Abstract
Transport of proteins through the ALP (alkaline phosphatase) pathway to the vacuole requires the function of the AP-3 adaptor complex and Vps41p. However, unlike other adaptor protein–dependent pathways, the ALP pathway has not been shown to require additional accessory proteins or coat proteins, such as membrane recruitment factors or clathrin. Two independent genetic approaches have been used to identify new mutants that affect transport through the ALP pathway. These screens yielded new mutants in both VPS41 and the four AP-3 subunit genes. Two new VPS41 alleles exhibited phenotypes distinct from null mutants of VPS41, which are defective in vacuolar morphology and protein transport through both the ALP and CPY sorting pathways. The new alleles displayed severe ALP sorting defects, normal vacuolar morphology, and defects in ALP vesicle formation at the Golgi complex. Sequencing analysis of these VPS41 alleles revealed mutations encoding amino acid changes in two distinct domains of Vps41p: a conserved N-terminal domain and a C-terminal clathrin heavy-chain repeat (CHCR) domain. We demonstrate that the N-terminus of Vps41p is required for binding to AP-3, whereas the C-terminal CHCR domain directs homo-oligomerization of Vps41p. These data indicate that a homo-oligomeric form of Vps41p is required for the formation of ALP containing vesicles at the Golgi complex via interactions with AP-3.
INTRODUCTION
The selective trafficking of proteins between organelles in the secretory and endocytic pathways is predominantly accomplished by membrane vesicles. In this system, newly synthesized proteins are actively selected and concentrated into distinct vesicle populations, which are subsequently targeted to a specific acceptor compartment. Vesicle formation is often mediated by adaptor and coat proteins that link cargo selection to vesicle budding by both binding and concentrating cargo proteins within the donor membrane as well as by deforming the membranes into nascent vesicles (Hirst and Robinson, 1998).
This mechanism for vesicle formation is conserved at multiple steps in vesicle-mediated transport pathways by related but distinct sets of adaptor and coat proteins. In the early secretory pathway, COPI and COPII coated vesicles direct transport of proteins between the endoplasmic reticulum and the Golgi complex (Robinson, 1997). In the endocytic and vacuolar/lysosomal pathways, three related heterotetrameric adaptor protein complexes, AP-1, AP-2, and AP-3 (Phan et al., 1994; Dell'Angelica et al., 1997; Simpson et al., 1997; Stepp et al., 1997) interact with either di-leucine or tyrosine-based sorting signals in the cytoplasmic tails of cargo proteins (Chen et al., 1990; Letourneur and Klausner, 1992; Marks et al., 1997). AP-2 is required for clathrin-mediated endocytosis at the plasma membrane, whereas AP-1 and -3 act at the Golgi and endosomal compartments to direct transport to both the vacuole/lysosome and the plasma membrane (Cowles et al., 1997a; Robinson, 1997; Faundez et al., 1998). A fourth adaptor protein complex, AP-4, has also been identified in mammalian cells. By homology, AP-4 is believed to act in vesicle formation, but the trafficking role of this complex has yet to be elucidated (Dell'Angelica et al., 1999; Hirst et al., 1999). Both AP-1 and AP-2 interact with the coat protein clathrin (Robinson, 1994), whereas to date, the role of clathrin in AP-3–dependent transport pathways has been controversial (Newman et al., 1995; Simpson et al., 1997; Dell'Angelica et al., 1998). In yeast there is strong evidence that AP-3 does not function with clathrin because clathrin mutants do not display defects similar to AP-3 mutants, and clathrin itself does not copurify with AP-3–coated vesicles (Vowels and Payne, 1998; Rehling et al., 1999; Yeung et al., 1999). This raises the possibility that AP-3 may interact with an unknown coat protein or proteins.
Biosynthetic transport of proteins to the yeast vacuole proceeds through two separate pathways, the CPY pathway and the ALP pathway (Burd et al., 1998). Many vacuolar resident proteins are delivered to the vacuole via the well-defined CPY pathway in which vacuolar hydrolases are transported from the Golgi compartment to an endosomal intermediate and then on to the vacuole (Stack et al., 1995). However, the membrane-bound vacuolar enzyme ALP and the vacuolar t-SNARE Vam3p are transported to the vacuole via the alternative ALP pathway that bypasses the prevacuolar endosome (Cowles et al., 1997b; Piper et al., 1997). Specific transport of proteins to the vacuole via the ALP pathway requires the function of the AP-3 adaptor protein complex (Cowles et al., 1997a; Stepp et al., 1997). In yeast, AP-3 binds to an acidic di-leucine sorting signal found in the cytoplasmic tails of cargo proteins (Darsow et al., 1998; Honing et al., 1998) and directs these cargoes into Golgi-derived vesicles that are then transported to the vacuole (Rehling et al., 1999). In other eukaryotes, the function of AP-3 is more complex. For example, mutations in human AP-3 result in a medically relevant disorder, Hermansky-Pudlack syndrome, in which patients have deficiencies in skin pigmentation and blood clotting and in the transport of resident proteins to the lysosome. In addition, mutations in AP-3 subunits in mice and Drosophila result in defects in coat color and eye pigmentation, respectively (Odorizzi et al., 1998). Together these data suggest that AP-3 in these organisms is required not only for transport of resident proteins to lysosomes, but also to lysosome-related organelles such as melanosomes and platelet storage granules.
In addition to the AP-3 adaptor protein complex, in yeast a number of vacuolar protein sorting (VPS) genes are also required for transport of ALP to the vacuole, including VAM3, the vacuolar t-SNARE, and VPS41/VAM2. Null mutations in genes such as VAM3 and VPS41 result in defects in both CPY and ALP transport to the vacuole (Radisky et al., 1997). Furthermore, both Vam3p and Vps41p are required for in vitro homotypic vacuole fusion (Nichols et al., 1997; Price et al., 2000b). However, unlike VAM3 and the other late-acting VPS genes, a temperature-conditional VPS41 mutant (vps41tsf) exhibits specific defects in transport of ALP to the vacuole (Cowles et al., 1997b). Furthermore, Vps41p is required for ALP pathway vesicle formation at the Golgi complex, and Vps41p physically associates with an AP-3 subunit (Rehling et al., 1999). Therefore, although Vps41p appears to act at an early transport step in the ALP pathway, the molecular function of Vps41p at this and additional steps in the pathway remains unclear.
To isolate new alleles of VPS41 and the AP-3 genes, as well as unidentified components of the ALP transport pathway, we undertook two genetic screens. Several new alleles of both VPS41 and the AP-3 adaptor genes were recovered from the screens. Analysis of two new constitutive loss-of-function VPS41 alleles revealed that these mutations cause phenotypes similar to AP-3 mutants, including strong, relatively specific defects in ALP transport, normal vacuolar morphology, and defects in the formation of ALP pathway intermediates. These alleles encode for proteins with mutations in either a novel N-terminal domain or the clathrin heavy-chain repeat (CHCR) domain of Vps41p. Analysis of these two protein domains show that they are required for Vps41p binding to AP-3 and homo-oligomerization of Vps41p, respectively. Both of these molecular interactions are essential for Vps41p function in the ALP pathway but seem to be dispensable for CPY pathway protein sorting. These results suggest that assembly of Vps41p into an oligomeric complex and its association with AP-3 are required at an early step of vesicle formation in the ALP pathway.
MATERIALS AND METHODS
Strains and Media
Yeast strains (Table 1) were grown in standard yeast extract-peptone-dextrose (YPD) or synthetic medium (YNB) supplemented with essential amino acids. Standard bacterial medium, containing 100 μg/ml ampicillin for plasmid retention, was used to propagate Escherichia coli. Transformation of Saccharomyces cerevisiae was done by the lithium acetate method (Ito et al., 1983). E. coli transformations were done by the method of Hanahan (1983).
Table 1.
S. cerevisiae strains used in this study
| Strain | Genotype | Reference |
|---|---|---|
| SEY6210 | MATα leu2-3,112 ura3-52 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9 | Robinson et al., 1988 |
| CCY250 | SEY6210; ste13Δ∷LEU2 | Cowles et al., 1997a |
| CBY31 | SEY6210; pep12Δ∷HIS3 | Burd et al., 1997 |
| TDY231 | SEY6210; pep12Δ∷HIS3; vps41-231 | This study |
| CCY4118 | SEY6210; ste13Δ∷LEU2; vps41-18 | This study |
| WSY41 | SEY6210; vps41Δ∷LEU2 | Radisky et al., 1997 |
| GOY8 | SEY6210; apl5Δ∷HIS3 | Cowles et al., 1997a |
| TDY27 | SEY6210; vam3tsf | This study |
| DKY25 | SEY6210; VPS41-HA∷HIS5MX6 | Rehling et al., 1999 |
| TDY30 | SEY6210; VPS41-231-HA∷HIS5MX6 | This study |
| PRY1 | SEY6210; APL5-HA∷HIS5MX6 | Rehling et al., 1999 |
Genetic Methods and EMS Mutagenesis
The ALP-Ste13 screen was performed exactly as previously described (Cowles et al., 1997a), except cells were mutagenized using ethyl methanesulfonate (EMS). For both screens, EMS mutagenesis, of either CCY250 harboring pAS13 (ALP-Ste13) (Cowles et al., 1997a) or CBY31 cells containing the plasmid pVAM3.416 (CEN, URA3, VAM3) (Darsow et al., 1997) was performed as described (Rose et al., 1990), resulting in ∼20–30% viability of mutagenized cells. For the Vam3p mislocalization screen, after EMS mutagenesis, the cells were diluted in YPD to recover for 1 h and then plated onto URA selective media and incubated at 38°C until colonies arose. Colonies that survived at 38°C were selected and cured of pVAM3.416 on plates containing 5-fluoroorotic acid and retested for temperature sensitivity at 38°C. Strains that were no longer temperature resistant without expression of VAM3 were selected. These strains were transformed with pPEP12.414 (CEN, TRP1, PEP12) and tested for CPY secretion by colony blot assays (Roberts et al., 1991). Cells that did not secrete CPY were then tested for ALP processing by Western blot analysis. Complementation analysis with known ALP pathway components was performed on all strains by individual transformation with characterized plasmids, followed by ALP and CPY pulse-chase assays.
Plasmid Construction and Nucleic Acid Manipulations
Restriction and modification enzymes were purchased from Boehringer Mannheim (Indianapolis, IN), New England Biolabs (Beverly, MA), or U.S. Biochemical Corporation (Cleveland, OH). pPEP12.414 was made by subcloning a ClaI-NsiI genomic fragment containing PEP12 into pRS414 vector (Sikorski and Hieter, 1989) digested with ClaI-PstI.
Cloned VPS41 Alleles.
The plasmid containing the vps41tsf allele was previously described (Cowles et al., 1997b). The vps41-18 allele was recovered from the chromosome using PCR with primers directed toward sequences 800 nucleotides upstream and 500 nucleotides downstream of the VPS41 open reading frame (ORF). PCR products were subsequently cloned with KpnI and SacI into pRS414 to yield pVPS41-18. The vps41-231 mutant gene, as well as wild-type chromosomal VPS41, were rescued from the chromosome by PCR with complementary primers directed 500 bp both upstream and downstream of the VPS41 ORF. PCR product was then cloned into TOPO-TA cloning vector (Invitrogen, San Diego, CA), digested with NsiI and SpeI, and subcloned into yeast expression vector by ligation into pRS414 vector digested with PstI and SpeI to yield pTD44 and pVPS41, respectively. Phenotypes of the mutants were confirmed by CPY and ALP pulse-chase assays.
Two-Hybrid Constructs.
Plasmid pPR15, encoding amino acids (aa) 729–932 of Apl5p in frame with the GAL4 DNA-binding domain in the pGBT9 vector was previously published (Rehling et al., 1999). A. Wurmser provided full-length VPS41 cloned into pGADGH (Clontech, Palo Alto, CA). A truncated form of VPS41 corresponding to the vps41-18 allele was produced by using the identical 5′ primer used for the full-length construct, in combination with a primer introducing the identical stop codon (5′-GGG GGG AGC TCT TAA TTT TCA TAA GGA CTT ATC ATG AAC G-3′). pTD41 containing the DNA encoding for Vps41p aa 1–570 in pGADGH was generated by PCR using primers containing in frame SmaI/XmaI sites at the start codon of VPS41 and primers containing an EcoRI site downstream in the VPS41 sequence. PCR products were cloned using the TOPO-TA cloning kit and then digested with XmaI and EcoRI and ligated into XmaI/EcoRI digested pGADGH vector. Plasmid pTD50, containing the full-length vps41-231 mutant in the pGADGH vector was constructed in two steps. First, the N-terminal domain of vps41-231 was amplified and constructed in the same manner as pTD41 to yield pTD49. The NdeI-XbaI C-terminal fragment of vps41-231 was then subcloned into pTD49 digested with NdeI-XbaI to yield pTD50.
GST Fusion Constructs.
GST-fusion protein plasmids were constructed as follows. Full-length VPS41 cloned into GST expression vector (pGST-VPS41) was constructed by subcloning the full-length fragment of VPS41 from the two-hybrid vector described above as an XmaI fragment into the bacterial expression vector pGEX-2T (Pharmacia, Piscataway, NJ) digested with the same enzyme. The GST-Vps41p truncation (pGST-VPS41T) was made by digesting pGST-VPS41 parent plasmid with XhoI, which cuts a single time in the C-terminus of VPS41 gene and filling the overhanging XhoI ends with T4 DNA polymerase. This manipulation introduced a frameshift resulting in a stop codon 7 amino acids downstream of the XhoI site at amino acid 714 of Vps41p. The GST fusion plasmid pPR22, containing aa 729–932 of Apl5p was described previously (Rehling et al., 1999)
Integrated HA Epitope Tags.
Tagging of VPS41 and vps41-231 with a triple HA sequence was performed by genomic integration at the 3′ end of the VPS41 ORF. Generation of PCR products containing an HIS3 marker gene and the tags, flanked by VPS41 homologous sequence, were performed as described earlier (Longtine et al., 1998) using the templates described therein.
Metabolic Labeling and Immunoprecipitation
To analyze the transport of vacuolar proteins, yeast cells were grown at 30°C in synthetic medium supplemented with amino acids. Cells at logarithmic growth phase were harvested and converted to spheroplasts as described previously (Paravicini et al., 1992). Spheroplasts were resuspended at a concentration of 3 OD600/ml in synthetic medium containing amino acids. Cultures were preincubated at 30°C for 5 min and then labeled for 10 min with 60 μCi [35S]cysteine and methionine/ml of cell suspension. After labeling, cultures were chased for 40 min with the addition of methionine, cysteine, yeast extract, and glucose to a final concentration of 5 mM, 1 mM, 0.4% and 0.2%, respectively. After chase, samples were harvested and precipitated by addition of trichloroacetic acid (TCA, 10% final concentration). Whole cells lysates were generated by glass bead disruption in urea buffer (50 mM Tris, pH 7.5, 1 mM EDTA, 1% SDS, and 6 M urea). Immunoprecipitated proteins were resolved by SDS-polyacrylamide gel electrophoresis (PAGE) and analyzed by autoradiography. Antibodies to CPY and ALP have been previously described (Klionsky and Emr, 1989).
Cell Fractionation
ALP intermediates were separated from Golgi membranes using a previously defined two-step gradient protocol (Rehling et al., 1999). In brief, 500-1000 ml of logarithmically growing cells (OD600 = 0.8) were harvested and softened for 10 min at room temperature (in 0.1 M Tris-HCl, pH 9.4, 10 mM DTT). Cells were then spheroplasted at 26°C in spheroplasting media (1× YNB, 2% glucose, amino acids, 1 M sorbitol, 20 mM Tris-HCl, pH 7.5, + 1 μg/OD600 zymolyase). Spheroplasts were harvested by centrifugation and resuspended in labeling medium (1× YNB, 2% glucose, amino acids, 1 M sorbitol) to a concentration of 3 OD600/ml and incubated at either 26°C (for constitutive alleles) or 38°C (for temperature-sensitive alleles) for 1 h. The spheroplasts were then chilled on ice for 5 min and harvested at 4°C. Spheroplasts were lysed in HEPES-KOH lysis buffer (20 mM HEPES-KOH, pH 6.8, 50 mM KOAc, 0.2 M sorbitol, 2 mM EDTA) containing protease inhibitors (to the following final concentration: 5 μg/ml antipain, 1 μg/ml aprotinin, 0.5 μg/ml leupeptin, 10 μg/ml α2-macroglobulin, and 0.1 mM AEBSF) with 15 strokes with a Kontes glass dounce homogenizer on ice. After a clearing spin at 300 × g for 5 min, the lysate was subjected to centrifugation at 13,000 × g (15 min). A 2-ml, 13,000 × g supernatant fraction was loaded on top of a sucrose step gradient consisting of 1.5 ml 30% (wt/vol) sucrose layered on top of 1 ml 60% (wt/vol) sucrose, in HEPES-KOH lysis buffer. The gradient was spun for 2 h at 150,000 × g at 4°C in a Beckman SW50.1 rotor (Fullerton, CA). The gradient was manually collected from the top into a 2.4-ml soluble fraction (S), a 1.2-ml membrane fraction (M), and a 0.9-ml pellet fraction (P). The membrane fraction was adjusted with HEPES-KOH lysis buffer to a sucrose concentration of 12% (wt/wt). Two milliliters of adjusted membrane fraction was loaded on top of a sucrose step gradient consisting of several concentration steps (wt/wt) that were from bottom to top as follows: 0.5 ml, 60%; 1 ml, 37%; 1.5 ml, 34%; 2 ml, 32%; 2 ml, 29%; 1 ml, 27%; and 1.5 ml 22%. This gradient was subjected to centrifugation at 160,000 × g for 18 h at 4°C. The gradient was harvested manually from the top into 14 fractions. The fractions were TCA precipitated and processed for SDS-PAGE and immunoblotting. Quantification of proteins from Western blots were made using NIH image software (Scion Image 1.62).
Fluorescence Microscopy
To examine vacuolar structures in live yeast cells, FM4-64 (Molecular Probes, Eugene, OR) labeling was done as previously described (Vida and Emr, 1995) except that the labeling was done at a concentration of 16 μM FM4-64 at 30°C for 15 min, and the cells were chased for a period of 1 h.
Protein Domain and Alignment Analysis
Analysis of the Vps41 protein domain structure was done using ProDom at SWISSPROT. Analysis of the N-terminal domain was done with PatMatch at the Saccharomyces Genome Database. Alignment of Vps41p and its homologues in other organisms was done using MegAlign in the DNA STAR software package.
Two-Hybrid Analysis
All two-hybrid plasmids were transformed into the yeast reporter strain PCY2 (Ito et al., 1983). Colonies were assayed for β-galactosidase activity by filter assay (Rehling et al., 1996). All assays were done in triplicate on at least three independent transformants.
Biochemical Assays
The full-length and truncated GST-Vps41 fusion proteins as well as the GST-Apl5p (aa 729–932) were transformed into E. coli. Expression and purification of GST fusion proteins were performed as described previously (Rehling et al., 1999). Yeast extracts for binding experiments were generated from 500-1000 OD600 of cells expressing the integrated Vps41p-HA or Apl5p-HA fusion proteins. Harvested cells were resuspended in 1–2 ml lysis buffer (20 mM HEPES-KOH, pH 6.8, 50 mM potassium acetate, 2 mM EDTA) plus 1× complete protease inhibitor cocktail (Boehringer Mannheim). Glass beads (0.5 g of 0.5-mm diameter) were added, and samples were alternatively vortexed for 30 s and cooled on ice for 1 min 15 times. The lysate was cleared for 10 min at 3000 × g at 4°C, and the supernatant was harvested, adjusted to 1% Triton X-100, and extracted on ice for 10 min. The lysate was then cleared for 10 min at 13,000 × g at 4°C, and the supernatant fraction was retained for binding experiments. Approximately 100–200 OD600 equivalents of the supernatant fraction (100–200 μl) were incubated for 1 h at 4°C with either GST or the various forms of Vps41p and Apl5p fused to GST that had been bound to glutathione-sepharose as described above. After incubation, sepharose beads were washed three times with lysis buffer containing 1% Triton X-100, three times with lysis buffer, and two times in final wash buffer (20 mM HEPES, 2 mM EDTA). Bound material was eluted with urea sample buffer (6 M urea, 100 mM Tris, pH 8, 4.5% SDS, 5% BME). For sizing column analysis, lysis protocols for making S100 supernatant fractions from bacteria and yeast differed slightly. The equivalant of 100 OD600 units of yeast expressing VPS41-HA were spheroplasted and lysed in 1.5 ml by dounce homogenization in PBS plus 1× complete protease inhibitor cocktail (Boehringer Mannheim) and subjected to a 30-min, 100,000 × g clearing spin. Equivalents at 20 OD600 of induced bacteria expressing either the full-length or truncated form of GST-Vps41 fusion proteins were lysed in 1.5 ml PBS plus 1× complete protease inhibitor cocktail (Boehringer Mannheim) by probe sonication (Branson, Danbury, CT) and subjected to a 30-min, 100,000 × g clearing spin. Cleared 1-ml supernatants were run over a Sephacryl S-300 16/60 column (Pharmacia) in PBS. Fractions of 1.4 ml were eluted at a flow rate of 0.4 ml/min and a portion of the resulting samples (1:1500 for bacteria and 1:40 for yeast extracts) were separated on SDS-PAGE and then immunoblotted with either anti-HA or anti-GST antisera and visualized by ECL (Amersham, Arlington Heights, IL). Sizing standards for the column were blue dextran, ferritin, catalase, and thyroglobulin.
RESULTS
Genetic Screen for Additional Components of the ALP Sorting Pathway
The ALP pathway in yeast directs the transport of two known cargo proteins to the vacuole: the membrane-bound vacuolar hydrolase ALP, and the vacuolar t-SNARE, Vam3p. Both of these cargo proteins contain acidic di-leucine sorting signals in their cytoplasmic domains (Darsow et al., 1998; Honing et al., 1998; Vowels and Payne, 1998) that are recognized by the AP-3 adaptor protein complex and are required for packaging these proteins into transport vesicle intermediates (Darsow et al., 1998; Honing et al., 1998). In addition to AP-3, the formation of ALP pathway intermediates depends on Vps41p, a protein that binds to Apl5p, the δ subunit of the AP-3 complex (Rehling et al., 1999). As in AP-1 and AP-2–mediated transport pathways, it is possible that other proteins that function specifically in the ALP pathway, involved in membrane recruitment of AP-3, budding, and uncoating of vesicles, remain to be identified. We undertook two different genetic approaches to identify such proteins.
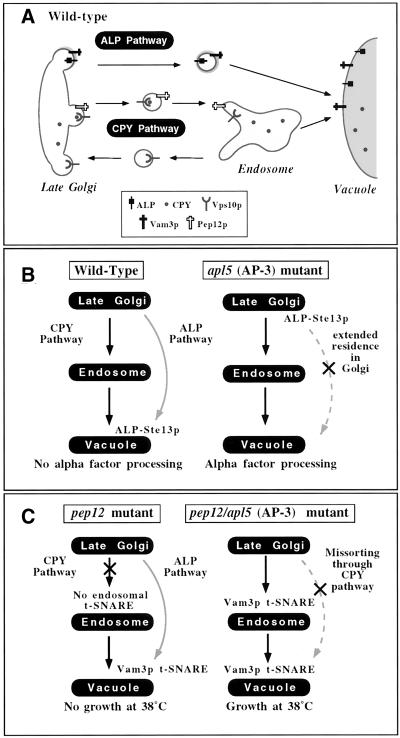
The first screen (Figure 1) was an extension of the previously published ALP-Ste13p screen (Cowles et al., 1997a). In brief, Ste13p is a Golgi-localized peptidase that is required for maturation of the secreted mating pheromone, alpha factor. The ALP-Ste13 fusion protein, unlike native Ste13p, is rapidly transported out of the Golgi complex, by virtue of the AP-3 sorting signal of ALP. In mating type alpha cells expressing the ALP-Ste13 fusion protein as the only form of Ste13p, the rapid transport of the fusion protein out of the Golgi complex to the vacuole separates Ste13p from its substrate, precursor alpha factor, and results in defects in alpha factor processing (Figure 1B). However, when transport through the ALP pathway is disrupted, residence of the ALP-Ste13 fusion protein in the Golgi complex is extended, where the Ste13p moiety can process alpha factor (Figure 1B). Binding of secreted mature alpha factor by receptors on the surface of cells of the opposite mating type results in G1 cell cycle arrest, which can be observed as a zone of growth inhibition when these cells are plated onto a lawn of opposite mating type cells. Therefore, the presence of a halo of growth inhibition can be used to visualize alpha factor processing and therefore, indirectly monitor ALP pathway sorting defects via Golgi localization of the ALP-Ste13 fusion protein. This approach was previously used to identify the AP-3 adaptor proteins in a screen for 2 μ dominant negative enhancers of halo formation (Cowles et al., 1997a) and was expanded in this study using EMS mutagenesis in an attempt to identify additional mutants defective in the delivery of ALP to the vacuole. Approximately 20,000 EMS-mutagenized colonies were screened for halo formation, of which ∼200 displayed a detectable halo phenotype. Secondary screening for lack of CPY secretion by CPY colony blotting and for ALP missorting by ALP pulse-chase immunoprecipitation experiments was performed. Six mutants with specific defects in ALP transport to the vacuole were recovered.
Figure 1.
Screens for ALP pathway components. (A) In wild-type yeast cells, protein trafficking between the Golgi and the vacuole proceeds through two parallel pathways. In the CPY pathway, cargo transits first from the Golgi to an endosomal compartment, where the endosomal t-SNARE, Pep12p, mediates docking and fusion with the endosomal membrane. From the endosome, the CPY pathway continues to the vacuole via a second step in which the vacuolar t-SNARE, Vam3p, mediates docking and fusion with the vacuolar membrane. In contrast, the ALP pathway appears to be a direct Golgi-to-vacuole pathway. Sorting signals in the cytoplasmic domains of the cargo proteins such as ALP and Vam3p are recognized by AP-3 and packaged into vesicles that are then directed from the Golgi to the vacuole without transit through an endosomal compartment. (B) Wild-type cells containing an ALP-Ste13p fusion protein are unable to process alpha factor because the fusion protein is directed to the vacuole through the ALP pathway by virtue of the AP-3 sorting signal of ALP. Disruption of trafficking through the ALP pathway by abrogation of AP-3 function results in retention of the ALP-Ste13p fusion protein in the Golgi and normal alpha factor processing. (C) pep12Δ cells disrupt trafficking through the CPY pathway and are temperature sensitive for growth at 38°C. The additional deletion of AP-3 in pep12Δ cells results in misrouting of ALP pathway cargoes such as Vam3p into the CPY pathway. Vam3p at the endosomal compartment can substitute for Pep12p and rescues the temperature sensitivity of pep12Δ cells.
The second genetic screen (Figure 1C) used the other known cargo of the ALP pathway, the vacuolar t-SNARE Vam3p. Mutations in the sorting signal of Vam3p or in the AP-3 adaptor proteins themselves prevent Vam3p entry into the ALP pathway, resulting in misrouting of Vam3p into the CPY pathway. At the CPY pathway endosome, Vam3p is able to functionally substitute for Pep12p, the endosomal t-SNARE (Darsow et al., 1998). Therefore, we reasoned that mutations in other genes required for transport in the ALP pathway may have similar effects on the trafficking of Vam3p and should also rescue pep12Δ mutant phenotypes (i.e., temperature-sensitive growth at 38°C). Therefore, we EMS-mutagenized pep12Δ cells and selected for temperature-resistant mutants. In the Vam3p mislocalization screen, ∼400,000 EMS-mutagenized colonies were screened for temperature resistance. Six hundred colonies that survived at 38°C were selected and subjected to secondary screens to determine whether the temperature resistance was dependent on the expression of VAM3. Clones that displayed temperature-resistant growth in a Vam3p-dependent manner were then screened for lack of CPY secretion by CPY colony blot and for ALP sorting by Western blotting. Nine mutants with ALP defects were recovered from the screen.
Thus, from both of these screens, a total of 15 mutants were isolated that displayed relatively specific defects in the transport of ALP to the vacuole. Of these 15 mutants, 12 of the mutant alleles were complemented by AP-3 genes. At least one representative allele of each of the four AP-3 subunits was identified. Interestingly, the remaining three mutants that were isolated from the screens were new missense alleles of VPS41, a gene that had been previously implicated as functioning at an early point in the ALP pathway through analysis of a temperature sensitive for function (tsf) allele of the gene (Cowles et al., 1997b; Rehling et al., 1999).
VPS41 Alleles Exhibit ALP Pathway Protein-sorting Defects
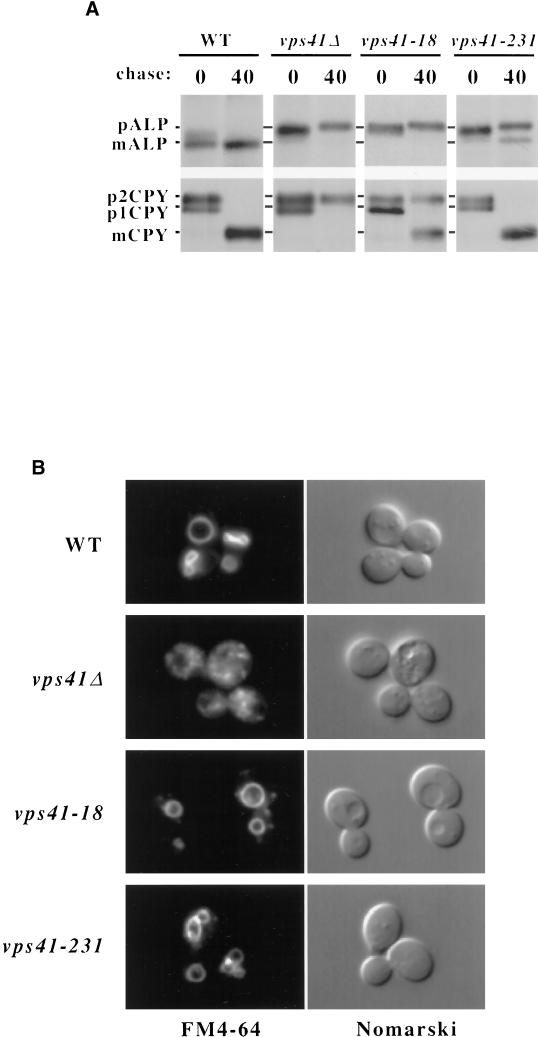
vps41tsf mutant cells display immediate defects in ALP transport to the vacuole and defects in budding of ALP pathway vesicles upon shift to nonpermissive temperature, suggesting that this protein may function in the formation of ALP pathway intermediates (Cowles et al., 1997b; Rehling et al., 1999). However, VPS41 deletion mutants have much more pleiotropic phenotypes than AP-3 subunit mutants, displaying defects in both vacuolar morphology and CPY sorting (Nakamura et al., 1997; Radisky et al., 1997), which suggests that Vps41p may also have a role in additional pathways or transport steps. Indeed, Vps41p seems to have a dual site of action as it is also part of the class C-Vps/HOPS protein complex (Seals et al., 2000; Wurmser et al., 2000) that directs SNARE-mediated fusion at the vacuole (Sato et al., 2000). The new constitutive loss-of-function alleles of VPS41 were selected on the basis of specific defects in ALP, and not CPY, transport to the vacuole, suggesting that these new mutants were distinct from null mutants of VPS41 in that that they were particularly defective in ALP pathway transport. Of the three new VPS41 alleles, two caused ALP sorting phenotypes similar in severity by both colony blot and Western blot analysis. We decided to further analyze one of these two mutants, vps41-18, and a third distinct mutant, vps41-231, by carefully examining their vacuolar protein–sorting defects by pulse-chase analysis. Wild-type, vps41Δ, vps41-18, and vps41-231 mutant cells were pulse-labeled with ]35S]cysteine/methionine for 10 min and then chased for 40 min with excess unlabeled cysteine/methionine at 30°C. Cells were harvested, and proteins were immunoprecipitated with specific antibodies and visualized by SDS-PAGE and autoradiography. Unlike wild-type cells in which both CPY and ALP were processed to their mature forms, in vps41Δ cells, both CPY and ALP were primarily found in their Golgi-modified precursor forms (Figure 2A), suggesting that transport through both biosynthetic pathways from the Golgi to the vacuole are disrupted in the deletion mutant (Radisky et al., 1997). As expected, in both of the new VPS41 mutants, ALP accumulated primarily in the precursor form after 40 min of chase (Figure 2). However, CPY was matured in the new VPS41 mutants, with ∼60% of CPY found in the mature form in vps41-18 and nearly 100% mature CPY in vps41-231 (Figure 2A).
Figure 2.
Vacuolar protein sorting and vacuolar morphology of VPS41 mutants. (A) vps41Δ (WSY41) cells transformed with either complementing VPS41 plasmid (pVPS41), or plasmids containing vps41-18 (pVPS41-18), or vps41-231 (pTD44), were pulse-labeled with [35S]cysteine/methionine and chased for 40 min. CPY and ALP were immunoprecipitated with polyclonal antibodies and analyzed by SDS-PAGE and autoradiography (B). The same strains shown in A were labeled with FM4-64 for 15 min at 30°C and then chased in fresh media for 1 h at 30°C.
Deletion of VPS41 results in a severely fragmented vacuolar morphology (Figure 2B) because of the accumulation of aberrant transport intermediates (Cowles et al., 1997b; Radisky et al., 1997). Because the VPS41 mutants have distinct vacuolar protein–sorting phenotypes when compared with the VPS41 deletion mutant, we were interested in examining the vacuolar morphology of these mutants. The vacuoles of wild-type, vps41Δ, vps41-18, and vps41-231 mutant cells were labeled with the vital stain FM4-64 for 15 min and then chased for 30 min at 30°C. The cells were then harvested and examined by fluorescence microscopy. Wild-type cells displayed typical vacuolar morphology, with one to three large, FM4-64–stained vacuolar compartments per cell, which could also be visualized by Nomarski optics (Figure 2B). In contrast, the vps41Δ cells displayed a highly dispersed and fragmented vacuolar morphology (Figure 2B), as has previously been reported (Radisky et al., 1997). Remarkably, although the new VPS41 mutant cells did contain some peripheral FM4-64–stained compartments not seen in wild-type cells, the vacuoles in these cells had relatively normal morphology (Figure 2B). Therefore, the new VPS41 mutants had phenotypes quite distinct from vps41Δ cells. Instead, these new VPS41 mutants were much more reminiscent of AP-3 mutants, which have ALP-specific sorting defects and normal vacuolar morphology (Cowles et al., 1997a; Stepp et al., 1997).
VPS41 Mutant Cells Are Defective in the Formation of ALP Transport Intermediates
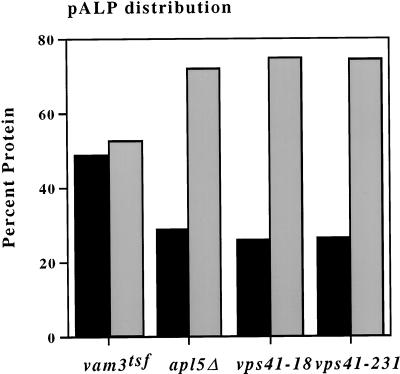
The relatively specific defects of the new VPS41 mutants suggested that they may, like AP-3, act at an early stage of transport intermediate formation at the Golgi complex. We have previously described an in vivo assay for the detection of ALP transport vesicle intermediates and demonstrated that both AP-3 and vps41tsf mutants are defective in the formation of these intermediates (Rehling et al., 1999). We examined the new VPS41 alleles in this assay to determine their effects on transport vesicle formation. Extracts were prepared from vam3tsf, apl5Δ vps41-18, and vps41-231 cells and were analyzed in the in vivo ALP vesicle formation assay (see MATERIALS AND METHODS). As expected, in vam3tsf cells, precursor ALP (pALP) was evenly distributed in two peaks, one at low sucrose concentrations (vesicle fraction) and another at higher sucrose concentration (Golgi fraction) (Figure 3), which is indicative of accumulation of transport vesicles (Rehling et al., 1999). In contrast to vam3tsf, in apl5Δ cells, pALP was found as a single peak, with ∼75% of the protein contained within the Golgi fractions (Figure 3), consistent with the previously reported defect in vesicle formation in apl5Δ cells. In both vps41-18 and vps41-231 strains, the pALP distribution was similar to that seen in apl5Δ cells (Figure 3). This result suggests that like AP-3 and vps41tsf (Rehling et al., 1999) mutant cells, these new VPS41 mutants also have defects early in the ALP pathway, specifically in the formation of vesicles from the Golgi complex.
Figure 3.
VPS41 alleles are defective in ALP intermediate formation. Five hundred OD600 equivalents of vam3tsf cells (TDY27), apl5Δ (GOY8), vps41-18 (WSY41 + pVPS41-18), and vps41-231(WSY41 + pTD44) were spheroplasted, temperature-shifted, lysed, and fractionated (see MATERIALS AND METHODS). Approximately 1 OD600 equivalent of material from each fraction was analyzed by SDS-PAGE and Western blot analysis. The distribution of pALP from each gradient was quantitated using Scion Image 1.62, and the relative amounts of protein contained within the Golgi and vesicle-enriched fractions were calculated. Legend: black bars = vesicle fraction; shaded gray bars = Golgi fraction.
VPS41 Alleles Encode Mutations in Distinct Domains of Vps41p
The phenotypes of the new VPS41 mutants in the formation of ALP transport vesicles at the Golgi suggested that these mutants are particularly defective in early events in the ALP pathway. We wanted to determine the nature and location of the mutations in the new VPS41 alleles, because they might indicate what domains of Vps41p are important for ALP pathway function. We rescued the mutant VPS41 genes from the chromosome by PCR and cloned them into yeast expression vectors. Phenotypes resulting from the cloned mutations were then confirmed by retransformation into vps41Δ yeast cells. We sequenced the mutant vps41-18, vps41-231, vps41tsf, and wild-type VPS41–containing plasmids for comparison.
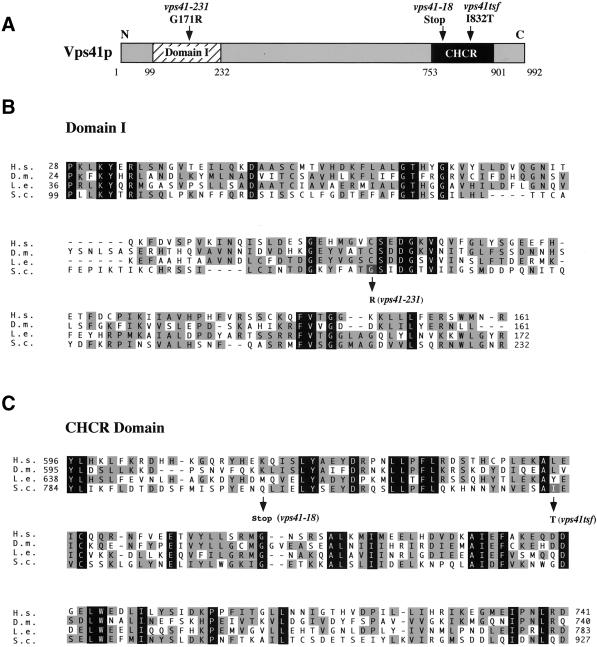
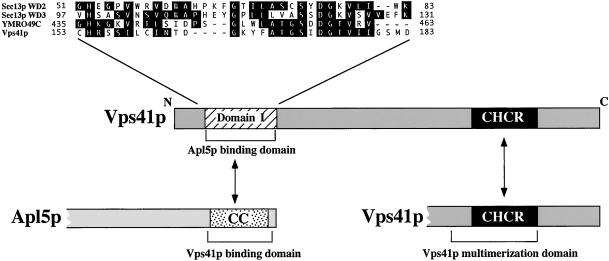
Sequence comparison of all known Vps41p sequences revealed only two conserved domains in the protein. First, an N-terminal domain (domain I) that is conserved between Vps41p homologues but is not found in other proteins, is located between amino acids 99 and 232 of Vps41p. Second, a C-terminal CHCR domain, which was originally identified in the clathrin heavy-chain molecule and can be found in other proteins, including a number of Vps proteins (Conibear and Stevens, 1998) is located between amino acids 753 and 901. Sequence analysis showed that vps41-18 and vps41tsf each contained mutations within the conserved CHCR domain in the C-terminus of Vps41p (Figure 4B). In clathrin, this domain is repeated seven times and is required for homo-oligomerization of the clathrin heavy-chain molecules to form the clathrin lattice structure (Ybe et al., 1999). vps41-18 contains a stop codon at amino acid 803 that truncates the C-terminal 20% of the protein and completely removes the CHCR domain. vps41tsf contains a single amino acid change from isoleucine to threonine at amino acid 832 (I832T) within the CHCR domain. Unlike the other alleles, sequencing of the vps41-231 mutant revealed that it did not contain a mutation in the CHCR domain. Instead, this gene had two single mutations coding for amino acid changes in the N-terminus of the protein. The mutations were separated by subcloning, and ALP sorting analysis was used to determine that the ALP missorting phenotype of the vps41-231 mutant was attributable to a single amino acid change of glycine to arginine at amino acid 171 (G171R) in domain I (Figure 4A). This mutation in vps41-231 falls within a particularly conserved region of domain I, suggesting that the mutation may act to disrupt this domain and that it may be important for Vps41p ALP pathway function.
Figure 4.
Alignment of Vps41p domains. (A) Vps41p contains two conserved domains, an N-terminal domain that is conserved between all of the Vps41p homologues, designated here as domain I, and a C-terminal clathrin heavy-chain repeat (CHCR) domain, which is found in clathrin heavy-chain , a number of Vps proteins, and in Vps41p and all of its homologues. (B) Domain I alignment with the Vps41p homologues from Homo sapiens (H.s.), Drosophila melanogaster (D.m.), and Lycopersicon esculentum (tomato) (L.e.). The amino acid change found in vps41-231 is indicated with an arrow, and the base change is noted. (C) CHCR alignment with Vps41p homologues. Amino acid changes in both vps41tsf and vps41-18 are blocked in gray and indicated with arrows. In B and C, identity between all sequences is blocked in black, and similarity between a subset of the sequences is blocked in light gray.
An N-terminal Region of Vps41p Interacts with Apl5p
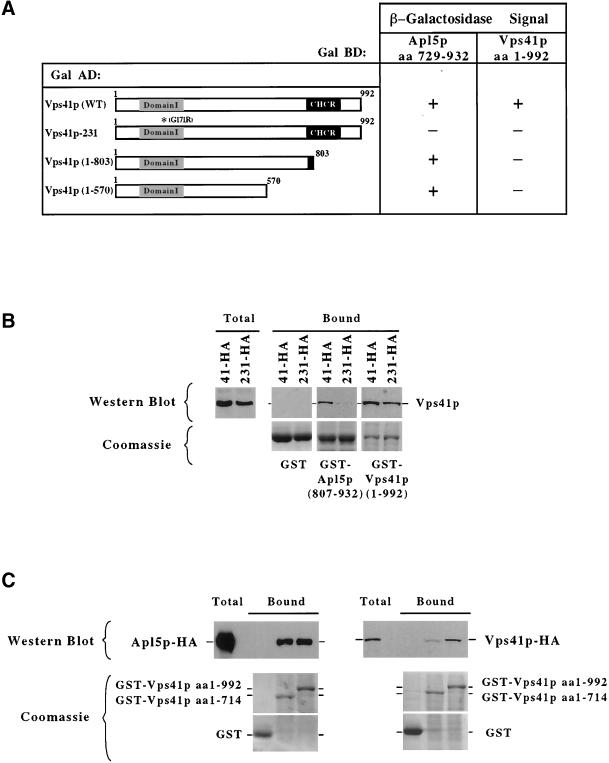
The extreme C-terminal portion of Apl5p has previously been shown be necessary and sufficient to bind to Vps41p (Rehling et al., 1999). However, the domain of Vps41p itself ,which is required for this interaction, has not been determined. We considered that the mutations in the new VPS41 alleles may potentially interfere with the ability of Vps41p to bind to the C-terminal domain of Apl5p. To examine this possibility, we performed two-hybrid analysis of Vps41p and Apl5p. As previously reported (Rehling et al., 1999), coexpression of full-length Vps41p fused to the GAL4 activation domain (AD) and a C-terminal fragment (aa 729–923) of Apl5p fused to the GAL4 DNA binding domain (BD) in the yeast reporter strain creates a positive two-hybrid interaction (Figure 5A). Truncations of Vps41p from the C-terminus were made to define the Apl5p interaction domain of Vps41p. These truncations revealed that the minimal domain of Vps41p that bound to Apl5p was the N-terminal 570 amino acids of the protein (Figure 5A). Further truncations of Vps41p from either the N- or the C-terminus were unable to bind to Apl5p (our unpublished observations). Interestingly, a Vps41p truncation in which the CHCR domain was removed, similar to the truncation mutation found in vps41-18 (aa 1–803), still efficiently bound to Apl5p (Figure 5A), suggesting that the CHCR domain is not directly required for AP-3 interaction. However, the mutation found in the vps41-231 allele encoded for an amino acid change in the N-terminus of the protein, within the minimal region required for binding to Apl5p. Indeed, when tested in the two-hybrid assay, Vps41-231 protein, which contains an amino acid substitution within domain I, no longer bound to Apl5p (Figure 5A), suggesting that disruption of domain I interferes with Vps41p binding to Apl5p.
Figure 5.
Analysis of Vps41p interactions. (A) GAL4-BD fusion with a C-terminal fragment of Apl5p (aa 729–932) and a GAL4-BD fusion with full-length Vps41p tested against the indicated Vps41p fragments fused in frame with the GAL4-AD. β-Galactosidase assays were done in duplicate on multiple transformants for each experiment. (B and C) The indicated GST fusion proteins were purified from E. coli on GSH-Sepharose. (A) Triton X-100–solubilized 13,000 × g supernatant fractions from yeast cells expressing either Apl5p-HA (PRY1), Vps41p-HA (DKY25), or Vps41-231-HA protein (TDY30) were incubated with the immobilized GST fusion proteins at 4°C and then washed. The bound proteins were eluted with sample buffer. For Western blotting, 20% of the eluate was loaded per lane. One percent of solubilized extract (total) was loaded as a reference. (B and C) Top panels: a Western blot probed with anti-HA antibodies; bottom panels: Coomassie-stained gels of 10% of the eluted sample indicate the relative amounts of the fusion proteins used in each experiment. The identity of each GST-fusion protein is indicated to the right of the bottom panels.
To confirm these results by an independent means, we made GST fusion constructs with either full-length Vps41p or a C-terminal domain of Apl5p containing aa 807–932. These proteins, as well as GST alone, were expressed in E. coli and purified onto glutathione sepharose beads for binding experiments. Glutathione sepharose beads containing either GST, GST-Apl5p (aa 807–932) or full-length GST-Vps41p (aa 1–992) were incubated with Triton X-100 solubilized total cell extracts made from cells expressing either Vps41-HA or Vps41-231-HA protein. Bound proteins were washed and eluted with SDS sample buffer and examined by Western blot analysis. Although Vps41p-HA bound to the GST-Apl5p fusion protein, theVps41-231-HA protein did not efficiently bind to the GST-Apl5 fusion protein (Figure 5B), suggesting, in concert with the two-hybrid data, that the amino acid change in domain I of the Vps41-231 protein interferes with the ability of Vps41p to bind to Apl5p. Interestingly, full-length GST-Vps41p bound to Vps41p-HA as well as to the Vps41-231-HA protein (Figure 5B), indicating that Vps41p is able to interact with itself and that the amino acid substitution within Vps41-231 protein does not affect this interaction. These results suggest that the C-terminal structure of the Vps41-231 protein is maintained and that the mutation selectively interferes with Apl5p interactions.
Vps41p Forms a Large Oligomeric Complex Both In Vitro and In Vivo
In clathrin, the CHCR domain is essential for assembly of clathrin heavy-chain into a homo-oligomeric complex. Because Vps41p binds to itself in vitro and the CHCR does not affect binding of Vps41p to Apl5p, we wanted to determine whether Vps41p was able to multimerize via the CHCR domain. We first tested for homotypic interactions between Vps41p by the two-hybrid assay. Coexpression of plasmids encoding for Vps41p GAL4-AD and Vps41p GAL4-BD in the yeast reporter strain resulted in reporter activation, suggesting, in agreement with the GST coprecipitation data (Figure 5B), that Vps41p may in fact associate with itself (Figure 5A). However, the vps41-18 mutant, which results in a truncation of the majority of the CHCR domain, does not associate with wild-type Vps41p (Figure 5A), suggesting that this domain is required for Vps41p homotypic interactions.
To examine the role of the CHCR domain in multimerization of Vps41p in vitro, GST, GST-Vps41p, or a GST-Vps41p truncation (aa 1–714) bound to glutathione sepharose beads were incubated for 1 h at 4°C with Triton X-100 solubilized cell extracts from strains expressing either Apl5p-HA or Vps41p-HA fusion proteins. After incubation, the beads were washed extensively, and the bound proteins were eluted with SDS sample buffer and examined by Western blotting with anti-HA antibodies. Again, in agreement with the two-hybrid data, Apl5p-HA bound to both GST-Vps41p and the GST-Vps41p (aa 1–714) truncation (Figure 5C). In contrast, although Vps41p-HA efficiently bound to full-length GST-Vps41p, Vps41p-HA displayed a dramatic (∼10-fold) decrease in binding to the GST-Vps41p (aa 1–714) truncation (Figure 5C), suggesting that the deletion of the CHCR domain results in a decreased ability of Vps41p to homo-oligomerize.
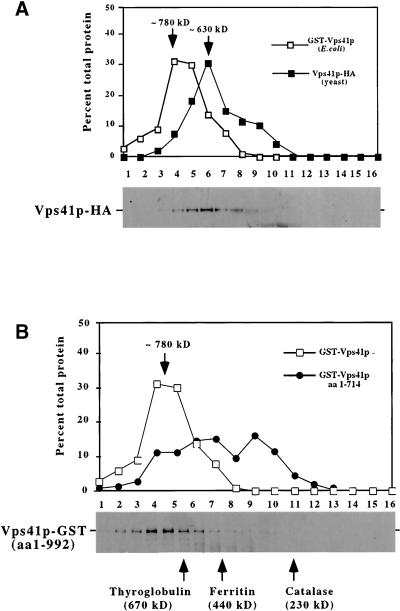
To determine whether Vps41p forms oligomeric structures in vivo, we examined the gel filtration characteristics of Vps41p from yeast extracts. A soluble cell extract from yeast cells expressing Vps41p-HA was generated and examined by gel filtration on an S300 sephacryl column. Vps41p-HA eluted at ∼630 kDa (Figure 6A), consistent with a complex containing approximately six Vps41p molecules if Vps41p is the sole component of the complex. However, because Vps41p also interacts with Apl5p, we considered that the AP-3 proteins may be associated with Vps41p in this high-molecular-weight complex. Surprisingly, the deletion of AP-3 complex components had no effect on the gel filtration characteristics of Vps41p-HA (our unpublished results), suggesting that these proteins were not stably associated with the soluble Vps41p complex in yeast. Furthermore, when the AP-3 proteins and the late-acting proteins such as Vps39p/Vam6p, a protein in the class C Vps/HOPS protein complex that has been previously reported to interact with Vps41p (Nakamura et al., 1997), were examined by gel filtration, they did not coelute with Vps41p (our unpublished results), suggesting that these proteins are not part of the soluble Vps41p complex.
Figure 6.
Analysis of Vps41p oligomeric complex. (A) Lysates were made from 100 OD600 equivalents of wild-type yeast expressing an integrated VPS41-HA fusion construct (DKY25) and centrifuged at 100,000 × g to remove insoluble proteins and membranes. The supernatant fraction was applied to the S300 sizing column. Proteins in the fractions were analyzed by Western blotting using antibodies against the HA epitope. The yeast Vps41p-HA profile is compared with recombinantly produced GST-Vps41p. (B) GST fused to either full-length Vps41p (pGSTVPS41) or a truncated form of Vps41p containing amino acids 1–714 (pGSTVPS41T) were induced in E. coli (XLIB). Lysates were made from 20 OD600 equivalents of E. coli and centrifuged at 100,000 × g to remove insoluble proteins and membranes. Supernatant fractions for both GST-Vps41p and GST-Vps41p (aa 1–714) truncation were subjected to fractionation on S300 sizing columns. In both A and B, the relative amounts of proteins in each fraction from the columns were compared using Scion Image 1.62 and plotted as a percentage of total protein. Bottom panels: Western blots of Vps41p-HA (A) and GST-Vps41p (B) from the columns. Arrows indicate the approximate size of the peak fractions in both A and B.
To further characterize the components and stoichiometry of the Vps41 protein complex, we recombinantly produced the full-length GST-Vps41 protein in E. coli and examined the mobility of the fusion protein on a sephacryl S300 sizing column. Interestingly, GST-Vps41p eluted at a calculated molecular weight of 780 kDa (Figure 6, A and B). Considering that the molecular weight of GST-Vps41p is ∼135 kDa, this large complex, like the Vps41p-HA from yeast extracts, would correspond to six Vps41p molecules. Furthermore, because the protein was produced in bacteria, the potential association of other yeast proteins is eliminated, suggesting that the soluble protein complex in both yeast and bacteria is most likely a homo-oligomer of Vps41p. However, we were concerned with the possibility that GST dimerization may be contributing to the size of the complex, so we also examined the mobility of the truncated GST-Vps41 protein (aa 1–714). If Vps41p oligomerization via the CHCR domain were responsible for the size of the complex, this truncated fusion protein would be expected to disrupt the formation of the complex. In fact, the recombinant GST-Vps41p truncation (aa 1–714) did not display a clear peak of protein from the gel filtration column and instead was distributed over a large range of smaller molecular weights (Figure 6B), indicating that deletion of the C-terminal portion of Vps41p dramatically destabilized homo-oligomeric complex formation. Therefore, several lines of evidence, both in vivo and in vitro, suggest that the soluble pool of Vps41p forms a homo-oligomeric complex and that the formation of this complex depends upon the CHCR domain in the C-terminus of Vps41p.
DISCUSSION
Using two distinct genetic screens, we attempted to identify new alleles of genes that function in the ALP transport pathway. From these screens, we recovered numerous alleles of the four AP-3 genes and VPS41, but did not uncover new complementation groups. It is possible that there are no additional genes required for ALP pathway transport. However, we think this is unlikely, as loading of AP-3 onto membranes is likely to require components other than the tails of cargo proteins. It is possible that additional components that function in the ALP pathway are redundant or have overlapping function with other ALP pathway components. For example, Arf1 has been shown in mammalian cells to be required for AP-3 membrane association (Ooi et al., 1998). In yeast there are several ARF genes that may have the capacity to substitute for one another. Furthermore, additional components that are required for ALP transport may not be specific to the ALP pathway. For example, the late-acting genes that direct the docking and fusion of ALP pathway vesicles with the vacuolar membrane are utilized by CPY pathway intermediates as well, and mutations in these genes result in defects in both ALP and CPY transport (Darsow et al., 1997; Rieder and Emr, 1997). Finally, it is possible that the core machinery necessary for vesicle formation has been identified and that other components may have regulatory roles, resulting in less severe ALP missorting phenotypes upon disruption. Though no additional ALP pathway components were identified, a pair of very informative new alleles of VPS41 were recovered from our screens, allowing us to dissect the function of Vps41p in the ALP pathway.
The role of Vps41p in the Vps pathway has been a controversial issue because of the complex phenotypes associated with mutants of this gene. Deletion of VPS41 results in defects in transport through both the ALP and CPY pathway and severely fragmented vacuolar morphology (Nakamura et al., 1997; Radisky et al., 1997), suggesting defects in fusion at the vacuole. Consistent with such vacuolar defects, recent studies have established Vps41p as a member of a vacuole-associated protein complex (Seals et al., 2000; Wurmser et al., 2000) required for vacuole fusion (Price et al., 2000a,b). Yet several other observations suggest that Vps41p also functions at an early step in the ALP pathway. First, a temperature-conditional allele of VPS41 (vps41tsf) has primary defects in ALP-specific trafficking to the vacuole, and only upon prolonged shifts to the nonpermissive temperature are CPY sorting and vacuolar morphology affected (Cowles et al., 1997b). Additional analysis of the same vps41tsf allele revealed that, much like AP-3 mutant cells, vps41tsf cells are defective in the formation of ALP pathway transport intermediates from the Golgi compartment (Rehling et al., 1999). Furthermore, protein interaction studies have revealed that Vps41p physically associates with the AP-3 complex subunit Apl5p (Rehling et al., 1999). Together, these results suggest that Vps41p has a role at two distinct points in the Vps pathway.
The recovery of constitutive VPS41 alleles from screens for ALP-specific transport components further suggests that Vps41p acts initially at an early stage in the ALP pathway. Although deletion mutants in late-acting genes such as the vacuolar t-SNARE VAM3 and the YPT7 Rab GTPase, which have identical phenotypes to vps41Δ mutants, were also recovered from the screen, these proteins were eliminated in secondary screens for CPY secretion as nonspecific (our unpublished observations). The new VPS41 mutants, however, did not display strong CPY secretion defects and therefore were clearly discernable from the late-acting genes. Careful analysis of vacuolar protein sorting in the new constitutive VPS41 mutants showed that although ALP was strongly blocked, CPY defects were weak to undetectable (Figure 2A). Surprisingly, these new VPS41 mutants displayed essentially wild-type vacuole morphology (Figure 2B), in contrast to the severely fragmented structures observed in vps41Δ mutant cells. Finally, like AP-3 mutants, the new VPS41 mutants were defective in formation of ALP transport intermediates (Figure 3B). Thus, the phenotypes of these alleles were much more similar to AP-3 deletion mutants than to vps41Δ mutant cells. Together, the analysis of the new VPS41 alleles provides a strong genetic argument for a dual role for Vps41p, both at an early step of vesicle formation in the ALP pathway at the Golgi complex and additionally as a component of the docking and fusion machinery at the vacuole (Price et al., 2000b; Sato et al., 2000; Wurmser et al., 2000). Coupling of budding and fusion events by a single protein is not a novel concept, since there is some precedent for such dual functions by the SNARE fusion machinery (Springer and Schekman, 1998) and Rab1 GTPase (Allan et al., 2000) in the formation of the COPII coat and in the docking and fusion of ER-derived transport vesicles at the Golgi. Reconciliation of these two activities of Vps41p will require additional work to determine relationships between Vps41p and the AP-3 adaptor complex as well as the molecular function of Vps41p in the late steps of vesicle docking and fusion at the vacuole.
Analysis of the mutations found in each of the new VPS41 alleles revealed that they encoded amino acid changes within either the CHCR domain (Figure 4B) or a novel N-terminal domain (domain I, Figure 4A), suggesting that these domains are particularly important for transport through the ALP pathway. Consistent with this, we have shown that both of these domains are protein interaction domains in Vps41p. The N-terminal half of Vps41p contains the minimal region that is required for binding to the Apl5p δ subunit of AP-3 (Figure 5, A and C). More importantly, mutations within a highly conserved domain of the N-terminus of the protein abolish the ability of Vps41p to interact with Apl5p (Figure 5, A and B). Interaction with AP-3 in particular would presumably be essential for Vps41p activity in the formation of ALP pathway vesicles at the Golgi membrane. Consistent with this, the vacuolar protein–sorting phenotype of vps41-231 is very specific for ALP transport (Figure 2A). Together, this data does not rule out an additional function for the N-terminal portion of the protein in later steps of transport to the vacuole, but it does strongly suggest that function of the N-terminal domain is required for the early function of vesicles formation at the Golgi membrane.
Our analysis of both recombinant Vps41p from bacteria and endogenous Vps41p from yeast extracts suggests that the soluble fraction of Vps41p exists as a homo-oligomer, most likely composed of six Vps41p molecules (Figure 6, A and B). Furthermore, our data strongly suggest that the CHCR domain is required for stable Vps41p oligomerization. The deletion of the CHCR domain results in a reduced ability of the truncated protein to bind to full-length Vps41p in vitro (Figure 5C) and results in a dramatic destabilization of the purified Vps41p complex (Figure 6A). Structural analysis of the CHCR domain in the clathrin molecule demonstrated that the CHCR domain mediates the homotypic interactions of clathrin to form triskelions (Ybe et al., 1999), consistent with the proposed function of the homologous domain in Vps41p. But what is the function of this oligomeric complex? It is interesting to note that the most highly conserved portion of the Vps41p N-terminal domain displays some similarity to WD40 repeat domains found in other yeast proteins such as Sec13p (Saxena et al., 1996) (Figure 7). WD40 repeats, although not similar at the sequence level, are highly conserved at the structural level with the beta propeller domains found in the N-terminal region of the clathrin heavy-chain molecule (ter Haar et al., 1998). The propeller domain in clathrin contains seven repeats that form the seven blades of the propeller and produces the binding site for the AP-2 adaptor complex and the arrestins (ter Haar et al., 2000). The Vps41p domain would roughly correspond to a single blade in the propeller structure. However, it is possible that the oligomerization of the protein may coordinate a number of the blade domains in concert to form a propeller-like structure in the oligomer. The deletion of the CHCR does not interfere with Vps41p binding to Apl5p in vitro, suggesting that oligomerization is not a prerequisite for association with AP-3. Nonetheless, the higher order structure could act to stabilize the interaction with AP-3 or to coordinate interactions with multiple AP-3 molecules to form clusters of AP-3 complexes that then could oligomerize further during the formation of the ALP transport vesicle. Like the vps41-231 mutant, the vps41-18 mutant results in a strong defect in ALP sorting, but the CHCR truncation results in a modest CPY sorting defect as well, suggesting that this domain may also affect the vacuolar fusion function of Vps41p, possibly by destabilizing the class C-Vps/HOPS complex. Clearly, additional analysis of Vps41p structure will be necessary to determine the molecular configuration of Vps41p and its interacting protein partners.
Figure 7.
Model for Vps41p protein domain function. Vps41p contains two regions that are particularly conserved between the known Vps41p homologues; an N-terminal domain required for binding of Vps41p to the C-terminal coiled coil containing domain of Apl5p, and a C-terminal clathrin heavy-chain repeat (CHCR) domain that mediates oligomerization of Vps41p. The N-terminal domain of Vps41p has homology to WD-40 repeats found in Sec13p and an unknown WD40 containing ORF, YMR049C. The sequence identity between the Vps41p domain and the WD-40 domains of Sec13p and the protein encoded by YMR049C is shown blocked in black.
Together, these data indicate that Vps41p function in the ALP pathway is dependent on both protein interactions with the AP-3 complex and oligomerization of Vps41p itself, which by analogy to clathrin, could suggest a coat-like function for Vps41p. However, Vps41p also seems to have a late function in docking and fusion at the vacuole, which makes a model for Vps41p performing the function of a traditional coat protein difficult to imagine. It is quite possible that Vps41p may perform dual functions, distinct at both early and late sites in the vacuolar protein-sorting pathway. In this model, Vps41p is incorporated onto the emerging ALP vesicles at the Golgi compartment and may act to stabilize AP-3 on the membrane via interactions with other proteins or lipids and in this capacity be required for efficient vesicle formation. In addition, once incorporated into the vesicle, Vps41p may form a docking site on the budded vesicle for the addition of the components of the class C Vps/HOPS complex, a necessary requirement for eventual docking and fusion at the vacuolar membrane. Consistent with this, the in vitro vacuole fusion assay requires Vps41p on both the acceptor and the donor compartment. By analogy, in heterotypic fusion of vesicles with the vacuolar membrane, Vps41p may also be required on both membranes (Price et al., 2000a,b). Interactions of Vps41p with both AP-3 and class C-Vps/HOPS complex proteins may act to bridge the ALP intermediate to its target destination, the vacuole. Interestingly, the Drosophila homologue of VPS41, light, results in defects in eye pigmentation, as do mutations in the Drosophila AP-3 adaptor subunit homologues, such as garnet (Simpson et al., 1997) and the VPS18 class C VPS gene homologue dor (Shestopal et al., 1997). Additionally, nonlethal mutations of light display genetic interactions in combination with alleles of both dor and garnet (Warner et al., 1998), suggesting that the functional relationships between VPS41, AP-3, and the class C VPS genes are evolutionarily conserved. Further characterization of Vps41p, its structure, localization, and functional organization should help to resolve the details of how this interesting and complex molecule executes its distinct roles in both vesicle formation and vesicle docking/fusion reactions.
ACKNOWLEDGMENTS
We thank A. Wurmser for providing the full-length VPS41 two-hybrid construct and P. Rehling for providing GST-Apl5p constructs. We are grateful to members of the Emr laboratory for helpful discussions. In particular, we thank T. Sato, M. Babst, and P. Rehling for critical reading of the manuscript. D.J.K is supported as a fellow of the American Cancer Society. This work was supported by National Institutes of Health grant CA58689 (to S.D.E.). S.D.E. is an Investigator with the Howard Hughes Medical Institute.
Abbreviations used:
- SNARE
SNAP receptor
- ALP
alkaline phosphatase
- CPY
carboxypeptidase Y
- FM4-64
N-(3-triethylammoniumpropyl)-4-(p-diethylaminophenylhexatrienyl) pyridinum dibromide
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- TCA
trichloroacetic acid
- CHCR
clathrin heavy-chain repeat
- BME
2-mercaptoethanol
- ORF
open reading frame, GST, glutathione S transferase
REFERENCES
- Allan BB, Moyer BD, Balch WE. Rab1 recruitment of p115 into a cis-SNARE complex: programming budding COPII vesicles for fusion [see comments] Science. 2000;289:444–448. doi: 10.1126/science.289.5478.444. [DOI] [PubMed] [Google Scholar]
- Burd CG, Babst M, Emr SD. Novel pathways, membrane coats and PI kinase regulation in yeast lysosomal trafficking. Semin Cell Dev Biol. 1998;9:527–533. doi: 10.1006/scdb.1998.0255. [DOI] [PubMed] [Google Scholar]
- Chen WJ, Goldstein JL, Brown MS. NPXY, a sequence often found in cytoplasmic tails, is required for coated pit-mediated internalization of the low density lipoprotein receptor. J Biol Chem. 1990;265:3116–3123. [PubMed] [Google Scholar]
- Conibear E, Stevens TH. Multiple sorting pathways between the late Golgi and the vacuole in yeast. Biochim Biophys Acta. 1998;1404:211–230. doi: 10.1016/s0167-4889(98)00058-5. [DOI] [PubMed] [Google Scholar]
- Cowles CR, Odorizzi G, Payne GS, Emr SD. The AP-3 adaptor complex is essential for cargo-selective transport to the yeast vacuole. Cell. 1997a;91:109–118. doi: 10.1016/s0092-8674(01)80013-1. [DOI] [PubMed] [Google Scholar]
- Cowles CR, Snyder WB, Burd CG, Emr SD. An alternative Golgi to vacuole delivery pathway in yeast: identification of a sorting determinant and required transport component. EMBO J. 1997b;16:2769–2782. doi: 10.1093/emboj/16.10.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darsow T, Burd CG, Emr SD. Acidic di-leucine motif essential for AP-3-dependent sorting and restriction of the functional specificity of the Vam3p vacuolar t-SNARE. J Cell Biol. 1998;142:913–922. doi: 10.1083/jcb.142.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darsow T, Rieder SE, Emr SD. A multispecificity syntaxin homologue, Vam3p, essential for autophagic and biosynthetic protein transport to the vacuole. J Cell Biol. 1997;138:517–529. doi: 10.1083/jcb.138.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Angelica EC, Klumperman J, Stoorvogel W, Bonifacino JS. Association of the AP-3 adaptor complex with clathrin. Science. 1998;280:431–434. doi: 10.1126/science.280.5362.431. [DOI] [PubMed] [Google Scholar]
- Dell'Angelica EC, Ohno H, Ooi CE, Rabinovich E, Roche KW, Bonifacino JS. AP-3: an adaptor-like protein complex with ubiquitous expression. EMBO J. 1997;16:917–928. doi: 10.1093/emboj/16.5.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Angelica EC, Shotelersuk V, Aguilar RC, Gahl WA, Bonifacino JS. Altered trafficking of lysosomal proteins in Hermansky-Pudlak syndrome due to mutations in the beta 3A subunit of the AP-3 adaptor. Mol Cell. 1999;3:11–21. doi: 10.1016/s1097-2765(00)80170-7. [DOI] [PubMed] [Google Scholar]
- Faundez V, Horng JT, Kelly RB. A function for the AP3 coat complex in synaptic vesicle formation from endosomes. Cell. 1998;93:423–432. doi: 10.1016/s0092-8674(00)81170-8. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hirst J, Bright NA, Rous B, Robinson MS. Characterization of a fourth adaptor-related protein complex. Mol Biol Cell. 1999;10:2787–2802. doi: 10.1091/mbc.10.8.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst J, Robinson MS. Clathrin and adaptors. Biochim Biophys Acta. 1998;1404:173–193. doi: 10.1016/s0167-4889(98)00056-1. [DOI] [PubMed] [Google Scholar]
- Honing S, Sandoval IV, von Figura K. A di-leucine-based motif in the cytoplasmic tail of LIMP-II and tyrosinase mediates selective binding of AP-3. EMBO J. 1998;17:1304–1314. doi: 10.1093/emboj/17.5.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H, Fukuda Y, Murata K, Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky DJ, Emr SD. Membrane protein sorting: biosynthesis, transport and processing of yeast vacuolar alkaline phosphatase. EMBO J. 1989;8:2241–2250. doi: 10.1002/j.1460-2075.1989.tb08348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneur F, Klausner RD. A novel di-leucine motif and a tyrosine-based motif independently mediate lysosomal targeting and endocytosis of CD3 chains. Cell. 1992;69:1143–1157. doi: 10.1016/0092-8674(92)90636-q. [DOI] [PubMed] [Google Scholar]
- Longtine MS, McKenzie A, 3rd, Demarini DJ, Shah NG, Wach A, Brachat A, Philippsen P, Pringle JR. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 1998;14:953–961. doi: 10.1002/(SICI)1097-0061(199807)14:10<953::AID-YEA293>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Marks MS, Ohno H, Kirchhausen T, Bonifacino JS. Protein sorting by tyrosine-based signals. Trends Cell Biol. 1997;7:124–128. doi: 10.1016/S0962-8924(96)10057-X. [DOI] [PubMed] [Google Scholar]
- Nakamura N, Hirata A, Ohsumi Y, Wada Y. Vam2/Vps41p and Vam6/Vps39p are components of a protein complex on the vacuolar membranes and involved in the vacuolar assembly in the yeast Saccharomyces cerevisiae. J Biol Chem. 1997;272:11344–11349. doi: 10.1074/jbc.272.17.11344. [DOI] [PubMed] [Google Scholar]
- Newman LS, McKeever MO, Okano HJ, Darnell RB. Beta-NAP, a cerebellar degeneration antigen, is a neuron-specific vesicle coat protein. Cell. 1995;82:773–783. doi: 10.1016/0092-8674(95)90474-3. [DOI] [PubMed] [Google Scholar]
- Nichols BJ, Ungermann C, Pelham HRB, Wickner WT, Haas A. Homotypic vacuolar fusion mediated by t- and v-SNAREs. Nature. 1997;387:199–202. doi: 10.1038/387199a0. [DOI] [PubMed] [Google Scholar]
- Odorizzi G, Cowles CR, Emr SD. The AP-3 complex: a coat of many colors. Trends Cell Biol. 1998;8:282–288. doi: 10.1016/s0962-8924(98)01295-1. [DOI] [PubMed] [Google Scholar]
- Ooi CE, Dell'Angelica EC, Bonifacino JS. ADP-ribosylation factor 1 (ARF1) regulates recruitment of the AP-3 adaptor complex to membranes. J Cell Biol. 1998;142:391–402. doi: 10.1083/jcb.142.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paravicini G, Horazdovsky BF, Emr SD. Alternative pathways for the sorting of soluble vacuolar proteins in yeast: a vps35 null mutant missorts and secretes only a subset of vacuolar hydrolases. Mol Biol Cell. 1992;3:415–427. doi: 10.1091/mbc.3.4.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan HL, Finlay JA, Chu DS, Tan PK, Kirchhausen T, Payne GS. The Saccharomyces cerevisiae APS1 gene encodes a homolog of the small subunit of the mammalian clathrin AP-1 complex: evidence for functional interaction with clathrin at the Golgi complex. EMBO J. 1994;13:1706–1717. doi: 10.1002/j.1460-2075.1994.tb06435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper RC, Bryant NJ, Stevens TH. The membrane protein alkaline phosphatase is delivered to the vacuole by a route that is distinct from the VPS-dependent pathway. J Cell Biol. 1997;138:531–545. doi: 10.1083/jcb.138.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price A, Seals D, Wickner W, Ungermann C. The docking stage of yeast vacuole fusion requires the transfer of proteins from a cis-SNARE complex to a Rab/Ypt protein. J Cell Biol. 2000a;148:1231–1238. doi: 10.1083/jcb.148.6.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price A, Wickner W, Ungermann C. Proteins needed for vesicle budding from the Golgi complex are also required for the docking step of homotypic vacuole fusion. J Cell Biol. 2000b;148:1223–1229. doi: 10.1083/jcb.148.6.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radisky DC, Snyder WB, Emr SD, Kaplan J. Characterization of VPS41, a gene required for vacuolar trafficking and high-affinity iron transport in yeast. Proc Natl Acad Sci USA. 1997;94:5662–5666. doi: 10.1073/pnas.94.11.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehling P, Darsow T, Katzmann DJ, Emr SD. Formation of AP-3 transport intermediates requires Vps41 function. Nat Cell Biol. 1999;1:346–353. doi: 10.1038/14037. [DOI] [PubMed] [Google Scholar]
- Rehling P, Marzioch M, Niesen F, Wittke E, Veenhuis M, Kunau WH. The import receptor for the peroxisomal targeting signal 2 (PTS2) in Saccharomyces cerevisiae is encoded by the PAS7 gene. EMBO J. 1996;15:2901–2913. [PMC free article] [PubMed] [Google Scholar]
- Rieder SE, Emr SD. A novel RING finger protein complex essential for a late step in protein transport to the yeast vacuole. Mol Biol Cell. 1997;8:2307–2327. doi: 10.1091/mbc.8.11.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts CJ, Raymond CK, Yamashiro CT, Stevens SH. Methods for studying the yeast vacuole. Meth Enzymol. 1991;194:644–661. doi: 10.1016/0076-6879(91)94047-g. [DOI] [PubMed] [Google Scholar]
- Robinson MS. Coats and vesicle budding. Trends Cell Biol. 1997;7:99–102. doi: 10.1016/S0962-8924(96)10048-9. [DOI] [PubMed] [Google Scholar]
- Robinson MS. The role of clathrin, adaptors, and dynamin in endocytosis. Curr Opin Cell Biol. 1994;6:538–544. doi: 10.1016/0955-0674(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Rose MD, Winston F, Hieter P. Methods in yeast genetics: a laboratory course manual, vol. 11–18. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1990. [Google Scholar]
- Sato TK, Rehling P, Peterson MR, Emr SD. Class C Vps protein complex regulates vacuolar SNARE pairing and is required for vesicle docking/fusion. Mol Cell. 2000;6:661–671. doi: 10.1016/s1097-2765(00)00064-2. [DOI] [PubMed] [Google Scholar]
- Saxena K, Gaitatzes C, Walsh MT, Eck M, Neer EJ, Smith TF. Analysis of the physical properties and molecular modeling of Sec13: a WD repeat protein involved in vesicular traffic. Biochemistry. 1996;35:15215–15221. doi: 10.1021/bi961616x. [DOI] [PubMed] [Google Scholar]
- Seals DF, Eitzen G, Margolis N, Wickner WT, Price A. A Ypt/Rab effector complex containing the Sec1 homolog Vps33p is required for homotypic vacuole fusion. Proc Natl Acad Sci USA. 2000;97:9402–9407. doi: 10.1073/pnas.97.17.9402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shestopal SA, Makunin IV, Belyaeva ES, Ashburner M, Zhimulev IF. Molecular characterization of the deep orange (dor) gene of Drosophila melanogaster. Mol Gen Genet. 1997;253:642–648. doi: 10.1007/s004380050367. [DOI] [PubMed] [Google Scholar]
- Sikorski RS, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson F, Peden AA, Christopoulou L, Robinson MS. Characterization of the adaptor-related protein complex, AP-3. J Cell Biol. 1997;137:835–845. doi: 10.1083/jcb.137.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer S, Schekman R. Nucleation of COPII vesicular coat complex by endoplasmic reticulum to Golgi vesicle SNAREs. Science. 1998;281:698–700. doi: 10.1126/science.281.5377.698. [DOI] [PubMed] [Google Scholar]
- Stack JH, Horazdovsky B, Emr SD. Receptor-mediated protein sorting to the vacuole in yeast. Annu Rev Cell Dev Biol. 1995;11:1–33. doi: 10.1146/annurev.cb.11.110195.000245. [DOI] [PubMed] [Google Scholar]
- Stepp JD, Huang K, Lemmon SK. The yeast adaptor protein complex, AP-3, is essential for the efficient delivery of alkaline phosphatase by the alternate pathway to the vacuole. J Cell Biol. 1997;139:1761–1774. doi: 10.1083/jcb.139.7.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Haar E, Harrison S C, Kirchhausen T. Peptide-in-groove interactions link target proteins to the beta-propeller of clathrin [see comments] Proc Natl Acad Sci USA. 2000;97:1096–1100. doi: 10.1073/pnas.97.3.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Haar E, Musacchio A, Harrison S C, Kirchhausen T. Atomic structure of clathrin: a beta propeller terminal domain joins an alpha zigzag linker. Cell. 1998;95:563–573. doi: 10.1016/s0092-8674(00)81623-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vida TA, Emr SD. A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J Cell Biol. 1995;128:779–792. doi: 10.1083/jcb.128.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vowels JJ, Payne GS. A dileucine-like sorting signal directs transport into an AP-3-dependent, clathrin-independent pathway to the yeast vacuole [published erratum appears in EMBO J. 1998;17(14):4211] EMBO J. 1998;17:2482–2493. doi: 10.1093/emboj/17.9.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner TS, Sinclair DA, Fitzpatrick KA, Singh M, Devlin RH, Honda BM. The light gene of Drosophila melanogaster encodes a homologue of VPS41, a yeast gene involved in cellular-protein trafficking. Genome. 1998;41:236–243. [PubMed] [Google Scholar]
- Wurmser AE, Sato TK, Emr SD. New component of the vacuolar class C-Vps complex couples nucleotide exchange on the Ypt7 GTPase to SNARE-dependent docking/fusion. J Cell Biol. 2000;151:551–562. doi: 10.1083/jcb.151.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ybe JA, Brodsky FM, Hofmann K, Lin K, Liu SH, Chen L, Earnest TN, Fletterick RJ, Hwang PK. Clathrin self-assembly is mediated by a tandemly repeated superhelix. Nature. 1999;399:371–375. doi: 10.1038/20708. [DOI] [PubMed] [Google Scholar]
- Yeung BG, Phan HL, Payne GS. Adaptor complex-independent clathrin function in yeast. Mol Biol Cell. 1999;10:3643–3659. doi: 10.1091/mbc.10.11.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]