Abstract
The simian virus 40 small t (SV40ST) oncoprotein interacts with protein phosphatase 2A (PP2A), an abundantly expressed family of serine-threonine phosphatases. This interaction is essential for the transformation of human cells by SV40, and several PP2A subunits have been implicated as tumor suppressor genes. However, the pathways controlled by specific PP2A complexes involved in cell transformation remain incompletely understood. Using a comprehensive loss-of-function approach, we identified four PP2A regulatory subunits (B56α, B56γ, PR72/PR130 and PTPA) that when suppressed replaced the expression of SV40ST in human cell transformation. We found that manipulation of complexes containing PP2A B56α, B56γ and PR72/PR130 activates the pathways regulated by c-Myc, Wnt, and PI3K/Akt in a manner that depends on their specific phosphatase activity. In contrast, suppression of PTPA disrupts the assembly of PP2A heterotrimeric complexes, which leads to activation of these same oncogenic pathways. These observations delineate the PP2A family members and pathways perturbed by SV40ST during human cell transformation.
Keywords: PP2A, SV40 small t antigen, cell transformation
Introduction
The aberrant activation of cellular signaling cascades contributes directly to human diseases. In particular, mutations that lead to constitutively active kinases are well-characterized drivers of cancer development and small molecule kinase inhibitors are now used as primary therapy for particular cancers (1). Moreover, inactivating mutations in phosphatases such as SHP2 contribute directly to malignant transformation. Although it is clear that phosphatases antagonize the action of kinases, the role of phosphatases in most human cancers remains undefined.
The study of transformation in human cell culture models has facilitated the identification of pathways related to cancer development. We previously showed that expression of the telomerase catalytic subunit (hTERT), the SV40 Early Region (SV40 ER), and an oncogenic allele of H-RAS confers a tumorigenic phenotype to several types of human cells (2, 3). The SV40 ER encodes two oncoproteins, the SV40 large T (SV40LT) and small t (SV40ST) antigens. SV40LT binds and inactivates the tumor suppressor proteins retinoblastoma and p53 [reviewed in (3)].
SV40ST binds to and inhibits the activity of the serine-threonine PP2A family (4, 5). Specifically, SV40ST mutants harboring amino acid substitutions that ablate the ability of SV40ST to bind PP2A also eliminate the ability of such mutants to transform rat and human cells (4, 6). Conversely, a SV40ST mutant that contains only the PP2A-inactivation domain retains the ability to induce transformation (4).
PP2A is also a major cellular binding partner for several other viral oncoproteins such as polyoma small and middle t antigens and the adenoviral E4orf4 protein [reviewed in (7)], suggesting that like other targets of viral oncoproteins, PP2A plays a important role in tumor suppression. Indeed, inactivating alterations of PP2A subunits have been found in human cancers (8–11), and suppression of these same PP2A components contributes to cell transformation (12, 13).
PP2A accounts for the majority of serine/threonine phosphatase activity in mammalian cells and has been implicated in the regulation of a wide diversity of signaling pathways [reviewed in (7)]. PP2A refers to a family of phosphatases that contain a common catalytic C subunit, whose activity is regulated by a diverse set of regulatory proteins. PP2A complexes usually contain an active core dimer composed of a catalytic C subunit (PP2AC/PPP2C) and a scaffolding A subunit (PR65/PPP2R1). In mammals, two distinct genes encode closely related versions of both the A and C subunits of PP2A. The AC dimer recruits a third regulatory B subunit, which dictates the substrate specificity and localization of the PP2A heterotrimeric complex. Four unrelated families of B subunits have identified to date: B/B55/PR55/PPP2R2, B'/B56/PR61/PPP2R5, B"/PR72/PPP2R3, and Striatin (14). At least 100 different PP2A heterotrimeric complexes are formed through combinatorial association of these subunits, and several lines of evidence suggest that particular PP2A holoenzyme complexes mediate specific physiological functions.
The PTPase activator (PTPA/PPP2R4) was initially described as a B regulatory subunit due to its ability to interact with the AC dimer. However, unlike other B rsubunits, which recruit the core enzyme to targeted PP2A substrates, PTPA may function as a chaperone that facilitates incorporation of divalent cations to the PP2A active site (15).
The diversity of PP2A complexes suggests that dysfunction of several distinct PP2A complexes may affect specific pathways and in turn contribute independently to transformation. In consonance with this idea, recent structural studies revealed that SV40ST competes for the binding site on AC dimer with multiple regulatory B subunits (16). We previously showed that suppression of the PP2A B56 γ subunit was able to partially replace SV40ST and induce cell transformation (17). However, the degree of cell transformation induced by PP2A B56γ suppression was quantitatively less than what we observed when we expressed SV40ST, suggesting that other PP2A subunits are involved in malignant transformation. Here we created and used a shRNA library targeting all known PP2A subunits to identify those subunits that are involved in human cell transformation.
Materials and Methods
Plasmids, cell lines, infections
pWZL-Blast c-MycT58A was the gift of Dr. Rosalie Sears (Oregon Health and Sciences University). pBABE-puro β-cateninS33Y, pBabe-hygro dominant-negative TCF (dnTCF), and pBabe-GFP myr-Akt have been described (18, 19). The pLKO.1-Puro shGFP and pLKO.1-puro vectors containing shRNAs targeting specific PP2A subunits (Table 1) were provided by the RNAi Consortium (20).
Table 1.
Suppression of PP2A subumts by shRNA
| Hairpin | Gene | Official name | Gene ID | Targeted sequence | Suppression, % |
|---|---|---|---|---|---|
| PPP2CA-1 | PP2A Cα | PPP2CA | NM_002715 | TGGAACTTGACGATACTCTAA | 88±8 |
| PPP2CA-2 | PP2A Cα | PPP2CA | NM_002715 | CACACAAGTTTATGGTTTCTA | 44±4 |
| PPP2CA-3 | PP2A Cα | PPP2CA | NM_002715 | GAGGGATATAACTGGTGCCAT | 15±3 |
| PPP2CB-1 | PP2A Cβ | PPP2CB | NM_004156 | TGTCTGCGAAAGTATGGGAAT | 62±5 |
| PPP2CB-3 | PP2A Cβ | PPP2CB | NM_004156 | AGGTTCTTCTTGGGAGTATGT | 81±7 |
| PPP2R2A-1 | B55α | PPP2R2A | NM_002717 | GTAGATGATGATGTAGCAGAA | 85±8 |
| PPP2R2A-2 | B55α | PPP2R2A | NM_002717 | GCAAGTGGCAAGCGAAAGAAA | 89±6 |
| PPP2R2D-1 | B55δ | PPP2R2D | NM_018461 | GTCCTTCTTCTCAGAAATAAT | 88±6 |
| PPP2R2D-2 | B55δ | PPP2R2D | NM_018461 | GCTCTGCTCTCTCTATGAGAA | 69±4 |
| PPP2R5A-1 | B56α | PPP2R5A | NM_006243 | CAGAGTGGTAACAGATGGGTA | 62±5 |
| PPP2R5A-2 | B56α | PPP2R5A | NM_006243 | GCTAACATCTTCCGTACACTT | 73±6 |
| PPP2R5B-1 | B56β | PPP2R5B | NM_006244 | GTTCACAGTAATCATGGTCTA | 67±5 |
| PPP2R5B-2 | B56β | PPP2R5B | NM_006244 | CCGCATGATCTCAGTGAATAT | 80±6 |
| PPP2R5C-1 | B56γ | PPP2R5C | NM_002719 | CCAGAAGTAGTCCATATGTTT | 86±5 |
| PPP2R5C-2 | B56γ | PPP2R5C | NM_002719 | TCCAGAAGTTACGTCAGTGTT | 70±6 |
| PPP2R5D-1 | B56δ | PPP2R5D | NM_006245 | GAGTTCTTCTTACGTTTCCTT | 96±5 |
| PPP2R5D-2 | B56δ | PPP2R5D | NM_006245 | TGCACTCTACAGGAACTCCAA | 82±6 |
| PPP2R5E-1 | B56ε | PPP2R5E | NM_006246 | CCTCCTAGTGACAGCAATGAA | 91±5 |
| PPP2R5E-2 | B56ε | PPP2R5E | NM_006246 | CCTCTGGATAACTTGACTGTA | 82±5 |
| PPP2R3B-1 | PR48 | PPP2R3B | NM_013239 | CGAGCATTTCTACGTCATCTA | 53±4 |
| PPP2R3B-2 | PR48 | PPP2R3B | NM_013239 | CGTCCACAAGTTCGTCGCCAT | 50±5 |
| PPP2R3A-1 | PR72/130 | PPP2R3A | NM_002718 | CCACACCTTCACACATGGAAT | 51±5 |
| PPP2R3A-2 | PR72/130 | PPP2R3A | NM_002718 | GCAGGATTATTGAAAGGATAT | 61±5 |
| STRN3-1 | PR93 | STRN3 | NM_014574 | CCTGTTACAATAACTGCTCAT | 67±4 |
| STRN3-2 | PR93 | STRN3 | NM_014574 | GCTGGCACTTTAGTTGGTCAT | 87±5 |
| STRN-1 | PR110 | STRN | NM_014574 | GCGGTGAAGATCGAGATACAA | 63±6 |
| STRN-2 | PR110 | STRN | NM_014574 | GCAAGGGATATACAAGCATTT | 84±5 |
| PTPA-1 | PR53 | PPP2R4 | NM_178001 | CCGTTTGATGAGAGGCTGTTT | 92±5 |
| PTPA-2 | PR53 | PPP2R4 | NM_178001 | GATCCACACAGTTCCAGACAT | 84±7 |
Note: Suppression levels of subunits were analyzed using qRT-PCR as described in Material and Methods. Data shown as mean ± SD of three independent experiments.
Cells were cultured in minimal Eagle medium alpha (MEMα) supplemented with 10% heat-inactivated fetal calf serum (IFS). All transfections were performed using Fugene 6 (Roche) and retroviral and lentiviral infections were performed as described (20). To generate stable cell lines, cells were selected using 2 μg ml−1 of puromycin for 3 d, 15 μg ml−1 of blastocidin for 10 d or sorted using BD FACSAria cell sorter.
Immunoblotting and immunoprecipitation
Cells were suspended in a lysis buffer [50mM Tris-HCl, pH 7.5, 150 mM NaCl, 1mM EDTA, protease inhibitor cocktail (Roche) and 0.5% NP-40] and cleared of insoluble material by centrifugation. Soluble proteins (100 μg) were subjected to SDS-PAGE followed by immunoblotting.
For immunoprecipitation, cells were lysed in a buffer containing 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1mM EDTA, protease inhibitor cocktail and 0.3% CHAPS. Cell lysates (2 mg) were incubated with a PP2A C (BD Biosciences) antibody overnight at 4°C, followed by the addition of protein G Sepharose beads (Roche) overnight at 4°C. The beads were washed three times with lysis buffer and eluted with 2XSDS sample buffer, followed by SDS-PAGE and immunoblotting.
The antibodies used included: PP2A Aα (6F9) (Covance), PP2A C (BD Biosciences) and methyl-PP2A C (2A10) (Santa Cruz Biotech), B56β (Santa Cruz Biotech), PR93 (S68) (Cell Signaling), FLAG (M2) (Sigma-Aldrich), β-actin (Sigma-Aldrich), HA (12C5) (Boehringer Mannheim), active-β-catenin (8E7) and total β-catenin (5H10) (Millipore), polyclonal phospho-Akt (Ser473) and Akt1 (Cell Signaling), c-Myc (D84C12) XP™ Rabbit mAb (Cell Signaling). Affinity-purified polyclonal antibodies were raised against B55α, B56γ, and SV40ST peptides as described (17).
Quantitative RT-PCR
For RT–PCR, total RNA was isolated using RNeasy (Qiagen) and cDNA synthesis was performed using the Advantage RT/PCR kit (Clontech). A list of primers used for qRT-PCR is presented in Supplementary Figure 2. Real-time PCR reactions were conducted in an ABI Prism 7700 Sequence Detection System (Applied Biosystems, Foster City, CA) using SYBR Green PCR Master Mix (Applied Biosystems).
Luciferase Assays
Luciferase assays were performed using the Dual luciferase system (Promega). 100 ng of Top-Glow or Fop-Glow luciferase vector was transfected in the presence of 5 ng of CMV-Wnt1 and 20 ng of CMV-Renilla, or 100 ng of pGL2-E2F2 was co-transfected with 20 ng of CMV-Renilla. The Firefly luciferase activity was normalized to the Renilla luciferase activity.
Anchorage-independent growth and tumor formation
For soft agar assays, 105 cells were plated in triplicate into 0.4% Noble agar supplemented with 10% IFS. Four wks after seeding, colonies were visualized and counted under microscope (10×magnification). For tumorigenicity assays, 2×106 cells were injected subcutaneously into female BALB/AnNTac-Foxn1nu/nu immunodeficient mice (2). The number of tumors formed was determined 40 d after injection.
Proliferation assays
1×104 cells were plated in triplicate. The number of viable cells was determined using a Z2 Particle Count and Size Analyzer (Beckman-Coulter). For population doubling determinations, a seeding density of 1×104 cells in a 10-cm plate was used, and triplicate plates were counted every 4 d.
Results
Identification of PP2A complexes involved in control of cell transformation
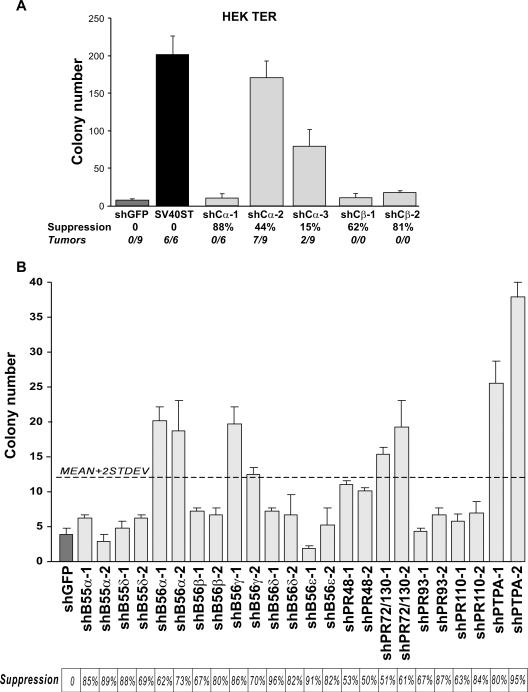
We and others previously found that SV40ST cooperates with SV40LT, the telomerase catalytic subunit (hTERT) and H-Ras-V12 to transform a wide range of human cells [reviewed in (3)]. The effect of SV40ST on cell transformation was only partially recapitulated by suppression of PP2A regulatory subunit B56γ, suggesting that other PP2A regulatory subunits might also be involved in control of cell transformation. To determine whether other PP2A complexes are involved in cell transformation induced by SV40ST, we assessed the consequences of suppressing the PP2A catalytic subunits Cα and Cβ in immortal, non-tumorigenic HEK cells expressing SV40LT, hTERT and H-Ras-V12 (HEK TER cells). By using several different shRNAs targeting PP2A Cα or Cβ subunits, we generated stable cell lines expressing a range of expression of the Cα or Cβ proteins. Suppression of the less abundant Cβ subunit by 50–80% failed to change the proliferation rate of HEK TER cells and did not affect their ability to grow in anchorage-independent AI manner and to form tumors in immunodeficient mice (Figure 1A; Supplement Figure 1). This observation suggests that inhibition of PP2A Cβ-specific complexes do not contribute to transformation in this assay.
Figure 1. shRNA screen to identify PP2A subunits involved in cell transformation.
A, AI growth and tumorigenicity of cell lines in which PP2A Cα or Cβ were suppressed or overexpressing SV40ST. The number of tumors formed per number of injection sites is shown. B, AI growth and tumor formation of HEK TER cells expressing shRNAs targeting PP2A B subunits. Results are shown as mean ± SD for 3 independent experiments. Dotted line indicates position of 2 SD from the mean of all samples. PP2A subunit suppression was assessed by qRT-PCR.
In contrast, HEK TER cells expressing very low levels of Cα proliferated poorly (Supplemental Figure 1) and failed to grow in an AI manner or to form tumors in immunodeficient hosts (Figure 1A). This phenotype is consistent with prior reports that demonstrated that deletion of Cα expression in yeast or mammalian cells leads to apoptosis (21, 22). However, HEK TER cells in which we partially suppressed PP2A Cα (50%) exhibited the ability to form AI colonies and to form tumors similar to that observed after the introduction of SV40ST (Figure 1A). These observations are consistent with notion that inhibition of PP2A by SV40ST is necessary for SV40ER mediated cell transformation (17). We noted that HEK TER cells in which PP2A Cα was suppressed to 20% of wild-type levels demonstrated an intermediate transforming phenotype. The correlation between levels of PP2A suppression and the transforming phenotypes suggests that other PP2A complexes in addition to PP2A B56γ–specific complexes contribute independently to cell transformation.
To identify PP2A regulatory B subunits in addition to B56γ that are involved in, we performed a loss-of-function screen using lentivirally delivered shRNAs targeting each of the known PP2A regulatory subunits. We introduced 5 shRNA targeting each of the known PP2A B subunits into HEK TER cells and determined the degree of gene suppression induced by each shRNA by quantitative RT-PCR (qRT-PCR) (Figure 1B; Supplemental Figure 2) and by immunoblotting (Supplemental Figure 3). Consistent with prior reports (23), we found that except for PP2A B55γ and B55β subunits (which are mostly expressed in the brain), each of the PP2A B subunits is expressed in these cells. For each of the PP2A B subunits, we identified at least two different shRNA that suppress each PP2A B subunit by at least 50% (Table 1). We selected these two B subunit-specific shRNAs to create HEK TER cell lines that stably express each of these shRNAs to assess the consequences of suppressing each of these PP2A B subunits on AI growth.
We found that in addition to B56γ, individually suppressing the expression of PR72/PR130, B56α or PTPA permitted HEK TER cells to grow in an AI manner (Figure 1B). The expression of two distinct shRNAs specific to each of these B subunits results in statistically significant (p<0.05) increases in AI colonies (Figure 1B). Since we used two shRNAs targeting different sequences in the same gene, it is unlikely that the observed effects are due to off-targets induced by these shRNAs. These observations implicate the PP2A regulatory subunits B56α, B56γ, PR72/PR130 and PTPA in human cell transformation.
Involvement of the PI3K/Akt, Wnt, and c-Myc pathways in SV40ST-induced transformation
The PP2A subunits, B56α, B56γ and PR72/PR130 have been implicated in the regulation of the PI3K/AKT, Wnt, and c-Myc pathways (12, 17, 24–27), suggesting that they are involved in transformation induced by suppression of specific PP2A complexes. Thus we analyzed activity of Akt, Wnt, and c-Myc pathways in HEK TER cells after suppression of specific PP2A subunits.
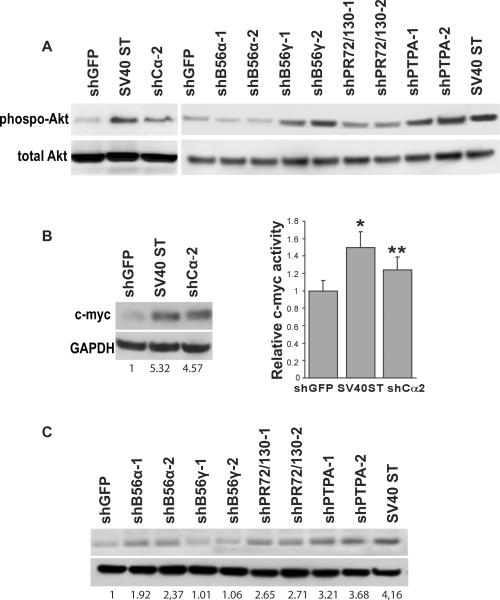
Consistent with previous reports (28–30), we found that expression of SV40ST, suppression of either PP2A Cα or B56γ resulted in elevated levels of Akt phosphorylation (Ser473). We also observed a similar increase of phosphorylated Akt in cells in which we suppressed PTPA expression (Figure 2A).
Figure 2. Inhibition of PP2A activates the PI3K/Akt and c-myc signaling pathways.
A, Immunoblot analysis of phospho-Akt and Akt1 expression in cells expressing control vector, SV40ST, or shRNAs specific for the indicated PP2A subunits. HEK TER cells were serum-starved for 24 h and then stimulated with 10% serum for 15 min. B, C, Expression of c-Myc in the HEK TER cells pre-treated with MG132 (10 μM) for 12 h. Transcriptional activation activity of c-Myc was assessed by its ability to activate the E2F2 promoter (E2F2(-Ebox)-Luc) *p<0.006 and **p<0.031. Mean±SD for 3 independent experiments is shown.
We also corroborated the observation that SV40ST overexpression or suppression of PP2A Cα resulted in increased expression of c-Myc protein and a small but reproducible increase in Myc-induced transactivation of the E2F2 promoter (31), which is dependent on the presence of c-Myc-binding sites (E-box elements) (28), in the cells expressing SV40ST or in which either PP2A Cα was suppressed (Figure 2B). We found that suppression of B56α, PR72/PR130 or PTPA led to a 2-fold increase in the steady state levels of c-Myc protein (Figure 2C). In contrast, suppression of B56γ was not accompanied with any change of c-Myc expression levels (Figure 2C).
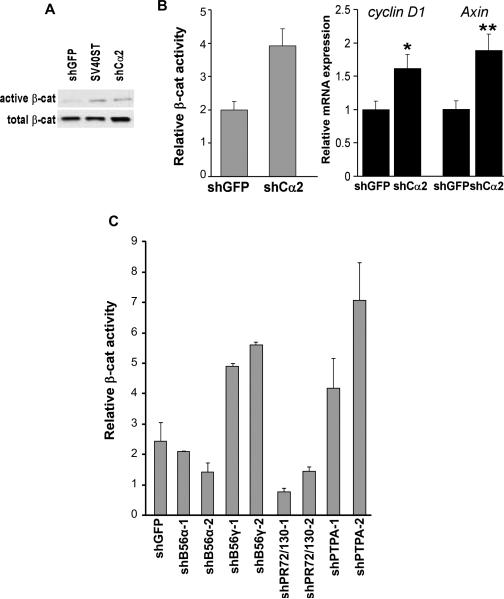
Moreover, we observed an increase in levels of the dephosphorylated (Ser37 and Thr41), active form of β-catenin after overexpression of SV40ST or suppression of PP2A Cα (Figure 3A). HEK TER cells in which PP2A Cα was partially suppressed also showed increased TOP/FOP luciferase reporter activity as well as statistically significant increased expression of β-catenin-driven genes, such as CCDN1 and AXIN (Figure 3B). As previously reported (27), we found that suppression of PP2A B56γ induced β-catenin-dependent transcription (Figure 3C). Suppression of PTPA also induced TOP/FOP luciferase reporter activity. In contrast, we observed a decrease of β-catenin activity after suppression of PR72/130 (Figure 3C).
Figure 3. Suppression of PP2A activates the Wnt pathway.
A, Expression of unphosphorylated and total β-catenin in cells expressing shGFP, SV40ST, or PP2A Cα–specific shRNA. Relative TOP/FOP activity and expression of β-catenin target genes are shown. *p<0.012, **p<0.004. B, C, Relative TOP/FOP activity in HEK TER cells expressing shGFP, SV40ST, or shRNAs targeting the indicated PP2A subunits. Mean ± SD for 3 independent experiments is shown.
These observations indicate that suppression of specific PP2A complexes resulted in up-regulation of the cancer-related signaling pathways PI3K/AKT, Wnt, and c-Myc. Depletion of specific PP2A B regulatory subunits resulted in activation of a subset of these signaling pathways while both inhibition of PP2A Cα and PTPA resulted in activation of all three pathways. Consistent with these observations, we found that suppression of B56α, B56γ or PR72/PR130 resulted in a small decrease in PP2A associated phosphatase activity while depletion of both PP2A Cα and PTPA inhibited PP2A phosphatase activity more dramatically (Supplemental Figure 4).
Manipulation of Akt, β-catenin, and c-Myc substitutes for SV40ST to induce transformation
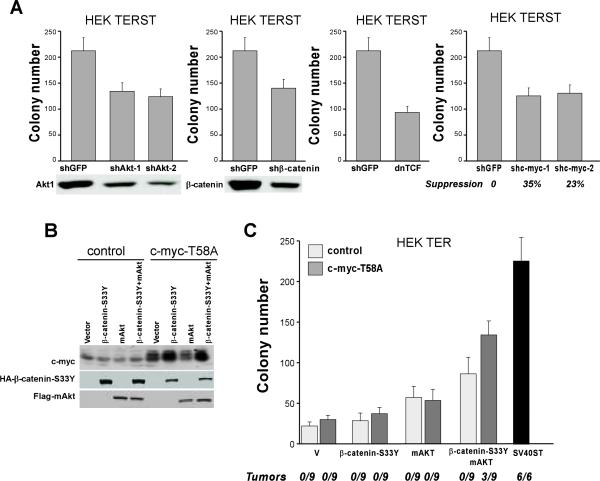
To study whether regulation of Akt, β-catenin, and c-Myc activity by specific PP2A complexes contributes to SV40ST-mediated transformation, we used both loss-of-function and gain-of-function approaches. Specifically, we suppressed the expression of Akt, β-catenin, and c-Myc in HEK TER cell transformed by SV40ST (HEK TERST cells). We found that partial suppression of Akt, c-Myc, or β-catenin by specific shRNAs did not significantly affect proliferation (Supplemental Figure 5) but did result in approximately 30% fewer AI colonies in cells dependent on SV40ST for transformation (Figure 4A). Moreover, overexpression of a dominantly interfering TCF mutant (dn-TCF) partially inhibited the ability of HEK TERST cells to grow in AI manner (Figure 4A). The suppression of the SV40ST transforming phenotype after inhibition of PI3K/Akt, c-Myc or Wnt signaling pathways confirmed that these oncogenic pathways contribute to SV40ST-induced transformation.
Figure 4. Cooperation between β-catenin, c-myc, and Akt in cell transformation.
A, AI growth of the HEK TERST cell lines expressing dnTCF or shRNAs specific for CTNNB1, AKT1, or MYC. Suppression of β-catenin and Akt1 was confirmed by immunoblotting. Inhibition of MYC was detected by qRT-PCR (lower panels). B, Combinational expression of c-MycT58A, β-cateninS33Y, and myr-Akt in HEK TER cells. C, Effect of a pairwise expression of the indicated genes on AI growth and tumorigenicity. Mean ± SD for 3 independent experiments. The number of formed tumors formed per number of injection sites is shown.
To complement these loss of function studies, we overexpressed pairwise combinations of constitutively active forms of Akt (myristoylated Akt) (33), β-catenin (β-cateninS33Y mutant) (34), and a degradation resistant c-Myc mutant (c-MycT58A mutant) (28) in immortalized but not tumorigenic HEK TER cells and assessed whether such cell lines were now capable of AI and tumorigenic growth (Figure 4B). We found that HEK TER expressing the stabilized and therefore activated mutants of β-catenin, Akt, or c-myc formed few AI colonies. Co-expression of activated Akt and β-catenin resulted in increased cell proliferation and an enhanced capacity to grow in AI fashion but did not allow such cells to form tumors in immunodeficient mice (Supplemental Figure 6, Figure 4C).
In contrast, the stabilized c-MycT58A mutant cooperated with activated Akt and β-catenin to increase cell proliferation to the same level as was found when we expressed SV40ST (Supplemental Figure 6). Co-expression of c-MycT58A with myristoylated Akt and β-cateninS33Y conferred the ability to grow in an AI manner (Figure 4C). Indeed, we observed that both the size and number of AI colonies was similar to that observed for SV40ST expressing cells (Supplementary Figure 7). Moreover, perturbation of all of these pathways was necessary to induce the formation of tumors in immunodeficient mice (Figure 4C). These observations demonstrate that simultaneously activating the PI3K/Akt, β-catenin, and c-Myc pathways cooperate to induce tumor formation similar to what we observed when PP2A Aα was suppressed.
Suppression of PTPA induces cell transformation through dysregulation of PP2A holoenzyme assembly
We assessed the consequences of suppressing the PP2A catalytic subunits Cα and Cβ on the composition of PP2A complexes in HEK TER cells. Suppression of PP2A Cα resulted in a substantial reduction of the steady state levels of the PP2A B55α, B56β, B56γ, and PR93 regulatory subunits and the PP2A A subunits (Figure 5A). These observations corroborate prior work that showed that the PP2A regulatory and structural subunits exhibit limited stability when not bound to a catalytic PP2A C subunit (12, 35), and suggest that loss of PP2A Cα induces cell transformation through disruption of multiple heterotrimeric PP2A complexes.
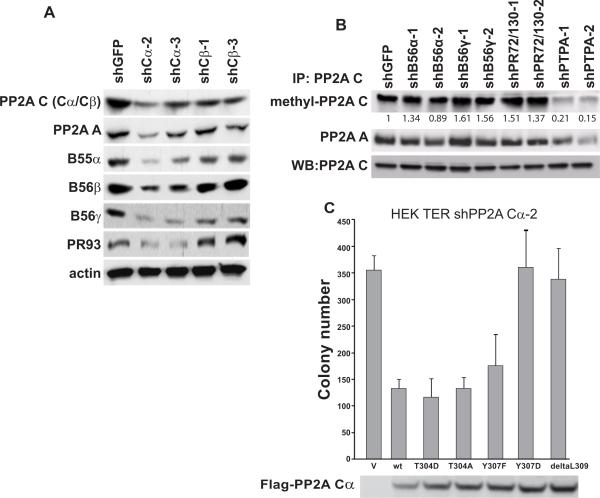
Figure 5. Loss of PTPA induces cell transformation by affecting PP2A C methylation.
A, Expression of the indicated PP2A subunits in cells in which Cα or Cβ was suppressed by the indicated shRNA. B, Methylation levels of PP2A Cα and Cβ and formation of AC dimers in cells expressing the indicated shRNAs. Methylation status of PP2Ac was detected by anti-methyl-PP2Ac antibody. C, The effects of expressing wild-type PP2ACα, phosphorylation-deficient (T304A and Y307F), phosphorylation-mimic (T304D and Y307D), and methylation-deficient (ΔL309) PP2A Cα mutants on AI growth. Flag-tagged wild-type PP2A Cα and the PP2A Cα mutants were introduced to HEK TER cells expressing shRNA specific for PP2A Cα. Expression of exogenous PP2A Cα was confirmed by immunoblotting using anti-Flag antibody. Mean ± SD for 3 independent experiments is shown.
In contrast, suppressing the expression of the PP2A Cβ subunit resulted in only a small decrease of the steady state levels of these PP2A subunits (Figure 5A), confirming that PP2A complexes containing Cβ represent a minor fraction of the total PP2A complexes in the cells (36, 37).
Previous reports revealed that Rrd1 and Rrd2, the PTPA orthologs in yeast, affect the methylation of the PP2A catalytic subunit and control the formation of PP2A heterotrimeric complexes (15, 38). Specifically, deletion of Rrd1 reduces yeast PP2A stability and alters its substrate specificity (15, 38). Mammalian PTPA can complement a mutant of the yeast PTPA genes Rrd1 and Rrd2, suggesting that the mammalian and yeast PTPA orthologs share functional domains (15). When we analyzed cells in which we had suppressed PTPA expression, we found that loss of PTPA dramatically abolished methylation of PP2A catalytic subunit. Because methylation of PP2AC could be also affected by its phosphorylation at Y307, we tested whether PTPA affects PP2AC phosphorylation and found no change in PP2A C phosphorylation in cells in which PTPA expression was suppressed (Supplemental Figure 8). In contrast, we observed a diminished ability of PP2AC to bind to the PP2A A subunit (Figure 5B) and found decreased PP2A A structural subunit expression in cells in which PTPA expression was suppressed (Supplemental Figure 8), consistent with the notion that PP2A A subunits are unstable when not bound to the PP2AC subunit. These observations suggest that PTPA suppression affects formation of heterotrimeric PP2A complexes.
Specifically, these observations suggest that suppression of PTPA induces cell transformation through the regulation of PP2AC methylation. To test whether methylation of PP2A C affected the tumor suppressive properties of PP2A, we generated a methylation-deficient ΔL309 PP2A Cα mutant (5). We also tested phosphorylation site mutants of PP2A Cα (T304A, T304D, Y307A, and Y307F), which have been shown to affect PP2A methylation. Specifically, the PP2A Cα phosphorylation-deficient (T304A and Y307F) and phosphorylation-mimic (T304D) mutants have been found to be methylated, while the PP2A Cα phosphorylation-mimic (Y307D) mutant fails to exhibit methylation (39, 40).
We introduced the wild-type or mutant PP2A Cα into HEK TER cells in which we had suppressed the endogenous PP2A Cα and analyzed AI growth of these cell lines. We found that wild-type PP2A Cα and the T304A, Y307F, and T304D mutants suppressed the transforming phenotype induced by partial suppression of PP2A Cα. In contrast, expression of the PP2A Cα methylation-deficient ΔL309 or Y307D mutants failed to inhibit the transformed phenotype (Figure 5C). These results suggest that methylation of PP2A Cα is crucial for tumor suppressive properties of PP2A and that PTPA subunit contributes to control of cell transformation through regulating the methylation status of PP2AC.
Discussion
DNA tumor viruses express dominant acting oncoproteins that exert their effects by binding to and deregulating cellular targets. In nearly all cases, the targets of viral oncoproteins have been subsequently shown to play important roles in spontaneously arising human cancers. Although PP2A is the primary host binding partner of SV40ST, the biochemical complexity of the PP2A family and the multitude of signaling pathways regulated by this abundant family of phosphatases have complicated efforts to establish the molecular mechanisms employed by SV40ST to induce cell transformation.
Prior work showed that SV40ST displaces multiple PP2A regulatory B subunits from the AC core dimer (16, 17, 41), suggesting that more than one PP2A specific complexes is involved in cell transformation. Indeed we found that partial suppression of the PP2A Cα catalytic subunit is accompanied by the degradation of multiple PP2A subunits and nearly completely recapitulates the transformation phenotype induced by the expression of SV40ST. Moreover, a number of other reports have shown that perturbation of several distinct PP2A complexes leads to the same phenotype. As an example, at least three different regulatory PP2A subunits, B56γ PR72, and PR130, are involved in regulation of the different components of the Wnt signaling pathway (25–27).
In addition, other PP2A subunits may contribute to cell transformation in a manner that is distinct from that induced by SV40ST. For example, we found that depletion of the PP2A Aβ structural subunit induced transformation through activation of the small GTPase RalA. Since SV40ST is not able to bind PP2A Aβ specific complexes, this mechanism of PP2A-mediated transformation is SV40ST independent (13).
Here we have identified several PP2A specific complexes involved in control of cell transformation. Suppression of at least three different B subunits, B56α, B56γ, and PR72/130 as well as a PP2A chaperone PTPA, induced a transformed phenotype, suggesting that these specific regulatory subunits contribute to cell transformation. Consistent with this idea, these same subunits have been implicated in the regulation of PI3K/AKT, Wnt and c-Myc signaling. Specifically, PP2A holoenzymes containing the B56α regulatory subunit associate with and directly dephosphorylate c-Myc at Ser62, a residue previously implicated in targeting c-Myc to proteosome degradation (28, 42). Moreover, loss of B56α or PR72/PR130 expression results in c-Myc overexpression, elevated levels of c-Myc Ser 62 phosphorylation, and increased c-Myc function (24).
We note that overexpression of the c-Myc-T58A mutant has been reported to replace SV40ST in cell transformation (28). We (43) and others (44) have reported that the combination of expressing hTERT and extended propagation of cells in culture led to amplifications of endogenous c-Myc. Herein, we used highly efficient retroviral infections to create cell lines and did not use cells that had been in culture for more than 30 population doublings.
Loss of B56γ has been shown to activate of the Akt pathway; however, it remains unclear whether AKT1 is a direct target of the PP2A B56γ-specific complex (17, 45). B56γ may also contribute to regulation of Wnt pathway through regulation of APC. By binding to APC, PP2A B56γ inhibits formation of APC-axin complexes leading destabilization of β-catenin (27). As a result, overexpression of B56γ reduces the abundance of β-catenin and inhibits transcription of β-catenin target genes (27).
The PR72 and PR130 isoforms, which share the same C terminus, have been shown to regulate activity of Naked cuticle, which is a Wnt antagonist. PR72 acts as a negative regulator of the Wnt signaling cascade through its interaction with Naked cuticle. On the other hand, PR130 modulates Wnt signal transduction by restricting the ability of Naked cuticle to function as a Wnt inhibitor (25, 26). As previously reported (46), we found that PR130 isoform is expressed at much higher levels compared to PR72 in HEK TER cells, and shRNAs specific for PR72/PR130 subunit disproportionally affected the expression of PR130 isoform (data not shown). Specifically, we used two independent shRNAs targeting PR72/130 subunit, of which shPR72/130-1 targets a sequence present only in PR130 isoform while shPR72/130-2 targets both PR72 and PR130. Since both shRNAs induced the same phenotypes, the observed results were specific for the PR130 isoform.
In contrast to the other PP2A regulatory B subunits identified in our screen, PTPA disrupts PP2A function through a distinct mechanism. The recent elucidation of the PP2A holoenzyme structure demonstrated that the highly conserved C-terminal tail of the PP2Ac subunit resides at a critical interface between the PP2A A structural subunit and the B subunit B56γ (14). As such, the recruitment of a B subunit to the core enzyme is tightly regulated by the methylation and phosphorylation patterns of the C-terminal tail. The formation of the holoenzyme complex and the activation of PP2AC are also restrained by a series of interlocking steps involving the dual action of the phosphatase methylesterase PME-1 and PTPA. The mechanism by which PTPA induces C subunit activity remains to be determined, but the PP2A A/PTPA complex may function partly by inhibiting the methylesterase activity of PME-1 (47). Consistent with this notion, we found that suppression of PTPA resulted in a marked decrease in PP2A C methylation.
We found that methylation of PP2AC is crucial for PP2A tumor suppressive activity. The highly conserved C-terminal 304TPDYFL309 tail of PP2A C is also phosphorylated at Y307 and T304, and phosphorylation of these residues affects methylation of PP2A Cα (39, 40). Indeed, the methylation-deficient ΔL309 and Y307D PP2A Cα mutants failed to reverse the tumorigenic phenotype induced by PP2A Cα suppression. This finding is consistent with the observation that phosphorylation at Y307 dramatically inhibits PP2A activity (48). PTPA suppression induced the strongest transforming phenotype among the B subunits tested in our screen, and overexpression of mouse PTPA induces apoptosis in HCT116 colon carcinoma cells (49). These observations implicate PTPA as a potential tumor suppressor that acts by modulating the assembly of PP2A heterotrimers through methylation of PP2A catalytic subunit. Indeed, a recent report demonstrated that suppression of the PP2A methylesterase PME-1 affects both cell proliferation and AI growth, and overexpression of PME-1 correlated with the stage of disease in gliomas (50).
As previously reported (7), we found that PP2A is implicated in regulation of a number of major signaling pathways, including Wnt, PI3K/Akt, and c-Myc. However, we note that direct substrates that modulate these signaling pathways have yet to be identified for the specific PP2A complexes. Further studies are necessary to dissect the mechanisms by which specific PP2A complexes affect these oncogenic pathways. However, the systematic identification of PP2A B subunits involved in control of cell transformation defines the PP2A-dependent pathways regulated by SV40ST and provides insight into how such pathways are perturbed in cell transformation.
Supplementary Material
Acknowledgements
Supported in part by grants from the U.S. National Cancer Institute P01 CA050661 (W.C.H.) and 1K99 CA125974-01A2 (A.A.S.).
References
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Hahn WC, Counter CM, Lundberg AS, Beijersbergen RL, Brooks MW, Weinberg RA. Creation of human tumour cells with defined genetic elements. Nature. 1999;400:464–8. doi: 10.1038/22780. [DOI] [PubMed] [Google Scholar]
- 3.Zhao JJ, Roberts TM, Hahn WC. Functional genetics and experimental models of human cancer. Trends Mol Med. 2004;10:344–50. doi: 10.1016/j.molmed.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Hahn WC, Dessain SK, Brooks MW, et al. Enumeration of the simian virus 40 early region elements necessary for human cell transformation. Mol Cell Biol. 2002;22:2111–23. doi: 10.1128/MCB.22.7.2111-2123.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu J, Boyapati A, Rundell K. Critical role for SV40 small-t antigen in human cell transformation. Virology. 2001;290:192–8. doi: 10.1006/viro.2001.1204. [DOI] [PubMed] [Google Scholar]
- 6.Mungre S, Enderle K, Turk B, et al. Mutations which affect the inhibition of protein phosphatase 2A by simian virus 40 small-t antigen in vitro decrease viral transformation. J Virol. 1994;68:1675–81. doi: 10.1128/jvi.68.3.1675-1681.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eichhorn PJ, Creyghton MP, Bernards R. Protein phosphatase 2A regulatory subunits and cancer. Biochim Biophys Acta. 2008 doi: 10.1016/j.bbcan.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Calin GA, di Iasio MG, Caprini E, et al. Low frequency of alterations of the alpha (PPP2R1A) and beta (PPP2R1B) isoforms of the subunit A of the serine-threonine phosphatase 2A in human neoplasms. Oncogene. 2000;19:1191–5. doi: 10.1038/sj.onc.1203389. [DOI] [PubMed] [Google Scholar]
- 9.Ruediger R, Pham HT, Walter G. Alterations in protein phosphatase 2A subunit interaction in human carcinomas of the lung and colon with mutations in the A beta subunit gene. Oncogene. 2001;20:1892–9. doi: 10.1038/sj.onc.1204279. [DOI] [PubMed] [Google Scholar]
- 10.Takagi Y, Futamura M, Yamaguchi K, Aoki S, Takahashi T, Saji S. Alterations of the PPP2R1B gene located at 11q23 in human colorectal cancers. Gut. 2000;47:268–71. doi: 10.1136/gut.47.2.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang SS, Esplin ED, Li JL, et al. Alterations of the PPP2R1B gene in human lung and colon cancer. Science. 1998;282:284–7. doi: 10.1126/science.282.5387.284. [DOI] [PubMed] [Google Scholar]
- 12.Chen W, Arroyo JD, Timmons JC, Possemato R, Hahn WC. Cancer-associated PP2A Aalpha subunits induce functional haploinsufficiency and tumorigenicity. Cancer Res. 2005;65:8183–92. doi: 10.1158/0008-5472.CAN-05-1103. [DOI] [PubMed] [Google Scholar]
- 13.Sablina AA, Chen W, Arroyo JD, et al. The Tumor Suppressor PP2A Abeta Regulates the RalA GTPase. Cell. 2007;129:969–82. doi: 10.1016/j.cell.2007.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mumby M. The 3D structure of protein phosphatase 2A: new insights into a ubiquitous regulator of cell signaling. ACS Chem Biol. 2007;2:99–103. doi: 10.1021/cb700021z. [DOI] [PubMed] [Google Scholar]
- 15.Fellner T, Lackner DH, Hombauer H, et al. A novel and essential mechanism determining specificity and activity of protein phosphatase 2A (PP2A) in vivo. Genes Dev. 2003;17:2138–50. doi: 10.1101/gad.259903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho US, Morrone S, Sablina AA, Arroyo JD, Hahn WC, Xu W. Structural basis of PP2A inhibition by small t antigen. PLoS Biol. 2007;5:e202. doi: 10.1371/journal.pbio.0050202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen W, Possemato R, Campbell KT, Plattner CA, Pallas DC, Hahn WC. Identification of specific PP2A complexes involved in human cell transformation. Cancer Cell. 2004;5:127–36. doi: 10.1016/s1535-6108(04)00026-1. [DOI] [PubMed] [Google Scholar]
- 18.Boehm JS, Zhao JJ, Yao J, et al. Integrative genomic approaches identify IKBKE as a breast cancer oncogene. Cell. 2007;129:1065–79. doi: 10.1016/j.cell.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 19.Firestein R, Bass AJ, Kim SY, et al. CDK8 is a colorectal cancer oncogene that regulates beta-catenin activity. Nature. 2008;455:547–51. doi: 10.1038/nature07179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moffat J, Grueneberg DA, Yang X, et al. A Lentiviral RNAi Library for Human and Mouse Genes Applied to an Arrayed Viral High-Content Screen. Cell. 2006;124:1283–98. doi: 10.1016/j.cell.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 21.Evans DR, Myles T, Hofsteenge J, Hemmings BA. Functional expression of human PP2Ac in yeast permits the identification of novel C-terminal and dominant-negative mutant forms. J Biol Chem. 1999;274:24038–46. doi: 10.1074/jbc.274.34.24038. [DOI] [PubMed] [Google Scholar]
- 22.Gotz J, Probst A, Ehler E, Hemmings B, Kues W. Delayed embryonic lethality in mice lacking protein phosphatase 2A catalytic subunit Calpha. Proc Natl Acad Sci U S A. 1998;95:12370–5. doi: 10.1073/pnas.95.21.12370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu P, Yu L, Zhang M, et al. Molecular cloning and mapping of the brain-abundant B1gamma subunit of protein phosphatase 2A, PPP2R2C, to human chromosome 4p16. Genomics. 2000;67:83–6. doi: 10.1006/geno.2000.6219. [DOI] [PubMed] [Google Scholar]
- 24.Arnold HK, Sears RC. Protein phosphatase 2A regulatory subunit B56alpha associates with c-myc and negatively regulates c-myc accumulation. Mol Cell Biol. 2006;26:2832–44. doi: 10.1128/MCB.26.7.2832-2844.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Creyghton MP, Roel G, Eichhorn PJ, et al. PR72, a novel regulator of Wnt signaling required for Naked cuticle function. Genes Dev. 2005;19:376–86. doi: 10.1101/gad.328905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Creyghton MP, Roel G, Eichhorn PJ, Vredeveld LC, Destree O, Bernards R. PR130 is a modulator of the Wnt-signaling cascade that counters repression of the antagonist Naked cuticle. Proc Natl Acad Sci U S A. 2006;103:5397–402. doi: 10.1073/pnas.0507237103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seeling JM, Miller JR, Gil R, Moon RT, White R, Virshup DM. Regulation of beta-catenin signaling by the B56 subunit of protein phosphatase 2A. Science. 1999;283:2089–91. doi: 10.1126/science.283.5410.2089. [DOI] [PubMed] [Google Scholar]
- 28.Yeh E, Cunningham M, Arnold H, et al. A signalling pathway controlling c-Myc degradation that impacts oncogenic transformation of human cells. Nat Cell Biol. 2004;6:308–18. doi: 10.1038/ncb1110. [DOI] [PubMed] [Google Scholar]
- 29.Yuan H, Veldman T, Rundell K, Schlegel R. Simian virus 40 small tumor antigen activates AKT and telomerase and induces anchorage-independent growth of human epithelial cells. J Virol. 2002;76:10685–91. doi: 10.1128/JVI.76.21.10685-10691.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao JJ, Gjoerup OV, Subramanian RR, et al. Human mammary epithelial cell transformation through the activation of phosphatidylinositol 3-kinase. Cancer Cell. 2003;3:483–95. doi: 10.1016/s1535-6108(03)00088-6. [DOI] [PubMed] [Google Scholar]
- 31.Sears R, Ohtani K, Nevins JR. Identification of positively and negatively acting elements regulating expression of the E2F2 gene in response to cell growth signals. Mol Cell Biol. 1997;17:5227–35. doi: 10.1128/mcb.17.9.5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arnold HK, Sears RC. A tumor suppressor role for PP2A-B56alpha through negative regulation of c-Myc and other key oncoproteins. Cancer Metastasis Rev. 2008;27:147–58. doi: 10.1007/s10555-008-9128-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kohn AD, Takeuchi F, Roth RA. Akt, a pleckstrin homology domain containing kinase, is activated primarily by phosphorylation. J Biol Chem. 1996;271:21920–6. doi: 10.1074/jbc.271.36.21920. [DOI] [PubMed] [Google Scholar]
- 34.Morin PJ, Sparks AB, Korinek V, et al. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–90. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 35.Silverstein AM, Barrow CA, Davis AJ, Mumby MC. Actions of PP2A on the MAP kinase pathway and apoptosis are mediated by distinct regulatory subunits. Proc Natl Acad Sci U S A. 2002;99:4221–6. doi: 10.1073/pnas.072071699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hemmings BA, Adams-Pearson C, Maurer F, et al. alpha- and beta-forms of the 65-kDa subunit of protein phosphatase 2A have a similar 39 amino acid repeating structure. Biochemistry. 1990;29:3166–73. doi: 10.1021/bi00465a002. [DOI] [PubMed] [Google Scholar]
- 37.Ory S, Zhou M, Conrads TP, Veenstra TD, Morrison DK. Protein phosphatase 2A positively regulates Ras signaling by dephosphorylating KSR1 and Raf-1 on critical 14-3-3 binding sites. Curr Biol. 2003;13:1356–64. doi: 10.1016/s0960-9822(03)00535-9. [DOI] [PubMed] [Google Scholar]
- 38.Hombauer H, Weismann D, Mudrak I, et al. Generation of active protein phosphatase 2A is coupled to holoenzyme assembly. PLoS Biol. 2007;5:e155. doi: 10.1371/journal.pbio.0050155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu XX, Du X, Moreno CS, et al. Methylation of the protein phosphatase 2A catalytic subunit is essential for association of Balpha regulatory subunit but not SG2NA, striatin, or polyomavirus middle tumor antigen. Mol Biol Cell. 2001;12:185–99. doi: 10.1091/mbc.12.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ogris E, Gibson DM, Pallas DC. Protein phosphatase 2A subunit assembly: the catalytic subunit carboxy terminus is important for binding cellular B subunit but not polyomavirus middle tumor antigen. Oncogene. 1997;15:911–7. doi: 10.1038/sj.onc.1201259. [DOI] [PubMed] [Google Scholar]
- 41.Sontag E, Fedorov S, Kamibayashi C, Robbins D, Cobb M, Mumby M. The interaction of SV40 small tumor antigen with protein phosphatase 2A stimulates the map kinase pathway and induces cell proliferation. Cell. 1993;75:887–97. doi: 10.1016/0092-8674(93)90533-v. [DOI] [PubMed] [Google Scholar]
- 42.Sears R, Leone G, DeGregori J, Nevins JR. Ras enhances Myc protein stability. Mol Cell. 1999;3:169–79. doi: 10.1016/s1097-2765(00)80308-1. [DOI] [PubMed] [Google Scholar]
- 43.Elenbaas B, Spirio L, Koerner F, et al. Human breast cancer cells generated by oncogenic transformation of primary mammary epithelial cells. Genes Dev. 2001;15:50–65. doi: 10.1101/gad.828901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang J, Hannon GJ, Beach DH. Risky immortalization by telomerase. Nature. 2000;405:755–6. doi: 10.1038/35015674. [DOI] [PubMed] [Google Scholar]
- 45.Chen Y, Xu Y, Bao Q, et al. Structural and biochemical insights into the regulation of protein phosphatase 2A by small t antigen of SV40. Nat Struct Mol Biol. 2007;14:527–34. doi: 10.1038/nsmb1254. [DOI] [PubMed] [Google Scholar]
- 46.Zwaenepoel K, Goris J, Erneux C, Parker PJ, Janssens V. Protein phosphatase 2A PR130/B”alpha1 subunit binds to the SH2 domain-containing inositol polyphosphate 5-phosphatase 2 and prevents epidermal growth factor (EGF)-induced EGF receptor degradation sustaining EGF-mediated signaling. FASEB J. 24:538–47. doi: 10.1096/fj.09-140228. [DOI] [PubMed] [Google Scholar]
- 47.Longin S, Jordens J, Martens E, et al. An inactive protein phosphatase 2A population is associated with methylesterase and can be re-activated by the phosphotyrosyl phosphatase activator. Biochem J. 2004;380:111–9. doi: 10.1042/BJ20031643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen J, Martin BL, Brautigan DL. Regulation of protein serine-threonine phosphatase type-2A by tyrosine phosphorylation. Science. 1992;257:1261–4. doi: 10.1126/science.1325671. [DOI] [PubMed] [Google Scholar]
- 49.Azam S, Drobetsky E, Ramotar D. Overexpression of the cis/trans isomerase PTPA triggers caspase 3-dependent apoptosis. Apoptosis. 2007;12:1243–55. doi: 10.1007/s10495-006-0050-8. [DOI] [PubMed] [Google Scholar]
- 50.Puustinen P, Junttila MR, Vanhatupa S, et al. PME-1 protects extracellular signal-regulated kinase pathway activity from protein phosphatase 2A-mediated inactivation in human malignant glioma. Cancer Res. 2009;69:2870–7. doi: 10.1158/0008-5472.CAN-08-2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.