Abstract
The Kit receptor tyrosine kinase functions in hemato– poiesis, melanogenesis and gametogenesis. Kit receptor-mediated cellular responses include proliferation, survival, adhesion, secretion and differentiation. In mast cells, Kit-mediated recruitment and activation of phosphatidylinositol 3′–kinase (PI 3–kinase) produces phosphatidylinositol 3′–phosphates, plays a critical role in mediating cell adhesion and secretion and has contributory roles in mediating cell survival and proliferation. To investigate the consequences in vivo of blocking Kit-mediated PI 3–kinase activation we have mutated the binding site for the p85 subunit of PI 3–kinase in the Kit gene, using a knock-in strategy. Mutant mice have no pigment deficiency or impairment of steady-state hematopoiesis. However, gametogenesis is affected in several ways and tissue mast cell numbers are affected differentially. While primordial germ cells during embryonic development are not affected, KitY719F/KitY719F males are sterile due to a block at the premeiotic stages in spermatogenesis. Furthermore, adult males develop Leydig cell hyperplasia. The Leydig cell hyperplasia implies a role for Kit in Leydig cell differentiation and/or steroido– genesis. In mutant females follicle development is impaired at the cuboidal stages resulting in reduced fertility. Also, adult mutant females develop ovarian cysts and ovarian tubular hyperplasia. Therefore, a block in Kit receptor-mediated PI 3–kinase signaling may be compensated for in hematopoiesis, melano– genesis and primordial germ cell development, but is critical in spermatogenesis and oogenesis.
Keywords: eKit receptor tyrosine kinase/Leydig cells/oogenesis/PI 3-kinase/spermatogenesis
Introduction
The receptor tyrosine kinase Kit and its only known ligand, Kit Ligand (KL), are encoded at the White spotting (W) and Steel (Sl) loci in the mouse, respectively (Chabot et al., 1988; Geissler et al., 1988; Copeland et al., 1990; Huang et al., 1990; Zsebo et al., 1990). Phenotypes of W and Sl mutations suggest roles for Kit signaling in melanogenesis, hematopoiesis and gametogenesis (Russell, 1979; Silvers, 1979). Many alleles of variable severity at both the W and Sl loci have been described and characterized (Besmer, 1997). W mutations either abolish or partially impair Kit receptor function. Whereas Kit receptor/W point mutations affect gametogenesis, melanogenesis and hematopoiesis during embryogenesis and in postnatal development to similar degrees, in contrast, mutations that affect Kit or KL expression may affect cellular targets of W and Sl mutations differentially. In hematopoiesis Kit function is critical in the stem cell hierarchy and in the erythroid and mast cell lineages; mutant animals have macrocytic anemia and lack tissue mast cells (Galli et al., 1994; Besmer, 1997). During embryonic development melanoblasts migrate from the neural crest to the periphery. They then enter the epidermal ectoderm and colonize hair follicles. Postnatally, amelanotic melanoblasts differentiate to become melanocytes. W and Sl mutations affect several aspects of melanogenesis, the early migratory phase in embryonic development, the time when melanoblasts reach the epidermal ectoderm as well as differentiated melanocytes in hair follicles causing differing degrees of white spotting (Silvers, 1979; Besmer et al., 1993).
The Kit receptor plays roles in Kit-mediated signaling events in primordial germ cells (PGCs), spermatogenesis and oogenesis (Bachvarova et al., 1993; Besmer et al., 1993). PGCs are derived from the posterior primitive streak and then migrate from the base of the allantois through the hindgut endoderm and the mesentery to the genital ridges; spermatogenesis and oogenesis then proceed following distinct, well studied developmental programs. During embryonic development the Kit receptor is expressed in PGCs from embryonic day 7.5 (E7.5) to E13.5 (Manova and Bachvarova, 1991). In the testis Kit expression starts at postnatal day 5 (P5) and is restricted to differentiating type A spermatogonia, type B spermatogonia, primary spermatocytes and Leydig cells (Manova et al., 1990; Yoshinaga et al., 1991). In the ovary the Kit receptor is expressed in primordial oocytes and growing oocytes throughout follicle development and in interstitial theca cells (Manova et al., 1990; Horie et al., 1991; Yoshinaga et al., 1991). KL is expressed in the microenvironment of Kit expressing cells along the migratory path of PGCs and in the gonads, in Sertoli cells of the testis and granulosa cells at all stages of follicle maturation. W and Sl mutations affect the survival, migration and proliferation of PGCs. The mutations also affect steps in spermatogenesis and oogenesis including the survival and proliferation of spermatogonia and oocyte growth, causing impaired fertility (Bennett, 1956; Kuroda et al., 1988; Nakayama et al., 1988; Bachvarova et al., 1993). In W and Sl mutant mice that lack Kit receptor function PGCs fail to proliferate and migrate, and none or only a few reach the gonad. In weak W and Sl alleles, effects on spermatogenesis and oogenesis are observed as well. In W/Wv mice only a few germ cells reach the gonad, and subsequent spermatogenesis is more severely affected than oogenesis. The KL expression mutations Slpanda, Slt and Slcon impair female fertility, while males are fertile. In contrast, a cytoplasmic domain mutation of the membrane growth factor KL, Sl17H, does not impair female fertility, but causes male sterility.
Taken together, the defects in W and Sl mutant mice are consistent with a role of the Kit receptor system in facilitating cell proliferation and survival of precursor cells as well as promoting cell migration, cell adhesion, secretion and other functions in differentiated cells (Galli et al., 1994; Besmer, 1997).
How does the Kit receptor mediate these diverse cellular responses in distinct cell populations during embryonic development and in the adult animal? Studies in bone marrow derived mast cells (BMMCs) have provided insight into the mechanism by which Kit mediates various cellular responses including cell proliferation, survival, adhesion, actin reorganization, membrane ruffling and secretion. Kit receptor activation leads to autophosphorylation, the phosphorylation of various substrates and the association with signaling molecules, thereby activating distinct signaling cascades. Molecules known to associate with the Kit receptor in vivo include the p85 subunit (p85α and p85β) of class IA phosphatidylinositol 3′–kinases (PI 3–kinases) (Blume-Jensen et al., 1994; Serve et al., 1994; Herbst et al., 1995), phospholipase Cγ–1 (Reith et al., 1991; Rottapel et al., 1991), the Grb2 adaptor protein, the Src kinase (Blume-Jensen et al., 1994) and the tyrosine phosphatases SHP1 and SHP2 (Yi and Ihle, 1993), but not the adaptor protein Shc and the exchange factor Nck (Blume-Jensen et al., 1994). In addition, Kit receptor activation causes phosphorylation and activation of the Shc adaptor protein (Cutler et al., 1993), Ras (Duronio et al., 1992) and the Vav GDP/GTP exchange factor (Alai et al., 1992). In BMMCs, mutation of the Kit receptor binding site for class IA PI 3–kinase adaptor proteins, KitY719, and for src, KitY567, was shown to affect cell proliferation, survival, adhesion and secretion to differing degrees (Serve et al., 1995; Vosseller et al., 1997; Timokhina et al., 1998). Whereas Kit-mediated PI 3–kinase activation contributes to the mitogenic and survival response in BMMCs, in the secretory response, cell adhesion response, actin polymerization and membrane ruffling responses, Kit-mediated PI 3–kinase activation is critical. Activation of PI 3–kinase results in the rapid accumulation of phosphatidylinositol-3,4–bisphosphate (PI 3,4–P2) and phosphatidylinositol-3,4,5–trisphosphate (PI 3,4,5–P3). PI 3,4–P2 and PI 3,4,5–P3 are important second messengers regulating the catalytic activity of downstream signaling molecules via binding to pleckstrin homology (PH) and FYVE domains, thereby mediating activation of a wide array of downstream targets including the protein kinases PDK1, Akt, PKCδ and the small GTPase Rac1 (Carpenter and Cantley, 1996; Toker and Cantley, 1997; Fruman et al., 1999a).
In order to investigate the mechanism of Kit receptor signaling and to elucidate the consequences of blocking signaling events mediated by the phosphorylation of Kit Tyr719 and the subsequent activation of PI 3–kinase in vivo, we mutated Tyr719 in the Kit receptor gene by substituting it with phenylalanine (Y719F) in the mouse genome by using a knock-in strategy. Analysis of homo– zygous mutant KitY719F/KitY719F mice reveals effects of the mutation on spermatogenesis, resulting in male sterility, on ovarian follicle development, severely impairing follicle maturation in females, and the development of hyperplastic changes in both the male and the female. This indicates an essential role for Kit Tyr719 and Kit-induced PI 3–kinase activity in mouse gametogenesis. Furthermore, an effect of the mutation is seen on peritoneal mast cell numbers but not on skin tissue mast cell numbers. Other Kit expressing lineages show no obvious phenotypes, suggesting that the cellular context is of great importance for the interpretation of the signal. Kit-induced PI 3–kinase activity in these tissues either is not required or can be compensated for most likely through synergy with other growth factor receptors.
Results
Point mutation in the Kit receptor (KitY719F) abolishes PI 3–kinase signaling in vivo
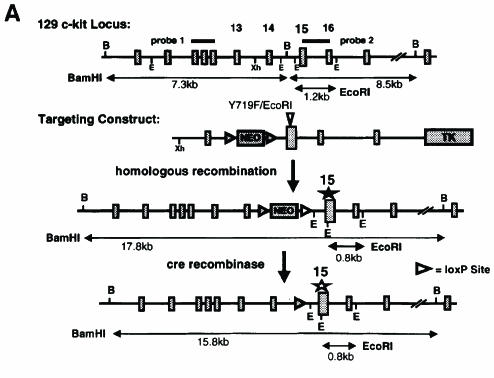
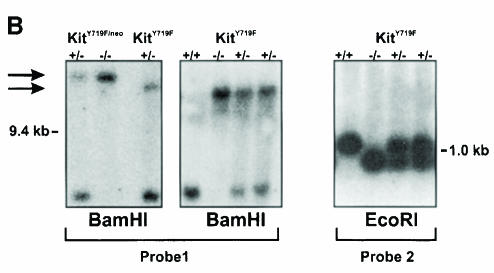
To gain insight into the role of Kit-induced PI 3–kinase activity in vivo we substituted tyrosine residue 719 of the c-kit gene with phenylalanine (Y719F) by using knock-in gene targeting technology (Figure 1). The Y719F mutation prevents the binding of SH2 domain proteins including the p85 subunit of PI 3–kinase and abolishes subsequent signaling events (Serve et al., 1994, 1995; Vosseller et al., 1997; Timokhina et al., 1998). In the targeting construct shown in Figure 1A the Y719F mutation in exon 15 produced a restriction site for EcoRI, thereby allowing easy identification of the mutant allele (Figure 1A). The neomycin resistance gene in intron 14 was flanked by two loxP sites for subsequent removal in vivo. Homologous replacement in ES cells produced neomycin-resistant colonies and seven correctly targeted ES cell clones were identified by Southern blot analysis. Two of these clones produced chimeric animals after injection into C57BL/6J blastocysts and successfully contributed to the germline, resulting in heterozygous KitY719F–neo/+ animals after mating with C57BL/6J mice (Figure 1B).
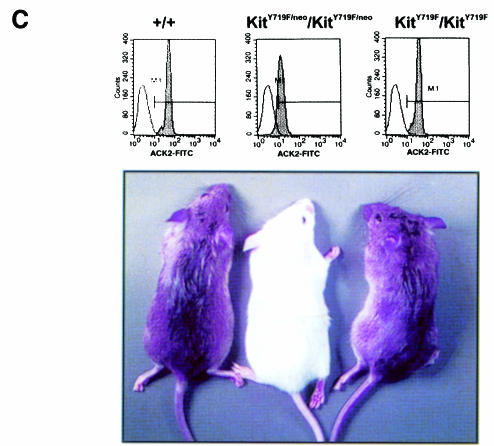
Fig. 1. Targeted mutation of Tyr719 in the 129/Sv Kit locus does not affect pigmentation in KitY719F/KitY719F mice. (A) Schematic representation of targeting strategy. B, BamHI; E, EcoRI; Xh, XhoI. LoxP sites are indicated by triangles. (B) Southern blot analysis of tail tips using BamHI and EcoRI digestion [see (A) for detail]. (C) Determination of cell surface expression of Kit in KitY719F/KitY719F, KitY719F/neo/KitY719F/neo and control BMMCs using FACS. The pigmentation phenotype of +/+, KitY719F/neo/KitY719F/neo and KitY719F/KitY719F mice is shown in the bottom panel.
It was previously shown that the inclusion of a neo gene in intronic sequences may interfere with the expression of the associated gene (McDevitt et al., 1997). KitY719F–neo/+ heterozygous mice displayed a pigmentation phenotype similar to the original W mutation, showing a white belly spot, white tail tips and white feet. Intercrossing of these mice produced KitY719F–neo/KitY719F–neo homozygous mutant mice. These mice were black-eyed whites and in addition displayed other characteristics of W mutant mice, including mast cell deficiency and male and female infertility. Analysis of Kit expression levels by fluorescence-activated cell sorter (FACS) in BMMCs revealed a 75% reduction compared with Kit expression levels in BMMCs from wild-type litter mates (Figure 1C). Therefore, placement of the neo expression cassette in intron 14 of the c-kit gene produced a hypomorphic allele. In order to obtain normal Kit expression levels in vivo we excised the neo expression cassette that is flanked by loxP sites by crossing the KitY719F–neo/+ mice with mice expressing the Cre recombinase under the direction of a suitable promoter. Heterozygous KitY719F–neo/+ mice were mated with transgenic mice in which expression of Cre was directed by the adenovirus EIIa promotor. In EIIa cre mice expression of the recombinase is restricted to the zygote stage of development (Lakso et al., 1996). Loss of the neo gene in the F1 generation was confirmed by Southern blotting using a BamHI restriction digest (probe 1; Figure 1B) and reprobing these filters with a neo-specific probe (not shown). Heterozygous KitY719F/+ mice were then intercrossed to generate homozygous mutant KitY719F/KitY719F animals. The presence of the Y719F point mutation was confirmed by digestion with EcoRI and Southern blotting (probe 2; Figure 1B). Excision of the neo cassette restored Kit expression levels in KitY719F/KitY719F BMMCs to wild-type levels, as shown by FACS analysis (Figure 1C).
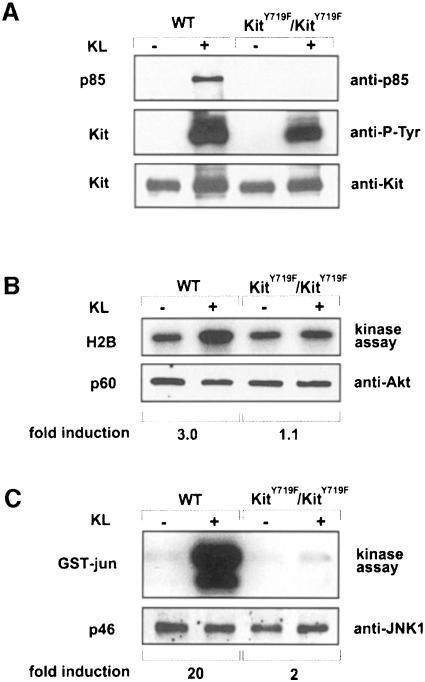
In order to establish that association of the p85 subunit of PI 3–kinase with the activated Kit receptor is abolished in BMMCs obtained from KitY719F/KitY719F mice, mutant and normal BMMCs were stimulated with KL/Mgf, cell extracts were prepared, and the Kit receptor was immunoprecipitated, fractionated by SDS–PAGE and immunoblotted using anti-p85 antibody. As shown in Figure 2A, no association of p85 with the activated KitY719F receptor was detected. In addition, blots were reprobed with anti-phosphotyrosine antibody. While both mutant and wild-type receptors were autophosphorylated in response to KL/Mgf (Figure 2A), phosphorylation levels in KitY719F/KitY719F BMMCs were slightly reduced, in agreement with published results (Timokhina et al., 1998).
Fig. 2.KL/Mgf-stimulated activation of Akt and JNK in KitY719F/KitY719F BMMCs. (A) Phosphorylation of Kit receptors in KitY719F/KitY719F BMMCs in response to KL/Mgf and association with the p85 subunit of PI 3–kinase. Cells were starved for 12 h in serum-free medium and then stimulated with KL/Mgf for 5 min. Middle panel: Kit proteins were immunoprecipitated, fractionated by SDS–PAGE and blotted with anti-phosphotyrosine antibody. Upper panel: membranes were stripped and reblotted with the anti-p85 antibody. Lower panel: Kit protein levels are shown. (B) KitY719F/KitY719F and wild-type BMMCs were starved in a serum-free medium for 12 h, followed by stimulation with KL/Mgf for 5 min. Cells were lysed and an Akt in vitro kinase assay was performed using histone H2B as a substrate. Lower panel: Akt protein (p60) levels are shown. (C) KitY719F/KitY719F and wild-type BMMCs were starved in a serum-free medium for 12 h and subsequently stimulated with KL/Mgf for 15 min and a JNK in vitro kinase assay was performed using GST–Jun fusion protein as a substrate. Lower panel: JNK1 protein (p46) levels are shown. The relative phosphorylation was quantitated using a phosphoimage analyzer (Fuji Mac Bas).
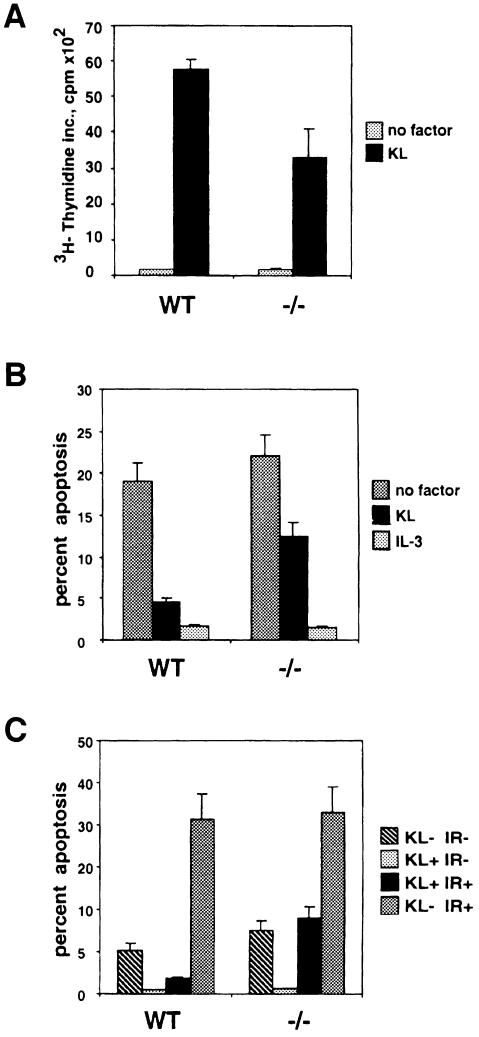
Next, KL/Mgf-stimulated activation of Akt and JNK in KitY719F/KitY719F BMMCs was analyzed. Kit-induced activation of Akt and JNK was determined using in vitro kinase assays with histone H2B and glutathione S–transferase (GST)–Jun proteins as substrates, respectively. Similarly to the results obtained with Kit negative Wsh/Wsh BMMCs reconstituted with mutant KitY719F receptor (our unpublished results), the PI 3–kinase binding site mutation abolished KL/Mgf-stimulated Akt activation (Figure 2B). However, reduction of KL/Mgf-stimulated JNK activity by the PI 3–kinase binding site mutation was somewhat stronger in BMMCs derived from the KitY719F/KitY719F mice (∼90% inhibition) (Figure 2C) compared with that observed previously in reconstituted BMMCs (Timokhina et al., 1998). Finally, KL/Mgf-induced proliferation and suppression of both irradiation- and deprivation-induced apoptosis in KitY719F/KitY719F BMMCs was evaluated (Figure 3). In all of the cellular assays, i.e. cell cycle progression determined by thymidine incorporation, protection from apoptosis by growth factor deprivation and irradiation, KitY719F/KitY719F BMMCs exhibited a 40–60% reduced response to KL/Mgf, in agreement with previous observations made in Kit negative BMMCs transduced to express KitY719F (Timokhina et al., 1998). Therefore, our results indicate critical roles for Kit-induced PI 3–kinase activity in mediating these mast cell responses, presumably by activating the Akt and Rac–JNK signaling pathways.
Fig. 3. Kit-mediated proliferation and suppression of apoptosis are affected in KitY719F/KitY719F BMMCs. (A) Proliferation. KitY719F/KitY719F and wild-type BMMCs were pretreated in a serum-free medium containing IL–3 (20 ng/ml) for 12 h, then starved for 1 h without factors; KL/Mgf (200 ng/ml) or IL–3 (20 ng/ml) was added and after 24 h [3H]thymidine incorporation was determined as described. (B) Deprivation-induced apoptosis. KitY719F/KitY719F and wild-type BMMCs were pretreated as in (A), deprived of growth factors for 50 h and analyzed as described. (C) Irradiation-induced apoptosis. KitY719F/KitY719F and wild-type BMMCs were pretreated as in (A), subjected to γ–irradiation (25 Gy) or left untreated in the presence or absence of KL/Mgf (200 ng/ml). After 24 h cells were harvested and analyzed for apoptosis.
Melanogenesis and steady-state hematopoiesis are normal in mutant mice
Intercrossing of KitY719F/+ heterozygous mice resulted in the expected distribution of genotypes (+/+, 28.9%, n =124; KitY719F/+, 46.2%, n = 198; KitY719F/KitY719F, 24.9%, n = 107), indicating that all mice were equally viable. The sex ratio of KitY719F/KitY719F mutants was not affected (50.5% male, n = 52; 49.5% female, n = 51). All mice developed normally and appeared healthy. No pigmentation phenotype is seen in KitY719F/KitY719F mutant mice. In contrast, homozygous mutant KitY719F–neo/KitY719F–neo mice are depigmented. The depigmentation phenotype in the KitY719F–neo/KitY719F–neo mice is comparable to that seen in mice heterozygous for the dominant-negative KitW42 allele (Tan et al., 1990) and this is in agreement with the notion that the KitY719F–neo allele is a hypomorph (Figure 1C).
In hematopoiesis, KL/Mgf and Kit have multiple roles. In mice carrying W mutations, effects are seen in erythropoiesis, the stem cell compartment and in mast cells (Galli et al., 1994; Besmer, 1997). Interestingly, hematocrit values and red blood cell, white blood cell, granulocyte and platelet numbers in mutant mice did not deviate from normal (Table I). These results suggest that Kit-induced PI 3–kinase signaling does not have an apparent role in melanogenesis and steady-state hematopoiesis.
Table I. Peripheral blood cell counts in +/+, KitY719F/+ and KitY719F/KitY719F mice.
| Genotype | Hematocrit (%) | RBC × 108/mm3 | WBC × 103/mm3 | ANC × 103/mm3 | PLT × 106/mm3 |
|---|---|---|---|---|---|
| +/+ (n = 5) | 51.2 ± 0.47 | 254 ± 19.5 (n = 2) | 6940 ± 2467 | 1390 ± 592 | 1.36 ± 025 |
| KitY719F/+ (n = 6) | 51.7 ± 5.08 | 291 (n = 1) | 6466 ± 700 | 1000 ± 357 | 1.55 ± 0.31 |
| KitY719F/KitY719F (n = 10) | 52.2 ± 2.2 | 275 ± 5.5 (n = 3) | 7190 ± 2777 | 1180 ± 940 | 1.4 ± 0.26 |
RBC, red blood cells; WBC, white blood cells; ANC, absolute neutrophil count; PLT, platelets.
Tissue mast cell numbers in mutant mice are differentially affected
W mutations affect the mast cell lineage (Kitamura et al., 1978; Galli et al., 1994; Besmer, 1997). Mutation of the Kit PI 3–kinase binding site, KitY719F, in BMMCs has been shown to affect several cellular responses that are mediated by stimulation of BMMCs with KL/Mgf, including adhesion to fibronectin, enhancement of IgE-triggered secretion, cell survival and proliferation (Serve et al., 1995; Vosseller et al., 1997; Timokhina et al., 1998). Interestingly, in KitY719F/KitY719F mice, mast cell numbers in dorsal skin sections were not reduced (Table II). In contrast, analysis of peritoneal mast cell numbers showed a 3.5–fold reduction in the mutant mice. Therefore, the KitY719F mutation exerts differential effects on mast cell development and/or survival in the peritoneum and the skin.
Table II. Mast cell numbers in the skin and peritoneum of +/+ and KitY719F/KitY719F mice.
| Genotype | Age (weeks) | Skin mast cells (CTMC/cm) | Peritoneal mast cells (% of total peritoneal cells) |
|---|---|---|---|
| +/+ | 10–16 | 325 ± 56 (n = 4) | 3.58 ± 1.69 (n = 6) |
| KitY719F/KitY719F | 10–16 | 300 ± 46 (n = 3) | 0.98 ± 0.64 (n = 5) |
| +/+ | 32 | 199 ± 18 (n = 2) | |
| KitY719F/KitY719F | 32 | 223 ± 13 (n = 2) |
Primordial germ cells are not affected by the mutation
Typically, W mutations affect male and female fertility (Besmer et al., 1993). The number of PGCs reaching the gonadal ridges is affected by W mutations, indicating an important role for Kit in survival, proliferation and/or migration of PGCs (Buehr et al., 1993). In the fetal gonad, Kit expression correlates with the proliferative state of both female and male germ cells (Manova and Bachvarova, 1991). At ∼E13.5 Kit expression ceases. Cessation of Kit expression coincides with germ cell entry into meiosis for the female and with entry into a quiescent phase for the male. To examine a possible effect of the KitY719F mutation on PGCs we compared the number of germ cells in the gonadal ridges of normal and homozygous mutant embryos at E12.5, a time when the majority of PGCs have completed the migratory phase. No differences were observed in KitY719F/KitY719F and wild-type mice when hematoxylin and eosin (H&E)-stained sections were compared (McLaren and Southee, 1997) (data not shown).
As a second approach to evaluate the number of PGCs, newborn ovaries were examined (Manova et al., 1990; Huang et al., 1993). Female germ cells undergo the last round of DNA replication at E13.5 before entering the first meiotic division. They pass through the leptotene, zygotene and pachytene stages and then arrest at the diplotene stage around the time of birth. Therefore, reduced numbers of germ cells at the time of birth may reflect reduced numbers of PGCs reaching the gonadal ridge. Again, examination of H&E-stained sections of three KitY719F/KitY719F and four heterozygous control ovaries at P0 did not reveal any differences (not shown). In contrast to other W mutations then, the KitY719F mutation does not affect the survival, proliferation and migration of PGCs.
Spermatogenesis is blocked at premeiotic stages in mutant mice
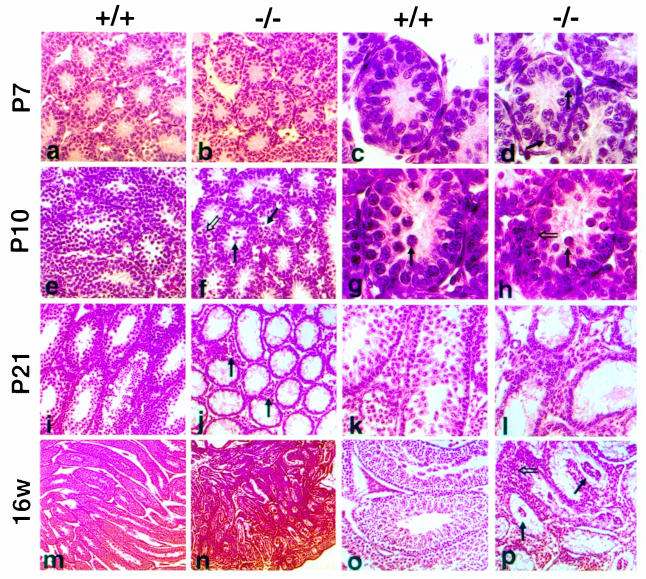
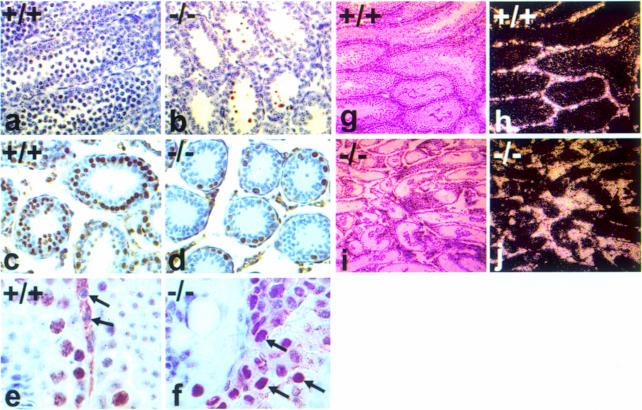
In the postnatal murine testis Kit expression is observed starting at P5 and is restricted to differentiating type A (A1–A4) and type B spermatogonia, preleptotene spermatocytes and the interstitial Leydig cells (Manova et al., 1990; Yoshinaga et al., 1991). Detailed histological analysis of P6 and P7 KitY719F/KitY719F mutant testis did not show any noticeable difference between mutant and control animals (Figure 4a–d). The seminiferous tubules have similar numbers of germ cells and the majority of spermatogonia have migrated to the periphery. Comparable frequencies of mitotic figures reflecting germ cell and Sertoli cell proliferation in normal and mutant tubules were observed. However, a striking difference between mutant (n = 6) and control testis [wild type (wt) n = 4 and heterozygotes n = 4] was evident at P10 (Figure 4e–h). In wild-type testis the cellularity of tubules is increased as a result of the proliferation of spermatogonia and the onset of meiosis, as shown by H&E staining and Ki–67 immunohistochemistry (Figures 4e and i and 5c). Ki–67 is a nuclear antigen that is expressed exclusively in the S, G2 and M phases of the cell cycle (Schluter et al., 1993). In contrast, we failed to observe germ cells that had entered meiosis in mutant testis at P10 (Figure 4g and h). Overall, the number of proliferating cells was significantly reduced and the thickness of the wall of the seminiferous epithelium of the mutant testis resembled that of immature tubules of wt animals at P6–P7. In conjunction with reduced proliferation of spermatogonia, an increase in the frequency of apoptotic germ cells was observed by the TUNEL method (Figure 5a and b). As a consequence, mutant tubules were rapidly depleted and their diameter did not increase compared with wild type. At P21 only a few germ cells remained at the basal membrane of the seminiferous tubules (Figure 4i–l). This phenotype was quite uniform in all mutant animals.
Fig. 4. Histological analysis of postnatal testis in KitY719F/KitY719F mice. Paraffin sections obtained from mutant (b, d, f, h, j, l, n and p) and wt (a, c, e, g, i, k, m and o) testis were stained with H&E. P7 (a and b) and (c and d); closed arrows identify spermatogonia at the basal membrane of tubules (d). Six mutant and four control animals were analyzed at P6/P7. At P10 no meiotic cells are found in mutant testis (e–h). The closed arrow in (g) identifies a primary spermatocyte. In mutant testis, spermatogonia at different stages of development are present. An open arrow identifies dividing spermatogonia in (f) and type A spermatogonia in (h). Mutant testis shows an increased frequency of apoptotic cells (closed arrows in f and h). At P10/P12 four mutant and three control animals were analyzed. At P21 KitY719F/KitY719F seminiferous tubules are empty and only a few germ cells are at the base of tubules (i–l). An increase in the interstitial space in mutant testis is detectable (closed arrows in j). At P21 two mutants and one control were analyzed. Adult KitY719F/KitY719F testis (16w) are significantly smaller than controls (m and n). Clusters of cells in the center of seminiferous tubules (closed arrows in p) are prominent and the interstitial space is enlarged by hyperplastic Leydig cells (open arrow in p). Five mutant adults and three controls were analyzed. Magnifications are 5× (m and n), 20× (i, j, o and p), 40× (a, b, e, f, k and l) and 100× (c, d, g and h).
Fig. 5. Identification of mitotically active and apoptotic germ cells in KitY719F/KitY719F and wt testis. TUNEL analysis was performed on paraffin sections of P10 wt (a) and mutant (b) testis. Ki–67 staining specific for proliferating cells was performed on paraffin sections of wt (c and e) and mutant (d and f) testis at P12 (c and d) and 16 weeks (e and f). Closed arrows in (e) and (f) show nuclei of Leydig cells in wt (e) and KitY719F/KitY719F (f) testis. Expression of Kit mRNA in 16 week wt and KitY719F/KitY719F testis examined by in situ hybridization (g–j). Dark field images of wt and KitY719F/KitY719F testis (g and i) show uniform Kit expression in the interstitial space (white grains). Corresponding bright field images are shown (h and j). Magnifications are 20× (a, b, c and d), 100× (e and f) and 10× (g, h, i and j).
The rapid depletion of the juvenile testis suggests an effect of the KitY719F mutation during the spermatogonial stages. However, at P10 some differentiating type A1–A4 spermatogonia were still observed (Figure 4h). The absence of meiotic cells and the localization of germ cells containing apoptotic bodies toward the lumen of the tubules might indicate either a block in the differentiation from type A to type B spermatogonia or failure of type B spermatogonia to initiate meiosis and form primary spermatocytes. Thus, it seems possible that the KitY719F mutation might affect more than one cellular response during the spermatogonial stages to cause the observed block in germ cell development. These observations indicate an absolute requirement for Kit-induced PI 3–kinase signaling during the early stages of spermatogenesis.
In the adult mutant testis we observed clusters of cells in the center of the seminiferous tubules (Figure 4p). Some of the cells in the clusters show germ cell characteristics. High magnification microscopy revealed intercellular bridges between cells within the clusters. In addition, individual cells in clusters were found to express Kit mRNA, a hallmark of spermatogonia (not shown). However, they do not express either KL/Mgf or AMH as determined by in situ hybridization and they do not express the mitosis-specific Ki–67 epitope (Figures 5f and 6c and g). It is possible that disruption of spermatogenesis in the mutant causes shedding of germ cells into the semini– ferous lumen.
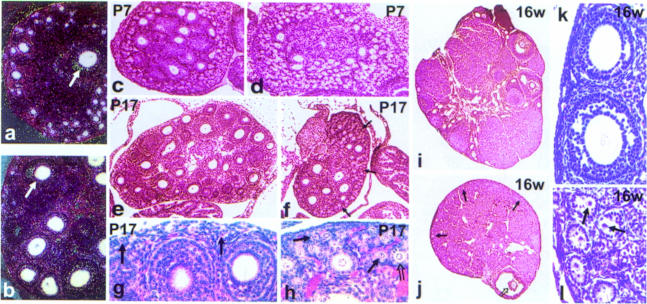
Fig. 6. Histological analysis of postnatal ovaries in KitY719F/KitY719F mice. Kit mRNA expression in wt and mutant ovaries was determined by in situ hybridization. The mutation does not affect Kit mRNA expression in oocytes of P17 ovaries and comparable levels of Kit are observed in oocytes of KitY719F/KitY719F (a) and wt (b) ovaries. In the dark field images (a and b) closed arrows show similar hybridization in antral follicles. Paraffin-embedded sections obtained from KitY719F/KitY719F (d, f, h, j and l) and wt (c, e, g, i and k) ovaries were stained with H&E. Follicle development in KitY719F/KitY719F mutant females is delayed at the cuboidal stages and the number of mature follicles is greatly reduced. At P7 there are fewer growing follicles in mutant ovaries (d) compared with wt (c). At P17 (e, f, g and h) an increase in the number of primordial/primary type 2 (closed arrow) and type 3a (open arrow) follicles is seen in the cortex of the ovary of mutant mice compared with wt. Growing follicles in KitY719F/KitY719F ovaries are typically surrounded by one or two layers of granulosa cells (f and h). A few follicles escape the defect and mature to antral stages (open arrow in j), as seen in the adult ovary (16 weeks) (i, j, k and l). Oocytes in the cortex of mutant ovaries are degenerated, acentric and follicle development is arrested at the type 3b stage (l). Follicles in the cortex of wt ovary are shown in (k). Solid arrows identify primordial and primary follicles in (j) and (l). The sections shown are representative of eight mutant and eight control animals at the juvenile stages and of six adult mutant and three adult control animals. Magnifications are 5× (i and j), 10× (e and f), 20× (a, b, c and d) and 40× (g, h, k and l).
Leydig cell hyperplasia in adult mutant mice
Adult mutant testes (20–24 weeks) are markedly reduced in size [0.0235 ± 0.003 g (n = 6) for KitY719F/KitY719F compared with 0.118 ± 0.03 g (n = 4) for controls], whereas the overall morphology of the reproductive tract appears normal and the size of the seminal vesicles is comparable to wild type (not shown). The seminiferous tubules in mutant adults have an aberrant structure, only a few germ cells remain at the basal membrane and spermatogonia could be identified occasionally (Figure 4m–p). The interstitial space in adult KitY719F/KitY719F testis is disproportionately increased and filled out with Leydig cells (Figure 4p). A first sign of the expansion of Leydig cells in the interstitial space is evident at P21. This increase of Leydig cell numbers does not appear to be caused by a significant reduction in the size of the seminiferous tubules but rather by mitotically active Leydig cells. In agreement with this hypothesis, Leydig cells in mutant testis were stained uniformly with the mitosis-specific antibody Ki–67 (Figure 5e and f). This phenotype was observed in all adult males (n = 5) examined. Despite the Leydig cell hyperplasia no tumor development in mutant males has been observed.
In situ hybridization of adult wild-type and KitY719F/KitY719F testis using a Kit-specific hybridization probe revealed significant Kit expression in interstitial Leydig cells but no Kit expression was detected in seminiferous tubules of mutant testis (Figure 5g–j). Examination of KL/Mgf expression by in situ hybridization at 16 weeks showed increased levels of KL/Mgf mRNA in Sertoli cells of mutant seminiferous tubules when compared with wild type (not shown). It has been reported previously that KL/Mgf mRNA expression in germ cell-depleted testis of Sld/Sld mice is elevated (Motro et al., 1991).
To analyze the functional state of the hyperplastic Leydig cells we determined serum testosterone and luteinizing hormone (LH) levels using radioimmunoassay (RIA) in adult mutant and control animals (Table III). While no significant differences in the testosterone levels in KitY719F/KitY719F and wild-type animals were found, LH levels were increased >7–fold in mutant animals. These results may be explained as a disruption in the negative feedback regulation of the hypothalamus and pituitary by androgen.
Table III. Serum levels of testosterone and luteinizing hormone (LH) in +/+ and KitY719F/KitY719F males.
| Genotype | Testosterone (ng/ml) | LH (ng/ml) |
|---|---|---|
| +/+ | 3.283 ± 1.703 (n = 5) | 0.137 ± 0.087 (n = 4) |
| KitY719F/KitY719F | 4.208 ± 1.586 (n = 6) | 1.022 ± 0.264 (n = 10) |
Values represent the mean ± SE. Statistical significance compared with +/+ controls was determined using Mann–Whitney U values with p ⩽0.05. Values were p = 0.357 for testosterone and p = 0.008 for LH.
Follicle development in mutant mice is impaired at the cuboidal stages
In the ovary, Kit expression is observed in oocytes from the time of birth until ovulation as well as in theca cells (Manova et al., 1990; Horie et al., 1991; Yoshinaga et al., 1991). Interaction of Kit with KL/Mgf expressed by granulosa cells is known to be essential for follicle development (Kuroda et al., 1988; Huang et al., 1993). We confirmed expression of Kit and KL/Mgf in mutant ovaries by in situ hybridization. Comparable levels of Kit or KL/Mgf expression were observed in KitY719F/KitY719F and wild-type ovaries (Figure 6a and b and not shown).
In juvenile KitY719F/KitY719F ovaries, follicle development is impaired and appears to be delayed when compared with wild-type ovaries (Figure 6). Analysis of H&E-stained paraffin sections at P7 (Figure 6c and d), P10 (not shown) and P17 (Figure 6e–h) showed greater numbers of small follicles in the cortex of the ovary than in wild-type controls. Some of these follicles are surrounded by a single layer of follicle cells [types 2 and 3a, respectively, according to the nomenclature of Peters and Pedersen (1967)], others contain a bigger ring of follicle cells (type 3b), and only very few follicles with two or more layers of follicle cells (types 4, 5a and 5b) are present in the ovarian medulla of the mutants. As a result of limited recruitment of follicles for growth, the number of pre-antral and antral follicles is greatly reduced (Figure 6e–h), as is the size of the entire mutant ovary. Adult mutant ovaries contain only occasional large and Graafian follicles (types 6–8, respectively) (Figure 6i and j), whereas the cortex is enriched with abnormal small follicles (types 2–3a) (Figure 6k and l). The follicle cells have lost their concentric orderly arrangement and the oocytes are not centrally placed and show signs of degeneration (arrow in Figure 6l). In contrast to the complete arrest in spermatogonial development in mutant mice, ovarian follicle development is not completely blocked, indicating that KitY719F mutation does not affect the later stages in follicle development. Superovulation experiments using adult females produced ova, although in low numbers, thus confirming the notion that later stages in oogenesis are not affected by the mutation (not shown). However, fertility of KitY719F/KitY719F females is greatly reduced.
Ovarian hyperplasia in adult KitY719F/KitY719F females
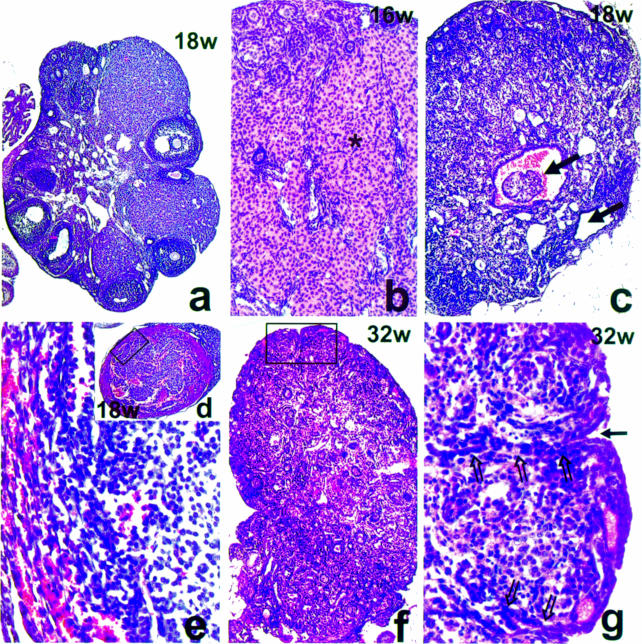
With increasing age there is a gradual depletion of follicles in KitY719F/KitY719F ovaries and pronounced abnormal development. At 6 weeks very few follicles continue to grow and develop into Graafian follicles in mutant ovaries. Pathological changes are evident with groups of small follicles showing irregularly shaped oocytes and the emergence of cyst-containing structures, possibly the remains of Graafian follicles (not shown). By 16–18 weeks interstitial tissue composed mostly of luteal-like cells occupies almost the entire ovary (Figure 7b). Only portions of the ovarian cortex contain follicles and follicle-like structures (Figure 7b and c). Anovular small follicles with disarranged granulosa cells are predominant. They represent a mixture of granulosa and interstitial-like cells. Gradually these follicle-like structures are fused into large masses by invasion of surrounding luteinized tissue. In addition, ovarian cysts develop in mutant females and in one case an encapsulated, hemorrhagic ovarian tumor was identified (Figure 7c–e). Analysis of Kit and KL/Mgf expression profiles by in situ hybridization showed regions of strong staining for both Kit and KL/Mgf in the tumor (not shown). This may suggest that the tumor originates from Kit expressing interstitial thecal or KL/Mgf expressing granulosa cells, and that Kit/KL might play an active role in ovarian tumor development. By 32 weeks mutant ovaries consist of a mixture of somatic cells surrounding disorganized groups of interstitial and luteal-like cells (Figure 7f). Invading tubules of the germinal epithelium penetrated the disorganized ovarian tissue (Figure 7g). Finally, by 16 months, complex tubular adenomas, similar to the ones described by Murphy in Wx/Wv, Wj/Wv and Wv/Wv mice were observed (data not shown) (Murphy, 1972). This phenotype was observed in all samples (n = 7) analyzed. These data imply that Kit-mediated PI 3–kinase signaling is indispensable for proper oocyte growth and development and its absence presumably leads to an abnormal relationship between oocyte and granulosa cell.
Fig. 7. Identification of ovarian cysts and tubular hyperplasia in adult KitY719F/KitY719F ovaries. H&E staining of paraffin sections of 18–week-old wt (a) and mutant ovaries at 16 (b), 18 (c–e) and 32 weeks (f and g) are shown. Only portions of the ovarian cortex at 16–18 weeks contained follicles and follicle-like structures (b and c). Large regions of the ovary are occupied by luteinized interstitial cells (asterisk in b). The oocyte-depleted ovaries have a high incidence in the development of ovarian cysts (closed arrows in c) and in one case an ovarian tumor has been observed (d); detail of the tumor (rectangle) is shown at high magnification in (e). At 32 weeks the ovary represents a mixture of follicle-like structures and somatic cells surrounding disorganized groups of interstitial and luteal-like cells (f). Invagination of the germinal epithelium (g, closed arrow) and tubular hyperplasia can be observed (f and g). Open arrows in (g) show invading epithelial cells. Magnifications are 5× (a), 10× (c, d and f), 20× (b) and 40× (e and g).
Discussion
In an attempt to understand the biological roles of PI 3–kinase signaling mediated by the Kit receptor we have characterized the phenotypes of mice expressing a mutant Kit receptor (KitY719F, obtained by a knock-in strategy) that fails to interact with PI 3–kinase. Whereas the Y719F mutation blocks the direct binding by Kit of the p85 regulatory subunit of PI 3–kinase and its activation, we cannot exclude the possibility that this mutation blocks other activating or inactivating interactions as well. The KitY719F mutation affects Kit function only in specific developmental processes and this is in contrast to other mutations in the Kit receptor gene that broadly affect Kit function in hematopoiesis, gametogenesis and melano– genesis. Thus, the mutation fails to affect steady-state hematopoiesis and tissue mast cell numbers, but substantially reduces peritoneal mast cell numbers. Furthermore, although Kit has important functions at multiple stages in embryonic and postnatal gametogenesis, our results show that PI 3–kinase signaling is critical only in a specific subset of the postnatal stages in the ovary and testis. We conclude, therefore, that in most cells that require Kit, there are redundant signaling pathways, but that in certain cell types the PI 3–kinase pathway is critical.
In vivo, in hematopoietic and melanogenic cell types and in skin mast cells the mutant defect may be compensated for by parallel Kit signaling pathways and/or other cytokine- or growth factor-mediated signaling involving PI 3–kinase and/or other mechanisms. Because of the intricate intracellular signaling networks, absence of a signal to activate or produce a second messenger may be redundantly produced by another cytokine- or growth factor-mediated signaling cascade. It is possible that the diversity of class IA PI 3–kinases contributes to this redundancy. For instance, there are at least three alternative catalytic subunits: p110α, β and δ, and an equal number of p85 adapter subunits: p85α, p85β and p85γ (Domin and Waterfield, 1997). Expression of some PI 3–kinase subunit isoforms is cell type specific and this may contribute to cell type-specific dependence on Kit activation, whereas others are expressed more ubiquitously. Consequently, mutations of p85 subunit and PI 3–kinase isoforms are expected to produce rather broad mutant phenotypes. In fact, recently it was shown that mutation of the p85α subunit of PI 3–kinase produced a perinatal phenotype in mutant mice (Fruman et al., 1999b; Suzuki et al., 1999). In contrast, our analysis of the phenotype of KitY719F/KitY719F mice was designed to investigate a specific subset of PI 3–kinase functions, those mediated by Kit receptor activation.
Another question concerns the downstream Kit/PI 3–kinase signaling pathways, which are crucial in different cell types affected by the KitY719F mutation. The phospholipid products of PI 3–kinase, PI 3,4–P2 and PI 3,4,5–P3, have pleiotropic roles in distinct signaling processes mediated primarily through the interaction of the phospholipids with PH and FYVE domain-containing proteins (Toker and Cantley, 1997; Rameh and Cantley, 1999). Our previous in vitro analysis of Kit signaling in BMMCs showed that downstream targets of phospholipid products produced by Kit-activated PI 3–kinase included Akt, Rac, JNK and PKCδ (Vosseller et al., 1997; Timokhina et al., 1998; I.Timokhina, unpublished; K.Vosseller, unpublished). Importantly our characterization of signaling in BMMCs obtained from KitY719F/KitY719F mice confirmed our previous findings. In contrast, downstream signaling mediated by Kit and PI 3–kinase in other cell types is less clear. In primary oocytes of cuboidal follicles perhaps Kit signaling through PI 3–kinase, Rac and JNK may activate transcription factors involved in the expression of growth factors critical in mediating follicle growth. In spermatogonia, Kit signaling through PI 3–kinase, Akt and suppression of the pro-apoptotic functions of Bad or the forkhead transcription factor FKHRL1 may be critical for cell survival (Datta et al., 1997, 1999; Brunet et al., 1999). Furthermore, signaling through PI 3–kinase, Rac and Jnk may be critical for proliferation of spermatogonia. However, in Leydig cells it is difficult to speculate about mechanisms for Kit signaling through PI 3–kinase, as a role for Kit in this cell type is not yet understood.
Although no defects were observed in steady-state hematopoiesis, skin tissue mast cells and melanogenesis in mutant animals, it is possible that the mutation affects stress hematopoiesis and/or homing properties of hematopoietic progenitors and possibly functional mast cell responses. A more detailed analysis of such questions is in progress. Many naturally occurring murine W and human Piedbald mutations have been identified (Besmer, 1997). However, mutations affecting Tyr719 of the Kit receptor have never been observed. In the light of our results this is not surprising: all W and Piedbald mutations have an easily observed pigmentation phenotype, while the KitY719 mutation apparently does not affect melano– genesis and instead may be associated more with infertility.
The effects of the KitY719F mutation on gametogenesis are quite impressive. Whereas analysis of gonads during embryonic development and in the newborn indicates no effect of the mutation on PGC proliferation and survival, strong effects are seen both on spermatogenesis and oogenesis. Since PGCs are not affected by the mutation and normal numbers of germ cells are present at the time of birth, mutant phenotypes in the postnatal gonads could be analyzed in detail. Two lines of evidence suggest a role for Kit signaling in follicle development. First, in Slpan mutant mice a severe phenotype is observed with follicle development arrested at the one-layered cuboidal stage (Huang et al., 1993). Secondly, in vivo use of antagonistic anti-Kit antibody suggests multiple roles for Kit in follicle development, at the one-layered cuboidal stage, the stage of follicular fluid formation of pre-antral follicles and at the penultimate stage of ovarian follicle development prior to ovulation (Yoshida et al., 1997). In the KitY719F/KitY719F mutant mice follicle development is delayed and impaired at the one- to three-layered cuboidal stages; however, the block is not complete and some follicles mature to become antral and ovulatory follicles. Thus, the KitY719F mutation affects early follicle growth and development but not the later stages. Furthermore, compared with the Slpan mutation the developmental block of the KitY719F mutation may take effect a little later, possibly because of Kit signaling pathways that are not affected by the mutation. The impairment/delay in follicle development at the cuboidal stages suggests a defect in the communication between germ cell and somatic cell (Bachvarova et al., 1993; Huang et al., 1993). Thus, Kit signaling may control the production of factor(s) involved in granulosa cell differentiation and proliferation. GDF–9 and BMP–15 are two transforming growth factor-β family growth and differentiation factors produced by oocytes that are thought to mediate proliferation and differentiation of granulosa cells in follicles (Dong et al., 1996; Dube et al., 1998). In fact GDF–9 function is required for follicle development and in mice carrying a GDF–9 loss-of-function mutation follicle development is arrested at the cuboidal stage (Dong et al., 1996). It had been proposed previously that Kit may control the expression of GDF–9 because of the similar mutant phenotypes of S1pan and GDF–9 knock-out mice (Yoshida et al., 1997). However, no significant differences in GDF–9 and BMP–15 mRNA expression levels determined by in situ hybridization were observed between control and KitY719F/KitY719F juvenile ovaries (G.Rothschild, H.Kissel and K.Manova, unpublished observation). Local autocrine and paracrine factors play key roles in pre-antral follicle development until hormonal mechanisms determine folliculogenesis (Gougeon, 1996; Eppig et al., 1997; Vanderhyden and Macdonald, 1998), and pre-antral oocytes from 12–day-old ovaries have been shown to secrete factors that prevent premature steroidogenic differentiation of granulosa cells (Vanderhyden and Tonary, 1995; Vanderhyden and Macdonald, 1998). In oocytes the KitY719F mutation could impair the production of such an inhibitory signal. As such, KitY719F/KitY719F ovaries contain anovular follicles with disorganized granulosa cells and interstitial cells.
Of interest also is the observation of ovarian cysts and tubular hyperplasia in KitY719F/KitY719F ovaries. This is reminiscent of Wx/Wv mice that have been shown to develop hyperplasia of the rete ovarii and complex tubular adenomas (Murphy, 1972). KitY719F/KitY719F ovaries are depleted of maturing follicles at an early age and hyper– plastic changes of the interstitium and the germinal epithelium can be detected. The expression of Kit and KL/Mgf in the ovarian tumor may suggest a role for granulosa and/or thecal cell hyperplasia and furthermore the absence of functional oocytes may upregulate steroidogenesis and contribute to the hyperplastic changes observed (Murphy and Beamer, 1973; Risma et al., 1995). At this time a functional relationship between gonadotropic hormones and Kit/KL signaling is not understood, although LH has been shown to downregulate Kit expression in theca and interstitial tissue without affecting Kit expression in oocytes (Motro and Bernstein, 1993). KL/Mgf expression is also found in some theca and interstitial cells (Motro and Bernstein, 1993). However, whether or not Kit or KL/Mgf plays an active role in the hyperplastic changes of the interstitium and in ovarian tumorigenesis remains to be determined.
In the postnatal testis the Kit receptor is expressed starting at P4–P6 in gonocytes and persists until the preleptotene spermatocyte stage (Manova et al., 1990; Yoshinaga et al., 1991). Several lines of evidence have suggested an essential role for Kit receptor signaling in spermatogenesis. Tubules of chimeric Sl/Sld // +/+ mice contain both differentiated and depleted tubule sections, possibly due to the lack of differentiation of spermatogonia in patches of Sl/Sld tubules (Nakayama et al., 1988). Regenerative differentiation after surgical reversal of Sl and W mutant cryptorchid testis was impaired at the level of proliferation and differentiation from spermatogonia A to B and at meiotic division (Nishimune et al., 1980; Koshimizu et al., 1991). A defect in germ cell differentiation is also evident in Sl17H mutant males (Brannan et al., 1992) and after administration of Kit receptor blocking antibody (Yoshinaga et al., 1991; Packer et al., 1995). Results from in vitro studies may suggest a role for Kit at the time of gonocyte migration from the center of tubules to the basal membrane at P4–P6 (Orth et al., 1997). Furthermore, progression of purified spermatocytes through meiosis mediated by the Sertoli cell line 15P is Kit dependent (Vincent et al., 1998). Our analysis of testis from KitY719F/KitY719F mice indicates normal numbers of germ cells at the time of birth and normal development until P6 and P7 when the majority of spermatogonia have migrated basally. This is important since studies in the rat have suggested a role in this early migratory phase of gonocytes from the adluminal compartment to the basal membrane (Orth et al., 1997). Subsequently, development in the mutant mice is impaired and at P10 the cellularity of tubules is reduced. There is a concomitant increase in the frequency of apoptotic germ cells and mutant germ cells fail to enter meiosis. Thus, tubules are rapidly depleted causing sterility. Accordingly, Kit-mediated PI 3–kinase signaling is critical for the development of male germ cells during the premeiotic stages. Whether the failure to enter meiosis may be attributed to a block in the proliferation and survival of spermatogonia, the differentiation of spermatogonia from type A to type B or failure of type B spermatogonia or preleptotene spermatocytes to initiate meiosis as reported by Vincent et al. (1998) is difficult to discern at this time. It is noteworthy that some type A spermatogonia are observed on P10 but the number of proliferating cells as determined by Ki–67 staining is reduced compared with controls. This may imply that the mutation affects both survival and proliferation of type A spermatogonia. Furthermore, the increase in apoptosis occurs when preparation for meiosis is under way, in agreement with the notion that the dying cells are primary spermatocytes.
Interstitial Leydig cells have a critical endocrine function in spermatogenesis and are the primary source of testicular testosterone. Leydig cells develop postnatally from mesenchymal progenitor cells and they proliferate and differentiate to form immature Leydig cells prepubertally (Ge et al., 1996). By the end of puberty, immature rat Leydig cells have divided once and have terminally differentiated into adult Leydig cells. Postnatal mouse Leydig cells express high levels of Kit receptor and Kit expression is maintained throughout postnatal life (Manova et al., 1990; Yoshinaga et al., 1991). However, little is known about a role for Kit in Leydig cell development and/or function. In mutant KitY719F/KitY719F mice starting at P21 Leydig cells are increased in number and they continue to proliferate as indicated by Ki–67 staining. This is in contrast to normal Leydig cells, which have a very low mitotic index. In adult mutant testis the interstitial space is expanded and filled out by proliferating Leydig cells. The Leydig cell hyperplasia may arise either from a cell autonomous effect of the mutant Kit receptor in Leydig cells or alternatively as the consequence of a secondary effect. Both AMH and AMH receptor knock-out mice develop Leydig cell hyperplasia at 10 weeks or older (Mishina et al., 1996). While AMH is expressed by Sertoli cells, the AMH receptor is expressed by Leydig cells (Racine et al., 1998; Lee et al., 1999). Thus, the Leydig cell hyperplasia suggests that AMH negatively regulates Leydig cell proliferation. While in KitY719F/KitY719F mice AMH expression in Sertoli cells is not appreciably affected (data not shown), KL/Mgf expression is upregulated in Sertoli cells. KL/Mgf over-expression in Sertoli cells therefore may stimulate mutant Kit receptors in Leydig cells inducing hyperplasia. Another issue concerns the endocrine function of the Leydig cells in the mutant mice. If the Leydig cells in adult mutant mice are mature, levels of serum testosterone should be higher than control, since Leydig cell numbers are increased greatly in these mice. However, serum testosterone levels are normal and LH serum levels are increased >7–fold. This could mean that on a per cell basis the Leydig cells in the mutant mice produce less testosterone than normal adult Leydig cells due to a failure to differentiate fully and/or to impairment of steroidogenesis. If the Leydig cells are deficient in androgen production prior to puberty, development of the normal feedback relationship will not occur and LH levels may become chronically elevated. Thus, the combination of hyperplasia and elevated LH levels caused by feedback as a result of subnormal testosterone levels may be responsible for the apparently normal testosterone levels in the adult mutant mice. Therefore, these results suggest that the Kit receptor may have a role in Leydig cell differentiation and/or steroidogenesis.
Materials and methods
Targeting construct to modify the 129/Sv Kit locus
An 18 kb fragment of the mouse c-kit gene (Gokkel et al., 1992) including exons 8–17 was isolated by screening a genomic 129/SvJ mouse library (ΘFixII; Stratagene) with a 32P-labeled PCR probe derived from the Kit kinase insert region. A replacement targeting vector was constructed using a 2.5 kb KpnI–BamHI fragment including exon 14 and a 4 kb BamHI fragment including exons 15–17. Site-directed mutagenesis was performed on a 0.6 kb BamHI–KpnI fragment containing exon 15, introducing the substitution mutation Y719F (GAATAT→ GAATTC) thereby creating a new EcoRI restriction site. A loxP flanked neomycin resistance gene expression cassette driven by the PGK promotor was isolated from the pKSloxPNT plasmid (kindly provided by A.Joyner, Skirball Institute, New York University Medical Center) inserted at a BamHI site in intron 14 thus deleting the restriction site. The presence of the Y719F mutation and exon 14–17 sequences in the targeting construct was confirmed by sequence analysis. A herpes simplex virus thymidine kinase expression cassette driven by polyoma enhancer sequences (Py-Tk) was attached to the long arm of homology to enrich for homlogous recombinants by negative selection using gancyclovir. The targeting construct was linearized using a unique NotI restriction site outside the region of homology for transfection of ES cells.
Isolation of recombinant ES cell clones and generation of KitY719F/KitY719F mutant mice
Transfection of ES cells (CJ7) with the targeting construct and ES cell culture were carried out essentially as recently described (Tajima et al., 1998b) following standard protocols. Recombinant ES cell clones were identified by Southern blot analysis using ES cell DNA digest with the restriction endonuclease BamHI and a 500 bp PCR-derived, 32P-labeled hybridization probe spanning exons 10–12 (probe 1; Figure 1A). In C57BL/6J and Balb/C DNA, probe 1 detects a polymorphic BamHI fragment of 5.5 kb instead of the 7.3 kb fragment found in 129/SvJ DNA. The presence of the mutation was confirmed by Southern blotting and digestion with EcoRI restriction using a 600 bp PCR-derived 32P probe including intron 15 (probe 2; Figure 1A). A total of 2400 W9.5 (Lau et al., 1994) and CJ7 (Swiatek and Gridley, 1993) ES cell clones were screened giving rise to five clones containing a correctly targeted allele carrying the mutation. One clone recombined correctly incorporating the neomycin cassette at the BamHI site but containing wt sequence at Y719.
Heterozygous mutant ES cells were injected into C57BL/6J blastocysts and chimeric males were backcrossed for germline transmission to C57BL/6J females. Two of the injected CJ7 clones successfully contri– buted to the germline and gave rise to KitY719F–neo/+ heterozygous animals (C57BL/6J × 129Sv). For excision of the neomycin resistance gene KitY719F–neo/+ heterozygous females were mated with EIIaCre (Lakso et al., 1996) transgenic males (BalbC) and the F1 generation was screened for loss of neo by using a BamHI restriction digest and Southern blotting (probe 1). Excision of the neo gene was confirmed by re-hybridizing the filters with a neo-specific probe and the presence of the Y719F point mutation by Southern blotting using EcoRI-digested DNA (probe 2). F1 KitY719F/+ heterozygous mice were then intercrossed to generate homozygous mutant KitY719F/KitY719F mice (C57BL/6J × 129Sv × BalbC).
Histological analysis and in situ hybridization
Testis and ovaries were dissected and fixed in Bouin's fixative at 4°C overnight. The tissues were dehydrated in rising concentrations of ethanol, embedded in paraffin, sectioned at 8 μm and stained in Gill's H&E. To evaluate mast cell numbers in the skin, dorsal skin pieces were briefly dried on a paper towel, fixed in Bouin's at 4°C overnight, dehydrated, embedded in paraffin, sectioned at 8 μm and stained with Toluidine Blue (pH 4) without H&E counterstaining to facilitate quantitation. The length of the counted section was determined and the number of cells/cm calculated.
RNA in situ hybridization, TUNEL method and Ki–67 staining
The tissues indicated were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) at 4°C overnight followed by ethanol dehydration the next day. Paraffin sections (8 μm) were prepared. Antisense probes were generated and hybridized essentially as described by Tomihara-Newberger et al. (1998). The following probes were used: Kit (Manova et al., 1990); KL/Mgf (Manova et al., 1993); GDF–9 (McGrath et al., 1995); and AMH (Mishina et al., 1996). The GDF–9 and AMH plasmids were kindly provided by Drs Se-Jin Lee and Robin Lovell-Badge.
For immunohistochemical analysis a Histomouse kit from Zymed (South San Francisco) was used following the manufacturer's instructions. Tissues were fixed in 4% paraformaldehyde or Bouin's fixative at 4°C overnight, dehydrated, embedded in paraffin and sectioned at 8 μm. Slides were deparaffinized, quenched in 1% H2O2 and washed in PBS. Antigen retrieval was performed for 10 min. After blocking, the primary antibody (1:200) was incubated in 2% bovine serum albumin (BSA)/PBS at 4°C overnight. The Ki–67 monoclonal antibody MM1 was from Novocastra Laboratories, Newcastle, UK.
For detection of apoptotic cells, tissues fixed in 4% paraformaldehyde and/or Bouin's were processed for the TUNEL reaction where biotinylated dUTP is added to DNA double-strand breaks using terminal transferase essentially as recently described (Manova et al., 1998). The procedure was based on the method of Gavrieli et al. (1992), with minor modifications (Manova et al., 1998).
Determination of peripheral blood parameters and mast cell numbers
Blood samples were drawn from the retro-orbital plexus or tail vein with a capillary pipet (Unopette; Becton-Dickinson, Rutherford, NJ). Platelet and white blood cell numbers were determined using a hemocytometer under a phase contrast microscope. The hematocrit was measured using a heparinized micro-hematocrit capillary tube (Fisher Scientific).
Determination of mast cell numbers in the skin of control and KitY719F/KitY719F mice was as described previously (Tajima et al., 1998a). Peritoneal mast cells were obtained from KitY719F/KitY719F and control mice by gentle lavage of the peritoneal cavity with 5 ml of PBS and mast cells were identified by staining with 0.1% Toluidine Blue.
Mast cell cultures
BMMCss from KitY719F/KitY719F and control mice were produced by culturing bone marrow in RPMI 1640 supplemented with 1 mM sodium pyruvate, 1 mM non-essential amino acids, 5.5 × 10–5 M β–mercaptoethanol, 0.075% sodium bicarbonate, 10% fetal bovine serum (RPMI complete) and 10% X63-derived interleukin (IL)–3 containing conditioned medium. Kit cell surface expression was monitored using anti-Kit monoclonal ACK2 antibody (1:60) coupled to fluorescein isothiocyanate (FITC) and FACS analysis.
Proliferation assay and determination of apoptosis
The proliferation assay was performed as described previously (Yee et al., 1994). Briefly, cells were starved of growth factors in complete RPMI for 12 h, 105 cells were seeded in 0.2 ml per well in triplicate in 96–well plates, followed by stimulation with KL/Mgf (200 ng/ml) or IL–3 (20 ng/ml) for 24 h. Cytokines were used at concentrations that produced a maximal proliferative response (data not shown). After 20 h, 0.5 μCi of [3H]thymidine was added for 4 h. Cells were harvested and 3H radioactivity was measured in a liquid scintillation counter.
For apoptosis assays 106 cells were seeded in 2 ml per well in six-well plates. After treatment with apoptotic stimuli as described in the figure legends, cells were collected into FACS tubes, washed once with Annexin V binding buffer (10 mM HEPES pH 7.4, 150 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, 0.5% BSA) and incubated for 30 min in 100 μl of binding buffer containing 10 μl of Annexin V– FITC (Biosource) in the dark. Cells were washed twice with the binding buffer without BSA, resuspended in PBS and analyzed by FACS. The fraction of green fluorescent cells represented the fraction of cells undergoing apoptosis.
JNK in vitro kinase assays
Cells (107) were collected in lysis buffer (50 mM HEPES pH 7.4, 10% glycerol, 150 mM NaCl, 1% Triton X–100, 0.5% deoxycholate, 1 mM EDTA, 1 mM EGTA, 50 μM ZnCl2, 25 mM NaF, 20 mM β–glycerophosphate, 50 μg/ml soybean trypsin inhibitor, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 1 mM phenylmethylsulfonyl fluoride and 1 mM sodium orthovanadate), cleared lysates were normalized for protein content and JNK was immunoprecipitated with antibodyprecoupled beads for 2 h at 4°C. The immunoprecipitates were washed three times in NP–40 buffer (1% NP–40, 2 mM sodium orthovanadate in PBS), once in LiCl buffer [10 mM Tris–HCl, 500 mM LiCl, 1 mM dithiothreitol (DTT)] and once in kinase buffer (12.5 mM MOPS pH 7.5, 12.5 mM β–glycerophosphate, 7.5 mM MgCl2, 0.5 mM EGTA, 0.5 mM NaF, 0.5 mM sodium orthovanadate, 1 mM DTT). JNK assays were performed as follows: 30 μl of kinase buffer containing 4 mg/ml GST–jun-1–135 (Verheij et al., 1996), 25 μM ATP and 1 μCi/ml [γ–32P]ATP were added to the beads and reactions were incubated for 20 min at 30°C. Reactions were stopped by addition of sample buffer and proteins fractionated by SDS–PAGE.
Akt in vitro kinase assay
Cells (107) were collected in a Triton X–100 lysis buffer containing 1 mM microcystin, cleared lysates were normalized for protein content and Akt proteins were immunoprecipitated with anti-Akt antibody-precoupled beads for 2 h at 4°C. Immunoprecipitates were washed three times with NP–40 buffer, once with LiCl and once in a kinase buffer (12.5 mM MOPS pH 7.5, 12.5 mM β–glycerophosphate, 5 mM MgCl2, 0.5 mM EGTA, 0.5 mM NaF, 0.5 mM sodium orthovanadate, 1 mM DTT), essentially as described previously (Franke et al., 1995), and Akt kinase assays were performed as follows: 30 μl of kinase buffer containing 4 mg/ml histone H2B (Boehringer Mannheim), 25 μM ATP and 1 μCi/ml [γ–32P]ATP were added to the beads and reactions were incubated for 20 min at 30°C. Reactions were stopped by addition of sample buffer and proteins fractionated by SDS–PAGE.
Immunoprecipitation and Western blotting
Cells were lysed in Triton X–100 lysis buffer, cleared lysates were normalized for protein content and proteins were immunoprecipitated with beads coupled with polyclonal antibodies specific for Kit, JNK and Akt for 2 h at 4°C. Immunoprecipitates were washed three times in lysis buffer, resuspended in sample buffer, boiled for 5 min, subjected to SDS–PAGE and transferred to nitrocellulose. The membranes were blocked overnight in TBS–Tween containing 5% non-fat milk or 5% BSA for anti-phosphotyrosine blotting. Membranes were incubated for 1 h with the following antibodies: anti-Kit polyclonal antibody (1:500), anti-phosphotyrosine antibody (1:1000), anti-phospho-JNK antibody (1:50) and anti-p85 antibody (1:500). Proteins were visualized by enhanced chemiluminescence (Pierce, Rockford, IL).
Measurement of serum LH and testosterone
Blood was allowed to stand at room temperature for 15 min. Serum was obtained by centrifugation of clotted blood and stored at –20°C until assay. The RIA for LH was performed according to a previously published procedure (Chandrashekar et al., 1988) using anti-rat LH antiserum (NIDDK-anti-rLH-S-11), rat LH standards (NIDDK-rLH-I-9) from the National Hormone and Pituitary Program, and radio-iodinated LH from Hazleton Washington (Catalog No. AG-0007; Vienna, VA). The tritium-based RIA for testosterone was performed as described previously (Cochran et al., 1981). The inter-assay variations of the LH and testosterone RIAs were 6.7 and 7.8%, respectively.
Acknowledgments
Acknowledgements
We would like to thank Dr Elizabeth Lacy for advice with the gene targeting experiments, Dr Alexandra Joyner for the pKSloxPNT plasmid, Dr Heiner Westphal for the EIIa-cre mice and Drs Robin Lovell-Badge and Se-Jin Lee for the AMH and GDF–9 probes, respectively. We would like to thank Drs John Eppig, Mary-Ann Handel and Rosemary Bachvarova for their help in evaluating testis and ovary sections and many insightful comments, Drs Carlos Cordon-Cardo and Patricia Saigo for their help with the evaluation of ovarian cysts and the Leydig cell hyperplasia, Dr Wolfram Ostertag for encouragement and Dr Dale Dorsett, Lee Niswander and Kathryn Anderson for constructive criticism of the manuscript. We would like to thank Dr Malcolm Moore and Harry Satterwhite for stimulating discussions and expert assistance with hematological determinations, Scott Kerns, Karen Witty and Alden Strock for help in various aspects of this study and Dr Keith Vosseller for discussions and encouragement. I.T. was supported by the Jack and Susan Rudin Educational and Scholarship Fund. This work was supported by grants from the National Institutes of Health, R37 CA32926 and HL/DK55748 (to P.B.).
References
- Alai M., Mui, A.L., Cutler, R.L., Bustelo, X.R., Barbacid, M. and Krystal, G. (1992) Steel factor stimulates the tyrosine phosphorylation of the proto-oncogene product, p95vav, in human hemopoietic cells. J. Biol. Chem., 267, 18021–18025. [PubMed] [Google Scholar]
- Bachvarova R.F., Manova,K. and Besmer,P. (1993) Role in gametogenesis of c-kit encoded at the W locus of mice. In Bernfield,M. (ed.), Molecular Basis of Morphogenesis. Wiley-Liss, New York, NY. [Google Scholar]
- Bennett D. (1956) Developmental analysis of a mutation with pleiotropic effects in the mouse. J. Morphol., 98, 199–229. [Google Scholar]
- Besmer P. (1997) Kit-ligand-stem cell factor. In Garland,J.M., Quesenberry,P.J. and Hilton,D.J. (eds), Colony-Stimulating Factors: Molecular and Cellular Biology. Marcel Dekker, New York, NY. [Google Scholar]
- Besmer P., Manova,K., Duttlinger,R., Huang,E.J., Packer,A., Gyssler,C. and Bachvarova,R.F. (1993) The kit-ligand (steel factor) and its receptor c-kit/W: pleiotropic roles in gametogenesis and melanogenesis. Dev. Suppl., 125–137. [PubMed] [Google Scholar]
- Blume-Jensen P., Ronnstrand, L., Gout, I., Waterfield, M.D. and Heldin, C.H. (1994) Modulation of Kit/stem cell factor receptor-induced signaling by protein kinase C. J. Biol. Chem., 269, 21793–21802. [PubMed] [Google Scholar]
- Brannan C.I. et al. (1992)Developmental abnormalities in Steel17H mice result from a splicing defect in the steel factor cytoplasmic tail. Genes Dev., 6, 1832–1842. [DOI] [PubMed] [Google Scholar]
- Brunet A. et al. (1999)Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell, 96, 857–868. [DOI] [PubMed] [Google Scholar]
- Buehr M., McLaren, A., Bartley, A. and Darling, S. (1993) Proliferation and migration of primordial germ cells in We/We mouse embryos. Dev. Dyn., 198, 182–189. [DOI] [PubMed] [Google Scholar]
- Carpenter C.L. and Cantley, L.C. (1996) Phosphoinositide kinases. Curr. Opin. Cell Biol., 8, 153–158. [DOI] [PubMed] [Google Scholar]
- Chabot B., Stephenson, D.A., Chapman, V.M., Besmer, P. and Bernstein, A. (1988) The proto-oncogene c-kit encoding a transmembrane tyrosine kinase receptor maps to the mouse W locus. Nature, 335, 88–89. [DOI] [PubMed] [Google Scholar]
- Chandrashekar V., Bartke, A. and Wagner, T.E. (1988) Endogenous human growth hormone (GH) modulates the effect of gonadotropin-releasing hormone on pituitary function and the gonadotropin response to the negative feedback effect of testosterone in adult male transgenic mice bearing human GH gene. Endocrinology, 123, 2717–2722. [DOI] [PubMed] [Google Scholar]
- Cochran R.C., Ewing, L.L. and Niswender, G.D. (1981) Serum levels of follicle stimulating hormone, luteinizing hormone, prolactin, testosterone, 5 α-dihydrotestosterone, 5 α-androstane-3 α, 17 β-diol, 5 α-androstane-3 β, 17 β-diol, and 17 β-estradiol from male beagles with spontaneous or induced benign prostatic hyperplasia. Invest. Urol., 19, 142–147. [PubMed] [Google Scholar]
- Copeland N.G., Gilbert, D.J., Cho, B.C., Donovan, P.J., Jenkins, N.A., Cosman, D., Anderson, D., Lyman, S.D. and Williams, D.E. (1990) Mast cell growth factor maps near the steel locus on mouse chromosome 10 and is deleted in a number of steel alleles. Cell, 63, 175–183. [DOI] [PubMed] [Google Scholar]
- Cutler R.L., Liu, L., Damen, J.E. and Krystal, G. (1993) Multiple cytokines induce the tyrosine phosphorylation of Shc and its association with Grb2 in hemopoietic cells. J. Biol. Chem., 268, 21463–21465. [PubMed] [Google Scholar]
- Datta S.R., Dudek, H., Tao, X., Masters, S., Fu, H., Gotoh, Y. and Greenberg, M.E. (1997) Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell, 91, 231–241. [DOI] [PubMed] [Google Scholar]
- Datta S.R., Brunet, A. and Greenberg, M.E. (1999) Cellular survival: a play in three Akts. Genes Dev., 13, 2905–2927. [DOI] [PubMed] [Google Scholar]
- Domin J. and Waterfield, M.D. (1997) Using structure to define the function of phosphoinositide 3-kinase family members. FEBS Lett., 410, 91–95. [DOI] [PubMed] [Google Scholar]
- Dong J., Albertini, D.F., Nishimori, K., Kumar, T.R., Lu, N. and Matzuk, M.M. (1996) Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature, 383, 531–535. [DOI] [PubMed] [Google Scholar]
- Dube J.L., Wang, P., Elvin, J., Lyons, K.M., Celeste, A.J. and Matzuk, M.M. (1998) The bone morphogenetic protein 15 gene is X-linked and expressed in oocytes. Mol. Endocrinol., 12, 1809–1817. [DOI] [PubMed] [Google Scholar]
- Duronio V., Welham, M.J., Abraham, S., Dryden, P. and Schrader, J.W. (1992) p21ras activation via hemopoietin receptors and c-kit requires tyrosine kinase activity but not tyrosine phosphorylation of p21ras GTPase-activating protein. Proc. Natl Acad. Sci. USA, 89, 1587–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppig J.J., Chesnel, F., Hirao, Y., O'Brien, M.J., Pendola, F.L., Watanabe, S. and Wigglesworth, K. (1997) Oocyte control of granulosa cell development: how and why. Hum. Reprod., 12, 127–132. [PubMed] [Google Scholar]
- Franke T.F., Yang, S.I., Chan, T.O., Datta, K., Kazlauskas, A., Morrison, D.K., Kaplan, D.R. and Tsichlis, P.N. (1995) The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell, 81, 727–736. [DOI] [PubMed] [Google Scholar]
- Fruman D.A., Rameh, L.E. and Cantley, L.C. (1999a) Phosphoinositide binding domains: embracing 3-phosphate. Cell, 97, 817–820. [DOI] [PubMed] [Google Scholar]
- Fruman D.A., Snapper, S.B., Yballe, C.M., Davidson, L., Yu, J.Y., Alt, F.W. and Cantley, L.C. (1999b) Impaired B cell development and proliferation in absence of phosphoinositide 3-kinase p85α. Science, 283, 393–397. [DOI] [PubMed] [Google Scholar]
- Galli S.J., Zsebo, K.M. and Geissler, E.N. (1994) The kit ligand, stem cell factor. Adv. Immunol., 55, 1–96. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y., Sherman, Y. and Ben-Sasson, S.A. (1992) Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J. Cell Biol., 119, 493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge R.S., Shan,L.X. and Hardy,M.P. (1996) Pubertal development of Leydig cells. In Payne,A.H., Hardy,M.P. and Russell,L.D. (eds), The Leydig Cell. Cache River Press, Vienna, IL, pp. 159–173. [Google Scholar]
- Geissler E.N., Ryan, M.A. and Housman, D.E. (1988) The dominant-white spotting (W) locus of the mouse encodes the c-kit proto-oncogene. Cell, 55, 185–192. [DOI] [PubMed] [Google Scholar]
- Gokkel E., Grossman, Z., Ramot, B., Yarden, Y., Rechavi, G. and Givol, D. (1992) Structural organization of the murine c-kit proto-oncogene. Oncogene, 7, 1423–1429. [PubMed] [Google Scholar]
- Gougeon A. (1996) Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr. Rev., 17, 121–155. [DOI] [PubMed] [Google Scholar]
- Herbst R., Shearman, M.S., Jallal, B., Schlessinger, J. and Ullrich, A. (1995) Formation of signal transfer complexes between stem cell and platelet-derived growth factor receptors and SH2 domain proteins in vitro.Biochemistry, 34, 5971–5979. [DOI] [PubMed] [Google Scholar]
- Horie K., Takakura, K., Taii, S., Narimoto, K., Noda, Y., Nishikawa, S., Nakayama, H., Fujita, J. and Mori, T. (1991) The expression of c-kit protein during oogenesis and early embryonic development. Biol. Reprod., 45, 547–552. [DOI] [PubMed] [Google Scholar]
- Huang E.J., Nocka, K., Beier, D.R., Chu, T.Y., Buck, J., Lahm, H.W., Wellner, D., Leder, P. and Besmer, P. (1990) The hematopoietic growth factor KL is encoded by the Sl locus and is the ligand of the c-kit receptor, the gene product of the W locus. Cell, 63, 225–233. [DOI] [PubMed] [Google Scholar]
- Huang E.J., Manova, K., Packer, A.I., Sanchez, S., Bachvarova, R.F. and Besmer, P. (1993) The murine steel panda mutation affects kit ligand expression and growth of early ovarian follicles. Dev. Biol., 157, 100–109. [DOI] [PubMed] [Google Scholar]
- Kitamura Y., Go, S. and Hatanaka, K. (1978) Decrease of mast cells in W/Wv mice and their increase by bone marrow transplantation. Blood, 52, 447–452. [PubMed] [Google Scholar]
- Koshimizu U., Sawada, K., Tajima, Y., Watanabe, D. and Nishimune, Y. (1991) White-spotting mutations affect the regenerative differentiation of testicular germ cells: demonstration by experimental cryptorchidism and its surgical reversal. Biol. Reprod., 45, 642–648. [DOI] [PubMed] [Google Scholar]
- Kuroda H., Terada, N., Nakayama, H., Matsumoto, K. and Kitamura, Y. (1988) Infertility due to growth arrest of ovarian follicles in Sl/Slt mice. Dev. Biol., 126, 71–79. [DOI] [PubMed] [Google Scholar]
- Lakso M., Pichel, J.G., Gorman, J.R., Sauer, B., Okamoto, Y., Lee, E., Alt, F.W. and Westphal, H. (1996) Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc. Natl Acad. Sci. USA, 93, 5860–5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau M.M., Stewart, C.E., Liu, Z., Bhatt, H., Rotwein, P. and Stewart, C.L. (1994) Loss of the imprinted IGF2/cation-independent mannose 6-phosphate receptor results in fetal overgrowth and perinatal lethality. Genes Dev., 8, 2953–2963. [DOI] [PubMed] [Google Scholar]
- Lee M.M., Seah, S.C., Masiakos, P.T., Sottas, C.M., Preffer, F.I., Donahoe, P.K., Maclaughlin, D.T. and Hardy, M.P. (1999) Mullerian-inhibiting substance type II receptor expression and function in purified rat Leydig cells. Endocrinology, 140, 2819–2827. [DOI] [PubMed] [Google Scholar]
- Manova K. and Bachvarova, R.F. (1991) Expression of c-kit encoded at the W locus of mice in developing embryonic germ cells and presumptive melanoblasts. Dev. Biol., 146, 312–324. [DOI] [PubMed] [Google Scholar]
- Manova K., Nocka, K., Besmer, P. and Bachvarova, R.F. (1990) Gonadal expression of c-kit encoded at the W locus of the mouse. Development, 110, 1057–1069. [DOI] [PubMed] [Google Scholar]
- Manova K., Huang, E.J., Angeles, M., De Leon, V., Sanchez, S., Pronovost, S.M., Besmer, P. and Bachvarova, R.F. (1993) The expression pattern of the c-kit ligand in gonads of mice supports a role for the c-kit receptor in oocyte growth and in proliferation of spermatogonia. Dev. Biol., 157, 85–99. [DOI] [PubMed] [Google Scholar]
- Manova K. et al. (1998)Apoptosis in mouse embryos: elevated levels in pregastrulae and in the distal anterior region of gastrulae of normal and mutant mice. Dev. Dyn., 213, 293–308. [DOI] [PubMed] [Google Scholar]
- McDevitt M.A., Shivdasani, R.A., Fujiwara, Y., Yang, H. and Orkin, S.H. (1997) A ‘knockdown’ mutation created by cis-element gene targeting reveals the dependence of erythroid cell maturation on the level of transcription factor GATA-1. Proc. Natl Acad. Sci. USA, 94, 6781–6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath S.A., Esquela, A.F. and Lee, S.J. (1995) Oocyte-specific expression of growth/differentiation factor-9. Mol. Endocrinol., 9, 131–136. [DOI] [PubMed] [Google Scholar]
- McLaren A. and Southee, D. (1997) Entry of mouse embryonic germ cells into meiosis. Dev. Biol., 187, 107–113. [DOI] [PubMed] [Google Scholar]
- Mishina Y., Rey, R., Finegold, M.J., Matzuk, M.M., Josso, N., Cate, R.L. and Behringer, R.R. (1996) Genetic analysis of the Mullerian-inhibiting substance signal transduction pathway in mammalian sexual differentiation. Genes Dev., 10, 2577–2587. [DOI] [PubMed] [Google Scholar]
- Motro B. and Bernstein, A. (1993) Dynamic changes in ovarian c-kit and Steel expression during the estrous reproductive cycle. Dev. Dyn., 197, 69–79. [DOI] [PubMed] [Google Scholar]
- Motro B., van der Kooy, D., Rossant, J., Reith, A. and Bernstein, A. (1991) Contiguous patterns of c-kit and steel expression: analysis of mutations at the W and Sl loci. Development, 113, 1207–1221. [DOI] [PubMed] [Google Scholar]
- Murphy E.D. (1972) Hyperplastic and early neoplastic changes in the ovaries of mice after genic deletion of germ cells. J. Natl Cancer Inst., 48, 1283–1295. [PubMed] [Google Scholar]
- Murphy E.D. and Beamer, W.G. (1973) Plasma gonadotropin levels during early stages of ovarian tumorigenesis in mice of the Wx-Wu genotype. Cancer Res., 33, 721–723. [PubMed] [Google Scholar]
- Nakayama H., Kuroda, H., Onoue, H., Fujita, J., Nishimune, Y., Matsumoto, K., Nagano, T., Suzuki, F. and Kitamura, Y. (1988) Studies of Sl/Sld in equilibrium with +/+ mouse aggregation chimaeras. II. Effect of the steel locus on spermatogenesis. Development, 102, 117–126. [DOI] [PubMed] [Google Scholar]
- Nishimune Y., Haneji, T. and Kitamura, Y. (1980) The effects of steel mutation on testicular germ cell differentiation. J. Cell Physiol., 105, 137–141. [DOI] [PubMed] [Google Scholar]
- Orth J.M., Qiu, J., Jester, W.F., Jr and Pilder, S. (1997) Expression of the c-kit gene is critical for migration of neonatal rat gonocytes in vitro. Biol. Reprod., 57, 676–683. [DOI] [PubMed] [Google Scholar]
- Packer A.I., Besmer, P. and Bachvarova, R.F. (1995) Kit ligand mediates survival of type A spermatogonia and dividing spermatocytes in postnatal mouse testes. Mol. Reprod. Dev., 42, 303–310. [DOI] [PubMed] [Google Scholar]
- Peters H. and Pedersen, T. (1967) Origin of follicle cells in the infant mouse ovary. Fertil. Steril., 18, 309–313. [DOI] [PubMed] [Google Scholar]
- Racine C., Rey, R., Forest, M.G., Louis, F., Ferre, A., Huhtaniemi, I., Josso, N. and di Clemente, N. (1998) Receptors for anti-mullerian hormone on Leydig cells are responsible for its effects on steroidogenesis and cell differentiation. Proc. Natl Acad. Sci. USA, 95, 594–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rameh L.E. and Cantley, L.C. (1999) The role of phosphoinositide 3-kinase lipid products in cell function. J. Biol. Chem., 274, 8347–8350. [DOI] [PubMed] [Google Scholar]
- Reith A.D., Ellis, C., Lyman, S.D., Anderson, D.M., Williams, D.E., Bernstein, A. and Pawson, T. (1991) Signal transduction by normal isoforms and W mutant variants of the Kit receptor tyrosine kinase. EMBO J., 10, 2451–2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risma K.A., Clay, C.M., Nett, T.M., Wagner, T., Yun, J. and Nilson, J.H. (1995) Targeted overexpression of luteinizing hormone in transgenic mice leads to infertility, polycystic ovaries, and ovarian tumors. Proc. Natl Acad. Sci. USA, 92, 1322–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottapel R., Reedijk, M., Williams, D.E., Lyman, S.D., Anderson, D.M., Pawson, T. and Bernstein, A. (1991) The Steel/W transduction pathway: kit autophosphorylation and its association with a unique subset of cytoplasmic signaling proteins is induced by the Steel factor. Mol. Cell. Biol., 11, 3043–3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell E.S. (1979) Hereditary anemias of the mouse: a review for geneticists. Adv. Genet., 20, 357–459. [PubMed] [Google Scholar]
- Schluter C., Duchrow, M., Wohlenberg, C., Becker, M.H., Key, G., Flad, H.D. and Gerdes, J. (1993) The cell proliferation-associated antigen of antibody Ki–67: a very large, ubiquitous nuclear protein with numerous repeated elements, representing a new kind of cell cycle-maintaining proteins. J. Cell Biol., 123, 513–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serve H., Hsu, Y.C. and Besmer, P. (1994) Tyrosine residue 719 of the c-kit receptor is essential for binding of the P85 subunit of phosphatidylinositol (PI) 3-kinase and for c-kit-associated PI 3–kinase activity in COS-1 cells. J. Biol. Chem., 269, 6026–6030. [PubMed] [Google Scholar]
- Serve H., Yee, N.S., Stella, G., Sepp-Lorenzino, L., Tan, J.C. and Besmer, P. (1995) Differential roles of PI3-kinase and Kit tyrosine 821 in Kit receptor-mediated proliferation, survival and cell adhesion in mast cells. EMBO J., 14, 473–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers W.K. (1979) The Coat Colors of Mice. Springer Verlag, New York, NY. [Google Scholar]
- Suzuki H., Terauchi, Y., Fujiwara, M., Aizawa, S., Yazaki, Y., Kadowaki, T. and Koyasu, S. (1999) Xid-like immunodeficiency in mice with disruption of the p85α subunit of phosphoinositide 3-kinase. Science, 283, 390–392. [DOI] [PubMed] [Google Scholar]
- Swiatek P.J. and Gridley, T. (1993) Perinatal lethality and defects in hindbrain development in mice homozygous for a targeted mutation of the zinc finger gene Krox20. Genes Dev., 7, 2071–2084. [DOI] [PubMed] [Google Scholar]
- Tajima Y., Huang, E.J., Vosseller, K., Ono, M., Moore, M.A. and Besmer, P. (1998a) Role of dimerization of the membrane-associated growth factor kit ligand in juxtacrine signaling: the Sl17H mutation affects dimerization and stability-phenotypes in hematopoiesis. J. Exp. Med., 187, 1451–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima Y., Moore, M.A.S., Soares, V., Ono, M., Kissel, H. and Besmer, P. (1998b) Consequences of exclusive expression in vivo of kit-ligand lacking the major proteolytic cleavage site. Proc. Natl Acad. Sci. USA, 95, 11903–11908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J.C., Nocka, K., Ray, P., Traktman, P. and Besmer, P. (1990) The dominant W42 spotting phenotype results from a missense mutation in the c-kit receptor kinase. Science, 247, 209–212. [DOI] [PubMed] [Google Scholar]
- Timokhina I., Kissel, H., Stella, G. and Besmer, P. (1998) Kit signaling through PI 3–kinase and Src kinase pathways: an essential role for Rac1 and JNK activation in mast cell proliferation. EMBO J., 17, 6250–6262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toker A. and Cantley, L.C. (1997) Signalling through the lipid products of phosphoinositide-3-OH kinase. Nature, 387, 673–676. [DOI] [PubMed] [Google Scholar]
- Tomihara-Newberger C., Haub, O., Lee, H.G., Soares, V., Manova, K. and Lacy, E. (1998) The amn gene product is required in extraembryonic tissues for the generation of middle primitive streak derivatives. Dev. Biol., 204, 34–54. [DOI] [PubMed] [Google Scholar]
- Vanderhyden B.C. and Macdonald, E.A. (1998) Mouse oocytes regulate granulosa cell steroidogenesis throughout follicular development. Biol. Reprod., 59, 1296–1301. [DOI] [PubMed] [Google Scholar]
- Vanderhyden B.C. and Tonary, A.M. (1995) Differential regulation of progesterone and estradiol production by mouse cumulus and mural granulosa cells by A factor(s) secreted by the oocyte. Biol. Reprod., 53, 1243–1250. [DOI] [PubMed] [Google Scholar]
- Verheij M. et al. (1996)Requirement for ceramide-initiated SAPK/JNK signalling in stress-induced apoptosis. Nature, 380, 75–79. [DOI] [PubMed] [Google Scholar]
- Vincent S., Segretain, D., Nishikawa, S., Nishikawa, S.I., Sage, J., Cuzin, F. and Rassoulzadegan, M. (1998) Stage-specific expression of the Kit receptor and its ligand (KL) during male gametogenesis in the mouse: a Kit–KL interaction critical for meiosis. Development, 125, 4585–4593. [DOI] [PubMed] [Google Scholar]
- Vosseller K., Stella, G., Yee, N.S. and Besmer, P. (1997) c-kit receptor signaling through its phosphatidylinositide-3′-kinase-binding site and protein kinase C: role in mast cell enhancement of degranulation, adhesion, and membrane ruffling. Mol. Biol. Cell, 8, 909–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee N.S., Paek, I. and Besmer, P. (1994) Role of kit-ligand in proliferation and suppression of apoptosis in mast cells: basis for radiosensitivity of white spotting and steel mutant mice. J. Exp. Med., 179, 1777–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi T. and Ihle, J.N. (1993) Association of hematopoietic cell phosphatase with c-Kit after stimulation with c-Kit ligand. Mol. Cell. Biol., 13, 3350–3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., Takakura, N., Kataoka, H., Kunisada, T., Okamura, H. and Nishikawa, S.I. (1997) Stepwise requirement of c-kit tyrosine kinase in mouse ovarian follicle development. Dev. Biol., 184, 122–137. [DOI] [PubMed] [Google Scholar]
- Yoshinaga K., Nishikawa, S., Ogawa, M., Hayashi, S., Kunisada, T. and Fujimoto, T. (1991) Role of c-kit in mouse spermatogenesis: identification of spermatogonia as a specific site of c-kit expression and function. Development, 113, 689–699. [DOI] [PubMed] [Google Scholar]
- Zsebo K.M. et al. (1990)Stem cell factor is encoded at the Sl locus of the mouse and is the ligand for the c-kit tyrosine kinase receptor. Cell, 63, 213–224. [DOI] [PubMed] [Google Scholar]