Abstract
The human amygdala is known to be involved in processing social, emotional, and reward-related information. Previous reports have indicated that the amygdala is involved in extracting trustworthiness information from faces. Interestingly, functional neuroimaging research using economic tasks that presumably require developing and/or expressing interpersonal trust, such as the Trust Game (TG), have not routinely identified involvement of the amygdala. The present study sought to explore the role of the amygdala in developing and expressing interpersonal trust, via a multi-round, multiplayer economic exchange, a version of the TG, in a large sample of participants with focal brain damage. Participants with unilateral damage to the amygdala displayed increased benevolent behavior in the TG, and specifically, they tended to increase trust in response to betrayals. On the other hand, neurologically-normal adults tended to repay trust in kind, i.e., they decreased interpersonal trust in response to betrayals or increased trust in response to increases from others. Comparison participants, with brain damage that does not include the amygdala, ventromedial prefrontal or insular cortices, tended to behave ambivalently to the expressed trust or betrayal of others. Our data suggest that the amygdala is necessary for developing and expressing normal interpersonal trust. This increased tendency to behave benevolently in response to defections from others may be related to the abnormal social behavior observed in this group. Moreover, increased benevolence may increase the likelihood or opportunity to be taken advantage of by others.
Keywords: amygdale, trust, decision-making, brain lesion, social cognition
Introduction
Convergent evidence from human and primate lesion studies, single-unit recordings, functional neuroimaging and other methodologies have shown that the amygdalae are intimately involved in social, emotional, and reward processing. The importance of the amygdalae for social functioning is particularly apparent following damage to this structure. Experimentally-induced, bilateral lesions to the amygdala of the rhesus macaque (Macaca mulatta), result in radically decreased social inhibition and increased social affiliation (Emery et al., 2001; Kluver & Bucy, 1937, 1939). Human studies of amygdala damage have revealed a similar pattern of social deficits. For example, patient SM, who has bilateral amygdala damage, exhibits impaired recognition of facial emotions and social disinhibition, and she is impaired in many aspects of social decision-making in the direction that would be predicted from the aforementioned macaque lesion studies (for a review see Adolphs, 2010).
The role of the amygdala in assessing the trustworthiness of others is of particular importance to the current discussion. An important component of social interaction is deciding with whom to interact, with whom to interact on multiple occasions, and how to behave when interacting with others based on prior experience and/or expectations of the encounter’s outcome. The amygdalae are known to be involved in processing of trustworthiness cues from faces, from both functional imaging studies (Engell, Haxby, & Todorov, 2007; Platek, Krill, & Wilson, 2009; Said, Baron, & Todorov, 2009; Winston, Strange, O’Doherty, & Dolan, 2002) and in human lesion research (Adolphs, Tranel, & Damasio, 1998). People with bilateral amygdala lesions (SM included) not only rate unfamiliar individuals to be more approachable and more trustworthy, but this effect is most pronounced for faces that normal subjects rate as very unapproachable and untrustworthy (Adolphs, et al., 1998). As a corollary, SM displays a closer preferred interpersonal distance (Kennedy, Gläscher, Tyszka, & Adolphs, 2009), possibly due to a damaged mechanism whereby individuals regulate their relationships with others, in terms of approach and avoidance behaviors. In a study designed to assess the emotional function of SM, experienced clinical psychologists blind to SM’s background, noted that “she did not seem to have a normal sense of distrust and ‘danger’” (Tranel, Gullickson, Koch, & Adolphs, 2006). On the flip side, increased amygdala activation has been associated with social phobia potentially characterized by social avoidance (Stein, Goldin, Sareen, Zorrilla, & Brown, 2002). Another pertinent finding is that the amygdala appears to be important for judging the actions of others as intentionally deceptive (Grezes, Berthoz, & Passingham, 2006; Grezes, Frith, & Passingham, 2004) and thus worthy of distrust. The amygdalae may play an important role in guiding behavior in social situations by assessing the value (not simply valence, but a non-linear combination of valence and magnitude) of social interaction partners, particularly in terms of acquiring the knowledge of whom to engage and whom to avoid. This view is consistent with the observed role of the amygdalae in reward processing (Gottfried, O’Doherty, & Dolan, 2003; O’Doherty, 2004) and emotion (for a review see Phelps, 2005).
A growing body of literature indicates that the amygdala is important for oxytocin-induced increases in trust. Intranasal administration of oxytocin has been shown to increase interpersonal trust in humans as measured by the Trust Game, below (Kosfeld, Heinrichs, Zak, Fischbacher, & Fehr, 2005). Oxytocin administration likewise increases ratings of trustworthiness of faces, in a manner reminiscent of that described following amygdala lesions (Theodoridou, Rowe, Penton-Voak, & Rogers, 2009). Moreover oxytocin is known strongly modulate amygdala activity in rodents (e.g., Terenzi & Ingram, 2005) and in humans (Gamer, Zurowski, & Büchel, 2010; Kirsch et al., 2005).
Given the role of the amygdalae in social evaluation and trust in particular, we expect that the amygdalae will play an integral role in forming normal relationships of mutual trust in real-life as well as in experimental paradigms that capture the development and expression of interpersonal trust in the laboratory. The Trust Game (TG), is a multi-round, multiplayer economic exchange that involves both interpersonal trust, i.e., trusting that the opponent will return a profit on an investment, and reciprocation, i.e., whether or not to betray or repay trust. The TG captures and quantifies behavior that intersects all of the purported amygdala functions, including social behavior and reward processing, given the interpersonal exchange aspect of the TG, the potential to develop a highly rewarding monetary gain by developing trust over time, and the potential for punishment and betrayal of trust.
Using various different versions of the TG, ranging from multiplayer, multi-round versions (e.g., King-Casas et al., 2005; Sripada et al., 2009) to one-shot versions (e.g., van den Bos, van Dijk, Westenberg, Rombouts, & Crone, 2009) to versions where roles are dynamically switched (e.g., Krueger et al., 2007), functional magnetic resonance imaging (fMRI) studies have not consistently shown changes in amygdala activation during the TG. One study reported a decrease in amygdala activation following oxytocin administration in relation to a lack of change in trust following betrayal (Baumgartner, Heinrichs, Vonlanthen, Fischbacher, & Fehr, 2008). Another study showed amygdala activation during a version of the TG in which investors were allowed to enact sanctions in the case of betrayals (Li, Xiao, Houser, & Montague, 2009). Other studies, with somewhat similar tasks, i.e., the Prisoner’s Dilemma or Ultimatum Game, have more consistently shown activation of the amygdalae during such tasks (Hsu, Bhatt, Adolphs, Tranel, & Camerer, 2005; Rilling et al., 2007; Rilling et al., 2008; Suzuki, Niki, Fujisaki, & Akiyama, 2010); however, these tasks have some very different rules and procedures than the TG.
These inconsistent (and with many null) findings may reflect the specific instructions and procedure, given the varied nature of different versions of the TG. However, we do not find this a parsimonious explanation, as all versions of the TG likely require many of the same cognitive operations, particularly within the realm of social evaluation. The lack of amygdala involvement may be due to investigators not probing the amygdala carefully, although this does not seem an important factor given that none of these studies identified brain regions of interest a priori. It may be that functional neuroimaging methods may not reveal brain regions that are activated in both conditions of the activation subtractions, i.e., chronically activated regions may be ‘washed out’ despite being integral throughout the task. Our study sought to help resolve some of these inconsistencies in the literature, by using a lesion-deficit approach to investigate whether the amygdala is necessary for normal interpersonal trust.
We predicted that the human amygdalae are necessary for developing normal social interactions that require the development of mutual interpersonal trust as in the TG. Previous reports of lesion populations described above (see Adolphs, et al., 1998) found a significant effect in cases of bilateral but not unilateral amygdala damage on trustworthiness ratings for faces. In the current study, we have a much larger sample size (32 as opposed to 7 unilateral cases of amygdala damage) and a potentially more ecologically valid, behavioral paradigm (the TG as opposed to facial judgments). We expected unilateral amygdala damage to affect trust behaviors in a qualitatively similar manner, though potentially modulated by both sex of the participant and laterality of the brain lesion. In order to simulate the development of trust over time, we employed a version of the TG in which participants play against the same opponent for multiple rounds. ‘Naïve’ trust may form based on an individual’s expectations when interacting with a new social partner—however, many social interactions, particularly those that are most relevant to our personal well-being, e.g., romantic partners, friendships, would-be cheaters, etc., occur through repeated, reciprocal interactions between the same individuals. We hypothesized that amygdala damage will interfere with normal patterns of interpersonal trust and reciprocity, such that betrayals of trust will not result in decreases in reciprocity as is expected in brain damaged and healthy comparison groups. Instead, amygdala damage will result in increases in reciprocity despite betrayals of trust, which could be taken as evidence of abnormal interpersonal trust.
Methods
Participants
Participants consisted of three main groups: men and women with adult-onset focal brain damage that includes the amygdala in one hemisphere (AMG group; N=32), men and women with adult-onset focal brain damage that excludes amygdala, ventromedial prefrontal and insular cortex (brain damage comparison participants, BDC group; N=48), and neurologically normal adults (normal comparison participants, NC group; N=59). Participants with damage to the insular or ventromedial prefrontal cortex were excluded, as damage to these regions may impact performance on the Trust Game, given the roles of these areas in social and emotional processing. All participant groups were comparable in age, were predominately right-handed, and had a similar level of education (see Table 1).
Table 1.
The BDC group is significantly older (p=0.014, indicated by the *) than the other groups. Years of education does not differ between groups (F=1.392, p=0.252). Groups with brain damage differ in terms of lesion etiology, where amygdala damage is most likely due to temporal lobe resection, whereas in the BDC group damage is most likely due to cerebral vascular accident.
| Demographics | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Age (years) | Handedness | Education (years) | Lesion Etiology | Years from Lesion Onset | ||||||||
| Right | Left | Mixed | ATL | CVA | Tumor | HSE | |||||||
| AMG | Overall | 32 | 48.5 | 84% | 9% | 6% | 14.3 | 88% | 3% | 3% | 6% | 7.5 | |
| Women | Overall | 21 | 49.2 | 81% | 14% | 5% | 14.2 | 95% | 5% | 0% | 0% | 6.2 | |
| Right | 7 | 49.5 | 86% | 14% | 0% | 15.3 | 86% | 14% | 0% | 0% | 5.1 | ||
| Left | 14 | 49.1 | 79% | 14% | 7% | 13.6 | 100% | 0% | 0% | 0% | 6.8 | ||
| Men | Overall | 11 | 47.1 | 91% | 0% | 9% | 14.4 | 73% | 0% | 9% | 18% | 10.0 | |
| Right | 3 | 56.0 | 67% | 0% | 33% | 14.7 | 33% | 0% | 0% | 67% | 13.3 | ||
| Left | 8 | 43.8 | 100% | 0% | 0% | 14.3 | 88% | 0% | 13% | 0% | 8.8 | ||
| BDC | Overall | 48 | 57.9* | 88% | 8% | 4% | 14.7 | 2% | 85% | 13% | 0% | 8.2 | |
| Women | 22 | 55.0 | 86% | 5% | 9% | 14.4 | 0% | 86% | 14% | 0% | 7.8 | ||
| Men | 26 | 60.3 | 88% | 12% | 0% | 15.0 | 4% | 85% | 12% | 0% | 8.6 | ||
| NC | Overall | 59 | 50.6 | 93% | 5% | 2% | 15.2 | - | - | - | - | - | |
| Women | 32 | 46.9 | 97% | 3% | 0% | 14.2 | - | - | - | - | - | ||
| Men | 27 | 55.1 | 89% | 7% | 4% | 16.4 | - | - | - | - | - | ||
| Overall | Women | 75 | 49.9 | 89% | 7% | 4% | 14.2 | 47% | 47% | 7% | 0% | 7.0 | |
| Men | 64 | 55.8 | 83% | 9% | 8% | 15.5 | 24% | 59% | 11% | 5% | 9.0 | ||
ATL = anterior temporal lobectomy, CVA = cerebrovascular accident (ruptured aneurysm, ischemic stroke, hemorrhage), HSE = herpes simplex encephalitis.
Participants with brain damage have, for the most part, intact psychometric intelligence, memory, executive function, and verbal ability (see Table 2). Likewise, time since lesion onset was similar for both AMG and BDC groups in the chronic phase of recovery from brain injury. Brain-damage groups differ in lesion etiology; in the AMG group lesions were most often due to medial temporal lobe resection for pharmacoresistant epilepsy, whereas the BDC group lesions were primarily due to cerebral vascular accident (including stroke, hemorrhage, and aneurysms). The remainder of the lesions were due to tumor resection (more common in the BDC group) or herpes simplex encephalitis (in some of the men in the AMG group) (see Table 1). All lesions were stable and could be clearly identified on magnetic resonance or computerized tomographic images. All lesions were visually inspected blind to the experimental results, lesion location, and lesion etiology. Participants were assigned to the amygdala group for analysis if at least one of their amygdalae was clearly damaged or removed entirely or if the amygdala was clearly and completely undercut of surrounding tissue. Participants were included in the BDC group if both amygdalae were completely intact and not undercut, and if there was no damage to ventromedial prefrontal cortex or insula.
Table 2.
Participants were equivalent on most neuropsychological measures. The AMG group had significantly lower score on the Boston Naming Test, though this is within normal range of performance.
| Neuropsychology | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WAIS |
BDI | BAI | AVLT | WCST | Facial Disc. | COWA | BNT | |||||
| FSIQ | VIQ | PIQ | ||||||||||
| AMG | Overall | 102 | 98 | 107 | 9.5 | 12.7 | 25.3 | 4.6 | 45.0 | 40.5 | 49.3* | |
| Women | Overall | 101 | 98 | 106 | 9.9 | 12.1 | 25.8 | 4.5 | 45.1 | 40.5 | 50.5 | |
| Right | 114 | 108 | 118 | 6.5 | 8.0 | 23.6 | 5.6 | 46.3 | 41.6 | 57.3 | ||
| Left | 97 | 94 | 102 | 11.7 | 14.1 | 27.2 | 4.0 | 44.5 | 39.9 | 46.5 | ||
| Men | Overall | 105 | 100 | 112 | 8.6 | 14.2 | 23.9 | 4.7 | 44.6 | 40.6 | 46.3 | |
| Right | 110 | 104 | 118 | 7.0 | - | 27.0 | 1.5 | 44.7 | 44.3 | 53.0 | ||
| Left | 102 | 98 | 108 | 9.6 | 14.2 | 22.6 | 6.0 | 44.5 | 38.4 | 43.6 | ||
| BDC | Overall | 103 | 104 | 103 | 9.8 | 6.9 | 25.9 | 5.2 | 45.8 | 35.8 | 54.9 | |
| Women | 101 | 102 | 102 | 11.1 | 8.9 | 28.4 | 4.9 | 44.2 | 35.0 | 55.5 | ||
| Men | 105 | 106 | 104 | 8.4 | 4.2 | 23.5 | 5.5 | 47.2 | 36.5 | 54.3 | ||
| Overall | Women | 101 | 100 | 104 | 10.6 | 10.2 | 27.2 | 4.7 | 44.6 | 37.6 | 53.2 | |
| Men | 105 | 105 | 106 | 8.4 | 7.0 | 23.6 | 5.3 | 46.6 | 37.5 | 52.5 | ||
WAIS = Wechsler Adult Intelligence Scale-III, FSIQ = Full Scale Intelligence Quotient, VIQ = Verbal Intelligence Quotient, PIQ = Performance Intelligence Quotient, BDI = Beck Depression Inventory, BAI = Beck Anxiety Inventory, AVLT = Rey Auditory Verbal Learning Test, 30-minute recognition score, correct + correct rejections, WCST = Wisconsin Card Sorting Test, number of categories completed, Facial Disc. = Facial Discrimination Test, COWA = Controlled Oral Word Association Test, BNT = Boston Naming Test. For all neuropsychological measures, references can be found in Tranel (2009).
We are particularly interested in the possible influences of the sex of the participant as well as which hemisphere is damaged. Previous research has suggested that the left amygdala may be critical for women and the right amygdala may be critical for men for social decision-making, emotions and emotional memory, and social conduct (Cahill, Uncapher, Kilpatrick, Alkire, & Turner, 2004; Kilpatrick, Zald, Pardo, & Cahill, 2006; Tranel & Bechara, 2009). Moreover, previous research suggests that men and women may differ in behavior when playing trust games; for example women may tend to show higher levels of reciprocity (Croson & Buchan, 1999). Participant groups consist of both men and women; the AMG group is further divided into men and women with damage to either the left or right amygdala, to examine the possibility that the right amygdala may be critical for men where the left amygdala may be critical for women for developing interpersonal trust. Participants with brain damage were selected from the Patient Registry of the Division of Behavioral Neurology and Cognitive Neuroscience in the Department of Neurology at the University of Iowa (Iowa City, IA). All participants were free of dementia, psychiatric disorder, substance abuse, and significant intellectual impairments. Normal comparison participants were recruited from the Iowa City area through advertisement, and were compensated for their participation. All participants provided informed consent prior to participation in accordance with the Institutional Review Board of the University of Iowa.
Procedure
All participants completed a multi-round, multiplayer version of the Trust Game. One player took the role of ‘Investor’ and the other player took the role of ‘Trustee.’ At the beginning of each round the Investor was given $20 and was asked to decide how much of this money to keep for themselves and to how much they would like to give to the other player. The Investor could divide this money any way they saw fit in whole dollar amounts. The money that they gave to the Trustee was tripled, then the Trustee decided how much of this tripled investment to keep for themself and how much to return to the Investor, again in whole dollar amounts. This version of the TG is adapted from the multi-round version of the TG used by King-Casas, et al. (2005), except that instead of playing a human opponent, the other player was simulated, and all participants played as both the Trustee and the Investor. During the game and the instructions leading up to the game, the opponent of the participant was always referred to as the ‘other player’. Participants were then informed of the Trustee’s decision, and a new round began with the Investor being given $20 more and was asked to divide it in the same way (see Fig. 1).
Fig. 1. The Trust Game.
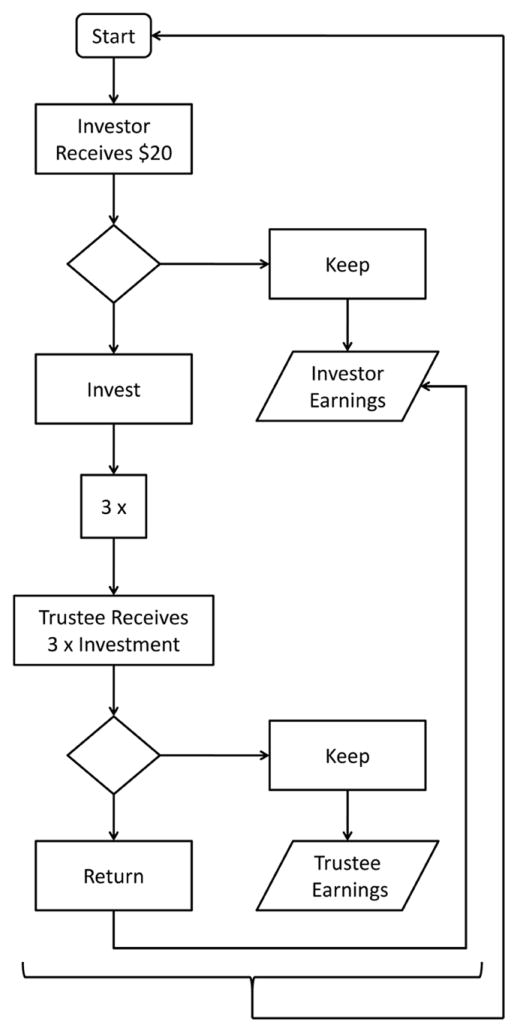
This flowchart depicts the order in which events occur during a given trial in this version of the Trust Game. Diamonds represent decisions of either the Investor or Trustee. Each round begins with the Investor receiving $20. The Investor decides how much to keep for themselves and how much to invest in the other player. The amount they invest is tripled, and then the Trustee decides how much of this tripled amount to keep for themselves and how much to return to the other player.
The participants first played the role of ‘Trustee’ for 20 rounds then were given a short break, where the instructions for the next version were given, and then played a further 20 rounds as the ‘Investor.’ Between versions, participants were instructed that they would “switch roles with the other player.” Given that behavior in the TG has a relatively high degree of inter-individual variability and that behavior is determined in large part by the actions of the other player, we chose to reduce the effect of inter-individual variability by having all participants have the same opponent such that we could isolate the behaviors of the participant while minimizing the variability from the opponent. This simulated opponent differs from the commonly used computer opponents in other research (e.g., McCabe, Houser, Ryan, Smith, & Trouard, 2001), in that it does not follow a pre-determined probabilistic response that is known to the participant. The simulated opponent responds dynamically in a ‘Tit-for-Tat’ manner to the behavior of the participant (see Appendix for simulated player rules). The simulated opponent was always referred to as “the other player;” and in cases where the participant inquired about the other player’s identity they were informed that they would be told following completion of the task.
Participants selected how much to keep for themselves and how much to give to the other player by sliding a horizontal scroll bar from left to right, varying from the default position at the far left to keep all of the available money to the opposite position at the far right to give all of the available money. In the first version, where the participant is the Trustee, the player saw a text description of how much they had given to the other player, how much this became after it was tripled, and asked to briefly wait while the other player decided how much to return to them. Once the other player had ‘decided’ how much to return (~6s), the participant received feedback including how much money the other player had returned, and how much money they earned on that round. On the screen there was a running tally for both the total amount earned by the Investor and Trustee, as well as the amount of the previous investment and previous return. The next round began when the participant clicked a button on the screen to continue.
The second version, where the participant was the Investor, each round began with the participant being given $20 and they were asked to divide this amount as they saw fit, using a horizontal slider similar to the one when deciding on their return amount in the first version of the task. They received feedback on how much they gave, and what the tripled amount was, then asked to wait for the other player to decide how much to return to them (~6s). After this had been decided, the participant was informed of the amount of money they earned in that round, including the amount returned to them from the other player, and then asked to press a button when they are ready to continue. The TG was implemented in Matlab® (r2007b, The Mathworks) and displayed on a 17” LCD monitor. All responses were made via mouse input using the dominant hand of the participant, except in cases where the dominant hand was limited in mobility as a result of their brain injury.
As a manipulation check following completion of both versions of the TG, participants answered a short questionnaire to assess their opinions and perceptions of the other player, using a series of 5-point Likert scales. These measures include: 1. how greedy or generous was the other player? 2. How human-like or computer-like was the other player (to assess how believable the simulated player responded)? 3. How trustworthy or deceitful was the other player? 4. How fair or unfair was the other player? 5. How quick or slow was the other player?
Measures
Given the complicated nature of the Trust Game, there are many variables of interest that can be extracted from the rich dataset that it provides. Based on previous literature, especially that of King-Casas, et al. (2005), using the TG and similar games, we derive two dependent variables that capture the nature of the financial interchange between the players over time. It is important to consider how one’s responses change in relation to changes in the other’s responses, as neither occur in isolation within this dyadic interaction. For example, a negative return or investment may be entirely appropriate and adaptive when used in conjunction or response to a decrease from the other player. Likewise an increase in investment or return may be maladaptive if it occurs in a fashion that allows the other player to take advantage of the other person’s kindness.
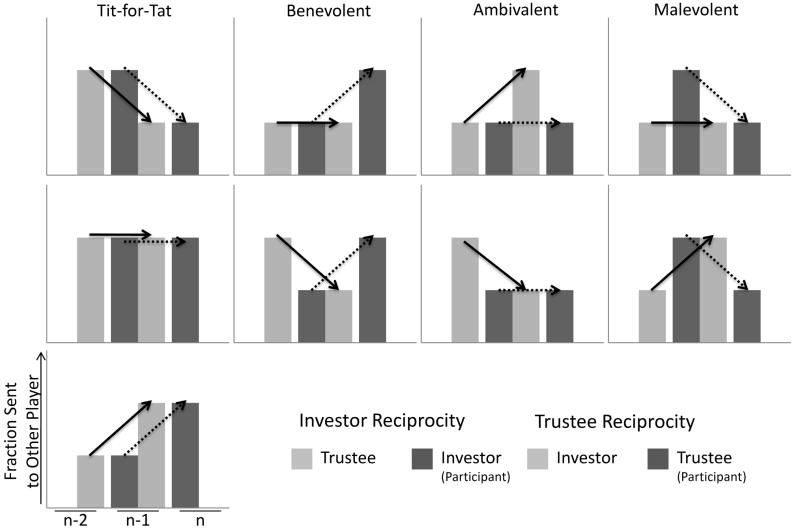
Our first dependent variable is the amount that the Investor changed their investment based on the change in the amount that the Trustee returned to them; referred to as ‘Investor reciprocity’ as defined in the research by King-Casas and colleagues (2005). Since our participants play both roles in the TG, we differentiate reciprocity when the participant was the Investor from reciprocity when the participant was the Trustee. We define our second dependent variable as the change in the Trustee’s return in response to the amount the Investor changed their investment, which we define as ‘Trustee reciprocity.’ We adapted and improved upon the classification schemes in the literature. Previous reports broke reciprocity into three bins, ‘Benevolent’, ‘Neutral’, and ‘Malevolent’ forms in contrast to a ‘Tit-for-Tat’ type of reciprocity (see King-Casas, et al., 2005). The definitions of these forms of reciprocity neglect an ‘ambivalent’ interaction type, where the participant did not change their response in relation to a change in response from the other player. Moreover, neutral reciprocity may be appropriately grouped with tit-for-tat reciprocity as the participant responds to the other player in kind. Here we define five types of reciprocity: 1. Tit-for-Tat reciprocity refers to participants’ change in money sent to the other player that mirror the changes in the amount sent from the other player. 2. Benevolent reciprocity refers to the cases where the amount of money sent by the participant increases in response to a decrease or no change in the amount of money sent by the other player. 3. Ambivalent reciprocity refers to no change in the amount of money sent by the participant despite changes in the amount sent from the other player. 4. Malevolent reciprocity refers to instances where the participants’ decrease the amount of money sent to the other player despite increases or no change in money sent from the other player (see Fig. 2).
Fig. 2. Reciprocity Types.
Tit-for-Tat: When the participant changes the amount they send in the same direction as the other player, either increased, decreased, or no change.
Benevolent: When the participant increases the amount sent to the other player despite a decrease or no change in the amount sent by the other player.
Ambivalent: The participant does not change the amount they send despite increases or decreases in the amount sent by the other player.
Malevolent: The participant decreases the amount sent to the other player despite an increase or no change in the amount sent by the other player.
n = the current trial.
Previous research suggests that tit-for-tat strategies are the normative response in the TG, e.g., King-Casas, and colleagues (2005) reported that reciprocity was the strongest predictor of subsequent changes in trust. From the TG we can thus derive an operational definition of interpersonal trust, where mutual trust is exemplified by mutual increases in the amount sent to the other player, mutual distrust as mutual decreases in the amount sent, both of which are manifest within a tit-for-tat strategy use. Misplaced or inappropriate trust may be exemplified by the use of benevolent reciprocity, where the person trusts the other player despite the other’s defection.
Here we define the observed, dominate, reciprocity strategy as responding with a certain type more the 30% of the time. In the cases where more than one type of reciprocity was observed on more than 30% of trials, the dominant strategy was classified according to the rules in Table 3.
Table 3.
Dominant reciprocity strategies were defined as follows. When a person used only one strategy more than 30% of the time, they were defined as using that strategy. In cases where more than one strategy was used more than 30% of the time theses rules were used to classify their strategy use. The rules written in bold and italicized, supersede the other rules.
| Reciprocity Strategy | Strategy Classification Rules (% of Trials) |
|---|---|
| Tit-for-Tat | > 50% Tit-for-Tat |
| Benevolent | > 50% Benevolent |
| > 30% Benevolent & >30% Tit-for-Tat | |
| > 30% Benevolent & >30% Ambivalent | |
| > 30% Benevolent & >30% Tit-for-Tat & >30% Ambivalent | |
| Ambivalent | > 50% Ambivalent |
| > 30% Ambivalent & >30% Tit-for-Tat | |
| Malevolent | > 50% Malevolent |
| > 30% Malevolent & >30% Tit-for-Tat | |
| > 30% Malevolent & >30% Ambivalent | |
| Sporadic | > 30% Benevolent & >30% Malevolent |
| > 30% Tit-for-Tat & >30% Benevolent & >30% Malevolent | |
| > 30% Benevolent & >30% Malevolent & >30% Ambivalent | |
| < 30% of any particular type | |
Statistical Analysis
To analyze our data, we used several approaches given the diverse nature of our variables of interest. To analyze group differences in demographic variables, we used one-way analysis of variance (ANOVA) where group was the independent variable and the demographic variable was the dependent variable. When comparing neuropsychological variables, we utilized independent samples t-tests to compare the AMG group to the BDC group. All ANOVAs were implemented in SPSS 17.0 (SPSS Inc., 2008). Many of our data consist of categorical variables, so we employed a two pronged approach to our analysis of categorical data. First, we constructed contingency tables for the variable in question, where the rows represented the groups and columns represented the categories of the dependent variable in question. Then we employed either a Chi-square test or a Fisher’s Exact Test if the assumptions of the Chi-square test were not met (e.g., <80% of cells are <5). All analyses on contingency tables were implemented in R (R Development Core Team, 2008). Second, since a Chi-square test does not provide any information on the relationship between variables, just that the whole pattern is different or not, we employed a Correspondence Analysis (Abdi & Williams, 2010) implemented in Matlab. The benefit of this method is that it provides a simple visualization of the relationship of the variables by allowing them to be plotted in a common space, such that variables (either from the rows or columns) that cluster together are related. The potential downside of correspondence analysis is that it does not provide a significance test, rather is a qualitative type of data exploration. By using a combination of traditional statistical methods that provide a significance test and with a method that provides a qualitative visualization of the relationship between variables, we can extract the most meaningful information from our categorical data.
Results
The groups did not differ significantly in level of education (F=1.392, p=0.252). The BDC group was significantly older than both AMG and NC groups (both p<0.017). The AMG group and the BDC group were not significantly different on most neuropsychological variables, including Full Scale IQ (t=-0.385, p=0.701), Performance IQ (t=1.173, p=0.245), Verbal IQ (t=-1.317, p=0.193), Beck Depression Inventory score (t=-0.124, p=0.901), Beck Anxiety Inventory score (equal variances not assumed, t=1.755, p=0.095), Rey Auditory-Verbal Learning Test 30-minute recognition score (equal variances not assumed, t=-0.390, p=0.698), Wisconsin Card Sorting Test number of categories completed (equal variances not assumed, t=-1.203, p=0.237), Facial Discrimination Test score (t=-0.604, p=0.548), and Controlled Oral Word Association Test score (t=1.602, p=0.114), equal variances were assumed unless otherwise specified. Participants with amygdala damage did score significantly lower on the Boston Naming Test compared to BDCs (t=2.558, p=0.015). BDC and AMG groups did not differ in time since lesion onset (t=-0.964, p=0.338).
In response to our manipulation check, the NC group rated the simulated player as significantly more computer-like than the BDC group (p=0.030) but not the AMG group (p=0.276). The BDC and AMG groups did not differ in how human- or computer-like they thought the other player was (p=0.354). The groups did not differ in how greedy/generous, trustworthy/deceitful, fair/unfair, quick/slow they thought the other player was (all p’s>0.2).
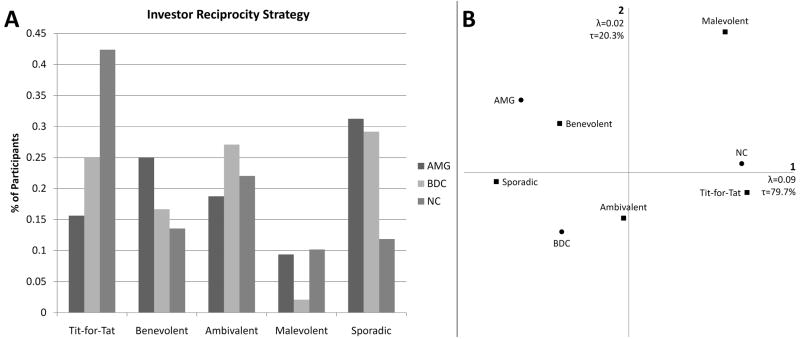
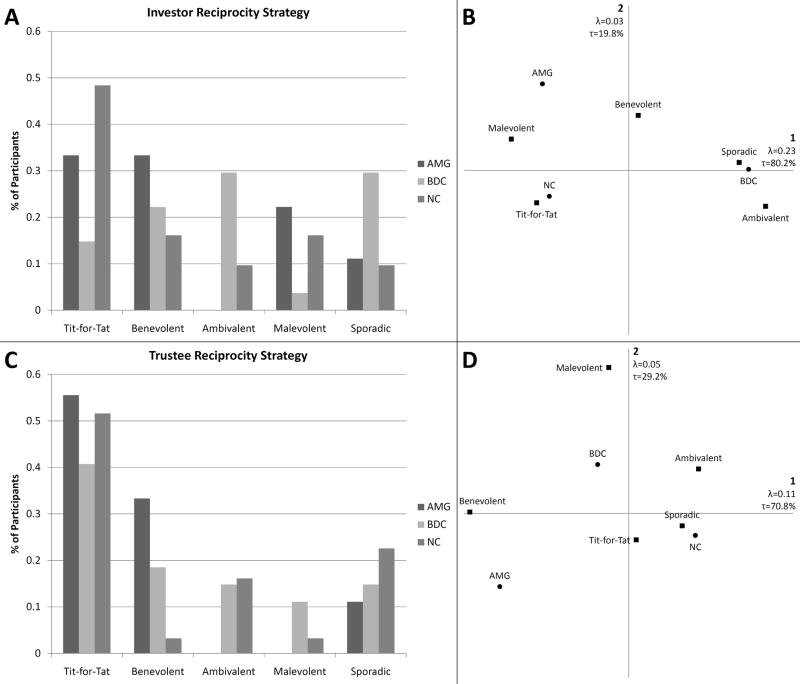
We observed significant differences between groups in terms of investor reciprocity strategy use (χ2=15.509, p=0.050) (see Fig. 3A). Correspondence analysis revealed that the AMG group uniquely clusters with a benevolent strategy and perhaps sporadic strategy use. The BDC group clustered with ambivalent strategy use and also close to sporadic strategy use. The NC group on the other hand clustered closely with use of a tit-for-tat strategy. Malevolent investor reciprocity strategy use distinctly clustered away from all groups and all other strategies (see Fig. 3B).
Fig. 3. Investor Reciprocity Strategy.
A. Chi-square test reveals significant differences between groups in terms of investor reciprocity strategy use (p=0.050). B. Correspondence analysis reveals a tight clustering of NCs with Tit-for-Tat strategy use, a loose relationship of BDCs somewhere between sporadic and ambivalent strategy use, and relationship between the AMG group and benevolent strategy use.
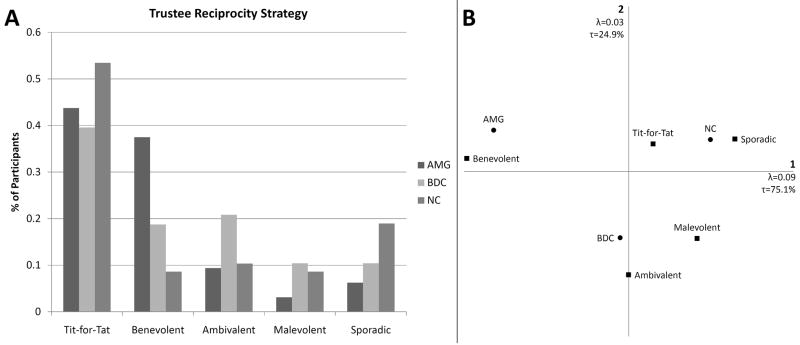
When we examined trustee reciprocity, we observed a significant difference in the pattern of strategy use between groups (χ2=17.242, p=0.028) (see Fig. 4A). Similar to what we observed for investor reciprocity, the AMG group uniquely clustered with benevolent strategy use. BDCs clustered with ambivalent strategy use, and NCs clustered with tit-for-tat and sporadic strategies (see Fig. 4B).
Fig. 4. Trustee Reciprocity Strategy.
A. Chi-square test reveals significant differences between groups in terms of trustee reciprocity strategy use (p=0.028). B. Correspondence analysis reveals a distinct clustering of the AMG group with a benevolent strategy, BDCs clustered closest to ambivalent strategy use, and NCs clustered somewhere between sporadic and tit-for-tat strategy use.
To summarize our main findings in the three groups: (1) Participants with amygdala damage displayed a tendency to utilize benevolent strategies. (2) The brain damage comparison group had a tendency toward ambivalent reciprocity strategies. (3) The normal comparison group tended to use tit-for-tat strategies. Given that the NC group rated the simulated opponent as being more computer-like than the BDC group, the NC group might be expected to be more ambivalent to the outcome for the computer player. This does not seem to be the case, since the NC group tends to use a tit-for-tat strategy and the BDC group tends to be more ambivalent.
For our secondary analyses, we were interested in examining the effects of sex of the participant and laterality of amygdala injuries. In addition, we were interested in exploring potential differences due to age given the observed difference in our BDC group. First, we divided the subjects into groups of men and women irrespective of brain injury status, and we found no significant differences between men and women on any variables. Likewise, we did not find any differences between the performances of men with amygdala damage and women with similar damage, nor did we find sex differences within BDC and NC groups. Within the AMG group we did not find any significant group effects for any of the variables when we compare men and women with left or right amygdala damage.
Since the BDC group differed in age, we examined whether or not age significantly affected strategy use. To analyze the possible effects of age on strategy use, we split the participants approximately at the median age (median=54.4 years old) such that we could compare the AMG, BDC, and NC groups within adults younger than 55 years old and within adults 55 years old or older.
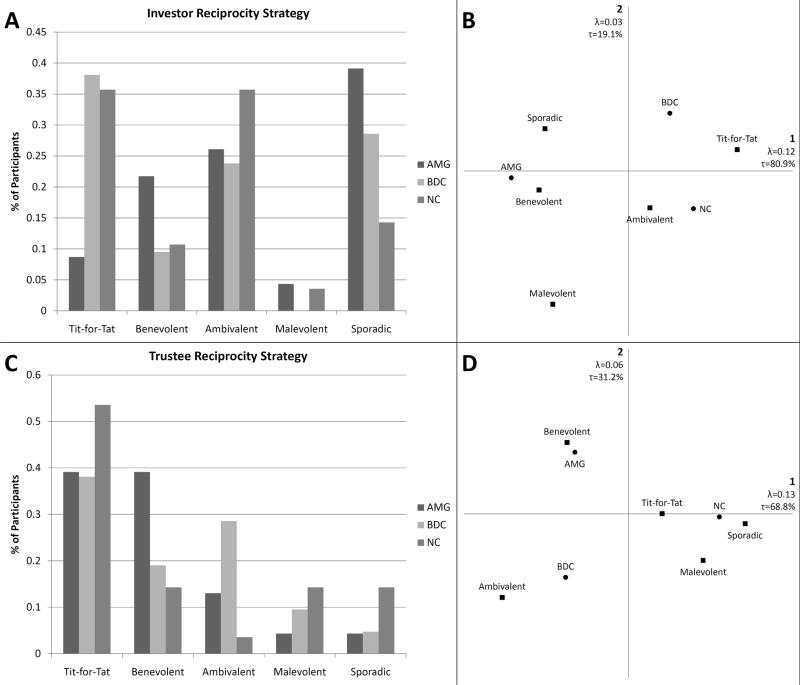
Younger adults did not display a significant effect of group on investor reciprocity (p=0.154) (see Fig. 5A). The AMG group clustered with a benevolent strategy; the BDC group did not cluster closely with any particular strategy but closest to tit-for-tat strategy use; and the NC group clustered nearest to ambivalent and tit-for-tat strategy use (see Fig. 5B). The older adults displayed a significant effect of group on investor reciprocity (p=0.017) (see Fig. 6A). In the older adults, we observed a tight cluster between the NC group and tit-for-tat strategy use, and BDCs and sporadic/ambivalent strategy use; whereas the AMG group did not cluster strongly with any strategy use but closest to malevolent and benevolent strategies (see Fig. 6B). The younger adults were significantly different from older adults in the AMG group (p=0.048), but not the BDC (p=0.331) or the NC group (p=0.100).
Fig. 5. Younger Adults.
A. There were no significant differences between groups within the younger adults on investor reciprocity strategy use or C. trustee reciprocity strategy use. However we see a consistent pattern where the AMG group forms a distinct cluster with benevolent reciprocity (both B. investor and D. trustee) strategy use.
Fig. 6. Older Adults.
A. We also observe significant between groups differences in terms of investor reciprocity strategy use (p=0.017), where B. NCs cluster closely with tit-for-tat strategy use, BDCs cluster with sporadic strategy use, and the AMG group clusters somewhere between malevolent and benevolent strategy use. In terms of trustee reciprocity C., older adults do not exhibit significant between groups differences (p=0.264). D. Correspondence analysis reveals that the basic pattern of the AMG group clustering closest to benevolent strategy use is retained in the older adults.
We did not find a significant effect of group on trustee reciprocity in either younger adults (p=0.130) (see Fig. 5C) or older adults (p=0.2638) (see Fig. 6C). Correspondence analysis revealed that the AMG groups retained a consistent relationship with benevolent strategy use, where the younger and older adults were not significantly different (p=0.744). Within the BDC group, the younger adults were most closely related to ambivalent strategy use, where the older adults did not cluster with any strategy, though the younger and older adults in the BDC groups were not significantly different (p=0.736). The NC group younger adults and older adults consistently clustered with tit-for-tat and sporadic strategies (p=0.170) (see Fig. 5D for younger adults and Fig. 6D for older adults).
In summary, younger BDC participants appeared to be more likely to use a tit-for-tat investor reciprocity strategy than older adults (making them consistent with the NC group as a whole) where the older BDC participants responded with a sporadic use of strategies. The AMG group retained a basic relationship with benevolent investor reciprocity strategy, but this association was weaker in older adults compared to younger adults. In terms of trustee reciprocity strategy use, the AMG group retained its closest relationship to benevolent strategy regardless of age, and the NC group clustered with a tit-for-tat strategy. The BDC group appeared to deploy trustee reciprocity strategies more randomly than the other groups regardless of age.
Discussion
Our data are consistent with a role for the amygdalae at the intersection of emotion and social behavior. At the heart of the Trust Game is a continually evolving relationship between Investor and Trustee. Each decision builds on the individual’s goals and strategies that are influenced by and deployed in relation to the behavior of the other player. There are several crucial factors in TG decision-making, including how much to ‘trust’ others, how to respond the actions of others, and how to develop a successful (if not short-term) social interaction. Similarly in real-life situations, proper appraisal of the value of a social interaction as well as production of appropriate and adaptive responses play important roles in creating, developing, and sustaining meaningful social relationships that have the potential for mutual benefit of all social partners.
We report here that normal healthy adults tend to use a Tit-for-Tat strategy in TG interactions. In other words, normal healthy adults tended to respond to the actions of others in kind, if someone’s trust is betrayed then they should be punished, conversely if someone expresses mutual trust this should be rewarded by a consistent expression of trust.
In contrast, participants with damage to the amygdala did not tend to respond in kind to betrayals or expressions of trust. Instead, following amygdala damage, participants tended to act in a benevolent manner and expressed their trust of another individual even when their trust had been betrayed. This potentially places these participants at risk of being taken advantage of by those who would abuse an overly trusting individual.
Participants with brain damage that excluded the amygdala (VMPC and insular cortex as well), displayed a different pattern than both participants with amygdala damage and those that are neurologically normal. These participants tended to be unresponsive, or ambivalent, to the betrayals or expressions of trust of the other player in the Trust Game. This does not necessarily put them at risk of being taken advantage of, as in the situation of benevolence observed for the participants with amygdala damage; however it may preclude the development of a mutually beneficial relationship. In this vein, such interpersonal behavior could encumber normal, productive, positive interpersonal exchanges, and in this way, contribute to general inadequacies and anomalies that typify many patients with brain damage. Further testing and more specific analysis of the underlying neuroanatomy of this ambivalent behavior may reveal other brain regions that are involved in social interactions.
Our results are consistent with the role the amygdala plays at the intersection of emotion and social decision-making. We speculate that damage to the amygdala results in a lack of proper evaluation of a social situation, i.e., not extracting the appropriate value information from the behaviors of others. By not attaching the appropriate value, in this case attaching a negative value to the betrayals of trust, the individual activates inappropriately positive responses. This view is consistent with the long-standing observations that the amygdala is involved in fear processing and response. Likewise, amygdala damage often results in inappropriate response to normally, fear-inducing stimuli, reductions in negative affect, and inappropriate social behavior (for reviews see Adolphs, 2010; Costafreda, Brammer, David, & Fu, 2008; Sergerie, Chochol, & Armony, 2008).
Our results strongly support the notion that the amygdala is a region which does much more than process the valence of faces. Much of the literature on trustworthiness involves extracting and processing cues to trustworthiness from the perception of faces (e.g., Adolphs, et al., 1998; Engell, et al., 2007). This is likely in part due to both the ease with which facial stimuli can be created and manipulated and the fact that paradigms that involve face viewing are relatively simple to implement within the technological confines of a magnetic resonance scanner. In contrast, the version of the TG presented here, provides no visual information as to the trustworthiness of others though trustworthiness can and should be inferred from the overt behavior of the interaction partner. Our findings, that the amygdala is necessary for developing interpersonal trust in the complete absence of visual social cues such as faces, extend previous research beyond a role for the amygdala in extracting social value from faces to inferring and computing social value from the behaviors of others. Despite the fact that the findings of the current study extend and support the literature on trustworthiness in facial processing, the discrepancy between our study and the fMRI literature on the TG, which has not routinely observed amygdala activation, remains puzzling.
To reconcile our results with the fMRI literature we will focus on the work of King-Casas and colleagues (2005) as our version of the TG most closely resembles the version used by this group. These authors suggested that the head of the caudate nucleus “receives or computes” whether or not the other player’s decision was fair and the individuals intention to trust. Our data are consistent with the caudate receiving this information but not in its computation.
There may be a logical reason to exclude the possibility that the caudate is computing the value of responses in the TG given the physical correlates of the blood-oxygen-level dependent (BOLD) signal itself. BOLD signal changes are related to synaptic activity in the dendrites of neurons and not their spiking activity (Logothetis, 2008; Logothetis, Pauls, Augath, Trinath, & Oeltermann, 2001). A brain region that computes the difference between A and B will not show a difference in the BOLD signal when contrasting A-B, since this region will have similar synaptic activity in the dendrites when computing A and computing B and differ only in spiking output activity. However, a region that differentially receives information of A or B can show a change in BOLD activation when contrasting A-B, since this will result in differential synaptic activity in the dendrites of this region. As an example, suppose we present a visual stimulus where in condition A, a series of lines move across the screen where in condition B, the same lines are static and do not move. A contrast of the activations of A-B would reveal increased activity in motion detection regions such as area MT where area V1 would be activated by both conditions and would not be activated in a contrast of A-B. However, it would be false to conclude that area V1 is not necessary for motion perception from the A-B contrast.
In the case of activations that are greater for benevolent than malevolent reciprocity, the brain region that computes this information will receive virtually identical information (i.e., the amount returned or invested, the change from previous rounds and the overall magnitude of the financial transaction) regardless of the categorization of benevolence or malevolence, and since the BOLD signal depends largely on dendritic activity, there will be no difference observed between benevolent or malevolent reciprocity in the region that actually computes these values. In contrast, in the regions that receive this information, for example the caudate, the signal that a given response was benevolent may impinge with more power on these targets than when the response is categorized as malevolent. This information of the value of the response, which our data suggest is computed by the amygdala, is then utilized by the caudate nucleus to compute the “intention to trust” the other player (King-Casas, et al., 2005). Our results suggest that the amygdalae play an integral role in the evaluation of the value of social information, and the caudate plays an important role downstream from social evaluation in determining behavior, consistent with a role for the caudate in learning contingencies between behavior and reward (Delgado, Miller, Inati, & Phelps, 2005).
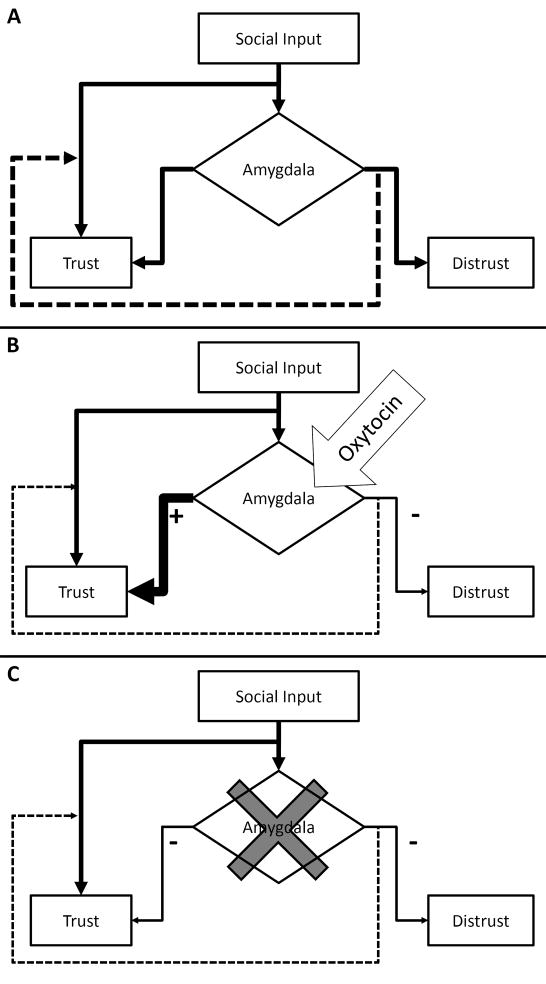
In terms of a model of the mechanisms behind deciding whether to trust or distrust another individual, our findings are consistent with a view that the amygdala evaluates incoming stimuli about a situation to decide whether to trust the individual in question or whether to distrust. However, to reconcile our findings with the literature on the role of oxytocin it may be necessary to include an alternative route to trust. Specifically, default behavior may be to trust, whereas distrust is dependent on amygdala evaluation of incoming stimuli as negative. Enhanced activations of the amygdala in relation to oxytocin administration inhibit evaluations of distrust and increase trust evaluations. On the other hand, lesions of the amygdala remove this evaluative step, leaving the default function of trust intact and dominant (see Fig. 7).
Fig. 7. A Model of the Role of the Amygdala in Trust.
A. In this model, people trust others as a default mode of behavior. The amygdala serves to evaluate incoming social stimuli to either enhance trust-related behaviors for positive evaluations, or to distrust the individual for negative evaluations. Dashed lines indicate inhibitory processes. B. This model is consistent with the literature on the effects of oxytocin, where amygdala activation and trust are enhanced by oxytocin administration. Oxytocin results in enhanced positive evaluations and inhibited negative evaluations. C. Amygdala lesions knock-out the evaluative process, resulting in default trust and lack of negative evaluations.
We did not observe any effects of sex or laterality of the damaged amygdala. This is surprising given the lines of converging evidence suggesting a potential right amygdala bias in men and left-amygdala bias in women (Koscik, Bechara, & Tranel, 2010). The main difficulty in the present study is the small numbers of participants in the groups once they are subdivided by sex and lesion side, e.g., leaving only 3 men with right amygdala damage. Moreover, of the men with right amygdala damage two of the three have damage due to herpes simplex encephalitis, which may affect other brain regions differently compared to a temporal lobectomy as in the majority of the other cases of amygdala damage. Similarly, matching groups versus matching individuals on demographic variables will potentially affect the results. Our groups are not significantly different neuropsychologically, i.e., between the amygdala and brain damaged comparison groups. However within each group there is a continuum of performance in each of these domains. To adequately compare within groups a case-matched approach would likely yield more valid results, however this is dependent on having more subjects in each subgroup. In future studies we hope to recruit and test an adequate number of men and women with left or right amygdala damage to satisfactorily test the sex related functional asymmetry hypothesis.
We did not expect any significant differences due to age, though we observe that age was negatively related to the amount of money earned. We do not find any overarching effects of age on strategy use except for a general weakening of our main group effects. Some of the effects were potentially approaching significance (p~0.1), and thus future studies focused on a representative sampling across the adult age-span might yield significant age effects on social strategy use.
In summary, we find that the amygdala is necessary for adaptive and appropriate social functioning at the intersection of evaluation and social interaction. Amygdala damage may result in maladaptive social behavior due to a lack of appropriate and accurate evaluation of social situations and social partners.
Acknowledgments
Supported by NINDS P50 NS19632 and NIDA R01 DA 022549
Appendix
The opponent in the TG was simulated, and responded to the input of the participant according to the following rules:
- When the other player was the Investor (i.e., the participant was the Trustee):
-
Base investment rate = 50% (of $20 possible)
- Every participant received $10 from the Investor on the first round.
- Maximum investment rate = 100%
- Minimum investment rate = 0%
- If Trustee returns $0, -30%
- If Investor total $ earned > Trustee total $ earned, +10% (Round 3+)
- If Investor total $ earned < Trustee total $ earned, -10% (Round 3+)
- Change proportional to the magnitude of investment, Previous Rate + Return Amount / (12 × Investment Amount)
- If Trustee increases return, +10%
-
- When the other player was the Trustee (i.e., the participant was the Investor):
-
Base return rate = 50%
- Every participant received 50% of the returnable amount on the first round.
- Maximum return rate = 75%
- Minimum return rate = 25%
- If investment = $0, -20%
- If Trustee total $ earned > Investor total $ earned, -10%
- If Trustee total $ earned < Investor total $ earned, +10%
- If Investor increases investment, +10%
- If Investor decreases investment, -10%
-
These rules were chosen such that the other player, the simulated player, would respond dynamically to the responses of the participant, in a ‘Tit-for-Tat’ manner without being overly harsh or drastic in the round-to-round changes in investment or return rates.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdi H, Williams L. Encyclopedia of research design. Thousand Oaks: Sage; 2010. Correspondence analysis. [Google Scholar]
- Adolphs R. What does the amygdala contribute to social cognition? Annals of the New York Academy of Sciences. 2010;1191:42–61. doi: 10.1111/j.1749-6632.2010.05445.x. The Year in Cognitive Neuroscience 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio A. The human amygdala in social judgment. Nature. 1998;393(6684):470–474. doi: 10.1038/30982. [DOI] [PubMed] [Google Scholar]
- Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, Fehr E. Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron. 2008;58(4):639–650. doi: 10.1016/j.neuron.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Cahill L, Uncapher M, Kilpatrick L, Alkire MT, Turner J. Sex-Related Hemispheric Lateralization of Amygdala Function in Emotionally Influenced Memory: An fMRI Investigation. Learning & Memory. 2004;11(3):261–266. doi: 10.1101/lm.70504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costafreda S, Brammer M, David A, Fu C. Predictors of amygdala activation during the processing of emotional stimuli: a meta-analysis of 385 PET and fMRI studies. Brain Research Reviews. 2008;58(1):57–70. doi: 10.1016/j.brainresrev.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Croson R, Buchan N. Gender and culture: International experimental evidence from trust games. American Economic Review. 1999;89(2):386–391. [Google Scholar]
- Delgado M, Miller M, Inati S, Phelps E. An fMRI study of reward-related probability learning. Neuroimage. 2005;24(3):862–873. doi: 10.1016/j.neuroimage.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Emery N, Capitanio J, Mason W, Machado C, Mendoza S, Amaral D. The effects of bilateral lesions of the amygdala on dyadic social interactions in rhesus monkeys (Macaca mulatta) Behavioral Neuroscience. 2001;115(3):515–544. [PubMed] [Google Scholar]
- Engell A, Haxby J, Todorov A. Implicit trustworthiness decisions: automatic coding of face properties in the human amygdala. Journal of Cognitive Neuroscience. 2007;19(9):1508–1519. doi: 10.1162/jocn.2007.19.9.1508. [DOI] [PubMed] [Google Scholar]
- Gamer M, Zurowski B, Büchel C. Different amygdala subregions mediate valence-related and attentional effects of oxytocin in humans. Proceedings of the National Academy of Sciences. 2010;107(20):9400. doi: 10.1073/pnas.1000985107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried J, O’Doherty J, Dolan R. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301(5636):1104. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- Grezes J, Berthoz S, Passingham R. Amygdala activation when one is the target of deceit: Did he lie to you or to someone else? Neuroimage. 2006;30(2):601–608. doi: 10.1016/j.neuroimage.2005.09.038. [DOI] [PubMed] [Google Scholar]
- Grezes J, Frith C, Passingham R. Brain mechanisms for inferring deceit in the actions of others. Journal of Neuroscience. 2004;24(24):5500. doi: 10.1523/JNEUROSCI.0219-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu M, Bhatt M, Adolphs R, Tranel D, Camerer CF. Neural systems responding to degrees of uncertainty in human decision-making. Science. 2005;310(5754):1680–1683. doi: 10.1126/science.1115327. [DOI] [PubMed] [Google Scholar]
- Kennedy D, Gläscher J, Tyszka J, Adolphs R. Personal space regulation by the human amygdala. Nature Neuroscience. 2009;12(10):1226–1227. doi: 10.1038/nn.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick LA, Zald DH, Pardo JV, Cahill LF. Sex-related differences in amygdale functional connectivity during resting conditions. Neuroimage. 2006;30(2):452–461. doi: 10.1016/j.neuroimage.2005.09.065. [DOI] [PubMed] [Google Scholar]
- King-Casas B, Tomlin D, Anen C, Camerer C, Quartz S, Montague P. Getting to know you: reputation and trust in a two-person economic exchange. Science. 2005;308(5718):78–83. doi: 10.1126/science.1108062. [DOI] [PubMed] [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, et al. Oxytocin modulates neural circuitry for social cognition and fear in humans. Journal of Neuroscience. 2005;25(49):11489. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluver H, Bucy P. Psychic blindness” and other symptoms following bilateral temporal lobectomy in rhesus monkeys. American Journal of Physiology. 1937;119:352–353. [Google Scholar]
- Kluver H, Bucy P. Preliminary analysis of functions of the temporal lobes in monkeys. Archives of Neurology & Psychiatry. 1939;42(6):979. doi: 10.1176/jnp.9.4.606. [DOI] [PubMed] [Google Scholar]
- Koscik T, Bechara A, Tranel D. Sex-related functional asymmetry in the limbic brain. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2010;35(1):340. doi: 10.1038/npp.2009.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak P, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435(7042):673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Krueger F, McCabe K, Moll J, Kriegeskorte N, Zahn R, Strenziok M, et al. Neural correlates of trust. Proceedings of the National Academy of Sciences. 2007;104(50):20084. doi: 10.1073/pnas.0710103104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Xiao E, Houser D, Montague P. Neural responses to sanction threats in two-party economic exchange. Proceedings of the National Academy of Sciences. 2009;106(39):16835. doi: 10.1073/pnas.0908855106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis N. What we can do and what we cannot do with fMRI. Nature. 2008;453(7197):869–878. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
- Logothetis N, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412(6843):150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- McCabe K, Houser D, Ryan L, Smith V, Trouard T. A functional imaging study of cooperation in two-person reciprocal exchange. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(20):11832. doi: 10.1073/pnas.211415698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty J. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Current Opinion in Neurobiology. 2004;14(6):769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Phelps E. Emotion and cognition: insights from studies of the human amygdala. 2005 doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- Platek S, Krill A, Wilson B. Implicit trustworthiness ratings of self-resembling faces activate brain centers involved in reward. Neuropsychologia. 2009;47(1):289–293. doi: 10.1016/j.neuropsychologia.2008.07.018. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2008. [Google Scholar]
- Rilling J, Glenn A, Jairam M, Pagnoni G, Goldsmith D, Elfenbein H, et al. Neural correlates of social cooperation and non-cooperation as a function of psychopathy. Biological Psychiatry. 2007;61(11):1260–1271. doi: 10.1016/j.biopsych.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Rilling J, Goldsmith D, Glenn A, Jairam M, Elfenbein H, Dagenais J, et al. The neural correlates of the affective response to unreciprocated cooperation. Neuropsychologia. 2008;46(5):1256–1266. doi: 10.1016/j.neuropsychologia.2007.11.033. [DOI] [PubMed] [Google Scholar]
- Said C, Baron S, Todorov A. Nonlinear amygdala response to face trustworthiness: contributions of high and low spatial frequency information. Journal of Cognitive Neuroscience. 2009;21(3):519–528. doi: 10.1162/jocn.2009.21041. [DOI] [PubMed] [Google Scholar]
- Sergerie K, Chochol C, Armony J. The role of the amygdala in emotional processing: A quantitative meta-analysis of functional neuroimaging studies. Neuroscience & Biobehavioral Reviews. 2008;32(4):811–830. doi: 10.1016/j.neubiorev.2007.12.002. [DOI] [PubMed] [Google Scholar]
- SPSS Inc. SPSS for Windows (Version 17.0.0) Chicago, Illinois: 2008. [Google Scholar]
- Sripada C, Angstadt M, Banks S, Nathan P, Liberzon I, Phan K. Functional neuroimaging of mentalizing during the trust game in social anxiety disorder. NeuroReport. 2009;20(11):984. doi: 10.1097/WNR.0b013e32832d0a67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein M, Goldin P, Sareen J, Zorrilla L, Brown G. Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Archives of General Psychiatry. 2002;59(11):1027. doi: 10.1001/archpsyc.59.11.1027. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Niki K, Fujisaki S, Akiyama E. Neural basis of conditional cooperation. Social Cognitive and Affective Neuroscience. 2010 doi: 10.1093/scan/nsq042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terenzi M, Ingram C. Oxytocin-induced excitation of neurones in the rat central and medial amygdaloid nuclei. Neuroscience. 2005;134(1):345–354. doi: 10.1016/j.neuroscience.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Theodoridou A, Rowe A, Penton-Voak I, Rogers P. Oxytocin and social perception: Oxytocin increases perceived facial trustworthiness and attractiveness. Hormones and Behavior. 2009;56(1):128–132. doi: 10.1016/j.yhbeh.2009.03.019. [DOI] [PubMed] [Google Scholar]
- Tranel D, Bechara A. Sex-related functional asymmetry of the amygdala: Preliminary evidence using a case-matched lesion approach. Neurocase. 2009;15(3):217–234. doi: 10.1080/13554790902775492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranel D, Gullickson G, Koch M, Adolphs R. Altered experience of emotion following bilateral amygdala damage. Cognitive neuropsychiatry. 2006;11(3):219–232. doi: 10.1080/13546800444000281. [DOI] [PubMed] [Google Scholar]
- van den Bos W, van Dijk E, Westenberg M, Rombouts SARB, Crone EA. What motivates repayment? Neural correlates of reciprocity in the Trust Game. Social Cognitive and Affective Neuroscience. 2009;4(3):294–304. doi: 10.1093/scan/nsp009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston J, Strange B, O’Doherty J, Dolan R. Automatic and intentional brain responses during evaluation of trustworthiness of faces. Nature Neuroscience. 2002;5(3):277–283. doi: 10.1038/nn816. [DOI] [PubMed] [Google Scholar]