Abstract
A wide diversity of adeno-associated virus (AAV) structural proteins uncovered from latent genomes in primate tissue has expanded the number of AAV vector serotypes, which can potentially confer unique cell tropism to the vector. We evaluated 17 of these vectors in the mouse brain using green fluorescent protein (GFP) as a reporter gene. A rapid initial evaluation was performed by neonatal lateral ventricle injections. Vectors made with capsids hu.32, hu.37, pi.2, hu.11, rh.8, hu.48R3, and AAV9 for comparison were selected for further analysis based on their ability to transduce large numbers of cells and result in novel patterns of cell transduction. These vectors were injected into adult brains in four major structures (cortex, striatum, hippocampus, and thalamus), and all were found to transduce neurons. In addition, hu.32, hu.11, pi.2, hu.48R3, and rh.8 resulted in GFP expression in some astrocytes or oligodendrocytes. AAVs rh.8, pi.2, hu.32, and hu.11 also appeared to result in neuronal transport of the vector genome. Vector transport was studied by a single unilateral injection into the hippocampus and vector genome was found in projection sites of the hippocampus. These unique patterns of cell transduction expand the potential repertoire for targeting AAV vectors to selected subsets of brain cells.
Introduction
Adeno-associated viruses (AAVs) are widely used vectors for gene therapy of the brain. Not only are AAV vectors nonpathogenic and result in long-term expression of the encoded gene, but they are also capable of transducing nondividing cells, which is necessary for treatment of the central nervous system (CNS). The prototype AAV2 vector results in relatively limited transduction of CNS cells, and many humans are seropositive for AAV2, limiting its use in clinical applications. However, the cross-packaging of the AAV2 genome with capsid proteins from alternative AAV serotypes has resulted in improved gene transfer in a variety of tissues, including the brain.1–5
Many AAV capsid sequences have been isolated from humans and nonhuman primates by molecular rescue of sequences of endogenous AAVs. Using this method, several novel serotypes and over 100 isolates have been identified and phylogenetically characterized into six clades, A through F.6–8 Some of these cap genes have been made into vectors6 and demonstrated high levels of transduction within the brain, including AAV7, 8, 9, and rh.10 (ref. 4). Most AAV vectors tested have a specific tropism for neurons and are unable to efficiently transduce other cell types within the brain such as astrocytes or oligodendrocytes.1,3,4 Certain AAV serotypes are able to undergo vector transport along neuronal projections,4,9–11 and this appears to be dependent on the capsid proteins expressed.4,11
Using structural diversity, high vector production yield, and gene transfer functionality in other settings as criteria for the selection (L.H.V., E. Brious, H.J. Nam, G. Gao, R. Xiao et al., manuscript submitted), we chose 17 isolates to evaluate in the murine CNS. This approach was aimed at identifying vectors capable of non-neuronal CNS cell targeting or transport along neuronal pathways. An initial screen, involving injection into the cerebral lateral ventricles of neonatal mice,12 resulted in widespread gene delivery with many of the AAV vectors. Based on the neonatal results, six capsid isolates were chosen for testing in four major regions of the adult mouse brain. All of these vectors were found to transduce a greater percentage of tissue in the mouse brain than the previously studied AAV serotype, AAV9. Five of the vectors transduced astrocytes and oligodendrocytes. Four of the vectors were also found in distal projection targets of the injected nuclei, suggesting axonal transport. When these vectors were injected unilaterally into the adult hippocampus, a region with well-defined neuronal projections, vector gene expression was found in projection sites, further evidence that these vectors are capable of axonal transport.
Over 80% of humans may be exposed to AAV2 (ref. 13), often resulting in a pre-existing humoral immunity that can affect the efficacy of AAV2-based gene therapies in the CNS.14,15 Immunogenicity of both capsid and transgene product has been reported to be dependant on the capsid structure, providing a rationale for the evaluation of different AAV capsid vectors.16,17 Our findings that some of these AAV vectors are capable of non-neuronal transduction and/or vector transport may be of benefit for the treatment of CNS diseases. AAV vector transport can be used to achieve improved dispersal of a therapeutic gene.4,11 Furthermore, within the CNS, many neurological diseases have been attributed to oligodendrocyte or astrocyte malfunctions rather than neuronal malfunction, making it necessary to identify gene-transfer vectors with alternative targeting properties.
RESULTS
The vectors tested were representative of all six primate dependoviridae clades (A–F) as well as two phylogenetic niches that were not assigned to clades (Figure 1).8 Some of the capsids tested (designated by R) underwent nonconservative site-directed mutagenesis to improve packaging into vectors and to increase transduction efficiency (L.H.V., E. Brious, H.J. Nam, G. Gao, R. Xiao et al., manuscript submitted). The number of mutations is given by the number after the R (e.g., R2 = 2 amino-acid changes). To evaluate the full potential of each vector, they were injected at the highest titer possible, as determined by the concentration of the vector preparation (Table 1). We tested three AAV isolates from clade A (hu.48R2, hu.48R3, and hu.44R3), which is related to AAV1 and AAV6, and one isolate from clade B (hu.29R), which is related to AAV2, but unlike AAV2, does not bind to heparan sulfate proteoglycans.16 We tested one natural isolate from clade C (hu.11), which is thought to arise through recombination of an AAV2 and AAV3-like virus,8 two isolates from clade D (cy.5R1 and cy.5R4), which are related to AAV7, seven isolates from clade E (rh.43, hu.37, rh.10, pi.2, rh.64R1, rh.64R2, and rh.2R), which are related to AAV8, and one isolate from clade F (hu32), which is related to AAV9. AAV9, which has been investigated previously,4 was used for comparison. Two additional capsids (rh.8 and rh.32.33) were evaluated that are not assigned to clades because they do not share close homology with other known AAVs.
Figure 1. Phylogenetic tree of all tested adeno-associated virus (AAV) isolates.
Dendrogram, using the Neighbor-Joining method and Poisson correction, was made with molecular evolutionary genetics analysis software (The Biodesign Institute, Tempe, AZ). AAVs are grouped in their respective clades as noted by brackets and the closest known serotype relative is shown in blue. Branch lengths correspond to the number of estimated amino-acid substitutions for that distance and the distance scale of branch length is noted on the bottom left.
Table 1.
AAV vectors used in this study
| Clade (related to) | Capsid isolate | Titer (in genome copy/ml) |
|---|---|---|
| A (AAV1/6) | hu.48R2 | 2.9 × 1013 |
| hu.48R3 | 1.3 × 1013 | |
| hu.44R3 | 2.7 × 1013 | |
| B (AAV2) | hu.29R | 1.4 × 1013 |
| C (AAV2–3) | hu.11 | 2.8 × 1013 |
| D (AAV7) | cy.5R1 | 8.7 × 1012 |
| cy.5R4 | 1.4 × 1013 | |
| E (AAV8) | hu.37 | 1.2 × 1013 |
| rh.10 | 4.4 × 1013 | |
| pi.2 | 2.4 × 1013 | |
| rh.64R1 | 4.7 × 1013 | |
| rh.64R2 | 2.4 × 1013 | |
| rh.2R | 7.6 × 1012 | |
| rh.43 | 4.0 × 1013 | |
| F (AAV9) | AAV9 | 5.7 × 1013 |
| hu.32 | 2.2 × 1013 | |
| Other | rh.32.33 | 1.0 × 1013 |
| rh.8 | 3.0 × 1013 |
Abbreviation: AAV, adeno-associated virus.
Two to three neonatal animals per group were injected into the lateral ventricles with 1 µl of vector per ventricle. This method provided a way to screen a large number of vectors due to the rapid injection procedure used for lateral ventricle injections into neonatal mice. Animals were killed 2 weeks after injection and analyzed for vector transduction. Slides were processed for in situ hybridization (ISH) using a riboprobe against the green fluorescent protein (GFP) RNA sequence because this provides information on the spread of vector genome itself, rather than GFP. We found that all 18 AAVs, including AAV9, were able to transduce cells of the neonatal CNS, but showed varying degrees of transduction efficiency (Figure 2). Because the neonatal injections were for screening purposes, a scoring system was used to estimate the quantity of ISH-positive cells in specific regions of the mouse brain (Table 2). Regions with very few ISH-positive cells were scored with (+), regions with many ISH-positive cells were scored with (++), and regions that were saturated with ISH-positive cells were given (+++). If no ISH-positive cells were found, the score was (−). This scoring system allowed a rapid analysis of the 18 vectors to focus on a smaller subset in a more detailed study. The variation in transduction did not correspond to the differences in titer (Tables 1 and 2). Many of the vectors tested in the neonatal animals resulted in dense transduction of regions of the cortex and olfactory bulbs. Most of the vectors resulted in moderate numbers of ISH-positive cells in the striatum, hippocampus, and prefrontal cortex. We were particularly interested in AAV vectors that resulted in high levels of gene expression in areas where other vectors showed little or no expression, which could be indicative of vector transport and/or novel tropism. A subset of the vectors tested also resulted in high numbers of ISH-positive cells in the mammillary bodies, hypothalamus, or thalamus (Table 2).
Figure 2. Comparison of vector distribution resulting from injections of adeno-associated virus (AAV) vectors into the lateral ventricles of neonatal mice.
Animals (n = 2–3 per vector) were injected with 1 µl of AAV vector into each lateral ventricle and killed 2 weeks after injection. The highest obtainable vector titers were used for all injections (Table 1). Frozen sections underwent in situ hybridization using a riboprobe against the green fluorescent protein sequence. Images shown are single-hemisphere representative examples of the transduction characteristics of the individual capsid isolates, grouped by clade. Vectors rh.32.33 and rh.8 do not fall into known clades, and hence they are classified as “Other.”
Table 2.
Transduction characteristics of novel AAV vectors after lateral ventricle injections into neonatal mouse brain
| Score in select brain regions | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Capsid (clade) |
Olfactory bulb |
Prefrontal cortex |
Striatum | Cortex | Rostral hippocampus |
Caudal hippocampus |
Thalamus | Amygdala | Mammillary bodies |
Hypothalamus |
| hu.48R2 (A) | ++abn = 2 | ++n = 2 | ++n = 2 | ++n = 1 +++n = 1 | ++n = 2 | ++n = 1 +++n = 1 | ++n = 2 | ++n = 2 | +n = 2 | +n = 2 |
| hu.48R3 (A) | ++n = 1 +++n = 1 | ++n = 2 | +n = 1 ++n = 1 | ++n = 2 | ++n = 2 | +n = 1 ++n = 1 | +n = 1 ++n = 1 | ++n = 2 | +n = 1 ++n = 1 | +n = 1 ++n = 1 |
| hu.44R3 (A) | +n = 1 ++n = 1 | ++n = 2 | −n = 1 +n = 1 | +n = 2 | ++n = 2 | ++n = 2 | ++n = 2 | −n = 1 +n = 1 | −n = 1 +n = 1 | +n = 2 |
| hu.29R (B) | ++n = 3 | ++n = 3 | +n = 1 ++n = 2 | ++n = 3 | +n = 3 | +n = 3 | −n = 2 +n = 1 | −n = 1 +n = 1 ++n = 1 | −n = 2 +n = 1 | −n = 3 |
| hu.11 (C) | ++n = 2 | ++n = 2 | +n = 2 | ++n = 1 +++n = 1 | ++n = 2 | ++n = 2 | +n = 2 | ++n = 1 | ++n = 2 | +n = 2 |
| cy.5R1 (D) | ++n = 1 +++n = 1 | ++n = 2 | ++n = 2 | ++n = 1 +++n = 1 | ++n = 2 | ++n = 2 | +n = 1 ++n = 1 | ++n = 2 | +n = 2 | +n = 2 |
| cy.5R4 (D) | ++n = 2 | ++n = 2 | ++n = 2 | ++n = 2 | ++n = 2 | ++n = 2 | +n = 2 | ++n = 1 +++n = 1 | +n = 2 | −n = 2 |
| hu.37 (E) | ++n = 2 | ++n = 2 | ++n = 2 | +++n = 2 | ++n = 2 | ++n = 2 | +n = 2 | ++n = 2 | +n = 1 +++n = 1 | +n = 1 ++n = 1 |
| rh.10 (E) | ++n = 3 | ++n = 3 | +n = 1 ++n = 2 | ++n = 3 | ++n = 3 | ++n = 3 | +n = 3 | ++n = 1 +++n = 1 | −n = 2 +n = 1 | −n = 3 |
| pi.2 (E) | ++n = 1 +++n = 1 | ++n = 2 | +n = 2 | ++n = 2 | +++n = 1 | ++n = 3 | ++n = 3 | +n = 1 ++n = 2 | ++n = 2 +++n = 1 | ++n = 2 +++n = 1 |
| rh.64R1 (E) | ++n = 1 +++n = 1 | ++n = 2 | +n = 2 | ++n = 1 +++n = 1 | ++n = 2 | ++n = 2 | +n = 2 | ++n = 3 | +n = 2 | +n = 2 |
| rh.64R2 (E) | +++n = 2 | ++n = 2 | −n = 1 ++n = 1 | ++n = 1 +++n = 1 | ++n = 2 | ++n = 2 | +n = 2 | ++n = 2 | −n = 2 | −n = 2 |
| rh.43 (E) | ++n = 3 | +n = 1 ++n = 2 | +n = 2 ++n = 1 | ++n = 2 +++n = 1 | ++n = 3 | +n = 1 ++n = 2 | +n = 2 ++n = 1 | +++n = 2 | −n = 3 | −n = 3 |
| rh.2R (E) | ++n = 2 +++n = 1 | ++n = 3 | +n = 2 ++n = 1 | ++n = 2 +++n = 1 | ++n = 3 | ++n = 3 | +n = 2 ++n = 1 | ++n = 2 +++n = 1 | −n = 2 +n = 1 | −n = 2 +n = 1 |
| hu.32 (F) | +++n = 2 | ++n = 2 | ++n = 2 | +++n = 2 | ++n = 2 | ++n = 2 | ++n = 2 | +n = 2 ++n = 1 | ++n = 1 +++n = 1 | ++n = 2 |
| AAV9 (F) | ++n = 1 +++n = 2 | ++n = 2 ++n =1 | ++n = 2 +++n 1 | ++n = 2 +++n = 1 | ++n = 3 | +++n = 3 | ++n = 2 | ++n = 2 | +n = 2 ++n = 1 | +n = 2 ++n = 1 |
| rh.8 (other) | ++n = 2 +++n = 1 | ++n = 3 | ++n = 3 | ++n = 3 | ++n = 3 | ++n = 3 | ++n = 3 | +n = 1 ++n = 2 | +n = 2 ++n = 1 | +n = 2 ++n = 1 |
| rh.32.33 (other) | ++n = 2 | ++n = 2 | ++n = 2 | ++n = 2 | ++n = 2 | ++n = 2 | ++n = 2 | ++n = 2 | −n = 2 | −n = 2 |
Abbreviation: AAV, adeno-associated virus.
Scoring: (−) no transduction, (+) very few positive cells, (++) many positive cells, and (+++) region is completely saturated with ISH-positive cells.
The number of animals (n) with the particular score is given to the right of the score.
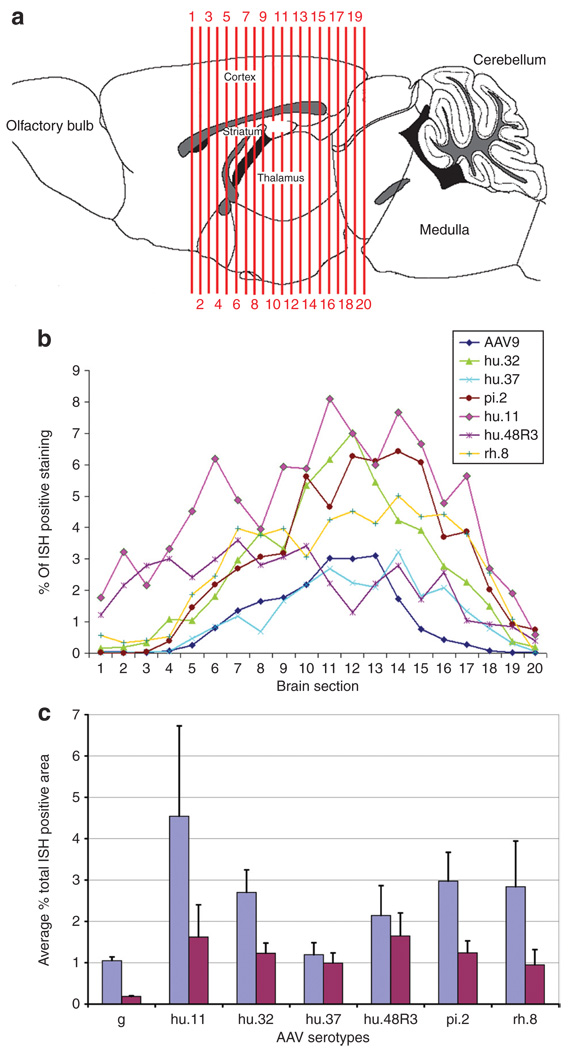
Six vectors were chosen for further analysis in adult animals, based on the performance scoring in the neonates. The vectors chosen were hu.32, hu.37, pi.2, hu.11, hu.48R3, and rh.8. We also injected AAV9 for comparison because we have previously characterized the transduction patterns of this serotype in adult mice.4 Vectors were injected unilaterally into the cortex, striatum, hippocampus, and thalamus (two injection tracks, two depths each, as in ref. 4) and analyzed 3 weeks after injection for expression by ISH against GFP mRNA. All of the vectors transduced cells in the injected structures, but to varying degrees (Figure 3a). ISH-stained cryosections from injected brains were scanned and sections were computationally analyzed using a custom-designed macro. The ratio of transduced area over total section area was determined, to calculate the percentage of ISH-positive tissue in 20 equally spaced sections spanning the transduced region of the brain (Figure 4a). All vectors resulted in a distribution of vector-positive area with the highest near the two injection tracts (Figure 4b). The caudal injection into the hippocampus and thalamus (section 12) resulted in the greatest percentage of ISH-positive area with most of the vectors. Vectors made with capsids hu.11, pi.2, and hu.32 had a greater percentage of ISH-positive area throughout most regions of the brain. Vectors made with capsids hu.11 and hu.48R3 had a higher percentage of ISH-positive area in sections in the rostral part of the brain, which correlates with the images shown in Figure 3a. The percent ISH-positive area across all the 20 sections was averaged for each animal and the averages for each serotype were compared. The averages (in percent ± SEM) for percent ISH-positive staining area were for AAV9 (1.05 ± 0.09), hu.11 (4.54 ± 2.19), hu.32 (2.70 ± 0.55), hu.37 (1.19 ± 0.29), hu48R3 (2.14 ± 0.72), pi.2 (2.97 ± 0.70), and rh.8 (2.83 ± 1.11).
Figure 3. Comparison of vector distribution resulting from injections of novel adeno-associated virus (AAV) vectors into adult mice.
AAV9, hu.32, hu.37, pi.2, hu.11, hu.48R3, and rh.8 were injected into the cortex, striatum, hippocampus, and thalamus (1 µ l per injection site) of adult mice (>2 months of age), and animals were killed 3 weeks after injection. The highest obtainable vector titers were used for all injections (Table 1). Frozen sections underwent in situ hybridization (ISH) using a riboprobe against the green fluorescent protein sequence. (a) Representative examples of the transduction capabilities of the individual vectors. The injection locations are shown with arrows on the AAV9 sections. The rostral-caudal level of the coronal section relative to bregma is given41 with (+) indicating that the location is rostral to bregma and a (−) indicating the section is caudal to bregma. Boxes in a, not drawn to scale, correspond to the panels in b. (b) ISH-positive cells can be found in the contralateral hippocampus of AAV9, hu.32, pi.2, hu.11, and rh.8 injected animals, indicating that these vectors were capable of axonal transport from the injected hippocampus.
Figure 4. Comparison of transduced area after adeno-associated virus (AAV) vector injections into adult animals.
The percentage of in situ hybridization (ISH) positive–stained area within 20 equally spaced sections (~240 µm apart) across the transduced region of the injected mouse brains was calculated using a custom-designed Image-Pro macro. (a) Locations of the 20 sections analyzed are shown, the numbers in a indicate the position of the section and correspond with numbers given along the x-axis in b. Drawing after Paxinos and Franklin.41 (b) The percentage of ISH-stained area for each section was calculated and the numbers were graphed across all 20 sections. (c) The average percent of ISH staining to total area of sections were averaged for all 20 sections and averaged across the three mice per group (blue bars are actual percentages, red bars are percentages normalized to 1 × 1013 genome equivalents/ml).
Using a measure of percent on samples taken at regular intervals through the area-of-interest accounted for variations in brain size from animal to animal. Using this method, there were no significant differences among the six novel serotypes. Because we used the highest titer of virus that could be generated for each serotype to maximize transduction (Table 1), we performed an alternative calculation of relative total areas by normalizing to a common titer (1 × 1013). The results among the six novel serotypes were even more similar to each other and showed a greater increase over AAV9 which had the highest titer (Figure 4c). The actual titers of the six vectors differed very little (1.2 × 1013 to 3.0 × 1013 genome equivalents/ml; differences in viral titers are expressed in log increments). We also analyzed the data by calculating the volume of transduction using the stereological estimation method of Cavalieri, as well as calculating the total ISH area relative to Nisslstained area, which is a measure of neuron cell bodies (the predominant transduced cell type). The relative relationship among the serotypes was the same (data not shown) as with the other methods, i.e., all six serotypes transduced more brain volume and cells than AAV9, which was among the best of the previously tested serotypes.4
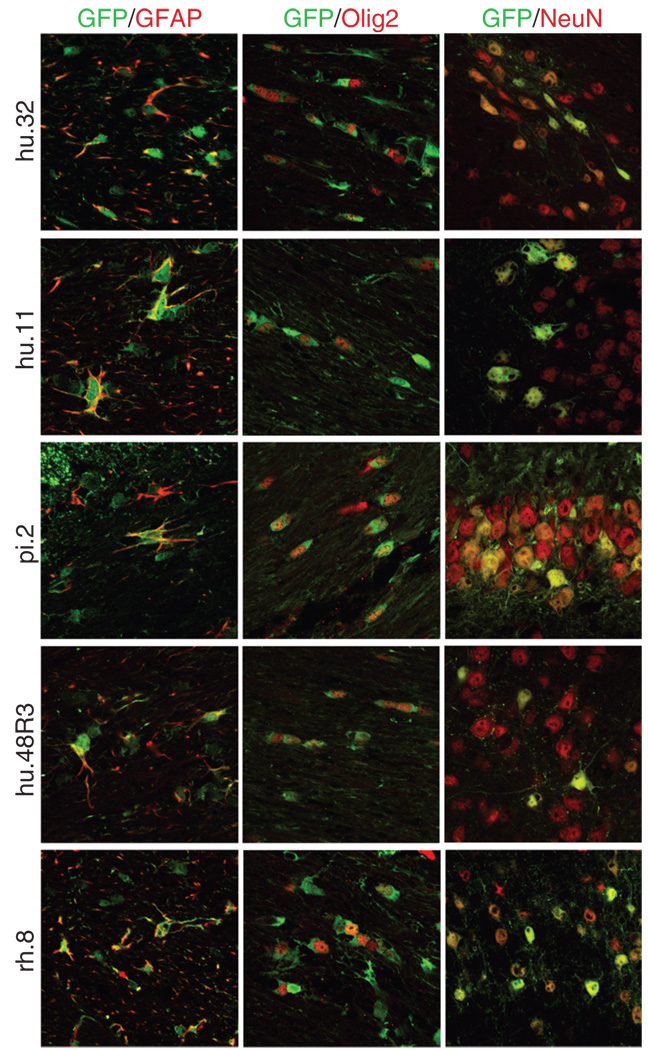
Four of the AAV vectors (hu.11, hu.48R3, pi.2, and hu.32) transduced regions rich in oligodendrocytes or astrocytes but with few neurons, such as the corpus callosum and external capsule, suggesting that non-neuronal cells were targeted. We thus co-labeled sections that were GFP-positive with antibodies to the cell-specific markers glial fibrillary acidic protein (astrocytes), Olig2 (oligodendrocytes), or NeuN (neurons). GFP-expressing astrocytes and oligodendrocytes were found in the corpus callosum and external capsule with all four vectors (Figure 5). In most of the brain, the transduced cells were neurons by morphology, but the high intensity of the GFP signal in the most highly transduced areas only allowed clear co-localization of GFP and NeuN near the edges of the transduced region.
Figure 5. Novel adeno-associated virus (AAV) vectors result in green fluorescent protein (GFP) expression in non-neuronal cells.
GFP-positive sections from brains injected with AAV hu.32, hu.11, pi.2, hu.48R3, and rh.8 were co-labeled with antibodies to the astrocytic marker glial fibrillary acidic protein (GFAP), the oligodendrocytic marker Olig2, or the neuronal marker NeuN. The GFP-positive cells stain green, whereas the cell-specific markers fluoresce red via conjugated secondary antibodies. All pictures were taken using confocal microscopy. GFAP and Olig2 pictures were taken with ×63 magnification and a ×2.3 zoom. NeuN pictures were taken with ×40 magnification and ×2 zoom. Pictures showing co-localization with GFAP or Olig2 were taken of the corpus callosum or external capsule. Pictures showing co-localization with NeuN were taken on the outer edges of the transduced regions and are of the hippocampus (AAV9 or pi.2), the thalamus (hu.32, hu.11, or hu.48R3), or of the cortex (rh.8).
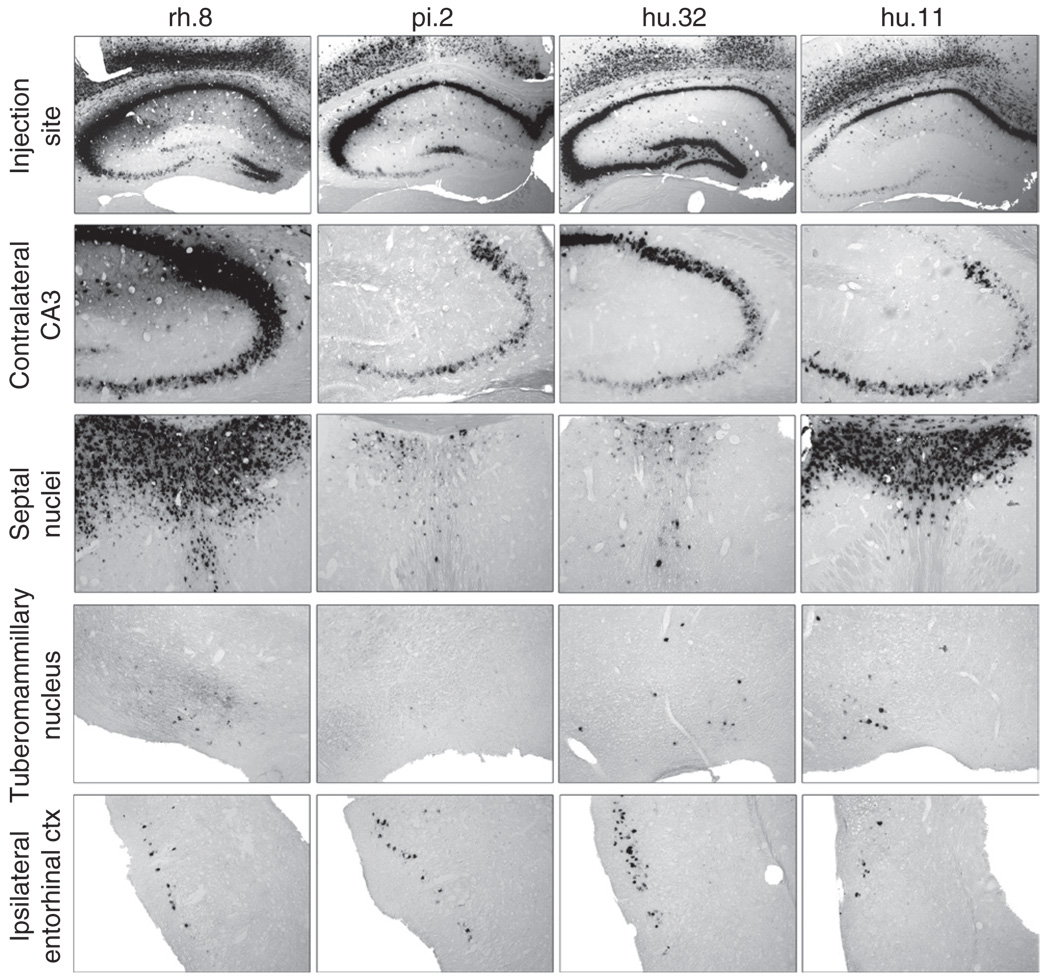
The presence of ISH-positive cells distal to the injection sites, in areas that have known connections with the injected sites, suggested that axonal transport of the vector had occurred, as seen with certain other serotypes.4,10,11 Four of the vectors (hu.32, rh.8, pi.2, and hu.11) resulted in ISH-positive cells in the CA3 region of the contralateral hippocampus, similar to that seen with AAV9 (Figure 3b), which has been shown to be transported in this structure.4,11 We tested the four vectors using a single, unilateral injection into the hippocampus, which has well-defined connections to the contralateral hippocampus,18,19 the entorhinal cortex, 20,21 the septal nuclei,22–25 and the tuberomammillary nuclei.26 All four vectors resulted in ISH-positive cells in the contralateral CA3 region, the lateral septal nuclei, the ipsilateral entorhinal cortex, and tuberomammillary nuclei (Figure 6, Supplementary Figure S1). AAV9 is closely related to hu.32 (ref. 8), and hu.32 resulted in ISH-positive cells in projection sites that were comparable to numbers seen after a single hippocampal injection of AAV9.4 Most of the ISH-positive cells seen were indicative of retrograde transport from the injected region, because the hippocampus receives projections from the contralateral hippocampus, entorhinal cortex, and tuberomammillary nuclei. A strong, bilateral ISH-positive signal was seen with rh.8 and hu.11 in the lateral septal nuclei (Figure 6) but not in the medial septal nucleus (not shown). This indicates that these serotypes can mediate anterograde transport because the septohippocampal pathway sends projections from one side of the hippocampus bilaterally to the lateral septal nuclei while the medial septal nucleus sends projections to the hippocampus.22–25 Because retrograde transport also occurred with these serotypes in other structures, e.g., entorhinal cortex, the direction of transport appears to depend on the specific neuronal pathway rather than being a sole property of the serotype. This is consistent with recent findings that both anterograde and retrograde transport appear to occur after injection of specific serotype vectors into the ventral tegmental area.11
Figure 6. Adeno-associated virus (AAV) vector transport following unilateral injection into the hippocampus.
The highest obtainable vector titers were used for the injections (Table 1). Animals (n = 3/group) were injected into the unilateral hippocampus with rh.8, pi.2, hu.32, or hu.11, and examined 3 weeks postinjection for vector distribution. Frozen sections underwent in situ hybridization (ISH) using a riboprobe against the green fluorescent protein sequence. The location of the injection is shown for the vectors in the first row of panels. ISH-positive cells could be found in projection sites of the hippocampus including the CA3 region of the contralateral hippocampus, the lateral septal nuclei, the entorhinal cortex, and the tuberomammillary nucleus.
DISCUSSION
The transduction capabilities of AAV vectors are directly related to the amino acid sequences that encode the proteins on the outer capsid of the AAV virion. Single amino acid changes can result in different host interactions of the AAV vector, which can alter the vector tropism at an entry or postentry level and thereby result in dramatically different transduction characteristics.27 We demonstrate that, depending on the structural composition of capsid, vectors are able to result in high levels of transduction in the brain, transduction of non-neuronal cells, or vector transport along neuronal projections. Analysis of the transduction properties of AAV variants is necessary to define an optimal vector, able to achieve a desired transduction pattern. In addition, humans may have pre-existing immunity to particular AAV capsids,28,29 even in the brain.30 Having a wide spectrum of vectors available from which to choose expands the therapeutic potential of AAV. Because many of the proposed capsid variants were derived from nonhuman primates, their seroprevalence in humans is likely reduced.
Most of the 18 vectors tested, including AAV9, resulted in high levels of transduction in the neonatal screen, and a subset appeared to have unique patterns of transduction compared to previously studied AAV serotypes. Interestingly, rh.10, which we have previously shown to result in very high levels of transduction after injections into the adult mouse brain,4 did not appear to result in as many transduced cells as other members of clade E after neonatal injections. Also interesting, hu.29R, which is closely related to AAV2 but lacks the heparin affinity of the AAV2 virion, resulted in transduction levels comparable to those previously shown for AAV2.2,12
We chose AAVs for further analysis based on their ability to transduce the neonatal brain after lateral ventricle injections. However, transduction of the neonatal brain does not necessarily correlate with transduction that can be expected in the adult brain. The best example of this is AAV5, which results in very low levels of transduction after injection into the lateral ventricles of neonates,31 but results in high levels of transduction when injected directly into the brain parenchyma of neonates31 or adult mice.1 Therefore, it is possible that AAV vectors that were initially excluded, due to low levels of transduction in the neonate, could have resulted in high levels of transduction in the adult animals. However, of the vectors tested in this study in adult animals, those that were found to result in high levels of transduction in the neonate animals also resulted in high levels of transduction in the adult mice.
When vectors were injected into adult mice, we found that each capsid isolate correlated with a distinct transduction profile. For the most part, AAVs that were structurally similar to previously characterized serotypes (e.g., hu.32 and AAV9 or hu.48R3 and AAV1) presented comparable transduction patterns. However, some unique transduction characteristics were found. For example, both hu.32 and hu.48R3 were shown to result in GFP expression in astrocytes, which has not been seen with their respective related serotypes AAV9 (ref. 4) and AAV1.2,3,5 Hu.32 was also found to result in greater transduction than AAV9, even though it had a lower titer, suggesting that the small differences between the capsids may provide a transduction advantage for hu.32 in the mouse brain.
Most AAV serotypes reported in the literature have an exclusively neuronal tropism, though AAV4 has been shown to transduce ependymal cells1,32 and may transduce astrocytes, depending on the site of injection.32 AAV5 can also transduce astrocytes, but the overall transduction of astrocytes is low and this also appears to be dependant on the site of injection.1 AAV1 can transduce oligodendrocytes within white matter tracts.33 Overall, AAV vectors appear to transduce neurons preferentially, and AAVs that do transduce non-neuronal cells result in only a low percentage of non-neuronal transduction compared to neuronal transduction. Astrocytes have been implicated in many CNS diseases (for reviews see refs. 34,35) and oligodendrocytes, as the myelinating cells of the CNS, are implicated in CNS diseases involving demyelination.37,38 Therefore, a vector that is able to transduce these non-neuronal cells to a greater degree than previously described serotypes may result in a greater therapeutic efficacy when used in many CNS diseases. We show that at least five of the vectors tested, hu.32, hu.11, pi.2, hu.48R3, and rh.8, were able to transduce both astrocytes and oligodendrocytes. Although it appears that these vectors were only able to transduce non-neuronal cells in white matter areas, this could be due to the limits of detection caused by the bright, saturating levels of GFP expression. The pattern of GFP expression detected by fluorescence (Supplementary Figure S2) corresponded to the pattern of gene expression detected by ISH (Figure 2).
We, and others, have shown that some AAV serotypes are able to undergo vector transport.4,9–11 In this study, at least four of the AAVs tested, rh.8, pi.2, hu.32, and hu.11, demonstrated neuronal transport, with rh.8 appearing to result in transport to greater levels than previously seen following a hippocampal injection with other transportable serotypes, AAV2 (ref. 10) or AAV9 (ref. 4). We chose 6 of the 17 AAV vectors tested in the neonate for injection into the adult mouse and found them to demonstrate new properties. There are also numerous other isolates that have been discovered that we did not test. It is possible that vectors made with the other capsids would also be able to result in high levels of transduction, non-neuronal transduction, or vector transport in an adult brain.
The information on the transduction characteristics of these AAV capsid variants tested can be used in a structure–function approach when comparing transduction characteristics with capsid architecture. Such a strategy could assist in the structural definition of domains on the AAV capsid relevant for its tropism. Future studies will look in more detail at the amino-acid sequence similarities among the capsids of the vectors in relation to the transduction patterns they confer, with the goal of optimizing the production of new vectors to select for specific patterns.
MATERIALS AND METHODS
Experimental animals
Normal C3H/HeOuJ mice were produced in our breeding colony. Neonatal injections were performed at P1. Adult injections were performed on mice >2 months of age. All procedures were approved by the Institutional Care and Use Committee at the Children’s Hospital of Philadelphia.
Design of vectors
AAV capsids were cloned into an AAV2-based packaging plasmid to obtain a hybrid construct with AAV2 rep and the alternative cap in frame as previously described.6 Capsid sequences were previously isolated from primate sources and were optimized for vector production by site-directed mutagenesis. Wild-type isolates were previously described7,8 and available through GenBank (rh.10: AY243015, rh32: AY243003, rh.33: AY243002, rh.8: AY242997, cy.5: AY243017, hu.48: AY530611, hu.11: AY530577, hu.29: AY530594, hu.44: AY530607, hu.32: AY530597, rh.64: AY530574, hu.37: AY530600, rh.43: AY530606, pi.2: AY530554, and rh.2: AY243007). In short, structure–function analysis highlighted atypical residues on the capsid that correlated with a loss of function. A mutagenesis approach was used that reverted these residues to a conserved state as predicted by homologous AAV capsid sequences. The first two letters of the nomenclature refer to the species of isolation followed by the number of the isolate from that species. This is followed by information on the number of amino-acid changes that have been introduced starting from the original isolate (indicated by R). The particular amino-acid changes that were introduced into the wild-type isolate sequence are the following: hu.44R3: G609D P446L E137K; hu.48R3: G277S E322K S552N; rh.64R2: R697W V686E; rh.2R: V651I; cy.5R1: G13D; cy.5R4: G13D D403N R51K N158K P161Q; and hu.29R: G396E.16 Rh.32.33 was generated by swapping a BsiWI–BbvCI fragment with the homologous fragment of rh.32 in the rh.32 capsid gene (L.H.V., E. Brious, H.J. Nam, G. Gao, R. Xiao et al., manuscript submitted).
AAV vector construction and packaging
All vectors expressed the cytomegalovirus promoter and enhanced GFP transgene and were cross-packaged into an AAV2 recombinant genome with heterologous cap sequence from the tested AAV variant using a triple-transfection procedure as previously described.6 The packaging, purification, and determination of vector titers were performed by the University of Pennsylvania Vector Core. All recombinant vectors were purified using the CsCl sedimentation method and genome copy titers were determined as described previously.39 For vector titers, see Table 1.
Intraventricular injections into neonatal mice
Neonatal animals were cryoanesthetized and injected with 1 µl of viral vector into each cerebral lateral ventricle with a finely drawn glass micropipette as described previously.12
Stereotaxic injections into adult mice
Under sterile conditions, the animals were anesthetized with isofluorane, secured in a stereotaxic frame (Kopf, Tujunga, CA), holes the size of the injection needle were drilled into the skull, and injections were done unilaterally with 1 µl of vector per brain region at a rate of 0.5 µl/minute. For the four site injections, two injection tracts were used, one for the cortex and striatum injection and one for the hippocampus and thalamus injection. For two injections along the same tract, the needle was lowered to the coordinates for the ventral site, the first injection was done, the needle was left in place for 3 minutes, then raised to the coordinates for the dorsal site where it was left in place for 2 minutes, the second injection was then done, and the needle was left in place for another 3 minutes before being slowly removed from the brain. Coordinates for injections were (in mm: caudal to bregma, left of midline, ventral to pial surface): cortex (0.1, 1.75, 0.9), striatum (0.1, 1.75, 2.1), hippocampus (2.1, 1.3, 1.7), and thalamus (2.1, 1.3, 3.2). For the vector transport experiment, a single injection was made into the hippocampus (coordinates 2.1, 1.3, 1.7) and the needle was withdrawn without stopping at the dorsal site. Animals were only included in the study if the needle track was in the target site on postmortem histological examination.
Tissue collection and preparation
At 2 weeks postinjection for neonatal injections and 3 weeks postinjection for adult injections, mice were anesthetized with a mixture of ketamine and xylazine (~0.15 ml per mouse) and perfused transcardially with a solution of phosphate-buffered saline followed by 4% paraformaldehyde. Brains from normal C3H animals were then removed and put in 4% paraformaldehyde overnight, following which they were transferred to 30% sucrose for cryoprotection. Once the brains sank in the sucrose, they were mounted in optimum cutting temperature solution (Sakura, Torrance, CA) and frozen at −20 °C until sectioning. Sectioning was done at a thickness of 20 µm using a cryostat (Leica Microsystems, Wetzlar, Germany) and the sections were mounted on three sets of slides which were then kept at −20 °C until staining.
Colorometric ISH
To generate digoxigenin (DIG)-labeled GFP cRNA riboprobes, the GFP sequence was obtained from the pZac2.1 plasmid, provided by the Vector Core of the University of Pennsylvania. The GFP sequence was cut out of the pZac plasmid using the NotI and HindIII restriction enzymes and inserted into the pBluescript II KS cloning plasmid (Stratagene, La Jolla, CA) in the forward or reverse orientation. The resulting plasmids were linearized using BssHII to generate sense and antisense templates for run-off transcription. Antisense and sense DIG-labeled cRNA probes were generated using a DIG labeling kit (Roche Biochemicals, Indianapolis, IN). Probe length and anti-DIG antibody reactivity were verified on a formaldehyde gel. Following incubation in DIG-labeled riboprobes, positive cells were identified using an anti-DIG-AP (Roche Biochemicals, Indianapolis, IN) antibody followed by NBP/BCIP (Roche Biochemicals, Indianapolis, IN) as previously reported.40 Control slides included AAV-GFP-injected and normal uninjected C3H/HeOuJ mouse sections labeled with sense and antisense probe.
Quantitation of GFP ISH-positive staining
Twenty ISH-stained sections, at 240 µm between each section were picked for each of the seven capsid variants injected into adult animals. Sections were aligned according to distance between sections and anatomical landmarks based on the Paxinos atlas of the mouse brain.41 The first section was from 1.2 mm rostral to bregma, and the last section was 4.1 mm caudal to bregma, so the total rostral to caudal distance analyzed was ~5.3 mm. Sections were scanned into the computer using a Microtek ArtixScan 4000tf (Microtek, Carson, CA) scanner at 4,000 dots per inch resolution. The green channel image was separated out from the color image for improved resolution of the ISH-positive areas, saved as a grayscale image, and used for analysis. Additionally, the grayscale levels were expanded to provide uniformity. The images were then analyzed with a custom-designed macro using Image-Pro software (Media Cybernetics, Bethesda, MD) by a blinded operator who, for each slide, selected a detection level that best represented the ISH-positive areas. The number of pixels in the section reaching the threshold was counted and converted to percent of area by using the total number of pixels for the whole section as the denominator as the ISH-positive area. The whole area of the section was also determined to calculate a percentage of the section that was ISH positive. The data were recorded and graphed in an Excel spreadsheet. The volume of transduction was calculated using the stereological estimation method of Cavalieri.42
Nissl staining
A mouse brain was cryosectioned and stained in a 0.1% cresyl violet/acetic acid solution for 2 minutes, followed by two 5-minute differentiation washes in 95% ethanol. They were then dehydrated in 100% ethanol followed by clearing in xylene and mounting in DPX mounting medium. The slides were then scanned similar to the method used for the ISH analysis. To generate the proportion of Nissl-positive staining area which corresponded to ISH-positive area, the ISH-positive slide was first registered to the corresponding Nissl section using the registration function of Image-Pro. Final checking and adjustment of the registration of the ISH-positive image was done in Adobe Photoshop. The area of Nissl staining was then compared in Image-Pro and Excel to the corresponding ISH brain sections.
Antibody staining
Co-labeling of GFP-positive cells with cell-specific markers was done using a chicken anti-GFP antibody (Invitrogen, Carlsbad, CA), rat anti-GFAP polyclonal antibody (gift from the laboratory of Judy Grinspan), mouse anti-NeuN monoclonal antibody (Chemicon, Temecula, CA), or rabbit-anti-Olig2 antibody (Chemicon, Temecula, CA). Secondary antibodies to rabbit, rat, or mouse were conjugated to Alexa Fluor 594 (Molecular Probes, Eugene, OR).
Statistics
Statistics were run using InStat (GraphPad Software, San Diego, CA) statistical software. Log values of the mean number of summed transduced pixels were compared using Student’s two-tailed t-tests. Statistical significance was set at a P value of <0.05.
SUPPLEMENTARY MATERIAL
Figure S1 Comparison of vector distribution resulting from unilateral injection of rh.8, pi.2, hu.32, or hu.11, into the hippocampus.
Figure S2 Examples of GFP fluorescence in brains injected neonatally.
ACKNOWLEDGMENTS
We thank Trena Clarke, Ara Polesky, Erlinda Cabacungan, and HuanYing Zhao for expert technical assistance; Julie Johnston and Guang-Ping Gao for help related to AAV production and advice. This research was supported by grants from National Institutes of Health (NS38690 and DK63690, J.H.W; DK47757, J.M.W.) and by Glaxo Smith Kline (J.M.W.). C.N.C. was supported by an NIH training grant (NS07180). J.M.W. and L.H.V. are inventors on patents licensed to various companies. This work was done in Philadelphia, PA, USA.
REFERENCES
- 1.Davidson BL, Stein CS, Heth JA, Martins I, Kotin RM, Derksen TA, et al. Recombinant adeno-associated virus type 2, 4, and 5 vectors: transduction of variant cell types and regions in the mammalian central nervous system. Proc Natl Acad Sci USA. 2000;97:3428–3432. doi: 10.1073/pnas.050581197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Passini MA, Watson DJ, Vite CH, Landsburg DJ, Feigenbaum AL, Wolfe JH. Intraventricular brain injection of adeno-associated virus type 1 (AAV1) in neonatal mice results in complementary patterns of neuronal transduction to AAV2 and total long-term correction of storage lesions in the brains of beta-glucuronidase-deficient mice. J Virol. 2003;77:7034–7040. doi: 10.1128/JVI.77.12.7034-7040.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burger C, Gorbatyuk OS, Velardo MJ, Peden CS, Williams P, Zolotukhin S, et al. Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Mol Ther. 2004;10:302–317. doi: 10.1016/j.ymthe.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 4.Cearley CN, Wolfe JH. Transduction characteristics of adeno-associated virus vectors expressing cap serotypes 7, 8, 9, and Rh10 in the mouse brain. Mol Ther. 2006;13:528–537. doi: 10.1016/j.ymthe.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 5.Taymans JM, Vandenberghe LH, Haute CV, Thiry I, Deroose CM, Mortelmans L, et al. Comparative analysis of adeno-associated viral vector serotypes 1, 2, 5, 7, and 8 in mouse brain. Hum Gene Ther. 2007;18:195–206. doi: 10.1089/hum.2006.178. [DOI] [PubMed] [Google Scholar]
- 6.Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci USA. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao G, Alvira MR, Somanathan S, Lu Y, Vandenberghe LH, Rux JJ, et al. Adeno-associated viruses undergo substantial evolution in primates during natural infections. Proc Natl Acad Sci USA. 2003;100:6081–6086. doi: 10.1073/pnas.0937739100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao G, Vandenberghe LH, Alvira MR, Lu Y, Calcedo R, Zhou X, et al. Clades of adeno-associated viruses are widely disseminated in human tissues. J Virol. 2004;78:6381–6388. doi: 10.1128/JVI.78.12.6381-6388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaspar BK, Llado J, Sherkat N, Rothstein JD, Gage FH. Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science. 2003;301:839–842. doi: 10.1126/science.1086137. [DOI] [PubMed] [Google Scholar]
- 10.Passini MA, Macauley SL, Huff MR, Taksir TV, Bu J, Wu IH, et al. AAV vector-mediated correction of brain pathology in a mouse model of Niemann-Pick A disease. Mol Ther. 2005;11:754–762. doi: 10.1016/j.ymthe.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 11.Cearley CN, Wolfe JH. A single injection of an adeno-associated virus vector into nuclei with divergent connections results in widespread vector distribution in the brain and global correction of a neurogenetic disease. J Neurosci. 2007;27:9928–9940. doi: 10.1523/JNEUROSCI.2185-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Passini MA, Wolfe JH. Widespread gene delivery and structure-specific patterns of expression in the brain after intraventricular injections of neonatal mice with an adeno-associated virus vector. J Virol. 2001;75:12382–12392. doi: 10.1128/JVI.75.24.12382-12392.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moskalenko M, Chen L, van Roey M, Donahue BA, Snyder RO, McArthur JG, et al. Epitope mapping of human anti-adeno-associated virus type 2 neutralizing antibodies: implications for gene therapy and virus structure. J Virol. 2000;74:1761–1766. doi: 10.1128/jvi.74.4.1761-1766.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mastakov MY, Baer K, Symes CW, Leichtlein CB, Kotin RM, During MJ. Immunological aspects of recombinant adeno-associated virus delivery to the mammalian brain. J Virol. 2002;76:8446–8454. doi: 10.1128/JVI.76.16.8446-8454.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peden CS, Burger C, Muzyczka N, Mandel RJ. Circulating anti-wild-type adeno-associated virus type 2 (AAV2) antibodies inhibit recombinant AAV2 (rAAV2)-mediated, but not rAAV5-mediated, gene transfer in the brain. J Virol. 2004;78:6344–6359. doi: 10.1128/JVI.78.12.6344-6359.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vandenberghe LH, Wang L, Somanathan S, Zhi Y, Figueredo J, Calcedo R, et al. Heparin binding directs activation of T cells against adeno-associated virus serotype 2 capsid. Nat Med. 2006;12:967–971. doi: 10.1038/nm1445. [DOI] [PubMed] [Google Scholar]
- 17.Zhang TP, Jin DY, Wardrop RM, 3rd, Gui T, Maile R, Frelinger JA, et al. Transgene expression levels and kinetics determine risk of humoral immune response modeled in factor IX knockout and missense mutant mice. Gene Ther. 2007;14:429–440. doi: 10.1038/sj.gt.3302881. [DOI] [PubMed] [Google Scholar]
- 18.Laurberg S. Commissural and intrinsic connections of the rat hippocampus. J Comp Neurol. 1979;184:685–708. doi: 10.1002/cne.901840405. [DOI] [PubMed] [Google Scholar]
- 19.Laurberg S, Sorensen KE. Associational and commissural collaterals of neurons in the hippocampal formation (hilus fasciae dentatae and subfield CA3) Brain Res. 1981;212:287–300. doi: 10.1016/0006-8993(81)90463-7. [DOI] [PubMed] [Google Scholar]
- 20.Steward O. Topographic organization of the projections from the entorhinal area to the hippocampal formation of the rat. J Comp Neurol. 1976;167:285–314. doi: 10.1002/cne.901670303. [DOI] [PubMed] [Google Scholar]
- 21.Wyss JM. An autoradiographic study of the efferent connections of the entorhinal cortex in the rat. J Comp Neurol. 1981;199:495–512. doi: 10.1002/cne.901990405. [DOI] [PubMed] [Google Scholar]
- 22.Meibach RC, Siegel A. Efferent connections of the septal area in the rat: an analysis utilizing retrograde and anterograde transport methods. Brain Res. 1977;119:1–20. doi: 10.1016/0006-8993(77)90088-9. [DOI] [PubMed] [Google Scholar]
- 23.Swanson LW. The anatomical organization of septo-hippocampal projections. Ciba Found Symp. 1977:25–48. doi: 10.1002/9780470720394.ch4. [DOI] [PubMed] [Google Scholar]
- 24.Swanson LW, Cowan WM. An autoradiographic study of the organization of the efferent connections of the hippocampal formation in the rat. J Comp Neurol. 1977;172:49–84. doi: 10.1002/cne.901720104. [DOI] [PubMed] [Google Scholar]
- 25.Swanson LW, Cowan WM. The connections of the septal region in the rat. J Comp Neurol. 1979;186:621–655. doi: 10.1002/cne.901860408. [DOI] [PubMed] [Google Scholar]
- 26.Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- 27.Wu Z, Asokan A, Grieger JC, Govindasamy L, Agbandje-McKenna M, Samulski RJ. Single amino acid changes can influence titer, heparin binding, and tissue tropism in different adeno-associated virus serotypes. J Virol. 2006;80:11393–11397. doi: 10.1128/JVI.01288-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chirmule N, Propert K, Magosin S, Qian Y, Qian R, Wilson J. Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther. 1999;6:1574–1583. doi: 10.1038/sj.gt.3300994. [DOI] [PubMed] [Google Scholar]
- 29.Erles K, Sebokova P, Schlehofer JR. Update on the prevalence of serum antibodies (IgG and IgM) to adeno-associated virus (AAV) J Med Virol. 1999;59:406–411. doi: 10.1002/(sici)1096-9071(199911)59:3<406::aid-jmv22>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 30.Hobbs JA. Detection of adeno-associated virus 2 and parvovirus B19 in the human dorsolateral prefrontal cortex. J Neurovirol. 2006;12:190–199. doi: 10.1080/13550280600827351. [DOI] [PubMed] [Google Scholar]
- 31.Watson DJ, Passini MA, Wolfe JH. Transduction of the choroid plexus and ependyma in neonatal mouse brain by vesicular stomatitis virus glycoprotein-pseudotyped lentivirus and adeno-associated virus type 5 vectors. Hum Gene Ther. 2005;16:49–56. doi: 10.1089/hum.2005.16.49. [DOI] [PubMed] [Google Scholar]
- 32.Liu G, Martins IH, Chiorini JA, Davidson BL. Adeno-associated virus type 4 (AAV4) targets ependyma and astrocytes in the subventricular zone and RMS. Gene Ther. 2005;12:1503–1508. doi: 10.1038/sj.gt.3302554. [DOI] [PubMed] [Google Scholar]
- 33.Wang C, Wang CM, Clark KR, Sferra TJ. Recombinant AAV serotype 1 transduction efficiency and tropism in the murine brain. Gene Ther. 2003;10:1528–1534. doi: 10.1038/sj.gt.3302011. [DOI] [PubMed] [Google Scholar]
- 34.Minagar A, Shapshak P, Fujimura R, Ownby R, Heyes M, Eisdorfer C. The role of macrophage/microglia and astrocytes in the pathogenesis of three neurologic disorders: HIV-associated dementia, Alzheimer disease, and multiple sclerosis. J Neurol Sci. 2002;202:13–23. doi: 10.1016/s0022-510x(02)00207-1. [DOI] [PubMed] [Google Scholar]
- 35.Maragakis NJ, Rothstein JD. Mechanisms of disease: astrocytes in neurodegenerative disease. Nat Clin Pract Neurol. 2006;2:679–689. doi: 10.1038/ncpneuro0355. [DOI] [PubMed] [Google Scholar]
- 36.He F, Sun YE. Glial cells more than support cells? Int J Biochem Cell Biol. 2007;39:661–665. doi: 10.1016/j.biocel.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 37.Baumann N, Pham-Dinh D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol Rev. 2001;81:871–927. doi: 10.1152/physrev.2001.81.2.871. [DOI] [PubMed] [Google Scholar]
- 38.Pleasure D, Soulika A, Singh SK, Gallo V, Bannerman P. Inflammation in white matter: clinical and pathophysiological aspects. Ment Retard Dev Disabil Res Rev. 2006;12:141–146. doi: 10.1002/mrdd.20100. [DOI] [PubMed] [Google Scholar]
- 39.Gao G, Qu G, Burnham MS, Huang J, Chirmule N, Joshi B, et al. Purification of recombinant adeno-associated virus vectors by column chromatography and its performance in vivo. Hum Gene Ther. 2000;11:2079–2091. doi: 10.1089/104303400750001390. [DOI] [PubMed] [Google Scholar]
- 40.Wolfe JH, Sands MS. Murine mucopolysaccharidosis type VII: a model system for somatic gene therapy of the central nervous system. In: Lowenstein RR, Enquist LW, editors. Protocols for Gene Transfer in Neuroscience: Towards Gene Therapy of Neurological Disorders. London: Wiley; 1996. pp. 263–274. [Google Scholar]
- 41.Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. San Diego, CA: Academic Press; 2001. [Google Scholar]
- 42.Mouton PR. Principles and Practices of Unbiased Stereology: An Introduction for Bioscientists. Baltimore, MD: Johns Hopkins University Press; 2002. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Comparison of vector distribution resulting from unilateral injection of rh.8, pi.2, hu.32, or hu.11, into the hippocampus.
Figure S2 Examples of GFP fluorescence in brains injected neonatally.