Abstract
Whilst being environmentally abundant, aluminum is not essential for life. On the contrary, aluminum is a widely recognized neurotoxin that inhibits more than 200 biologically important functions and causes various adverse effects in plants, animals, and humans. The relationship between aluminum exposure and neurodegenerative diseases, including dialysis encephalopathy, amyotrophic lateral sclerosis and Parkinsonism dementia in the Kii Peninsula and Guam, and Alzheimer's disease (AD) has been suggested. In particular, the link between aluminum and Alzheimer's disease has been the subject of scientific debate for several decades. However, the complex characteristics of aluminum bioavailability make it difficult to evaluate its toxicity and therefore, the relationship remains to be established. Mounting evidence has suggested that significance of oligomerization of β-amyloid protein and neurotoxicity in the molecular mechanism of AD pathogenesis. Aluminum may play crucial roles as a cross-linker in β-amyloid oligomerization. Here, we review the detailed characteristics of aluminum neurotoxicity based on our own studies and the recent literatures. Our aim is to revisit the link between aluminum and AD and to integrate aluminum and amyloid cascade hypotheses in the context of β-amyloid oligomerization and the interactions with other metals.
1. Introduction
Aluminum (Al) is abundantly distributed in our environment, and compounds containing Al have been used in manufacturing (e.g., clays, glasses, and alum) for centuries. Despite its abundance, Al was first isolated as an element in 1827, and its use as being a silvery metal began only after 1886. Al is a new metal in this context. Because of its beneficial characteristics such as a lightweight, nonmagnetic, malleable, and ductile element, Al has a widespread and important use in industrial applications and consumer products. Al is also used in cooking utensils and in pharmacological agents including antacids and antiperspirants from which the element enters the human body.
Al is not essential for life. On the contrary, Al is a well established neurotoxin and is suspected to be linked with various neurodegenerative diseases including Alzheimer's disease (AD), amyotrophic lateral sclerosis (ALS), and Parkinsonism dementia in the Kii Peninsula and Guam [1], and the Gulf War syndrome [2].
In particular, a possible relationship between Al and the pathogenesis of AD has been discussed for several decades [3–7]. AD is a severe senile type of dementia first reported in 1906. The pathological hallmarks of AD are the deposition of extracellular senile plaques, intracellular neurofibrillary tangles (NFTs), and the selective loss of synapses and neurons in the hippocampal and cerebral cortical regions. The major component of NFTs is the phosphorylated tau protein. Senile plaques are largely comprised of β-amyloid protein (AβP) [8]. The hypothesis that Al is an environmental contributor to the pathogenesis of AD, termed the “aluminum hypothesis”, was proposed in the 1960s based on various neurotoxicological, analytical, and epidemiological findings [9–11]. In spite of these findings, the aluminum hypothesis has been the subject of much debate and criticism for several decades. During this period, great progress was made in AD research. Particularly, numerous studies have supported the idea termed “amyloid cascade hypothesis”, namely that the conformational changes of AβP and its neurotoxicity play a central role in AD pathogenesis [12, 13]. Al3+ and other metals including Zn2+, Cu2+, and Fe3+ influence the oligomerization and conformational changes of AβP as cross-linkers, and, therefore, their implications are important in this context. Furthermore, increasing evidence suggests the implication of these metals in the pathogenesis of AD [14–16]. Al binds to various metal-binding proteins and influences homeostasis of other metals.
We review here the detailed characteristics of aluminum neurotoxicity based on our own studies and the recent literature. Our aim is to update the various adverse effects of Al and revisit the link between Al and AD based on new findings on Al-induced conformational changes and metal-metal interactions.
2. Neurotoxicity of Aluminum Update
2.1. Effects of Al on the Memory Disorder of Human: Historical Overview
An association between Al poisoning and memory disorder in humans was first reported in 1921 [17]. Later, it was shown that the intracerebral administration of Al induced epilepsy in experimental animals [18]. As a component of dialysis solutions or Al-containing pharmacological compounds, Al is known to cause various dialysis-related disorders, including osteomalacia (aluminum bone disease), microcytic anemia, β2-microglobulin-associated amyloidosis [19], and dialysis encephalopathy in hemodialysis patients [20].
The accidental contamination of Al into drinking water occurred and more than 20,000 persons were exposed to high level of Al at 1988 in Camelford (Cornwall, UK). Residents exposed to contaminated Al exhibited various symptoms related to cerebral impairments such as loss of concentration and short term memory in a 10-year follow-up study [21].
Martyn et al. reported a high incidence of AD in areas with a high level of Al in the drinking water in England and Wales [11]. A considerable number of studies have provided evidence to support an association between AD and Al in drinking water after this initial report [22]. Frecker reported on a Norwegian area where high Al concentrations in drinking water were linked with high dementia mortality [23]. Neri and Hewitt found a positive relationship between Al in drinking water and AD risk in Canada [24]. Forbes and McLachlan demonstrated a greater risk of AD in Canadian areas where concentrations of Al are high and those of fluoride are low [25]. Rondeau et al. demonstrated that high daily intake of Al was correlated with increased risk of dementia or cognitive decline in a 15-year follow-up French cohort study [26–28]. These studies suggest that Al has adverse effects on human memories and causes dementia when it enters the brain.
2.2. Effects of Al on the Central Nervous System In Vitro or In Vivo
Despite it's environmental abundance, Al is not an essential element for living organisms, and no enzymatic reaction requires Al. Al is reported to influence more than 200 biologically important reactions and to cause various adverse effects on the mammalian central nervous system (CNS) (Table 1). These include crucial reactions for brain development such as the axonal transport, neurotransmitter synthesis, synaptic transmission, phosphorylation or dephosphorylation of proteins, protein degradation, gene expression, and inflammatory responses.
Table 1.
Effects of aluminum on the central nervous system.
| References | |
|---|---|
| (1) Nucleus and gene expression | |
| Binding to DNA | |
| Binds to histone-DNA complex and induces conformational changes of chromatin. | [29] |
| Induces topological changes of DNA. | [30, 31] |
| Altered gene expression | |
| Induces decreased expression of neurofilament and tubulin. | [32] |
| Induces altered expression of genes of neurofilament, APP, and neuron specific enolase. | [33] |
| Induces decreased expression of transferrin receptor. | [34] |
| Induces altered expression of RNA polymerase I. | [35] |
| Induces downregulation of mitochondrial cytochrome c oxidase. | [36] |
| Induces altered expression of calbindin-D28k. | [37] |
| Induces decrease in the expression of nerve growth factor (NGF) and brain derived neurotrophic factor (BDNF). | [38] |
| Induces expression of pro-inflammatory genes and pro-apoptotic genes. | [39] |
| Induces elevated expression of APP. | [40, 41] |
| Induces altered expression of oxidative stress marker genes (SOD1, glutathione reductase, etc.). | [42] |
| Induces decreased expression of neprilysin. | [43] |
| Induces altered expression of β-APP secretase (BACE1 and BACE2). | [40, 44] |
| (2) Cellular functions | |
| Energy metabolism | |
| Inhibits the activity of hexokinase | [45] |
| Inhibits the activity of phosphofructokinase | [46] |
| Inhibits the activity of glucose-6-phosphate dehydrogenase | [47] |
| Causes mitochondrial dysfunction and depletion of ATP | [48, 49] |
| Decreases in activity and expression of TCA-cycle related enzymes (succinate dehydrogenase (SDH), alpha-ketoglutarate dehydrogenase (KGDH), isocitrate dehydrogenase-NAD+ (IDH), fumarase (FUM), aconitase (ACN), and cytochrome c oxidase (Cyt C Ox)). | [50] |
| Phosphorylation and dephosphorylation | |
| Inhibits the activity of protein phosphatase. | [51] |
| Increases the activity of protein kinase C and cytoskeleton proteins. | [52] |
| Accelerates phosphorylation and accumulation of neurofilament. | [53] |
| Enhances Ca2+/Calmodulin dependent protein kinase activity. | [54] |
| Accelerates phosphorylation of MAP 2 and neurofilament. | [55] |
| Inhibits dephosphorylation of tau. | [56] |
| Induces nonenzymatic phosphorylation of tau. | [57] |
| Abnormal accumulation of proteins | |
| Causes the conformational change and the accumulation of neurofilament and MAP1A, MAP1B. | [58] |
| Accelerates the phosphorylation of tau and its accumulation. | [59] |
| Causes the accumulation of tau protein in neuroblastoma cells or in primary cultured neurons. | [60, 61] |
| Causes the accumulation of tau protein in experimental animals. | [33, 62, 63] |
| Causes neurofibrillary degeneration in vivo. | [9] |
| Causes the accumulation of AβP in cultured neurons or in neuroblastoma cells. | [64, 65] |
| Causes the accumulation of AβP in vivo. | [44, 66, 67] |
| Neurotransmitter release | |
| Inhibits glutamate release. | [68] |
| Impairs synaptic transmission. | [69, 70] |
| Inactivates glutamate dehydrogenase. | [71] |
| Inhibits NMDA-type glutamate receptor. | [72] |
| Inhibits choline acetyl transferase and tyrosine hydroxylase, glutamate decarboxylase. | [73, 74] |
| Influences acetyl-CoA and inhibits acetylcholine release. | [75] |
| Activates monoamine oxidase. | [76, 77] |
| Inhibits dopamine beta-hydroxylase. | [78] |
| Inhibits uptake of serotonin and noradrenalin in synaptosomes. | [79] |
| Channel inhibition | |
| Influences the activities of Na+ channels and K+ channels. | [80] |
| Enhances the voltage-activated Na+ channels. | [81] |
| Inhibits the voltage-gated calcium channel. | [70, 82] |
| Inhibits the IP3-mediated Ca2+ release. | [83] |
| Others | |
| Influences GTP binding proteins as aluminum fluoride. | [84] |
| Inhibits GAP junction. | [85] |
| Inhibits axonal transports. | [86] |
| Binds to calmodulin and inhibition of calmodulin-binding enzymes. | [87] |
| Induces inflammatory responses. | [88] |
| (3) Membrane lipids | |
| Peroxidation | |
| Accelerates iron-induced membrane lipid peroxidation. | [89] |
| Enhances lipid peroxidation in liposomes. | [90] |
| Induces peroxidation of myelin lipids in vivo. | [91] |
| Increases peroxidation products (malondialdehyde). | [59] |
| Membrane properties | |
| Causes the change the lipid/phospholipids profiles of myelin in vivo. | [92] |
| Induces the change in membrane physical properties (surface potential, lipid fluidity, and lipid arrangement). | [91] |
| Induces the change of membrane fluidity. | [93] |
| (4) Higher functions | |
| Cell death | |
| Causes the apoptotic neuronal death. | [94, 95] |
| Causes the apoptosis of astrocytes. | [96] |
| Causes the death of motor neuron. | [97, 98] |
| Behavior, learning, and memory, others | |
| Inhibits long term potentiation (LTP). | [99, 100] |
| Causes learning disorder or memory deficit in experimental animals. | [101–103] |
| Influences electrical activity in hippocampus and inhibits spatial learning memory deficit in aging rats. | [104] |
| Causes memory deficit in AD model mice. | [105, 106] |
| Causes encephalopathy in dialysis patients. | [20] |
| Causes encephalopathy in patients with renal failure. | [107] |
Al exhibits only one oxidation state, Al3+. Al3+ has affinity for negatively charged, oxygen-donor ligands. Inorganic and organic phosphates, carboxylate, and deprotonated hydroxyl groups form strong bonds with Al3+. Owing to these chemical characteristics, Al3+ binds to the phosphate groups of DNA and RNA, affecting DNA topology and influencing the expression of various genes essential for brain functions. Lukiw et al. reported that nanomolar levels of Al3+ were sufficient to influence neuronal gene expression [33, 35].
Al3+ also binds to the phosphate groups of nucleoside di- and triphosphates, such as ATP and can thus influence energy metabolism. Furthermore, Al inhibits the functions of various protein kinases and phosphatases.
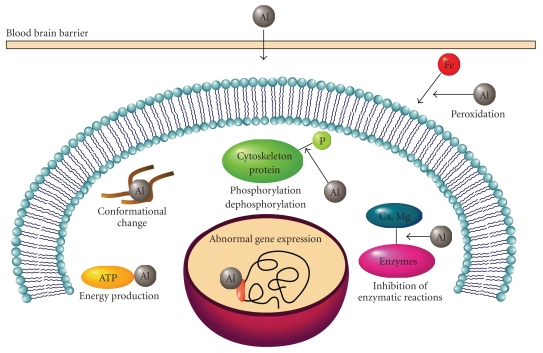
Al3+ has very low ligand-exchange rate in comparison to other metals. For example, the ligand-exchange rate of Mg2+ is 105 times faster than that of Al3+, and therefore, Al3+ inhibits enzymes with Mg2+ cofactors. Al3+ also inhibits biological processes involving rapid Ca2+ exchange: the exchange rate for Al3+ is 108 times slower than that of Ca2+. These properties make Al useless in enzymatic reactions and increase its half-life in the human body. We show the typical effects of Al in Figure 1.
Figure 1.
Effects of aluminum on the central nervous system. Major biological effects of Al on the central nervous system are depicted.
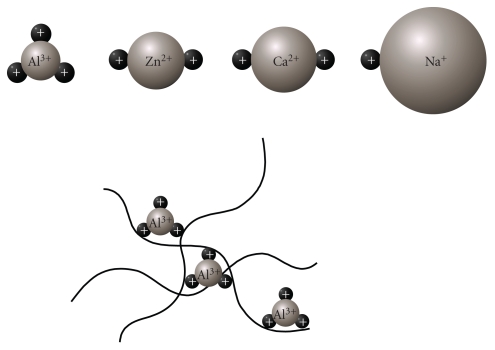
Al3+ has strong positive charges and a relatively small ionic radius in comparison to other metal ions such as Ca2+, Zn2+, and Na+ (Figure 2). Thus, Al3+ firmly binds to metal-binding amino acids (histidine (His), tyrosine (Tyr), arginine (Arg) etc.) or phosphorylated amino acids and acts as a cross-linker; this property has made it useful as a leather tanning agent. By binding to various proteins, Al can cause the oligomerization of proteins, inducing conformational changes that can inhibit their degradation by proteases. Strong binding of Al3+ to phosphorylated amino acids promotes the self-aggregation and accumulation of highly phosphorylated cytoskeleton proteins, including neurofilament and microtubule-associated proteins (MAPs), and so forth [58].
Figure 2.
Cross-linking of protein by Al3+. Al3+ has a relatively small ionic radius (50 pm) with 3 positive charges; here it is compared to other metal ions such as Zn2+ (74 pm), Ca2+ (99 pm), and Na+ (95 pm). These characteristics enable Al to be an effective cross-linker of proteins.
Consequently, Al causes apoptotic death of neurons and glial cells. Chronic administration of Al impairs long-term potentiation (LTP), which is a form of synaptic information storage well-known as a paradigm of memory mechanisms. Al also impairs various enzymes including those related to neurotransmitter synthesis and thus affects the neurotransmitter content. Al3+ also inhibits voltage-gated Ca2+ channels and neurotransmitter receptors, and impairs synaptic transmission. Finally, Al causes spatial memory deficit, influences emotional reactivity, and impairs various brain functions related to learning and memory. These adverse effects may be involved in the mechanisms that underlie Al-induced memory disorder.
3. Link between Al and AD
3.1. Historical Overview of Aluminum Hypothesis and Arguments
A link between Al and AD is supported on many fronts, beginning in 1965 with the finding of Klatzo et al. that the intracerebral administration of Al to experimental animals induced neurofibrillary degeneration and the appearance of tangle-like structures that were similar to the NFTs found in the brains of AD patients [9]. Crapper et al. reported an increased level of Al in the brains of AD patients [10]. In the 1970s, Al in dialysis solutions or pharmacological compounds was found to cause dementia in dialysis patients (dialysis encephalopathy) [20]. As noted previously, several epidemiological studies reported a high percentage of AD cases in areas with high Al level in drinking water [11, 19].
Despite supporting evidence, the aluminum hypothesis of AD remains controversial and has been the subject of much debate in the past few decades. There were at least three arguments against the aluminum hypothesis. First, it has been argued that neurofibrillary changes in Al-intoxicated animals (Al-NFTs) are different from those in AD patients (AD-NFTs) [108]. Arguments cite morphological and biochemical differences such as the lack of paired helical filamental (PHF) structures, their different distributions in nerve terminals, and the absence of immunoreactivity for tau protein, which is the main component of NFTs in AD patients. Second, there is no significant difference in Al levels of AD patients and age-matched controls [109]. Third, the epidemiological studies on Al in drinking water are immature and inconclusive [110]. However, most of these criticisms were made in the 1990s. We would like to reinvestigate these early arguments in the context of new findings in the study of AD.
Regarding the first argument, more recent immunohistochemical studies have indicated that depositions in the brains of Al-intoxicated animals are stained with the anti-tau antibody [62, 63]. The accumulation of tau protein was reported in patients with dialysis encephalopathy [111], and in Al-intoxicated cultured neuronal cells [60, 61]. Al inhibits the dephosphorylation of tau [56] and enhances its aggregation in vitro [112]. Furthermore, NFTs in some AD patients have been shown to be composed of straight-type filaments rather than PHF-type filaments as is observed in Al-NFT [113]. These data indicate that attempts to discredit the aluminum hypothesis on the basis of differences between Al-NFTs and AD-NFTs are no longer tenable.
3.2. Accumulation of Al in AD Brain
Another argument cites a lack of significant difference between Al levels in AD patients and age-matched controls. One reason for the controversy may be Al contamination of the solutions used in the process of tissue fixation and staining. Therefore, prior studies in fixed tissues cannot be relied upon for precise measures of Al; quantitative analysis of nonfixed and freshly frozen tissues is necessary. One such study showed that the amount of Al in whole brains of AD patients was not significantly different in comparison to controls [114]. Landsberg et al. claimed that they could not detect Al in senile plaques or NFTs using nuclear microscopy [115]. However, this failure could simply be due to low detection limits of their analytical method. Bouras et al. used highly sensitive laser microprobe mass analysis (LAMMA) with nonfixed brain samples and reported an accumulation of Al in NFT-bearing neurons of AD brains [116]. An accumulation of Al in both senile plaques and NFTs has been reported in renal failure patients [117]. Recently, Yumoto et al. analyzed Al using energy-dispersive X-ray spectroscopy combined with transmission electron microscopy (TEM-EDX), a method which yields a high-resolution and low detection limit. Their detailed analysis demonstrated that Al was present in cores of senile plaques at a concentration of 35–50 ppm [118].
3.3. Epidemiological Studies of AD and Al in Drinking Water
Some epidemiological studies have failed to demonstrate the relationship between Al and AD [119, 120]. However, there are a number of possible explanations for this inconsistency, particularly when considering the difficulty in making side-by-side comparisons of epidemiological studies of Al (e.g., intake estimations, effect of move, changes in water-treatment processes, etc.). Using strict neuropathological criteria to discriminate between AD patients and controls (including histopathological verification), McLachlan et al. found an elevated risk of histopathologically verified AD to be associated with the consumption of higher concentrations of Al in drinking water [121]. More detailed analysis revealed an association between exposure to organic monomeric Al and AD, even after adjustment for education level, family history and presence of the apoE4 allele [122].
The amount of Al consumed in drinking water is approximately 5% of the total daily intake. Thus, it is possible that some factors that prevent or accelerate Al absorption may exist in drinking water. Silicate in the water was reported to interact with Al and prevent Al toxicity to fish [123, 124]. Therefore, the level of silicate in drinking water may also be important. In a French cohort study, the relationship between Al and cognitive impairment is suggested to be influenced by the silica concentration [29]. Cognitive impairment among women was correlated with low concentrations of silica in drinking water [125].
In considering the above new lines of evidence about the neurotoxicity and epidemiology of Al, it is difficult to agree with the early criticisms of the aluminum hypothesis.
3.4. Effects of Al on the Oligomerization of AβP
In the 1990s when the early arguments were claimed, Al-induced Alzheimer-like pathological changes were first attributed to tau proteins (NFT). However, numerous biochemical, toxicological, cell biological, and genetic studies have supported the “amyloid cascade hypothesis”, namely, that the accumulation of AβP and its neurotoxicity play a central role in the pathogenesis of AD [12, 13].
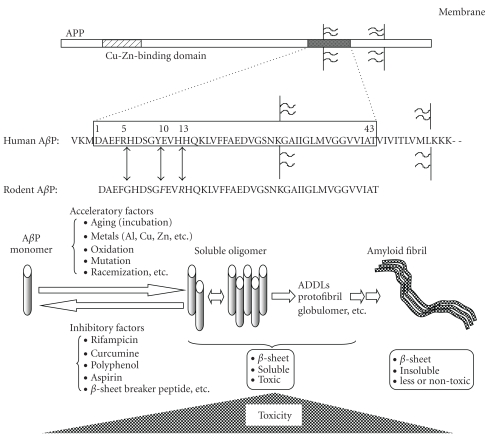
AβP is a small peptide of 39–43 amino acid residues, secreted by cleavage of the amyloid precursor protein (APP) N-terminus by β-APP cleaving enzyme (BACE) and intramembrane cleavage of its C-terminus by γ-secretase. Genetic studies of early-onset cases of familial AD indicated that APP mutations and AβP metabolism are associated with AD [126]. Yankner et al. reported that the first 40 amino acid residues of AβP (AβP(1–40)) caused the death of cultured rat hippocampal neurons or neurodegeneration in the brains of experimental animals [127]. AβP is a hydrophobic peptide with an intrinsic tendency to self-assemble and form SDS-stable oligomers in aqueous solution. The monomeric form of AβP has a random coiled structure. Oligomeric AβPs have β-pleated sheet structures and form insoluble aggregates, termed amyloid fibrils. Neurotoxicity of AβP(1–40) peptides was enhanced by the process of “aging” (aggregated under incubation at 37°C for several days) compared to freshly prepared AβP(1–40) in cultured neurons [128], and were correlated with its β-sheet contents [129]. Recent approaches using size-exclusion chromatography, gel electrophoresis, and atomic force microscopy have demonstrated that the soluble oligomers are synaptotoxic and neurotoxic [130]. Figure 3 exhibits the oligomerization of AβP and its neurotoxicity.
Figure 3.
Secretion of AβP from APP and its oligomerization. AβP is secreted by the cleavage of the APP N-terminus by β-secretase (BACE), followed by the intramembrane cleavage of the C-terminus by γ-secretase. APP also binds to Cu or Zn. Human AβP and rodent AβP differ by 3 amino acids (Arg5, Tyr10, and His13). AβP monomers form random-coil structures. However, under aging conditions or the existence of trace metals such as Al, Zn, and Cu, AβP self-aggregates and oligomerizes (dimmer to protofibrils), and then forms insoluble amyloid fibrils. Although monomeric AβPs are not toxic, oligomeric AβPs induce marked neuronal death.
Considering that AβP is secreted in the cerebrospinal fluid (CSF) of young individuals as well as in aged or dementia patients [146], factors that accelerate or inhibit oligomerization may play essential roles in the pathogenesis of AD. Several factors such as peptide concentration, pH or composition of solvents, and temperature can influence the oligomerization processes [147].
Interestingly, rodent AβP exhibits less tendency to oligomerization than human AβP in vitro [148] and the accumulation of AβP is rarely observed in the brains of rodents (rats or mice) as compared to primates (humans or monkeys). As shown in Figure 3, the amino acid sequences of human and rodent AβP are similar, but rodent AβP differs from primate only 3 amino acids (Arg5, Tyr10, and His13) from primate AβP. All 3 amino acids have the ability to bind metals. Therefore, trace elements including Al3+ are of particular interest as potential acceleratory factors and may play important roles in the accumulation of AβP in the human brain.
Table 2 summarizes the effects of Al3+ on conformational changes of AβP and other various disease-related proteins. Exley et al. first demonstrated by CD spectroscopy that Al induces a conformational change in AβP(1–40) [131]. Al has also been shown to promote the aggregation of 125I-labelled AβP(1–40), with similar findings for Fe and Zn [132]. Bush et al. demonstrated that Zn2+ and Cu2+ caused the oligomerization of AβP [149, 150]. However, role of Zn2+ in AD is complex and enigmatic. Lovell et al. reported that zinc has the protective effects against AβP-induced neurotoxicity [151]. We have demonstrated that Zn2+ blocks AβP-channels formed on membranes and inhibits the neurotoxicity [152].
Table 2.
Al-induced conformational changes of various proteins.
| Proteins | References |
|---|---|
| Disease-related proteins | |
| Alzheimer's disease | |
| AβP (1-40) | [64, 131–134] |
| DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVV | |
| AβP (1-42): | [135, 136] |
| DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVVIA | |
| AβP (25-35): | [137] |
| GSNKGAIIGLMV | |
| APP | [138] |
| Tau or hyperphosphorylated tau (PHF-tau) | [32, 57, 139] |
| Perkinson's disease and other diseases with Lewy body | |
| α-synuclein (NACP) | [140, 141] |
| Type 2 diabetes mellitus | |
| Amylin: | [142] |
| KCNTATCATQRLANFLVHSSNNFGAILSSTNVGSNTY | |
| Familial British dementia | |
| ABri: | [143] |
| ASNCPAIRHPGNKPAVGTLICSRTVKKNIIGGN | |
| Spinocerebellar ataxia | |
| Ataxin 3 | [144] |
| Dialysis-related arthropathy | |
| β2-microglobulin | [145] |
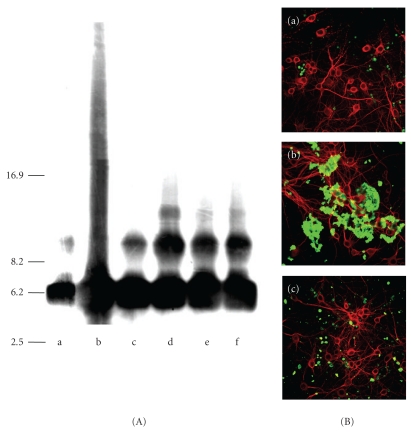
We have developed a system for investigating AβP oligomerization that involves immunoblotting and precipitation. Using this system, we have demonstrated that Al enhances the polymerization of AβP(1–40) and forms SDS-stable oligomers in vitro [64, 133, 134]. The aggregated AβP(1–40) is redissolved by adding deferoxamine (DFO), an Al chelator. The oligomerization induced by Al is more marked than that induced by other metals, including Zn2+, Fe3+, Cu2+, and Cd2+(Figure 4(A)). Furthermore, while Al-aggregated AβPs bind tightly to the surface of cultured neurons and form fibrillar deposits, Zn-aggregated AβPs are rarely observed on the surface of cultured neurons (Figure 4(B)). These results suggest that Al-aggregated AβPs have a strong affinity for membrane surfaces as a result of minimal degradation by proteases. Indeed, Al has been shown to inhibit the degradation of AβP as the result of conformational changes [43, 153]. Furthermore, AβP coupled with Al is more toxic than normal AβP causing membrane disruption or perturbation of neural Ca2+ homeostasis and mitochondrial respiration [154–156].
Figure 4.
Aggregation of AβP by Al and other metals. (A) Immunoblotting of AβP preincubated with Al and other metals. The solutions of AβP(1–40) were incubated at 37°C for 24 h with or without 1 mM of various metals, and were analyzed by SDS-PAGE and immunoblotting using an antibody to AβP. Each lane contained 4 μg AβP(1–40). Lane a: control, b: AlCl3, c: ZnCl2, d: CuCl2, e: FeCl3, f: CdCl2, Modified from [134]. (B) Deposition of AβP on surfaces of cultured neurons. Solutions of AβP(1–40) preincubated at 37°C for 24 h (a), with 1 mM AlCl3 (b), or 1 mM ZnCl2 (c) were applied to cultured rat cortical neurons. After 2 days of exposure, cells were washed and double immunostained with a polyclonal antibody to AβP (green) and a monoclonal antibody to MAP2 (red), and observed by laser confocal microscope. Scale bar: 50 μm, modified from [64].
The chronic application of Al caused the accumulation of AβP in cultured neurons of rat cerebral cortex [64] and in neuroblastoma cells [65]. Praticó et al. (2002) found that orally administered Al caused a marked increase in the amount of AβP both in its secreted and accumulated forms, and increased deposition of senile plaques in AD-model mice transfected with the human APP gene (Tg 2576) [66]. These results are consistent with other studies demonstrating that oral Al exposure causes the accumulation of AβP and impairs spatial learning memory in AD-model mice [67].
Exposure to Al causes the accumulation of AβP and induces adverse effects in humans as seen in the aftermath of the accidental Al exposure in 1988 at Camelford [157]. The neuropathological case study of a 58 year-old woman who was exposed to Al and died 15 years later with unspecified neurological symptoms demonstrated the rare form of sporadic cerebral amyloid angiopathy, which is characterized by the deposition of AβP in blood vessels and has a causative link with AD [158]. The deposition of high amounts of Al in the patient's brain was also observed.
Al has also been reported to bind and cause conformational changes in other AD-related proteins, including APP [138], tau protein [32, 57], and PHF-tau protein [139] and in proteins related to other neurodegenerative diseases such as α-synuclein (Parkinson's disease (PD) and dementia with Lewy bodies; DLB) [140, 141], amylin (diabetes mellitus) [142], ABri (familial British dementia) [143], and ataxin 3 (spinocerebellar ataxia type 3) [144], β2-microglobulin (dialysis-related arthropathy) [145] (Table 2).
3.5. Metal-Metal Interactions in the Pathogenesis of AD
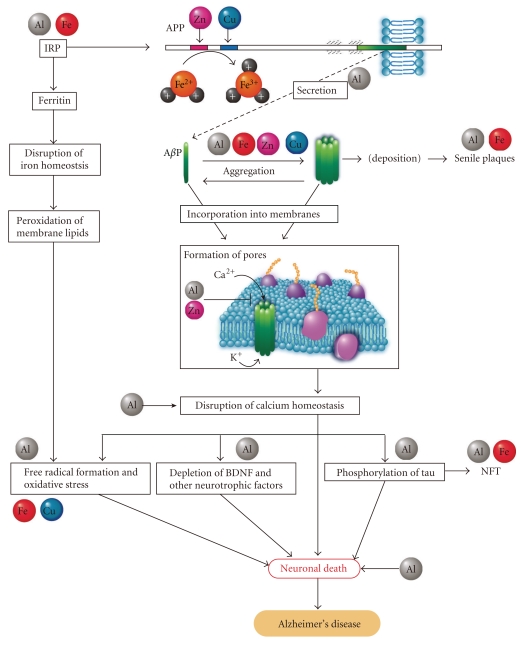
The evidence now suggests that the significance of Al in the pathogenesis of AD should be concerned. Other metals usually share the binding site of one metal ion, although their binding constants differ. Al binds to various metal-binding proteins and influences metal homeostasis. The interactions between Al and other metals should be considered owing to the implications of various trace elements in the pathogenesis of AD. Figure 5 illustrates the modified aluminum hypothesis that accounts for the implications of Al and other trace metals in AD pathology from the secretion of AβP to its neurotoxicity as mentioned below.
Figure 5.
Modified aluminum hypothesis addressing the implications of Al and other trace metals in the pathogenesis of Alzheimer's disease. This model describes the implication of Al and other trace metals including Fe, Cu, and Zn in APP processing, generation and oligomerization of AβP, and the neurotoxic effects caused by AβP. Details are descried in the text.
3.5.1. Al3+ Affects Iron-Homeostasis and Generates Free Radicals
Al has similar characteristics to iron (Fe) and binds to Fe-binding proteins such as ferritin, transferrin, iron regulatory protein (IRP) or to iron chelators such as DFO. The iron responsive element/iron regulatory protein (IRE/IRP) network regulates the production of iron binding proteins which prevent the formation of free Fe2+, which causes toxic free radicals [159]. In iron-deficient conditions, IRP binds to IRE and regulates the expression of genes that contain IREs in their mRNA, such as ferritin or transferrin. As the concentration of free Fe2+ increases, the binding of iron to IRP, expression of transferrin is downregulated and that of ferritin is upregulated, and the amount of free Fe2+ is thereby decreased. Al3+ also binds to IRP [34, 160], and thus influences the expression of Fe-binding proteins with IREs in their mRNA causing an elevated Fe concentration [161]. Al also influences the uptake of iron into cultured neurons or glial cells [34, 162]. Thus, Al3+ affects iron homeostasis and the expression of various iron-regulated proteins with IREs. Important findings are that APP mRNA contains an IRE as well as ferritin, and its expression is regulated by iron [163]. Indeed, Al caused elevated expression of APP in experimental animals [40, 41]. Recently, Duce et al. demonstrated that APP has ferroxidase activity, which converts Fe2+ to Fe3+ and regulates free pro-oxidant Fe2+ concentrations [164]. They also found that Zn2+ inhibits the ferroxidase activity of APP. APP also possesses copper/zinc binding sites in its amino-terminal domain and in the AβP domain and may be involved in homeostasis of these metals [165]. Al3+ stimulates Fe-induced membrane lipid peroxidation and causes oxidative damage in vitro and in vivo, although Al3+ does not directly affect peroxidation [89, 90]. There are other important findings implicating iron homeostasis in AD pathogenesis. Iron related genes such as transferrin C2 or hemochromatosis were revealed to be risk factors for AD [166, 167]. Imagawa et al. (1992) reported that iron supplementation was effective for the recovery of cognitive functions in AD patients [168].
3.5.2. Al3+ and Other Metals Enhance the Oligomerization of AβP
An abnormal expression of APP could lead to an increased secretion of AβP, and then enhance its accumulation. Secreted AβP is usually degraded by various proteases such as neprilysin within a short period. The downregulation of neprilysin induced by Al can cause the accumulation AβP [43]. Furthermore, AβP becomes oligomerized in the presence of trace metals such as Al3+, Zn2+, Fe3+, and Cu2+, could be resistant to proteases, and thus accumulates in the brain.
3.5.3. Al3+ Impairs Calcium Homeostasis
AβP oligomers could be readily incorporated into cell membranes, resulting in the formation of ion channels [147]. A subsequent influx of Ca2+ through these amyloid channels would lead to the phosphorylation of tau, depletion of neurotrophic factors, and the formation of free radicals, and so forth, with the outcome of these effects being neuronal death. Al3+ blocks various Ca2+ channels and influences Ca2+ homeostasis. We found that Al also inhibits the increase in Ca2+ levels induced by brain-derived neurotrophic factor (BDNF) [94]. As described previously, Al is implicated in most of these neurodegenerative pathways such as dephosphorylation of tau [56], depletion of neurotrophic factor [38], formation of free radicals [89], and induction of neuronal death.
This working hypothesis may be useful in developing an understanding of the link between AD and trace elements including Al, Zn, Cu, and Fe. Considering the implications of metals in AD pathogenesis, chelation therapy for AD treatment is of great interest [169]. Clioquinol (quinoform), a chelator of Cu2+ or Zn2+, inhibits oligomerization of AβP and attenuates the accumulation of amyloid in the brains of experimental animals. Clinical trials using its analogue PBT2 are under investigation [170]. DFO, a chelator of Al and Fe, attenuates the decline of daily living skills in AD patients [171]. Silicates, which couple with Al and reduce its toxicity, are also candidates for chelation therapy in AD [172].
4. Conclusion: Al and Human Health
In this review, we have summarized the properties associated with various aspects of Al neurotoxicity. There is growing evidence for a link between Al and AD, and between other metals and AD. Nevertheless, because the precise mechanism of AD pathogenesis remains unknown, this issue is controversial. However, it is widely accepted that Al is a recognized neurotoxin, and that it could cause cognitive deficiency and dementia when it enters the brain and may have various adverse effects on CNS. In general, the absorption of metals by the gastrointestinal tract is widely variable and is influenced by various factors including an individual difference, age, pH, stomach contents [173]. Recent studies using mass spectrometry of 26Al have demonstrated that small, but a considerable amount of Al crosses the blood brain barrier, enters into the brain, and accumulates in a semipermanent manner [174, 175]. Therefore, Al can cause severe health problems in particular populations, including infants, elderly people, and patients with impaired renal functions, and unnecessary exposure to Al should be avoided for such patients [176].
In 1989, a joint FAO/WHO Expert Committee on Food Additives (JECFA) recommended a provisional tolerable weekly intake (PTWI) of 7.0 mg/kg body weight Al; however, this was changed in 2007 to 1.0 mg/kg body weight because of potential effects on the reproductive system and the developing nervous system. The characteristics of Al neurotoxity are complex, and further research is needed especially in relation to bioavailability, cellular effects, metabolism, and metal-metal interactions.
Acknowledgments
This work was partially supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan and by a Grant from Cooperation for Innovative Technology and Advanced Research in Evolutional Area (CITY AREA) from the Miyazaki Prefectural Industrial Support Foundation.
Abbreviations
- AD:
Alzheimer's disease
- AβP:
β-amyloid protein
- Al:
Aluminum
- ALS:
Amyotrophic lateral sclerosis
- APP:
Amyloid precursor protein
- BACE:
β-APP cleaving enzyme
- BDNF:
Brain derived neurotrophic factor
- CSF:
Cerebrospinal fluid
- CNS:
Central nervous system
- DFO:
Deferoxamine
- DLB:
Dementia with Lewy bodies
- JECFA:
FAO/WHO Expert Committee on Food Additives
- IRE:
Iron responsive element
- IRP:
Iron regulatory protein
- LAMMA:
Laser microprobe mass analysis
- LTP:
Long-term potentiation
- MAP:
Microtubule-associated protein
- NFT:
Neurofibrillary tangle
- NGF:
Nerve growth factor
- PD:
Parkinson's disease
- PHF:
Paired helical filament
- PTWI:
Provisional tolerable weekly intake
- TEM-EDX:
Energy-dispersive X-ray spectroscopy combined with transmission electron microscopy.
References
- 1.Shiraki H, Yase Y. Amyotrophic lateral sclerosis and Parkinsonism-dementia in the Kii peninsula: comparison with the same disorders in Guam and with Alzheimer’s disease. Handbook of Clinical Neurology. 1991;15:273–300. [Google Scholar]
- 2.Petrik MS, Wong MC, Tabata RC, Garry RF, Shaw CA. Aluminum adjuvant linked to Gulf War illness induces motor neuron death in mice. NeuroMolecular Medicine. 2007;9(1):83–100. doi: 10.1385/nmm:9:1:83. [DOI] [PubMed] [Google Scholar]
- 3.Campbell A. The potential role of aluminium in Alzheimer’s disease. Nephrology Dialysis Transplantation. 2002;17(2):17–20. doi: 10.1093/ndt/17.suppl_2.17. [DOI] [PubMed] [Google Scholar]
- 4.McLachlan DR. Aluminium and the risk for Alzheimer’s disease. Environmetrics. 1995;6(3):233–275. [Google Scholar]
- 5.Zatta P, editor. Recent topics in aluminium chemistry. Coordination Chemistry Reviews. 2002;228(2) [Google Scholar]
- 6.Kawahara M. Effects of aluminum on the nervous system and its possible link with neurodegenerative diseases. Journal of Alzheimer’s Disease. 2005;8(2):171–182. doi: 10.3233/jad-2005-8210. [DOI] [PubMed] [Google Scholar]
- 7.Zatta P, Lucchini R, van Rensburg SJ, Taylor A. The role of metals in neurodegenerative processes: aluminum, manganese, and zinc. Brain Research Bulletin. 2003;62(1):15–28. doi: 10.1016/s0361-9230(03)00182-5. [DOI] [PubMed] [Google Scholar]
- 8.Selkoe DJ. The molecular pathology of Alzheimer’s disease. Neuron. 1991;6(4):487–498. doi: 10.1016/0896-6273(91)90052-2. [DOI] [PubMed] [Google Scholar]
- 9.Klatzo I, Wisniewski H, Streicher E. Experimental production of neurofibrillary degeneration I. Light microscopic observation. Journal of Neuropathology & Experimental Neurology. 1965;24:187–199. doi: 10.1097/00005072-196504000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Crapper DR, Krishnan SS, Dalton AJ. Brain aluminum distribution in Alzheimer’s disease and experimental neurofibrillary degeneration. Science. 1973;180(4085):511–513. doi: 10.1126/science.180.4085.511. [DOI] [PubMed] [Google Scholar]
- 11.Martyn CN, Osmond C, Edwardson JA, Barker DJP, Harris EC, Lacey RF. Geographical relation between Alzheimer’s disease and aluminium in drinking water. The Lancet. 1989;1(8629):59–62. [PubMed] [Google Scholar]
- 12.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 13.Wirths O, Multhaup G, Bayer TA. A modified β-amyloid hypothesis: intraneuronal accumulation of the β-amyloid peptide—the first step of a fatal cascade. Journal of Neurochemistry. 2004;91(3):513–520. doi: 10.1111/j.1471-4159.2004.02737.x. [DOI] [PubMed] [Google Scholar]
- 14.Sensi SL, Paoletti P, Bush AI, Sekler I. Zinc in the physiology and pathology of the CNS. Nature Reviews Neuroscience. 2009;10(11):780–791. doi: 10.1038/nrn2734. [DOI] [PubMed] [Google Scholar]
- 15.Zatta P, Drago D, Bolognin S, Sensi SL. Alzheimer’s disease, metal ions and metal homeostatic therapy. Trends in Pharmacological Sciences. 2009;30(7):346–355. doi: 10.1016/j.tips.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Hung YH, Bush AI, Cherny RA. Copper in the brain and Alzheimer’s disease. Journal of Biological Inorganic Chemistry. 2010;15(1):61–76. doi: 10.1007/s00775-009-0600-y. [DOI] [PubMed] [Google Scholar]
- 17.Spofforth J, Edin lRC, Eng MRC. Case of aluminium poisoning. The Lancet. 1921;197(5103):p. 1301. [Google Scholar]
- 18.Chusid JG, Pacella BL, Kopeloff LM, Kopeloff N. Chronic epilepsy in the monkey following multiple intracerebral injection of alumina cream. Proceedings of the Society for Experimental Biology and Medicine. 1951;78:53–54. doi: 10.3181/00379727-78-18970. [DOI] [PubMed] [Google Scholar]
- 19.Wills MR, Savory J. Aluminum and chronic renal failure: sources, absorption, transport, and toxicity. Critical Reviews in Clinical Laboratory Sciences. 1989;27(1):59–107. doi: 10.3109/10408368909106590. [DOI] [PubMed] [Google Scholar]
- 20.Alfrey AC, LeGendre GR, Kaehny D. The dialysis encephalopathy syndrome. Possible aluminium intoxication. The New England Journal of Medicine. 1976;294(4):184–188. doi: 10.1056/NEJM197601222940402. [DOI] [PubMed] [Google Scholar]
- 21.Altmann P, Cunningham J, Dhanesha U, Ballard M, Thompson J, Marsh F. Disturbance of cerebral function in people exposed to drinking water contaminated with aluminium sulphate: retrospective study of the Camelford water incident. British Medical Journal. 1999;319(7213):807–811. doi: 10.1136/bmj.319.7213.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flaten TP. Aluminium as a risk factor in Alzheimer’s disease, with emphasis on drinking water. Brain Research Bulletin. 2001;55(2):187–196. doi: 10.1016/s0361-9230(01)00459-2. [DOI] [PubMed] [Google Scholar]
- 23.Frecker MF. Dementia in Newfoundland: identification of a geographical isolate? Journal of Epidemiology and Community Health. 1991;45(4):307–311. doi: 10.1136/jech.45.4.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neri LC, Hewitt D. Aluminium, Alzheimer’s disease, and drinking water. The Lancet. 1991;338(8763):p. 390. doi: 10.1016/0140-6736(91)90531-s. [DOI] [PubMed] [Google Scholar]
- 25.Forbes WF, McLachlan DRC. Further thoughts on the aluminum—Alzheimer’s disease link. Journal of Epidemiology and Community Health. 1996;50(4):401–403. doi: 10.1136/jech.50.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacqmin H, Commenges D, Letenneur L, Barberger-Gateau P, Dartigues JF. Components of drinking water and risk of cognitive impairment in the elderly. American Journal of Epidemiology. 1994;139(1):48–57. doi: 10.1093/oxfordjournals.aje.a116934. [DOI] [PubMed] [Google Scholar]
- 27.Rondeau V, Commenges D, Jacqmin-Gadda H, Dartigues JF. Relation between aluminum concentrations in drinking water and Alzheimer’s disease: an 8-year follow-up study. American Journal of Epidemiology. 2000;152(1):59–66. doi: 10.1093/aje/152.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rondeau V, Jacqmin-Gadda H, Commenges D, Helmer C, Dartigues JF. Aluminum and silica in drinking water and the risk of Alzheimer’s disease or cognitive decline: findings from 15-year follow-up of the PAQUID cohort. American Journal of Epidemiology. 2009;169(4):489–496. doi: 10.1093/aje/kwn348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lukiw WJ, Kruck TPA, Crapper McLachlan DR. Alterations in human linker histone-DNA binding in the presence of aluminum salts in vitro and in Alzheimer’s disease. NeuroToxicology. 1987;8(2):291–301. [PubMed] [Google Scholar]
- 30.Bharathi KS, Jagannatha R, Stein R. First evidence on induced topological changes in supercoiled DNA by an aluminium D-aspartate complex. Journal of Biological Inorganic Chemistry. 2003;8(8):823–830. doi: 10.1007/s00775-003-0484-1. [DOI] [PubMed] [Google Scholar]
- 31.Latha KS, Anitha S, Rao KSJ, Viswamitra MA. Molecular understanding of aluminum-induced topological changes in (CCG)12 triplet repeats: relevance to neurological disorders. Biochimica et Biophysica Acta. 2002;1588(1):56–64. doi: 10.1016/s0925-4439(02)00133-3. [DOI] [PubMed] [Google Scholar]
- 32.Muma NA, Singer SM. Aluminum-induced neuropathology: transient changes in microtubule-associated proteins. Neurotoxicology and Teratology. 1996;18(6):679–690. doi: 10.1016/s0892-0362(96)00126-2. [DOI] [PubMed] [Google Scholar]
- 33.Parhad IM, Krekoski CA, Mathew A, Tran PM. Neuronal gene expression in aluminum myelopathy. Cellular and Molecular Neurobiology. 1989;9(1):123–138. doi: 10.1007/BF00711449. [DOI] [PubMed] [Google Scholar]
- 34.Oshiro S, Kawahara M, Mika S, et al. Aluminum taken up by transferrin-independent iron uptake affects the iron metabolism in rat cortical cells. Journal of Biochemistry. 1998;123(1):42–46. doi: 10.1093/oxfordjournals.jbchem.a021914. [DOI] [PubMed] [Google Scholar]
- 35.Lukiw WJ, LeBlanc HJ, Carver LA, McLachlan DRC, Bazan NG. Run-on gene transcription in human neocortical nuclei: inhibition by nanomolar aluminum and implications for neurodegenerative disease. Journal of Molecular Neuroscience. 1998;11(1):67–78. doi: 10.1385/JMN:11:1:67. [DOI] [PubMed] [Google Scholar]
- 36.Bosetti F, Solaini G, Tendi EA, Chikhale EG, Chandrasekaran K, Rapoport SI. Mitochondrial cytochrome c oxidase subunit III is selectively down-regulated by aluminum exposure in PC12S cells. NeuroReport. 2001;12(4):721–724. doi: 10.1097/00001756-200103260-00021. [DOI] [PubMed] [Google Scholar]
- 37.Cox KA, Dunn MA. Aluminum toxicity alters the regulation of calbindin-D28k protein and mRNA expression in chick intestine. Journal of Nutrition. 2001;131(7):2007–2013. doi: 10.1093/jn/131.7.2007. [DOI] [PubMed] [Google Scholar]
- 38.Johnson VJ, Sharma RP. Aluminum disrupts the pro-inflammatory cytokine/neurotrophin balance in primary brain rotation-mediated aggregate cultures: possible role in neurodegeneration. NeuroToxicology. 2003;24(2):261–268. doi: 10.1016/S0161-813X(02)00194-8. [DOI] [PubMed] [Google Scholar]
- 39.Lukiw WJ, Percy ME, Kruck TP. Nanomolar aluminum induces pro-inflammatory and pro-apoptotic gene expression in human brain cells in primary culture. Journal of Inorganic Biochemistry. 2005;99(9):1895–1898. doi: 10.1016/j.jinorgbio.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 40.Lin R, Chen X, Li W, Han Y, Liu P, Pi R. Exposure to metal ions regulates mRNA levels of APP and BACE1 in PC12 cells: blockage by curcumin. Neuroscience Letters. 2008;440(3):344–347. doi: 10.1016/j.neulet.2008.05.070. [DOI] [PubMed] [Google Scholar]
- 41.Walton JR, Wang MX. APP expression, distribution and accumulation are altered by aluminum in a rodent model for Alzheimer’s disease. Journal of Inorganic Biochemistry. 2009;103(11):1548–1554. doi: 10.1016/j.jinorgbio.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 42.García T, Esparza JL, Giralt M, Romeu M, Domingo JL, Gómez M. Protective role of melatonin on oxidative stress status and RNA expression in cerebral cortex and cerebellum of aβpp transgenic mice after chronic exposure to aluminum. Biological Trace Element Research. 2010;135(1–3):220–232. doi: 10.1007/s12011-009-8490-y. [DOI] [PubMed] [Google Scholar]
- 43.Luo Y, Niu F, Sun Z, et al. Altered expression of Aβ metabolism-associated molecules from d-galactose/AlCl3 induced mouse brain. Mechanisms of Ageing and Development. 2009;130(4):248–252. doi: 10.1016/j.mad.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 44.Castorina A, Tiralongo A, Giunta S, Carnazza ML, Scapagnini G, D'Agata V. Early effects of aluminum chloride on beta-secretase mRNA expression in a neuronal model of β-amyloid toxicity. Cell Biology and Toxicology. 2010;26(4):367–377. doi: 10.1007/s10565-009-9149-3. [DOI] [PubMed] [Google Scholar]
- 45.Socorro JM, Olmo R, Teijon C, Blanco MD, Teijon JM. Analysis of aluminum-yeast hexokinase interaction: modifications on protein structure and functionality. Journal of Protein Chemistry. 2000;19(3):199–208. doi: 10.1023/a:1007055719926. [DOI] [PubMed] [Google Scholar]
- 46.Lai JCK, Blass JP. Inhibition of brain glycolysis by aluminum. Journal of Neurochemistry. 1984;42(2):438–446. doi: 10.1111/j.1471-4159.1984.tb02697.x. [DOI] [PubMed] [Google Scholar]
- 47.Cho SW, Joshi JG. Inactivation of bakers’ yeast glucose-6-phosphate dehydrogenase by aluminum. Biochemistry. 1989;28(8):3613–3618. doi: 10.1021/bi00434a069. [DOI] [PubMed] [Google Scholar]
- 48.Kumar V, Bal A, Gill KD. Impairment of mitochondrial energy metabolism in different regions of rat brain following chronic exposure to aluminium. Brain Research. 2008;1232:94–103. doi: 10.1016/j.brainres.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 49.Lemire J, Mailloux R, Puiseux-Dao S, Appanna VD. Aluminum-induced defective mitochondrial metabolism perturbs cytoskeletal dynamics in human astrocytoma cells. Journal of Neuroscience Research. 2009;87(6):1474–1483. doi: 10.1002/jnr.21965. [DOI] [PubMed] [Google Scholar]
- 50.Mailloux RJ, Hamel R, Appanna VD. Aluminum toxicity elicits a dysfunctional TCA cycle and succinate accumulation in hepatocytes. Journal of Biochemical and Molecular Toxicology. 2006;20(4):198–208. doi: 10.1002/jbt.20137. [DOI] [PubMed] [Google Scholar]
- 51.Shetty K, Veeranna T, Guru SC. Phosphatase activity against neurofilament proteins from bovine spinal cord: effect of aluminium and neuropsychoactive drugs. Neuroscience Letters. 1992;137(1):83–86. doi: 10.1016/0304-3940(92)90304-p. [DOI] [PubMed] [Google Scholar]
- 52.Johnson GVW, Cogdill KW, Jope RS. Oral alumimum alters in vitro protein phosphorylation and kinase activities in rat brain. Neurobiology of Aging. 1990;11(3):209–216. doi: 10.1016/0197-4580(90)90547-d. [DOI] [PubMed] [Google Scholar]
- 53.Julka D, Gill KD. Involvement of altered cytoskeletal protein phosphorylation in aluminum-induced CNS dysfunction. Journal of Biochemical Toxicology. 1996;11(5):227–233. doi: 10.1002/(SICI)1522-7146(1996)11:5<227::AID-JBT3>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 54.Kaur A, Joshi K, Minz RW, Gill KD. Neurofilament phosphorylation and disruption: a possible mechanism of chronic aluminium toxicity in Wistar rats. Toxicology. 2006;219(1–3):1–10. doi: 10.1016/j.tox.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 55.Troncoso JC, March JL, Haner M, Aebi U. Effect of aluminum and other multivalent cations on neurofilaments in vitro: an electron microscopic study. Journal of Structural Biology. 1990;103(1):2–12. doi: 10.1016/1047-8477(90)90080-v. [DOI] [PubMed] [Google Scholar]
- 56.Yamamoto H, Saitoh Y, Yasugawa S, Miyamoto E. Dephosphorylation of τ factor by protein phosphatase 2A in synaptosomal cytosol fractions, and inhibition by aluminum. Journal of Neurochemistry. 1990;55(2):683–690. doi: 10.1111/j.1471-4159.1990.tb04187.x. [DOI] [PubMed] [Google Scholar]
- 57.El-Sebae AH, Abdel-Ghany ME, Shalloway D, Zeid MMA, Blancato J, Saleh MA. Aluminum interaction with human brain tau protein phosphorylation by various kinases. Journal of Environmental Science and Health Part B. 1993;28(6):763–777. doi: 10.1080/03601239309372852. [DOI] [PubMed] [Google Scholar]
- 58.Diaz-Nido J, Avila J. Aluminum induces the in vitro aggregation of bovine brain cytoskeletal proteins. Neuroscience Letters. 1990;110(1-2):221–226. doi: 10.1016/0304-3940(90)90815-q. [DOI] [PubMed] [Google Scholar]
- 59.Abd-Elghaffar SK, El-Sokkary GH, Sharkawy AA. Aluminum-induced neurotoxicity and oxidative damage in rabbits: protective effect of melatonin. Neuroendocrinology Letters. 2005;26(5):609–616. [PubMed] [Google Scholar]
- 60.Guy SP, Jones D, Mann DMA, Itzhaki RF. Human neuroblastoma cells treated with aluminium express an epitope associated with Alzheimer’s disease neurofibrillary tangles. Neuroscience Letters. 1991;121(1-2):166–168. doi: 10.1016/0304-3940(91)90676-k. [DOI] [PubMed] [Google Scholar]
- 61.Kawahara M, Muramoto K, Kobayashi K, Kuroda Y. Functional and morphological changes in cultured neurons of rat cerebral cortex induced by long-term application of aluminum. Biochemical and Biophysical Research Communications. 1992;189(3):1317–1322. doi: 10.1016/0006-291x(92)90217-9. [DOI] [PubMed] [Google Scholar]
- 62.Savory J, Huang Y, Herman MM, Reyes MR, Wills MR. Tau immunoreactivity associated with aluminum maltolate-induced neurofibrillary degeneration in rabbits. Brain Research. 1995;669(2):325–329. doi: 10.1016/0006-8993(94)01297-u. [DOI] [PubMed] [Google Scholar]
- 63.Kihira T, Yoshida S, Yase Y, Ono S, Kondo T. Chronic low-Ca/Mg high-Al diet induces neuronal loss. Neuropathology. 2002;22(3):171–179. doi: 10.1046/j.1440-1789.2002.00441.x. [DOI] [PubMed] [Google Scholar]
- 64.Kawahara M, Kato M, Kuroda Y. Effects of aluminum on the neurotoxicity of primary cultured neurons and on the aggregation of β-amyloid protein. Brain Research Bulletin. 2001;55(2):211–217. doi: 10.1016/s0361-9230(01)00475-0. [DOI] [PubMed] [Google Scholar]
- 65.Campbell A, Kumar A, La Rosa FG, Prasad KN, Bondy SC. Aluminum increases levels of β-amyloid and ubiquitin in neuroblastoma but not in glioma cells. Proceedings of the Society for Experimental Biology and Medicine. 2000;223(4):397–402. doi: 10.1046/j.1525-1373.2000.22356.x. [DOI] [PubMed] [Google Scholar]
- 66.Praticò D, Uryu K, Sung S, Tang S, Trojanowski JQ, Lee VM. Aluminum modulates brain amyloidosis through oxidative stress in APP transgenic mice. The FASEB Journal. 2002;16(9):1138–1140. doi: 10.1096/fj.02-0012fje. [DOI] [PubMed] [Google Scholar]
- 67.Rodella LF, Ricci F, Borsani E, et al. Aluminium exposure induces Alzheimer’s disease-like histopathological alterations in mouse brain. Histology and Histopathology. 2008;23(4-6):433–439. doi: 10.14670/HH-23.433. [DOI] [PubMed] [Google Scholar]
- 68.Provan SD, Yokel RA. Aluminum inhibits glutamate release from transverse rat hippocampal slices: role of G proteins, Ca channels and protein kinase C. NeuroToxicology. 1992;13(2):413–420. [PubMed] [Google Scholar]
- 69.Canales JJ, Corbalán R, Montoliu C, et al. Aluminium impairs the glutamate-nitric oxide-cGMP pathway in cultured neurons and in rat brain in vivo: molecular mechanisms and implications for neuropathology. Journal of Inorganic Biochemistry. 2001;87(1):63–69. doi: 10.1016/s0162-0134(01)00316-6. [DOI] [PubMed] [Google Scholar]
- 70.Meiri H, Banin E, Roll M, Rousseau A. Toxic effects of aluminium on nerve cells and synaptic transmission. Progress in Neurobiology. 1993;40(1):89–121. doi: 10.1016/0301-0082(93)90049-x. [DOI] [PubMed] [Google Scholar]
- 71.Yang SJ, Huh JW, Lee JE, Choi SY, Kim TU, Cho SW. Inactivation of human glutamate dehydrogenase by aluminum. Cellular and Molecular Life Sciences. 2003;60(11):2538–2546. doi: 10.1007/s00018-003-3298-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nday CM, Drever BD, Salifoglou T, Platt B. Aluminium interferes with hippocampal calcium signaling in a species-specific manner. Journal of Inorganic Biochemistry. 2010;104:919–927. doi: 10.1016/j.jinorgbio.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 73.Hofstetter JR, Vincent I, Bugiani O, Ghetti B, Richter JA. Aluminum-induced decreases in choline acetyltransferase, tyrosine hydroxylase, and glutamate decarboxylase in selected regions of rabbit brain. Neurochemical Pathology. 1987;6(3):177–193. doi: 10.1007/BF02834199. [DOI] [PubMed] [Google Scholar]
- 74.Zatta P, Ibn-Lkhayat-Idrissi M, Zambenedetti P, Kilyen M, Kiss T. In vivo and in vitro effects of aluminum on the activity of mouse brain acetylcholinesterase. Brain Research Bulletin. 2002;59(1):41–45. doi: 10.1016/s0361-9230(02)00836-5. [DOI] [PubMed] [Google Scholar]
- 75.Bielarczyk H, Tomaszewicz M, Szutowicz A. Effect of aluminum on acetyl-CoA and acetylcholine metabolism in nerve terminals. Journal of Neurochemistry. 1998;70(3):1175–1181. doi: 10.1046/j.1471-4159.1998.70031175.x. [DOI] [PubMed] [Google Scholar]
- 76.Rao KSJ, Rao GV. Effect of aluminium (Al) on brain mitochondrial monoamine oxidase-A (MAO-A) activity—an in vitro kinetic study. Molecular and Cellular Biochemistry. 1994;137(1):57–60. doi: 10.1007/BF00926039. [DOI] [PubMed] [Google Scholar]
- 77.Zatta P, Zambenedetti P, Milanese M. Activation of monoamine oxidase type-B by aluminum in rat brain homogenate. NeuroReport. 1999;10(17):3645–3648. doi: 10.1097/00001756-199911260-00033. [DOI] [PubMed] [Google Scholar]
- 78.Milanese M, Lkhayat MI, Zatta P. Inhibitory effect of aluminum on dopamine β-hydroxylase from bovine adrenal gland. Journal of Trace Elements in Medicine and Biology. 2001;15(2-3):139–141. doi: 10.1016/s0946-672x(01)80057-2. [DOI] [PubMed] [Google Scholar]
- 79.Lai JCK, Lim L, Davison AN. Effects of Cd2+, Mn2+, and AI2+ on rat brain synaptosomal uptake of noradrenaline and serotonin. Journal of Inorganic Biochemistry. 1982;17(3):215–225. doi: 10.1016/s0162-0134(00)80100-2. [DOI] [PubMed] [Google Scholar]
- 80.Kanazirska M, Vassilev PP, Birzon SY, Vassilev PM. Voltage-dependent effect of AI3+ on channel activities in hippocampal neurons. Biochemical and Biophysical Research Communications. 1997;232(1):84–87. doi: 10.1006/bbrc.1997.6226. [DOI] [PubMed] [Google Scholar]
- 81.Csóti T, Gyri J, Salánki J, Erdélyi L. pH-dependent actions of aluminum on voltage-activated sodium currents in snail neurons. NeuroToxicology. 2001;22(1):109–116. doi: 10.1016/s0161-813x(00)00006-1. [DOI] [PubMed] [Google Scholar]
- 82.Platt B, Busselberg D. Combined actions of Pb2+, Zn2+, and AI2+ on voltage-activated calcium channel currents. Cellular and Molecular Neurobiology. 1994;14(6):831–840. doi: 10.1007/BF02088688. [DOI] [PubMed] [Google Scholar]
- 83.Pentyala S, Ruggeri J, Veerraju A, et al. Microsomal Ca2+ flux modulation as an indicator of heavy metal toxicity. Indian Journal of Experimental Biology. 2010;48(7):737–743. [PubMed] [Google Scholar]
- 84.Li L. The biochemistry and physiology of metallic fluoride: action, mechanism, and implications. Critical Reviews in Oral Biology and Medicine. 2003;14(2):100–114. doi: 10.1177/154411130301400204. [DOI] [PubMed] [Google Scholar]
- 85.Theiss C, Meller K. Aluminum impairs gap junctional intercellular communication between astroglial cells in vitro. Cell and Tissue Research. 2002;310(2):143–154. doi: 10.1007/s00441-002-0639-3. [DOI] [PubMed] [Google Scholar]
- 86.Bizzi A, Crane RC, Autilio Gambetti L, Gambetti P. Aluminum effect on slow axonal transport: a novel impairment of neurofilament transport. Journal of Neuroscience. 1984;4(3):722–731. doi: 10.1523/JNEUROSCI.04-03-00722.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Siegel N, Suhayda C, Haug A. Aluminum changes the conformation of calmodulin. Physiological Chemistry and Physics. 1982;14(2):165–167. [PubMed] [Google Scholar]
- 88.Campbell A, Becaria A, Lahiri DK, Sharman K, Bondy SC. Chronic exposure to aluminum in drinking water increases inflammatory parameters selectively in the brain. Journal of Neuroscience Research. 2004;75(4):565–572. doi: 10.1002/jnr.10877. [DOI] [PubMed] [Google Scholar]
- 89.Oteiza PI. A mechanism for the stimulatory effect of aluminum on iron-induced lipid peroxidation. Archives of Biochemistry and Biophysics. 1994;308(2):374–379. doi: 10.1006/abbi.1994.1053. [DOI] [PubMed] [Google Scholar]
- 90.Kaneko N, Sugioka T, Sakurai H. Aluminum compounds enhance lipid peroxidation in liposomes: insight into cellular damage caused by oxidative stress. Journal of Inorganic Biochemistry. 2007;101(6):967–975. doi: 10.1016/j.jinorgbio.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 91.Verstraeten SV, Oteiza PI. Al-mediated changes in membrane physical properties participate in the inhibition of polyphosphoinositide hydrolysis. Archives of Biochemistry and Biophysics. 2002;408(2):263–271. doi: 10.1016/s0003-9861(02)00557-x. [DOI] [PubMed] [Google Scholar]
- 92.Pandya JD, Dave KR, Katyare SS. Effect of long-term aluminum feeding on lipid/phospholipid profiles of rat brain myelin. Lipids in Health and Disease. 2004;3, article no. 13 doi: 10.1186/1476-511X-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Silva VS, Cordeiro JM, Matos MJ, Oliveira CR, Gonçalves PP. Aluminum accumulation and membrane fluidity alteration in synaptosomes isolated from rat brain cortex following aluminum ingestion: effect of cholesterol. Neuroscience Research. 2002;44(2):181–193. doi: 10.1016/s0168-0102(02)00128-1. [DOI] [PubMed] [Google Scholar]
- 94.Ghribi O, Herman MM, Forbes MS, DeWitt DA, Savory J. GDNF protects against aluminum-induced apoptosis in rabbits by upregulating Bcl-2 and Bcl-X and inhibiting mitochondrial Bax translocation. Neurobiology of Disease. 2001;8(5):764–773. doi: 10.1006/nbdi.2001.0429. [DOI] [PubMed] [Google Scholar]
- 95.Kawahara M, Kato-Negishi M, Hosoda R, Imamura L, Tsuda M, Kuroda Y. Brain-derived neurotrophic factor protects cultured rat hippocampal neurons from aluminum maltolate neurotoxicity. Journal of Inorganic Biochemistry. 2003;97(1):124–131. doi: 10.1016/s0162-0134(03)00255-1. [DOI] [PubMed] [Google Scholar]
- 96.Guo GW, Liang YX. Aluminum-induced apoptosis in cultured astrocytes and its effect on calcium homeostasis. Brain Research. 2001;888(2):221–226. doi: 10.1016/s0006-8993(00)03057-2. [DOI] [PubMed] [Google Scholar]
- 97.Strong MJ, Garruto RM. Neuron-specific thresholds of aluminum toxicity in vitro: a comparative analysis of dissociated fetal rabbit hippocampal and motor neuron-enriched cultures. Laboratory Investigation. 1991;65(2):243–249. [PubMed] [Google Scholar]
- 98.Shaw CA, Petrik MS. Aluminum hydroxide injections lead to motor deficits and motor neuron degeneration. Journal of Inorganic Biochemistry. 2009;103(11):1555–1562. doi: 10.1016/j.jinorgbio.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Platt B, Carpenter DO, Busselberg D, Reymann KG, Riedel G. Aluminum impairs hippocampal long-term potentiation in rats in vitro and in vivo. Experimental Neurology. 1995;134(1):73–86. doi: 10.1006/exnr.1995.1038. [DOI] [PubMed] [Google Scholar]
- 100.Wang M, Chen JT, Ruan DY, Xu YZ. The influence of developmental period of aluminum exposure on synaptic plasticity in the adult rat dentate gyrus in vivo. Neuroscience. 2002;113(2):411–419. doi: 10.1016/s0306-4522(02)00193-8. [DOI] [PubMed] [Google Scholar]
- 101.Yokel RA, Allen DD, Meyer JJ. Studies of aluminum neurobehavioral toxicity in the intact mammal. Cellular and Molecular Neurobiology. 1994;14(6):791–808. doi: 10.1007/BF02088685. [DOI] [PubMed] [Google Scholar]
- 102.Kaneko N, Takada J, Yasui H, Sakurai H. Memory deficit in mice administered aluminum-maltolate complex. BioMetals. 2006;19(1):83–89. doi: 10.1007/s10534-005-6965-7. [DOI] [PubMed] [Google Scholar]
- 103.Walton JR. A longitudinal study of rats chronically exposed to aluminum at human dietary levels. Neuroscience Letters. 2007;412(1):29–33. doi: 10.1016/j.neulet.2006.08.093. [DOI] [PubMed] [Google Scholar]
- 104.Sethi P, Jyoti A, Singh R, Hussain E, Sharma D. Aluminium-induced electrophysiological, biochemical and cognitive modifications in the hippocampus of aging rats. NeuroToxicology. 2008;29(6):1069–1079. doi: 10.1016/j.neuro.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 105.Ribes D, Colomina MT, Vicens P, Domingo JL. Effects of oral aluminum exposure on behavior and neurogenesis in a transgenic mouse model of Alzheimer’s disease. Experimental Neurology. 2008;214(2):293–300. doi: 10.1016/j.expneurol.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 106.Ribes D, Colomina MT, Vicens P, Domingo JL. Impaired spatial learning and unaltered neurogenesis in a transgenic model of alzheimer’s disease after oral aluminum exposure. Current Alzheimer Research. 2010;7(5):401–408. doi: 10.2174/156720510791383840. [DOI] [PubMed] [Google Scholar]
- 107.Zatta P, Zambenedetti P, Reusche E, et al. A fatal case of aluminium encephalopathy in a patient with severe chronic renal failure not on dialysis. Nephrology Dialysis Transplantation. 2004;19(11):2929–2931. doi: 10.1093/ndt/gfh439. [DOI] [PubMed] [Google Scholar]
- 108.Wisniewski HM, Wen GY. Aluminium and Alzheimer’s disease. Ciba Foundation Symposium. 1992;169:142–154. doi: 10.1002/9780470514306.ch9. [DOI] [PubMed] [Google Scholar]
- 109.Shore D, Wyatt RJ. Aluminum and Alzheimer’s disease. Journal of Nervous and Mental Disease. 1983;171(9):553–558. doi: 10.1097/00005053-198309000-00005. [DOI] [PubMed] [Google Scholar]
- 110.Doll R. Review: Alzheimer’s disease and environmental aluminium. Age and Ageing. 1993;22(2):138–153. doi: 10.1093/ageing/22.2.138. [DOI] [PubMed] [Google Scholar]
- 111.Harrington CR, Wischik CM, McArthur FK, Taylor GA, Edwardson JA, Candy JM. Alzheimer’s-disease-like changes in tau protein processing: association with aluminium accumulation in brains of renal dialysis patients. The Lancet. 1994;343(8904):993–997. doi: 10.1016/s0140-6736(94)90124-4. [DOI] [PubMed] [Google Scholar]
- 112.Scott CW, Fieles A, Sygowski LA, Caputo CB. Aggregation of tau protein by aluminum. Brain Research. 1993;628(1-2):77–84. doi: 10.1016/0006-8993(93)90940-o. [DOI] [PubMed] [Google Scholar]
- 113.Crowther RA. Straight and paired helical filaments in Alzheimer disease have a common structural unit. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(6):2288–2292. doi: 10.1073/pnas.88.6.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bjertness E, Candy JM, Torvik A, et al. Content of brain aluminum is not elevated in Alzheimer disease. Alzheimer Disease and Associated Disorders. 1996;10(3):171–174. doi: 10.1097/00002093-199601030-00006. [DOI] [PubMed] [Google Scholar]
- 115.Landsberg JP, McDonald B, Watt F. Absence of aluminium in neuritic plaque cores in Alzheimer’s disease. Nature. 1992;360(6399):65–68. doi: 10.1038/360065a0. [DOI] [PubMed] [Google Scholar]
- 116.Bouras C, Giannakopoulos P, Good PF, Hsu A, Hof PR, Perl DP. A laser microprobe mass analysis of brain aluminum and iron in dementia pugilistica: comparison with Alzheimer’s disease. European Neurology. 1997;38(1):53–58. doi: 10.1159/000112903. [DOI] [PubMed] [Google Scholar]
- 117.Candy JM, McArthur FK, Oakley AE, et al. Aluminium accumulation in relation to senile plaque and neurofibrillary tangle formation in the brains of patients with renal failure. Journal of the Neurological Sciences. 1992;107(2):210–218. doi: 10.1016/0022-510x(92)90291-r. [DOI] [PubMed] [Google Scholar]
- 118.Yumoto S, Kakimi S, Ohsaki A, Ishikawa A. Demonstration of aluminum in amyloid fibers in the cores of senile plaques in the brains of patients with Alzheimer’s disease. Journal of Inorganic Biochemistry. 2009;103(11):1579–1584. doi: 10.1016/j.jinorgbio.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 119.Wettstein A, Aeppli J, Gautschi K, Peters M. Failure to find a relationship between mnestic skills of octagenarians and aluminum in drinking water. International Archives of Occupational and Environmental Health. 1991;63(2):97–103. doi: 10.1007/BF00379071. [DOI] [PubMed] [Google Scholar]
- 120.Martyn CN, Coggon DN, Inskip H, Lacey RF, Young WF. Aluminum concentrations in drinking water and risk of Alzheimer’s disease. Epidemiology. 1997;8(3):281–286. doi: 10.1097/00001648-199705000-00009. [DOI] [PubMed] [Google Scholar]
- 121.McLachlan DRC, Bergeron C, Smith JE, Boomer D, Rifat SL. Risk for neuropathologically confirmed Alzheimer’s disease and residual aluminum in municipal drinking water employing weighted residential histories. Neurology. 1996;46(2):401–405. doi: 10.1212/wnl.46.2.401. [DOI] [PubMed] [Google Scholar]
- 122.Gauthier E, Fortier I, Courchesne F, Pepin P, Mortimer J, Gauvreau D. Aluminum forms in drinking water and risk of Alzheimer’s disease. Environmental Research. 2000;84(3):234–246. doi: 10.1006/enrs.2000.4101. [DOI] [PubMed] [Google Scholar]
- 123.Birchall JD, Chappell JS. The chemistry of aluminum and silicon in relation to Alzheimer’s disease. Clinical Chemistry. 1988;34(2):265–267. [PubMed] [Google Scholar]
- 124.Edwardson JA, Moore PB, Ferrier IN, et al. Effect of silicon on gastrointestinal absorption of aluminium. The Lancet. 1993;342(8865):211–212. doi: 10.1016/0140-6736(93)92301-9. [DOI] [PubMed] [Google Scholar]
- 125.Gillete-Guyonnet S, Andrieu S, Nourhashemi F, de la Guéronnière V, Grandjean H, Vellas B. Cognitive impairment and composition of drinking water in women: findings of the EPIDOS study. American Journal of Clinical Nutrition. 2005;81(4):897–902. doi: 10.1093/ajcn/81.4.897. [DOI] [PubMed] [Google Scholar]
- 126.Goate A, Chartier-Harlin MC, Mullan M, et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer’s disease. Nature. 1991;349(6311):704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- 127.Yankner BA, Duffy LK, Kirschner DA. Neurotrophic and neurotoxic effects of amyloid β protein: reversal by tachykinin neuropeptides. Science. 1990;250(4978):279–282. doi: 10.1126/science.2218531. [DOI] [PubMed] [Google Scholar]
- 128.Pike CJ, Burdick D, Walencewicz AJ, Glabe CG, Cotman CW. Neurodegeneration induced by β-amyloid peptides in vitro: the role of peptide assembly state. Journal of Neuroscience. 1993;13(4):1676–1687. doi: 10.1523/JNEUROSCI.13-04-01676.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Simmons LK, May PC, Tomaselli KJ, et al. Secondary structure of amyloid β peptide correlates with neurotoxic activity in vitro. Molecular Pharmacology. 1994;45(3):373–379. [PubMed] [Google Scholar]
- 130.Selkoe DJ. Soluble oligomers of the amyloid β-protein impair synaptic plasticity and behavior. Behavioural Brain Research. 2008;192(1):106–113. doi: 10.1016/j.bbr.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Exley C, Price NC, Kelly SM, Birchall JD. An interaction of β-amyloid with aluminium in vitro. FEBS Letters. 1993;324(3):293–295. doi: 10.1016/0014-5793(93)80137-j. [DOI] [PubMed] [Google Scholar]
- 132.Mantyh PW, Ghilardi JR, Rogers S, et al. Aluminum, iron, and zinc ions promote aggregation of physiological concentrations of β-amyloid peptide. Journal of Neurochemistry. 1993;61(3):1171–1174. doi: 10.1111/j.1471-4159.1993.tb03639.x. [DOI] [PubMed] [Google Scholar]
- 133.Kawahara M, Muramoto K, Bobayashi K, Mori H, Kuroda Y. Aluminum promotes the aggregation of Alzheimer’s amyloid β-protein in vitro. Biochemical and Biophysical Research Communications. 1994;198(2):531–535. doi: 10.1006/bbrc.1994.1078. [DOI] [PubMed] [Google Scholar]
- 134.Kuroda Y, Kawahara M. Aggregation of amyloid beta-protein and its neurotoxicity: enhancement by aluminum and other metals. Tohoku Journal of Experimental Medicine. 1994;174(3):263–268. doi: 10.1620/tjem.174.263. [DOI] [PubMed] [Google Scholar]
- 135.Fasman GD, Perczel A, Moore CD. Solubilization of β-amyloid-(1-42)-peptide: reversing the β-sheet conformation induced by aluminum with silicates. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(2):369–371. doi: 10.1073/pnas.92.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.House E, Collingwood J, Khan A, Korchazkina O, Berthon G, Exley C. Aluminium, iron, zinc and copper Influence the in vitro formation of amyloid fibrils of Aβ42 in a manner which may have consequences for metal chelation therapy in Alzheimer’s disease. Journal of Alzheimer’s Disease. 2004;6(3):291–301. doi: 10.3233/jad-2004-6310. [DOI] [PubMed] [Google Scholar]
- 137.Bondy SC, Truong A. Potentiation of beta-folding of β-amyloid peptide 25–35 by aluminum salts. Neuroscience Letters. 1999;267(1):25–28. doi: 10.1016/s0304-3940(99)00307-9. [DOI] [PubMed] [Google Scholar]
- 138.Chong YH, Suh YH. Aggregation of amyloid precursor proteins by aluminum in vitro. Brain Research. 1995;670(1):137–141. doi: 10.1016/0006-8993(94)01304-z. [DOI] [PubMed] [Google Scholar]
- 139.Murayama H, Shin R-W, Higuchi J, Shibuya S, Muramoto T, Kitamoto T. Interaction of aluminum with PHFtau in Alzheimer’s disease neurofibrillary degeneration evidenced by desferrioxamine-assisted chelating autoclave method. American Journal of Pathology. 1999;155(3):877–885. doi: 10.1016/s0002-9440(10)65187-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Paik SR, Lee JH, Kim DH, Chang CS, Kim J. Aluminum-induced structural alterations of the precursor of the non-Aβ component of Alzheimer’s disease amyloid. Archives of Biochemistry and Biophysics. 1997;344(2):325–334. doi: 10.1006/abbi.1997.0207. [DOI] [PubMed] [Google Scholar]
- 141.Uversky VN, Li J, Fink AL. Metal-triggered structural transformations, aggregation, and fibrillation of human α-synuclein: a possible molecular link between parkinson’s disease and heavy metal exposure. Journal of Biological Chemistry. 2001;276(47):44284–44296. doi: 10.1074/jbc.M105343200. [DOI] [PubMed] [Google Scholar]
- 142.Ward B, Walker K, Exley C. Copper(II) inhibits the formation of amylin amyloid in vitro. Journal of Inorganic Biochemistry. 2008;102(2):371–375. doi: 10.1016/j.jinorgbio.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 143.Khan A, Ashcroft AE, Korchazhkina OV, Exley C. Metal-mediated formation of fibrillar ABri amyloid. Journal of Inorganic Biochemistry. 2004;98(12):2006–2010. doi: 10.1016/j.jinorgbio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 144.Ricchelli F, Fusi P, Tortora P, et al. Destabilization of non-pathological variants of ataxin-3 by metal ions results in aggregation/fibrillogenesis. International Journal of Biochemistry and Cell Biology. 2007;39(5):966–977. doi: 10.1016/j.biocel.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 145.Chaussidon M, Netter P, Kessler M, et al. Dialysis-associated arthropathy: secondary ion mass spectrometry evidence of aluminum silicate in β-microglobulin amyloid synovial tissue and articular cartilage. Nephron. 1993;65(4):559–563. doi: 10.1159/000187563. [DOI] [PubMed] [Google Scholar]
- 146.Fukuyama R, Mizuno T, Mizuno T, et al. Age-dependent change in the levels of Aβ40 and Aβ42 in cerebrospinal fluid from control subjects, and a decrease in the ratio of Aβ42 to Aβ40 level in cerebrospinal fluid from Alzheimer’s disease patients. European Neurology. 2000;43(3):155–160. doi: 10.1159/000008156. [DOI] [PubMed] [Google Scholar]
- 147.Kawahara M. Role of calcium dyshomeostasis via amyloid channels in the pathogenesis of Alzheimer’s disease. Current Pharmaceutical Design. 2010;16:2779–2789. doi: 10.2174/138161210793176545. [DOI] [PubMed] [Google Scholar]
- 148.Dyrks T, Dyrks E, Masters CL, Beyreuther K. Amyloidogenicity of rodent and human βA4 sequences. FEBS Letters. 1993;324(2):231–236. doi: 10.1016/0014-5793(93)81399-k. [DOI] [PubMed] [Google Scholar]
- 149.Bush AI, Pettingell WH, Multhaup G, et al. Rapid induction of Alzheimer Aβ amyloid formation by zinc. Science. 1994;265(5177):1464–1467. doi: 10.1126/science.8073293. [DOI] [PubMed] [Google Scholar]
- 150.Atwood CS, Moir RD, Huang X, et al. Dramatic aggregation of alzheimer Aβ by Cu(II) is induced by conditions representing physiological acidosis. Journal of Biological Chemistry. 1998;273(21):12817–12826. doi: 10.1074/jbc.273.21.12817. [DOI] [PubMed] [Google Scholar]
- 151.Lovell MA, Xie C, Markesbery WR. Protection against amyloid beta peptide toxicity by zinc. Brain Research. 1999;823(1-2):88–95. doi: 10.1016/s0006-8993(99)01114-2. [DOI] [PubMed] [Google Scholar]
- 152.Kawahara M, Arispe N, Kuroda Y, Rojas E. Alzheimer’s disease amyloid β-protein forms Zn2+-sensitive, cation-selective channels across excised membrane patches from hypothalamic neurons. Biophysical Journal. 1997;73(1):67–75. doi: 10.1016/S0006-3495(97)78048-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sakamoto T, Saito H, Ishii K, Takahashi H, Tanabe S, Ogasawara Y. Aluminum inhibits proteolytic degradation of amyloid β peptide by cathepsin D: a potential link between aluminum accumulation and neuritic plaque deposition. FEBS Letters. 2006;580(28-29):6543–6549. doi: 10.1016/j.febslet.2006.10.075. [DOI] [PubMed] [Google Scholar]
- 154.Ricchelli F, Drago D, Filippi B, Tognon G, Zatta P. Aluminum-triggered structural modifications and aggregation of β-amyloids. Cellular and Molecular Life Sciences. 2005;62(15):1724–1733. doi: 10.1007/s00018-005-5141-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Drago D, Cavaliere A, Mascetra N, et al. Aluminum modulates effects of βamyloid1-42 on neuronal calcium homeostasis and mitochondria functioning and is altered in a triple transgenic mouse model of Alzheimer’s disease. Rejuvenation Research. 2008;11(5):861–871. doi: 10.1089/rej.2008.0761. [DOI] [PubMed] [Google Scholar]
- 156.Drago D, Bolognin S, Zatta P. Role of metal ions in the Aβ oligomerization in Alzheimer’s disease and in other neurological disorders. Current Alzheimer Research. 2008;5(6):500–507. doi: 10.2174/156720508786898479. [DOI] [PubMed] [Google Scholar]
- 157.Exley C, Esiri MM. Severe cerebral congophilic angiopathy coincident with increased brain aluminium in a resident of Camelford, Cornwall, UK. Journal of Neurology, Neurosurgery and Psychiatry. 2006;77(7):877–879. doi: 10.1136/jnnp.2005.086553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Dickstein DL, Walsh J, Brautigam H, Stockton SD, Jr., Gandy S, Hof PR. Role of vascular risk factors and vascular dysfunction in Alzheimer’s disease. Mount Sinai Journal of Medicine. 2010;77(1):82–102. doi: 10.1002/msj.20155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Muckenthaler MU, Galy B, Hentze MW. Systemic iron homeostasis and the iron-responsive element/iron-regulatory protein (IRE/IRP) regulatory network. Annual Review of Nutrition. 2008;28:197–213. doi: 10.1146/annurev.nutr.28.061807.155521. [DOI] [PubMed] [Google Scholar]
- 160.Yamanaka K, Minato N, Iwai K. Stabilization of iron regulatory protein 2, IRP2, by aluminum. FEBS Letters. 1999;462(1-2):216–220. doi: 10.1016/s0014-5793(99)01533-1. [DOI] [PubMed] [Google Scholar]
- 161.Crichton RR, Wilmet S, Legssyer R, Ward RJ. Molecular and cellular mechanisms of iron homeostasis and toxicity in mammalian cells. Journal of Inorganic Biochemistry. 2002;91(1):9–18. doi: 10.1016/s0162-0134(02)00461-0. [DOI] [PubMed] [Google Scholar]
- 162.Oshiro S, Kawahara M, Kuroda Y, et al. Glial cells contribute more to iron and aluminum accumulation but are more resistant to oxidative stress than neuronal cells. Biochimica et Biophysica Acta. 2000;1502(3):405–414. doi: 10.1016/s0925-4439(00)00065-x. [DOI] [PubMed] [Google Scholar]
- 163.Cho H-H, Cahill CM, Vanderburg CR, et al. Selective translational control of the Alzheimer amyloid precursor protein transcript by iron regulatory protein-1. Journal of Biological Chemistry. 2010;285(41):31217–31232. doi: 10.1074/jbc.M110.149161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Duce JA, Tsatsanis A, Cater MA, et al. Iron-export ferroxidase activity of β-amyloid precursor protein is inhibited by zinc in Alzheimer’s disease. Cell. 2010;142(6):857–867. doi: 10.1016/j.cell.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kong GKW, Miles LA, Crespi GAN, et al. Copper binding to the Alzheimer’s disease amyloid precursor protein. European Biophysics Journal. 2008;37(3):269–279. doi: 10.1007/s00249-007-0234-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Namekata K, Imagawa M, Terashi A, Ohta S, Oyama F, Ihara Y. Association of transferrin C2 allele with late-onset Alzheimer’s disease. Human Genetics. 1997;101(2):126–129. doi: 10.1007/s004390050600. [DOI] [PubMed] [Google Scholar]
- 167.Lehmann DJ, Schuur M, Warden DR, et al. Transferrin and HFE genes interact in Alzheimer’s disease risk: the Epistasis Project. doi: 10.1016/j.neurobiolaging.2010.07.018. Neurobiology of Aging. In press. [DOI] [PubMed] [Google Scholar]
- 168.Imagawa M, Naruse S, Tsuji S, Fujioka A, Yamaguchi H. Coenzyme Q10, iron, and vitamin B6 in genetically-confirmed Alzheimer’s disease. The Lancet. 1992;340(8820):p. 671. doi: 10.1016/0140-6736(92)92203-r. [DOI] [PubMed] [Google Scholar]
- 169.Hegde ML, Bharathi P, Suram A, et al. Challenges associated with metal chelation therapy in alzheimer’s disease. Journal of Alzheimer’s Disease. 2009;17(3):457–468. doi: 10.3233/JAD-2009-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bush AI, Tanzi RE. Therapeutics for Alzheimer’s disease based on the metal hypothesis. Neurotherapeutics. 2008;5(3):421–432. doi: 10.1016/j.nurt.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yokel RA. Aluminum chelation: chemistry, clinical, and experimental studies and the search for alternatives to desferrioxamine. Journal of Toxicology and Environmental Health. 1994;41(2):131–174. doi: 10.1080/15287399409531834. [DOI] [PubMed] [Google Scholar]
- 172.Exley C. Organosilicon therapy in Alzheimer’s disease? Journal of Alzheimer’s Disease. 2007;11(3):301–302. doi: 10.3233/jad-2007-11305. [DOI] [PubMed] [Google Scholar]
- 173.Kawahara M, Konoha K, Nagata T, Sadakane Y. Aluminum and human health: its intake, bioavailability and neurotoxicity. Biomedical Research on Trace Elements. 2007;18:111–120. [Google Scholar]
- 174.Yumoto S, Nagai H, Kobayashi K, Tamate A, Kakimi S, Matsuzaki H. Al incorporation into the brain of suckling rats through maternal milk. Journal of Inorganic Biochemistry. 2003;97(1):155–160. doi: 10.1016/s0162-0134(03)00246-0. [DOI] [PubMed] [Google Scholar]
- 175.Priest ND. The biological behaviour and bioavailability of aluminium in man, with special reference to studies employing aluminium-26 as a tracer: review and study update. Journal of Environmental Monitoring. 2004;6(5):375–403. doi: 10.1039/b314329p. [DOI] [PubMed] [Google Scholar]
- 176.Kawahara M. Auminum and human health: possible link with neurodegenerative disorders. In: Huang S, editor. Metals and Neurodegeneration. Research Signpost; 2010. pp. 15–56. [Google Scholar]