Summary
The hair follicle bulge in the epidermis associates with the arrector pili muscle (APM) that is responsible for piloerection (commonly called "goosebumps"). We show that deposition of nephronectin into the basement membrane by bulge stem cells mediates selective adhesion of α8β1 integrin-expressing mesenchymal cells, including APM progenitors. Nephronectin induces α8 integrin-positive cells to upregulate smooth muscle markers. In nephronectin-null skin, the number of APM is reduced and those that form insert above the bulge, where there is compensatory upregulation of the nephronectin family member EGFL6. Deletion of α8 integrin also abolishes the selectivity of APM anchorage to the bulge. Nephronectin is a Wnt target gene; epidermal β-catenin activation upregulates epidermal nephronectin and dermal α8 integrin expression. We conclude that by expressing nephronectin bulge stem cells provide a smooth muscle cell niche and act as tendon cells for the APM. The results also reveal a functional role for basement membrane heterogeneity in tissue patterning.
Introduction
The epidermis is maintained through self-renewal of stem cells and differentiation of their progeny to form the lineages of the interfollicular epidermis and adnexal structures, including the hair follicles and sebaceous glands (Watt et al., 2006). There are several distinct populations of stem cells in adult epidermis. Their properties are regulated by intrinsic transcriptional programs in response to signals from the external microenvironment, or niche (Watt and Hogan, 2000; Watt et al., 2006).
One location of epidermal stem cells is the permanent portion of the hair follicle, known as the bulge. During the resting phase of the hair growth cycle (telogen), bulge cells are in close contact with a cluster of mesenchymal cells known as the dermal papilla, and reciprocal interactions between epidermal stem cells and dermal papilla cells are essential for hair follicle formation and maintenance (Millar, 2002; Yang and Cotsarelis, 2010). A second close association between the bulge and the adjacent mesenchyme involves a smooth muscle, called the arrector pili muscle (APM), which is responsible for raising the hair follicles (piloerection) to trap body heat and express emotions. Unlike the association between the bulge and the dermal papilla, which is lost during the growth (anagen) phase of the hair growth cycle, the bulge maintains contact with the APM throughout the hair cycle (Muller-Rover et al., 2001). Although it is well known that the bulge is the permanent attachment site of the APM, how attachment of the muscle is established and maintained in the bulge is entirely unknown.
We hypothesised that bulge-arrector pili muscle interactions might involve epidermal basement membrane components. Laminin-511 has been shown to mediate epidermal-dermal papilla signalling during hair development (Gao et al., 2008), and in a range of other tissues, such as kidney and pancreas, the basement membrane is involved in bidirectional cellular interactions (Linton et al., 2007; Nikolova et al., 2006). Furthermore, the basement membrane of the skin exhibits local variation in structure and composition (Timpl, 1996). Such basement membrane heterogeneity would not only provide a potential mechanism for anchoring different stem cell populations in different regions of the epidermis, but could also result in local differences in signalling with adjacent mesenchymal cells (Akiyama et al., 1995; Fuchs, 2008; Spradling et al., 2001; Watt and Hogan, 2000).
Gene expression profiling of the bulge compartment has revealed that bulge stem cells express a number of extracellular matrix (ECM) proteins that are distinct from those of other epidermal cells (Morris et al., 2004; Ohyama et al., 2006; Tumbar et al., 2004). One of these is nephronectin, an ECM protein with five EGF-like repeats, an RGD sequence, and a COOH-terminal MAM domain (Brandenberger et al., 2001). Nephronectin is an interesting candidate mediator of epidermal interactions with mesenchymal cells, since the nephronectin receptor is α8β1 (Brandenberger et al., 2001; Sato et al., 2009), an integrin that is expressed in the dermal papilla but not by adult epidermal keratinocytes (Driskell et al., 2009; Watt, 2002). Furthermore, the epithelial-mesenchymal interactions required for kidney organogenesis are disrupted in mice lacking α8β1 or nephronectin (Brandenberger et al., 2001; Linton et al., 2007; Muller et al., 1997).
In this study, we show that bulge stem cells create a specialized basement membrane containing nephronectin, which induces arrector pili muscle differentiation and anchorage to the bulge. Loss of nephronectin or α8 integrin expression causes delocalization of the APM. Thus the bulge ECM not only contributes to the specialized niche of hair follicle stem cells but also provides a niche for smooth muscle progenitors.
Results
Bulge stem cells deposit nephronectin
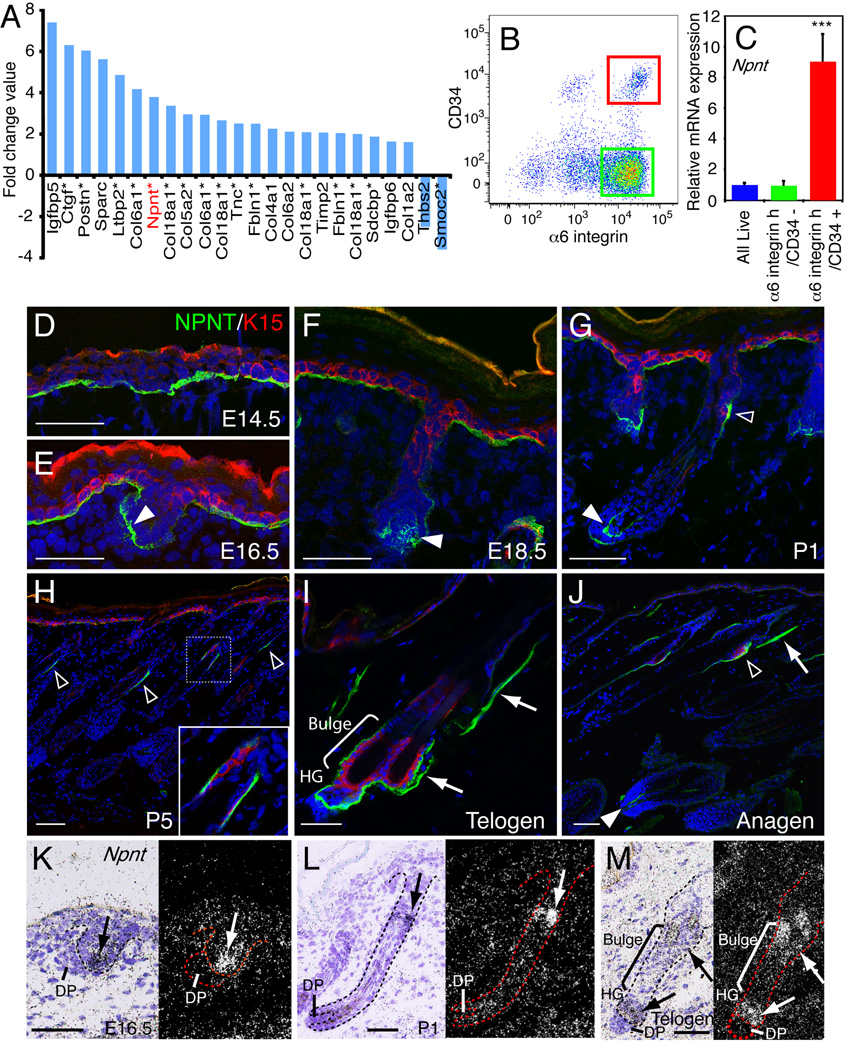
By examining published microarray data, we identified a range of ECM genes that were upregulated in bulge stem cells, including nephronectin (Npnt) (Figure 1A, Table S1, S2, S3). To confirm that nephronectin was upregulated in bulge cells, Q-PCR was performed on mRNA from disaggregated dorsal skin keratinocytes that had been FACS-sorted on the basis of the expression of bulge stem cell markers, CD34 and α6 integrin (Figure 1B). CD34+/α6 integrinhigh cells were enriched for expression of the additional bulge maker Sox9 and expressed low levels of Sca1, a marker of interfollicular epidermal cells (Figure S1A; Jensen et al., 2009; Trempus et al., 2003). These cells had high levels of Npnt in comparison with unfractionated basal cells (All Live; cells with low forward and side scatter) and CD34−/α6 integrinhigh non-bulge stem cells (Figure 1C). Expression levels of other ECM genes identified from the microarrays were also confirmed by Q-PCR (Figure 1A, Figure S1A).
Figure 1. Nephronectin expression in skin.
(A) ECM genes that are upregulated or downregulated in bulge stem cells relative to other basal keratinocytes, ranked based on log2 fold change value (see Table S1). Asterisks indicate the genes that are also upregulated or downregulated in mouse label retaining cells (Tables S2, S3). Some genes are listed more than once due to their multiple spots on the array. (B, C) Adult telogen dorsal keratinocytes were FACS-sorted according to α6 integrin and CD34 expression (B). Bulge stem cells (red gate; α6 integrinhigh/CD34+), non-bulge basal stem cells (green gate; α6 integrinhigh/CD34−) and all live basal cells were sorted and Npnt mRNA levels were determined by Q-PCR (C). Data are mean ± SEM from three mice. (D–J) Sections of E14.5 (D), E16.5 (E), E18.5 (F), P1 (G), P5 (H), adult telogen (I) and anagen (J) skin were immunostained for nephronectin (NPNT; green) and bulge stem cell marker K15 (red), with DAPI counterstain (blue). Note nephronectin deposition in hair germ (white arrowheads), bulge (open arrowheads) and APM (arrows). (K–M) In situ hybridization with Npnt probe on E16.5 (K), P1 (L), and adult telogen skin (M). Arrows indicate the strong nephronectin expression. Scale bars: 50 µm. See also Figure S1.
We next examined the distribution of nephronectin protein in embryonic and adult skin. At E14.5, nephronectin was detected throughout the epidermal-dermal basement membrane, where it was colocalized with the ubiquitous basement membrane component laminin γ1 chain (Figure 1D; Figure S1B). However, at E16.5 and E18.5, when hair follicle morphogenesis had begun, nephronectin accumulated between the hair germ and dermal condensate, but was hardly detectable along the rest of the hair follicle (Figure 1E, F, Figure S1B). Just after birth (P1, P5), nephronectin was detected in the basement membrane of keratin 15 (K15)- and Sox9-positive early bulge stem cells as well as at the base of the follicle (Figure 1G, Figure S1B, C). At this time, nephronectin deposition in the bulge was asymmetrically distributed at the posterior side (Figure 1G, H, Figure S1B).
In adult telogen and anagen skin, nephronectin was localized to the basement membrane of the bulge, hair bulb, and APM (Figure 1I, J, Figure S1B). Other ECM proteins that localised to the bulge rather than along the entire outer root sheath were periostin and fibulin-1 (Figure S1D). Tenascin-C was confined to the bulge in telogen follicles but showed more widespread distribution in anagen (Figure S1D).
In situ hybridization for Npnt mRNA in embryonic and adult skin confirmed that nephronectin is expressed by epidermal cells. Epithelial cells of the bulge and hair germ were strongly positive for Npnt mRNA, whereas the dermis was negative (Figure 1K–M). We conclude that expression of nephronectin by bulge stem cells and hair germ cells results in heterogeneity in epidermal basement membrane composition from early hair morphogenesis to adulthood.
α8 integrin is specifically expressed by cells of the dermal papilla and arrector pili muscle and colocalizes with nephronectin
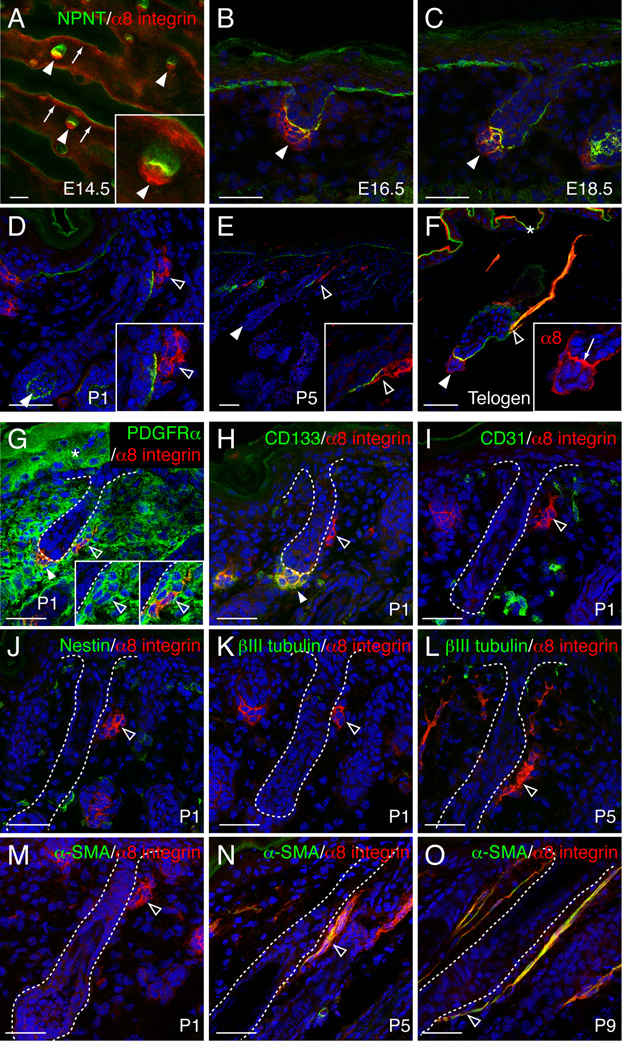
Since α8β1 integrin is the major nephronectin receptor (Brandenberger et al., 2001; Sato et al., 2009), we examined whether α8β1 integrin colocalised with nephronectin in embryonic and adult skin. Whole-mount immunostaining of E14.5 dorsal skin revealed that α8 integrin was strongly expressed in dermal condensates (arrowheads in Figure 2A) and also widely expressed in the superficial dermis, correlating with the widespread distribution of nephronectin in the basement membrane (arrows in Figure 2A). Between E16.5–E18.5, α8 integrin expression in the superficial dermis was downregulated, but it was highly expressed in the dermal condensates and dermal papillae of developing hair follicles and colocalized with nephronectin (arrowheads in Figure 2B, C).
Figure 2. Nephronectin colocalizes with α8 integrin-positive dermal cells in dermal papilla and arrector pili muscle.
(A–F) Immunostaining for nephronectin (green) and α8 integrin (red) in developing and adult dorsal skin. Arrows: α8 expression in superficial dermis (A) or at nephronectin-positive basement membrane between hair germ and dermal papilla (F); white arrowheads: dermal papilla cells; open arrowheads: α8 integrin-positive cells around bulge. (G–O) Expression of α8 integrin (red) and other dermal markers (green) in postnatal dorsal skin. Open arrowheads: α8 integrin-positive dermal cells adjacent to bulge. White arrowhead in G and H: dermal papilla. All sections were counterstained with DAPI (blue). Asterisk indicates non-specific staining. Scale bars: 50 µm. See also Figure S2.
At P1 and P5, α8 integrin-positive dermal cell clusters were detected in association with nephronectin in the early bulge (open arrowheads in Figure 2D, E) and elongated towards the interfollicular epidermis (Figure 2E), while α8 integrin was downregulated in dermal papillae of hair follicles in late anagen (Figure 2D, E). In adult telogen skin, α8 integrin accumulated at the interface between hair germ and dermal papilla, colocalizing with nephronectin (arrow in Figure 2F). The arrector pili muscles co-expressed α8 integrin and nephronectin and inserted into the nephronectin-positive bulge (Figure 2F). In addition to nephronectin α8β1 binds to several ECM proteins, including osteopontin, tenascin-C, fibronectin, and vitronectin (Brandenberger et al., 2001; Denda et al., 1998; Schnapp et al., 1995). However, none of these proteins showed specific colocalization with α8 integrin (Figure S2A–D).
To identify the α8 integrin-positive cells associated with the early bulge cells, we stained newborn skin sections with a panel of antibodies. At P1, the α8 integrin-positive population expressed dermal fibroblast markers PDGFRα and HSP47 (Figure 2G, Figure S2E; Erez et al., 2010; Kuroda and Tajima, 2004). α8 integrin-positive cells in the dermal papilla expressed CD133 (white arrowhead in Figure 2H; (Driskell et al., 2009), but CD133 was not expressed by α8 integrin-positive cells at the bulge (open arrowhead in Figure 2H). α8 integrin-positive cells did not express the endothelial cell marker CD31, the neural crest cell derivative marker nestin or the neuron specific marker βIII tubulin (Figure 2I–L). At P1, bulge-associated α8 integrin-positive cells did not express smooth muscle actin α-SMA (Figure 2M). However, from P5 onwards, they were strongly positive for α-SMA (Figure 2N, O) and dystrophin (Figure S2F). These results suggest that the α8 integrin-positive fibroblasts associated with the bulge in P1 skin are progenitors of the APM.
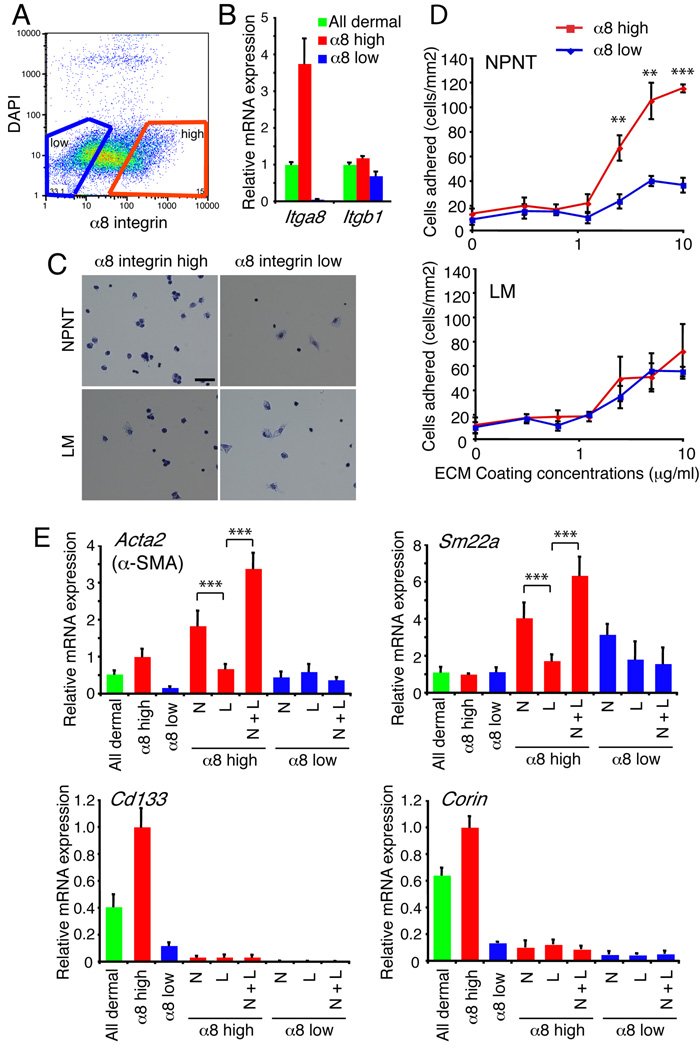
α8 integrin-positive dermal cells adhere strongly to nephronectin and upregulate smooth muscle markers
The specific colocalization of nephronectin and α8 integrin led us to hypothesize that nephronectin mediates adhesion of α8 integrin-positive dermal cells to the bulge basement membrane. To examine this, we performed solid-phase cell adhesion assays with purified nephronectin. Disaggregated P1 dorsal dermal cells were sorted on the basis of surface α8 integrin expression (Figure 3A). Q-PCR analysis confirmed that α8 integrin mRNA (Itga8) was upregulated in the α8high population, whereas cells with high or low α8 integrin levels showed little variation in β1 integrin (Itgb1) levels (Figure 3B). Solid-phase cell adhesion assays revealed that α8 integrinhigh dermal cells adhered strongly to nephronectin-coated substrates in a concentration-dependent manner, whereas α8 integrinlow dermal cells did not (Figure 3C, D). In contrast, both populations adhered equally well to laminin-coated substrates (Figure 3C, D).
Figure 3. Adhesion and gene expression of dermal cells plated on nephronectin.
(A, B) Disaggregated P1 dermal cells were FACS-sorted on the basis of α8 integrin levels (A), confirmed by Q-PCR for α8 integrin (Itga8) and β1 integrin (Itgb1) (B). Data are means ± SEM from three mice. (C, D) Solid-phase cell adhesion assays with FACS-sorted dermal cells plated on nephronectin (NPNT)- or laminin (LM)-coated dishes (10 µg/ml). Cells were stained with Diff Quik (C) and quantitated (D). (C) Scale bar: 50 µm. (D) Data are means ± SEM from triplicate wells. (E) Q-PCR of smooth muscle (Acta2 and Sm22a) and dermal papilla marker (Cd133 and Corin) expression in unfractionated (all dermal), α8 integrin-high or -low populations seeded on ECM protein-coated dishes (10 µg/ml of each protein) for 12 hours. N: nephronectin; L: laminin; N+L: nephronectin and laminin. Data are means ± SEM from three independent wells.
Unfractionated, α8high and α8low cells were plated on nephronectin, laminin or a mixture of both for 12 hours and then collected for Q-PCR analysis (Figure 3E). We examined expression of two smooth muscle cell marker genes, α-smooth muscle actin (α-SMA; Acta2) and smooth muscle protein 22-α (Sm22a), and two dermal papilla cell marker genes, Cd133 and Corin. Prior to plating, α8high cells expressed high levels of Cd133 and Corin, consistent with their presence in dermal papillae (Figure 3E). However, expression of these markers was downregulated, regardless of whether the cells were plated on nephronectin or laminin (Figure 3E). In contrast, the α8 integrinhigh population showed significant upregulation of Acta2 and Sm22a when plated on nephronectin or nephronectin and laminin, but not on laminin alone (Figure 3E).
We conclude that nephronectin mediates adhesion of α8 integrin-positive dermal cells and stimulates expression of APM markers.
Nephronectin is required to anchor arrector pili muscles to the bulge
To investigate the function of nephronectin in vivo, we analyzed Npnt knockout mice (Linton et al., 2007). Analysis of haematoxylin and eosin (H&E)-stained sections of adult dorsal telogen skin did not reveal any gross abnormalities and there was no significant difference in the size of hair bulbs and dermal papillae (Figure 4A, B and data not shown). However, in Npnt −/− skin, α8 integrin was absent from the basement membrane separating the dermal papilla and hair germ and was more prominently distributed at the periphery of the dermal papilla (Figure S3A, B, C). The effect was specific to α8 integrin, because the distribution of the phylogenetically related RGD-binding integrin subunits, αv and α5, in dermal papillae was not altered (Figure S3D, E). In addition, lack of nephronectin did not affect the distribution of the ubiquitous basement membrane component, laminin γ1 chain (Figure S3B).
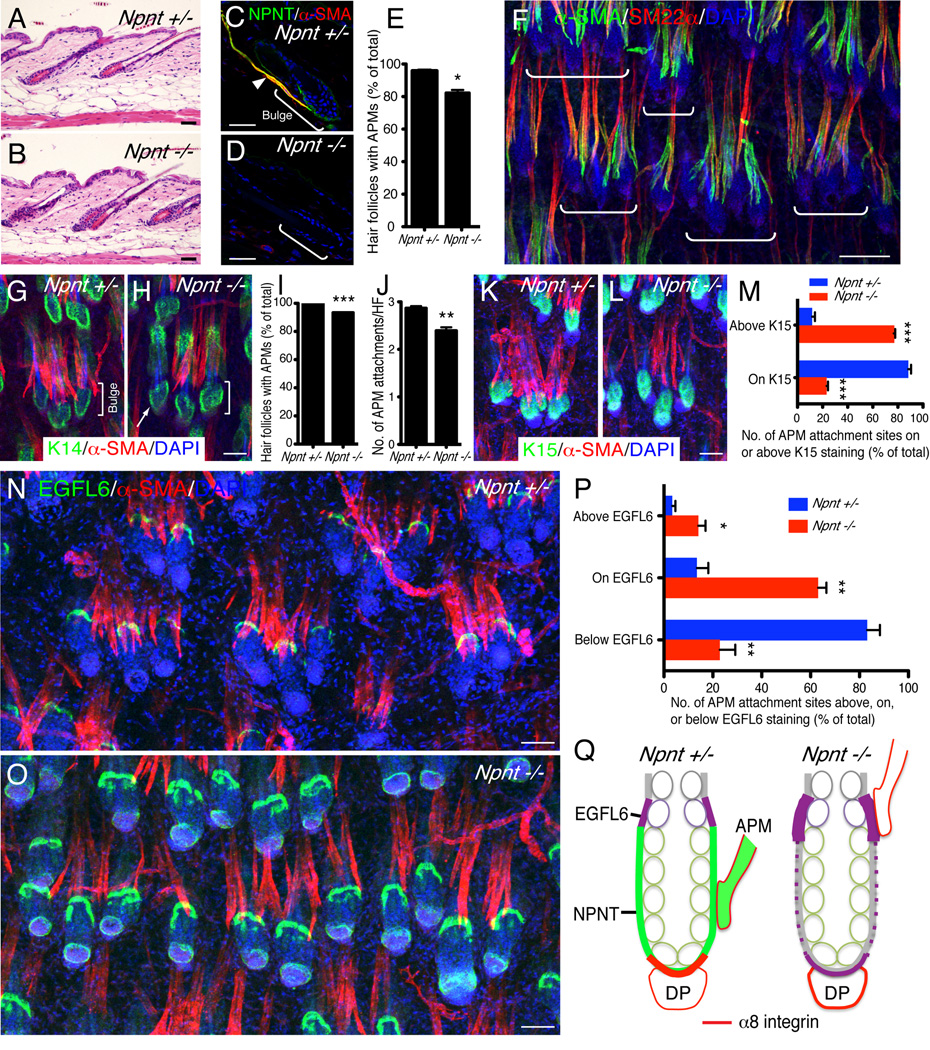
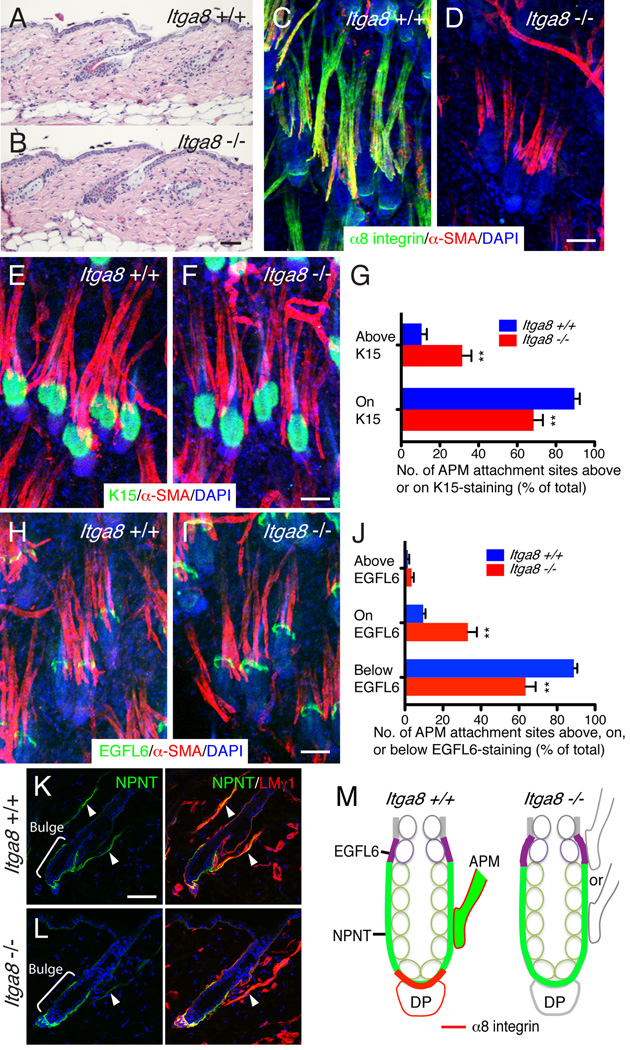
Figure 4. Nephronectin is required to anchor arrector pili muscles to the bulge.
(A, B) H&E-stained adult dorsal telogen skin. (C–E) Adult dorsal skin immunostained for nephronectin (green) and α-SMA (red) and counterstained with DAPI (blue). Arrowhead indicates APM. (E) % hair follicles with arrector pili muscles. (F) Maximum projection image of dorsal wild type skin whole-mount stained for α-SMA (green), SM22α (red), and nuclei (blue). White brackets indicate groups of hair follicles that share arrector pili muscles. (G–J) Whole-mounts (G, H) were immunostained for K14 (green) and α-SMA (red), with DAPI counterstain (blue). Arrow (H) indicates hair follicle without associated APM. Brackets indicate bulge. % follicles with arrector pili muscles (I) and number of APM attachments per hair follicle (J). (K–M) Whole-mounts were immunostained for K15 (green) and α-SMA (red), with DAPI counterstain (blue). Position of APM attachment sites relative to K15-positive bulge was quantified (M). (N–P) Whole-mounts were immunostained for EGFL6 (green) and α-SMA (red), with DAPI counterstain (blue). Position of APM attachment sites relative to EGFL6-positive zone was quantified (P). (Q) Schematic summary of data. Green: nephronectin; purple: EGFL6; red: α8 integrin; green circles: K15-positive bulge cells. In the presence of nephronectin, arrector pili muscles insert at the bulge. In the absence of nephronectin, EGFL6 expression in the upper bulge is increased and muscles insert in that region. α8 integrin colocalises with nephronectin in the basement membrane of the hair germ adjacent to the dermal papilla (DP). In the absence of nephronectin EGFL6 is expressed in that region, but there is no colocalisation with α8 integrin. All skin samples were from the back of 7 week old telogen mice. (E, I, J, M, P) Data are means ± SEM from three mice, 100 follicles per mouse. Scale bars: 50 µm; except for F (100 µm). See also Figure S3.
To examine whether loss of nephronectin affected the APM, we stained sections of adult telogen dorsal skin with anti-α-SMA. In control skin, muscles were detected in association with 95.9±1.2% of hair follicles. In Npnt −/− skin, there was a small but significant decrease in the percentage of hair follicles with arrector pili muscles (82.2±3.3%) (Figure 4C–E).
Given the obvious limitations of conventional histology for analysing the spatial relationship between the APM and the hair follicle, we developed a whole-mount labelling technique in which we could observe the interaction in three-dimensions. Arrector pili muscles were visualized by staining for α-SMA and SM22α and hair follicles were visualised by DAPI labelling or Keratin 14 (K14) staining. There was an inverse gradient of α-SMA and SM22α, with α-SMA being more abundant next to the bulge and SM22α closer to the interfollicular epidermis (Figure 4F, Figure S3F). Individual muscle bundles usually branched to insert into the bulges of several neighbouring hair follicles and each follicle typically had one associated muscle bundle (Figure 4F).
Using whole-mount visualisation, we confirmed the reduced number of hair follicles with associated arrector pili muscles in Npnt −/− skin: 99.7% of control follicles had an associated muscle, compared with 93.3% in knockout skin (Figure 4G–I). There was also a significant reduction in the number of muscle attachment sites per hair follicle (Figure 4J). However, the major effect of Npnt deletion was to change the site of muscle attachment to the follicle. In control mice, the APM attached to the K15-positive bulge, whereas in Npnt knockout mice, the attachment was higher up the follicle (Figure 4G, H, K–M). In Npnt +/− skin, 88.3% of muscle attachments located on the bulge. However, in the knockout, only 23.2% were on the bulge, and 76.3% located above the bulge. These results show that nephronectin is required to anchor the APM to the bulge.
The observation that loss of nephronectin led to a specific upward shift in APM insertion point, rather than randomising the insertion sites, led us to investigate whether there was compensatory change in the distribution of the nephronectin family member EGFL6/MAEG. Like nephronectin, EGFL6 is an α8β1 ligand that is expressed in mouse skin (Osada et al., 2005). In wild type and Npnt +/− skin EGFL6 was expressed in dermal condensates at E14.5 and in the dermal sheath at E16.5–E18.5 (Figure S3G, H). In newborn skin (P1 and P5), EGFL6, like nephronectin, localized to the K15-positive bulge (Figure S3G and Figure 1). However, in adult skin EGFL6 was only deposited in the basement membrane of the upper bulge, which is CD34-positive and K15-negative (Figure S3G). Egfl6 mRNA was highly upregulated in CD34+α6high cells relative to other basal keratinocytes (Figure S3I), indicating that EGFL6 in the upper bulge is of epidermal origin.
In control skin, the arrector pili muscles inserted below the EGFL6-positive zone of the hair follicles (Figure 4N, P). However, in Npnt −/− skin, EGFL6 deposition in the upper bulge was increased and the muscles inserted into the EGFL6-positive zone (Figure 4O, P; see also Figure S3G, J–L). Although the site of insertion of the APM was altered in Npnt −/− skin, piloerection could still be induced by treatment with the α1-adrenergic receptor agonist phenylephrine, which induces smooth muscle contraction (Figure S3M, N).
Our data reveal that nephronectin anchors the APM to the bulge and that in the absence of nephronectin the site of insertion shifts upwards to the EGFL6-positive zone (Figure 4Q).
α8 integrin determines specificity of arrector pili muscle attachment site to the bulge
Since the α8β1 integrin mediates nephronectin binding, we examined whether APM anchorage to the bulge was also disrupted in α8 integrin (Itga8) knockout mice (Muller et al., 1997). H&E-stained sections of adult dorsal telogen Itga8 −/− skin, like Npnt −/− skin, did not show any gross abnormalities (Figure 5A, B). Nephronectin deposition in the bulge was normal, but nephronectin was lacking in the APM, demonstrating that the α8 integrin is essential for deposition of nephronectin in the APM, but not in the bulge (Figure 5K, L). αv integrins, which, like α8, mediate nephronectin adhesion (Brandenberger et al., 2001), showed normal expression in the dermal papilla and APM of Itga8 −/− skin (Figure S4A–D).
Figure 5. Deletion of α8 integrin disrupts specificity of APM attachment site to the bulge.
(A, B) H&E-stained adult dorsal telogen skin. (C, D) Skin whole-mounts were immunostained for α8 integrin (green) and α-SMA (red), with DAPI counterstain (blue). (E–G) Whole-mounts (E, F) were immunostained for K15 (green) and α-SMA (red), with DAPI counterstain (blue). Position of APM attachment sites relative to K15-positive bulge was quantified (G). (H–J) Whole-mounts were immunostained for EGFL6 (green) and α-SMA (red), with DAPI counterstain (blue). Position of APM attachment sites relative to EGFL6-positive zone was quantified (J). (K) Conventional cryo-sections were immunostained for nephronectin (green) and laminin γ1 chain (red), with DAPI counterstain (blue). Arrowheads indicate APM. (M) Schematic summary of data. Green: nephronectin; purple: EGFL6; red: α8 integrin; green circles: K15-positive bulge cells. In the presence of α8 integrin, the APM is anchored to the bulge. In the absence of α8 integrin, muscles lose specificity for nephronectin and can anchor to both the nephronectin-positive bulge and the EGFL6-positive upper bulge regions. In the absence of α8 integrin, nephronectin deposition in the APM is disrupted, but that in the bulge is unaffected. All skin samples were from the back of 7–11 week old telogen mice. (G, J) Data are means ± SEM from four mice, 100 follicles per mouse. Scale bars: 50 µm. See also Figure S4.
Arrector pili muscles were still associated with hair follicles in Itga8 −/− skin with a small reduction in the number of APM (Figure 5C, D, S4E), but, as in the case of Npnt −/− skin, their selectivity for the bulge was disrupted. The proportion of muscles that attached to the K15-positive, nephronectin-positive, bulge region was decreased (Figure 5E–G) and the proportion that inserted above the bulge, in the EGFL6-positive region, was increased (Figure 5H–J). The striking difference between Npnt −/− and Itga8 −/− skin was that whereas in the absence of nephronectin α8β1-positive APM cells showed selectivity for the EGFL6-positive region, APM lacking α8β1 lost their selectivity for nephronectin-positive basement membrane and inserted into both nephronectin- and EGFL6-positive regions (Figure 4Q, Figure 5M).
We conclude that the site of APM attachment to the hair follicle is determined by the combination of α8β1 expression on smooth muscle cells and nephronectin deposition by bulge stem cells.
Epidermal Wnt/β-catenin signalling determines regional nephronectin and α8 integrin expression
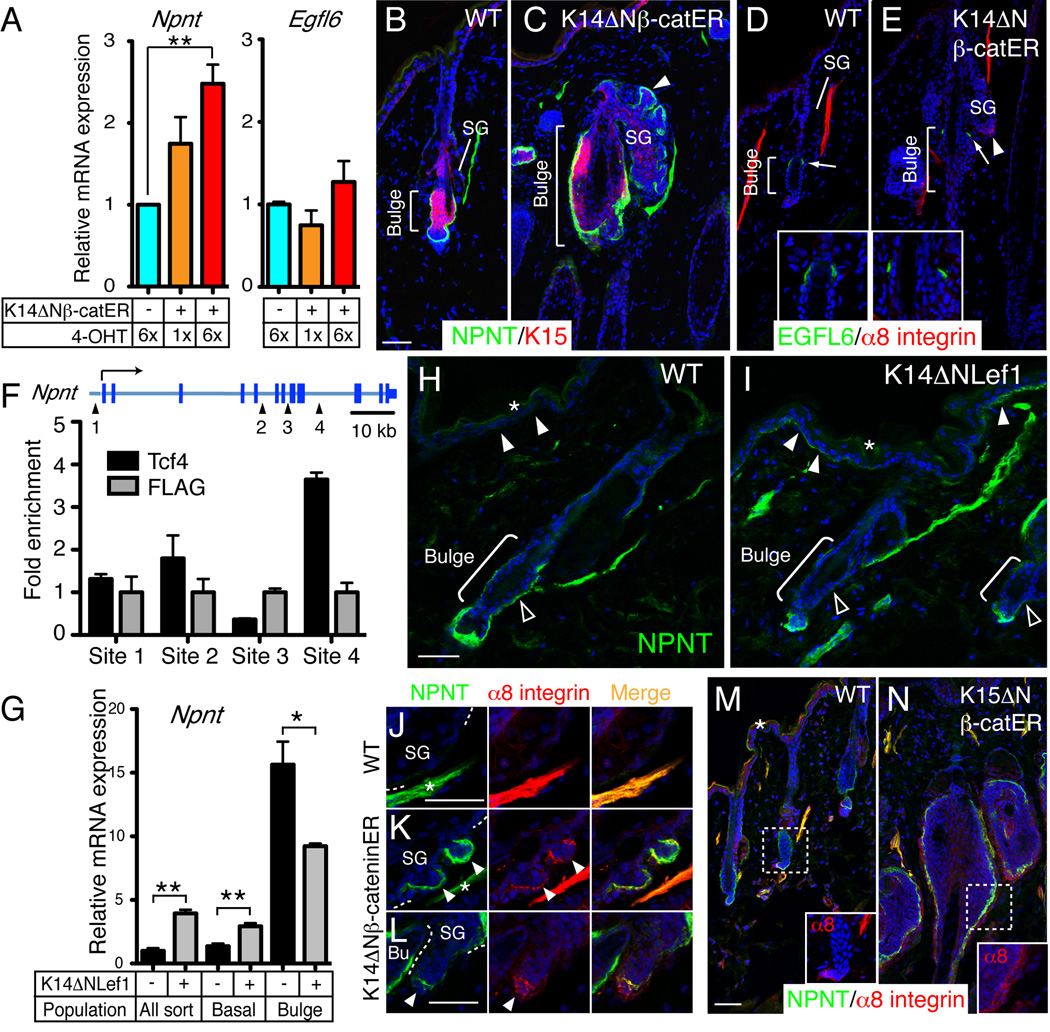
Since bulge-specific expression of nephronectin creates the niche for APM cell anchorage and differentiation, we next investigated the molecular mechanism of regional nephronectin expression. The two sites of epidermal nephronectin deposition, the bulge and hair germ, are sites of active Wnt/β-catenin signalling (Lowry et al., 2005; Nguyen et al., 2009), leading us to investigate whether nephronectin is a Wnt target gene. We activated Wnt/β-catenin signalling by topical application of 4-hydroxytamoxifen (4-OHT) to the back skin of adult telogen K14ΔNβ-cateninER mice, which express stabilised β-catenin fused with the C-terminus of a mutant oestrogen receptor under the control of the K14 promoter (Lo Celso et al., 2004). This resulted in a 4-OHT dose-dependent increase in epidermal mRNA encoding nephronectin (Figure 6A) and the bulge Wnt effectors Tcf3 and Tcf4 (Figure S5A). In contrast, Egfl6 mRNA levels did not change (Figure 6A). Upregulation of nephronectin protein was observed in the bulge and the ectopic hair follicles of 4-OHT-treated K14ΔNβ-cateninER mice (Figure 6B, C; see also Lo Celso et al., 2004), whereas deposition of EGFL6 was unaffected (Figure 6D, E).
Figure 6. Regulation of regional nephronectin-α8 integrin interaction by Wnt/β-catenin signalling.
(A) Q-PCR of Npnt and Egfl6 mRNA in keratinocytes isolated from skin of wild type (ΔNβ-catER −) and K14ΔNβ-cateninER mice (ΔNβ-catER +) that had been treated with 4-OHT for the number of times indicated. Data are means ± SEM from three mice. (B–E) Dorsal skin of wild type and K14ΔNβ-cateninER mice treated 6× with 4-OHT and immunostained for nephronectin (green; B, C) or EGFL6 (green; D, E) and K15 (red; B, C) or α8 integrin (red; D, E), with DAPI counterstain (blue). Arrowheads indicate ectopic hair follicle arising from sebaceous gland (SG). Arrows indicate EGFL6- staining. Inserts show higher magnification views of EGFL6 staining around the bulge. (F) ChIP using antibodies against Tcf4 and FLAG in K14ΔNβ-cateninER keratinocytes. Primers surrounding four conserved putative binding sites for Lef/Tcfs at the Npnt locus were used to detect the precipitated DNA fragments. Data are means ± SEM of two independent experiments. (G) Q-PCR of mRNA from FACS-isolated bulge, non-bulge (basal) and total basal (All sort) keratinocytes from wild type and K14ΔNLef1 adult telogen skin. Data are means ± SEM from three mice. (H, I) Wild type and K14ΔNLef1 skin immunostained for nephronectin (green) with DAPI counterstain (blue). Basement membrane of interfollicular epidermis (white arrowheads) and bulge (open arrowheads) is indicated. (J–N) Sections of wild type (J, M), K14ΔNβ-cateninER (K, L) and K15ΔNβ-cateninER (N) skin treated 6× (J–L) or 9× (M, N) with 4-OHT and immunostained for nephronectin (green) and α8 integrin (red), with DAPI counterstain (blue). Arrowheads indicate ectopic hair follicles. Asterisks in J and K indicate arrector pili muscles. Inserts in M, N show magnified images of α8 integrin staining around the bulge. Asterisks in H, I and M indicate non-specific staining. Scale bars: 50 µm. See also Figure S5.
Using UCSC Genome Browser, several conserved putative binding sites for Lef/Tcfs in the Npnt locus were identified. We therefore performed chromatin immunoprecipitation (ChIP) assays with an antibody to Tcf4, which, together with Tcf3, is specifically expressed in the bulge (Nguyen et al., 2009), and chromatin from cultured 4-OHT-treated K14ΔNβ-cateninER keratinocytes. One of the conserved sites (site 4) showed consistent enrichment for Tcf4 relative to the control FLAG antibody (Figure 6F), demonstrating that nephronectin is a direct target of Tcf4.
We then analyzed the expression of nephronectin in K14ΔNLef1 mice that express N-terminally deleted Lef1, which lacks the β-catenin-binding site, under the control of the K14 promoter (Niemann et al., 2002). ΔNLef1 acts as a dominant-negative inhibitor of Wnt/β-catenin signalling by blocking formation of β-catenin/Lef/Tcf complexes. We separated adult bulge and other basal keratinocytes from K14ΔNLef1 transgenic mice by FACS and examined the expression levels of nephronectin by Q-PCR. In bulge keratinocytes, nephronectin expression was decreased, whereas in non-bulge keratinocytes, expression was upregulated (Figure 6G). Consistent with this, in K14ΔNLef1 skin, nephronectin deposition in the bulge and hair germ was decreased, while nephronectin deposition in the interfollicular epidermis was increased (Figure 6H, I).
We also examined the effects of BMP and Notch signalling on nephronectin expression (Figure S5B, C). Inhibition of BMP signalling by expression of the BMP antagonist Noggin under the control of the K14 promoter (Sharov et al., 2009) increased nephronectin expression in the bulge. However, activation of Notch signalling by expression of the Notch intracellular domain via the K14 promoter (Estrach et al., 2006) did not affect nephronectin expression. The upregulation of nephronectin in K14Noggin skin fits well with the upregulation of Wnt signalling that occurs on BMP inhibition in this model (Sharov et al., 2009). These results indicate that activation of Wnt/β-catenin signalling in the bulge and hair germ induces nephronectin expression, whereas Wnt/β- catenin signalling in the interfollicular epidermis normally suppresses nephronectin.
To determine whether epidermal β-catenin activation was sufficient to induce α8β1 expression in adjacent dermal cells, we examined 4-OHT treated mice expressing the ΔNβ-cateninER transgene. When Wnt/β-catenin signalling was activated in K14ΔNβ-cateninER transgenic mice, α8 integrin was ectopically expressed by dermal cells adjacent to ectopic hair follicles, co-localizing with nephronectin (Figure 6J–L). When β-catenin was selectively activated in bulge stem cells by 4-OHT-treatment of K15ΔNβ-cateninER mice (Baker et al., 2010), nephronectin was upregulated in the bulge and there was a corresponding increase in bulge-associated α8-integrin expressing dermal cells (Figure 6M, N). These observations establish that epidermal Wnt/β-catenin signalling induces expression of α8 integrin in adjacent dermal cells.
Taken together, our data show that nephronectin is a Wnt/β-catenin target gene and that Wnt/β-catenin signalling in epidermis determines not only region-specific nephronectin deposition but also, as a result, the location of α8 integrin expressing mesenchymal cells.
Discussion
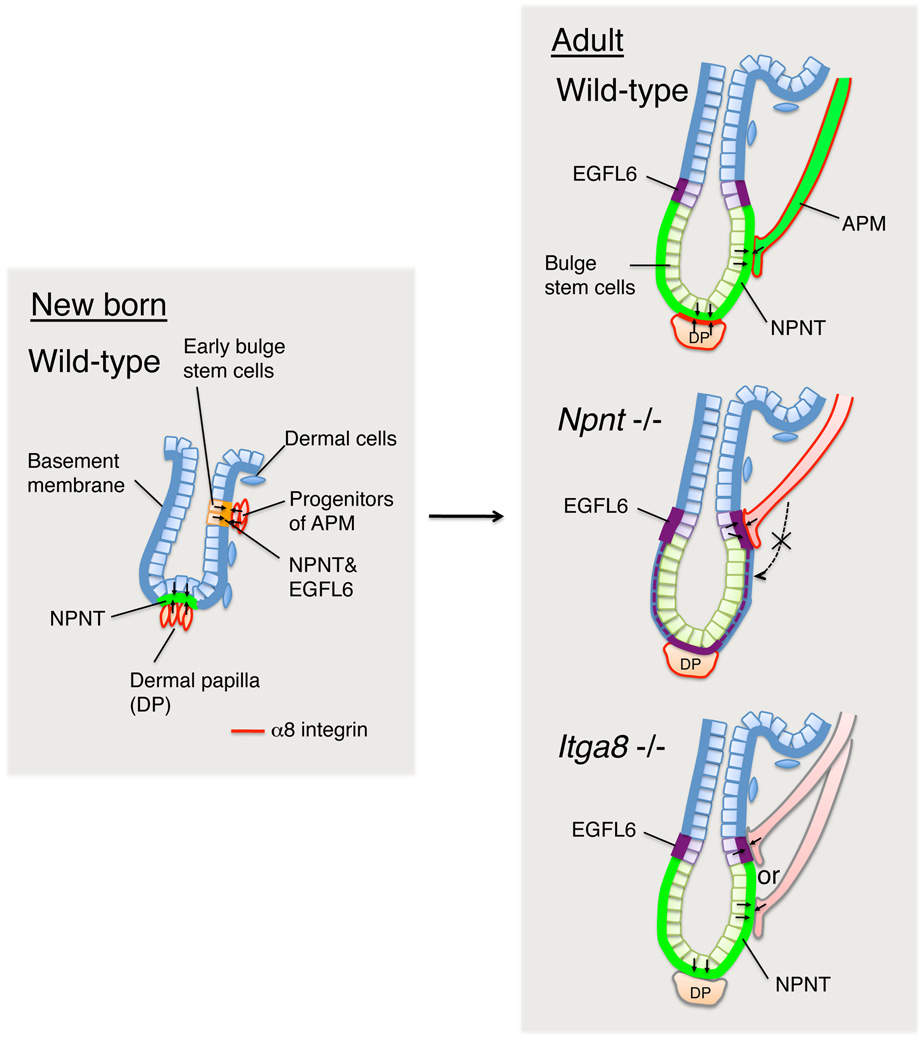
It has previously been suggested that heterogeneity in basement membrane composition may help to establish distinct stem cell niches; however, direct evidence has been lacking (Akiyama et al., 1995; Fuchs, 2008; Hall and Watt, 1989; Scadden, 2006; Spradling et al., 2001; Watt and Hogan, 2000). Our study is the first to identify how local variation in epidermal basement membrane composition is established and to demonstrate that the specific composition of the bulge ECM not only provides a specialized environment for bulge stem cells, but also for adjacent mesenchymal cells (Figure 7).
Figure 7. Model depicting role for nephronectin-α8β1 interactions in creating the niche for the arrector pili muscle at the hair follicle bulge.
During hair morphogenesis in neonatal skin, early bulge stem cells locally deposit nephronectin in the bulge basement membrane. Nephronectin induces neighbouring mesenchymal progenitors to differentiate into α8 integrin-positive APM cells, which adhere specifically to nephronectin, establishing a stable anchorage to the bulge that is maintained throughout adult life. In the absence of nephronectin, the APM is not anchored to the bulge, but attaches above the bulge, where there is compensatory upregulation of EGFL6. Lack of nephronectin also disturbs α8 integrin-mediated hair follicle-dermal papilla interactions (Figure S3). In the absence of α8 integrin, nephronectin is still deposited in the bulge, but the selectivity of the APM interaction is lost and muscles are anchored both to the nephronectin-positive bulge and the EGFL6-positive upper bulge.
Sox9-positive bulge stem cells are specified in early hair follicle morphogenesis, just after birth (Nowak et al., 2008). This coincides with restriction of nephronectin deposition to the bulge basement membrane and an associated accumulation of α8 integrin-positive mesenchymal cells, precursors of the APM. Thus, the bulge and the niche for smooth muscle progenitors are established simultaneously. Nephronectin expression is confined to the bulge basement membrane throughout adult life, irrespective of the hair cycle, and consistent with the permanent association of the APM with the bulge.
We found that recombinant nephronectin not only supported adhesion of α8 integrin-positive fibroblasts from neonatal dermis, but also induced expression of smooth muscle differentiation markers, consistent with reports that α8 integrin signalling maintains differentiation of vascular smooth muscle cells (Zargham and Thibault, 2006; Zargham et al., 2007). It therefore seems likely that during skin development APM cells differentiate from mesenchymal progenitors through adhesion to nephronectin deposited in the early bulge. The synergistic effect of nephronectin and laminin, another component of the bulge basement membrane (Figure S1B), in inducing smooth muscle markers is consistent with the positive role of laminin in intestinal smooth muscle differentiation (Bolcato-Bellemin et al., 2003).
Deletion of nephronectin resulted in upregulation of the nephronectin related protein, EGFL6, and a corresponding change in the arrector pili muscle insertion site from the bulge to the EGFL6-positive zone above. The LFEIFEIER motif that mediates the high affinity interaction of nephronectin with α8β1 integrin is lacking in EGFL6 (Osada et al., 2005; Sato et al., 2009) and this is the likely explanation for the specific association of α8-positive cells with nephronectin in wild type skin. On deletion of the α8 integrin, nephronectin was still expressed in the bulge, but the specificity of APM association with nephronectin was lost, and muscles inserted into both the nephronectin-positive and EGFL6-positive zones. This is probably because, in the absence of α8β1, adhesion to nephronectin is mediated via the αv integrins, which is a lower affinity interaction (Brandenberger et al., 2001; Figure S4).
While the APM is known to attach to the bulge via a tendon, the tendon cells have not been identified (Barcaui et al., 2002; Guerra Rodrigo et al., 1975). We showed that the tendon/ligament extracellular matrix protein periostin (Horiuchi et al., 1999; Norris et al., 2007) was strongly expressed in bulge stem cells and deposited locally around the bulge. Furthermore, the gene signature of bulge stem cells includes many tendon-related genes, such as Scx (scleraxis), Mitf, Igfbp5, Fbln1 (fibulin-1), Postn (periostin), Tnc (tenascin-C), Sparc, Igfbp6, and Fgf18 (Brent et al., 2003; Jelinsky et al., 2010; Morris et al., 2004; Tumbar et al., 2004). We therefore propose that bulge stem cells function as tendon cells in providing a physical connection for the APM. The immobility of bulge stem cell compartment assures that APM attachment is stable regardless of the stage of the hair growth cycle.
We did not obtain any evidence that, as has been suggested, the APM determines the location of bulge stem cells (Akiyama et al., 1995; Christiano, 2004). The delocalisation of the muscle that occurred on loss of nephronectin had no clear effect on the bulge, as judged by bulge morphology and expression of keratin 15 and CD34. Thus nephronectin expression by bulge stem cells provides a niche for APM cells, but is not an essential component of the epidermal stem cell niche.
Wnt/β-catenin signalling is well known to play a role in controlling epidermal stem cell renewal and lineage selection and in reciprocal interactions with the dermal papilla (Alonso and Fuchs, 2003; Watt and Collins, 2008). However, a role for Wnt/β-catenin signalling in regulating the APM was previously unknown. We demonstrate that nephronectin, unlike EGFL6, is a direct target of Wnt/β-catenin signalling. Nephronectin expression is upregulated by Wnt/β-catenin activation, directly or via inhibition of BMP signalling, in the epidermis and, as a result, there is a corresponding upregulation of α8 integrin expression in the adjacent dermis.
The consequences of activating Wnt/β-catenin signalling were context-dependent. Activation in the bulge upregulated nephronectin, whereas activation in the interfollicular epidermis did not. Conversely, expression of ΔNLef1, which inhibits Wnt signalling, stimulated nephronectin expression in the interfollicular epidermis and downregulated expression in the bulge. A likely explanation for the region-specific effects of ΔNLef1 is that ΔNLef1 expression in the bulge inhibits β-catenin-dependent induction of nephronectin expression by Tcf3 or Tcf4, whereas in the interfollicular epidermis Tcf/Lef transcription factors are not expressed and so ΔNLef1 regulates nephronectin expression independently of the Wnt pathway (Nguyen et al., 2006).
The APM plays an important role in thermoregulation, since it is responsible for piloerection, which traps warm air at the skin surface. Piloerection is also believed to cause constriction of the sebaceous glands, aiding release of sebum onto the skin surface (Poblet et al., 2004). Furthermore, as Charles Darwin noted in 1872, involuntary erection of hairs, feathers and other dermal appendages is an evolutionarily conserved response to emotional disturbance. Malignant tumours of the arrector pili have been reported and are known as cutaneous leiomyosarcoma (Fons et al., 2009). Our results provide new insights into the mechanism of arrector pili muscle morphogenesis and answer the long-standing question of why the arrector pili muscle is constantly attached to the hair follicle bulge (Akiyama et al., 1995).
Experimental Procedures
Generation and experimental treatment of mice
Npnt knockout mice, Itga8 knockout mice, K14ΔNLef1, K14ΔNβ-cateninER (D2 line) and K15ΔNβ-cateninER transgenic mice have been described previously (Baker et al., 2010; Linton et al., 2007; Lo Celso et al., 2004; Muller et al., 1997; Niemann et al., 2002). The ΔNβ-cateninER transgene was activated by topical application of 4-hydroxytamoxifen (4-OHT; Sigma) dissolved in acetone. Shaved back skin was treated topically with 1.5 mg of 4-OHT in 200 µl of acetone three times per week for 2 weeks unless otherwise specified.
Gene expression analysis
For microarray analysis, CEL format files of the gene expression profiles of K15-positive mouse bulge stem cells (GSE1096) were obtained from the NCBI’s GEO (Gene Expression Omnibus) web site (Morris et al., 2004) and analyzed with Genespring X10.0 (Agilent Technologies).
Immunohistochemistry and whole-mount preparations
Immunofluorescence staining of tissue sections was performed by conventional methods.
Whole-mount immunostaining of mouse dorsal skin was performed by applying the methods established for whole-mount immunostaining of mouse tail epidermis, with some modifications (Braun et al., 2003). Embryonic and adult dorsal skin was dissected and the subcutaneous fat tissue was removed. Skin was fixed with 4% paraformaldehyde/PBS for 1 hour at room temperature and blocked with a blocking buffer for 1 hour. Skin samples were incubated with primary antibodies diluted in blocking buffer overnight at room temperature, washed with 0.2% Tween 20/PBS for 4 hours and then incubated with DAPI and secondary antibodies diluted in blocking buffer overnight at room temperature. Finally, skin samples were washed with 0.2% Tween 20/PBS for 4 hours at room temperature and mounted.
Images were acquired using a Leica TCS SP5 Tandem Scanner confocal microscope. Z-stack maximum projection images of whole-mount preparations were produced using LAS AF software (Leica).
Quantitative RT-PCR
Total RNA was isolated using an RNeasy Mini kit (Qiagen) with on-column DNase I digestion. cDNA was synthesized using SuperScript III (Invitrogen). RT-PCR was performed using SYBR green super mix (ABI). The expression levels of target genes were normalized by Gapdh levels utilizing a standard curve method. Primers used in this study are listed in Supplemental Information.
FACS
Mouse adult dorsal telogen keratinocytes were isolated, stained for α6 integrin and CD34, and sorted as described previously (Silva-Vargas et al., 2005).
For dermal cell sorting, P1 newborn dorsal skin was treated with 5 mM EDTA/PBS at 37°C for 3 hours. The epidermal sheet, including hair follicles, was peeled off and discarded. The dermis was incubated in FAD medium containing 0.2% collagenase (Gibco) and 2U/ml of DNase I (Sigma) at 37°C for 1 hour. The dermal cell suspension was stained for α8 integrin. Cells were sorted with a FACSAria according to α8 integrin expression, after gating out dead cells and cells with high forward and side scatter.
Additional Experimental Procedures
These are described in Supplemental Information.
Supplementary Material
Acknowledgements
We thank Vladimir Botchkarev for K14-Noggin skin and Takako Sasaki for Fibulin-1 antibody. We gratefully acknowledge the technical assistance of the core resources of the CRUK Cambridge Research Institute. We thank the entire Watt lab for valuable reagents, suggestions, and advice. This work was funded by Cancer Research UK and supported by the University of Cambridge and Hutchison Whampoa Ltd. We also gratefully acknowledge financial support from the Uehara Memorial Foundation (H.F.) and EU FP7 (Optistem).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akiyama M, Dale BA, Sun TT, Holbrook KA. Characterization of hair follicle bulge in human fetal skin: the human fetal bulge is a pool of undifferentiated keratinocytes. J Invest Dermatol. 1995;105:844–850. doi: 10.1111/1523-1747.ep12326649. [DOI] [PubMed] [Google Scholar]
- Alonso L, Fuchs E. Stem cells in the skin: waste not, Wnt not. Genes Dev. 2003;17:1189–1200. doi: 10.1101/gad.1086903. [DOI] [PubMed] [Google Scholar]
- Baker CM, Verstuyf A, Jensen KB, Watt FM. Differential sensitivity of epidermal cell subpopulations to beta-catenin-induced ectopic hair follicle formation. Dev Biol. 2010;343:40–50. doi: 10.1016/j.ydbio.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcaui CB, Pineiro-Maceira J, de Avelar Alchorne MM. Arrector pili muscle: evidence of proximal attachment variant in terminal follicles of the scalp. Br J Dermatol. 2002;146:657–658. doi: 10.1046/j.1365-2133.2002.04541.x. [DOI] [PubMed] [Google Scholar]
- Bolcato-Bellemin AL, Lefebvre O, Arnold C, Sorokin L, Miner JH, Kedinger M, Simon-Assmann P. Laminin alpha5 chain is required for intestinal smooth muscle development. Dev Biol. 2003;260:376–390. doi: 10.1016/s0012-1606(03)00254-9. [DOI] [PubMed] [Google Scholar]
- Brandenberger R, Schmidt A, Linton J, Wang D, Backus C, Denda S, Muller U, Reichardt LF. Identification and characterization of a novel extracellular matrix protein nephronectin that is associated with integrin alpha8beta1 in the embryonic kidney. J Cell Biol. 2001;154:447–458. doi: 10.1083/jcb.200103069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun KM, Niemann C, Jensen UB, Sundberg JP, Silva-Vargas V, Watt FM. Manipulation of stem cell proliferation and lineage commitment: visualisation of label-retaining cells in wholemounts of mouse epidermis. Development. 2003;130:5241–5255. doi: 10.1242/dev.00703. [DOI] [PubMed] [Google Scholar]
- Brent AE, Schweitzer R, Tabin CJ. A somitic compartment of tendon progenitors. Cell. 2003;113:235–248. doi: 10.1016/s0092-8674(03)00268-x. [DOI] [PubMed] [Google Scholar]
- Christiano AM. Epithelial stem cells: stepping out of their niche. Cell. 2004;118:530–532. doi: 10.1016/j.cell.2004.08.024. [DOI] [PubMed] [Google Scholar]
- Darwin C. The expression of the emotions in man and animals. London: J. Murray; 1872. [Google Scholar]
- Denda S, Reichardt LF, Muller U. Identification of osteopontin as a novel ligand for the integrin alpha8 beta1 and potential roles for this integrin-ligand interaction in kidney morphogenesis. Mol Biol Cell. 1998;9:1425–1435. doi: 10.1091/mbc.9.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driskell RR, Giangreco A, Jensen KB, Mulder KW, Watt FM. Sox2-positive dermal papilla cells specify hair follicle type in mammalian epidermis. Development. 2009;136:2815–2823. doi: 10.1242/dev.038620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erez N, Truitt M, Olson P, Hanahan D. Cancer-Associated Fibroblasts Are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NF-kappaB-Dependent Manner. Cancer Cell. 2010;17:135–147. doi: 10.1016/j.ccr.2009.12.041. [DOI] [PubMed] [Google Scholar]
- Estrach S, Ambler CA, Lo Celso C, Hozumi K, Watt FM. Jagged 1 is a beta-catenin target gene required for ectopic hair follicle formation in adult epidermis. Development. 2006;133:4427–4438. doi: 10.1242/dev.02644. [DOI] [PubMed] [Google Scholar]
- Fons ME, Bachhuber T, Plaza JA. Cutaneous leiomyosarcoma originating in a symplastic pilar leiomyoma: a rare occurrence and potential diagnostic pitfall. J Cutan Pathol. 2009 doi: 10.1111/j.1600-0560.2009.01420.x. [DOI] [PubMed] [Google Scholar]
- Fuchs E. Skin stem cells: rising to the surface. J Cell Biol. 2008;180:273–284. doi: 10.1083/jcb.200708185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, DeRouen MC, Chen CH, Nguyen M, Nguyen NT, Ido H, Harada K, Sekiguchi K, Morgan BA, Miner JH, et al. Laminin-511 is an epithelial message promoting dermal papilla development and function during early hair morphogenesis. Genes Dev. 2008;22:2111–2124. doi: 10.1101/gad.1689908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra Rodrigo F, Cotta-Pereira G, David-Ferreira JF. The fine structure of the elastic tendons in the human arrector pili muscle. Br J Dermatol. 1975;93:631–637. doi: 10.1111/j.1365-2133.1975.tb05112.x. [DOI] [PubMed] [Google Scholar]
- Hall PA, Watt FM. Stem cells: the generation and maintenance of cellular diversity. Development. 1989;106:619–633. doi: 10.1242/dev.106.4.619. [DOI] [PubMed] [Google Scholar]
- Horiuchi K, Amizuka N, Takeshita S, Takamatsu H, Katsuura M, Ozawa H, Toyama Y, Bonewald LF, Kudo A. Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J Bone Miner Res. 1999;14:1239–1249. doi: 10.1359/jbmr.1999.14.7.1239. [DOI] [PubMed] [Google Scholar]
- Jelinsky SA, Archambault J, Li L, Seeherman H. Tendon-selective genes identified from rat and human musculoskeletal tissues. J Orthop Res. 2010;28:289–297. doi: 10.1002/jor.20999. [DOI] [PubMed] [Google Scholar]
- Jensen KB, Collins CA, Nascimento E, Tan DW, Frye M, Itami S, Watt FM. Lrig1 expression defines a distinct multipotent stem cell population in mammalian epidermis. Cell Stem Cell. 2009;4:427–439. doi: 10.1016/j.stem.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda K, Tajima S. HSP47 is a useful marker for skin fibroblasts in formalin-fixed, paraffin-embedded tissue specimens. J Cutan Pathol. 2004;31:241–246. doi: 10.1111/j.0303-6987.2003.00166.x. [DOI] [PubMed] [Google Scholar]
- Linton JM, Martin GR, Reichardt LF. The ECM protein nephronectin promotes kidney development via integrin alpha8beta1-mediated stimulation of Gdnf expression. Development. 2007;134:2501–2509. doi: 10.1242/dev.005033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Celso C, Prowse DM, Watt FM. Transient activation of beta-catenin signalling in adult mouse epidermis is sufficient to induce new hair follicles but continuous activation is required to maintain hair follicle tumours. Development. 2004;131:1787–1799. doi: 10.1242/dev.01052. [DOI] [PubMed] [Google Scholar]
- Lowry WE, Blanpain C, Nowak JA, Guasch G, Lewis L, Fuchs E. Defining the impact of beta-catenin/Tcf transactivation on epithelial stem cells. Genes Dev. 2005;19:1596–1611. doi: 10.1101/gad.1324905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar SE. Molecular mechanisms regulating hair follicle development. J Invest Dermatol. 2002;118:216–225. doi: 10.1046/j.0022-202x.2001.01670.x. [DOI] [PubMed] [Google Scholar]
- Morris RJ, Liu Y, Marles L, Yang Z, Trempus C, Li S, Lin JS, Sawicki JA, Cotsarelis G. Capturing and profiling adult hair follicle stem cells. Nat Biotechnol. 2004;22:411–417. doi: 10.1038/nbt950. [DOI] [PubMed] [Google Scholar]
- Muller U, Wang D, Denda S, Meneses JJ, Pedersen RA, Reichardt LF. Integrin alpha8beta1 is critically important for epithelial-mesenchymal interactions during kidney morphogenesis. Cell. 1997;88:603–613. doi: 10.1016/s0092-8674(00)81903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Rover S, Handjiski B, van der Veen C, Eichmuller S, Foitzik K, McKay IA, Stenn KS, Paus R. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J Invest Dermatol. 2001;117:3–15. doi: 10.1046/j.0022-202x.2001.01377.x. [DOI] [PubMed] [Google Scholar]
- Nguyen H, Merrill BJ, Polak L, Nikolova M, Rendl M, Shaver TM, Pasolli HA, Fuchs E. Tcf3 and Tcf4 are essential for long-term homeostasis of skin epithelia. Nat Genet. 2009;41:1068–1075. doi: 10.1038/ng.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen H, Rendl M, Fuchs E. Tcf3 governs stem cell features and represses cell fate determination in skin. Cell. 2006;127:171–183. doi: 10.1016/j.cell.2006.07.036. [DOI] [PubMed] [Google Scholar]
- Niemann C, Owens DM, Hulsken J, Birchmeier W, Watt FM. Expression of DeltaNLef1 in mouse epidermis results in differentiation of hair follicles into squamous epidermal cysts and formation of skin tumours. Development. 2002;129:95–109. doi: 10.1242/dev.129.1.95. [DOI] [PubMed] [Google Scholar]
- Nikolova G, Jabs N, Konstantinova I, Domogatskaya A, Tryggvason K, Sorokin L, Fassler R, Gu G, Gerber HP, Ferrara N, et al. The vascular basement membrane: a niche for insulin gene expression and Beta cell proliferation. Dev Cell. 2006;10:397–405. doi: 10.1016/j.devcel.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Norris RA, Damon B, Mironov V, Kasyanov V, Ramamurthi A, Moreno-Rodriguez R, Trusk T, Potts JD, Goodwin RL, Davis J, et al. Periostin regulates collagen fibrillogenesis and the biomechanical properties of connective tissues. J Cell Biochem. 2007;101:695–711. doi: 10.1002/jcb.21224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak JA, Polak L, Pasolli HA, Fuchs E. Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell. 2008;3:33–43. doi: 10.1016/j.stem.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama M, Terunuma A, Tock CL, Radonovich MF, Pise-Masison CA, Hopping SB, Brady JN, Udey MC, Vogel JC. Characterization and isolation of stem cell-enriched human hair follicle bulge cells. J Clin Invest. 2006;116:249–260. doi: 10.1172/JCI26043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osada A, Kiyozumi D, Tsutsui K, Ono Y, Weber CN, Sugimoto N, Imai T, Okada A, Sekiguchi K. Expression of MAEG, a novel basement membrane protein, in mouse hair follicle morphogenesis. Exp Cell Res. 2005;303:148–159. doi: 10.1016/j.yexcr.2004.04.053. [DOI] [PubMed] [Google Scholar]
- Poblet E, Jimenez F, Ortega F. The contribution of the arrector pili muscle and sebaceous glands to the follicular unit structure. J Am Acad Dermatol. 2004;51:217–222. doi: 10.1016/j.jaad.2004.01.054. [DOI] [PubMed] [Google Scholar]
- Sato Y, Uemura T, Morimitsu K, Sato-Nishiuchi R, Manabe R, Takagi J, Yamada M, Sekiguchi K. Molecular basis of the recognition of nephronectin by integrin alpha8beta1. J Biol Chem. 2009;284:14524–14536. doi: 10.1074/jbc.M900200200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441:1075–1079. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- Schnapp LM, Hatch N, Ramos DM, Klimanskaya IV, Sheppard D, Pytela R. The human integrin alpha 8 beta 1 functions as a receptor for tenascin, fibronectin, and vitronectin. J Biol Chem. 1995;270:23196–23202. doi: 10.1074/jbc.270.39.23196. [DOI] [PubMed] [Google Scholar]
- Sharov AA, Mardaryev AN, Sharova TY, Grachtchouk M, Atoyan R, Byers HR, Seykora JT, Overbeek P, Dlugosz A, Botchkarev VA. Bone morphogenetic protein antagonist noggin promotes skin tumorigenesis via stimulation of the Wnt and Shh signaling pathways. Am J Pathol. 2009;175:1303–1314. doi: 10.2353/ajpath.2009.090163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Vargas V, Lo Celso C, Giangreco A, Ofstad T, Prowse DM, Braun KM, Watt FM. Beta-catenin and Hedgehog signal strength can specify number and location of hair follicles in adult epidermis without recruitment of bulge stem cells. Dev Cell. 2005;9:121–131. doi: 10.1016/j.devcel.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Spradling A, Drummond-Barbosa D, Kai T. Stem cells find their niche. Nature. 2001;414:98–104. doi: 10.1038/35102160. [DOI] [PubMed] [Google Scholar]
- Timpl R. Macromolecular organization of basement membranes. Curr Opin Cell Biol. 1996;8:618–624. doi: 10.1016/s0955-0674(96)80102-5. [DOI] [PubMed] [Google Scholar]
- Trempus CS, Morris RJ, Bortner CD, Cotsarelis G, Faircloth RS, Reece JM, Tennant RW. Enrichment for living murine keratinocytes from the hair follicle bulge with the cell surface marker CD34. J Invest Dermatol. 2003;120:501–511. doi: 10.1046/j.1523-1747.2003.12088.x. [DOI] [PubMed] [Google Scholar]
- Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt FM. Role of integrins in regulating epidermal adhesion, growth and differentiation. EMBO J. 2002;21:3919–3926. doi: 10.1093/emboj/cdf399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt FM, Collins CA. Role of beta-catenin in epidermal stem cell expansion, lineage selection, and cancer. Cold Spring Harb Symp Quant Biol. 2008;73:503–512. doi: 10.1101/sqb.2008.73.011. [DOI] [PubMed] [Google Scholar]
- Watt FM, Hogan BL. Out of Eden: stem cells and their niches. Science. 2000;287:1427–1430. doi: 10.1126/science.287.5457.1427. [DOI] [PubMed] [Google Scholar]
- Watt FM, Lo Celso C, Silva-Vargas V. Epidermal stem cells: an update. Curr Opin Genet Dev. 2006;16:518–524. doi: 10.1016/j.gde.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Yang CC, Cotsarelis G. Review of hair follicle dermal cells. J Dermatol Sci. 2010;57:2–11. doi: 10.1016/j.jdermsci.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zargham R, Thibault G. Alpha 8 integrin expression is required for maintenance of the smooth muscle cell differentiated phenotype. Cardiovasc Res. 2006;71:170–178. doi: 10.1016/j.cardiores.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Zargham R, Touyz RM, Thibault G. alpha 8 Integrin overexpression in de-differentiated vascular smooth muscle cells attenuates migratory activity and restores the characteristics of the differentiated phenotype. Atherosclerosis. 2007;195:303–312. doi: 10.1016/j.atherosclerosis.2007.01.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.