Abstract
Vitamin D insufficiency is common globally and low levels are linked to higher cancer incidence. Although vitamin D insufficiency is related to inferior prognosis in some cancers, no data exist for chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL). We evaluated the relationship of 25(OH)D serum levels with time-to-treatment (TTT) and overall survival (OS) in newly diagnosed CLL patients participating in a prospective cohort study (discovery cohort) and a separate cohort of previously untreated patients participating in an observational study (confirmation cohort). Of 390 CLL patients in the discovery cohort, 119 (30.5%) were 25(OH)D insufficient. After a median follow-up of 3 years, TTT (hazard ratio[HR] = 1.66; P = .005) and OS (HR = 2.39; P = .01) were shorter for 25(OH)D-insufficient patients. In the validation cohort, 61 of 153 patients (39.9%) were 25(OH)D insufficient. After a median follow-up of 9.9 years, TTT (HR = 1.59; P = .05) and OS (HR 1.63; P = .06) were again shorter for 25(OH)D-insufficient patients. On pooled multivariable analysis of patients in both cohorts adjusting for age, sex, Rai stage, CD38 status, ZAP-70 status, immunoglobulin heavy chain variable (IGHV) gene mutation status, CD49d status, and cytogenetic abnormalities assessed by interphase fluorescent in situ hybridization testing, 25(OH)D insufficiency remained an independent predictor of TTT (HR = 1.47; P = .008), although the association with OS was not significant (HR = 1.47; P = .07). Vitamin D insufficiency is associated with inferior TTT and OS in CLL patients. Whether normalizing vitamin D levels in deficient CLL patients would improve outcome merits clinical testing.
Introduction
Vitamin D insufficiency is common globally and in the United States. Approximately 25%-50% of patients seen in routine clinical practice have vitamin D levels below the optimal range, and it is estimated that up to 1 billion people worldwide have vitamin D insufficiency.1–3 Vitamin D is obtained from skin exposure to sunlight (ie, ultraviolet B radiation) and through dietary sources including supplementation. Serum levels of 25-hydroxyvitamin D (25[OH]D) reflect whole-body vitamin D stores and are used to assess individual vitamin D adequacy or insufficiency.3 25(OH)D is converted to 1,25-dihydroxyvitamin D (1,25[OH]2D), the physiologically active form of vitamin D, via the action of 1-α-hydroxylase primarily in the kidney. Once formed, 1,25(OH)2D exerts its biologic effects by binding to the vitamin D nuclear transcription factor receptor, which regulates the expression of nearly 200 genes.4
Vitamin D has a central role in maintaining serum calcium and skeletal homeostasis, as well as multiple other cellular effects, including regulation of differentiation, proliferation, apoptosis, metastatic potential, and angiogenesis.5 Several reports now suggest that low serum 25(OH)D levels may be associated with increased incidence of colorectal,6,7 breast,8,9 and other cancers.10 Consistent with these results, one population-based, double-blind, randomized placebo-controlled trial found that women who increased their daily vitamin D intake by 1100 IU reduced their risk of cancer by 60%-77%.11 In addition to the risk of developing malignancy, recent data suggest that low 25(OH)D levels at diagnosis may be associated with poorer prognosis in colorectal,12 breast,13 melanoma,14 and lung15 cancer, although these data have not yet been replicated in independent cohorts.
Despite growing evidence for a relationship between vitamin D levels and solid tumor risk, far less is known about the relationship between vitamin D and the risk of hematologic malignancy. A pooled analysis of 10 studies found that higher levels of recreational sun exposure, which would be anticipated to increase vitamin D levels, was associated with a lower risk for non-Hodgkin lymphoma.16 Furthermore, data from 2 prospective cohort studies17,18 provide suggestive evidence that low serum 25(OH)D levels are associated with increased incidence of NHL; however, these studies did not report on chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) specifically, and one recent case-control study did not find a relationship between 25(OH)D levels and risk of developing CLL/SLL.19
To our knowledge, there are no data on the relationship between vitamin D and prognosis in patients with CLL. Because CLL is currently incurable, the identification of remediable factors that relate to disease progression would be valuable. To test the hypothesis that vitamin D levels predict outcome in patients with CLL, we examined the association of serum 25(OH)D levels with time to treatment (TTT) and overall survival (OS) in a prospective cohort of consecutively enrolled patients with newly diagnosed CLL, and validated these findings in a second, independent CLL cohort.
Methods
Discovery cohort
Patients in the discovery cohort were participants in the ongoing prospective cohort study of patients with NHL from the Molecular Epidemiology Resource of the University of Iowa/Mayo Clinic Lymphoma Specialized Program of Research Excellence (SPORE).20 This study was reviewed and approved by the Human Subjects Institutional Review Board at the Mayo Clinic and the University of Iowa, and written informed consent was obtained from all participants in accordance with the Declaration of Helsinki. Since September 2002, we have offered enrollment to consecutive, newly diagnosed patients with CLL who were evaluated at Mayo Clinic Rochester or the University of Iowa within 9 months of diagnosis, were age 18 years and older, and were a US resident at diagnosis (data on NHL will be reported separately). Exclusion criteria included known HIV infection and unwillingness or inability to provide written in-ormed consent.
All leukemia cell immunophenotype and pathology analyses were reviewed to confirm that each case met the criteria for a diagnosis of CLL. All patients had an absolute lymphocyte count (ALC) > 5.0 × 109/L and fulfilled the 1996 criteria for CLL in effect throughout the study interval21 and/or fulfilled the World Health Organization criteria for the SLL variant of CLL.22 Baseline clinical, laboratory, and treatment data were abstracted from medical records using a standardized protocol. Participants provided peripheral blood serum samples and were systematically followed every 6 months for the first 3 years, and then annually thereafter. Disease progression (ie, requirement for treatment) and deaths were verified through medical record review. We also verified patients' reports of no disease progression on an annual basis. For decedents, we obtained a copy of the death certificate and medical records associated with the death.
Confirmation cohort
Between January 1994 and October 2002, we enrolled a separate cohort of 159 patients with previously untreated CLL seen at Mayo Clinic Rochester or Mayo Clinic Jacksonville in a prospective trial evaluating the prognostic importance of cytogenetic abnormalities and clonal evolution as evaluated by fluorescence in situ hybridization (FISH). The characteristics of this cohort have been previously described in detail.23–25 The trial was approved by the Mayo Clinic Human Subjects Institutional Review Board, and all patients provided written informed consent.
Prognostic parameters
Prognostic testing (immunoglobulin heavy chain variable [IGHV] region gene mutation analysis, ZAP-70 status, CD38 status, CD49d status, and cytogenetic abnormalities assessed by interphase FISH testing) was performed as part of clinical or research studies using methods previously described by our group.23,26,27
Vitamin D measurements
Vitamin D insufficiency was defined as a serum 25(OH)D level < 25 ng/mL (62.5 nmol/L). Although consensus guidelines for the diagnosis of vitamin D insufficiency have not been established, this is an accepted level for the establishment of hypovitaminosis D3 and is the threshold used in routine clinical practice to identify individuals with 25(OH)D insufficiency by Mayo Clinic Medical Laboratories (http://www.mayomedicallaboratories.com). To avoid assay variability, which can confound vitamin D determinations made using radioimmunoassay methods,28 all vitamin D measurements were made by liquid chromatography-tandem mass spectrometry (LC-MS/MS). Measurements of 25(OH)D were made by deuterated stable isotope (d6-25[OH]D)-dilution LC-MS/MS on an API 4000 instrument (Applied Biosystems). Calibration used a 6-point standard curve over a concentration range of 0-200 ng/mL. Intra- and inter-assay coefficients of variation were all < 7% (data not shown). All vitamin D assays were performed on stored research samples; results were not included in the clinical record and were not known to the treating physicians in either study.
Statistical analysis
Fisher exact and χ2 tests, where appropriate, were used to assess the association of 25(OH)D insufficiency and clinical, prognostic, and demographic factors in CLL patients. TTT was defined as the time from diagnosis to disease progression requiring treatment. OS was defined as the time from diagnosis to death due to any cause. Patients without an event or death were censored at time of last known follow-up. Kaplan-Meier29 curves and Cox proportional hazards regression models30 were used to assess the association of vitamin D levels and outcome. Cox models adjusted for demographic and CLL prognostic factors were also assessed. We also analyzed the associations using the actual, continuously distributed 25(OH)D levels values via penalized smoothing splines, or P-splines.31 Analyses were performed using SAS Version 9.1.3 and R Version 2.7.1.
Results
Patients
From September 2002 through February 2008, 610 CLL patients seen within 9 months of diagnosis at the Mayo Clinic or University of Iowa were enrolled in an ongoing prospective observational cohort study. Of these 610 patients, 220 (36%) did not have serum samples available and were excluded from analysis. The remaining 390 patients constituted the discovery cohort used to explore the relationship between vitamin D insufficiency and clinical outcome. Serum samples were collected a median of 2.5 months after diagnosis (range 0-73, 293/390 [75%] within 12 months of diagnosis).
A separate cohort of 159 patients with previously untreated CLL seen at Mayo Clinic was enrolled in a prospective observational trial between January 1994 and October 2002.23–25 Of these 159 patients, 153 (96%) had serum samples stored and constituted the confirmation cohort. All 153 patients had serum samples drawn within 12 months of study registration, with a median time from diagnosis to study enrollment of 3.2 months (range 0-236, 94/152 [61%] within 12 months of diagnosis). The clinical characteristics of the patients in these 2 prospective observational cohort studies are shown in Table 1. A majority of patients (> 80%) in both cohorts had Rai stage 0 or I disease at diagnosis.
Table 1.
Patient characteristics
| Discovery cohort (n = 390) | Confirmation cohort (n = 153) | |
|---|---|---|
| Median age at diagnosis, y | 63 | 67 |
| Male sex | 267 (68.5%) | 108 (70.6%) |
| Rai stage at diagnosis | ||
| 0 | 180 (46.3%) | 79 (51.6%) |
| I | 169 (43.4%) | 48 (31.4%) |
| II | 29 (7.5%) | 17 (11.1%) |
| III | 6 (1.5%) | 2 (1.3%) |
| IV | 5 (1.3%) | 7 (4.6%) |
| Missing | 1 | 0 |
| CD38 | ||
| Negative | 234 (71.8%) | 104 (68%) |
| Positive | 92 (28.2) | 49 (32%) |
| Missing | 64 | 0 |
| ZAP-70 | ||
| Negative | 215 (69.1%) | 64 (41.8%) |
| Positive | 96 (30.9%) | 89 (58.2%) |
| Missing | 79 | 0 |
| IGHV | ||
| Mutated | 109 (38.2%) | 70 (56.5%) |
| Unmutated | 176 (61.8%) | 54 (43.5%) |
| Missing | 105 | 29* |
| CD49d | ||
| Negative | 134 (67.3%) | 109 (71.2%) |
| Positive | 65 (32.7%) | 44 (28.8%) |
| Missing | 195 | 0 |
| FISH (prior to treatment) | ||
| 13q− | 126 (40.6%) | 69 (45.1%) |
| Normal | 83 (26.8%) | 36 (23.5%) |
| +12 | 57 (18.4%) | 22 (14.4%) |
| 11q− | 26 (8.4%) | 13 (8.5%) |
| 17p− | 12 (3.9%) | 8 (5.2%) |
| Other | 6 (1.9%) | 5 (3.3%) |
| Missing | 80 | 0 |
| 25 OH vitamin D levels | ||
| Mean (SD) | 30.6 (10.65) | 26.6 (9.27) |
| % insufficient (< 25 ng/mL) | 119 (30.5%) | 61 (39.9%) |
IGHV sequencing was attempted in all 153 patients, with 124 (81%) patients classifiable as mutated or unmutated.
Vitamin D insufficiency and clinical outcome in discovery cohort
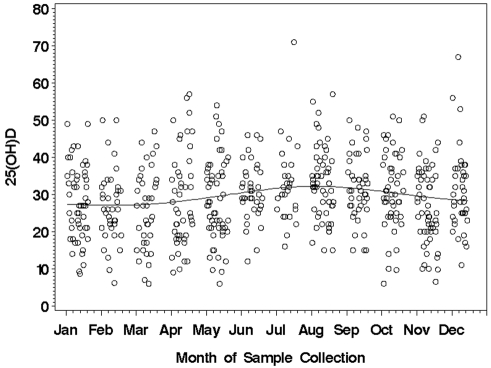
The mean 25(OH)D level for the 390 patients in the discovery cohort was 30.6 ± 10.7 ng/mL (range 6.0-71.0). Because vitamin D levels can be related to sun exposure, we evaluated the relationship between vitamin D levels and the month of sample collection. Minimal relationship between the month of sample collection and 25(OH)D level was observed (Figure 1). ALCs obtained within 2 months of the vitamin D assay did not correlate with 25(OH)D level (n = 229; Spearman correlation = −0.03; P = .65). Overall, 9 patients (2.3%) had severe vitamin D insufficiency (< 10 ng/mL), 110 (28.2%) had mild to moderate insufficiency (10-24 ng/mL), and 271 (69.5%) had 25(OH)D levels within the optimal range(25-80 ng/mL). In aggregate, 119 (30.5%) were 25(OH)D insufficient. No difference in the prevalence of vitamin D insufficiency was observed based on whether samples were collected ≤ 12 months or > 12 months from diagnosis (31.5% vs 27.6%, respectively; P = .46). 25(OH)D insufficiency was not correlated with age, sex, Rai stage, or season of CLL diagnosis (Table 2). 25(OH)D insufficiency had no relationship with CD38, ZAP-70, CD49d, or IGHV mutation status and had no relationship with FISH risk category as classified using the Dohner system.32
Figure 1.
Month of sample collection and 25(OH)D levels (ng/mL) in the discovery cohort (n = 390).
Table 2.
Patient characteristics and vitamin D insufficiency discovery cohort
| Vitamin D sufficient (n = 271) | Vitamin D insufficient (n = 119) | P | |
|---|---|---|---|
| Median age at diagnosis, y | 62 | 64 | .38 |
| Male | 178 (65.7%) | 89 (74.8%) | .07 |
| Rai stage at diagnosis | |||
| 0 | 131 (48.3%) | 49 (41.5%) | trend P = .23 |
| I | 111 (41.0%) | 58 (49.2%) | |
| II | 25 (9.2%) | 4 (3.4%) | |
| III | 1 (0.4%) | 5 (4.2%) | |
| IV | 3 (1.1%) | 2 (1.7%) | |
| Season at CLL diagnosis | |||
| Spring | 60 (22.1%) | 31 (26.1%) | .75 |
| Summer | 84 (31.0%) | 34 (28.6%) | |
| Fall | 66 (24.4%) | 25 (21.0%) | |
| Winter | 61 (22.5%) | 29 (24.4%) | |
| CD38 | |||
| Negative | 171 (74.0%) | 63 (66.3%) | .16 |
| Positive | 60 (26.0%) | 32 (33.7%) | |
| ZAP-70 | |||
| Negative | 153 (69.9%) | 62 (67.4%) | .67 |
| Positive | 66 (30.1%) | 30 (32.6%) | |
| IGHV | |||
| Mutated | 129 (63.9%) | 47 (56.6%) | .25 |
| Unmutated | 73 (36.1%) | 36 (43.4%) | |
| CD49d | |||
| Negative | 96 (68.1%) | 38 (65.5%) | .73 |
| Positive | 45 (31.9%) | 20 (34.5%) | |
| FISH (prior to treatment) | |||
| 13q− | 96 (43.8%) | 30 (33.0%) | .10 |
| Normal | 53 (24.2%) | 30 (33%) | |
| +12 | 41 (18.7%) | 16 (17.6%) | |
| 11q− | 19 (8.7%) | 7 (7.7%) | |
| 17p− | 5 (2.3%) | 7 (7.7%) | |
| Other | 5 (2.3%) | 1 (1.1%) |
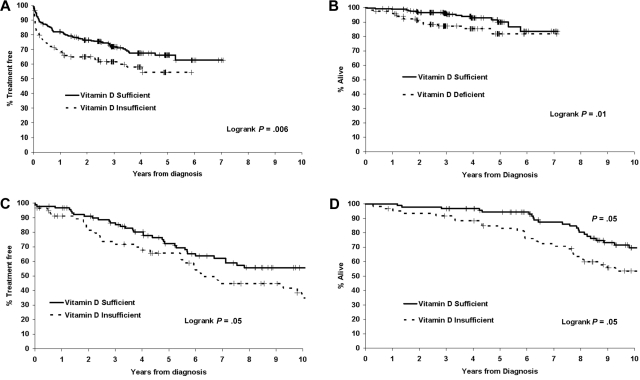
Follow-up for vital status was available for all patients, with a median follow-up time from diagnosis for living patients in the discovery cohort of 36 months (range 1-86 months). As of the last follow-up, 131 (33.6%) patients had received treatment for progressive disease and 34 (8.7%) had died. Both TTT and OS were shorter in 25(OH)D-insufficient patients (Figure 2A-B). The HR for TTT among 25(OH)D-insufficient patients was 1.66 (95% confidence interval [CI] = 1.16-2.37; P = .005), while the HR for death was 2.39 (95% CI = 1.21-4.70; P = .01). The HRs for TTT and OS were similar when the outcome was measured from the date of sample (rather than diagnosis) or limited to patients assayed ≤ 12 months from diagnosis (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Figure 2.
TTT and OS based on vitamin D insufficiency. (A) TTT in the discovery cohort (n = 390). (B) OS in the discovery cohort (n = 390). (C) TTT in the confirmation cohort (n = 152). (D) OS in the confirmation cohort (n = 153).
Vitamin D insufficiency and clinical outcome in confirmation cohort
We next attempted to validate these findings in an independent group of previously untreated CLL patients. The mean 25(OH)D level in the confirmation cohort was 26.6 ± 9.3 ng/mL (range 6.0-54.0). Overall, 6 patients (3.9%) had severe insufficiency (< 10 ng/mL), 55 (36.0%) had mild to moderate insufficiency (10-24 ng/mL), and 92 (60.1%) had 25(OH)D levels in the optimal range (25-80 ng/mL). In aggregate, 61 (39.9%) patients had 25(OH)D insufficiency. No difference in the prevalence of vitamin D insufficiency was observed based on whether samples were collected ≤ 12 months versus > 12 months from diagnosis (43.6% vs 33.9%; P = .23). 25(OH)D insufficiency was not correlated with age, sex, season of diagnosis, or disease stage in the confirmation cohort. 25(OH)D insufficiency also showed no correlation with ZAP-70, CD49d, IGHV mutation status or FISH risk category in the confirmation cohort, but was associated with CD38 status: 57.1% of CD38-positive patients had 25(OH)D insufficiency compared with 31.7% of CD38-negative patients (P = .003).
Follow-up for vital status was available for all patients, with a median follow-up from diagnosis for living patients in the confirmation cohort of 118 months (range 10-275 months). As of the last follow-up, 70 (45.8%) patients had been treated and 62 (40.5%) had died. TTT and OS were shorter for 25(OH)D-insufficient patients in the confirmation cohort (Figure 2C-D). The HR for TTT among 25(OH)D-insufficient patients was 1.59 (95% CI = 0.99-2.56; P = .05), while the HR for OS was 1.63 (95% CI = 0.99-2.69; P = .06). HRs for TTT and OS were similar when outcome was measured from the date of sample (rather than diagnosis) or limited to patients assayed ≤ 12 months from diagnosis (supplemental Table 1).
Interactions between vitamin D insufficiency and prognostic parameters
Based on the similar associations of vitamin D insufficiency with TTT and OS in these 2 cohorts, patients from both cohorts were pooled to allow assessment of the severity of vitamin D insufficiency and clinical outcome, interactions with prognostic variables, and for multivariable analysis. With respect to continuous 25(OH)D levels and clinical outcome, TTT was shorter as 25(OH)D decreased until 25(OH)D reached a level of ∼15 ng/mL, at which point the association of 25(OH)D and TTT remained constant. Thus, patients with mild insufficiency had better TTT than patients with severe insufficiency; however, patients below a 25(OH)D level of 15 ng/mL had a poor TTT that was similar to those with a level of 15 ng/mL. The association between 25(OH)D and survival increased consistently as 25(OH)D increased. Thus, patients with severe insufficiency had worse survival than patients with mild or moderate insufficiency (supplemental Figure 1).
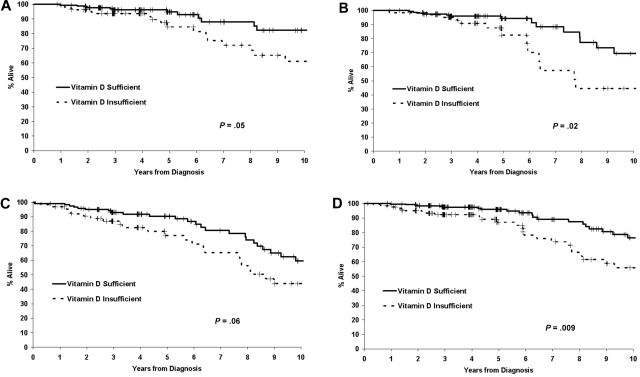
We next explored interactions of vitamin D insufficiency with other prognostic variables. The association of 25(OH)D insufficiency with OS among Rai stage 0 patients was HR = 1.92 (95% CI = 0.99-3.73; P = .05), while the association with TTT was HR = 1.72 (95% CI = 0.96-3.10; P = .07; Figure 3A). Among Rai stage I or greater patients, 25(OH)D insufficiency was also associated with both OS (HR = 1.86, 95% CI = 1.11-3.12; P = .02) and TTT (HR = 1.59, 95% CI = 1.15-2.22; P = .005). 25(OH)D insufficiency appeared to be a better predictor of OS among IGHV-unmutated patients (HR = 2.20; 95% CI = 1.08-4.78; P = .02) than in IGHV-mutated (HR = 1.32; 95% CI = 0.68-2.57; P = .41) patients, and in ZAP-70-positive (HR = 1.68; 95% CI = 0.98-2.87; P = .06) patients than in ZAP-70-negative (HR = 1.56; 95% CI = 0.72-3.36; P = .26) patients (Figure 3B-C). For FISH risk categories, 25(OH)D insufficiency was associated with OS among patients with standard-risk (eg, normal, trisomy 12, del 13q14) FISH (HR = 2.02; 95% CI = 1.19-3.43; P = .009; Figure 3D), but not in those with high-risk FISH (HR = 0.65; 95% CI = 0.27-1.56; P = .34) as defined by the presence of del(17p13) or del(11q22).32
Figure 3.
Overall survival based on vitamin D insufficiency in both cohorts. (A) OS among Rai 0 patients (n = 259). (B) OS of IGHV-unmutated patients based on vitamin D insufficiency (n = 230). (C) OS of ZAP-70–positive patients based on vitamin D insufficiency (n = 185). (D) OS of patients with standard-risk FISH (eg, normal, trisomy 12, 13q−) based on vitamin D insufficiency (n = 393).
On multivariable analysis in all patients adjusting for age, sex, Rai stage, CD38, ZAP-70, IGHV, CD49d, and FISH, 25(OH)D insufficiency was an independent predictor of TTT (HR = 1.47; 95% CI = 1.11-1.96; P = .008). On multivariable analysis for OS, the HR for death associated with 25(OH)D insufficiency was similar to univariate findings, although the P value did not reach the threshold of statistical significance (HR = 1.47; 95% CI = 0.97-2.23; P = .07).
Discussion
Consistent with the prevalence of hypovitaminosis D in the general population,1–3 30%-40% of CLL patients in the 2 observational cohorts studied had vitamin D insufficiency. Vitamin D insufficiency was associated with inferior TTT and a 2-fold increased risk of death in the discovery cohort. The association between vitamin D insufficiency and clinical outcome was confirmed in an independent cohort of CLL patients and persisted in a pooled multivariable analysis of both cohorts after adjusting for age, sex, stage, CD38, ZAP-70, IGHV, CD49d, and FISH analysis. These findings suggest that vitamin D insufficiency may be the first potentially modifiable host factor associated with prognosis in newly diagnosed CLL.
Our finding that 25(OH)D insufficiency is associated with inferior TTT (a disease-specific end point) also suggests that the prognostic effect of vitamin D may be directly related to its impact on CLL and may not be simply a general host effect. This possibility could be explained by biologic characteristics of the CLL disease process. The vitamin D receptor is highly expressed by CLL B cells relative to normal B and T cells, and pharmacologic doses of vitamin D derivatives developed as therapeutic compounds have been shown to induce caspase 3- and 9-dependent apoptosis (ie, the mitochondrial pathway) of CLL B cells in vitro.33 Treatment of CLL B cells with these vitamin D analogs was also associated with mitogen-activated protein kinase (MAPK) pathway activation and suppression of extracellular signal-regulated kinase (ERK) activity.33 An effect of serum vitamin D levels on tumor-specific outcomes is also consistent with the recently reported effects of vitamin D in other cancers12–15 and with the documented effects of vitamin D on cellular processes of particular relevance to leukemogenesis.5,10,34 In addition, vitamin D has been shown to inhibit proliferation and induce differentiation of both lymphocytes35 and lymphoma cell lines36 on in vitro testing.
One question that arises is whether serum vitamin D levels could be influenced by tumor burden (ie, higher ALC or greater nodal disease could lead to vitamin D binding and lower serum levels).37–40 In this regard, serum vitamin D levels had no relationship with Rai stage in either the discovery cohort or the confirmation cohort and also had no correlation with ALC in the 229 patients in the discovery cohort who had an ALC measured within 2 months of the vitamin D measurement. Furthermore, serum vitamin D levels remained a predictor for TTT among both Rai 0 patients and patients with stage Rai > I when these groups were evaluated separately and on the multivariable analysis controlling for disease stage. Thus, while it is an important subject for future investigations, it does not appear that the observed relationship between serum vitamin D levels and clinical outcome is related to an interaction between vitamin D levels and tumor burden.
The strengths of our study include the large, prospective design of consecutively enrolled, newly diagnosed CLL patients, the availability of key baseline clinical and prognostic information, nearly complete follow-up of patients to define TTT and OS, and confirmation of the association between 25(OH)D insufficiency and clinical outcome in an independent CLL cohort. In addition, the LC-MS/MS method used to measure serum 25(OH)D levels is considered the most reliable and accurate method for 25(OH)D determination.28 The major limitation of the study is the use of an observational study design, which is susceptible to confounding and selection biases. Nonetheless, we used a cohort design, adjusted for known CLL prognostic factors, and confirmed the association between 25(OH)D insufficiency and clinical outcome in an independent cohort. While the association of 25(OH)D insufficiency with OS in the multivariable analysis was above the threshold of statistical significance (P = .07), the HR was of similar magnitude to the univariate analysis, suggesting weaker power to assess this end point.
Because our observational study design does not provide definitive evidence for a causal relationship between lower vitamin D levels and poorer outcomes in CLL, it is unknown whether normalizing vitamin D levels in CLL patients with documented insufficiency would improve outcome. Previous observational findings exploring the potential effects of other vitamins on cancer prevention have not always proven beneficial in interventional studies.41 For example, observational data suggesting that B-carotene levels were associated with lung cancer were not substantiated in randomized trials,42,43 although it is important to note that these studies tested supplementation rather then selective repletion in patients with documented deficiency, and evaluated vitamins for chemoprevention rather than therapeutic effects in patients with cancer. One study of 34 patients with low-grade NHL treated with a synthetic analog of 1,25(OH)2D reported tumor regression in 24% of patients (4 complete remissions; 4 partial remissions).44 It is also important to note that clinical recommendations exist for vitamin D testing in routine clinical practice, and these sugest replacement in patients with 25(OH)D levels below the optimal range.45
In conclusion, our data provide the first direct evidence that 25(OH)D levels may be an important host factor influencing prognosis in CLL patients and, to our knowledge, it is the first study evaluating the effects of 25(OH)D insufficiency on cancer outcome to include a validation cohort confirming the results in an independent patient sample. The effect of 25(OH)D insufficiency on cancer outcome may be particularly relevant in CLL because it is a disease with a long natural history and patients are often observed for years prior to the initiation of therapy. Future studies dissecting the mechanism(s) through which the vitamin D pathway influences leukemogenesis, host immunity, and leukemia cell biology are needed and may provide insight into the mechanisms of disease progression that identify potential therapeutic strategies. Future trials evaluating the impact of vitamin D repletion in CLL patients with documented 25(OH)D insufficiency are needed to determine whether this strategy improves outcome in patients with CLL.
Supplementary Material
Acknowledgments
This work was supported in part by the National Institutes of Health (P50 CA97274 and CA 113408); by Gabrielle's Angel Foundation for Cancer Research; by the Henry J. Predolin Foundation; by Vysis Inc; and by the Mayo Hematologic Malignancies Fund.
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: T.D.S., M.T.D., and J.R.C. provided the conception, design, and financial support; J.R.C. provided administrative support; T.D.S., T.G.C., C.S.Z., N.E.K., T.E.W., G.J.W., B.K.L., and C.A.H. provided study materials or patients; T.D.S., M.T.D., C.A., K.G.R., M.J.M., S.L.S., and J.R.C. collected and assembled the data; C.A., M.J.M., K.G.R., T.D.S., S.L.S., and J.R.C. performed data analysis and interpretation; T.D.S. and J.R.C. co-wrote the manuscript; and all authors provided final approval of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Tait D. Shanafelt, MD, 200 First St, Rochester, MN 55902; e-mail: shanafelt.tait@mayo.edu.
References
- 1.Thomas MK, Lloyd-Jones DM, Thadhani RI, et al. Hypovitaminosis D in medical inpatients. N Engl J Med. 1998;338(12):777–783. doi: 10.1056/NEJM199803193381201. [DOI] [PubMed] [Google Scholar]
- 2.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81(3):353–373. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- 3.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 4.Carlberg C. Current understanding of the function of the nuclear vitamin D receptor in response to its natural and synthetic ligands. Recent Results Cancer Res. 2003;164:29–42. doi: 10.1007/978-3-642-55580-0_2. [DOI] [PubMed] [Google Scholar]
- 5.Bikle D. Nonclassic actions of vitamin D. J Clin Endocrinol Metab. 2009;94(1):26–34. doi: 10.1210/jc.2008-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gorham ED, Garland CF, Garland FC, et al. Vitamin D and prevention of colorectal cancer. J Steroid Biochem Mol Biol. 2005;97(1-2):179–194. doi: 10.1016/j.jsbmb.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 7.Yin L, Grandi N, Raum E, Haug U, Arndt V, Brenner H. Meta-analysis: longitudinal studies of serum vitamin D and colorectal cancer risk. Aliment Pharmacol Ther. 2009;30(2):113–125. doi: 10.1111/j.1365-2036.2009.04022.x. [DOI] [PubMed] [Google Scholar]
- 8.Crew KD, Shane E, Cremers S, McMahon DJ, Irani D, Hershman DL. High prevalence of vitamin D deficiency despite supplementation in premenopausal women with breast cancer undergoing adjuvant chemotherapy. J Clin Oncol. 2009;27(13):2151–2156. doi: 10.1200/JCO.2008.19.6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen P, Hu P, Xie D, Qin Y, Wang F, Wang H. Meta-analysis of vitamin D, calcium and the prevention of breast cancer. Breast Cancer Res Treat. 2010;121(2):469–477. doi: 10.1007/s10549-009-0593-9. [DOI] [PubMed] [Google Scholar]
- 10.Garland CF, Gorham ED, Mohr SB, Garland FC. Vitamin D for cancer prevention: global perspective. Ann Epidemiol. 2009;19(7):468–483. doi: 10.1016/j.annepidem.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 11.Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr. 2007;85(6):1586–1591. doi: 10.1093/ajcn/85.6.1586. [DOI] [PubMed] [Google Scholar]
- 12.Ng K, Meyerhardt JA, Wu K, et al. Circulating 25-hydroxyvitamin d levels and survival in patients with colorectal cancer. J Clin Oncol. 2008;26(18):2984–2991. doi: 10.1200/JCO.2007.15.1027. [DOI] [PubMed] [Google Scholar]
- 13.Goodwin PJ, Ennis M, Pritchard KI, Koo J, Hood N. Prognostic effects of 25-hydroxyvitamin D levels in early breast cancer. J Clin Oncol. 2009;27(23):3757–3763. doi: 10.1200/JCO.2008.20.0725. [DOI] [PubMed] [Google Scholar]
- 14.Newton-Bishop JA, Beswick S, Randerson-Moor J, et al. Serum 25-hydroxyvitamin D3 levels are associated with breslow thickness at presentation and survival from melanoma. J Clin Oncol. 2009;27(32):5439–5444. doi: 10.1200/JCO.2009.22.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou W, Heist RS, Liu G, et al. Circulating 25-hydroxyvitamin D levels predict survival in early-stage non-small-cell lung cancer patients. J Clin Oncol. 2007;25(5):479–485. doi: 10.1200/JCO.2006.07.5358. [DOI] [PubMed] [Google Scholar]
- 16.Kricker A, Armstrong BK, Hughes AM, et al. Personal sun exposure and risk of non Hodgkin lymphoma: a pooled analysis from the Interlymph Consortium. Int J Cancer. 2008;122(1):144–154. doi: 10.1002/ijc.23003. [DOI] [PubMed] [Google Scholar]
- 17.Giovannucci E, Liu Y, Rimm EB, et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst. 2006;98(7):451–459. doi: 10.1093/jnci/djj101. [DOI] [PubMed] [Google Scholar]
- 18.Lim U, Freedman DM, Hollis BW, et al. A prospective investigation of serum 25-hydroxyvitamin D and risk of lymphoid cancers. Int J Cancer. 2009;124(4):979–986. doi: 10.1002/ijc.23984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Purdue MP, Freedman DM, Gapstur SM, et al. Circulating 25-hydroxyvitamin D and risk of non-hodgkin lymphoma: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol. 1010;172(1):58–69. doi: 10.1093/aje/kwq117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiner G, Witzig TE, Link BK. The University of Iowa/Mayo Clinic Lymphoma SPORE: SPORE update. Clin Adv Hematol Oncol. 2004;2:57–59. [Google Scholar]
- 21.Cheson BD, Bennett JM, Grever M, et al. National Cancer Institute-sponsored Working Group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood. 1996;87(12):4990–4997. [PubMed] [Google Scholar]
- 22.Harris NL, Jaffe ES, Diebold J, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting-Airlie House, Virginia, November 1997. J Clin Oncol. 1999;17(12):3835–3849. doi: 10.1200/JCO.1999.17.12.3835. [DOI] [PubMed] [Google Scholar]
- 23.Shanafelt TD, Witzig TE, Fink SR, et al. Prospective evaluation of clonal evolution during long-term follow-up of patients with untreated early-stage chronic lymphocytic leukemia. J Clin Oncol. 2006;24(28):4634–4641. doi: 10.1200/JCO.2006.06.9492. [DOI] [PubMed] [Google Scholar]
- 24.Shanafelt TD, Geyer SM, Bone ND, et al. CD49d expression is an independent predictor of overall survival in patients with chronic lymphocytic leukaemia: a prognostic parameter with therapeutic potential. Br J Haematol. 2008;140(5):537–546. doi: 10.1111/j.1365-2141.2007.06965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shanafelt TD, Hanson C, Dewald GW, et al. Karyotype evolution on fluorescent in situ hybridization analysis is associated with short survival in patients with chronic lymphocytic leukemia and is related to CD49d expression. J Clin Oncol. 2008;26(14):e5–e6. doi: 10.1200/JCO.2008.16.7874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dewald G, Brockman S, Paternoster S, et al. Chromosome anomalies detected by interphase fluorescence in hybridization: correlation with significant biological features of chronic lymphocytic leukemia. Br J Haematol. 2003;121(2):287–295. doi: 10.1046/j.1365-2141.2003.04265.x. [DOI] [PubMed] [Google Scholar]
- 27.Jelinek DF, Tschumper RC, Geyer SM, et al. Analysis of clonal B-cell CD38 and immunoglobulin variable region sequence status in relation to clinical outcome for B-chronic lymphocytic leukaemia. Br J Haematol. 2001;115(4):854–861. doi: 10.1046/j.1365-2141.2001.03149.x. [DOI] [PubMed] [Google Scholar]
- 28.Binkley N, Krueger D, Gemar D, Drezner MK. Correlation among 25-hydroxy-vitamin D assays. J Clin Endocrinol Metab. 2008;93(5):1804–1808. doi: 10.1210/jc.2007-2340. [DOI] [PubMed] [Google Scholar]
- 29.Kaplan E, Meier P. Nonparametric estimation for incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 30.Cox DR. Regression models and life tables (with discussion). J R Stat Soc Series B Stat Methodol. 1972;34(2):187–220. [Google Scholar]
- 31.Eilers P, Marx B. Flexible smoothing with B-splines and penalties. Stat Sci. 1996;11(2):89–121. [Google Scholar]
- 32.Döhner H, Stilgenbauer S, Benner A, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med. 2000;343(26):1910–1916. doi: 10.1056/NEJM200012283432602. [DOI] [PubMed] [Google Scholar]
- 33.Pepper C, Thomas A, Hoy T, Milligan D, Bentley P, Fegan C. The vitamin D3 analog EB1089 induces apoptosis via a p53-independent mechanism involving p38 MAP kinase activation and suppression of ERK activity in B-cell chronic lymphocytic leukemia cells in vitro. Blood. 2003;101(7):2454–2460. doi: 10.1182/blood-2002-07-1984. [DOI] [PubMed] [Google Scholar]
- 34.Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer. 2007;7(9):684–700. doi: 10.1038/nrc2196. [DOI] [PubMed] [Google Scholar]
- 35.Provvedini DM, Tsoukas CD, Deftos LJ, Manolagas SC. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983;221(4616):1181–1183. doi: 10.1126/science.6310748. [DOI] [PubMed] [Google Scholar]
- 36.Hickish T, Cunningham D, Colston K, et al. The effect of 1,25-dihydroxyvitamin D3 on lymphoma cell lines and expression of vitamin D receptor in lymphoma. Br J Cancer. 1993;68(4):668–672. doi: 10.1038/bjc.1993.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petrini M, Carulli G, Ambrogi F, Galbraith RM. Phenotypic markers in chronic lymphocytic leukemia. N Engl J Med. 1986;314(23):1514–1515. [PubMed] [Google Scholar]
- 38.Petrini M, Emerson DL, Galbraith RM. Linkage between surface immunoglobulin and cytoskeleton of B lymphocytes may involve Gc protein. Nature. 1983;306(5938):73–74. doi: 10.1038/306073a0. [DOI] [PubMed] [Google Scholar]
- 39.Petrini M, Galbraith RM, Werner PA, Emerson DL, Arnaud P. Gc (vitamin D binding protein) binds to cytoplasm of all human lymphocytes and is expressed on B-cell membranes. Clin Immunol Immunopathol. 1984;31(2):282–295. doi: 10.1016/0090-1229(84)90248-4. [DOI] [PubMed] [Google Scholar]
- 40.Constans J, Oksman F, Viau M. Binding of the apo and holo forms of the serum vitamin D-binding protein to human lymphocyte cytoplasm and membrane by indirect immunofluorescence. Immunol Lett. 1981;3(3):159–162. doi: 10.1016/0165-2478(81)90120-6. [DOI] [PubMed] [Google Scholar]
- 41.Greenwald P, Anderson D, Nelson SA, Taylor PR. Clinical trials of vitamin and mineral supplements for cancer prevention. Am J Clin Nutr. 2007;85(1):314S–317S. doi: 10.1093/ajcn/85.1.314S. [DOI] [PubMed] [Google Scholar]
- 42.The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. N Engl J Med. 1994;330(15):1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 43.Omenn GS, Goodman G, Thornquist M, et al. The beta-carotene and retinol efficacy trial (CARET) for chemoprevention of lung cancer in high risk populations: smokers and asbestos-exposed workers. Cancer Res. 1994;54(7 suppl):2038s–2043s. [PubMed] [Google Scholar]
- 44.Raina V, Cunningham D, Gilchrist N, Soukop M. Alfacalcidol is a nontoxic, effective treatment of follicular small-cleaved cell lymphoma. Br J Cancer. 1991;63(3):463–465. doi: 10.1038/bjc.1991.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heaney RP. The Vitamin D requirement in health and disease. J Steroid Biochem Mol Biol. 2005;97(1-2):13–19. doi: 10.1016/j.jsbmb.2005.06.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.