet oxygen generated from irradiation in the presence of methylene blue or riboflavin (a singlet oxygen sensitizer) was shown to generate a slight depolymerization of HA, but did induce substantial changes in the HA tertiary structure (Andley and Chakrabarti 1983)
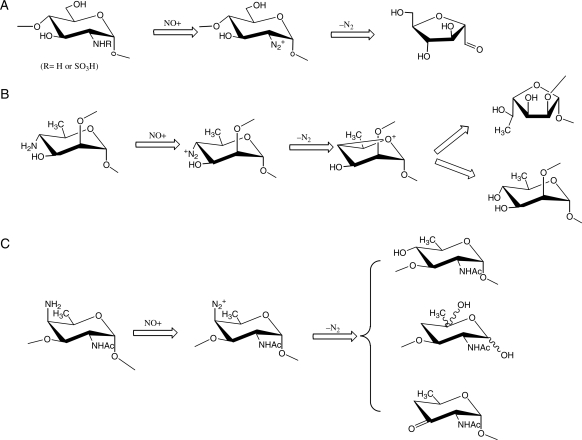
NO and nitrous acid
NO and HNO2 share one common intermediate, the nitrosonium cation (NO+) to depolymerize GAGs (Shively and Conrad 1976; Vilar et al. 1997; Hassan et al. 1998). The nitrosium cation nitrosates free amino groups at ∼pH 4 or N-sulfo groups at ∼pH 1.5, respectively, but not N-acetyl groups. The nitrosation of amino groups or N-sulfo groups causes loss of nitrogen gas with a ring contraction of 2-amino-2-deoxy sugars to 2,5-anhydro sugars coupled to elimination of the aglycone (Scheme 3a). In addition, 4-amino-4-deoxy sugars are also amenable to the deamination reaction through a reaction sequence similar to 2-amino-2-deoxy sugars (Lindberg et al. 1980; Kenne et al. 1982). However, whether NO-mediated deamination of 4-amino sugars results in the cleavage of the glycosidic bonds depends on the favored conformation of the 4-amino group and the glycosidic linkage of 4-amino-4-deoxy sugars in the carbohydrate polymers. For example, the deamination of 4-amino groups of 2-linked 4-amino-4, 6-deoxymannopyranosyl residues yields 2-linked rhamnopyranosyl and 2-linked 6-deoxyallofuranosyl residues, but the glycosidic linkages stay intact (Scheme 3b; Kenne et al. 1982). In contrast, the deamination of 4-amino groups of 3-linked 2-acetamido-4-amino-2,4,6-trideoxygalactopyranosyl (AATp) residues yields cleavage at both C-3 and C-1 of AATp (Scheme 3c; Lindberg et al. 1980).
Scheme 3.
The deamination of 2-amino or 4-amino hexamine residues (adapted from Lindberg et al. 1980; Kenne et al. 1982; Vilar et al. 1997).
Polysaccharide depolymerization by cell-derived ROS/RNS
Although numerous individual ROS/RNS have been shown to depolymerize polysaccharides in vitro, there are at least three factors to take into account when considering the damage to biological substrates including carbohydrates in vivo (Kohen and Nyska 2002; Winterbourn 2008): (1) whether substrates are located at adjacent ROS/RNS production sites, since the high reactivity of these species usually has a relatively short life span; (2) whether the concentration of ROS/RNS is high enough; and (3) whether the levels of antioxidant are efficient to decompose ROS/RNS at the attack sites. The following section will describe the depolymerization of various polysaccharides by ROS/RNS generated from different cell types.
Neutrophils
During defense against infections, neutrophils survey, ingest, and destroy invading microorganisms in mammals, and secret potent antimicrobial products that includes ROS/RNS, proteinases, and antimicrobial peptides (Fialkow et al. 2007). These ROS/RNS are produced intracellularly and can be released extracellularly (Freitas et al. 2009).
The GAGs, such as hyaluronan, chondroitin 4-sulfate, and dermatan sulfate, were shown to be degraded with loss of both uronic acid and hexosamine residues when cultured with PMN stimulated by phorbol myristyl acetate (PMA). The degradation pattern of GAG was very similar to the pattern of degradation observed following hydroxyl radical attack (Moseley et al. 1997). The ability to degrade hyaluronan appears to be specific to neutrophils stimulated with PMA, not with fMLP, concanavalin-A, or digitonin (Saari et al. 1993; Rees et al. 2008). Degradation of hyaluronan of joint synovial fluid has been linked to rheumatoid arthritis. In the synovial fluid of rheumatoid arthritis, the fragmented hyaluronan was considered to be due to the attack by ROS such as the hydroxyl radical (McCord 1974; Grootveld et al. 1991), and activated neutrophils were considered as the major cell types to produce ROS degrading hyaluronan (Greenwald and Moak 1986). Furthermore, ROS-mediated hyaluronan degradation generated products with a polydisperse size and possibly with different conformational characteristics due to radical-featured repolymerization (McNeil et al. 1985). Interestingly, rat mast cell granules-associated heparin could even be degraded by normal neutrophils. Moreover, neutrophils from patients with chronic granulomatous disease [deficiency of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase] had a dramatic decrease in their ability to degrade heparin (Metcalfe et al. 1990). This provided direct evidence demonstrating that ROS in neutrophils are the major reactive species responsible for heparin degradation.
Antigen presenting cells
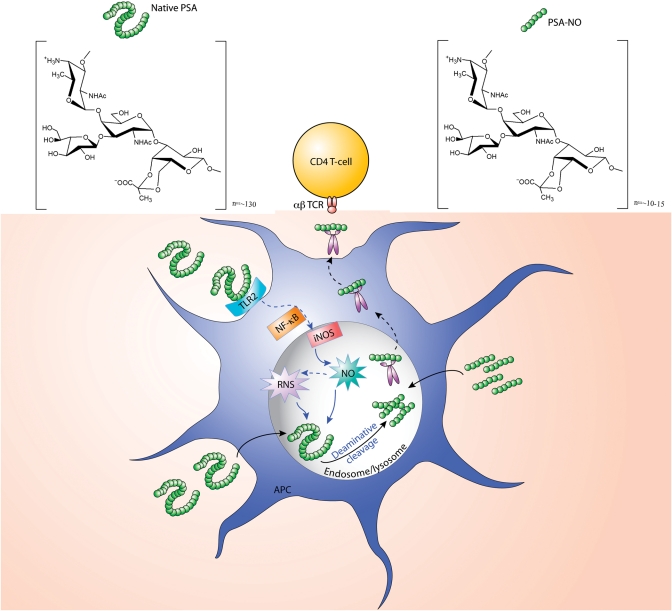
Polysaccharide A (PSA, see structure in Figure 1), the most immunodominant and highly conserved zwitterionic polysaccharide from the anaerobic bacterium Bacteroides fragilis plays a key role in both the pathogenic and the beneficial biological effects of this microbe. On the one hand, PSA facilitates the induction of abscesses when B. fragilis enters a normally sterile site (Tzianabos et al. 2000); on the other hand, PSA specifically directs maturation of the mammalian immune system and protects animals from inflammatory bowel disease through the induction of interleukin-10-producing CD4+ T cells (Mazmanian et al. 2005, 2008). Both of these important outcomes are directly attributable to the ability of PSA to activate CD4+ T cells. T cell activation requires presentation of PSA by antigen presenting cells (APCs) through the major histocompatibility complex II (MHCII) pathway (Cobb et al. 2004). In marked contrast to the processing of protein antigens in the acidic endolysosomal compartment by proteases, processing of PSA depended on the presence of inducible NO synthase (iNOS), but not NADPH oxidase in the APCs (Cobb et al. 2004). PSA induces iNOS mRNA expression and NO radical production in dendritic cells (DCs) through toll-like receptor 2 activation (Wang et al. 2006; Duan et al. 2008). The degradation of PSA was greatly suppressed in iNOS-deficient DCs when compared with wild type (WT) DCs. In contrast, N-acetylated PSA (complete N-acetylation of all free amino groups in PSA) was degraded equally between WT and iNOS-deficient DCs (Duan et al. 2008). In addition, native PSA, but not N-acetylated PSA was significantly degraded with NO in MPO [myeloperoxidase, which restricts the production of NO (Kumar et al. 2005)] deficient macrophages when compared with WT macrophages. These observations suggested that iNOS-derived RNS is the predominant species to degrade the native PSA molecule with deamination probably being the only responsible mechanism. As opposed to the native PSA, a partially deaminated product PSA-NO (∼16 kDa), which has an intact zwitterionic motif bypasses this cleavage step and can directly bind to MHCII for presentation to the αβ T cell receptor (Figure 1). Similarly, capsular polysaccharide SP1 form type I Streptococcus pneumoniae was also degraded in endsome/lysosome of APCs in an NO-dependent manner (Velez et al. 2009). In contrast to PSA degradation by RNS, dextran was depolymerized in the endolysosome, and this degradation attributed to hydrogen peroxide- and superoxide-derived ROS (Duan et al. 2008).
Fig. 1.
A proposed model of APC processing and presentation of B. fragilis PSA and its pre-processed products. The native PSA molecule (∼130 repeating units) produced by B. fragilis comes into contact with a DC and initially activates the cell through toll-like receptor 2-mediated signaling resulting in increased NF-kB-mediated transcription of iNOS. iNOS upregulation results in enhanced production of NO by the cell. NO is the chemical responsible for the deaminative cleavage of PSA to its product PSA-NO (∼10 repeating units). Native PSA requires deaminative cleavage prior to binding to MHCII and subsequent presentation to the αβ T cell receptor of CD4+ T cells. If experimentally, PSA is pre-processed by reacting it with NO in vitro, the product PSA-NO bypasses this cleavage step and can directly bind to MHCII for presentation to the αβ-T cell receptor.
Endothelial cells
After incubation of heparin with human umbilical vein endothelial cells (HUVECs), the degradative products of heparin were recovered in the medium (Vilar et al. 1997). This degradation depended on NO, which is synthesized constitutively by endothelial NO synthase. In contrast, hyaluronan stayed intact when cultured with HUVECs, although other reactive species, such as hydroxyl radical and peroxynitrite, can chemically depolymerize hyaluronan (Li et al. 1997). Deamination is one possible mechanism for NO-mediated degradation of heparin, but not hyaluronan because in the latter molecule all free amino groups are N-acetylated.
Cell-associated proteoglycans containing heparan sulfate (HS) are involved in the development, tissue repair, and tumorigenesis (Bernfield et al. 1999). In particular, HS side chains are capable of binding and/or activating and/or transporting many growth factors, cytokines, enzymes, viral proteins, and polyamines (Bernfield et al. 1999). HS proteoglycans on the surface are continuously endocytosed, degraded, and newly resynthesized through a recycling process in vascular endothelial cells (Mani et al. 2000), normal fibroblasts (Fransson et al. 1995), and carcinoma cells (Mani et al. 2007). During recycling, in addition to heparanase-catalyzed degradation, the HS side chains are degraded via an NO-dependent deaminative cleavage at N-unsubstituted glucosamine residues. Deaminative cleavage will generate a reducing terminal sugar anhydromannose, which could be visualized by a specific fluorophore-labeled monoclonal antibody (Cheng et al. 2002). In this process, NO/HNO is originated from preformed S-nitroso groups in the core protein of HS proteoglycans, and the degradation is found to start in early endosomes and complete in late endosomes (Mani et al. 2007).
Plant cells
Cellulose, hemicelluloses (mainly xyloglucan in the plant), and pectin are the major carbohydrates making up the primary (growing) plant cell wall. Cell wall loosening is an important developmental process in key stages (seed germination, elongation growth, and fruit ripening) of the plant life cycle (Muller et al. 2009). This process requires structural changes in the cell wall. Enzymatic hydrolysis of cell wall polysaccharides by glycosidases, such as β-1,3-glucanase, β-1,4-mannanase, xyloglucan endotransglucosylase/hydrolases, etc., is essential for wall modification (Chen et al. 2002; Leubner-Metzger 2002; da Silva et al. 2004). As an additional plant cell wall-loosening agent, ROS-like hydroxyl radical (·OH) presumably can target cell wall components including polysaccharides, leading to the breakage of cell wall polysaccharides (Muller et al. 2009). In fact, apoplastic hydroxyl radical and superoxide radicals were detected in cress caps and radicles in vivo during seed germination. By ways of electron paramagnetic resonance spectroscopy and 3H fingerprinting, it was found that hydroxyl radical attacks tissue-specific, not random cell wall polysaccharides (Schopfer 2001). In addition to ROS, plant tissues also synthesize NO via the nonenzymatic reduction in apoplastic nitrite (Bethke et al. 2004), whereas it remains unknown whether NO-derived RNS such as peroxynitrite is involved in the depolymerization of cell wall polysaccharides in vivo.
Polysaccharide depolymerization by ROS/RNS in concert with enzymes
The catabolism of polysaccharide is mainly achieved by glycoside hydrolases in vivo. Genetic deficiency in enzymes essential for cleaving carbohydrate chains, for example, mucopolysaccharidoses, will cause serious diseases characteristic of polysaccharide accumulation in mammalian tissues (Dorfman and Matalon 1976). However, as an alternative and supplementary mechanism, especially in inflammatory conditions, ROS/RNS might play a role in the depolymerization of polysaccharides. In fact, there is evidence suggesting that the oxidatively cleaved polysaccharides by ROS/RNS could serve as the substrates for the enzymatic cleavage that follows, thus facilitating depolymerization of the polysaccharide (Greenwald and Moy 1980; Metcalfe et al. 1990). For instance, prior treatment with ROS/RNS rendered hyaluronan more susceptible to degradation by lysosomal carbohydrases such as β-glucuronidase or β-N-acetylglucosaminidase (Greenwald and Moy 1980). One explanation is the partial degradation of polysaccharides by ROS/RNS would overcome steric factors which prevent the interaction between enzymes and highly polymerized substrates (Greenwald and Moy 1980). Notably, the substantial alternation in the structure of unfragmented polysaccharide by ROS/RNS was shown to slow down the cleavage of glycosidic bonds by enzymes with high specificity. Compared with original hyaluronan having a similar molecular size, the polymer modified by ROS/RNS originating from ultrasonic treatment was more resistant to enzymatic cleavage by specific hyaluronidase (Chabrecek et al. 1991; Kohen and Nyska 2002).
Brown rot basidiomycetes, the highly destructive wood decay fungi, such as Gloeophyllum trabeum and Postia placenta, can produce significant quantities of extracellular ROS to degrade cellulose (Hammel et al. 2002; Baldrian and Valaskova 2008). In the case of P. placenta, endoglucanases were only active on cellulose after oxidative pretreatment (Ratto et al. 1997). Thus it was proposed that the initial depolymerization probably facilitates the penetration and entry of oxidative and hydrolytic enzymes which further degrade celluloses (Hammel et al. 2002). Actually, one recent study (Vaaje-Kolstad et al. 2010) had discovered that a chitin-binding protein CBP21 oxidatively catalyzed the cleavage of glycosidic bonds in crystalline chitin in the presence of an external electron donor. The resulting oxidized chain ends effectively promoted further degradation of chitin by chitinases.
Potential outcome of ROS/RNS-mediated scission of polysaccharide chains
The classical immunological paradigms of antigen presentation has MHCII molecules presenting extracellular antigens, whereas MHCI molecules presenting antigens located in the intracellular compartment (Cresswell 1994; Pamer and Cresswell 1998). The molecules that these pathways are known to present are proteins or protein conjugates. In the MHCI pathway, intracellular protein antigens are processed by the cytosolic proteasome in APCs and subsequently presented to CD8+ T cells. Similarly, extracellular protein antigens are degraded by endosomal and lysosomal proteases in APCs before being presented by MHCII molecules to CD4+ T cells (Abbas and Lichtman 2005). Glycolipids have been shown to be presented by CD1 molecules directly or after being degraded by an enzymatic process (Schaible and Kaufmann 2000; Prigozy et al. 2001). Interestingly, polysaccharides are depolymerized via a ROS/RNS-dependent manner in APCs (Duan et al. 2008). In particular, zwitterionic polysaccharides, i.e. PSA from commensal bacteria B. fragilis, are processed to a significantly smaller molecular size (∼10–15 kDa) within endosome/lysosome through a deaminative mechanism. Thus, MHCII pathway-mediated carbohydrate antigen processing in APCs could be achieved by ROS/RNS-mediated oxidative reactions.
In mammals, hyaluronan, which is an important component of the extracellular matrix involved in the structure of connective tissues, can modulate a variety of cellular and tissue functions (Raines et al. 2000). Hyaluronan is a linear polymer composed of repeating units of the disaccharide [-d-glucuronic acid-β-1,3-N-acetyl-d-glucosamine-β-1,4-]. Differently sized hyaluronans trigger different signal transduction pathways (Jiang et al. 2005; Jiang et al. 2007). For example, tetrasaccharides are antiapoptotic and inducers of heat shock proteins (Jiang et al. 2007). Hyaluronan oligomers with 8–16 repeating units, stimulated angiogenesis in vivo, and endothelial proliferation in vitro (Stern et al. 2006); smaller hyaluronans (<500 kDa), but not the native hyaluronans (>1000 kDa) induce proinflammatory responses in macrophages (Jiang et al. 2005). Larger hyaluronan (1000–5000 kDa) suppress angiogenesis, immune responses, and phagocytosis (Jiang et al. 2007). In rheumatoid arthritis, neutrophil-derived ROS attack has been shown to be responsible for the fragmentation of hyaluronan in joint synovial fluid (McCord 1974; Grootveld et al. 1991). The differently sized hyaluronans might be important in regulating the disease process.
In plants, intact chain length of cell wall polysaccharides is a requisite for structural integrity of the plant cell wall (Herron et al. 2000). The depolymerization of these polysaccharides leads to loosening of the cell wall and plays a major role in fruit softening during ripening (Prasanna et al. 2007) in which ROS/RNS-like hydroxyl radical were shown be involved (Schopfer 2001; Muller et al. 2009).
Conclusions and future directions
Oxidative degradation of polysaccharides might result in nonspecific and/or specific scission of carbohydrate chains. This process could be an alternative and/or supplementary way for glycoside hydrolases-mediated hydrolysis to depolymerize polysaccharides. Indeed, ROS/RNS can degrade polysaccharides and lead to the subsequent enzymatic cleavage of glycosidic bonds. Cellular levels of ROS/RNS can effectively breakdown polysaccharides, and the data suggest that oxidative cleavage of carbohydrate chains is biologically relevant. It appears that ROS/RNS can also modify the monosaccharide composition of polysaccharides and potentially generate polymer fragments possibly having properties substantially different from those of the original macromolecule. However, in a biological context, the consequence of this change remains unclear.
In encapsulated pathogenic bacteria, polysaccharides on their surfaces contribute to host virulence by many mechanisms including: antiphagocytic and antibacteriolytic activity; immune evasion; immune modulation; and biofilm formation (Comstock and Kasper 2006). These capsular polysaccharides always have very large molecular size, and their immunogenicity has been found to be directly related to the chain length (Kabat and Bezer 1958; Howard et al. 1971; Kasper et al. 1982; Kalka-Moll et al. 2000). For instance, dextran with a molecular mass <80,000–90,000 Da does not elicit an antibody response in humans (Kabat and Bezer 1958). Given ROS/RNS are released massively during the crosstalk between host and invading organism, the fragmented products of capsular polysaccharides presumably would be generated intracellularly and/or extracellularly. It remains unknown whether these decomposed carbohydrates could elicit more progressive or suppressive humoral immune responses when compared with those elicited by native polysaccharides. In APCs, it has been found that the predominant products (∼10 kDa) of the degradation of polysacccharides by RNS/ROS within endsome/lysosome are produced over a period of time. However, either prolonging treatment of ZPS or dextran with exogenous ROS/RNS or increasing the ROS/RNS concentration results in formation of smaller degraded products in vitro. These data suggested that the ROS/RNS-induced depolymerization of polysaccharide is finely controlled in APCs (Duan et al. 2008). More recently, it has been shown that the oxidation of endocytosed PSA release protons which in turn inhibits the breakdown of PSA. That is, the oxidative processing of carbohydrate antigens is operated in a self-regulatory feedback mechanism (Lewis and Cobb 2010). At the present, it awaits to be understood whether this feedback mechanism is universal to the processing of other carbohydrates in APCs.
Funding
This work was supported by National Institute of Allergy and Infectious Diseases (NIAID) (RO1 AI039576) to D.L.K., and in part by special talent recruitment fund of Northwest A&F University (to J.D).
Abbreviation
AATp, 3-linked 2-acetamido-4-amino-2,4,6-trideoxygalactopyranosyl; APCs, antigen presenting cells; DC, dendritic cell; GAG, glycosaminoglycan; HA, hyaluronic acid; HS, heparan sulfate; HUVECs, heparin with human umbilical vein endothelial cells; iNOS, inducible NO synthase; MHCII, major histocompatibility complex II; MPO, myeloperoxidase; NADPH, nicotinamide adenine dinucleotide phosphate; NO, nitric oxide; PMA, phorbol myristyl acetate; PSA, polysaccharide A; RNS, reactive nitrogen species; ROS, reactive oxygen species WT, wild type.
Acknowledgements
We appreciate Ms. Julie McCoy for helpful editorial assistance.
References
- Abbas AK, Lichtman AH. Cellular and Molecular Immunology. 5th ed. USA: Elsevier Saunders; 2005. [Google Scholar]
- Al-Assaf S, Navaratnam S, Parsons BJ, Phillips GO. Chain scission of hyaluronan by peroxynitrite. Arch Biochem Biophys. 2003;411:73–82. doi: 10.1016/s0003-9861(02)00724-5. doi:10.1016/S0003-9861(02)00724-5. [DOI] [PubMed] [Google Scholar]
- Andley UP, Chakrabarti B. Role of singlet oxygen in the degradation of hyaluronic acid. Biochem Biophys Res Commun. 1983;115:894–901. doi: 10.1016/s0006-291x(83)80019-9. doi:10.1016/S0006-291X(83)80019-9. [DOI] [PubMed] [Google Scholar]
- Baldrian P, Valaskova V. Degradation of cellulose by basidiomycetous fungi. FEMS Microbiol Rev. 2008;32:501–521. doi: 10.1111/j.1574-6976.2008.00106.x. doi:10.1111/j.1574-6976.2008.00106.x. [DOI] [PubMed] [Google Scholar]
- Bernfield M, Gotte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, Zako M. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem. 1999;68:729–777. doi: 10.1146/annurev.biochem.68.1.729. doi:10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- Bethke PC, Badger MR, Jones RL. Apoplastic synthesis of nitric oxide by plant tissues. Plant Cell. 2004;16:332–341. doi: 10.1105/tpc.017822. doi:10.1105/tpc.017822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabrecek P, Sÿoltes L, Orvisky E. Comparative depolymerization of sodium hyaluronate by ultrasonic and enzymatic treatments. J Appl Polym Sci, Appl Polym Symp. 1991;48:233. doi:10.1002/app.1991.070480019. [Google Scholar]
- Chen F, Nonogaki H, Bradford KJ. A gibberellin-regulated xyloglucan endotransglycosylase gene is expressed in the endosperm cap during tomato seed germination. J Exp Bot. 2002;53:215–222. doi: 10.1093/jexbot/53.367.215. doi:10.1093/jexbot/53.367.215. [DOI] [PubMed] [Google Scholar]
- Cheng F, Mani K, van den Born J, Ding K, Belting M, Fransson LA. Nitric oxide-dependent processing of heparan sulfate in recycling S-nitrosylated glypican-1 takes place in caveolin-1-containing endosomes. J Biol Chem. 2002;277:44431–44439. doi: 10.1074/jbc.M205241200. doi:10.1074/jbc.M205241200. [DOI] [PubMed] [Google Scholar]
- Cobb BA, Wang Q, Tzianabos AO, Kasper DL. Polysaccharide processing and presentation by the MHCII pathway. Cell. 2004;117:677–687. doi: 10.016/j.cell.2004.05.001. doi:10.1016/j.cell.2004.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comstock LE, Kasper DL. Bacterial glycans: Key mediators of diverse host immune responses. Cell. 2006;126:847–850. doi: 10.1016/j.cell.2006.08.021. doi:10.1016/j.cell.2006.08.021. [DOI] [PubMed] [Google Scholar]
- Cresswell P. Assembly, transport, and function of MHC class II molecules. Annu Rev Immunol. 1994;12:259–293. doi: 10.1146/annurev.iy.12.040194.001355. doi:10.1146/annurev.iy.12.040194.001355. [DOI] [PubMed] [Google Scholar]
- da Silva EA, Toorop PE, van Aelst AC, Hilhorst HW. Abscisic acid controls embryo growth potential and endosperm cap weakening during coffee (Coffea arabica cv. Rubi) seed germination. Planta. 2004;220:251–261. doi: 10.1007/s00425-004-1344-0. doi:10.1007/s00425-004-1344-0. [DOI] [PubMed] [Google Scholar]
- Deslongchamps P, Moreau C. Ozonolysis of acetals. (1) Ester synthesis, (2) THP ether cleavage, (3) selective oxidation of β-glycoside, (4) oxidative removal of benzylidene and ethylidene protecting groups. Can J Chem. 1971;49:2465–2467. doi:10.1139/v71-405. [Google Scholar]
- Dorfman A, Matalon R. The mucopolysaccharidoses (a review) Proc Natl Acad Sci USA. 1976;73:630–637. doi: 10.1073/pnas.73.2.630. doi:10.1073/pnas.73.2.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan J, Avci FY, Kasper DL. Microbial carbohydrate depolymerization by antigen-presenting cells: Deamination prior to presentation by the MHCII pathway. Proc Natl Acad Sci USA. 2008;105:5183–5188. doi: 10.1073/pnas.0800974105. doi:10.1073/pnas.0800974105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fialkow L, Wang Y, Downey GP. Reactive oxygen and nitrogen species as signaling molecules regulating neutrophil function. Free Radic Biol Med. 2007;42:153–164. doi: 10.1016/j.freeradbiomed.2006.09.030. doi:10.1016/j.freeradbiomed.2006.09.030. [DOI] [PubMed] [Google Scholar]
- Fransson LA, Edgren G, Havsmark B, Schmidtchen A. Recycling of a glycosylphosphatidylinositol-anchored heparan sulphate proteoglycan (glypican) in skin fibroblasts. Glycobiology. 1995;5:407–415. doi: 10.1093/glycob/5.4.407. doi:10.1093/glycob/5.4.407. [DOI] [PubMed] [Google Scholar]
- Freitas M, Lima JL, Fernandes E. Optical probes for detection and quantification of neutrophils’ oxidative burst. A review. Anal Chim Acta. 2009;649:8–23. doi: 10.1016/j.aca.2009.06.063. doi:10.1016/j.aca.2009.06.063. [DOI] [PubMed] [Google Scholar]
- Gilbert BC, King DM, Thomas B. Radical reactions of carbohydrates. Part 2. An electron spin resonance study of the oxidation of D-glucose and related compounds with the hydroxyl radical. J Chem Soc Perkin Trans. 1981;2:1186–1199. [Google Scholar]
- Greenwald RA, Moak SA. Degradation of hyaluronic acid by polymorphonuclear leukocytes. Inflammation. 1986;10:15–30. doi: 10.1007/BF00916037. doi:10.1007/BF00916037. [DOI] [PubMed] [Google Scholar]
- Greenwald RA, Moy WW. Effect of oxygen-derived free radicals on hyaluronic acid. Arthritis Rheum. 1980;23:455–463. doi: 10.1002/art.1780230408. doi:10.1002/art.1780230408. [DOI] [PubMed] [Google Scholar]
- Grootveld M, Henderson EB, Farrell A, Blake DR, Parkes HG, Haycock P. Oxidative damage to hyaluronate and glucose in synovial fluid during exercise of the inflamed rheumatoid joint. Detection of abnormal low-molecular-mass metabolites by proton-n.m.r. spectroscopy. Biochem J. 1991;273(Pt 2):459–467. doi: 10.1042/bj2730459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammel KE, Kapich AN, Jensen KA, Jr, Ryan ZC. Reactive oxygen species as agents of wood decay by fungi. Enzyme Microb Technol. 2002;30:445–453. doi:10.1016/S0141-0229(02)00011-X. [Google Scholar]
- Hassan MS, Mileva MM, Dweck HS, Rosenfeld L. Nitric oxide products degrade chondroitin sulfates. Nitric oxide. 1998;2:360–365. doi: 10.1006/niox.1998.0198. doi:10.1006/niox.1998.0198. [DOI] [PubMed] [Google Scholar]
- Hawkins CL, Davies MJ. Direct detection and identification of radicals generated during the hydroxyl radical-induced degradation of hyaluronic acid and related materials. Free Radic Biol Med. 1996;21:275–290. doi: 10.1016/0891-5849(96)00042-1. doi:10.1016/0891-5849(96)00042-1. [DOI] [PubMed] [Google Scholar]
- Heinze T, Liebert T, Koschella A. Esterification of Polysaccharides. New York: Springer; 2006. Introduction and objectives. [Google Scholar]
- Herron SR, Benen JA, Scavetta RD, Visser J, Jurnak F. Structure and function of pectic enzymes: Virulence factors of plant pathogens. Proc Natl Acad Sci USA. 2000;97:8762–8769. doi: 10.1073/pnas.97.16.8762. doi:10.1073/pnas.97.16.8762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard JG, Zola H, Christie GH, Courtenay BM. Studies on immunological paralysis. V. The influence of molecular weight on the immunogenicity, tolerogenicity and antibody-neutralizing activity of the 3 pneumococcal polysaccharide. Immunology. 1971;21:535–546. [PMC free article] [PubMed] [Google Scholar]
- Jan F, Rabek JF, Ranby B. Role of singlet oxygen in photo-oxidation of polymers. J Photochem Photobiol. 1974;28:557–569. [Google Scholar]
- Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, et al. Regulation of lung injury and repair by toll-like receptors and hyaluronan. Nat Med. 2005;11:1173–1179. doi: 10.1038/nm1315. doi:10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- Jiang D, Liang J, Noble PW. Hyaluronan in tissue injury and repair. Annu Rev Cell Dev Biol. 2007;23:435–461. doi: 10.1146/annurev.cellbio.23.090506.123337. doi:10.1146/annurev.cellbio.23.090506.123337. [DOI] [PubMed] [Google Scholar]
- Kabat EA, Bezer AE. The effect of variation in molecular weight on the antigenicity of dextran in man. Arch Biochem Biophys. 1958;78:306–318. doi: 10.1016/0003-9861(58)90354-0. doi:10.1016/0003-9861(58)90354-0. [DOI] [PubMed] [Google Scholar]
- Kalka-Moll WM, Tzianabos AO, Wang Y, Carey VJ, Finberg RW, Onderdonk AB, Kasper DL. Effect of molecular size on the ability of zwitterionic polysaccharides to stimulate cellular immunity. J Immunol. 2000;164:719–724. doi: 10.4049/jimmunol.164.2.719. [DOI] [PubMed] [Google Scholar]
- Kasper DL, Baker CJ, Edwards MS, Nicholson-Weller A, Jennings HJ. In: Seminars in Infectious Diseases. Weinstein L, Fields BM, editors. New York: Thieme-Stratton; 1982. p. 275. [Google Scholar]
- Kasper DL, Paoletti LC, Wessels MR, Guttormsen HK, Carey VJ, Jennings HJ, Baker CJ. Immune response to type III group B streptococcal polysaccharide-tetanus toxoid conjugate vaccine. J Clin Invest. 1996;98:2308–2314. doi: 10.1172/JCI119042. doi:10.1172/JCI119042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenne L, Lindberg B, Unger P, Gustafsson B, Holme T. Structural studies of the Vibrio cholerae O-antigen. Carbohydr Res. 1982;100:341–349. doi: 10.1016/s0008-6215(00)81047-2. doi:10.1016/S0008-6215(00)81047-2. [DOI] [PubMed] [Google Scholar]
- Kennett EC, Davies MJ. Degradation of matrix glycosaminoglycans by peroxynitrite/peroxynitrous acid: Evidence for a hydroxyl-radical-like mechanism. Free Radic Biol Med. 2007;42:1278–1289. doi: 10.1016/j.freeradbiomed.2007.01.030. doi:10.1016/j.freeradbiomed.2007.01.030. [DOI] [PubMed] [Google Scholar]
- Kohen R, Nyska A. Oxidation of biological systems: Oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol Pathol. 2002;30:620–650. doi: 10.1080/01926230290166724. doi:10.1080/01926230290166724. [DOI] [PubMed] [Google Scholar]
- Kon S, Schwimmer S. Depolymerization of polysaccharides by active oxygen species derived from a xanthine oxidase system. J Food Biochem. 1977;1:141–152. doi:10.1111/j.1745-4514.1977.tb00177.x. [Google Scholar]
- Kumar AP, Ryan C, Cordy V, Reynolds WF. Inducible nitric oxide synthase expression is inhibited by myeloperoxidase. Nitric oxide. 2005;13:42–53. doi: 10.1016/j.niox.2005.04.002. doi:10.1016/j.niox.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Leubner-Metzger G. Seed after-ripening and over-expression of class I β-1,3-glucanase confer maternal effects on tobacco testa rupture and dormancy release. Planta. 2002;215:959–968. doi: 10.1007/s00425-002-0837-y. doi:10.1007/s00425-002-0837-y. [DOI] [PubMed] [Google Scholar]
- Lewis CJ, Cobb BA. Carbohydrate oxidation acidifies endosomes, regulating antigen processing and TLR9 signaling. J Immunol. 2010;184:3789–3800. doi: 10.4049/jimmunol.0903168. doi:10.4049/jimmunol.0903168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Rosenfeld L, Vilar RE, Cowman MK. Degradation of hyaluronan by peroxynitrite. Arch Biochem Biophys. 1997;341:245–250. doi: 10.1006/abbi.1997.9970. doi:10.1006/abbi.1997.9970. [DOI] [PubMed] [Google Scholar]
- Lindberg B, Lindqvist B, Lonngren J, Powell DA. Structural studies of the capsular polysaccharide from Streptococcus pneumoniae type 1. Carbohydr Res. 1980;78:111–117. doi: 10.1016/s0008-6215(00)83664-2. doi:10.1016/S0008-6215(00)83664-2. [DOI] [PubMed] [Google Scholar]
- Mani K, Cheng F, Fransson LA. Heparan sulfate degradation products can associate with oxidized proteins and proteasomes. J Biol Chem. 2007;282:21934–21944. doi: 10.1074/jbc.M701200200. doi:10.1074/jbc.M701200200. [DOI] [PubMed] [Google Scholar]
- Mani K, Jonsson M, Edgren G, Belting M, Fransson LA. A novel role for nitric oxide in the endogenous degradation of heparan sulfate during recycling of glypican-1 in vascular endothelial cells. Glycobiology. 2000;10:577–586. doi: 10.1093/glycob/10.6.577. doi:10.1093/glycob/10.6.577. [DOI] [PubMed] [Google Scholar]
- Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–118. doi: 10.1016/j.cell.2005.05.007. doi:10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–625. doi: 10.1038/nature07008. doi:10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- McCord JM. Free radicals and inflammation: Protection of synovial fluid by superoxide dismutase. Science. 1974;185:529–531. doi: 10.1126/science.185.4150.529. doi:10.1126/science.185.4150.529. [DOI] [PubMed] [Google Scholar]
- McNeil JD, Wiebkin OW, Betts WH, Cleland LG. Depolymerisation products of hyaluronic acid after exposure to oxygen-derived free radicals. Ann Rheum Dis. 1985;44:780–789. doi: 10.1136/ard.44.11.780. doi:10.1136/ard.44.11.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe DD, Thompson HL, Klebanoff SJ, Henderson WR., Jr Oxidative degradation of rat mast-cell heparin proteoglycan. Biochem J. 1990;272:51–57. doi: 10.1042/bj2720051. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley R, Waddington RJ, Embery G. Degradation of glycosaminoglycans by reactive oxygen species derived from stimulated polymorphonuclear leukocytes. Biochim Biophys Acta. 1997;1362:221–231. doi: 10.1016/s0925-4439(97)00083-5. [DOI] [PubMed] [Google Scholar]
- Moseley R, Waddington R, Evans P, Halliwell B, Embery G. The chemical modification of glycosaminoglycan structure by oxygen-derived species in vitro. Biochim Biophys Acta. 1995;1244:245–252. doi: 10.1016/0304-4165(95)00010-9. [DOI] [PubMed] [Google Scholar]
- Muller K, Linkies A, Vreeburg RA, Fry SC, Krieger-Liszkay A, Leubner-Metzger G. In vivo cell wall loosening by hydroxyl radicals during cress seed germination and elongation growth. Plant Physiol. 2009;150:1855–1865. doi: 10.1104/pp.109.139204. doi:10.1104/pp.109.139204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamer E, Cresswell P. Mechanisms of MHC class I–restricted antigen processing. Annu Rev Immunol. 1998;16:323–358. doi: 10.1146/annurev.immunol.16.1.323. doi:10.1146/annurev.immunol.16.1.323. [DOI] [PubMed] [Google Scholar]
- Paoletti LC, Kasper DL, Michon F, DiFabio J, Jennings HJ, Tosteson TD, Wessels MR. Effects of chain length on the immunogenicity in rabbits of group B Streptococcus type III oligosaccharide-tetanus toxoid conjugates. J Clin Invest. 1992;89:203–209. doi: 10.1172/JCI115564. doi:10.1172/JCI115564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasanna V, Prabha TN, Tharanathan RN. Fruit ripening phenomena–an overview. Crit Rev Food Sci Nutr. 2007;47:1–19. doi: 10.1080/10408390600976841. doi:10.1080/10408390600976841. [DOI] [PubMed] [Google Scholar]
- Prigozy TI, Naidenko O, Qasba P, Elewaut D, Brossay L, Khurana A, Natori T, Koezuka Y, Kulkarni A, Kronenberg M. Glycolipid antigen processing for presentation by CD1d molecules. Science. 2001;291:664–667. doi: 10.1126/science.291.5504.664. doi:10.1126/science.291.5504.664. [DOI] [PubMed] [Google Scholar]
- Raines EW, Koyama H, Carragher NO. The extracellular matrix dynamically regulates smooth muscle cell responsiveness to PDGF. Ann N Y Acad Sci. 2000;902:39–51. doi: 10.1111/j.1749-6632.2000.tb06299.x. Discussion 51–52 doi:10.1111/j.1749-6632.2000.tb06299.x. [DOI] [PubMed] [Google Scholar]
- Rao MV, Davis KR. The physiology of ozone induced cell death. Planta. 2001;213:682–690. doi: 10.1007/s004250100618. doi:10.1007/s004250100618. [DOI] [PubMed] [Google Scholar]
- Ratto M, Ritschkoff AC, Viikari L. The effect of oxidative pretreatment on cellulose degradation by Poria placenta and Trichoderma reesei cellulases. Appl Microbiol Biotechnol. 1997;48:53–57. doi:10.1007/s002530051014. [Google Scholar]
- Rees MD, Davies MJ. Heparan sulfate degradation via reductive homolysis of its N-chloro derivatives. J Am Chem Soc. 2006;128:3085–3097. doi: 10.1021/ja0577239. doi:10.1021/ja0577239. [DOI] [PubMed] [Google Scholar]
- Rees MD, Hawkins CL, Davies MJ. Hypochlorite-mediated fragmentation of hyaluronan, chondroitin sulfates, and related N-acetyl glycosamines: Evidence for chloramide intermediates, free radical transfer reactions, and site-specific fragmentation. J Am Chem Soc. 2003;125:13719–13733. doi: 10.1021/ja0370591. doi:10.1021/ja0370591. [DOI] [PubMed] [Google Scholar]
- Rees MD, Hawkins CL, Davies MJ. Hypochlorite and superoxide radicals can act synergistically to induce fragmentation of hyaluronan and chondroitin sulphates. Biochem J. 2004;381:175–184. doi: 10.1042/BJ20040148. doi:10.1042/BJ20040148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees MD, Kennett EC, Whitelock JM, Davies MJ. Oxidative damage to extracellular matrix and its role in human pathologies. Free Radic Biol Med. 2008;44:1973–2001. doi: 10.1016/j.freeradbiomed.2008.03.016. doi:10.1016/j.freeradbiomed.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Saari H, Konttinen YT, Friman C, Sorsa T. Differential effects of reactive oxygen species on native synovial fluid and purified human umbilical cord hyaluronate. Inflammation. 1993;17:403–415. doi: 10.1007/BF00916581. doi:10.1007/BF00916581. [DOI] [PubMed] [Google Scholar]
- Schaible UE, Kaufmann SH. CD1 molecules and CD1-dependent T cells in bacterial infections: A link from innate to acquired immunity? Semin Immunol. 2000;12:527–535. doi: 10.1006/smim.2000.0272. doi:10.1006/smim.2000.0272. [DOI] [PubMed] [Google Scholar]
- Schopfer P. Hydroxyl radical-induced cell-wall loosening in vitro and in vivo: Implications for the control of elongation growth. Plant J. 2001;28:679–688. doi: 10.1046/j.1365-313x.2001.01187.x. doi:10.1046/j.1365-313x.2001.01187.x. [DOI] [PubMed] [Google Scholar]
- Shively JE, Conrad HE. Formation of anhydrosugars in the chemical depolymerization of heparin. Biochemistry. 1976;15:3932–3942. doi: 10.1021/bi00663a005. doi:10.1021/bi00663a005. [DOI] [PubMed] [Google Scholar]
- Stern R, Asari AA, Sugahara KN. Hyaluronan fragments: An information-rich system. Eur J Cell Biol. 2006;85:699–715. doi: 10.1016/j.ejcb.2006.05.009. doi:10.1016/j.ejcb.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Tzianabos AO, Finberg RW, Wang Y, Chan M, Onderdonk AB, Jennings HJ, Kasper DL. T cells activated by zwitterionic molecules prevent abscesses induced by pathogenic bacteria. J Biol Chem. 2000;275:6733–6740. doi: 10.1074/jbc.275.10.6733. doi:10.1074/jbc.275.10.6733. [DOI] [PubMed] [Google Scholar]
- Vaaje-Kolstad G, Westereng B, Horn SJ, Liu Z, Zhai H, Sorlie M, Eijsink VG. An oxidative enzyme boosting the enzymatic conversion of recalcitrant polysaccharides. Science. 2010;330:219–222. doi: 10.1126/science.1192231. doi:10.1126/science.1192231. [DOI] [PubMed] [Google Scholar]
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. doi:10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Velez CD, Lewis CJ, Kasper DL, Cobb BA. Type I Streptococcus pneumoniae carbohydrate utilizes a nitric oxide and MHC II-dependent pathway for antigen presentation. Immunology. 2009;127:73–82. doi: 10.1111/j.1365-2567.2008.02924.x. doi:10.1111/j.1365-2567.2008.02924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilar RE, Ghael D, Li M, Bhagat DD, Arrigo LM, Cowman MK, Dweck HS, Rosenfeld L. Nitric oxide degradation of heparin and heparan sulphate. Biochem J. 1997;324(Pt 2):473–479. doi: 10.1042/bj3240473. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Hollingsworth RI, Kasper DL. Ozonolysis for selectively depolymerizing polysaccharides containing β-d-aldosidic linkages. Proc Natl Acad Sci USA. 1998;95:6584–6589. doi: 10.1073/pnas.95.12.6584. doi:10.1073/pnas.95.12.6584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Hollingsworth RI, Kasper DL. Ozonolytic depolymerization of polysaccharides in aqueous solution. Carbohydr Res. 1999;319:141–147. doi: 10.1016/s0008-6215(99)00113-5. doi:10.1016/S0008-6215(99)00113-5. [DOI] [PubMed] [Google Scholar]
- Wang Q, McLoughlin RM, Cobb BA, Charrel-Dennis M, Zaleski KJ, Golenbock D, Tzianabos AO, Kasper DL. A bacterial carbohydrate links innate and adaptive responses through toll-like receptor 2. J Exp Med. 2006;203:2853–2863. doi: 10.1084/jem.20062008. doi:10.1084/jem.20062008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels MR, Paoletti LC, Guttormsen HK, Michon F, D'Ambra AJ, Kasper DL. Structural properties of group B streptococcal type III polysaccharide conjugate vaccines that influence immunogenicity and efficacy. Infect Immun. 1998;66:2186–2192. doi: 10.1128/iai.66.5.2186-2192.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterbourn CC. Reconciling the chemistry and biology of reactive oxygen species. Nat Chem Biol. 2008;4:278–286. doi: 10.1038/nchembio.85. doi:10.1038/nchembio.85. [DOI] [PubMed] [Google Scholar]
- Yao H, Yang SR, Kode A, Rajendrasozhan S, Caito S, Adenuga D, Henry R, Edirisinghe I, Rahman I. Redox regulation of lung inflammation: Role of NADPH oxidase and NF-kappaB signalling. Biochem Soc Trans. 2007;35:1151–1155. doi: 10.1042/BST0351151. doi:10.1042/BST0351151. [DOI] [PubMed] [Google Scholar]