Abstract
Granulosa cells of mammalian Graafian follicles maintain oocytes in meiotic arrest, which prevents the precocious maturation. We show that mouse mural granulosa cells, which line the follicle wall, express natriuretic peptide precursor type C, Nppc, mRNA while cumulus cells surrounding oocytes express mRNA of the NPPC receptor NPR2, a guanylyl cyclase. NPPC elevated cGMP levels in cumulus cells and oocytes and inhibited meiotic resumption in vitro. Meiotic arrest was not sustained in most Graafian follicles of Nppc or Npr2 mutant mice, and meiosis resumed precociously. Oocyte-derived paracrine factors promoted cumulus cell expression of Npr2 mRNA. Therefore, the granulosa cell ligand NPPC and its receptor NPR2 in cumulus cells prevent precocious meiotic maturation, which is critical for maturation and ovulation synchrony and for normal female fertility.
Meiosis is a germ cell-specific process that reduces the number of chromosomes from the diploid to the haploid number. It begins in human and mouse ovaries during fetal life but meiotic progression becomes arrested for prolonged periods at the diplotene stage of meiotic prophase. Fully-grown mammalian oocytes in Graafian follicles are maintained in meiotic prophase arrest until the preovulatory surge of luteinizing hormone (LH) triggers the resumption of meiosis and ovulation. The mature oocytes (eggs) are then available for fertilization within the oviduct. The somatic cell compartment of Graafian follicles, consisting of mural granulosa cells lining the inside of the follicle wall and cumulus cells surrounding the oocyte, plays a crucial role in maintaining oocyte meiotic arrest in mammals since removal of the oocyte-cumulus cell complex from these follicles results in gonadotropin-independent meiotic resumption in culture (1, 2). Cyclic nucleotides cAMP and cGMP are crucial to the maintenance of meiotic arrest. Cyclic AMP is generated within oocytes downstream of GPR3 and GPR12, regulators of Gs proteins controlling adenylyl cyclase (3, 4). Inability to sustain oocyte cAMP levels leads to precocious gonadotropin-independent resumption of meiosis, which interrupts the synchrony between oocyte maturation and ovulation and compromises female fertility (3-5). PDE3A, an oocyte-specific phosphodiesterase, becomes activated after the LH-surge to decrease cAMP levels in oocytes and thereby initiates pathways governing meiotic resumption (6). Before the LH-surge, cGMP, originating in granulosa cells of the follicular somatic compartment and transferred to the oocyte via gap junctions, inhibits activity of PDE3A in the oocyte (7, 8). Therefore, control of cGMP production by granulosa cells is crucial for maintaining meiotic arrest in fully-grown oocytes.
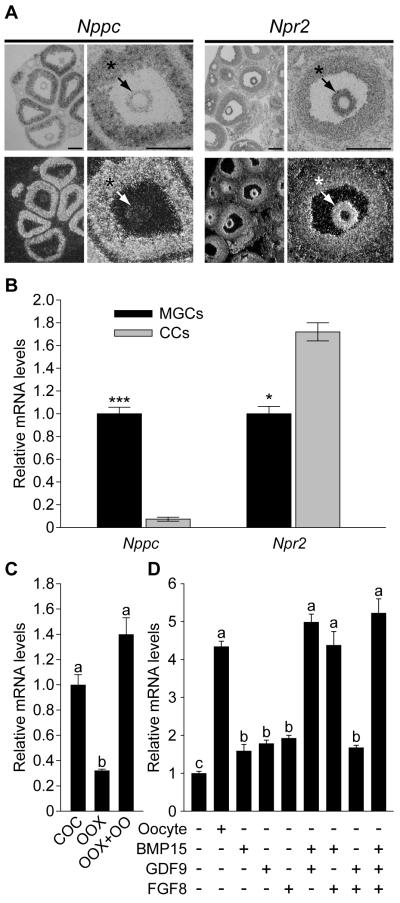
Exploration of the mouse cumulus cell transcriptome for mRNAs encoding guanylyl cyclases using microarray analysis (9) revealed abundant expression of natriuretic peptide receptor 2 (Npr2, also called GC-B) mRNA. The presence of this guanylyl cyclase was reported in rat follicles and the binding of its ligand, NPPC, also known as CNP, to granulosa cells varied during the estrous cycle (10). Moreover, NPPA (ANP) was reported to inhibit resumption of meiosis in rat oocytes (11). Therefore, expression of Nppc and Npr2 mRNAs in mouse ovarian follicles was determined by in situ hybridization. Nppc mRNA was expressed predominantly by mural granulosa cells, which line the inside of the follicular wall, and, in contrast, Npr2 mRNA was expressed predominantly by cumulus cells; outwardly decreasing levels of expression included some periantral mural granulosa cells (Fig. 1A). QRT-PCR confirmed that the steady-state levels of Nppc mRNA were at least 10-fold higher in mural granulosa cells than in cumulus cells and levels of Npr2 mRNA were about twice as high in cumulus cells versus mural granulosa cells (Fig. 1B). Because the isolation and collection of mural granulosa cells from follicles is unavoidably biased to those that line the antrum, and as shown by in situ hybridization these periantral mural granulosa cells express Npr2 mRNA at levels much higher than the larger population of outermost mural granulosa cells (Fig. 1A), this difference in Npr2 expression is really much greater than 2-fold.
Fig. 1.
Expression of Nppc and Npr2 mRNA by granulosa cells. (1A) In situ hybridization showing localization of Nppc and Npr2 mRNA expression in prepubertal mouse ovaries 48 hr after eCG injection. Histology is shown in bright field and localization of specific mRNAs is shown in dark field images. * indicates mural granulosa cells; arrows indicate cumulus cells. Bar = 200 μm. (1B) Comparison of steady-state levels of Nppc and Npr2 mRNAs by mural and cumulus cells by QRT-PCR. Levels of transcripts in cumulus cells are expressed relative to that measured in mural granulosa cells, mean of 3 experiments was set to a value of 1. *** indicates P < 0.001; * indicates P < 0.05. (1C) Effect of oocytes on Npr2 expression in cumulus cells. Levels of Npr2 mRNA in cumulus cells cultured as intact COCs (COC), oocytectomized (OOX) cumulus cells, or OOX cumulus cells co-cultured with fully-grown oocytes (OOX+OO; 2 oocytes/μl) for 14 hr were determined. The mean value in COC group was set as 1. (1D) Effect of GDF9, BMP15, and FGF8 on Npr2 mRNA levels in OOX cumulus cells. OOX cumulus cells were co-cultured with either denuded oocytes (Oocyte) or 500 ng/ml mouse GDF9, 500 ng/ml human BMP15, 100 ng/ml mouse FGF8B, or the various combinations indicated for 14 hr and levels of Npr2 mRNA determined. The mean value in the control (no-treatment) group was set as 1. Values indicated by different letters are significantly different (P < 0.05).
The intriguing juxtaposition of the cell types expressing the ligand mRNA (Nppc) and its cognate receptor (Npr2) suggests a functional relationship. Supporting this idea, we found that NPPC peptide, but not the related NPPA or NPPB prevented spontaneous (gonadotropin-indpendent) resumption of cumulus cell-enclosed (Fig. 2A), but not denuded (Fig. S1), mouse oocytes in vitro. Likewise, NPPC, but not NPPA or NPPB, elevated cumulus cell and oocyte cGMP levels in cultured cumulus cell-oocyte complexes (Fig. 2B). Cyclic AMP levels were elevated in oocytes but not in cumulus cells (Fig. 2C). Since oocytes do not express detectable NPR2 receptors, effects of NPPC on cGMP levels in oocytes probably result from the generation of cGMP by cumulus cells and subsequent transfer to oocytes via gap junctions. Most likely increased oocyte cAMP resulted from higher levels of oocyte cGMP suppressing oocyte cAMP phosphodiesterase activity.
Fig. 2.
Effect of natriuretic peptides on gonadotopin-independent (spontaneous) resumption of meiosis and production of cAMP and cGMP in vitro (2A) Effect of natriuretic peptides on spontaneous maturation of cumulus cell-enclosed oocytes in vitro. Oocytes were cultured 4 hr in medium containing human NPPA, NPPB, or NPPC and assessed for germinal vesicle breakdown (GVBD) indicative of the resumption of meiosis. NPPC dose-dependently inhibited resumption of cumulus cell-enclosed oocytes with a maximum effect at 100 nM. Neither NPPA or NPPB had detectable activity in preventing GVBD at 100 nM. Effect of natriuretic peptides on levels of cGMP (2B) and cAMP (2C) in cumulus and oocyte compartments of cumulus-oocyte complexes (COCs). COCs were incubated in medium containing 100 nM human NPPA, NPPB or NPPC for 1 hr, and levels of cGMP and cAMP in oocytes and cumulus cells were evaluated by ELISA. NPPC, but not NPPA or NPPB, promoted increased cGMP levels in both oocytes and cumulus cells but an increase in cAMP was seen only in oocytes. CC indicates the total cumulus cells surrounding one oocyte. (*P < 0.05 compared with control).
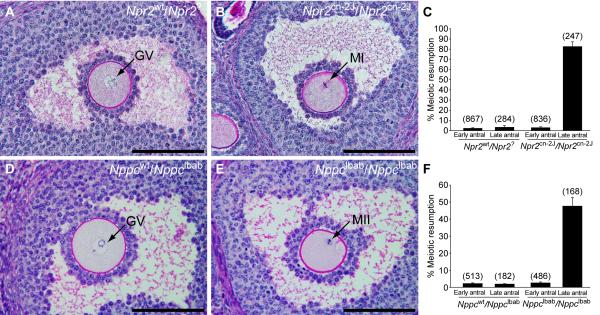
If the function of the NPPC/NPR2 pathway is to participate in the maintenance of meiotic arrest, then oocytes within Graafian (large antral) follicles of Nppc and Npr2 mutant mice should exhibit precocious gonadotropin-independent resumption of meiosis. Ovaries were removed from 22-24 day old Npr2cn-2J/Npr2cn-2J homozygous mutant and Npr2wt/Npr2? control mice 44 hr after injection of eCG to promote follicular development and prepared for histological analysis. Because homozygous Nppclbab/Nppclbab mice exhibit early postnatal lethality, ovaries of 5-day old mutant and wild-type control mice were grafted to the kidney capsules of immunodeficient CBySmn.CB17-Prkdcscid/J ovariectomized adult mice, a procedure that supports ovarian growth and development. Ovaries were removed thirty days later. The percentages of germinal vesicle (GV) intact (meiosis arrested) and germinal vesicle breakdown (GVBD, meiosis resumed) oocytes in antral follicles were determined in serial sections. There were no major morphological differences in ovarian histology in wild-type or mutant ovaries (see low magnification images in Fig. S2). Oocytes in early antral follicles of both mutant ovaries were at the GV-stage, the same as in the wild-type controls. However, while oocytes in Graafian (late antral) follicles in control ovaries were maintained at the GV-stage, 50% of oocytes in Nppclbab/Nppclbab and 80% of oocytes in Npr2cn-2J/Npr2cn-2J mutant late antral follicles had resumed meiosis (Fig. 3). The finding that the precocious resumption of meiosis phenotype is expressed by only 50% of the oocytes in ovaries of the Nppc mutant is likely because NppcIbab is a hypomorphic mutation, producing a peptide with slight guanylyl cyclase-stimulating activity (12). Also, NPPC may circulate in the normal mouse recipients for the mutant ovarian grafts. Cumulus cells in mutant ovaries were tightly packed around maturing oocytes and showed no evidence of cumulus expansion, which would be indicative of gonadotropin-stimulated maturation. Therefore, NPPC and its receptor NPR2 play a major role in the maintenance of meiotic arrest.
Fig. 3.
Failure to maintain meiotic arrest in Graafian (late antral) follicles of Npr2cn-2J/Npr2cn-2J and Nppclbab/Nppclbab mutant mouse ovaries. (A) A prophase-arrested oocyte (GV indicates germinal vesicle) within a late antral follicle of a control Npr2wt/Npr2? ovary. (B) An oocyte with metaphase I (MI) chromosomes within a late antral follicle of an Npr2cn-2J/Npr2cn-2J mutant ovary. (C) Percentages of oocytes that had resumed meiosis, counted in sections of ovaries from control Npr2wt/Npr2? and mutant Npr2cn-2J/Npr2cn-2J mice that were treated with eCG for 44 hr. Graph shows mean ± SEM of 6 ovaries; numbers above bars indicate number of follicles examined. (D-E) Ovarian follicles after development as grafts under kidney capsules of immunodeficient hosts for 30 days. (D) A prophase-arrested oocyte within a late antral follicle of a heterozygous control Nppcwt/Nppclbab ovary. (E) An oocyte with metaphase II (MII) chromosomes and a polar body, within a late antral follicle of a mutant Nppclbab/Nppclbab ovary. (F) Percentages of oocytes that had resumed meiosis, counted in sections of Nppcwt/Nppclbab and Nppclbab/Nppclbab grafted ovaries. Graph shows mean ± SEM of 6 ovaries; numbers above bars indicate number of follicles examined. The terms “Early” and “Late” antral follicles are used as defined by Pedersen and Peters (15). Bars = 100 μm.
Higher expression of transcripts in cumulus cells than mural granulosa cells often indicates that oocyte-derived paracrine factors promote cumulus cell expression (13). Therefore, the possible role of oocyte-derived paracrine factors in regulating levels of Npr2 mRNA in cumulus cells was determined. Microsurgical extirpation of oocytes from complexes (oocytectomy, OOX) significantly reduced Npr2 mRNA in cumulus cells. Co-culture of cumulus cells with fully-grown denuded oocytes (2 oocytes/μl) restored levels of Npr2 mRNA to that observed in intact complexes (Fig. 1C). Therefore, levels of Npr2 mRNA in cumulus cells are regulated, at least in part, by oocyte-derived paracrine factors. GDF9, BMP15, and FGF8B are paracrine growth factors secreted by oocytes (14). Each of these alone only slightly promoted expression of Npr2 mRNA by cumulus cells in vitro (Fig. 1D). However, combinations of BMP15 + GDF9, BMP15 + FGF8B, or all three proteins promoted levels of Npr2 mRNA expression in cumulus cells equivalent to those promoted by co-culture with cumulus cell-denuded oocytes (Fig. 1D).
Cumulus cells function to support oocyte development while mural granulosa cells have important endocrine functions and become corpora luteal cells after ovulation. Oocyte-derived paracrine factors regulate cumulus cell expression of transcripts at levels different from those in mural granulosa cells (13). Thus, the two populations of granulosa cells have distinct roles, and the oocyte profoundly affects the differentiation of cumulus cells to promote its own development. This study demonstrates a complex regulatory network among mural granulosa cells, cumulus cells, and oocytes before the LH-surge, one that is essential for maintaining oocyte meiotic arrest.
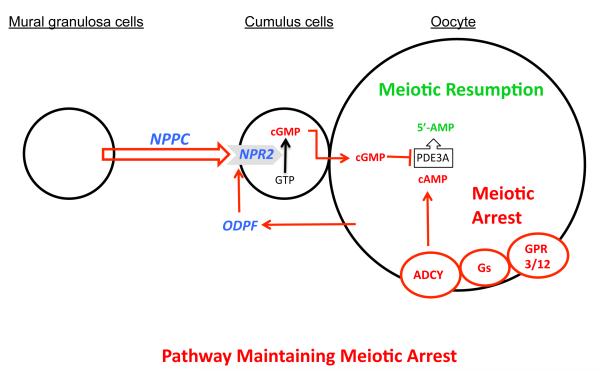
Based on findings presented here and key studies by others, we put forward the following model for the maintenance of meiotic arrest in fully-grown mammalian oocytes (Fig. 4). Oocyte cAMP is crucial for maintenance of meiotic arrest and is generated by oocyte adenylyl cyclase, which is controlled by the constitutive action of GPR3 and GPR12 via Gs protein (3, 4). Inhibition of oocyte cAMP-phosphodiesterase (PDE3A) activity is essential for sustaining elevated cAMP levels (6). Cyclic GMP diffuses into the oocyte from companion cumulus cells via gap junctions and inhibits oocyte PDE3A activity and cAMP hydrolysis and maintains meiotic arrest (7, 8). We show here that NPPC produced by follicular mural granulosa cells stimulates the generation of cGMP by cumulus cell NPR2. Remarkably, oocytes themselves participate in this meiosis-arresting pathway not only by producing cAMP, but also by promoting cumulus cell expression of NPR2 receptors, which generate the cGMP needed to inhibit oocyte PDE3A activity and thereby maintain meiotic arrest.
Fig. 4.
Model depicting the role of NPPC and NPR2 and oocyte-derived paracrine factors (ODPF) in the maintenance of oocyte meiotic arrest. See text for details. ADCY, adenylyl cyclase; GPR3/12, G-protein coupled receptors 3 and 12; Gs, GNAS (guanine nucleotide binding protein, alpha stimulating) complex; ODPF, oocyte-derived paracrine factors.
Supplementary Material
Acknowledgments
16. We thank Drs. Greg Cox, Tom Gridley and Mary Ann Handel for comments in preparation of the manuscript and Dr. Laurinda Jaffe for helpful discussions. JJE, KS, and Y-QS were supported by grants HD23839 and HD21970 from Eunice Kennedy Shriver NICHD. MZ and GX received support from National Basic Research Program of China (Nos. 2007CB947401 and 2006CB504003) and 2009SKLAB07-5 from State Key Laboratory for Agrobiotechnology of China. We also thank Dr. Martin Mazuk, Bayor College of Medicine, for providing GDF9 and BMP15 and Marilyn O’Brien and Karen Wigglesworth for technical assistance.
References
- 1.Pincus G, Enzmann EV. J. Exp. Med. 1935;62:655. doi: 10.1084/jem.62.5.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edwards RG. Nature. 1965;208:349. doi: 10.1038/208349a0. [DOI] [PubMed] [Google Scholar]
- 3.Mehlmann LM, et al. Science. 2004;306:1947. doi: 10.1126/science.1103974. [DOI] [PubMed] [Google Scholar]
- 4.Hinckley M, Vaccari S, Horner K, Chen R, Conti M. Dev Biol. 2005;287:249. doi: 10.1016/j.ydbio.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 5.Ledent C, et al. Proc Natl Acad Sci U S A. 2005;102:8922. doi: 10.1073/pnas.0503840102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richard FJ, Tsafriri A, Conti M. Biol Reprod. 2001;65:1444. doi: 10.1095/biolreprod65.5.1444. [DOI] [PubMed] [Google Scholar]
- 7.Vaccari S, Weeks Ii JL, Hsieh M, Menniti FS, Conti M. Biol. Reprod. 2009;81:595. doi: 10.1095/biolreprod.109.077768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Norris RP, et al. Development. 2009;136:1869. doi: 10.1242/dev.035238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Su YQ, et al. Development. 2008;135:111. doi: 10.1242/dev.009068. [DOI] [PubMed] [Google Scholar]
- 10.Jankowski M, et al. Biol. Reprod. 1997;56:59. doi: 10.1095/biolreprod56.1.59. [DOI] [PubMed] [Google Scholar]
- 11.Tornell J, Carlsson B, Billig H. Endocrinology. 1990;126:1504. doi: 10.1210/endo-126-3-1504. [DOI] [PubMed] [Google Scholar]
- 12.Tsuji T, et al. Biochem Biophys Res Commun. 2008;376:186. doi: 10.1016/j.bbrc.2008.08.139. [DOI] [PubMed] [Google Scholar]
- 13.Diaz FJ, Wigglesworth K, Eppig JJ. J. Cell Sci. 2007;120:1330. doi: 10.1242/jcs.000968. [DOI] [PubMed] [Google Scholar]
- 14.Sugiura K, et al. Development. 2007;134:2593. doi: 10.1242/dev.006882. [DOI] [PubMed] [Google Scholar]
- 15.Pedersen T, Peters H. J. Reprod. Fert. 1968;17:555. doi: 10.1530/jrf.0.0170555. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.