Abstract
The prion protein (PrP) binds copper and has antioxidant activity enhancing the survival of neurones in culture. The ability of the PrP to bind other cations was tested and it was found that only manganese could substitute for copper. Although initially manganese-loaded PrP exhibited similar structure and activity to copper-loaded PrP, after aging, manganese-loaded PrP became proteinase resistant and lost function. It was also found that manganese could be incorporated into PrP expressed by astrocytes and that this PrP was partially proteinase resistant. These results show that it is possible to generate proteinase-resistant PrP from cells and suggest a possible mechanism for the formation of the scrapie isoform of the PrP as generated in sporadic prion disease.
Keywords: copper/manganese/oxidative stress/prion/proteinase K
Introduction
The majority of cases of naturally occurring prion diseases (transmissible spongiform encephalopathies) arise sporadically with no known cause (Prusiner, 1991). These include the majority of human cases of Creutzfeldt–Jakob disease (CJD), scrapie of sheep, chronic wasting disease of deer and others. Other human diseases are associated with point mutations in the prion protein (PrP) gene (Hsiao and Prusiner, 1990; Prusiner and Hsaio, 1994; Ghetti et al., 1996). Bovine spongiform encephalopathy may have been spread among cattle by the feeding of infected offal but the original disease may also have arisen sporadically.
Prion diseases are characterized by the conversion of the normal cellular form of the prion protein (PrPc) to an altered isoform (PrPSc). Theoretically, transmission of disease can be induced by introduction of PrPSc into the brain, which catalyses conversion of the host's normal PrPc to the abnormal isoform (Cohen and Prusiner, 1998). It is characteristic of all prion diseases that PrPSc accumulates in the central nervous system in infected individuals and it probably has a causal role in inducing neurodegeneration and gliosis, the pathological hallmarks of these diseases (DeArmond et al., 1992).
PrPSc unlike PrPc is resistant to digestion with proteinase K (PK) and aggregates to form fibrils (Bolton et al., 1982; Prusiner, 1982). It is high in β–sheet content. In vitro fibril formation from PrPc using PrPSc has been demonstrated (Kocisko et al., 1994; Bessen et al., 1997). However, the mechanism by which PrPSc is formed in vivo from PrPc is unknown. The seeding theory (Cohen and Prusiner, 1998) is one popular theory, which suggests that a number of ‘seeds’ of PrPSc are formed slowly from host protein. Once such nuclei of PrPSc are formed large amounts of PrPSc can form more rapidly. There is some evidence to suggest that caveolae-like domains in cell membranes are the possible point in which PrPSc and PrPc may interact (Vey et al., 1996). There is also a strong argument that another protein, ‘factor X’, may be necessary for the formation of PrPSc from PrPc (Kaneko et al., 1997).
The cellular isoform of the PrP is a 30–35 kDa glycoprotein that is anchored to the cell membrane via a GPI anchor. PrPc binds copper (Brown et al., 1997a, 1999; Stöckel et al., 1998) and its expression appears to aid resistance to oxidative stress and copper toxicity at least in vitro (Brown et al., 1996a, 1997b, 1998). PrPc has been shown to influence copper uptake into neurones (Brown, 1999a). Copper taken up in association with PrPc can be utilized at the synapse (Brown et al., 1997a; Brown, 1999a) or can be incorporated in Cu/Zn superoxide dismutase (Brown and Besinger, 1998). More importantly, PrPc with copper bound to it can act as a superoxide dismutase (Brown et al., 1999), possibly explaining why cells deficient in PrPc expression are sensitive to stress from cell culturing conditions (Brown et al., 1997b; Kuwahara et al., 1999).
Although it has been established that PrPc is a copper-binding protein it is unknown whether the protein can bind other divalent cations. In this report we found that recombinant PrPc will bind manganese and nickel. Of these, manganese appeared to alter PrPc to a proteinase-resistant form that forms fibrils. Furthermore, PrPc expression influences uptake of manganese into cells. Therefore, we speculate that incorporation of manganese into PrPc may be one way in which PrPSc can be formed in vivo.
Results
Binding of copper to PrP
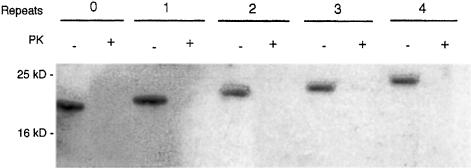
We have shown previously that full-length PrP binds up to four copper atoms to the octameric repeat region (Brown et al., 1999). PrP refolded with copper was demonstrated to have an antioxidant activity like that of superoxide dismutase. It has been suggested from studies with peptides based on the octameric repeat region of PrP (Viles et al., 1999) that the four histidines in the repeat region coordinate four copper atoms, each one associated with two histidines. Alternatively, each histidine could bind one copper atom each. To test this possibility we prepared mutants of mouse PrP with none, one, two or three repeats. The mutants and full-length PrP were expressed in bacteria, extracted from inclusion bodies and refolded in urea in the presence of 5 mM CuSO4. The resulting proteins could be detected following Western blotting with a specific PrP antibody and were PK sensitive (Figure 1). The copper not bound to PrP was removed by dialysis. The histidine tag was removed as previously described (Brown et al., 1999). The resulting protein was analysed by total X–ray reflection fluorescence (TXRF) spectroscopy (Table I). The mutant PrP with no octameric repeats bound a residual level of copper. In comparison, the other mutants bound the equivalent of one, two or three atoms of copper depending on how many octameric repeats were left in the protein. These results suggest that the four histidines in the repeat region can bind one copper atom each.
Fig. 1. Western blot of mutant PrPs. Protein from different deletion mutants of recombinant mouse PrP following refolding with copper was electrophoresed and blotted using the Western technique. Parallel samples were treated with 2 μg/ml proteinase K (PK+). PrP was detected using a polyclonal antibody specific for PrP (see Materials and methods). Wild-type PrP (four octameric repeats) showed a band at 25 kDa. The deletion mutants, with three, two, one and no (0) octameric repeats showed bands of decreasing size.
Table I. Copper binding to recombinant PrP with different numbers of octameric repeats.
| No. of repeats | μg Cu/mg protein | Atoms/molecule |
|---|---|---|
| 0 | 0.2 ± 0.2 | 0.1 ± 0.1 |
| 1 | 2.6 ± 0.4 | 1.0 ± 0.2 |
| 2 | 6.2 ± 0.6 | 2.4 ± 0.3 |
| 3 | 8.3 ± 0.5 | 3.2 ± 0.2 |
| 4 | 11.2 ± 0.7 | 4.4 ± 0.3 |
Values of μg metal ion/mg of protein were obtained from TXRF analysis of different PrP proteins with 0–4 octameric repeats.
Atoms/molecule indicates the molar ratio of copper atoms to PrP molecule. No. of repeats indicates the number of octameric repeats in the PrP molecule studies. All PrP proteins were refolded with CuCl2. Values were determined for a minimum of six measurements; the mean and SEM are shown.
The activity of the different octameric repeat mutants was assessed using an assay for superoxide dismutase based on the production of oxygen radicals by xanthine oxidase and the formation of formazan from nitro-blue tetrazolium (NBT).
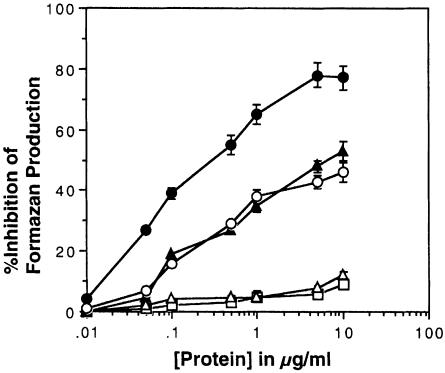
The mutants containing one or no octameric repeats showed no activity in the assay (Figure 2). The minimal requirement for superoxide dismutase activity was two octameric repeats. A mutant with three octameric repeats binding three copper atoms had no more activity than (not significant by Student's t-test, p >0.05) a mutant with two. The activity of wild-type PrP (binding four atoms of copper to the octameric repeats) was the highest, and was significantly greater than that of PrP with only two or three repeats (Figure 2).
Fig. 2. Activity of octameric repeat mutants of PrP. The superoxide dismutase activity of PrP refolded with copper was assessed using a standard NBT/xanthine oxidase-based assay. Increasing concentrations of PrP with four (•), three (○), two (▴), one (▵) and no (□) octameric repeats were added to individual assays. Values indicate the ability of the added protein to inhibit the formation of formazan. Inhibition of formazan in the reaction is indicative of superoxide dismutase activity. Shown are the mean and SEM for four assays.
Refolding of PrP with other divalent cations
Recombinant full-length and deleted PrP was refolded as described with other divalent cations including calcium, magnesium, manganese, nickel, iron and zinc at concentrations of 5 mM. Following refolding and dialysis to remove excess cations the protein was examined with TXRF analysis to determine which cations were retained. Values shown in Table II indicate the number of atoms per molecule of PrP. The deletion mutant did not retain any of the divalent cations, indicating that any retention by the full-length protein was a result of binding to the octarepeat region. Of the cations tested only nickel, zinc and manganese were retained by PrP. Of these, zinc was poorly retained. In order to determine whether manganese and nickel could replace copper when in competition, full-length PrP was refolded with either nickel and copper or manganese and copper in equimolar concentrations (5 mM). In these cases (Table II) copper was preferentially bound in place of nickel but manganese was bound equivalently with copper (i.e. two atoms each per molecule). When the concentration of manganese was decreased during refolding (5 mM copper, 0.5 mM manganese) the amount of manganese binding was reduced to one-tenth that of copper. These results suggest that manganese can substitute equivalently for copper in the holo-form of PrP.
Table II. Binding of divalent cations to full-length recombinant PrP.
| Refolding cation |
Atoms/molecule |
|
|
|
|
|
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cu | Mn | Zn | Mg | Ni | Ca | Fe | |||||||
| Copper | 4.4 ± 0.3 | nd | nd | nd | nd | nd | nd | ||||||
| Manganese | nd | 4.2 ± 0.4 | nd | nd | nd | nd | nd | ||||||
| 1:1 copper:manganese | 2.1 ± 0.1 | 2.2 ± 0.2 | nd | nd | nd | nd | nd | ||||||
| 1:10 copper:manganese | 0.5 ± 0.2 | 3.9 ± 0.3 | nd | nd | nd | nd | nd | ||||||
| Nickel | nd | nd | nd | nd | 4.6 ± 0.3 | nd | nd | ||||||
| 1:1 copper:nickel | 3.4 ± 0.4 | nd | nd | nd | 0.6 ± 0.4 | nd | nd | ||||||
| Zinc | nd | nd | 1.8 ± 0.2 | nd | nd | nd | nd | ||||||
| Magnesium | nd | nd | nd | 0.3 ± 0.2 | nd | nd | nd | ||||||
| Iron | nd | nd | nd | nd | nd | nd | 0.8 ± 0.4 | ||||||
| Calcium | nd | nd | nd | nd | nd | 0.03 ± 0.03 | nd | ||||||
The cation content of wild-type recombinant PrP determined after refolding with different cations (refolding cation) was determined using TXRF analysis. The values are expressed as the number of atoms of the cation bound to each molecule of PrP (atoms/molecule). nd indicates that levels were below detection with this method. Values were determined for a minimum of six measurements; the mean and SEM are shown.
Analysis of activity of manganese- and nickel–loaded PrP
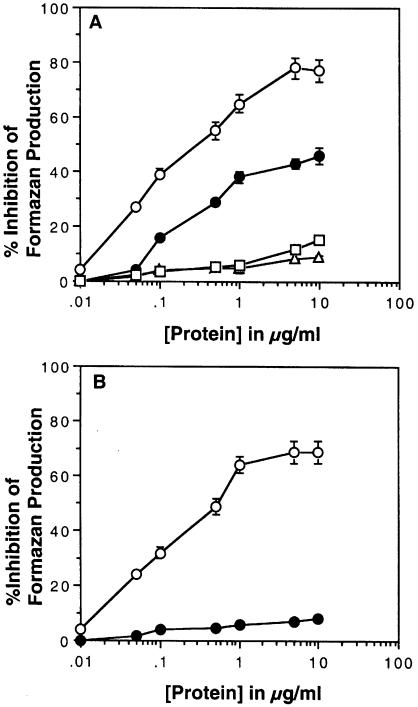
Given that only manganese and nickel substituted for copper in refolded PrP, samples refolded only with these cations were used for assaying superoxide dismutase activity of these proteins. As indicated above, copper-loaded PrP has substantial activity in this assay. PrP loaded with nickel showed no activity in the same assay (Figure 3A). However, manganese-loaded PrP showed some activity, plateauing at ∼50% of that seen for the same mass of copper-loaded PrP. The samples for the studies were used directly after completion of the refolding and dialysis procedure.
Fig. 3. Activity of full-length PrP refolded with different cations. (A) The superoxide dismutase activity of PrP refolded with either copper (○), nickel (▵), manganese (•) or zinc (□) from chloride salts was assessed using a standard NBT/xanthine oxidase-based assay. Increasing concentrations of PrP were added. (B) The superoxide dismutase activity of aged PrP refolded with either copper or manganese was assessed using the NBT/xanthine oxidase assay. After 2 weeks of aging, PrP refolded with copper (○) retained activity but that refolded with manganese did not (•). Shown are the mean and SEM for four assays.
Copper- and manganese-loaded PrP that had been left to age for 2 weeks at 4°C were assayed again for superoxide dismutase activity using the same assay. In this case, copper-loaded PrP showed the same activity as fresh material. However, the manganese-loaded PrP showed almost no activity, suggesting that some change had occurred in the protein during aging (Figure 3B).
Analysis of protease resistance
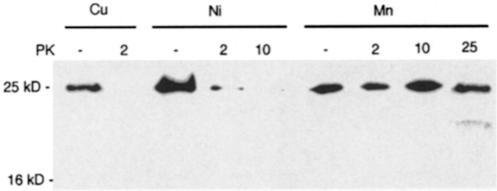
Recombinant PrP refolded with either copper, manganese or nickel was treated with increasing concentrations of PK. The digested protein was electrophoresed on a polyacrylamide gel, blotted and PrP detected with a specific antibody. All freshly refolded PrP was PK sensitive to low concentrations (0.2 μg/ml) (data not shown). However, when PrP that had been aged for 2 weeks was treated with PK, manganese-refolded PrP showed increased PK resistance up to 25 μg/ml (Figure 4). Copper-refolded PrP could be completely degraded with 2 μg/ml PK and nickel-refolded PrP could be mostly degraded with 2 μg/ml PK and completely degraded with 10 μg/ml PK. This suggests that the binding of manganese in place of copper may alter its PK sensitivity, possibly due to changes in its secondary structure.
Fig. 4. PK resistance of recombinant PrP. Protein from wild-type recombinant mouse PrP following refolding with either copper, nickel or manganese was aged for 2 weeks and then electrophoresed and blotted using the Western technique. Parallel samples were treated with increasing concentrations of PK (μg/ml). PrP was detected using a polyclonal antibody as described in Materials and methods. The experiment was repeated four times.
CD analysis
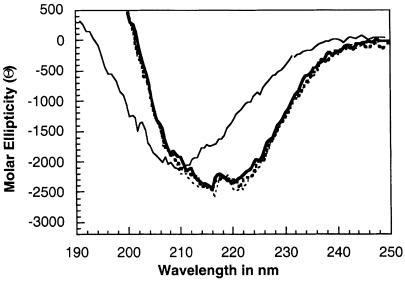
Previously we have shown that there is a shift in circular dichroism (CD) spectra when PrP is refolded in the presence of copper. The CD spectra for PrP freshly refolded with copper or manganese were identical (Figure 5). Also, the CD spectrum of copper-refolded PrP that was aged for 2 weeks was no different to that of freshly prepared PrP. However, when manganese-refolded PrP was aged for 2 weeks, the CD spectrum showed strong changes at ∼210 nm, consistent with greatly increased β–sheet content. This suggests that manganese-refolded PrP underwent a spontaneous change in secondary structure when manganese was bound.
Fig. 5. Far UV CD spectra of recombinant PrP were determined for PrP refolded with either copper or manganese. Freshly prepared samples were compared with those for samples that had been aged for 2 weeks. Samples of copper-refolded protein showed an identical spectrum when prepared fresh (thick dotted line) or aged (thin dotted line). Fresh PrP refolded with manganese (thick line) showed the same spectrum. However, PrP refolded with manganese and aged showed a different spectrum (thin line) indicative of increased β–sheet content and decreased α–helical content.
Analysis of the turbidity of manganese- and copper-refolded aged protein indicated that there was no change in measured turbidity (n = 4; data not shown). Furthermore, analysis of the manganese content of aged manganese-refolded PrP by TXRF analysis indicated that this material retained the same amount of manganese (3.9 ± 0.4 atoms per molecule; n = 4). These results indicate that the change in activity and structure of aged manganese-refolded PrP is not due to loss of manganese content or aggregation.
Cell culture studies
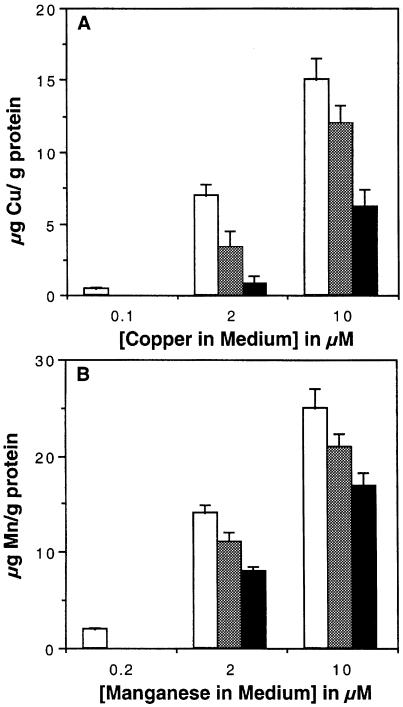
Experiments were carried out to determine whether cells grown in a manganese-rich medium would retain levels of manganese that might then be incorporated into PrP. Manganese is very toxic to neurones, therefore astrocytes were used. Monolayer cultures of pure murine astrocytes were prepared. The astrocytes were grown for 7 days in serum-free medium. The astrocytes were exposed to either copper or manganese or both in varying concentrations for 2 days. The concentrations used were either equivalent to or slightly higher than the levels of these cations found in serum (Rukgauer et al., 1997). The cells were then thoroughly washed before harvesting. The cation content of the cells was assessed using TXRF analysis (Figure 6). According to this analysis the serum-free medium used contained 0.1 μM copper and 0.2 μM manganese (n = 3). Increasing the manganese or copper in the medium increases the amount of either metal in the astrocytes. Addition of a high concentration of manganese in the presence of added copper decreased the copper content of the cells (Figure 6A). However, added copper had only a minor effect on the manganese content of cells (Figure 6B). This suggests that manganese can enter cells through competition with copper at more sites than copper can compete with manganese. These experiments show that astrocytes can retain high levels of copper or manganese.
Fig. 6. Manganese uptake by cells. TXRF analysis was used to determine the cation concentration in extracts of cells. (A) Astrocytes were treated with various concentrations of copper (chloride) alone (open bars) or with addition of either 2 μM (grey bars) or 5 μM (black bars) manganese (chloride). Determination of copper content is shown. (B) Astrocytes were treated with various concentrations of manganese alone (open bars) or with addition of either 2 μM (grey bars) or 5 μM (black bars) copper. Determination of manganese content is shown. The mean and SEM of six measurements are shown. Values are based on the protein content of the starting material.
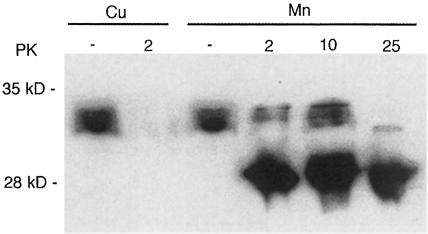
Astrocytes grown in the presence of manganese or copper were collected and a protein extract made by homogenization in phosphate-buffered saline (PBS) containing 0.1% NP–40. PrP was immunoprecipitated from the extracts using affinity chromatography based on a specific antibody to PrP. Analysis of the affinity-purified protein by TXRF analysis indicated that native PrP isolated from cells grown in the presence of manganese could incorporate manganese (Table III). Other immunoprecipitated PrP was digested with PK and electrophoresed on a polyacrylamide gel. After Western blotting, PrP was detected with a specific antibody. It was found that PrP from manganese-treated cells was more resistant to PK treatment than that from cells grown in the presence of copper (Figure 7). It should be noted that PrP from astrocytes has only glycosylated forms of PrP, of which the double glycosylated form predominates (Brown, 1999b). The bands seen after PK treatment were more reminiscent of the bands seen after PK treatment of PrPSc than of those for the recombinant protein seen in Figure 4. These results suggest that cells can incorporate manganese into PrP and that some of the PrP is PK resistant.
Table III. Cation content of immunoprecipitated PrP from astrocytes.
| Treatment | Cu | Mn |
|---|---|---|
| Medium | 5.2 ± 0.8 | 0.8 ± 0.2 |
| 2 μM Cu | 15.1 ± 2.1 | 0.01 ± 0.01 |
| 10 μM Cu | 22.0 ± 2.2 | nd |
| 2 μM Mn | 3.2 ± 2.2 | 4.3 ± 1.2 |
| 10 μM Mn | 1.2 ± 0.5 | 11.2 ± 1.2 |
Values for the cation content of immunoprecipitated protein are in
μg cation/mg protein. Shown are the mean and SEM for three determinations each. Treatment indicates the conditions that the cells were grown in (see Materials and methods; Figure 6).
Fig. 7. PK resistance of PrP expressed by cells. Extracts were made from astrocytes grown in the presence of high manganese or copper concentrations. The PrP content was immunoprecipitated. Samples of the immunoprecipitants were treated with PK and studied using Western blotting. Samples from cells grown with added copper were sensitive to PK but the extract from cells grown with manganese contained PK-resistant PrP. Undigested lanes (PK–) were loaded with one-tenth of the amount loaded for PK-digested samples. PrP was detected on the blot using a specific polyclonal antiserum to PrP. Values for PK are in μg/ml.
Discussion
There is increasing evidence that PrPc has a function in the regulation of cellular resistance to oxidative stress and that it is involved in or dependent upon copper metabolism in the brain. PrPc expression regulates uptake of copper into neurones and its utilization at the synapse (Brown, 1999a). Copper taken up in association with PrPc can be utilized intracellularly by enzymes such as Cu/Zn superoxide dismutase (Brown and Besinger, 1998) or can be utilized by PrPc itself for superoxide dismutase activity. Therefore, not only does PrPc have a molecular function but also its expression influences copper utilization by cells.
In the present work we demonstrate that the minimum number of copper ions required for superoxide dismutase activity is two and that the molecule shows increased activity when a further two atoms of copper bind. As binding of copper to PrP induces a more ordered structure (Miura et al., 1996, 1999; Wong et al., 1999), it is likely that the increased activity on binding of two further atoms results from increased order in the molecule. Increased redox potential resulting from increased binding of copper is insufficient to explain this as binding of three copper atoms did not produce a corresponding increase in activity.
Previous experiments with peptides related to the octameric repeat region suggested that binding of each copper atom required coordination between two histidine residues (Viles et al., 1999). However, we found that full-length PrP with two octameric repeats and thus two histidines was able to bind two copper atoms. Viles et al. (1999) found that peptides with three repeats did bind three copper atoms. Similarly, PrP with three repeats bound three copper atoms. The binding of this third copper atom did not add activity to the protein as described above. In studies using equilibrium dialysis (Brown et al., 1997a) based on an N-terminal fragment of PrP it was found that there was cooperativity in the binding atoms. This implies that once one atom of copper is bound further atoms can bind more easily. Therefore, it is likely that the binding of one copper atom changes the conformation of PrP to allow the binding of other copper ions. In a short peptide it is unlikely that binding of one copper ion would induce sufficient changes in the molecule to allow binding of the second atom of copper. Therefore, although coordination of copper between two histidines might facilitate copper binding to peptides and might even be involved in binding of copper to the whole molecule, it is likely that the copper ions bind principally to one histidine each.
It is known that copper-binding proteins can often bind other divalent cations such as zinc under certain circumstances. However, recombinant PrP did not show high retention of zinc. PrP did bind nickel and manganese. This is in contrast to the work of Stöckel et al. (1998) who did not find that recombinant PrP binds manganese. However, the same study also indicated that PrP binds only two atoms of copper. We and others (Viles et al., 1999) have provided evidence that PrP can bind four copper atoms. Therefore, it is possible that the methods used by Stöckel et al. (1998) to incorporate copper or manganese into PrP were not adequate to saturate the molecule. Refolding the protein in the presence of cations possibly represents the optimal method for cation incorporation. Furthermore, we have shown that manganese is incorporated into native PrP from cells cultured with manganese in the medium. Therefore, it is possible that manganese can be incorporated into or binds to PrPc in vivo.
It has previously been shown that copper bound to PrP can be taken up by cells (Brown, 1999a). It is possible that PrPc could influence uptake of other cations. Indeed, incorporation of manganese into PrPc from cells that take up manganese as we have demonstrated here would suggest that PrPc may also influence manganese metabolism. Manganese is used as an electron acceptor in manganese superoxide dismutase, which is found in mitochondria, and it is interesting that recombinant PrP showed some superoxide dismutase activity on binding manganese.
Studies on replacement of cations in superoxide dismutase have indicated that replacement can occur. In copper-containing proteins the copper can be replaced by cobalt with subsequent changes in activity and structure (O'Neill et al., 1982; Salvato et al., 1989). Similarly, studies of manganese-containing superoxide dismutase indicate that the manganese can be replaced by iron and the enzyme retains activity. Manganese superoxide dismutase is a potent enzyme but the lower redox potential for manganese probably means that it cannot be as active as the copper-containing enzyme. However, more significant for this study is the finding that replacement of copper with other cations can lead to changes in secondary structure. It appears that PrP is less stable on binding manganese and quickly converts to a misfolded form.
The observation that on binding manganese PrPc becomes proteinase resistant is probably the most important finding in the present report. Proteinase resistance, along with fibril formation, is one of the most important characteristics distinguishing PrPSc from PrPc. Although there are now many reports of the generation of proteinase-resistant recombinant protein, this is the first report of the formation of proteinase-resistant protein from native PrPc from cells without the interaction of those cells with PrPSc. Cells expressing PrP carrying mutations associated with inherited prion disease have been known to generate proteinase-resistant PrP but this does not occur with cells expressing the native protein. This therefore represents de novo formation of PK-resistant PrP.
When the mechanism of infection in prion disease is understood there will still remain sporadic prion disease, which occurs without any known infection event. The cause of sporadic prion disease is a mystery, and attempts to explain human CJD by suggesting that it comes from eating infected sheep have been disreputed. Therefore, an environmental cause for sporadic disease remains a possibility. Recent investigations of scrapie, CJD and chronic wasting disease clusters in Iceland, Slovakia and Colorado, respectively, have indicated that the soil in these regions is low in copper and higher in manganese (Purdy, 2000). The possibility that imbalances in environmental cations entering the food chain may induce conditions favouring the formation of proteinase-resistant PrP is a controversial but intriguing possibility. This idea is supported by the findings presented in this paper.
The finding that incorporation of manganese into PrP makes it proteinase resistant and abolishes its function is a long way from explaining sporadic prion disease. However, a current favourite among hypotheses concerning conversion of PrPc to PrPSc requires the formation of ‘seeds’ or ‘nuclei’ of proteinase-resistant PrP (Cohen and Prusiner, 1998). The formation of sufficient proteinase-resistant PrP to create such a seed is the limiting step in this model of PrPSc formation. Incorporation of manganese in place of copper in the holo-form of PrPc may represent one possible way in which the substance of a ‘seed’ could form.
In summary, the work presented here confirms that copper is the preferred cation incorporated into PrPc at the octameric repeat region. This copper can be replaced by manganese but the consequence was found to be loss of function and increased proteinase resistance. Each atom of copper incorporated into PrP is coordinated by the histidine in a single octameric repeat. Although PrP can bind the same number of manganese atoms as copper atoms the resulting protein is aberrant. These results show for the first time a mechanism by which proteinase-resistant native PrPc can be expressed by cells.
Materials and methods
Protein purification and refolding
The production of recombinant mouse PrP has been described previously (Wong et al., 1999). Briefly, amplified product was cloned in the expression vector pET–23 (Novagen) and transformed in Escherichia coli AD494(DE3). The expressed proteins were recovered from urea-solubilized bacterial lysate after sonication by immobilized nickel-based affinity chromatography (Invitrogen). The eluted material was refolded by several successive rounds of dilution in either deionized water, 5 mM CuCl2 or 5 mM CuSO4 followed by ultrafiltration and dialysis to remove unbound copper. The final protein was typically >95% pure and was concentrated to ∼1–2 mg/ml. Its identity was confirmed by N-terminal sequencing and Western blotting using the polyclonal antibody to mouse PrP (142–160). Protein concentration was determined using the Bio-Rad protein assay reagent or as previously described (Brown et al., 1999). Refolding with other divalent cations was carried out similar to that described above. The same concentration (5 mM) of the chloride salt of each cation was used in place of CuCl2.
Some samples were stored for 2 weeks at 4°C. These samples are referred to as ‘aged’. Aggregation of protein was assessed using spectrophotometric determination of turbidity (n = 4) per sample. The method used was as previously described for amyloid proteins (Evans et al., 1995). Samples showing increased turbidity were not used for further study.
Site-directed mutagenesis
Specific deletions of one, two, three or four of the octameric repeats present in the mouse prion sequence were accomplished by site-directed mutagenesis (QuickChange; Stratagene) using paired splint oligonucleotides to remove residues 51–68 (one repeat plus the incomplete repeat), 51–76 (two repeats), 51–82 (three repeats) or 51–90 (four repeats). The mutation was confirmed by DNA sequence analysis across the deletion site and the encoded protein purified and refolded as above.
TXRF analysis
Metal ion content of protein samples was determined using TXRF as described previously (Brown et al., 1999). Briefly, elemental determinations were carried out using an EXTRA II Energy Dispersive X-ray fluorescence spectrometer by operating the X-ray tube at 50 keV and 10 mA with a count time of 200 s. A 10 ml aliquot of each protein sample together with an equal volume of internal standard (10 p.p.m. cobalt) was placed directly onto the surface of a quartz reflector and dried in air by placing the reflector in a positive pressure filtered air cabinet. Background readings for the quartz reflectors were also recorded.
Superoxide dismutase assay
The assay was performed as described previously (Brown et al., 1999) employing superoxide production from xanthine oxidase and detection of a coloured formazan product formed from NBT. Inhibition of formazan production by added protein is equivalent to the superoxide dismutase activity. Formazan production was measured spectrophotometrically at 560 nm on a U1100 spectrophotometer (Hitachi).
CD analysis
Samples were dissolved at a concentration of 10 μM in 1 mM MES pH 5.5, as determined using the Bio-Rad protein assay. CD spectra were recorded in a 0.1 cm path length quartz cell at room temperature under constant nitrogen flush using the Jasco J720 spectropolarimeter. At least four spectra were accumulated, and the appropriate blanks were subtracted. The values were expressed as molar ellipticity (θ).
PK analysis
Purified recombinant proteins or immunoprecipitated native protein were assessed for proteinase sensitivity by treatment with 2–25 μg/ml PK (Boehringer Mannheim) at 37°C for 1 h (Herrmann and Caughey, 1998). The reaction was stopped by adding Perfabloc (Boehringer Mannheim) to 2 mM and the products of digestion were analysed by Western blotting using a rabbit polyclonal antibody against mouse PrP (142–160) as previously described (Brown et al., 1999).
Cell culture
Pure cultures of astrocytes from wild-type mice were prepared as previously described (Brown et al., 1996b). Astrocytes were cultured in serum-free medium for experiments involving treatment with divalent cations. Astrocytes were kept in serum-free culture for 7 days with changes of medium every third day. Copper or manganese was added to cultures in the form of a chloride salt and as a histidine chelate. Treatment with cations was for 2 days; after this time the cells were scraped off the flasks and collected for TXRF analysis of cellular metal content for immunoprecipitation of the PrP. Immunoprecipitated PrP from astrocytes was used for TXRF analysis of manganese and copper content of or PK resistance of the PrP. The cells were collected and digested in PBS containg 0.1% NP–40. Immunoprecipitation was as previously described (Brown et al., 1999).
Acknowledgments
Acknowledgements
This work was supported by a grant (976051) from the EU Biomed Commission (D.R.B.) and a grant (8/BS410551) and fellowship from the BBSRC (D.R.B.).
References
- Bessen R.A., Raymond,G.J. and Caughey,B. (1997) In situ formation of protease-resistant prion protein in transmissible spongiform encephalopathy-infected brain slices. J. Biol. Chem., 272, 15227–15231. [DOI] [PubMed] [Google Scholar]
- Bolton D.C., McKinley, M.P. and Prusiner, S.B. (1982) Identification of a protein that purifies with the scrapie prion. Science, 218, 1309–1311. [DOI] [PubMed] [Google Scholar]
- Brown D.R. (1999a) Prion protein expression aids cellular uptake and veratridine-induced release of copper. J. Neurosci. Res., 58, 717–725. [PubMed] [Google Scholar]
- Brown D.R. (1999b) Prion protein peptide neurotoxicity can be mediated by astrocytes. J. Neurochem., 73, 1105–1113. [DOI] [PubMed] [Google Scholar]
- Brown D.R. and Besinger, A. (1998) Prion protein expression and superoxide dismutase activity. Biochem. J., 334, 423–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D.R., Schmidt, B. and Kretzschmar, H.A. (1996a) Role of microglia and host prion protein in neurotoxicity of a prion protein fragment. Nature, 380, 345–347. [DOI] [PubMed] [Google Scholar]
- Brown D.R., Schmidt, B. and Kretzschmar, H.A. (1996b) A neurotoxic prion protein fragment enhances proliferation of microglia but not astrocytes in culture. Glia, 18, 59–67. [DOI] [PubMed] [Google Scholar]
- Brown D.R., et al. (1997a)The cellular prion protein binds copper in vivo.Nature, 390, 684–687. [DOI] [PubMed] [Google Scholar]
- Brown D.R., Schultz-Schaeffer, W.J., Schmidt, B. and Kretzschmar, H.A. (1997b) Prion protein-deficient cells show altered response to oxidative stress due to decreased SOD-1 activity. Exp. Neurol., 146, 104–112. [DOI] [PubMed] [Google Scholar]
- Brown D.R., Schmidt, B. and Kretzschmar, H.A. (1998) Effects of copper on survival of prion protein knockout neurones and glia. J. Neurochem., 70, 1686–1693. [DOI] [PubMed] [Google Scholar]
- Brown D.R., Wong, B.-S., Hafiz, F., Clive, C., Haswell, S. and Jones, I.M. (1999) Normal prion protein has superoxide dismutase-like activity. Biochem. J., 344, 1–5. [PMC free article] [PubMed] [Google Scholar]
- Cohen F.E. and Prusiner, S.B. (1998) Pathological conformations of prion proteins. Annu. Rev. Biochem., 67, 793–819. [DOI] [PubMed] [Google Scholar]
- DeArmond S.J., Kristensson, K. and Bowler, R.P. (1992) PrPSc causes nerve cell death and stimulates astrocyte proliferation: a paradox. Prog. Brain Res., 94, 437–446. [DOI] [PubMed] [Google Scholar]
- Evans K.C., Berger, E.P., Cho, C.-G., Weisgraber, K.H. and Lansbury, P.T., Jr (1995) Apolipoprotein E is a kinetic but not a thermodynamic inhibitor of amyloid formation: implications for the pathogenesis and treatment of Alzheimer's disease. Proc. Natl Acad. Sci. USA, 92, 763–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghetti B., Piccardo, P., Frangione, B., Giaccone, G., Young, K., Prelli, F., Farlow, M.R., Dlouhy, S.R. and Tagliavini, F. (1996) Prion protein amyloidosis. Brain Pathol., 6, 127–145. [DOI] [PubMed] [Google Scholar]
- Herrmann L.M. and Caughey, B. (1998) The importance of the disulfide bond in prion protein conversion. Neuroreport, 9, 2457–2461. [DOI] [PubMed] [Google Scholar]
- Hsiao K. and Prusiner, S.B. (1990) Inherited human prion diseases. Neurology, 40, 1820–1827. [DOI] [PubMed] [Google Scholar]
- Kaneko K., Zulianello, L., Scott, M., Cooper, C.M., Wallace, A.C., James, T.L., Cohen, F.E. and Prusiner, S.B. (1997) Evidence for protein X binding to a discontinuous epitope on the cellular prion protein during scrapie prion propagation. Proc. Natl Acad. Sci. USA, 94, 10067–10074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocisko D.A., Come, J.H., Priola, S.A., Chesebro, B., Raymond, G.J., Lansbury, P.T. and Caughey, B. (1994) Cell-free formation of protease resistant prion protein. Nature, 370, 471–474. [DOI] [PubMed] [Google Scholar]
- Kuwahara C., et al. (1999)Prions prevent neuronal cell-line death. Nature, 400, 225–226. [DOI] [PubMed] [Google Scholar]
- Miura T., Hori-i, A. and Takeuchi, H. (1996) Metal dependent α-helix formation promoted by the glycine-rich octapeptide region of prion protein. FEBS Lett., 396, 248–252. [DOI] [PubMed] [Google Scholar]
- Miura T., Hori-i, A., Mototani, H. and Takeuchi, H. (1999) Raman spectroscopic study on the copper(II) binding mode of prion octapeptide and its pH dependence. Biochemistry, 38, 11560–11569. [DOI] [PubMed] [Google Scholar]
- O'Neill P., Fielden, E.M., Cocco, D., Rotilio, G. and Calabrese, L. (1982) Evidence for catalytic dismutation of superoxide by cobalt(II) derivatives of bovine superoxide dismutase in aqueous solution as studied by pulse radiolysis. Biochem. J., 205, 181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner S.B. (1982) Novel proteinaceous infectious particles cause scrapie. Science, 216, 136–144. [DOI] [PubMed] [Google Scholar]
- Prusiner S.B. (1991) Molecular biology of prion disease. Science, 252, 1515–1522. [DOI] [PubMed] [Google Scholar]
- Prusiner S.B. and Hsiao, K. (1994) Human prion disease. Ann. Neurol., 35, 385–395. [DOI] [PubMed] [Google Scholar]
- Purdy M. (2000) Ecosystems supporting clusters of sporadic TSEs demonstrate excesses of the radical generating divalent cation manganese and deficiencies of antioxidant co-factors: Cu, Se, Fe, Zn. Does a foreign cation substitution at PrP's Cu domain initiate TSE? Med. Hypotheses, 54, 278–306. [DOI] [PubMed] [Google Scholar]
- Rukgauer M., Klein, J. and Kruse-Jarres, J.D. (1997) Reference values for the trace elements copper, manganese, selenium and zinc in the serum/plasma of children, adolescents and adults. J. Trace Elem. Med. Biol., 11, 92–98. [DOI] [PubMed] [Google Scholar]
- Salvato B., Beltramini, M., Ricchelli, F. and Tallandini, L. (1989) Cobalt substitution studies on bovine erythrocyte superoxide dismutase: evidence for a novel cobalt–superoxide dismutase derivative. Biochim. Biophys. Acta, 998, 14–20. [DOI] [PubMed] [Google Scholar]
- Stöckel J., Safar, J., Wallace, A.C., Cohen, F.E. and Prusiner, S.B. (1998) Prion protein selectively binds copper(II) ions. Biochemistry, 37, 7185–7193. [DOI] [PubMed] [Google Scholar]
- Vey M., Pilkuhn, S., Wille, H., Nixon, R., DeArmond, S.J., Smart, E.J., Anderson, R.G.W., Taraboulos, A. and Prusiner, S.B. (1996) Subcellular colocalization of the cellular and scrapie prion proteins in caveolae-like membranous domains. Proc. Natl Acad. Sci. USA, 93, 14945–14949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viles J.H., Cohen, F.E., Prusiner, S.B., Goodin, D.B., Wright, P.E. and Dyson, H.J. (1999) Copper binding to the prion protein: structural implications of four identical co-operative binding sites. Proc. Natl Acad. Sci. USA, 96, 2042–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong B.-S., Wang, H., Brown, D.R. and Jones, I.M. (1999) Selective oxidation of methionine residues in prion proteins. Biochem. Biophys. Res. Commun., 279, 352–355. [DOI] [PubMed] [Google Scholar]