Abstract
Based on the hypothesis that soy food consumption may influence breast tissue activity, we examined its effect on the production of nipple aspirate fluid (NAF), a possible indicator of breast cancer risk. Of 310 premenopausal women screened, 112 (36%) produced at least 10 μL of NAF, the minimum for study participation. In a crossover design, we randomized 96 women to 2 groups who, in reverse order, consumed a high-soy diet with 2 soy servings/d (1 serving = 177 mL soy milk, 126 g tofu, or 23 g soy nuts) and a low-soy diet with <3 servings/wk of soy for 6 mo each separated by a 1-mo washout period. During each diet period, 3 NAF samples were obtained (baseline and 3 and 6 mo) using a FirstCyte Aspirator and 4 urine samples (baseline and 1, 3, and 6 mo) were analyzed for isoflavonoids by liquid chromatography tandem MS. Adherence to the study protocol according to 24-h dietary recalls and urinary isoflavonoid excretion was high. The drop-out rate was 15% (n = 14); 82 women completed the intervention. The 2 groups produced similar mean NAF volumes at baseline (P = 0.95) but differed in age and previous soy intake and in their response to the intervention (P = 0.03). In both groups, NAF volume decreased during the first 3 mo of the high-soy diet period and returned to baseline at 6 mo, but there was no effect of the high-soy diet on NAF volume (P = 0.50 for diet; P-interaction = 0.21 for diet with time). Contrary to an earlier report, soy foods in amounts consumed by Asians did not increase breast tissue activity as assessed by NAF volume.
Introduction
A number of epidemiologic studies support the hypothesis that soy foods protect against breast cancer, in particular among Asian populations with high intake (1, 2). Because of their estrogen-like structure and properties, isoflavones as the active ingredients present in soybeans have been the focus of clinical and experimental investigations (3). Depending on dose, isoflavones may act as agonists or antagonists through competitive binding to estrogen receptors, but other mechanisms of action have also been described (4). Although the findings from randomized trials show little effect on breast cancer risk as assessed by serum estrogens (5) and breast density (6–9), it is possible that isoflavones act directly on breast tissue.
Nipple aspirate fluid (NAF)5 collection is a noninvasive method to obtain breast fluid and cells in a large proportion of, but not all, women (10–12). In an uncontrolled trial, the administration of soy protein isolate appeared to stimulate NAF production in premenopausal women (13). This raised concern, because, in large prospective investigations of pre- and postmenopausal women after as many as 25 y of follow-up, participants who produced any NAF experienced a 1.5- to 2-fold higher breast cancer risk than those who did not (14–16). The only other report so far, a small pilot study, did not observe an increase in NAF volume among 11 women after 1 mo of administering soy milk (17). The objective of the current report was to examine the effect of soy foods on NAF volume in premenopausal women who participated in a nutritional intervention that administered soy foods for 6 mo.
Methods
Study design.
We conducted a randomized, crossover soy intervention consisting of 6 mo each of a low-soy and high-soy diet period with a 1-mo washout between diets (Supplemental Fig. 1). The study protocol for this intervention was approved by the Committee on Human Subjects at the University of Hawaii and the Institutional Review Boards at the participating clinics. All participants signed an informed consent form. A Data Safety Monitoring Committee annually reviewed study progress, reasons for drop-outs, and any reported adverse health effects.
Interested women were recruited through multiple sources, including emails to students at the University of Hawaii, letters to patients who had undergone breast or cervical cancer screening at Kaiser Permanente Hawaii and Hawaii Pacific Health, and advertisements in newsletters and newspapers. After mailing 16,306 invitation emails and letters, 825 women responded and were prescreened by telephone. Of these, 310 (38%) were eligible to proceed for a screening visit (Supplemental Fig. 1). The rates of women who attended a screening visit were 21–30% for previous studies and word of mouth, 35–40% for advertisements, flyers, and emails, and 43% for clinics, but the randomization rates were lower for volunteers recruited through the university (19%) and media (23%) than for women identified through clinics (37%). We excluded 515 women who consumed >5 soy servings/wk, had breast implants, used estrogen-containing oral contraceptives or supplements containing isoflavones, were pregnant or breast-feeding, had been diagnosed with cancer, or did not have a uterus or regular menstrual periods. Potential participants also had to be able to donate at least 10 μL NAF, the minimal amount needed for planned laboratory analyses; only 112 of 310 women were able to do so at the screening visit.
As part of the screening visit, all women completed a 26-page questionnaire that included a dietary history, weight and height measurements, a 24-h diet recall, soy questionnaires, and a NAF collection. After screening, 96 participants proceeded to randomization; the 48 women in Group H-L started with the high-soy diet, while the 48 women in Group L-H consumed the low-soy diet first (Supplemental Table 1). All women participated in 5 follow-up visits: mo 3 and 6 of the first diet, after the washout, and mo 10 and 13 of the second diet. Altogether, 14 women (15%) dropped out; all but one left the study during the first 3 mo.
Soy intervention and compliance.
During the high-soy diet period, participants were counseled to consume 2 servings of soy foods/d. This amount provides ∼50 mg of isoflavones (18), a dose similar to daily intake levels reported for Asian populations (19). One serving was defined as 177 mL of soy milk, 126 g of tofu, or 23 g of soy nuts. Women were allowed to choose foods and most women preferred soymilk and tofu. A 3-mo supply of soy foods was provided at the beginning and the middle of the high-soy diet. The participants were free to consume the 2 soy servings at any time of the day as single doses or all at once; there was no restriction to consume additional soy foods. During the low-soy diet period, the women were instructed to maintain their usual diet and consume <3 servings of soy/wk and no soy-containing supplements. As recognition of their effort, they obtained monthly grocery store coupons. No additional changes in lifestyle were recommended. As part of monthly contacts, participants reported changes in health status, if any, for monitoring of adverse health effects.
Adherence to the intervention strategy was assessed by urinary isoflavonoid excretion and by 7 unannounced 24-h dietary recalls. Trained staff collected the first recall during the screening visit and 3 recalls during each diet by telephone. Information on macro- and micronutrients, including isoflavones, was obtained from the food composition tables developed at our center using published data and our own analyses (18, 20).
Sample collection.
NAF and overnight urine samples were collected from each woman at the study visits (Supplemental Table 1) planned to occur during the mid-luteal phase, 3–11 d before the next menstruation, based on self-reported information on previous menstruation dates and cycle length. In a follow-up phone call, the actual date of the next menstruation after the visit was recorded to determine the number of days between NAF collection and the next menstruation. However, due to scheduling problems, only 59% of visits actually took place within that time frame; 9% were closer to the next menstruation and 32% occurred at an earlier time. For NAF collection, a trained staff member demonstrated the collection technique using a FirstCyte Aspirator, a device similar to a manual breast pump consisting of a 10- or 20-cc syringe attached to a small suction cup (10). After cleansing, warming, and massaging, the subject compressed the breast with both hands while the coordinator applied negative pressure to the cup over the nipple by withdrawing the plunger of the syringe halfway until fluid appeared at the nipple surface. After collecting all droplets, the woman expressed additional fluid through massage and pressure behind the nipple area. A maximum of 3 attempts per breast were made. All research assistants adopted the same collection method, but it is possible that some of the high volume producers were able to obtain more NAF by expression without suction. The NAF was collected with microcapillary tubes (10, 20, and 50 μL) and the total amount was recorded. The first 20 μL of NAF were pooled in PBS in a dilution of 1:11, well mixed, aliquoted, and stored at −80°C. The next 5–20 μL were combined with Shandon Cytospin Collection Fluid to preserve breast cells for cytologic analysis. If more NAF was obtained, it was diluted in PBS again. For the 15% of women who were able to produce >90 μL NAF, the collection was usually terminated at 120 μL.
During each diet, the women donated 3 overnight urine samples and 1 mail-in morning urine sample. For overnight urine, participants voided their bladders before going to bed and then collected all urine during the night and in the morning. A mixture of boric and ascorbic acid was added to the air-tight plastic containers to reduce the pH and stabilize the isoflavones without interfering with the assay. Mail-in urine samples were sent at room temperature and overnight samples were transported in an ice box. The urine samples were analyzed for creatinine levels with a Roche-Cobas MiraPlus chemistry analyzer using a kit from Randox Laboratories that is based on a kinetic modification of the Jaffé reaction and for isoflavonoid levels (daidzein, genistein, equol, and O-desmethylangolensin) using liquid chromatography tandem MS; isoflavonoid excretion was expressed as nmol/mmol creatinine (17, 21).
Statistical analysis.
The statistical analysis was performed using the SAS statistical software package version 9.2. (SAS Institute). Normality of distributions was assessed by visual inspection of the frequency curves and non-normally distributed data were log-transformed. Differences were considered significant at a P-value of 0.05. All women were classified into 4 ethnic categories: 48 Caucasians, 26 Asians (4 Chinese, 6 Filipino, 14 Japanese, and 2 Korean), and 22 Others (16 Native Hawaiian/Pacific Islander, 2 African American, 1 American Indian, and 3 Others). Separate compliance indicators based on the 24-h recalls were computed for the 2 diets: >40 mg/d of isoflavones for the high-soy diet and <10 mg/d of isoflavones during the low-soy diet period. Based on a lifetime soy questionnaire that inquired about soy intake during childhood, adolescence, and early and late adulthood (22), we estimated mean soy servings per week and a summary score for lifetime soy intake, which was classified into low (<2 servings/wk) and high (≥2 servings/wk).
All analyses followed the intent-to-treat principle. To assess differences in baseline characteristics between the 2 groups, Student’s t tests were performed for continuous variables and χ2 tests for categorical variables. We calculated unadjusted means and SD for NAF volume by group and time of collection. We computed a 1-way intraclass correlation coefficient (ICC) according to the Shrout and Fleiss convention (23) as a measure of stability of NAF volume over time. To assess the effect of the soy diet on NAF volume, we examined overall group mean differences using the Proc Mixed procedure in SAS with the log-transformed NAF volume as the dependent variable. Mixed models address the dependence of observations in a repeated measurement design by simultaneously modeling the within-person and between-person variances (24, 25). We examined the influence of the soy diet, change over time, interaction between soy diet and time, and effect of group assignment. Analyses were repeated after stratification by ethnicity (Asian vs. Caucasian), compliance, and lifetime soy intake, as well as after restriction to midluteal samples, i.e. 3–11 d before the next menstruation and after removing the drop-outs.
Results
Of the 310 women attending a screening visit, 112 (36%) produced at least 10 μL NAF. The success rate differed by ethnicity (P = 0.01); whereas 47% (44 of 93) of Caucasians produced a sufficient amount, only 26% (33 of 128) of Asians and 39% (35 of 89) of Others were successful. Randomization resulted in 2 groups that did not differ by ethnicity, BMI, reproductive characteristics, or drop-out rate (Table 1). However, women in group L-H were younger by 3 y (P = 0.02), reported a higher soy intake throughout life (P = 0.01), and excreted lower levels of urinary isoflavonoids (P = 0.03) than group H-L. The mean NAF volume at randomization was ∼33 μL and slightly lower at subsequent collection times, but it never differed by group. Not all women produced at least 10 μL NAF at all visits; the respective proportions were 87, 85, 77, 76, 78, and 78% from baseline to mo 13. As indicated by the ICC of 0.58, the amount of NAF produced was relatively stable over time.
TABLE 1.
Characteristics of the study participants by group1
| Characteristic | All | Group H-L | Group L-H | P-value2 |
| n | 96 | 48 | 48 | |
| Drop-outs, n (%) | 14 (15) | 8 (17) | 6 (13) | 0.56 |
| Ethnicity, n (%) | ||||
| White | 48 (50) | 23 (48) | 25 (52) | |
| Asian | 26 (27) | 14 (29) | 12 (25) | |
| Other | 22 (23) | 11 (23) | 11 (23) | 0.89 |
| Age at screening, y | 39.1 ± 6.4 | 40.7 ± 6.0 | 37.6 ± 6.4 | 0.02 |
| BMI, 2 | 25.9 ± 5.6 | 25.7 ± 5.1 | 26.0 ± 6.1 | 0.82 |
| Age at menarche, y | 12.5 ± 1.4 | 12.6 ± 1.4 | 12.4 ± 1.5 | 0.67 |
| Number of children | 1.5 ± 1.3 | 1.6 ± 1.1 | 1.4 ± 1.4 | 0.30 |
| Age at first live birth,3y | 28 ± 6.7 | 27.9 ± 7.3 | 28.2 ± 6.1 | 0.86 |
| Breastfeeding duration,3mo | 16.5 ± 26.2 | 15.3 ± 22.2 | 17.7 ± 30.1 | 0.66 |
| NAF volume, μL | ||||
| Baseline | 33 ± 38 | 33 ± 41 | 33 ± 34 | 0.95 |
| mo 3 | 24 ± 22 | 25 ± 18 | 24 ± 24 | 0.73 |
| mo 6 | 32 ± 36 | 37 ± 40 | 28 ± 32 | 0.27 |
| mo 7 | 24 ± 25 | 25 ± 21 | 23 ± 28 | 0.67 |
| mo 10 | 24 ± 29 | 27 ± 32 | 21 ± 24 | 0.39 |
| mo 13 | 29 ± 33 | 29 ± 36 | 29 ± 30 | 0.98 |
| Soy intake,4servings/wk | ||||
| Early life | 1.5 ± 3.4 | 0.6 ± 0.04 | 2.3 ± 1.1 | 0.02 |
| Adulthood | 3.9 ± 4.5 | 2.8 ± 3.3 | 5.0 ± 5.2 | 0.01 |
| Lifetime | 2.6 ± 3.6 | 1.7 ± 2.4 | 3.5 ± 4.3 | 0.01 |
| Urinary isoflavones, μmol/mmol creatinine | 0.564 ± 1.046 | 0.823 ± 1.281 | 0.317 ± 0.687 | 0.03 |
| Self-reported isoflavone intake, mg/d | 20.6 ± 39.4 | 15.5 ± 4.7 | 25.7 ± 13.5 | 0.21 |
Data are (%) or means ± SD.
P-values for comparison between groups by Student's t test, 2-tailed, 2-sample, unequal variance for numerical variables or χ2-test for categorical variables.
n = 70 for parous women: 38 in group H-L and 32 in group L-H.
Based on a lifetime soy questionnaire that inquired about soy intake during childhood, adolescence, and early and late adulthood; 1 serving = 177 mL soy milk, 126 g tofu, or 23 g soy nuts.
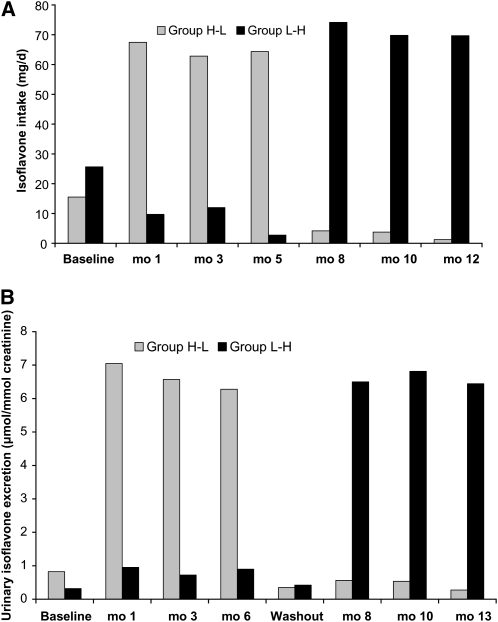
According to the 24-h recalls, mean daily isoflavone consumption during the high soy diet was consistently >60 mg (Fig. 1A). Adherence during the high-soy diet period based on the indicator variable (>40 mg/d isoflavones) was 75–86% and during the low-soy diet period (<10 mg/d isoflavones) 74–98%. The strong increase in urinary isoflavone excretion confirmed the dietary recall data (Fig. 1B); mean values were >10-fold higher during the high-soy than the low-soy diet period. The correlation of urinary isoflavone values with isoflavones assessed by 24-h recalls was highly significant (r = 0.41; P < 0.0001). The number of self-reported adverse health effects did not differ by diet; there was a total of 31 reports during the high-soy diet period, 40 reports during the low-soy diet period, and 27 reports during the washout period. None of the conditions were serious.
FIGURE 1.
Isoflavone intakes (A) and urinary excretion (B) in premenopausal women who consumed low- and high-soy diets for 6 mo each in a crossover design. Values are means, n = 40 (H-L) or 42 (L-H).
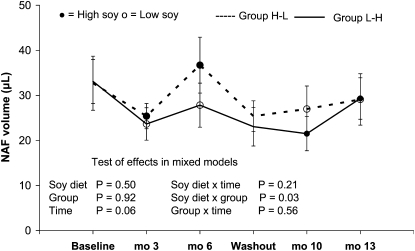
A comparison of the treatment effect by group (Fig. 2) suggested a weak carryover effect (P = 0.11). Therefore, we kept the groups separate in a first analysis. In this mixed model, no effect of the high-soy diet on NAF volume was observed (P = 0.50 for diet and P = 0.21 for the interaction of diet with time). Although the mean NAF volume collected in group H-L was not higher than in group L-H (P = 0.92), there was an interaction effect between group and high-soy diet (P = 0.03) and a borderline significant effect of time (P = 0.06). In both groups, NAF volume was lower after 3 mo than at baseline and returned to a level close to baseline after 6 mo. Further stratification indicated that the differential group effect was present only among women with Asian ancestry and among women with high lifetime soy intake.
FIGURE 2.
NAF volume in premenopausal women who consumed low- and high-soy diets for 6 mo each in a crossover design. Values are means ± SEM, n = 40 (H-L) or 42 (L-H).
When we combined groups H-L and L-H as commonly done in crossover interventions, the effect of the soy diet and the interaction with time remained nonsignificant (P = 0.52 and P-interaction = 0.21), while the effect of time was borderline (P = 0.07). The combined means for the low-soy diet were 30 ± 29, 25 ± 28, and 28 ± 34 μL at baseline and mo 3 and 6, whereas they were 28 ± 36, 23 ± 21, and 33 ± 35 μL during the high-soy diet.
NAF volume did not differ by time within the menstrual cycle (P = 0.33); the respective mean NAF volumes during the follicular, midcyle, midluteal, and late luteal phase were 23 ± 26, 30 ± 34, 28 ± 32, and 31 ± 28 μL. When we repeated the mixed model with only the 289 midluteal (3–11 d before the next menstruation) NAF collections, there was no effect of the soy diet and the interaction of diet with group assignment was no longer significant. After excluding the 14 drop-outs or the noncompliant participants as indicated by the 24-h recalls, the results remained the same, i.e. the soy diet did not affect NAF volume.
Discussion
The results of this randomized crossover soy intervention with excellent compliance as evaluated by 24-h dietary recalls and urinary isoflavonoid excretion did not indicate a change in NAF volume as a result of 2 soy servings/d. Unfortunately, the randomization did not lead to completely balanced groups: Group H-L was 3 y older and reported lower soy intake during early life and adulthood. In contrast, group L-H had lower excretion of urinary isoflavones. This discrepancy might be due to questionnaire data reflecting long-term soy intake information, whereas urinary isoflavones reflect soy consumption 1–2 d prior to urine collection (26). The 2 groups also reacted differently to the intervention as shown by the significant interaction effect between group and dietary assignment. Despite these differences and the fact that not all NAF collections occurred during the midluteal phase as planned, the different mixed models were consistent with one another. In both groups, NAF volume temporarily decreased during the first 3 mo of the high-soy diet and returned to baseline at the end of the 6-mo intervention, but there was no effect of the soy diet on NAF volume.
The findings agree with our pilot study that did not find an increase in NAF volume among 11 women after 1 mo of administering soy milk (17), but disagree with the only previous report measuring changes in NAF volume over 1 y (13). In that study, NAF volume increased 2- to 6-fold compared to baseline during the intervention phase in mo 4–9: 11.5, 18.2, 28.4, and 32.6 μL for mo 1–3, 4–6, 7–9, and 10–12, respectively (P = 0.03). However, this observation was limited to the 14 premenopausal of 24 participants. Also, the women served as their own controls and, therefore, temporal improvements in NAF production or collection cannot be excluded. Our study also agrees with previous reports that fewer Asian than Caucasian women are able to produce NAF (11, 27, 28).
There is limited evidence whether NAF producers have a higher breast cancer risk. In a follow-up of 2 large cohorts (14), the RR were 1.6 (95% CI = 1.1–2.3) and 1.2 (95% CI = 0.8–2.0), respectively, for those with normal cytology in aspirates compared with women from whom no fluid was obtained. A 25-y follow-up study (15) described a higher RR only for women with epithelial cells in NAF (RR = 1.92; 95% CI = 1.22–3.01), whereas the risk for women without epithelial cells was not significantly elevated (RR = 1.05; 95% CI = 0.71–1.57). Similar findings were reported from a later cohort (16); the respective risk estimates were 1.4 (95% CI = 0.32–6.4) for fluid without epithelial cells and 1.7 (95% CI = 0.87–3.5) for normal epithelial, but only epithelial cells that had hyperplasia or atypia were associated with an elevated risk of 2.0 (95% CI = 1.1–3.6).
This study had a number of limitations. The randomization was not perfect; therefore, some unknown difference between the 2 groups may be responsible for the interaction between dietary and group assignment. The NAF collection process poses many problems and the absolute amount of fluid collected is difficult to measure accurately. The number of attempts and the amount of time spent to obtain fluid considerably influence the final collection volume, in particular for women who produce large amounts of NAF. If a participant was pressed for time, the collection may have been terminated early and the success rate decreased over time. Individual reports indicate that the ability to produce NAF may be reduced under stress or during illness, but the ICC of 0.58 indicated that NAF volume was relatively stable within women. Given the small number of women who converted from NAF producers to nonproducers, we were not able to examine reasons for a conversion.
The difficulties of collecting these small amounts of fluid in capillaries are numerous. Sometimes the NAF is very aqueous, whereas at other times, it can be quite viscous; both types are difficult to capture in a capillary. Measuring the total amount can be complicated by air bubbles in the capillaries. Given these issues, cell cytology and composition of NAF might be better indicators of breast tissue activity. Moreover, we were not able to ascertain whether the menstrual cycles were ovulatory, because the determination of midluteal was based on the time between the visit and the next menstruation date. Because we tried to enroll participants with regular cycles, the number of anovulatory cycles is not expected to be higher than the 10–15% observed in a previous study using ovulation kits (29). Also, we did not observe differences in NAF volume by menstrual cycle phase (data not shown). Finally, our findings may not apply to women with high soy consumption throughout life, because the inclusion criteria required low soy intake.
On the other hand, this trial has many strengths. For a nutritional study, it was relatively long term. The women represented several ethnic groups, the soy foods in the study were similar to traditional Asian foods and administered in comparable amounts (19), the adherence to the dietary protocol was very high, the drop-out rate was acceptably low, and the analysis was based on multiple collections including NAF and urinary isoflavonoid levels. The crossover randomized design allowed for assessing both between- and within-woman effects.
Although not measured in this study, isoflavones are undoubtedly present in NAF. As shown in our pilot study, median isoflavone levels were 180 nmol/L in 10 women consuming 2 servings/d of soy milk (17), which is ∼10–20% of the concentration typically found in serum given the same exposure (17, 30). In a recent report analyzing breast tissue from reduction surgeries, genistein and dadzein levels were also found to be higher in serum than in breast tissue (31). Although the findings of this crossover randomized study with soy foods in the range of Asian diets does not suggest any effects on breast tissue activity as assessed by NAF volume, future analyses will address estrogen concentrations and cell cytology of the breast fluid in this study population. Given the widespread concern about possible adverse effects of soy foods (32–34), the results of this study add to the literature that indicates the safety of consuming soy foods in amounts comparable to traditional Asian diets.
Supplementary Material
Acknowledgments
We thank Dr. Nicholas Petrakis, Professor Emeritus at the University of California at San Francisco, who kindly provided expertise and training in many aspects of NAF research. G.M. and A.A.F. designed the research project; I.S.P. and Y.M. managed data and performed statistical data analysis; G.M. and Y.M. wrote the paper; S.M.C., I.S.P., and A.A.F. critically revised the paper; G.M. and A.A.F. had primary responsibility for final content. All authors read and approved the final manuscript.
Footnotes
Supported by grants from the National Cancer Institute (R01 CA 80843 and P30 CA 71789) and from the National Center for Research Resources (S10 RR 020890).
This trial was registered at clinicaltrials.gov as NCT00513916.
Supplemental Table 1 and Figure 1 are available with the online posting of this paper at jn.nutrition.org.
Abbreviations used: Group H-L, high-soy diet first, then low-soy diet; Group L-H, low-soy diet first, then high-soy diet; ICC, intraclass correlation coefficient; NAF, nipple aspirate fluid.
Literature Cited
- 1.Trock BJ, Hilakivi-Clarke L, Clarke R. Meta-analysis of soy intake and breast cancer risk. J Natl Cancer Inst. 2006;98:459–71 [DOI] [PubMed] [Google Scholar]
- 2.Wu AH, Yu MC, Tseng CC, Pike MC. Epidemiology of soy exposures and breast cancer risk. Br J Cancer. 2008;98:9–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Setchell KD. Phytoestrogens: the biochemistry, physiology, and implications for human health of soy isoflavones. Am J Clin Nutr. 1998;68:S1333–46 [DOI] [PubMed] [Google Scholar]
- 4.Barnes S, Boersma B, Patel R, Kirk M, Darley-Usmar VM, Kim H, Xu J. Isoflavonoids and chronic disease: mechanisms of action. Biofactors. 2000;12:209–15 [DOI] [PubMed] [Google Scholar]
- 5.Hooper L, Ryder JJ, Kurzer MS, Lampe JW, Messina MJ, Phipps WR, Cassidy A. Effects of soy protein and isoflavones on circulating hormone concentrations in pre- and post-menopausal women: a systematic review and meta-analysis. Hum Reprod Update. 2009;15:423–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maskarinec G, Takata Y, Franke AA, Williams AE, Murphy SP. A 2-year soy intervention in premenopausal women does not change mammographic densities. J Nutr. 2004;134:3089–94 [DOI] [PubMed] [Google Scholar]
- 7.Maskarinec G, Verheus M, Tice J. Epidemiologic studies of isoflavones and mammographic density. Nutrients. 2010;2:35–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maskarinec G, Verheus M, Steinberg FM, Amato P, Cramer MK, Lewis RD, Murray MJ, Young RL, Wong WW. Various doses of soy isoflavones do not modify mammographic density in postmenopausal women. J Nutr. 2009;139:981–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hooper L, Madhavan G, Tice JA, Leinster SJ, Cassidy A. Effects of isoflavones on breast density in pre- and post-menopausal women: a systematic review and meta-analysis of randomized controlled trials. Hum Reprod Update. 2010;16:745–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petrakis NL. Nipple aspirate fluid in epidemiologic studies of breast disease. Epidemiol Rev. 1993;15:188–95 [DOI] [PubMed] [Google Scholar]
- 11.Wrensch MR, Petrakis NL, Gruenke LD, Ernster VL, Miike R, King EB, Hauck WW. Factors associated with obtaining nipple aspirate fluid: analysis of 1428 women and literature review. Breast Cancer Res Treat. 1990;15:39–51 [DOI] [PubMed] [Google Scholar]
- 12.Baltzell KA, Wrensch M, Sison JD. A descriptive study of variables associated with obtaining nipple aspirate fluid in a cohort of non-lactating women. BMC Womens Health. 2006;6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petrakis NL, Barnes S, King EB, Lowenstein J, Wiencke J, Lee MM, Miike R, Kirk M, Coward L. Stimulatory influence of soy protein isolate on breast secretion in pre-and postmenopausal women. Cancer Epidemiol Biomarkers Prev. 1996;5:785–94 [PubMed] [Google Scholar]
- 14.Wrensch MR, Petrakis NL, Miike R, King EB, Chew K, Neuhaus J, Lee MM, Rhys M. Breast cancer risk in women with abnormal cytology in nipple aspirates of breast fluid. J Natl Cancer Inst. 2001;93:1791–8 [DOI] [PubMed] [Google Scholar]
- 15.Buehring GC, Letscher A, McGirr KM, Khandhar S, Che LH, Nguyen CT, Hackett AJ. Presence of epithelial cells in nipple aspirate fluid is associated with subsequent breast cancer: a 25-year prospective study. Breast Cancer Res Treat. 2006;98:63–70 [DOI] [PubMed] [Google Scholar]
- 16.Baltzell KA, Moghadassi M, Rice T, Sison JD, Wrensch M. Epithelial cells in nipple aspirate fluid and subsequent breast cancer risk: a historic prospective study. BMC Cancer. 2008;8:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maskarinec G, Hebshi S, Custer L, Franke AA. The relation of soy intake and isoflavone levels in nipple aspirate fluid. Eur J Cancer Prev. 2008;17:67–70 [DOI] [PubMed] [Google Scholar]
- 18.Franke AA, Hankin JH, Yu MC, Maskarinec G, Low SH, Custer LJ. Isoflavone levels in soy foods consumed by multiethnic populations in Singapore and Hawaii. J Agric Food Chem. 1999;47:977–86 [DOI] [PubMed] [Google Scholar]
- 19.Messina M, Nagata C, Wu AH. Estimated Asian adult soy protein and isoflavone intakes. Nutr Cancer. 2006;55:1–12 [DOI] [PubMed] [Google Scholar]
- 20.Murphy SP. Unique nutrition support for research at the Cancer Research Center of Hawaii. Hawaii Med J. 2002;61:15, 17 [PubMed] [Google Scholar]
- 21.Franke AA, Halm BM, Custer LJ, Tatsumura Y, Hebshi S. Isoflavones in breastfed infants after mothers consume soy. Am J Clin Nutr. 2006;84:406–13 [DOI] [PubMed] [Google Scholar]
- 22.Maskarinec G, Aylward AG, Erber E, Takata Y, Kolonel LN. Soy intake is related to a lower body mass index in adult women. Eur J Nutr. 2008;47:138–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86:420–8 [DOI] [PubMed] [Google Scholar]
- 24.Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS system for mixed models. Cary (NC): SAS Institute Inc.; 1996 [Google Scholar]
- 25.Singer JD, Willett JB. Applied longitudinal data analysis: modeling change and event occurrence. Oxford: Oxford University Press; 2003 [Google Scholar]
- 26.Franke AA, Halm BM, Kakazu K, Li X, Custer LJ. Phytoestrogenic isoflavonoids in epidemiologic and clinical research. Drug Test Anal. 2009;1:14–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petrakis NL, Lee MM, Wrensch MR, Ernster VL, Miike R, Koo LC, Ho JC. Birthplace and yield of nipple aspirate fluid in Chinese women. Cancer Epidemiol Biomarkers Prev. 1998;7:835–9 [PubMed] [Google Scholar]
- 28.Zhao YS, Pang D, Wang F, Xue YW, Gao DN, Li H, Li K, Wang BY, Wang D, et al. Nipple aspirate fluid collection, related factors and relationship between carcinoembryonic antigen in nipple aspirate fluid and breast diseases in women in Harbin, PRC. Cancer Epidemiol Biomarkers Prev. 2009;18:732–8 [DOI] [PubMed] [Google Scholar]
- 29.Maskarinec G, Franke AA, Williams AE, Hebshi S, Oshiro C, Murphy SP, Stanczyk FZ. Effects of a 2-year randomized soy intervention on sex hormone levels in premenopausal women. Cancer Epidemiol Biomarkers Prev. 2004;13:1736–44 [PubMed] [Google Scholar]
- 30.Gardner CD, Chatterjee LM, Franke AA. Effects of isoflavone supplements vs. soy foods on blood concentrations of genistein and daidzein in adults. J Nutr Biochem. 2009;20:227–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bolca S, Urpi-Sarda M, Blondeel P, Roche N, Vanhaecke L, Possemiers S, Al-Maharik N, Botting N, De KD, et al. Disposition of soy isoflavones in normal human breast tissue. Am J Clin Nutr. 2010;91:976–84 [DOI] [PubMed] [Google Scholar]
- 32.Messina M, Caskill-Stevens W, Lampe JW. Addressing the soy and breast cancer relationship: review, commentary, and workshop proceedings. J Natl Cancer Inst. 2006;98:1275–84 [DOI] [PubMed] [Google Scholar]
- 33.Messina MJ, Loprinzi CL. Soy for breast cancer survivors: a critical review of the literature. J Nutr. 2001;131:S3095–108 [DOI] [PubMed] [Google Scholar]
- 34.Hilakivi-Clarke L, Andrade JE, Helferich W. Is soy consumption good or bad for the breast? J Nutr. 2010;140:S2326–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.