Abstract
Excitatory amino acid transporters (EAAT) transport glutamate into cells to regulate glutamate neurotransmission and to maintain nontoxic extracellular glutamate levels for neurons. We showed previously that the commonly used volatile anesthetic isoflurane increases the transporting activity of EAAT3, the major neuronal EAAT. This effect requires a protein kinase C (PKC) α-mediated and S465-dependent EAAT3 redistribution to the plasma membrane. Thus, we hypothesize that specific peptides can be designed to block this effect. We conjugated a 10-amino acid synthetic peptide with a sequence identical to that of EAAT3 around the S465 to a peptide that can facilitate permeation of the plasma membrane. This fusion peptide inhibited the isoflurane-increased EAAT3 activity and redistribution to the plasma membrane in C6 cells and hippocampus. It did not affect the basal EAAT3 activity. This peptide also attenuated isoflurane-induced increase of PKCα in the immunoprecipitates produced by an anti-EAAT3 antibody. A scrambled peptide that has the same amino acid composition as the S465 sequence-specific peptide but has a random sequence did not change the effects of isoflurane on EAAT3. The S465 sequence-specific peptide, but not the scrambled peptide, is a good PKCα substrate in in vitro assay. These peptides did not affect cell viability. These results, along with our previous findings, strongly suggest that PKCα interacts with EAAT3 to regulate its functions. The S465 sequence-specific peptide may interrupt this interaction and is an effective inhibitor for the regulation of EAAT3 activity and trafficking by PKCα and isoflurane.
Keywords: glutamate transporters, isoflurane, neurons, peptide inhibitor, protein kinase C, trafficking
1. Introduction
Glutamate transporters (also named excitatory amino acid transporters, EAAT)2 transport glutamate, the major excitatory neurotransmitter, from extracellular space into cells under physiological conditions (Danbolt, 2001). Through this basic function, EAAT prevents extracellular glutamate accumulation and regulates glutamate neurotransmission. Five EAATs have been identified so far. EAAT1 and EAAT2 are mainly expressed in glial cells. EAAT3 and EAAT4 are predominantly in neurons. EAAT5 is found in the neurons and glial cells of retina (Danbolt, 2001). EAAT1-3 are distributed in many brain regions; whereas EAAT4 is mostly expressed in the cerebellum (Danbolt et al., 1998). Thus, EAAT3 is the major neuronal EAAT in the brain.
A distinct feature of EAAT3 is that there is a significant pool of EAAT3 in the intracellular space (Gonzalez et al., 2007). This pool of EAAT3 resides in multiple cellular compartments including small vesicles and endosomes and can be trafficked to the plasma membrane where EAAT performs its function of glutamate transport. Regulation of EAAT trafficking can modulate the transporting activity of EAATs (Davis et al., 1998; Gonzalez et al., 2007; Huang and Zuo, 2005). Our laboratory and others have provided evidence that protein kinase C (PKC) can regulate EAAT3 trafficking/redistribution to the plasma membrane (Baik et al., 2009; Davis et al., 1998; Huang et al., 2006; Huang and Zuo, 2005).
We have shown that isoflurane, a commonly used volatile anesthetic, induces a PKCα-mediated increase of EAAT3 activity and redistribution of EAAT3 to the plasma membrane (Huang and Zuo, 2005). Up to 74% of intracellular EAAT3 can be redistributed to the plasma membrane in C6 cells after isoflurane exposure (Huang and Zuo, 2005). This effect may require phosphorylation of S465 in the EAAT3 molecule (Huang et al., 2006). Thus, we hypothesize that an S465 sequence-specific peptide can work as an inhibitor to reduce the isoflurane-induced, PKCα-mediated increase of EAAT3 activity and redistribution to the plasma membrane. To test this hypothesis, we conjugated S465 sequence-specific peptides with the trans-activating transcriptional activator protein transduction domain (TAT PTD). TAT PTD consists of 11 amino acids and does not participating in transcriptional activation of any genes. It has been used frequently to facilitate movement of peptides and proteins across cell plasma membranes (Fawell et al., 1994; Schwarze et al., 1999). Our results suggest that the S465 sequence-specific peptide is an effective inhibitor to attenuate isoflurane-increased EAAT3 activity and redistribution to the plasma membrane in C6 cells and hippocampus. The peptide also inhibits the interaction between PKCα and EAAT3 and is a good phosphorylation substrate for PKCα.
2. Materials and Methods
The animal protocol was approved by the institutional Animal Care and Use Committee of the University of Virginia (Charlottesville, VA). All animal experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publications number 80-23) revised in 1996.
2.1. Cell culture
Rat C6 glioma cells (American Type Culture Collection; Manassas, VA) that express endogenous EAAT3 were cultured in flasks in F-10 nutrient mixture (Ham) containing 15% horse serum and 2.5% fetal bovine serum at 37°C in a 95% air-5% CO2 incubator. When cells reached 50 - 60% confluence, the culture medium was replaced with a serum-free medium (F-10 mixture only) for 2 h before isoflurane incubation.
2.2. Preparation of hippocampal slices
Similar to the method reported before (Zhao et al., 2006), hippocampal slices were freshly prepared from 18- to 35-day-old Sprague-Dawley rats (Hilltop, Scotdale, PA). Rats were anesthetized with isoflurane and then decapitated. Brains were removed rapidly and placed in ice-cold artificial cerebrospinal fluid (aCSF) bubbled with 5% CO2 and 95% O2. The aCSF contained 116 mM NaCl, 26.2 mM NaHCO3, 5.4 mM KCl, 1.8 mM CaCl2, 0.9 mM MgCl2, 0.9 mM NaH2PO4 and 5.6 mM glucose, pH 7.4. Hippocampi were immediately dissected out and sliced into 400 μm thick slices using a vibrating tissue slicer in ice-cold cutting solution containing 260 mM sucrose, 26.2 mM NaHCO3, 3 mM KCl, 1.2 mM NaH2PO4, 5 mM MgCl2 and 9 mM glucose, pH 7.4 and bubbled with 5% CO2 and 95% O2. These slices were immersed in circulating aCSF continuously bubbled with 5% CO2 and 95% O2 (oxygenated aCSF) at room temperature for at least 1 h for recovery of the synaptic function (Popovic et al., 2000) and were then transferred to oxygenated aCSF at 37°C for 15 min before they were used for experiments.
2.3. Preparation of crude synaptosomes
The preparation of crude synaptosomes was as we described before (Huang and Zuo, 2005). All steps were done at 4°C. Briefly, adult Sprague-Dawley rats were transcardially perfused with normal saline under isoflurane anesthesia. Their hippocampi were immediately dissected on ice and weighed (usually ∼50 mg/each side). They were then homogenized in 20 volumes of ice-cold 0.32 M sucrose solution with 8 strokes in a glass Dounce homogenizer. Each stroke lasted for 5 s. The solution was centrifuged at 800 g for 10 min. The supernatant was centrifuged again at 20,000 g for 20 min. The pellet was resuspended in sucrose solution and repelleted again by centrifugation at 20,000 g for 20 min. This washed P2 pellet was crude synaptosomes that were resuspended in phosphate-buffered saline (PBS)-Ca/Mg containing 10 mM dextrose for isoflurane exposure.
2.4. Isoflurane incubation
C6 cells, hippocampal slices and hippocampal synaptosomes were incubated with isoflurane in an open system as follows. Fresh serum-free medium (50 to 200 ml), aCSF or PBS-Ca/Mg containing 10 mM dextrose that had been gassed with 95% air-5% CO2 through or not through an isoflurane vaporizer delivering 2% isoflurane at a flow rate 3 1/min for 20 min was added to the cells, hippocampal slices or synaptosomes for 5 min at 37°C. Preliminary experiments with gas chromatography showed that isoflurane concentrations in the solutions reached equilibrium 5 min after the onset of gassing under these experimental conditions. During the incubation, the solutions were continuously gassed with the carrier gases containing or not containing isoflurane to compensate for isoflurane loss from the solutions to air. We chose the exposure condition (2% isoflurane for 5 min at 37°C) because this condition induced a significant increase of EAAT3 activity and redistribution to the plasma membrane in our previous studies (Huang et al., 2006; Huang and Zuo, 2005). Although 1% isoflurane also significantly increased EAAT3 activity and redistribution to the plasma membrane, its effects were not as big as those induced by 2% isoflurane (Do et al., 2002; Huang and Zuo, 2003).
2.5. Peptide preparation and application
The TAT PTD peptide (YGRKKRRQRRR) was conjugated directly to the peptides that we designed based on the sequence of EAAT3 around the S465. The sequences of the TAT PTD-conjugated and S465 sequence-specific peptides are as follows: 1) TAT PTD conjugated and S465 sequence-specific peptide: TAT PTD-VEKLSKKELE; 2) TAT PTD conjugated, S465 sequence-specific and S465-phosphorylated peptide (abbreviated as the phospho-peptide in the following sections): TAT PTD-VEKL(pS)KKELE; and 3) TAT PTD conjugated scrambled peptide: TAT PTD-VEKKKLLEES. The S465 is bolded in the sequence. The S465 sequence-specific peptide has a sequence identical to EAAT3 of all mammalian species identified so far including rat, human, mouse and rabbit EAAT3 (Arriza et al., 1994; Kanai and Hediger, 1992; Mukainaka et al., 1995). The scrambled peptide has an identical proportion of each amino acid to that of the S465 sequence-specific peptide but the sequence is scrambled. These peptides were synthesized in vitro by the Biomolecular Research Facility, University of Virginia (Charlottesville, VA).
To test the effects of these peptides, C6 cells or hippocampal slices were incubated with or without the peptides (10 μM except for the dose-response experiments) for 30 min at 37°C before the cells were exposed to 2% isoflurane for 5 min. The peptides were also in the incubation solution during the isoflurane exposure. Dose-response experiments were performed by incubating C6 cells with 0, 1.25, 2.5, 5, 10 and 20 μM S465 sequence-specific peptide for 30 min before the isoflurane exposure and during the isoflurane exposure.
2.6. Biotinylation
Biotinylation of cell surface proteins was performed as we described previously (Huang et al., 2006; Huang and Zuo, 2005). C6 cells in 75-cm2 tissue culture flasks were used for the experiments. After incubation with or without isoflurane for 5 min, the cells were rinsed twice with ice-cold PBS, and then were incubated with 2 ml of sulfo-NHS-SS-Biotin solution (Pierce Biotechnology, Rockford, IL) for 30 min at 4°C with gentle shaking. The biotinylation reaction was terminated by washing the cells three times with ice-cold PBS-Ca/Mg containing 100 mM glycine. After the cells were incubated in this wash solution for 45 min at 4°C with gentle agitation, they were lysed in 2 ml of lysis buffer containing 100 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 1 μg/ml leupeptin, 250 μM phenylmethylsulfonyl fluoride, 1 μg/ml aprotinin, 1 mg/ml trypsin inhibitor, and 1 mM iodoacetamide for 1 h at 4°C with vigorous shaking. The total lysates were centrifuged at 20,000 g for 20 min at 4°C to remove nuclei and debris. The resulting supernatants were incubated with equal volumes of suspension of avidin-conjugated beads (600 μl of bead suspension to 600 μl of lysates) for 1 h at room temperature with occasional stirring. The mixture was then centrifuged at 16,500 g for 15 min at 4°C. The pellet was then washed four times, each time with 1 ml of lysis buffer. The pellet that contained the biotinylated cell surface proteins was resuspended in 500 μl of Laemmli buffer containing 62.5 mM Tris-HCl, pH 6.8, 2% SDS, 20% glycerol, and 5% 2-mercaptoethanol for 30 min to dissolve the biotinylated proteins. The mixture was centrifuged at 16,500 g for 10 min at 4 °C and this third supernatant was kept for Western blotting as the biotinylated fraction.
For experiments using hippocampal synaptosomes, immediately after isoflurane exposure sulfo-NHS-SS-Biotin was added to the incubation buffer to achieve a concentration of 1 mg/ml. This buffer was kept for 20 min at 4°C. The biotinylated proteins in the synaptosomes were then separated from non-biotinylated proteins as described above.
2.7. Western blotting
After protein content in samples was quantitated by the Lowry assay using a protein assay kit (catalogue number: 690-A; Sigma Chemical, St. Louis, MO), 25 μg of proteins per lane were subjected to Western analysis as described before (Huang and Zuo, 2003; Zuo and Johns, 1997). Briefly, proteins were separated by electrophoresis through 10% sodium dodecyl sulfate-polyacrylamide gels and then were electrotransferred to polyvinylidene fluoride membranes. The protein bands were probed with primary antibodies (rabbit polyclonal anti-EAAT3 at 0.5 μg/ml or mouse monoclonal anti-PKCα at 1 μg/ml) and then a horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse IgG antibody, and finally visualized by the enhanced chemiluminescence method with multiple exposures of films due to the limited linear range of intensity produced by this method. Quantitative analysis of the protein bands was performed using an ImageQuant 5.0 Molecular Dynamics Densitometer (Molecular Dynamics, Sunnyvale, CA). The relationship between the protein band signal and exposure time of the heaviest band on the films was established. Protein bands on a film where the intensity of the heaviest band was still within the linear range were measured to generate the data reported here.
2.8. Immunoprecipitation
As we described previously (Huang and Zuo, 2005), C6 cells cultured in 75-cm2 dishes were lysed in 2 ml of buffer containing 50 mM Tris-HCl, 1% NP-40, 0.5% sodium deoxycholate, 50 mM NaCl, Halt phosphatase inhibitor cocktail and complete protease inhibitors for 1 h at 4°C. The lysates were centrifuged at 14,000 g for 15 min to remove cell debris. The resulting supernatants were incubated overnight with 2 μg of affinity-purified polyclonal rabbit anti-EAAT3 antibody at 4°C. The mixture was then incubated with 40 μl of protein A/G plus-agarose beads for 1 h at 4°C with gentle shaking. The sample was then centrifuged at 500 g for 2 min at 4°C. The pellet containing bead-bound immune complexes was washed four times with the lysis buffer and the immune complexes were then eluted by incubation with 100 μl of Laemmli buffer at 90 – 95°C for 5 min. Control experiments using beads alone or rabbit IgG to replace the rabbit anti-EAAT3 antibody were performed to show the specificity of the anti-EAAT3 antibody.
2.9. Glutamate uptake assay
As described before (Huang and Zuo, 2003; Zuo, 2001), C6 cells grown in 25-cm2 flasks were washed twice with wash buffer containing 10 mM HEPES, 140 mM NaCl, 5 mM Tris-base, 2.5 mM KCl, 2.5 mM CaCl2, 1.2 mM MgCl2, 1.2 mM K2HPO4, 10 mM dextrose, pH 7.2. The C6 cells or hippocampal slices were then incubated with 10 μM L-[3H]-glutamate in the wash buffer (C6 cells) or aCSF (hippocampal slices) in the presence or absence of 2% isoflurane for 5 min at 37°C. The incubation solution for hippocampal slices also contained 300 μM dihydrokainate, a relatively selective EAAT2 inhibitor (Huang and Zuo, 2003; Shimamoto et al., 1998). Since hippocampus also expressed other EAATs in addition to EAAT3 (Rothstein et al., 1994), the use of dihydrokainate was to reduce the activity of EAATs other than EAAT3 in the samples. The incubation with [3H]-glutamate was terminated by removing the incubation buffer and washing the cells three times with ice-cold wash buffer or aCSF. The cells or hippocampal slices were lysed with 0.2 M NaOH and radioactivity was measured in a liquid scintillation counter. An aliquot of hippocampal slice lysis solution was used to measure protein concentrations.
2.10. Phosphorylation of EAAT3 peptides by PKC
The S465 sequence-specific peptide and the scrambled peptide were phosphorylated in vitro with purified PKCα as described previously for phosphorylation of a neuromodulin-based peptide (Vinton et al., 1998) or the peptide hormone ghrelin (Dehlin et al., 2008). Reactions were carried out at 30°C in 100 μl solution containing 20 mM 4-morpholinepropanesulfonic acid (MOPS) (pH 7.4), 5 mM MgCl2, 133 μM CaCl2, 40 μM ATP spiked with 1-2 μCi [γ-32P]ATP, 25 nM PKC isozyme and 22 μM peptide substrate with or without 200 μM bovine brain phosphatidylserine with 5 mol % diolein. Reactions with histone (0.15 mg/ml) instead of peptide substrates were included as controls. After 10 min of incubation, 60 μl aliquots were removed and spotted onto P-81 ion exchange paper (Whatman, Piscataway, NJ). Papers were washed three times in 50 mM NaCl to remove unincorporated ATP, dried and counted in a scintillation counter. PKCα was expressed in Sf9 insect cells infected with a recombinant baculovirus construct and purified through ion exchange and hydrophobic columns as previously described (Sando and Chertihin, 1996; Sando et al., 1998).
2.11. Cell viability
To determine the toxicity of our peptides, we incubated C6 cells with the S465 sequence-specific peptide or scrambled peptide at 10 μM or 0.5 mM for 6 h or 24 h at 37°C. Cell viability was then determined with a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay according to the manufacturer's procedure and as we described before (Xu et al., 2008). Briefly, 10 μl of the MTT labeling reagent was added to each well (96-well plates) that contained 100 μl of culture medium and cells. Four hours later, 100 μl of the solubilization solution was added to each well. The plate was kept overnight at 37°C. Absorbance of the samples was measured at 570 nm with the reference wavelength of 650 nm using a microplate reader (Bio-Rad Laboratories, Hercules, CA). In each experiment, the results of the MTT measurements from the controls without any treatments were set as 100%. The results from the sister cultures, subjected to various treatments, were then calculated as a percentage of the controls.
2.12. Statistical analysis
In the experiments analyzing EAAT3 expression in the plasma membrane, the intensity of EAAT3 protein bands in the isoflurane and peptide treatment groups was normalized to that of EAAT3 bands from cells under control condition. In the immunoprecipitation experiments, the intensity of PKCα protein bands was normalized to that of EAAT3 protein bands in the same sample and this intensity ratio was further normalized by the intensity ratio derived from the corresponding control sample. Results are means ± S.D. of the fold changes over the controls, with controls being set as 1. Data of cell viability are means ± S.D. of the percentage changes over the controls, with controls being set as 100%. Results of glutamate uptake assay are means ± S.D. of the measured numbers in each sample. Statistical analysis was performed by one-way analysis of variance followed by the Tukey test for post hoc comparison as appropriate. A P < 0.05 was considered significant.
3. Results
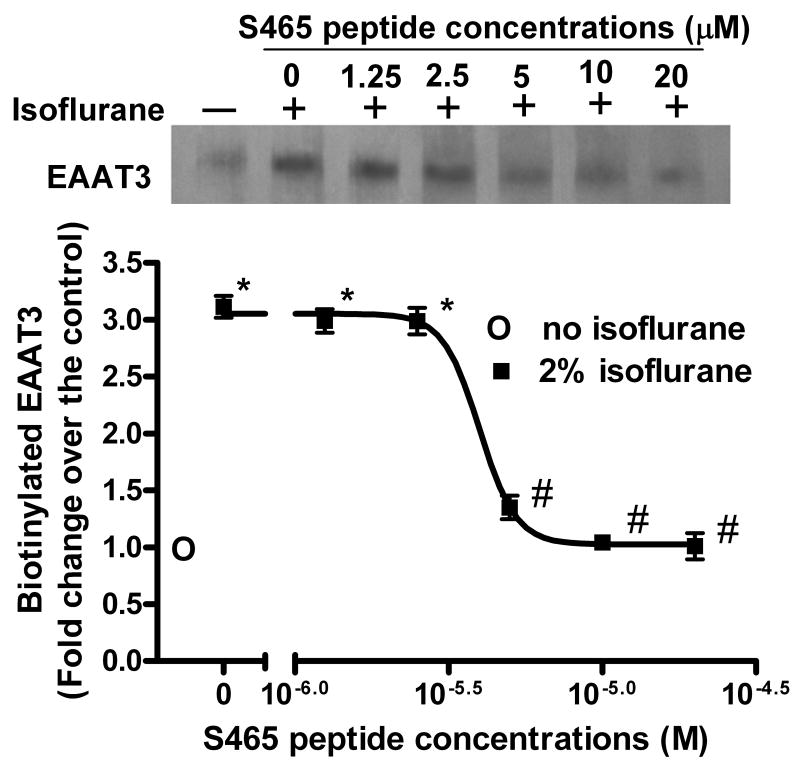
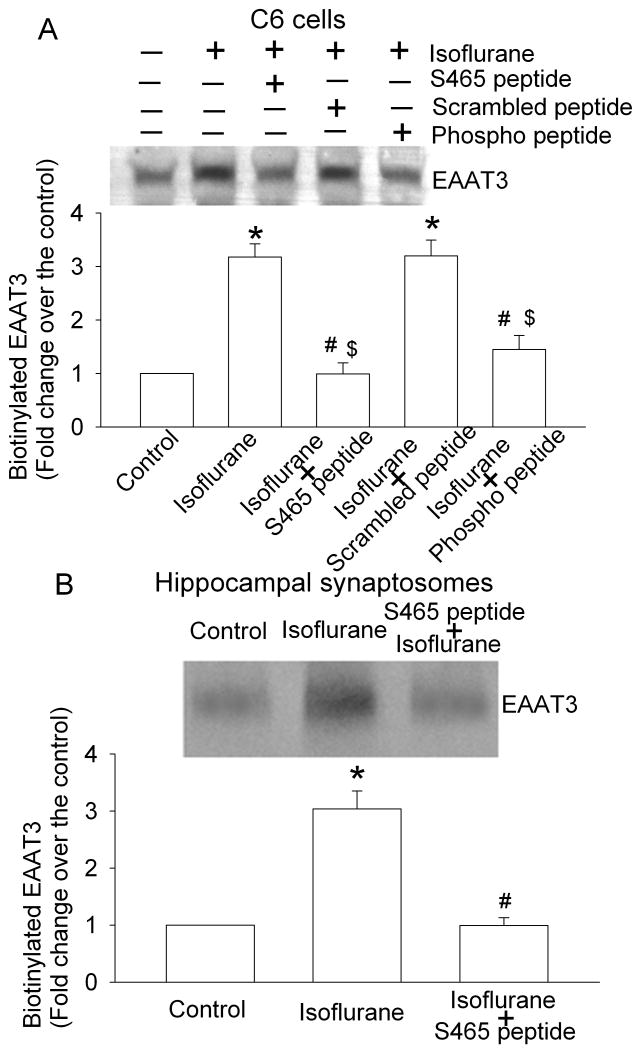
As we have shown previously (Huang et al., 2006; Huang and Zuo, 2005), a 5-min exposure of C6 cells to 2% isoflurane significantly increased the amount of EAAT3 in the plasma membrane (Figs. 1 and 2). This increase was not due to increased total EAAT3 expression because of the short experimental duration (5 min) and our previous data showing that an identical isoflurane exposure condition does not affect the total EAAT3 expression (Huang and Zuo, 2005). This increase also was not due to cell lysis during the isoflurane exposure and biotinylation procedure because there was a very low amount of actin, an intracellular protein, in the biotinylated fraction of C6 cells and hippocampal synaptosomes (Huang and Zuo, 2005). This isoflurane-induced increase of EAAT3 expression in the plasma membrane of C6 cells was dose-dependently blocked by the S465 sequence-specific peptide with an EC50 4.0 μM (Fig. 1). This increased EAAT3 redistribution also was blocked by the corresponding phospho-peptide, but was not affected by the scrambled peptide (Fig. 2A). Similarly, the S465 sequence-specific peptide inhibited the isoflurane-induced redistribution of EAAT3 to the plasma membrane in rat hippocampus (Fig. 2B). These results suggest that the S465 sequence-specific peptide is an effective inhibitor for isoflurane-induced increase of EAAT3 redistribution to the plasma membrane in C6 cells and hippocampus.
Fig. 1. Dose-dependent inhibition of isoflurane-induced redistribution of EAAT3 to the plasma membrane by a synthetic peptide.
C6 cells were incubated with or without 2% isoflurane in the presence or absence of the S465 sequence-specific peptides for 5 min at 37°C. Cell surface proteins were separated from intracellular proteins by the biotinylation method. A representative Western blot is shown in the top panel and the graphic presentation of the EAAT3 protein abundance quantified by integrating the volume of autoradiograms from four separate experiments is shown in the bottom panel. * P < 0.05 compared to control (no isoflurane exposure), # P < 0.05 compared with isoflurane only.
Fig. 2. Inhibition of isoflurane-induced redistribution of EAAT3 to the plasma membrane by synthetic peptides.
C6 cells (Panel A) or hippocampal synaptosomes (Panel B) were incubated with or without 2% isoflurane in the presence or absence of 10 μM peptides for 5 min at 37°C. Cell surface proteins were separated from intracellular proteins by the biotinylation method. A representative Western blot is shown in the top panel and the graphic presentation of the EAAT3 protein abundance quantified by integrating the volume of autoradiograms from four separate experiments is shown in the bottom panel. * P < 0.05 compared to control, # P < 0.05 compared with isoflurane only, $ P < 0.05 compared with isoflurane + scrambled peptide.
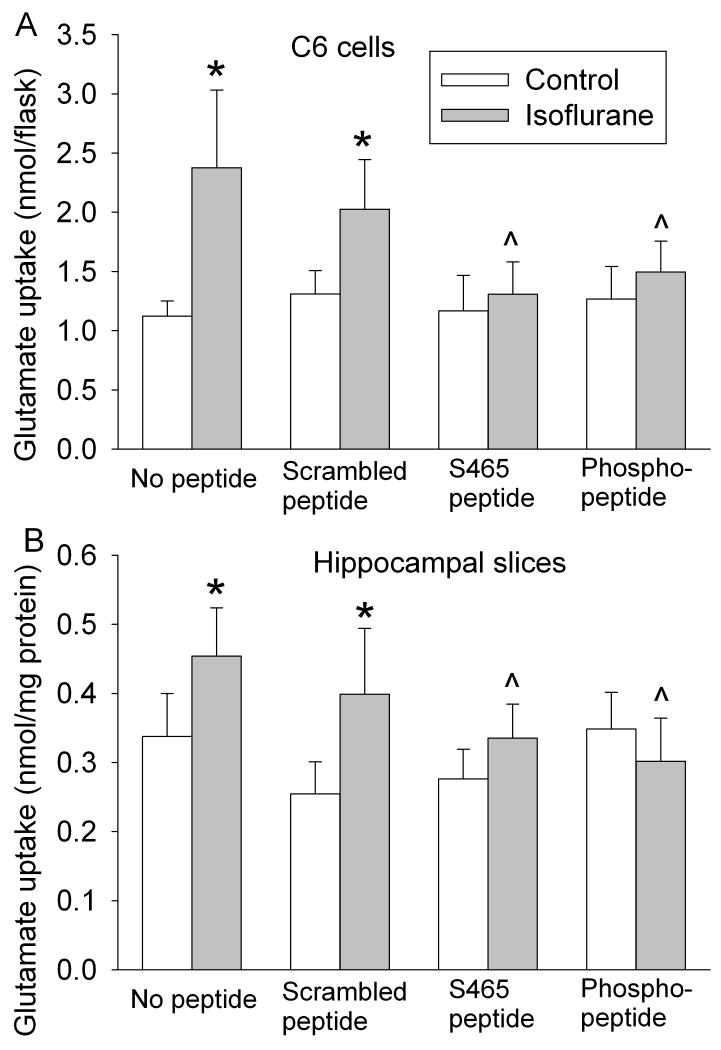
Consistent with these EAAT3 plasma membrane expression data, the isoflurane-induced increase of EAAT3 activity in C6 cells also was blocked by the S465 sequence-specific peptide and the corresponding phospho-peptide. The isoflurane-stimulated glutamate uptake appeared slightly lower with the scrambled peptide but this decrease was not statistically significant (Fig. 3A). None of the peptides affected the baseline EAAT3 activity (Fig. 3A). These results suggest that the S465 sequence-specific peptide is an effective inhibitor for isoflurane-induced increase of EAAT3 activity in C6 cells. Similarly, isoflurane also induced a relatively small but significant increase of dihydrokainate-insensitive glutamate transport activity in the hippocampal slices. This increase was inhibited by the S465 sequence-specific peptide and the corresponding phospho-peptide and was not altered by the scrambled peptide (Fig. 3B). Since hippocampus expresses abundant EAAT3 (Rothstein et al., 1994), the isoflurane-induced increase of dihydrokainate-insensitive glutamate transport activity may reflect the increase of EAAT3 activity in these brain slices. These results, along with the data showing inhibition of isoflurane-induced redistribution of EAAT3 to the plasma membrane of hippocampal neurons by the S465 sequence-specific peptide (Fig. 2B), suggest that this peptide is effective in inhibiting the isoflurane-increased EAAT3 activity in the neurons of hippocampal slices.
Fig. 3. Inhibition of isoflurane-increased EAAT3 activity by synthetic peptides in C6 cells (Panel A) and hippocampal slices (Panel B).
Glutamate uptake was performed by incubating C6 cells or hippocampal slices with 10 μM L-[3H] glutamate in the presence or absence of 2% isoflurane and 10 μM synthetic peptides for 5 min at 37°C (n = 4 - 7). * P < 0.05 compared with the corresponding control. ˆ P < 0.05 compared with cells incubated with isoflurane in the absence of any peptide.
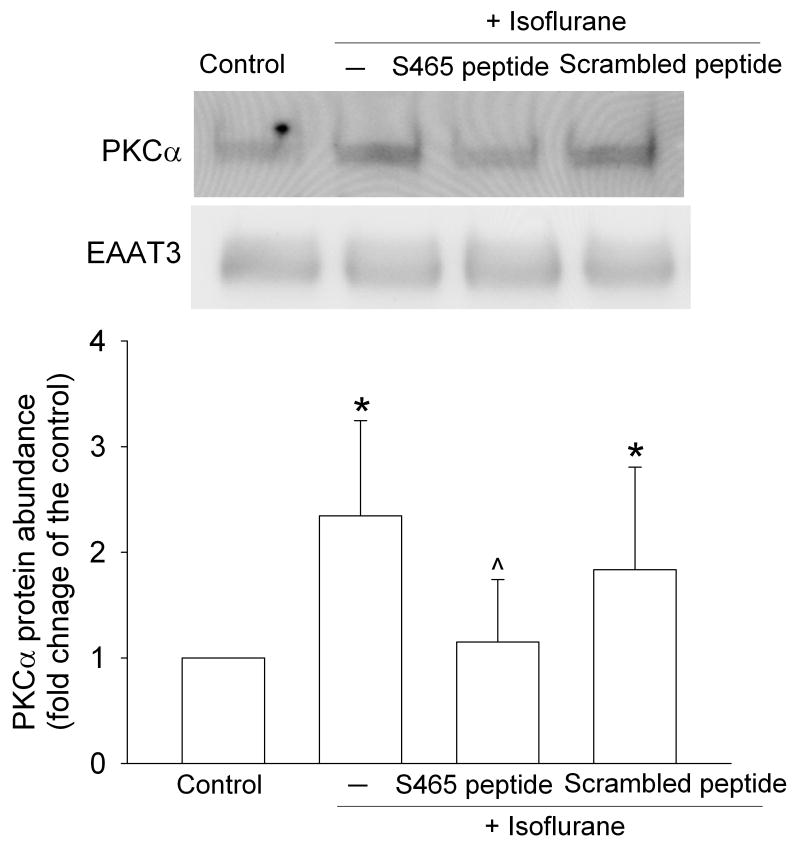
We have shown previously that isoflurane increased the expression of PKCα in immunoprecipitates with an EAAT3 antibody (Huang and Zuo, 2005). In this study, this increased PKCα expression was reduced by the S465 sequence-specific peptide but the small decrease affected by the scrambled peptide was not significant (Fig. 4). These results suggest that the S465 sequence-specific peptide interferes with the interaction between PKCα and EAAT3.
Fig. 4. Inhibition of isoflurane-increased co-immunoprecipitation of PKCα with EAAT3 by synthetic peptides.
C6 cells were incubated with or without 2% isoflurane in the presence or absence of 10 μM peptides for 5 min at 37°C and the total lysates were immunoprecipitated with an anti-EAAT3 antibody. A representative Western blot of the precipitates is shown in the top panel and the graphic presentation of the PKCα protein abundance quantified by integrating the volume of autoradiograms from four separate experiments is shown in the bottom panel. * P < 0.05 compared with the control. ˆ P < 0.05 compared with isoflurane alone.
The ability of the EAAT3 S465 to serve as a direct substrate for the calcium-dependent PKCα was assessed using the S465 sequence-specific peptide. S465 is the only phosphorylatable residue in this peptide. Maximal phosphorylation by PKCα was achieved after 10 min at 30°C to a stoichiometry of 0.45 mol phosphate per mol peptide in the presence of phosphaticylserine plus diacylglycerol vs. 0.085 mol phosphate/mol peptide without the phospholipid PKC activators. PKCα exhibits some slower, lipid-independent activity with peptide substrates as noted previously (Dehlin et al., 2008; Vinton et al., 1998). In contrast, the scrambled peptide incorporated 0.12 mol phosphate per mol peptide in the presence of the activating lipids and 0.004 mol without lipid.
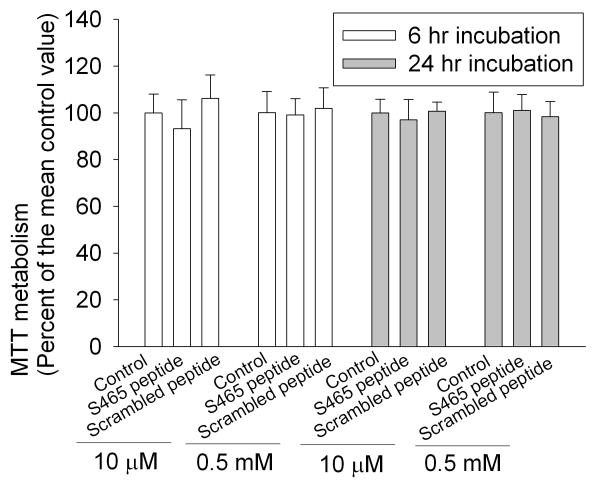
The S465 sequence-specific peptide may not cause significant toxicity to cells because the incubation of C6 cells with these peptides at high concentrations and for a long time did not affect the viability of these cells as measured by the MTT assay (Fig. 5), a commonly used cell survival assay.
Fig. 5. No change in C6 cell viability after application of synthetic peptides.
The cells were incubated with the peptides at 10 μM or 0.5 mM for 6 h or 24 h. Cell viability was assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (n = 10).
4. Discussion
We have shown in our previous studies that the commonly used volatile anesthetic isoflurane increases EAAT3 activity via an increase of EAAT3 expression in the plasma membrane (Huang and Zuo, 2005). These effects may be mediated by PKCα and depend on the phosphorylation of S465, a potential PKC phosphorylation site in the EAAT3 molecule, because specific down-regulation of PKCα, but not PKCβ, and single amino acid mutation (S465A) in the EAAT3 molecule block isoflurane-increased EAAT3 activity, phosphorylation and redistribution to the plasma membrane (Huang et al., 2006; Huang and Zuo, 2005). These results suggest that PKCα may directly interact with EAAT3. In this study, we designed a peptide based on the sequence of EAAT3 around the S465. We conjugated this peptide to the TAT PTD, a highly positively charged peptide that has been used to carry peptides and proteins into cells under in vitro and in vivo conditions (Fawell et al., 1994; Schwarze et al., 1999). This translocation is fast (within a few min) (Chellaiah et al., 2000) and does not appear to cause any disruption of the plasma membrane because it does not promote the uptake of non-conjugated peptides present in the incubation media (Wadia and Dowdy, 2002). We show here that the S465 sequence-specific peptide inhibits the isoflurane-increased EAAT3 activity and redistribution to the plasma membrane (Figs 1, 2 and 3). These results suggest that the S465 sequence-specific peptide competes with EAAT3 for PKCα. Consistent with this suggestion, our results showed that the S465 sequence-specific peptide reduced the amount of PKCα in the immunoprecipitates produced by an anti-EAAT3 antibody (Fig. 4). In addition, the S465 sequence-specific peptide-induced inhibition of isoflurane effects on EAAT3 may be specific because this peptide did not inhibit the basal EAAT3 activity (Fig. 3). In supporting this finding, our previous study showed that PKCα down-regulation or inhibition did not affect the basal EAAT3 activity and expression in the plasma membrane, implying that the basal EAAT3 activity does not require PKCα (Huang and Zuo, 2005).
Although it is not yet possible to demonstrate that regulation of intact EAAT3 by PKCα is mediated by direct phosphorylation at S465 in cells, several observations strongly support that probability: a) isoflurane-increased EAAT3 activity and redistribution to the plasma membrane are blocked by specific PKCα down-regulation (Huang and Zuo, 2005); b) the amount of PKCα, but not many other PKC isozymes including PKCβ, PKCδ and PKCε, in the immunoprecipitates produced by an anti-EAAT3 antibody is increased after C6 cells are exposed to isoflurane (Huang and Zuo, 2005); c) the S465A mutation abolishes isoflurane-increased EAAT3 activity, phosphorylation and redistribution to the plasma membrane (Huang et al., 2006) and d) we have shown here that PKCα can directly phosphorylate a peptide substrate encompassing this site in a lipid-dependent manner and that the S465 sequence-specific peptide inhibits the co-immunoprecipitation of EAAT3 and PKCα.
To determine whether our results obtained with C6 cells are relevant to brain, we tested the effects of the S465 sequence-specific peptide on hippocampal slices and synaptosomes of rats. Our results show that the S465 sequence-specific peptide inhibits the isoflurane-increased and dihydrokainate-insensitive glutamate transporter activity and did not affect the basal dihydrokainate-insensitive glutamate transport activity, a pattern that is similar to that from C6 cells, a cell line that expresses EAAT3 only (Davis et al., 1998; Huang and Zuo, 2005). In addition, the S465 sequence-specific peptide also blocked the isoflurane-increased EAAT3 redistribution to the hippocampal synaptosomes. These results suggest that the S465 sequence-specific peptide also can inhibit isoflurane-increased EAAT3 activity and plasma membrane redistribution in the hippocampus.
We observed a large increase of EAAT3 redistribution to the plasma membrane after isoflurane exposure. However, the increase of EAAT3 activity by isoflurane in the hippocampal slices was relatively small. This seemingly inconsistent result may be due to factors such as: a) multiple EAATs contribute to the basal EAAT activity shown in figure 3B and the basal EAAT3 activity is not known; and b) it is difficult to exclude that some of the glutamate molecules transported by EAATs have been released into extracellular space by neurons during the activity assay even though the assay time was short (5 min). Although the isoflurane-induced increase of EAAT3 activity was small in the hippocampus, the effects of isoflurane on EAAT3 redistribution and activity may still have significant biological effects because it has been shown that the binding of glutamate to cell surface EAAT3 before glutamate is transported into cells contributes significantly to the regulation of glutamate neurotransmission (Grewer et al., 2000; Scimemi et al., 2009).
A 14-amino acid peptide that contains the TAT PTD has been shown to inhibit protein kinases including PKC through a substrate competition mechanism (Ekokoski et al.). This inhibition may also occur in our study because the isoflurane-stimulated EAAT3 activity in the presence of scrambled peptide trended to be less than that in the presence of isoflurane only (Fig. 3). However, the possible PKC inhibition by TAT PTD may not be very significant in the C6 cells and hippocampus because there was no statistically significant difference between the EAAT3 activities in the presence of isoflurane alone and with the scrambled peptides.
The phospho-peptide was as effective as non-phospho-S465 sequence-specific peptide in inhibiting isoflurane-increased EAAT3 activity and redistribution. This phospho-peptide can be dephosphorylated by intracellular phosphatases and, therefore, may work through the same mechanisms as the non-phospho-S465 sequence-specific peptide. Alternatively, our result may suggest that the phospho-peptide competes with the phosphorylated EAAT3 for association with proteins that are necessary for EAAT3 redistribution to the plasma membrane. The constitutive recycling of EAAT3 to the plasma membrane has been shown to be Rab11-dependent (Gonzalez et al., 2007). The mechanisms and proteins that are involved in PKCα-mediated EAAT3 redistribution to the plasma membrane are not known. Further studies are needed to reveal the mechanisms by which the phospho-peptide inhibits the isoflurane-increased EAAT3 activity and trafficking to the plasma membrane and in identifying the proteins involved in the EAAT3 redistribution to the plasma membrane.
A few general EAAT inhibitors are available in the market. However, very few inhibitors are EAAT type specific and there are no EAAT3 specific inhibitors yet. Type specific inhibitors are very much needed for identifying EAAT type specific functions. Our study suggests that the S465 sequence-specific peptide may be a specific inhibitor for the PKC-mediated increase in EAAT3 activity and may be a good research tool because it appears that this peptide does not cause significant toxicity to cells and does not affect the basal EAAT3 activity. Our study also may provide an example of a novel approach for designing inhibitors for proteins whose functions can be regulated by PKC.
Our collective results suggest that isoflurane can activate PKCα to phosphorylate EAAT3, which then leads to EAAT3 redistribution to the plasma membrane. This effect also may occur in the hippocampus, a brain region that is involved in learning and memory functions. EAAT3 trafficking to the plasma membrane has been proposed to play a role in learning and memory function and synaptic plasticity (Levenson et al., 2002). Isoflurane can enhance synaptogenesis (Briner et al., 2010). Thus, our findings on the isoflurane effects on EAAT3 activity and trafficking may be relevant to understanding the mechanisms of isoflurane-induced changes in synaptic plasticity. Also, many studies have shown that isoflurane has neuroprotective effects (Li and Zuo, 2009; Sakai et al., 2007). It has been shown that EAAT3 can transport not only glutamate but also cysteine very effectively (Lee et al., 2009; Zerangue and Kavanaugh, 1996). Cysteine is the rate-limiting substrate for the synthesis of glutathione, the major intracellular anti-oxidant (Dringen et al., 1999). EAAT3 knockout mice have decreased glutathione and increased oxidative stress markers in their brains (Aoyama et al., 2006). Our recent data suggest that these EAAT3 knockout mice have decreased tolerance for brain ischemia (Li and Zuo, 2010). We also have shown that volatile anesthetics including isoflurane can maintain EAAT3 function to transport cysteine even under oxidative stress (Lee et al., 2009). Thus, the isoflurane-induced increase in EAAT3 trafficking to the plasma membrane may be an underlying mechanism for isoflurane neuroprotective effects. Reducing energy utilization has been considered as a major mechanism for volatile anesthetics to provide neuroprotection (Nakashima et al., 1995). The enhancement of an energy-required glutamate uptake via EAATs seems to be on the contrary to this mechanism. However, volatile anesthetics have been known to influence activities of many proteins. This influence can lead to increased activity of the proteins, such as ATP-activated potassium channels, or decreased activity of the proteins, such as glutamate receptors (Campagna et al., 2003; Zuo et al., 1999). Volatile anesthetics reduce the overall energy consumption of tissues and organs but do not need to reduce every process that is energy-required. The effects of isoflurane on EAAT3 may limit excitatory glutamate neurotransmission, which may result in reduction of overall energy consumption in the tissues.
Acknowledgments
This study was supported by grants (R01 GM065211 to Z Zuo and R01 GM31184 to J Sando) from the National Institute of Health, Bethesda, Maryland, by a grant from the International Anesthesia Research Society (2007 Frontiers in Anesthesia Research Award to Z Zuo), Cleveland, Ohio, by a Grant-in-Aid from the American Heart Association Mid-Atlantic Affiliate (0755450U to Z Zuo) and the Department of Anesthesiology, University of Virginia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aoyama K, Suh SW, Hamby AM, Liu J, Chan WY, Chen Y, Swanson RA. Neuronal glutathione deficiency and age-dependent neurodegeneration in the EAAC1 deficient mouse. Nat Neurosci. 2006;9:119–126. doi: 10.1038/nn1609. [DOI] [PubMed] [Google Scholar]
- Arriza JL, Fairman WA, Wadiche JI, Murdoch GH, Kavanaugh MP, Amara SG. Functional comparisons of three glutamate transporter subtypes cloned from human motor cortex. J Neurosci. 1994;14:5559–5569. doi: 10.1523/JNEUROSCI.14-09-05559.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baik HJ, Huang Y, Washington JM, Zuo Z. Critical role of s465 in protein kinase C-increased rat glutamate transporter type 3 activity. Int J Neurosci. 2009;119:1419–1428. doi: 10.1080/00207450903014783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briner A, De Roo M, Dayer A, Muller D, Habre W, Vutskits L. Volatile Anesthetics Rapidly Increase Dendritic Spine Density in the Rat Medial Prefrontal Cortex during Synaptogenesis. Anesthesiology. 2010;112:546–556. doi: 10.1097/ALN.0b013e3181cd7942. [DOI] [PubMed] [Google Scholar]
- Campagna J, Miller K, Forman S. Mechanisms of action of inhaled anesthetics. N Engl J Med. 2003;348:2110–2124. doi: 10.1056/NEJMra021261. [DOI] [PubMed] [Google Scholar]
- Chellaiah MA, Soga N, Swanson S, McAllister S, Alvarez U, Wang D, Dowdy SF, Hruska KA. Rho-A is critical for osteoclast podosome organization, motility, and bone resorption. J Biol Chem. 2000;275:11993–12002. doi: 10.1074/jbc.275.16.11993. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Danbolt NC, Chaudhry FA, Dehnes Y, Lehre KP, Levy LM, Ullensvang K, Storm-Mathisen J. Properties and localization of glutamate transporters. Prog Brain Res. 1998;116:23–43. doi: 10.1016/s0079-6123(08)60428-8. [DOI] [PubMed] [Google Scholar]
- Davis KE, Straff DJ, Weinstein EA, Bannerman PG, Correale DM, Rothstein JD, Robinson MB. Multiple signaling pathways regulate cell surface expression and activity of the excitatory amino acid carrier 1 subtype of Glu transporter in C6 glioma. J Neurosci. 1998;18:2475–2485. doi: 10.1523/JNEUROSCI.18-07-02475.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehlin E, Liu J, Yun SH, Fox E, Snyder S, Gineste C, Willingham L, Geysen M, Gaylinn BD, Sando JJ. Regulation of ghrelin structure and membrane binding by phosphorylation. Peptides. 2008;29:904–911. doi: 10.1016/j.peptides.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do SH, Kamatchi GL, Washington JM, Zuo Z. Effects of volatile anesthetics on glutamate transporter, excitatory amino acid transporter type 3. Anesthesiology. 2002;96:1492–1497. doi: 10.1097/00000542-200206000-00032. [DOI] [PubMed] [Google Scholar]
- Dringen R, Pfeiffer B, Hamprecht B. Synthesis of the antioxidant glutathione in neurons: supply by astrocytes of CysGly as precursor for neuronal glutathione. J Neurosci. 1999;19:562–569. doi: 10.1523/JNEUROSCI.19-02-00562.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekokoski E, Aitio O, Tornquist K, Yli-Kauhaluoma J, Tuominen RK. HIV-1 Tat-peptide inhibits protein kinase C and protein kinase A through substrate competition. Eur J Pharm Sci. 2010;40:404–411. doi: 10.1016/j.ejps.2010.04.013. [DOI] [PubMed] [Google Scholar]
- Fawell S, Seery J, Daikh Y, Moore C, Chen LL, Pepinsky B, Barsoum J. Tat-mediated delivery of heterologous proteins into cells. Proc Natl Acad Sci U S A. 1994;91:664–668. doi: 10.1073/pnas.91.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez MI, Susarla BT, Fournier KM, Sheldon AL, Robinson MB. Constitutive endocytosis and recycling of the neuronal glutamate transporter, excitatory amino acid carrier 1. J Neurochem. 2007;103:1917–1931. doi: 10.1111/j.1471-4159.2007.04881.x. [DOI] [PubMed] [Google Scholar]
- Grewer C, Watzke N, Wiessner M, Rauen T. Glutamate translocation of the neuronal glutamate transporter EAAC1 occurs within milliseconds. Proc Natl Acad Sci U S A. 2000;97:9706–9711. doi: 10.1073/pnas.160170397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Feng X, Sando JJ, Zuo Z. Critical Role of Serine 465 in Isoflurane-induced Increase of Cell-surface Redistribution and Activity of Glutamate Transporter Type 3. J Biol Chem. 2006;281:38133–38138. doi: 10.1074/jbc.M603885200. [DOI] [PubMed] [Google Scholar]
- Huang Y, Zuo Z. Isoflurane enhances the expression and activity of glutamate transporter type 3 in C6 glioma cells. Anesthesiology. 2003;99:1346–1353. doi: 10.1097/00000542-200312000-00016. [DOI] [PubMed] [Google Scholar]
- Huang Y, Zuo Z. Isoflurane induces a protein kinase C alpha-dependent increase in cell surface protein level and activity of glutamate transporter type 3. Mol Pharmacol. 2005;67:1522–1533. doi: 10.1124/mol.104.007443. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Hediger MA. Primary structure and functional characterization of a high-affinity glutamate transporter. Nature. 1992;360:467–471. doi: 10.1038/360467a0. see comments. [DOI] [PubMed] [Google Scholar]
- Lee SA, Choi JG, Zuo Z. Volatile anesthetics attenuate oxidative stress-reduced activity of glutamate transporter type 3. Anesth Analg. 2009;109:1506–1510. doi: 10.1213/ANE.0b013e3181b6709a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson J, Weeber E, Selcher JC, Kategaya LS, Sweatt JD, Eskin A. Long-term potentiation and contextual fear conditioning increase neuronal glutamate uptake. Nat Neurosci. 2002;5:155–161. doi: 10.1038/nn791. [DOI] [PubMed] [Google Scholar]
- Li L, Zuo Z. Isoflurane preconditioning improves short-term and long-term neurological outcome after focal brain ischemia in adult rats. Neuroscience. 2009;164:497–506. doi: 10.1016/j.neuroscience.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Zuo Z. Glutamate transporter type 3 knockout reduces brain tolerance to focal brain ischemia in mice. J Cereb Blood Flow Metab. 2010 doi: 10.1038/jcbfm.2010.222. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukainaka Y, Tanaka K, Hagiwara T, Wada K. Molecular cloning of two glutamate transporter subtypes from mouse brain. Biochim Biophys Acta. 1995;1244:233–237. doi: 10.1016/0304-4165(95)00062-g. [DOI] [PubMed] [Google Scholar]
- Nakashima K, Todd MM, Warner DS. The relation between cerebral metabolic rate and ischemic depolarization. A comparison of the effects of hypothermia, pentobarbital, and isoflurane. Anesthesiology. 1995;82:1199–1208. doi: 10.1097/00000542-199505000-00015. [DOI] [PubMed] [Google Scholar]
- Popovic R, Liniger R, Bickler PE. Anesthetics and mild hypothermia similarly prevent hippocampal neuron death in an in vitro model of cerebral ischemia. Anesthesiology. 2000;92:1343–1349. doi: 10.1097/00000542-200005000-00024. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Martin L, Levey AI, Dykes-Hoberg M, Jin L, Wu D, Nash N, Kuncl RW. Localization of neuronal and glial glutamate transporters. Neuron. 1994;13:713–725. doi: 10.1016/0896-6273(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Sakai H, Sheng H, Yates RB, Ishida K, Pearlstein RD, Warner DS. Isoflurane Provides Long-term Protection against Focal Cerebral Ischemia in the Rat. Anesthesiology. 2007;106:92–99. doi: 10.1097/00000542-200701000-00017. [DOI] [PubMed] [Google Scholar]
- Sando JJ, Chertihin OI. Activation of protein kinase C by lysophosphatidic acid: dependence on composition of phospholipid vesicles. Biochem J. 1996;317(Pt 2):583–588. doi: 10.1042/bj3170583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sando JJ, Chertihin OI, Owens JM, Kretsinger RH. Contributions to maxima in protein kinase C activation. J Biol Chem. 1998;273:34022–34027. doi: 10.1074/jbc.273.51.34022. [DOI] [PubMed] [Google Scholar]
- Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science. 1999;285:1569–1572. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- Scimemi A, Tian H, Diamond JS. Neuronal transporters regulate glutamate clearance, NMDA receptor activation, and synaptic plasticity in the hippocampus. J Neurosci. 2009;29:14581–14595. doi: 10.1523/JNEUROSCI.4845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamoto K, Lebrun B, Yasuda-Kamatani Y, Sakaitani M, Shigeri Y, Yumoto N, Nakajima T. DL-threo-beta-benzyloxyaspartate, a potent blocker of excitatory amino acid transporters. Mol Pharmacol. 1998;53:195–201. doi: 10.1124/mol.53.2.195. [DOI] [PubMed] [Google Scholar]
- Vinton BB, Wertz SL, Jacob J, Steere J, Grisham CM, Cafiso DS, Sando JJ. Influence of lipid on the structure and phosphorylation of protein kinase C alpha substrate peptides. Biochem J. 1998;330:1433–1442. doi: 10.1042/bj3301433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadia JS, Dowdy SF. Protein transduction technology. Curr Opin Biotechnol. 2002;13:52–56. doi: 10.1016/s0958-1669(02)00284-7. [DOI] [PubMed] [Google Scholar]
- Xu X, Feng J, Zuo Z. Isoflurane preconditioning reduces the rat NR8383 macrophage injury induced by lipopolysaccharide and interferon gamma. Anesthesiology. 2008;108:643–650. doi: 10.1097/ALN.0b013e318167aeb4. [DOI] [PubMed] [Google Scholar]
- Zerangue N, Kavanaugh MP. Interaction of L-cysteine with a human excitatory amino acid transporter. J Physiol. 1996;493(Pt 2):419–423. doi: 10.1113/jphysiol.1996.sp021393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao P, Huang Y, Zuo Z. Opioid preconditioning induces opioid receptor-dependent delayed neuroprotection against ischemia in rats. J Neuropathol Exp Neurol. 2006;65:945–952. doi: 10.1097/01.jnen.0000235123.05677.4b. [DOI] [PubMed] [Google Scholar]
- Zuo Z. Isoflurane enhances glutamate uptake via glutamate transporters in rat glial cells. Neuroreport. 2001;12:1077–1080. doi: 10.1097/00001756-200104170-00042. [DOI] [PubMed] [Google Scholar]
- Zuo Z, Johns RA. Inhalational anesthetics up-regulate constitutive and lipopolysaccharide-induced inducible nitric oxide synthase expression and activity. Mol Pharmacol. 1997;52:606–612. doi: 10.1124/mol.52.4.606. [DOI] [PubMed] [Google Scholar]
- Zuo Z, Tichotsky A, Johns RA. Inhibition of excitatory neurotransmitter-nitric oxide signaling pathway by inhalational anesthetics. Neuroscience. 1999;93:1167–1172. doi: 10.1016/s0306-4522(99)00194-3. [DOI] [PubMed] [Google Scholar]