Abstract
Prior studies exploring the mechanisms controlling erythroid gene regulation implicated MARE (Maf recognition element) cis–elements as crucial to the transcriptional activity of many erythroid genes. Numerous transcription factors can elicit responses through MAREs, including not only the AP-1 family proteins, but also a growing list of factors composed of Cap-N-Collar (CNC)–small Maf heterodimers. While these factors can activate transcription from MAREs in co-transfection assays, mouse germline mutations in cnc genes tested to date have failed to reveal primary erythroid phenotypes. Here we report that after combining the mafK and mafG targeted null alleles, mutant animals display several synthetic phenotypes, including erythroid deficiencies. First, compound homozygous small maf gene mutants survive embryogenesis, but die postnatally. Secondly, compound mutant animals develop severe neurological disorders. Thirdly, they exhibit an exacerbated mafG deficiency in megakaryopoiesis, specifically in proplatelet formation, resulting in profound thrombocytopenia. Finally, the compound mutant animals develop severe anemia accompanied by abnormal erythrocyte morphology and membrane protein composition. These data provide direct evidence that the small Maf transcription factors play an important regulatory role in erythropoiesis.
Keywords: anemia/cytoskeleton/platelet/small Maf/spherocytosis
Introduction
The chicken ɛ/β-globin gene enhancer was the first distant transcriptional control element identified that was proven to be crucial for tissue-specific control over erythroid gene regulation (Choi and Engel, 1986; Hesse et al., 1986). Analysis of enhancer activity by transfection of clustered array mutants into erythroid cells showed that one particular DNA sequence motif, specifying an AP-1 binding site, conferred the greatest contribution to enhancer activity (Reitman and Felsenfeld, 1988). The protein that bound to this element, referred to as nuclear factor-erythroid 2 (NF-E2), was shown to be distinct from AP-1 through analysis of the erythroid-specific porphobilinogen deaminase gene promoter (Mignotte et al., 1989). Over the past decade, this same sequence motif has been identified within cis regulatory elements of numerous erythroid genes. Despite the accumulated wealth of information demonstrating the importance of this binding site in erythroid gene regulation, there has been no formal demonstration of which transcription factor actually elicits responses from this cis–element in erythroid cells.
After its purification and cloning by reverse genetics, NF-E2 was found to be a heterodimeric basic region plus leucine zipper transcription factor. The larger subunit of this complex, called p45 (Andrews et al., 1993a), displayed all the hallmarks of a hematopoietic cell-restricted transcription factor, while the smaller subunit, called p18 or MafK (Andrews et al., 1993b; Igarashi et al., 1994), consisted of little more than an amphipathic dimerization motif and a positively charged domain. MafK shared greatest sequence homology with the family of proteins related to the chicken v-Maf oncoprotein (Nishizawa et al., 1989), while the p45 subunit was most closely related to the Drosophila nuclear regulatory Cap-N-Collar (CNC) protein (Mohler et al., 1991).
Since those early observations, the number of proteins has expanded considerably that can form (either productive or unproductive) homo- or heterodimers, or that can specifically bind to the extended AP-1 sequence motif (called a MARE, for Maf recognition element; Kataoka et al., 1994b; Motohashi et al., 1997) present in the regulatory sequences of most erythroid as well as many non-erythroid genes. This group of MARE binding factors now includes four large Maf proteins (Swaroop et al., 1992; Kataoka et al., 1993, 1994a; Ogino and Yasuda, 1998), six CNC family members (Chan et al., 1993, 1996; Itoh et al., 1995; Johnsen et al., 1996; Oyake et al., 1996; Kobayashi et al., 1999) and three small Maf proteins (Fujiwara et al., 1993; Kataoka et al., 1995), in addition to all of the previously identified members of the AP-1 (Jun/Fos) transcription factor families. Thus, the complexity of regulatory responses that can be controlled through the MARE element is vast: the factors capable of binding to these sites are pervasive, and the consequences of their binding have been shown to elicit transcriptional responses ranging from activation to repression (Engel, 1994; Kataoka et al., 1995; Motohashi et al., 1997).
Three of the six currently known cnc family members have been examined by germline mutagenesis. p45 mutation conferred permanently impaired platelet formation as well as transient neonatal erythrocyte abnormalities, which were proposed to be an indirect consequence of thrombocytopenia since adult mutant animals had normal erythropoiesis (Shivdasani and Orkin, 1995; Shivdasani et al., 1995). Nrf1 mutant mice had defective definitive hematopoiesis, although it was non-cell autonomous (Farmer et al., 1997), while Nrf2 mutants displayed no erythroid phenotype (Itoh et al., 1997; Kuroha et al., 1998). Thus, no clear relationship between CNC family member–small Maf heterodimers and hematopoiesis had been established. The ominous question naturally arose as to whether or not the bona fide regulatory protein(s) that activates transcription from erythroid MAREs was among this group of transcription factors.
We recently embarked on an analogous strategic approach to this same question, initiating experiments to determine whether or not any of the heterodimeric partners of the CNC proteins, the small Maf transcription factors (MafF, MafG and MafK), exhibited erythroid phenotypes after germline gene targeted ablation. We (Shavit et al., 1998) and others (Kotkow and Orkin, 1996) reported that targeted disruption of mafK led to no discernible phenotype, and very recently we also found that mafF germline ablation similarly caused no apparent disturbance in embryonic or adult development (Onodera et al., 1999). However, mafG germline mutation led to mild thrombocytopenia, weakly phenocopying the p45 CNC mutation (Shavit et al., 1998). Once again, the small maf mutant animals displayed no erythroid deficiencies.
In order to characterize pathologies engendered by small maf gene mutations more fully, we wished to determine whether mafG mutant phenotypes were exacerbated by loss of other small maf alleles. If more profound deficiencies were encountered in the compound mutants than in either single gene homozygous mutant animal, this would constitute strong prima-facie evidence for interallelic complementation among this family of factors. To this end, we intercrossed the small maf germline mutant animals to generate compound mutants.
The results of the compound mutant loss-of-function analyses shed significant new insight into the cellular processes controlled by small Maf–CNC heterodimer activity. First, compound homozygous maf mutant animals survive gestation. Animals missing both the mafF and mafK genes suffer virtually no abnormalities, while compound mutants missing both mafF and mafG differ only slightly from those missing mafG alone (our unpublished observations). However, unlike any of the individual small maf mutant or the p45 mutant animals, mafK::mafG compound homozygous mutants succumb immediately after birth to postnatal lethality, which appears to be a consequence of differential contributions from three separate pathological deficiencies. First, compound homozygous mutants exhibit profound thrombocytopenia, thus exaggerating the homozygous mafG–/– mutant phenotype to phenocopy precisely the p45 mutation in its effects on megakaryopoiesis. Secondly, compound mutant animals bearing only one active mafK allele (e.g. mafG–/–::mafK+/–) display chronic posterior ataxia, exacerbating the previously documented mafG–/– homozygous deficiency. Finally, homozygous compound mutants suffer from anemia, red cell fragility and erythroid cytoskeletal defects that resemble hereditary spherocytosis. Unlike the p45 mutants, the mafK::mafG compound homozygous mutants display red cell morphological abnormalities at all developmental stages, including in definitive erythroid cells prior to birth. Furthermore, the small maf compound mutants never survive postnatally. These experiments show for the first time an in vivo requirement for the small Maf family proteins in erythropoiesis, and they thus provide a key link in identifying trans-acting factor(s) that acts at this demonstrably crucial cis regulatory transcriptional control element.
Results
Synthetic perinatal lethality as a consequence of combining homozygous mafK and mafG mutant alleles
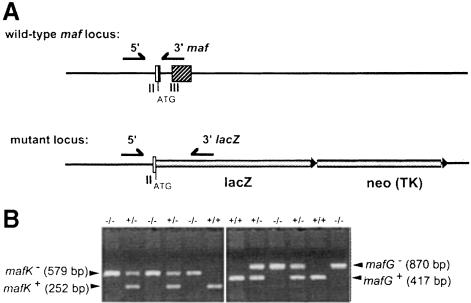
We first intercrossed mafG+/– with mafK–/– mutant mice (129/CD1 mixed hybrid background) to generate mafG+/–::mafK+/– compound mutants; the compound heterozygotes were recovered at the expected Mendelian frequencies. Since those animals exhibited no apparent dysfunction, we intercrossed them and genotyped progeny between postnatal day 7 (P7) and P14. Genomic tail DNA was analyzed by PCR using common 5′ mafG- and mafK-specific as well as separate 3′ primers to distinguish between the wild-type and mutant alleles (Figure 1). Of 279 pups, all but one of the anticipated intercross genotypes were recovered at the expected Mendelian frequency (Table IA). Only four compound homozygous mutant (CM) animals were recovered, of 17 expected statistically. Although we increased the number of CM pups by initiating intercrosses with (phenotypically normal) mafG+/–::mafK–/– breeding pairs, we still recovered only four CM pups out of 122 progeny from the latter intercrosses (of 30.5 expected; Table IB). Both results were statistically significant (Table IA, p <0.05; Table IB, p <0.01), demonstrating minimally that CM animals fail to thrive after birth. These CM pups were quite small compared with their littermates (Figure 2A) and did not survive to weaning. Therefore, we decided to kill CM pups at or before P14 to investigate further the consequences of combining these two null mutant alleles.
Fig. 1. Small maf mutant detection strategy. PCR screening of the mafG and mafK loci. Owing to the high similarity between the mafG and mafK loci, both are represented on a single diagram. (A) A common 5′ primer was used with two distinct 3′ primers, one corresponding to sequences within the second intron of the wild-type allele (top) and a 3′ lacZ primer for the mutant allele (bottom). (B) Two percent agarose gel electrophoresis showing a typical distribution of wild-type, heterozygous and homozygous mutant animals from a compound heterozygous mutant intercross.
Table I. Genotypes recovered from compound heterozygous mutant intercrosses.
| (A) mafG+/–::mafK+/– × mafG+/–::mafK+/– | ||
|---|---|---|
| Genotype | No. of 18.5 d.p.c. embryos (observed/expected) | No. of P14 pups (observed/expected) |
| mafG+/+::mafK+/+ | 6/6.4 | 12/17.4 |
| mafG+/+::mafK+/– | 10/12.9 | 47/34.8 |
| mafG+/+::mafK–/– | 9/6.4 | 16/17.4 |
| mafG+/–::mafK+/+ | 15/12.9 | 40/34.8 |
| mafG+/–::mafK+/– | 21/25.8 | 69/69.7 |
| mafG+/–::mafK–/– | 18/12.9 | 40/34.8 |
| mafG–/–::mafK+/+ | 4/6.4 | 21/17.4 |
| mafG–/–::mafK+/– | 13/12.9 | 30/34.8 |
| mafG–/–::mafK–/– | 7/6.4 | 4/17.4* |
| Total | n = 103 | n = 279 |
| (B) mafG+/–::mafK–/– × mafG+/–::mafK–/– | ||
|---|---|---|
| Genotype | No. of P14 pups (observed/expected) | |
| mafG+/+::mafK–/– | 38/30.5 | |
| mafG+/–::mafK–/– | 80/61.0 | |
| mafG–/–::mafK–/– | 4/30.5** | |
| Total | n = 122 | |
Interbreeding was performed between animals of mixed 129/CD1 background. Pups were genotyped between P7 and P14. The number of progeny of a specific genotype (observed) is compared with the frequency at which they would be expected given a normal Mendelian distribution (expected). The observed/expected difference is statistically significant: * p<0.05; ** p <0.01.
Fig. 2. Physiological characteristics of mafG::mafK CM pups. (A) The appearance of newborn CM pups. The CM pup (on the right) is both smaller and more pale than its control littermate (left); pups of this genotype most often suffer from hemorrhage in the lower abdomen [arrowhead in (B)] and head (not shown). (C) Small maf multiple allele mutant pups exhibit fully penetrant dyskinesia. The two pups shown are a control compound heterozygote (mafG+/–::mafK+/–; right) and a three-allele mutant (mafG–/–::mafK+/–; left) animal. The three-allele mutant adult was unable to right itself or control involuntary convulsions in its spastic hindquarters, and was therefore extremely ataxic.
The identification of surviving postnatal CM animals suggested that the lethality of this genotype was probably not embryonic in origin, but rather occurred at around the time of birth. We thus analyzed intercross progeny at 18.5 days post-coitus (d.p.c.), 1 day before birth, to determine whether or not CM embryos normally complete gestation. We recovered an expected number of CM pups (Table IA), which appeared grossly normal. Therefore, the loss of mafG and mafK together does not significantly affect embryonic development, and validates the hypothesis that the death of CM animals is a postnatal phenotype. Since none of the single gene small maf homozygous mutants had a shortened lifespan (Shavit et al., 1998; Onodera et al., 1999), the consequences of combining two of the mutant alleles resulted in this profoundly altered property, demonstrating that the origin of this perinatal CM attribute was due to the combination of the mutant mafG and mafK alleles, by definition a synthetic lethal phenotype.
Compound small maf mutants display dosage-dependent phenotypes and reveal in vivo functions for mafK
We previously reported platelet reduction and behavioral defects in mafG–/– homozygous mutant animals (Shavit et al., 1998). We therefore wondered whether there would be greater severity in these phenotypes if the animals were missing additional mafK alleles.
CM pups were bruised and had an ashen appearance (Figure 2A and B), possibly indicating that they might suffer from platelet deficiency and anemia. We therefore drew peripheral blood from 18.5 d.p.c., P14 and adult animals of various genotypes and performed complete blood analysis. As shown in Table II, all of the animals bearing at least one active mafG allele had similar hematological parameters. The blood data for mafG–/–::mafK+/– animals were also normal except for a markedly reduced platelet count, which was more severe than in mafG–/– homozygous mutant animals (Table IIC). Since deletion of the second active mafK allele in the mafG–/– mutant background reduces the platelet count essentially to zero (Table IIB), we conclude that consecutive deletion of mafK alleles in the mafG homozygous mutant background leads to a small Maf dosage-dependent loss of platelets.
Table II. Blood parameters in small maf compound mutant mice.
| (A) 18.5 d.p.c. Genotype | No. of RBC (× 106/mm3) | Hemoglobin (g/dl) | Hematocrit (%) | MCV (fl) | MCH (pg) | MCHC (%) | No. of platelets (× 103/mm3) |
|---|---|---|---|---|---|---|---|
| mafG+/–::mafK+/– | 3.35 ± 0.27 | 12.1 ± 1.3 | 29.5 ± 3.2 | 88.0 ± 3.6 | 36.1 ± 1.1 | 41.0 ± 0.9 | 334 ± 45 |
| mafG–/–::mafK+/– | 3.32 ± 0.20 | 11.8 ± 0.5 | 28.8 ± 1.5 | 87.0 ± 10.1 | 35.7 ± 3.8 | 41.0 ± 1.0 | 78 ± 12 |
| mafG–/–::mafK–/– | 3.03 ± 0.57 | 10.9 ± 0.9 | 25.8 ± 2.1 | 86.6 ± 10.7 | 36.6 ± 4.6 | 42.2 ± 0.2 | 98 ± 16 |
| (B) P14 | |||||||
|---|---|---|---|---|---|---|---|
| Genotype | No. of RBC (× 106/mm3) | Hemoglobin (g/dl) | Hematocrit (%) | MCV (fl) | MCH (pg) | MCHC (%) | No. of platelets (× 103/mm3) |
| mafG+/+::mafK–/– | 6.18 ± 0.09 | 12.9 ± 0.2 | 37.0 ± 1.1 | 59.8 ± 0.8 | 20.9 ± 0.0 | 35.0 ± 0.4 | 654 ± 51 |
| mafG+/–::mafK–/– | 6.03 ± 0.20 | 11.9 ± 0.7 | 33.6 ± 1.6 | 55.2 ± 1.0 | 19.8 ± 0.6 | 35.8 ± 1.0 | 635 ± 64 |
| mafG–/–::mafK+/– | 6.34 ± 0.11 | 12.8 ± 0.4 | 34.4 ± 0.6 | 54.3 ± 0.0 | 20.2 ± 0.3 | 37.3 ± 0.6 | 164 ± 7 |
| mafG–/–::mafK–/– | 3.50 ± 0.47 | 7.1 ± 1.6 | 16.7 ± 2.0 | 49.5 ± 2.0 | 17.9 ± 2.0 | 36.1 ± 2.6 | 20 ± 6 |
| (C) Adult | |||
|---|---|---|---|
| Genotype | No. of RBC (× 106/mm3) | Hemoglobin (g/dl) | No. of platelets (× 103/mm3) |
| mafG+/+::mafK+/+ | 8.34 ± 0.60 | 15.7 ± 0.5 | 1191 ± 80 |
| mafG+/+::mafK–/– | 8.91 ± 0.19 | 14.4 ± 0.3 | 1095 ± 48 |
| mafG+/–::mafK–/– | 9.29 ± 0.98 | 15.1 ± 0.8 | 1087 ± 79 |
| mafG–/–::mafK+/+ | 8.99 ± 0.56 | 15.1 ± 0.7 | 529 ± 40 |
| mafG–/–::mafK+/– | 8.79 ± 0.63 | 14.5 ± 0.5 | 164 ± 25 |
| mafG–/–::mafK–/– | ND | ND | ND |
Each value represents the mean ± SD of measurements of blood samples from at least three different animals. ND, not determined.
We next examined the neurological phenotype of the mutants. Hind leg clasping was a late-onset symptom in mafG–/– mutant mice, observed only after 5 or 6 months of age, and was not fully penetrant (Shavit et al., 1998). In stark contrast, the onset of the same behavior in mafG–/–::mafK+/– animals occurred 3–4 weeks after birth, and was fully penetrant (n = 14/14). In addition, these same mutants displayed severe motor ataxia after 2 months of age, with intermittently spastic hind legs (Figure 2C), which appeared to be an exacerbated neurological defect previously documented in the mafG–/– animals. Importantly, these findings showed that the loss of MafK in the mafG-null mutant background results in MafK-dependent neuromuscular phenotypes.
Perhaps most surprisingly, mafG+/–::mafK–/– mutants exhibited hematological parameters that were indistinguishable from those of wild-type mice and the animals displayed no neurological symptoms. After prolonged observation, we determined that no defects were observed in mafG+/–::mafK–/– mice. Analysis of the mafG+/–::mafK–/– mutants indicated that one active allele of mafG in a homozygous mafK-null mutant background was sufficient for the development, survival and fertility of these laboratory mice.
Small maf mutant megakaryocytes are defective in proplatelet formation
Since thrombocytopenia was markedly exacerbated in both the mafG–/–::mafK+/– and the CM mice, we set out to clarify the nature of the small maf mutant-induced developmental block in platelet formation.
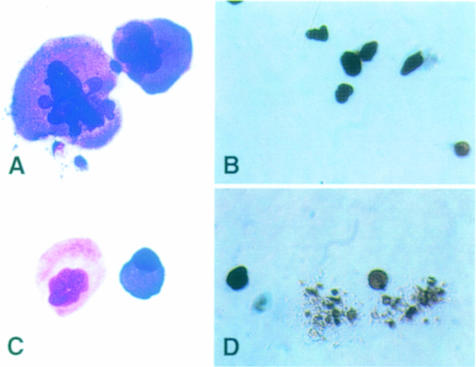
Megakaryocytes were isolated from the bone marrow of 6- to 8-week-old mice (Nagahisa et al., 1996; Osada et al., 1999). Most of the megakaryocytes purified from the mafG–/–::mafK+/– mutant mice were large and appeared to be mature (Figure 3A). In contrast, megakaryocytes from control mice represented different stages of maturation, ranging from small immature cells to large mature cells (Figure 3C). Thus, relatively mature megakaryocytes seemed to dominate in the bone marrow of mafG–/–::mafK+/– mice, which could reflect changes in their proliferation and/or differentiation in the mafG–/–::mafK+/– genetic background.
Fig. 3. Megakaryocyte morphology and in vitro platelet formation potential in mafG+/–::mafK+/– and mafG–/–::mafK+/– mice. Megakaryocytes were isolated from the bone marrow of mafG–/–::mafK+/– mice (A and B) or mafG+/–::mafK+/– mice (C and D). The morphology of both was examined by Giemsa staining (A and C). After plating into culture dishes, control megakaryocytes formed proplatelet projections after 24 h in culture (D), while megakaryocytes recovered from mafG–/–::mafK+/– mutants showed no propensity for megakaryocyte fragmentation or proplatelet formation (B).
When cultured in vitro, ∼20% of normal megakaryocytes develop proplatelets, arrays of filamentous cell projections. We quantified the number of purified megakaryocytes with proplatelets after 1 day of in vitro culture, and calculated the incidence of proplatelet formation (PPF). Importantly, mafG–/–::mafK+/– megakaryocytes did not form proplatelets, in contrast to megakaryocytes of other genotypes having normal PPF incidence (Figure 3B and D; Table III). Thus, the results of inactivating one mafK allele and the consequent reduction in small Maf activity result in the complete loss of PPF in mafG–/–::mafK+/– animals. We concluded that one of the primary defects in megakaryocytes isolated from mafG–/–::mafK+/– mice was the loss of PPF activity, suggesting that two of the small Maf proteins, MafG and MafK, might act as direct regulators of the PPF process in megakaryocytes.
Table III. No proplatelet formation in small maf compound mutant mice.
| Genotype | No. of megakaryocytes with PPF/total megakaryocytes |
|||
|---|---|---|---|---|
| Experiment No. |
Average PPF (%) | |||
| 1 | 2 | 3 | ||
| mafG+/+::mafK+/+ | 59/634 | 122/380 | 130/311 | 27.7 ± 16.7 |
| mafG+/+::mafK–/– | 53/344 | 161/440 | 70/226 | 27.6 ± 11.0 |
| mafG+/–::mafK–/– | 73/535 | 76/264 | 126/392 | 24.8 ± 9.8 |
| mafG–/–::mafK+/+ | 20/1333 | 4/420 | 2/413 | 0.98 ± 0.51 |
| mafG–/–::mafK+/– | 0/268 | 0/828 | 0/866 | 0 |
Small maf CM animals have irregular RBC morphology and increased RBC heterogeneity
In addition to bruising, the ashen appearance of the P0–P3 CM pups (Figure 2A) indicated that they might also be anemic. As shown in Table IIB, P14 CM pups had fewer red blood cells (RBCs) as well as lowered hemoglobin concentration accompanied by an extremely low platelet count, indicating that the CM animals indeed suffered from severe anemia. CM pup RBCs displayed reduced mean corpuscular volume (MCV) and mean cell hemoglobin (MCH), but mean corpuscular hemoglobin concentration (MCHC) was normal, which differs from the parameters observed in typical iron-deficient anemia caused by frequent hemorrhage. Curiously, CM mice at 18.5 d.p.c. exhibited virtually normal erythroid parameters (Table IIA). We noticed that the P14 CM pups had slight splenomegaly and unusual, persistent fetal liver hematopoiesis (Figure 4A and B; the control is shown in Figure 4C). Histological examination did not distinguish these hematopoietic cells from those in 18.5 d.p.c. CM fetal liver (data not shown), and both appeared normal except for megakaryocyte accumulation. We thus speculated that P14 CM pups displayed reactive extramedullary hematopoiesis due to anemia and that morphological changes of erythroid cells would be more apparent in peripheral blood.
Fig. 4. Persistent fetal liver hematopoiesis in CM pups. Livers from P14 pups were examined. Hematopoietic foci [white arrowheads in (A)] are observed in P14 CM pup liver, while the liver of a littermate mafG+/–::mafK+/– pup harbors few hematopoietic cells (C). (B) Higher magnification of persistent hematopoietic cells of (A). The scale bar corresponds to 33 μm (A and C) and 83 μm (B).
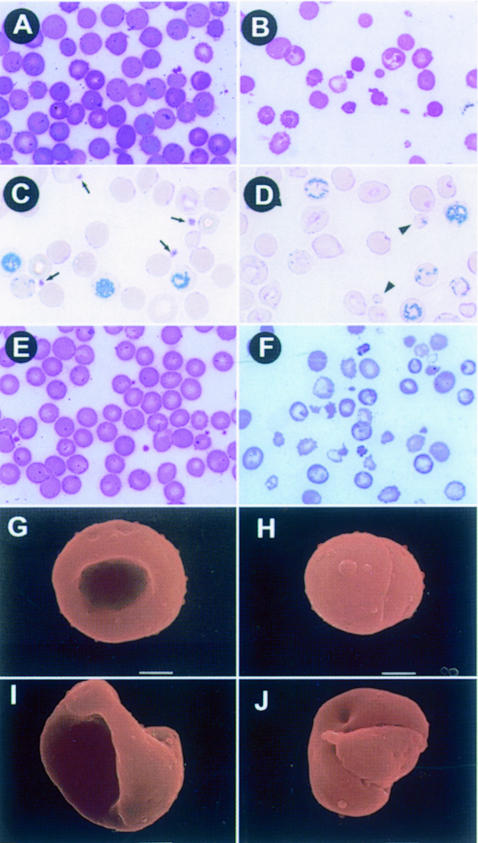
We therefore prepared peripheral blood smears from 18.5 d.p.c. (Figure 5A and B), P10 (Figure 5C and D) and P14 (Figure 5E and F) littermates of compound heterozygous mutant intercrosses. The RBCs in CM pups displayed aberrant morphology at all stages of definitive erythropoiesis (Figure 5B, D and F). As anticipated from MCV values, many of the CM RBCs were small and round with irregular shapes. By scanning electron microscopic (SEM) analysis, the aberrant RBCs were found to be spherocytes as well as other irregularly shaped cells (Figure 5G–J). It is clear that the erythroid abnormality is not caused by acute blood loss alone, since a majority of the RBCs were misshapen even in 18.5 d.p.c. embryos that displayed no anemia (Figure 5A and B; Table IIA). When we stained reticulocytes taken from the peripheral blood of CM pups and compared it with that of various other genotype littermates, not only had the reticulocyte frequency increased, but there were also many that were small and round, or irregularly shaped (Figure 5C and D). These abnormal reticulocytes strongly supported the hypothesis that an RBC deformity was a primary effect of compound disruption of the mafG and mafK genes, while leaving open the two possibilities that erythropoiesis is defective and generates misshapen RBCs or that fragility of CM RBCs ends in fragmentation during circulation. Thus, this phenotype differs from that of the p45 mutants, which appear to suffer no permanent erythroid deficits (Shivdasani and Orkin, 1995).
Fig. 5. Irregular RBCs in CM mice. Smears of peripheral blood from mafG+/–::mafK+/– (A, C and E) or mafG–/–::mafK–/– (CM; B, D and F) 18.5 d.p.c. embryos (A and B) or P10 (C and D) or P14 (E and F) pups. In the CM blood smears, numerous spherocytes and poikilocytes (irregularly shaped RBCs) are observed. The P10 CM pups have an increased number of reticulocytes (C and D). Small and deformed reticulocytes are also observed [arrowheads in (D)], which are clearly distinguishable from densely stained platelets [arrows in (C)]. Note the absence of platelets in CM blood. Scanning electron micrographs of mafG+/– (G) or CM (H, I and J) circulating erythroid cells from P14 pups are shown. The CM erythroid cells display spherocyte (H) or poikilocyte (I and J) morphologies.
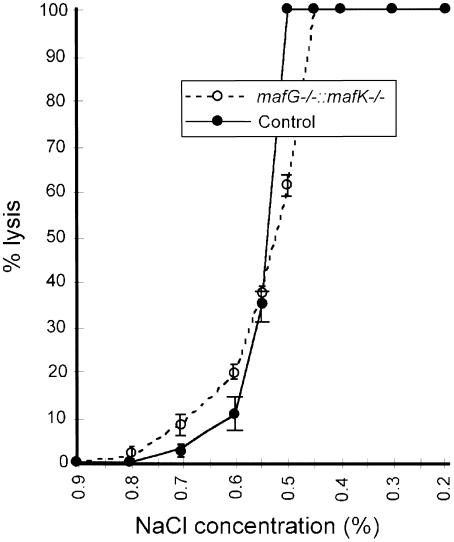
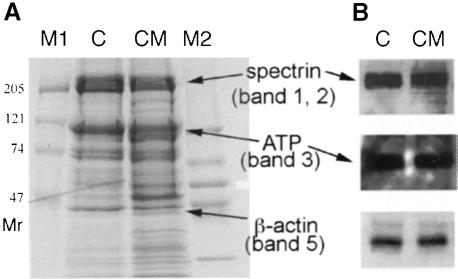
The microscopic results indicated that CM mice might have disorganized erythroid membranes that are unable to maintain normal erythroid cell cytoskeletal morphology, thereby leading to hemolysis and anemia. In accord with this hypothesis, osmotic fragility tests showed that half of the RBCs taken from P14 CM blood were less stable than those from normal littermates (Figure 6). Interestingly, the other half of the CM RBCs were more stable. This divergent CM RBC stability may originate from alteration in RBC membrane composition, also reflected in an increased number of reticulocytes, which are more resistant to osmolarity change. This result revealed that CM RBCs displayed increased heterogeneity and that blood from CM pups contains RBCs that are more sensitive to osmolarity changes. To clarify the biochemical origins of the hypothetical erythroid membrane defect(s), SDS–PAGE and Western blot analyses were performed to examine the membrane protein constituents of mutant and control erythroid cell ghosts (gently lysed RBCs) (Figure 7). When compared with ghosts prepared from the blood of normal littermates, CM cytoskeletons had relatively elevated levels of two proteins migrating with a mobility of apparent molecular sizes of 95 and 48 kDa (Figure 7A). The former (95 kDa) was similar to the electrophoretic mobility of kidney band 3 (Brosius et al., 1989), which is an alternatively spliced kidney-specific isoform of the anion transport protein. The latter (48 kDa) was similar in size to band 4.9, dematin (Rana et al., 1993). However, these proteins that had accumulated to abnormal abundance in the P14 CM RBC ghosts did not correspond to either the kidney-specific splice variant of band 3 or to band 4.9, as demonstrated on Western blots (Figure 7B). Other major membrane proteins, such as α- and β-spectrin (band 1 and band 2; 210–220 kDa) as well as erythroid band 3 (100 kDa) and actin (band 5; 42 kDa) were expressed at approximately normal levels. We also analyzed the abundance of a number of other erythroid tissue-specific mRNAs that might be affected by the mutations using semi-quantitative RT–PCR (α- and β–globin, porphobilinogen deaminase, heme oxygenase, erythroid 5-aminolevulinate synthase and ferrochelatase), but no differences were detected in the abundance of these mRNAs recovered from CM versus normal animals (data not shown).
Fig. 6. CM blood contains erythrocytes more sensitive to alterations in osmolarity. Control and CM red blood cells were incubated at varying salt concentrations (abscissa) for 5 min. The hemoglobin concentration in 0% NaCl solution was taken as 100% lysis, and each percentage lysis value was determined. Half of the RBCs from CM pups were more sensitive to osmolarity changes than control RBCs. Another half were more resistant. Each value represents an average from two different animals, and the SD for each experiment is shown.
Fig. 7. Cytoskeletal defects in CM erythrocyte ghosts. (A) SDS–PAGE analysis of erythroid membrane protein recovered from P14 control (C; mafG+/–::mafK+/+) and CM (mafG–/–::mafK–/–) peripheral blood. The same number of cells were lysed and loaded on a 5–15% gradient polyacrylamide gel. Proteins were visualized by staining with Coomassie Blue. In the CM erythroid membranes, two proteins appeared to be overexpressed, and migrated with apparent Mr of 95 and 48 kDa. (B) Western blot analysis with anti-spectrin, anti-band 3 or anti-dematin antibodies. No difference was observed in the abundance of these three proteins in CM versus control RBC ghosts.
We conclude that the anemia observed in CM mice is a small maf mutant synthetic phenotype, and thus detectable only after combining two null mafG and mafK alleles. The erythroid cell spherocytosis and anemia were not observed if even one small mafG or mafK allele was active. Therefore, small maf CM pups appear to display severe anemia in part as a consequence of increased fragility of a significant fraction of their RBCs. We assume that the aberrant accumulation of 95 and/or 48 kDa erythrocyte membrane-associated proteins may cause the defect in RBC membrane integrity, which in turn leads to increased heterogeneity in RBC morphology and in osmotic fragility.
No cross-regulation between the three small maf genes
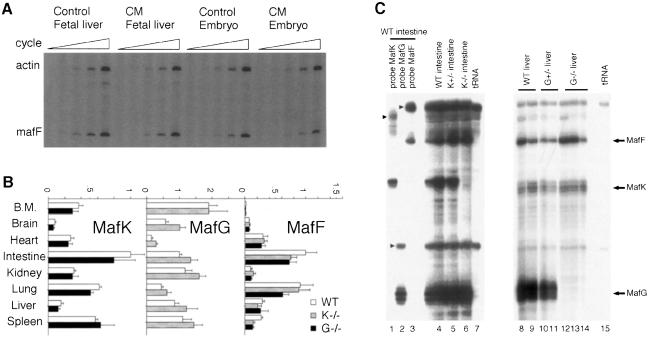
The observation that CM embryos can complete normal prenatal development led to the hypothesis that expression of the remaining small Maf protein, MafF, might be upregulated in CM mice to compensate for loss of the other two gene products (e.g. Foley et al., 1998). To test this hypothesis, we determined the abundance of MafF mRNA in 14.5 d.p.c. fetal livers and embryos using semi-quantitative RT–PCR. As shown in Figure 8A, no increase in MafF accumulation was observed in CM fetal liver or whole embryos, suggesting that there is no compensating upregulation of the mafF gene even when MafG and MafK are absent. Similarly, we found no evidence for upregulation of either of the remaining two small maf mRNAs in any single maf mutant when analyzed by real-time quantitative RT–PCR analysis (Figure 8B). These results were further supported by RNase protection assays also showing that there was no increase in the remaining small maf mRNAs in either mafG or mafK mutant mice (Figure 8C). These results therefore demonstrate that the transcription of the three small maf genes is not influenced by the loss of others, and thus that the abundance of small Maf transcript is determined by small Maf-autonomous mechanisms.
Fig. 8. Maf-independent transcription of the small maf genes. (A) Semi-quantitative RT–PCR analysis of RNAs recovered from 14.5 d.p.c. fetal liver and whole embryo of control and CM mice. The number of PCR cycles was 16, 18, 20, 22 or 24 for β-actin, and 24, 26, 28, 30 or 32 for MafF, shown by increasing triangle size. (B) MafK, MafG and MafF mRNA expression profiles were examined in mafK-null mutant, mafG-null mutant and wild-type adult mice by real-time quantitative RT–PCR. Expression levels of small maf genes were normalized for rRNA expression level in the same cDNA preparation. The expression levels were normalized for mRNA levels in the wild-type intestine (set as 1). Each value represents the mean ± SD of the relative levels from four independent experiments. Open bars, shaded bars and closed bars indicate wild-type, mafK-null mutant and mafG-null mutant mice, respectively. B.M. is bone marrow. (C) MafK, MafG and MafF mRNA levels were also analyzed by RNase protection assays. Antisense probes for MafK (lane 1), MafG (lane 2) or MafF (lane 3) were hybridized to 50 μg each of total RNA from the intestine of a wild-type adult mouse. The three probes were mixed and hybridized to RNA samples from wild-type intestine (lane 4), mafK+/– intestine (lane 5), mafK–/– intestine (lane 6), wild-type liver (lanes 8 and 9), mafG+/– liver (lanes 10 and 11), mafG–/– liver (lanes 12–14) or tRNA (lanes 7 and 15). The migration positions of undigested probes are indicated by arrowheads, while the positions of protected fragments are indicated by arrows.
Discussion
This study vividly demonstrates that the small Maf proteins function in overlapping fashion in several developmental pathways in vivo. CM mice die perinatally from loss of both MafG and MafK, presumably due to absolute thrombocytopenia (exacerbation of a mafG-specific phenotype) and anemia (a unique mafG::mafK synthetic compound phenotype). mafG–/–::mafK+/– mutants display more severe manifestations of mafG-specific phenotypes, in a dosage-dependent manner, and thereby demonstrate that MafK functions in both hematopoietic and neuronal lineages, as we anticipated from their expression profiles (Motohashi et al., 1996; Shavit et al., 1998). Finally, analysis of mafG+/–::mafK–/– compound mutant animals demonstrates that mafG is the more critical gene for survival, and that only one mafG allele is both necessary and sufficient for a semblance of normal reproduction and existence.
The observation that small maf CM mice exhibited severe thrombocytopenia and deformed erythrocytes was in part similar to the phenotypes exhibited by p45 homozygous mutant mice (Shivdasani et al., 1995) confirms the fact that the small Maf proteins and p45 are bona fide heterodimeric partners, certainly in megakaryocytes. However, the CM phenotype was clearly more severe than the p45 mutation since no CM newborn ever survived to weaning. In contrast, once p45 mutant animals survive beyond a specific critical point in neonatal development, they recover from neonatal injuries and develop normally (Shivdasani et al., 1995). We suspect, but have no data to support the hypothesis, that the difference in transcription factor requirements between fetal liver and adult (bone marrow or spleen) hematopoiesis might account for the difference in the survivability of p45 versus maf CM mice, and that the loss of small Maf proteins causes a broader and more severe defect in bone marrow hematopoiesis than does the p45 mutation.
Embryonic analysis showed that mafG–/–::mafK–/– mutants could survive through gestation, and were recovered at normal Mendelian frequencies at 18.5 d.p.c. Therefore, the compound mutant lethality observed is peri- or postnatal. This was unexpected considering the dynamic expression patterns displayed by both mafG and mafK, with complementary expression patterns as early as 6.5 d.p.c., including only few apparent sites of overlapping expression from 8.5 d.p.c. onwards (Shavit et al., 1998). Nonetheless, prominent sites of co-expression included pre-gastrulation mesenchyme, the ectoplacental cone, the yolk sac endoderm and the fetal liver. This expression pattern led us to hypothesize that compound mutant lethality, if encountered, would occur during one of these early critical stages during which those organs or tissue functions become important to the progress of embryogenesis (Shavit et al., 1998). The experimental results that we described here suggest several alternative possibilities. One possible explanation is that MafF is able to compensate for the loss of both MafG and MafK in these tissues. The expression of the mafF gene in the developing embryo is relatively limited in comparison to mafG (Onodera et al., 1999) and is not subject to up-regulation (Figure 7A), and therefore MafF seems unlikely to be a candidate for a factor that is able to compensate for the loss of mafG and mafK. It is nonetheless possible that minuscule levels of MafF, levels that are undetectable by β-galactosidase staining, could compensate for the loss of MafG and MafK. To test this hypothesis, we are currently attempting to generate animals in which all three small maf genes are ablated. An alternative explanation for normal embryonic development in the CM embryos is that other MARE and/or TPA-responsive element (TRE) binding factors, including large Mafs and Fos or Jun family members, may also compensate for MafG and MafK loss. Finally, it is also conceivable that other small maf genes are yet to be cloned, and that these overlap with mafG and mafK in their expression patterns or are induced in the mutants. However, since there is no evidence from numerous direct homology screens, two-hybrid screens or any other functional assay that a fourth small Maf protein exists, we feel that this final possibility is unlikely.
The biochemical origin of poikilocytes in the CM mice is not clear. It is well known that deficiencies in major erythroid membrane skeletal proteins can elicit multiple disease phenotypes (spherocytosis, eliptocytosis, poikilocytosis or erythroid cell fragmentation; Clark and Wagner, 1989). It was reported earlier that disruption of the band 3 gene caused spherocytosis and hemolytic anemia (Southgate et al., 1996). Based on the size of the proteins, we originally suspected that misregulation of the anion transporter (band 3) or dematin (band 4.9) proteins could be the cause of the strange RBC morphology. However, this hypothesis was definitively rejected by Western blot analysis, which showed that the band 3 and band 4.9 proteins were unchanged in abundance or size. Nonetheless, it is clear from SDS–PAGE that at least two proteins are markedly overexpressed in CM erythroid ghosts, suggesting that inappropriate excess of one or both of these proteins leads to pathophysiological disruption of the erythroid cytoskeleton.
These observations on mutant RBC ghosts are most consistent with the hypothesis that MafG and MafK homo- or heterodimers normally repress transcription of specific RBC target genes by virtue of small Maf binding to target MARE sites, and that in the absence of these small Maf proteins (in CM animals) these target genes are transcriptionally de-repressed and thereby inappropriately expressed. The consequences of this hypothetical overly abundant expression would then lead to the observed defective membrane integrity, suggesting that erythroid cytoskeletal proteins are among those Maf-repressed target genes. Small Maf proteins were shown previously to be effective repressors in vitro (Igarashi et al., 1995a, b) and in vivo (H.Motohashi and M.Yamamoto, unpublished observations), thereby indirectly supporting this hypothesis, while the present data show that RBC ghosts in CM animals abnormally accumulate proteins associated with RBC ghosts, also supporting a ‘Maf repressor’ hypothesis.
Analyses of the mafG–/–::mafK+/– mutant animals were particularly informative in that they revealed the dosage-dependent phenotypes and demonstrated an in vivo role for MafK that was undetectable in the single small maf mutant animals (Kotkow and Orkin, 1996; Shavit et al., 1998). The dosage-dependent decrease in platelet number with successive loss of MafK in the mafG-null background is particularly suggestive for the mechanism by which these proteins function in vivo: namely, that a single transcriptional event leading to platelet maturation requires, in a dosage-dependent fashion, small Maf activity. These multiple allele small maf mutant mice also exhibited exaggerated manifestations of the behavioral defects observed in mafG–/– animals. The hind leg clasping phenotype was particularly obvious and was 100% penetrant in mafG–/–::mafK+/– animals. Finally, mafG–/–::mafK+/– mutants were 10–20% smaller than mafG–/– littermates, which were themselves almost half the size of control animals. The dosage-dependent nature of all these behavioral and physiological deficits was further supported by analysis of the sole CM pup that survived until P19. It clasped its hind legs even before weaning and was half the size again of its mafG–/– littermates. While CM animals might have overcome the profound thrombocytopenia (as do 10% of the p45 mutants; Shivdasani et al., 1995), the additional stress from anemia due to the small maf erythroid deficit would certainly prevent them from surviving beyond adolescence.
Finally, we specifically note that the mafG+/–::mafK–/– mice were normal. This was quite surprising, since the data therefore imply that only one allele of mafG is necessary for all functions normally fulfilled by two wild-type alleles of mafK and mafG. This result suggests that MafK is less important to survival and reproductive capacity than is MafG in laboratory mice. However, the expression patterns of these two genes imply that MafK has unique roles unrelated to MafG, and that these roles may be compensated for by MafF or some other MARE binding protein(s).
Materials and methods
Genotyping of pups and embryos from compound heterozygous intercrosses
The individual mafK and mafG mutants (Shavit et al., 1998) were intercrossed to generate the compound heterozygous mutants; these were then interbred for compound mutant analysis. Tails for genotyping were digested overnight in lysis buffer (100 mM NaCl, 1.0% SDS, 50 mM Tris–HCl pH 8.0, 100 mM EDTA pH 8.0, 0.35 mg/ml fresh proteinase K) at 55°C, phenol–chloroform extracted and precipitated.
Genotyping was performed by PCR. The sequences of the primers used for this analysis were: 5′ mafG primer, MafG36: GCATGACTCGCCAGGAACAG; 3′ mafG primer, MafG433: CCCAAGCCCAGCCTCTCTAC; 5′ mafK primer, MafKEx2.1: CCTACCGTTTCTGTCTTTCCAG; 3′ mafK primer, MafKIn346: AATTCCTGAGGACAAAGCTGAC; 3′ mut. primer, LacZ4: CCTGTAGCCAGCTTTCATCAAC.
Preparation of peripheral blood smears
Litters of newborn pups were decapitated and a drop of blood was put onto a glass slide. The blood drop was spread with the edge of another glass slide, and then air-dried. The slides were subsequently fixed in methanol and processed with a Diff-quik™ (American Scientific Products) kit, which produces results similar to Wright–Giemsa staining. Peripheral blood from P10 pups (Figure 5C and D) was stained with Brecher's new methylene blue solution (Muto, Tokyo) for the detection of reticulocytes.
Hematological analysis
Blood was drawn into a heparinized capillary from newly isolated and freshly decapitated 18.5 d.p.c. embryos for hematological analysis; yolk sacs were genotyped later. Live mice were killed with CO2 followed by cervical dislocation and 100–200 μl (2-week-old) or 500 μl (adult) of blood were drawn from the inferior vena cava into a syringe containing 2 μl (2-week-old) or 5 μl (adult) of 0.5 M EDTA. The blood samples from adults were analyzed by the Biological Research Laboratory of the University of Illinois at Chicago or by Anaylitics, Inc. (Gaithersburg, MD).
For 2-week old mice, it was difficult to determine precisely the platelet number in the compound mutant animals, since it appeared that small, fragmented RBCs might erroneously contribute to the automated platelet determination by cell size (see Figure 5). Therefore, the platelet:erythrocyte ratio was determined microscopically first from blood, then the actual platelet number was determined by counting the number of erythrocytes. The control platelet number quantified in this manner was ∼75% of the value obtained by automated counting.
For histological analysis of persistent fetal liver hematopoiesis, the livers of P14 pups and neonates were fixed with 3.7% formaldehyde, embedded in paraffin, and then stained with hematoxylin and eosin.
Proplatelet formation assay
Bone marrow cells were collected from femurs and tibias of 6- to 8-week-old mice. Bone marrows were flushed with 2 ml of CATCH medium (Hanks' balanced salt solution, 1 mM adenosine, 2 mM theophyline, 0.38% sodium citrate pH 7.2) into a plastic dish. To enrich the megakaryocytes, marrow cells were centrifuged in 50% Percoll/CATCH solution (density 1.065 g/ml) at 1100 r.p.m. at 20°C for 30 min. The intermediate layer was recovered, put on top of a bovine serum albumin (BSA) density gradient (16% BSA/CATCH at the bottom, 4% BSA/CATCH in the middle and 2% BSA/CATCH at the top) and allowed to stand at room temperature for 1 h. Cells were collected from the bottom and resuspended in Iscove's modified Dulbecco's medium (Gibco) supplemented with 1× Nutridoma-SP (Boehringer Mannheim). These enriched megakaryocytes were cultured in 5% CO2 at 37°C for 24–36 h. An aliquot of the cells was mounted on slides by cytospin (Shandon) and stained with Giemsa.
Acetylcholinesterase activity was detected before counting megakaryocytes for PPF. Cells were fixed with 0.05% paraformaldehyde/phosphate-buffered saline (PBS) at room temperature for 30 min. After washing with PBS, 0.05% acetylthiocholine iodide in 75 mM sodium phosphate buffer pH 6.0, 5 mM sodium citrate, 3 mM CuSO4, 0.5 mM KCNFe3+ was applied to the cells and incubated at 37°C for 30 min. The number of acetylcholinesterase-positive cells displaying obvious filamentous cell projections (e.g. Figure 3D) was counted and the PPF ratio was calculated (Table III).
Scanning electron microscopy
Washed RBCs were attached to a coverslip coated with poly-l-lysine by gravity and then fixed with 1% glutaraldehyde, 150 mM NaCl, 20 mM Tris–HCl pH 7.4 for 1 h. After dehydration with graded ethanol solutions and surface coating with Au/Pd, samples were imaged using a JSM-35CF JEOL SEM. The SEM images shown were pseudocolored in red using Adobe Photoshop.
Osmotic fragility tests
RBCs were recovered from 2-week-old CM or littermate control pups (mafG+/–::mafK+/+ or mafG+/+::mafK–/–) and placed for 5 min in iso-osmotic (0.9%) to extreme hypo-osmotic (0.2%) NaCl solution. After removing unlysed RBCs by centrifugation, the hemoglobin concentration of the supernatant solution was determined by color reaction and the percentage lysis was determined (Briegel et al., 1993).
SDS–PAGE and Western blot analysis
Blood was drawn from P14 animals and RBC numbers were determined using a hemocytometer to normalize for cell number. A 20–30 μl volume (depending on cell number) of drawn blood was placed in an Eppendorf tube and after washing three times with 0.15 M NaCl, 5 mM sodium phosphate pH 8.0, 1 mM EDTA, cells were lysed by the addition of 0.5 ml of 0°C lysing buffer (10 mM sodium phosphate pH 8.0, 1 mM EDTA, 0.15 mM phenylmethylsulfonyl fluoride, 0.04 mM diisopropylfluorophosphate). After 5 min on ice, erythroid membrane proteins were isolated by centrifugation and subsequent washing. These erythroid membranes were electrophoresed on a 5–15% gradient polyacrylamide gel, and proteins were visualized by standard Coomassie Blue staining.
For Western blot analysis, anti-human band 3 polyclonal antibody, anti-mouse dematin polyclonal antibody and anti-human spectrin polyclonal antibody (Sigma) were used. Anti-human band 3 polyclonal antibody is reactive to both erythroid and kidney band 3 proteins (Southgate et al., 1996). The dilution was 1:1000 for primary antibodies and 1:2000 for the appropriate secondary antibodies. Antibody reactivity was visualized by ECL color development (Amersham).
RT–PCR and RNase protection assay
The expression profiles of the mafF, mafG and mafK genes were detected by real-time quantitative PCR (ABI PRISM 7700 Sequence Detection System). RNAs were extracted using ISOGEN (Nippon Gene) from tissues of 8-week-old mice obtained from the same mating colony. cDNAs were synthesized from these RNAs, and real-time PCR was performed as described previously (Onodera et al., 1999). Radiolabeled MafF PCR was performed using conditions described previously (Onodera et al., 1999).
For the RNase protection assays, RNA samples were isolated from 10-week-old mice by ultracentrifugation with guanidine isothiocyanate–cesium chloride. To prepare RNA probes for MafK, MafG and MafF mRNAs, pmMafK5, pmMafG2 and pmMafF2 were constructed. pmMafK5 was prepared by inserting a 0.3 kbp NcoI fragment of mouse MafK cDNA into the NcoI site of pGEM-5Zf(+) (Promega). pmMafG2 was made by inserting a 0.2 kbp PvuII–ApaI fragment of mouse MafG cDNA into EcoRV–ApaI sites of pBluescript SK(+) (Stratagene). pmMafF2 was made by inserting a 0.4 kbp ApaI fragment of mouse MafF cDNA into the ApaI site of pBluescript SK(+). Each of these plasmids was digested with a restriction enzyme recognizing a unique site and then transcribed with Sp6 polymerase (pmMafK5) or with T7 polymerase (pmMafG2 and pmMafF2) in the presence of [α-32P]CTP. The probes were hybridized to 50 μg of total RNA, digested with RNase A and T1, and electrophoresed as described previously (Motohashi et al., 1996).
Acknowledgments
Acknowledgements
We are grateful to Weimin Song for excellent technical assistance and Kim-Chew Lim for critical discussions and advice. We thank Brenda Riley and Athar Chishti for the gift of the anti-band 3 and anti-band 4.9 antisera, Ruby MacDonald for advice in preparing erythroid cell ghosts, Eugene Minner for assistance with SEM and Takuya Komeno for help with the PPF assay. This work was supported by a JSPS postdoctoral fellowship for research abroad (K.O.) and an MSTP training grant to Northwestern University (T32 GM08152; J.A.S.). Research infrastructure support was provided by the Robert H.Lurie Comprehensive Cancer Center (P30 CA60553), an NIH grant (R01 CA80088; J.D.E.) and grants from the Ministry of Education, Science, Sports and Culture (H.M. and M.Y.), JSPS-RFTF and CREST (M.Y.).
References
- Andrews N.C., Erjument-Bromage, H., Davidson, M.B., Tempst, P. and Orkin, S.H. (1993a) Erythroid transcription factor NF-E2 is a haematopoietic-specific basic-leucine zipper protein. Nature, 362, 722–728. [DOI] [PubMed] [Google Scholar]
- Andrews N.C., Kotkow, K.J., Ney, P.A., Erdjument-Bromage, H., Tempst, P. and Orkin, S.H. (1993b) The ubiquitous subunit of erythroid transcription factor NF-E2 is a small basic-leucine zipper protein related to the v-maf oncogene. Proc. Natl Acad. Sci. USA, 90, 11488–11492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briegel K., Lim, K.-C., Plank, C., Beug, H., Engel, J.D. and Zenke, M. (1993) Ectopic expression of a conditional GATA-2/estrogen receptor chimera arrests erythroid differentiation in a hormone-dependent manner. Genes Dev., 7, 1097–1109. [DOI] [PubMed] [Google Scholar]
- Brosius F.C., Alper, S.L., Garcia, A. and Lodish, H. (1989) The major kidney band 3 gene transcript predicts an amino-terminal truncated band 3 polypeptide. J. Biol. Chem., 264, 7784–7787. [PubMed] [Google Scholar]
- Chan J.Y., Han, X.-L. and Kan, Y.-W. (1993) Cloning of Nrfl, an NF-E2-related transcription factor, by genetic selection in yeast. Proc. Natl Acad. Sci. USA, 90, 11371–11375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K., Lu, R., Chan, J.C. and Kan, Y.W. (1996) NRF2, a member of the NFE2 family of transcription factors, is not essential for murine erythropoiesis, growth, and development. Proc. Natl Acad. Sci. USA, 93, 13943–13948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi O.-R. and Engel, J.D. (1986) A 3′ enhancer is required for temporal and tissue-specific transcriptional activation of the chicken adult β–globin gene. Nature, 323, 731–734. [DOI] [PubMed] [Google Scholar]
- Clark M. and Wagner,G.M. (eds) (1989) The Hereditary Hemolytic Anemias. Churchill Livingstone, New York, NY. [Google Scholar]
- Engel J.D. (1994) Meticulous AP-1 factors [Maf]. Nature, 367, 516–517. [DOI] [PubMed] [Google Scholar]
- Farmer S.C., Sun, C.W., Winnier, G.E., Hogan, B.L. and Townes, T.M. (1997) The bZIP transcription factor LCR-F1 is essential for mesoderm formation in mouse development. Genes Dev., 11, 786–798. [DOI] [PubMed] [Google Scholar]
- Foley K.P., McArthur, G.A., Queva, C., Hurlin, P.J., Soriano, P. and Eisenman, R.N. (1998) Targeted disruption of the MYC antagonist MAD1 inhibits cell cycle exit during granulocyte differentiation. EMBO J., 17, 774–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara K., Kataoka, T.K. and Nishizawa, M. (1993) Two new members of the maf oncogene family, mafK and mafF, encode nuclear b–Zip proteins lacking putative trans-activator domain. Oncogene, 8, 2371–2380. [PubMed] [Google Scholar]
- Hesse J.E., Nickol, J.M., Lieber, M.R. and Felsenfeld, G. (1986) Regulated gene expression in transfected primary chicken erythrocytes. Proc. Natl Acad. Sci. USA, 83, 4312–4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi K., Kataoka, K., Itoh, K., Hayashi, N., Nishizawa, M. and Yamamoto, M. (1994) Regulation of transcription by dimerization of erythroid factor NF-E2 p45 with small Maf proteins. Nature, 367, 568–572. [DOI] [PubMed] [Google Scholar]
- Igarashi K., Itoh, K., Hayashi, N., Nishizawa, M. and Yamamoto, M. (1995a) Conditional expression of the ubiquitous transcription factor MafK induces erythroleukemia cell differentiation. Proc. Natl Acad. Sci. USA, 92, 7445–7449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi K., Itoh, K., Motohashi, H., Hayashi, N., Matuzaki, Y., Nakauchi, H., Nishizawa, M. and Yamamoto, M. (1995b) Activity and expression of murine small Maf family protein mafK. J. Biol. Chem., 270, 7615–7624. [DOI] [PubMed] [Google Scholar]
- Itoh K., Igarashi, K., Hayashi, N., Nishizawa, M. and Yamamoto, M. (1995) Cloning and characterization of a novel erythroid cell-derived CNC family transcription factor heterodimerizing with the small Maf family proteins. Mol. Cell. Biol., 15, 4184–4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K. et al. (1997)An Nrf2/small maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun., 236, 313–322. [DOI] [PubMed] [Google Scholar]
- Johnsen O., Skammelsrud, N., Luna, L., Nishizawa, M., Prydz, H. and Kolsto, A.B. (1996) Small Maf proteins interact with the human transcription factor TCF11/Nrf1/LCR-F1. Nucleic Acids Res., 24, 4289–4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka K., Nishizawa, M. and Kawai, S. (1993) Structure–function analysis of the maf oncogene product, a member of the b-zip protein family. J. Virol., 67, 2133–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka K., Fujiwara, K.T., Noda, M. and Nishizawa, M. (1994a) MafB, a new maf family transcription activator that can associate with Maf and Fos, but not with Jun. Mol. Cell. Biol., 14, 7581–7591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka K., Noda, M. and Nishizawa, M. (1994b) Maf nuclear oncoprotein recognizes sequences related to an AP-1 site and forms heterodimers with fos and jun. Mol. Cell. Biol., 14, 700–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka K., Igarashi, K., Itoh, K., Fujiwara, K.T., Noda, M., Yamamoto, M. and Nishizawa, M. (1995) Small Maf proteins heterodimerize with Fos and may act as competitive repressors of the NF-E2 transcription factor. Mol. Cell. Biol., 15, 2180–2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi A., Itoh, E., Toki, T., Takahashi, S.-i., Igarashi, K., Hayashi, N. and Yamamoto, M. (1999) Molecular cloning and functional characterization of a new CNC family transcription factor Nrf3. J. Biol. Chem., 274, 6443–6452. [DOI] [PubMed] [Google Scholar]
- Kotkow K.J. and Orkin, S.H. (1996) Complexity of the erythroid transcription factor in NF-E2 as revealed by gene targeting of the mouse p18 locus. Proc. Natl Acad. Sci. USA, 93, 3514–3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroha T., Takahashi, S., Komeno, T., Itoh, K., Nagasawa, T. and Yamamoto, M. (1998) Ablation of Nrf2 function does not embellish erythroid or megakaryocytic cell lineage dysfunction caused by p45 NF-E2 gene disruption. J. Biochem., 123, 376–379. [DOI] [PubMed] [Google Scholar]
- Mignotte V., Eleouet,J.F., Raich,N. and Romeo,P.-H. (1989) Cis- and trans-acting elements involved in the regulation of the erythroid promoter of the human porphobilinogen deaminase gene. Proc. Natl Acad. Sci. USA, 86, 6548–6552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler J., Vani, K., Leung, S. and Epstein, A. (1991) Segmentally restricted, cephalic expression of a leucine zipper gene during Drosophila embryogenesis. Mech. Dev., 34, 3–9. [DOI] [PubMed] [Google Scholar]
- Motohashi H., Igarashi, K., Onodera, K., Takahashi, S., Ohtani, H., Nakafuku, M., Nishizawa, M., Engel, J.D. and Yamamoto, M. (1996) Mesodermal- vs. neuronal-specific expression of MafK is elicited by different promoters. Genes Cells, 1, 223–238. [DOI] [PubMed] [Google Scholar]
- Motohashi H., Shavit, J.A., Igarashi, K., Yamamoto, M. and Engel, J.D. (1997) The world according to Maf. Nucleic Acids Res., 25, 2953–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahisa H., Nagata, Y., Ohnuki, T., Osada, M., Nagasawa, T., Abe, T. and Todokoro, K. (1996) Bone marrow stromal cells produce thrombopoietin and stimulate megakaryocyte growth and maturation but suppress proplatelet formation. Blood, 87, 1309–1316. [PubMed] [Google Scholar]
- Nishizawa M., Kataoka, K., Goto, N., Fujiwara, K. and Kawai, S. (1989) v-maf, a viral oncogene that encodes a ‘leucine zipper’ motif. Proc. Natl Acad. Sci. USA, 86, 7711–7715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogino H. and Yasuda, K. (1998) Induction of lens differentiation by activation of a bZIP transcription factor, L-Maf. Science, 280, 115–118. [DOI] [PubMed] [Google Scholar]
- Onodera K., Shavit, J.A., Motohashi, H., Katsuoka, F., Akasaka, J., Engel, J.D. and Yamamoto, M. (1999) Characterization of the murine mafF gene. J. Biol. Chem., 274, 21162–21169. [DOI] [PubMed] [Google Scholar]
- Osada M., Komeno, T., Todokoro, K., Takizawa, M., Kojima, H., Suzukawa, K., Ninomiya, H., Abe, T. and Nagasawa, T. (1999) Immature megakaryocytes undergo apoptosis in the absence of thrombopoietin. Exp. Hematol., 27, 131–138. [DOI] [PubMed] [Google Scholar]
- Oyake T., Itoh, K., Motohashi, H., Hayashi, N., Hoshino, H., Nishizawa, M., Yamamoto, M. and Igarashi, K. (1996) Bach proteins belong to a novel family of BTB-basic leucine zipper transcription factors that interact with mafK and regulate transcription through the NF-E2 site. Mol. Cell. Biol., 16, 6083–6095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana A.P., Ruff, P., Maalouf, G.J., Speicher, D.W. and Chishti, A.H. (1993) Cloning of human erythroid dematin reveals another member of the villin family. Proc. Natl Acad. Sci. USA, 90, 6651–6655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitman M. and Felsenfeld, G. (1988) Mutational analysis of the chicken β-globin enhancer reveals two positive-acting domains. Proc. Natl Acad. Sci. USA, 85, 6267–6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shavit J.A., Motohashi, H., Onodera, K., Akasaka, J., Yamamoto, M. and Engel, J.D. (1998) Impaired megakaryopoiesis and behavioral defects in mafG-null mutant mice. Genes Dev., 12, 2164–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivdasani R.A. and Orkin, S.H. (1995) Erythropoiesis and globin gene expression in mice lacking the transcription factor NF-E2. Proc. Natl Acad. Sci. USA, 92, 8690–8694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivdasani R.A., Rosenblatt, M.F., Zucker-Franklin, D.C., Jackson, C.W., Hunt, P., Saris, C.J. and Orkin, S.H. (1995) Transcription factor NF-E2 is required for platelet formation independent of the actions of thrombopoietin/MGDF in megakaryocyte development. Cell, 81, 695–704. [DOI] [PubMed] [Google Scholar]
- Southgate C.D., Chishti, A.H., Mitchell, B., Yi, S.J. and Palek, J. (1996) Targeted disruption of the murine erythroid band 3 gene results in spherocytosis and severe haemolytic anemia despite a normal membrane skeleton. Nature Genet., 14, 227–230. [DOI] [PubMed] [Google Scholar]
- Swaroop A., Xu, J., Pawar, H., Jackson, A., Scolnick, C. and Agarwal, N. (1992) A conserved retina-specific gene encodes a basic motif/leucine zipper protein. Proc. Natl Acad. Sci. USA, 89, 266–270. [DOI] [PMC free article] [PubMed] [Google Scholar]