Abstract
With the emergences of engineered devices at microscale and nanoscale dimensions, there is a growing need for controlled actuation and transport at these length scales. The kinesin–microtubule system provides a highly evolved biological transport system well suited for these tasks. Accordingly, there is an ongoing effort to create hybrid nanodevices that integrate biological components with engineered materials for applications such as biological separations, nanoscale assembly, and sensing. Adopting microtubules for these applications generally requires covalent attachment of biotin, fluorophores, or other biomolecules to tubulin enable surface or cargo attachment, or visualization. This review summarizes different strategies for functionalizing microtubules for application-focused as well as basic biological research. These functionalization strategies must maintain the integrity of microtubule proteins so that they do not depolymerize and can be transported by kinesin motors, while adding utility such as the ability to reversibly bind cargo. The relevant biochemical and electrical properties of microtubules are discussed, as well as strategies for microtubule stabilization and long-term storage. Next, attachment strategies, such as antibodies and DNA hybridization that have proven useful to date, are discussed in the context of ongoing hybrid nanodevice research. The review concludes with a discussion of less explored opportunities, such as harnessing the utility of tubulin posttranslational modifications and the use of recombinant tubulin that may enable future progress in nanodevice development.
Keywords: Fluorescence, Biotinylation, Microfabrication, Nanotechnology, Kinesin, Cytoskeleton
Introduction
Eukaryotic cells utilize a complex system of motor proteins and cytoskeletal filaments to ensure proper transport of intracellular cargo. Microtubules are integral components of this transport system as well as important mechanical elements that contribute to cell shape and stiffness. Kinesin motor proteins bind to a variety of cargo, including vesicles, chromosomes, and other microtubules, and utilize the chemical energy from ATP hydrolysis to transport these cargo along microtubules. At present, there is an ongoing effort to develop hybrid nanodevices for various applications in biotechnology, such as nanoscale devices that can transport, sort, and/or organize proteins, DNA, and nanoparticles. The kinesin–microtubule system is an ideal biological transport system to integrate into such hybrid devices, and significant progress has been made in this area in recent years.
The first synthetic systems incorporating kinesin motors and microtubules were envisioned as “molecular shuttles”—simple transport or assembly devices integrating engineered materials and biological components (Dennis et al. 1999; Hess and Vogel 2001). Several features characterize these devices, including directional guidance, cargo loading and unloading, and regulation of movement (Hess and Vogel 2001). Research in this area has focused on improving these features and has progressed such that molecular shuttles are now envisioned as “pharmacytes” (i.e., self-powered drug delivery devices) (Freitas 2006), “smart dust” biosensors (Bachand et al. 2009; Fischer et al. 2009), and lab-on-a-chip systems (Hiyama et al. 2010).
Microtubule functionalization has played, and continues to play, a critical role in making the jump from in vivo intracellular transport to in vitro nanoscale device applications. Functionalization is defined as the addition of a functional chemical group, typically through covalent linkages, resulting in improved utility of the final product. In the case of microtubules, such chemical functionalization produces tubulin that can be easily visualized and can be further manipulated to improve applicability to nanoscale devices. By covalently labeling tubulin with biotin, many other molecules such as DNA and antibodies can then be noncovalently linked to microtubules; because of their broad utility, we include these combined covalent/noncovalent attachment strategies here under the general definition of “microtubule functionalization.” Most importantly, functionalized tubulin frequently functions quite similarly to native tubulin, thereby allowing the in vivo kinesin/microtubule transport system to be exploited for in vitro applications.
The ability to modify tubulin is an important enabling step for many nanoscale engineering applications, and improvements in techniques for functionalizing microtubules have contributed significantly to advancements in this field. The focus of this review is the role of microtubule functionalization in the development of hybrid nanoscale devices. Because this application-directed research is inextricably linked to fundamental research on cytoskeletal function, some of the important fundamental work on which this recent work rests will also be covered. After describing characteristics of microtubules and nanoscale devices, we discuss common approaches to microtubule functionalization and current applied research. We conclude with a discussion of potential future directions to maximize the functionality of microtubules in hybrid devices.
Biochemical properties of microtubules
Microtubules consist of α and β heterodimers of tubulin that polymerize to form hollow tube-like filaments with 25-nm diameters and lengths of up to tens of microns (Ledbetter and Porter 1963; Nogales et al. 1999). Microtubules undergo dynamic instability—alternating phases of slow growth and rapid shrinkage—by the addition and loss of tubulin subunits from the microtubule ends (Cassimeris et al. 1987). Microtubule plus-ends, which are more dynamic, are generally found at the cell periphery while microtubule minus-ends are anchored in or near the centrosome. In cells, tubulin associates with a variety of proteins (i.e., microtubule associated proteins—MAPs), including the two main categories of structural MAPs and motor proteins, such as kinesins (Mandelkow and Mandelkow 1995).
Tubulin is enriched in neurons and can be purified from brain tissue, typically from cows and pigs. Several protocols exist outlining this process, mainly using cycles of polymerization and depolymerization to ensure removal of MAPs and isolation of functional dimers (Williams and Lee 1982; Castoldi and Popov 2003; Uppalapati et al. 2009). The tubulin can be polymerized in vitro and microtubules stabilized by the addition of taxol (Schiff et al. 1979).
Purified native tubulin can then be further functionalized through a variety of labeling processes, thereby providing the raw material necessary for nanoscale device transport applications. Labeling amine groups on exposed lysine residues by small molecules containing reactive succinimidyl ester groups has proven very successful (Hyman et al. 1991; Uppalapati et al. 2009), while labeling cysteine residues with sulfhydryl-reactive compounds has been shown to be detrimental to microtubules (Boal et al. 2006). Tubulin labeled at low stoichiometries generally functions similarly to native tubulin with respect to kinesin interactions. However, at high labeling stoichiometries, functionalization can decrease motility speed depending on the motor density and specific kinesin isoforms used (Korten and Diez 2006). It should be noted that labeling reactions often result in low yields (less than 10%), so there is clearly room for improvement in tubulin labeling strategies. Purified native and labeled tubulin are also commercially available from Cytoskeleton, Inc. (Denver, CO).
Electrical properties of microtubules
Tubulin dimers and assembled microtubules have an overall negative charge at physiological pH due to a glutamate-rich C-terminal extension (Vassilev and Kanazirska 1985; Lowe et al. 2001; Stracke et al. 2002). The negative charge has two implications for in vitro applications. First, it allows microtubules to undergo electrophoresis in DC electrical fields. During electrophoresis, microtubules can be aligned or directed towards the positive electrode (Stracke et al. 2002; van den Heuvel et al. 2005). However, ions in the buffer can also be subjected to electrophoresis, which can result in damage to the electrodes and depolymerization of the microtubules (Uppalapati et al. 2008a).
The second implication of the negative charge of microtubules is that it attracts counter ions present in the buffer solution, creating a cation shield (Minoura and Muto 2006; Uppalapati et al. 2008b). Because these electrostatic interactions are nonspecific and noncovalent, microtubules can be polarized in AC electric fields. Through this polarization process, microtubules can be subjected to positive dielectrophoretic forces and manipulated using microfabricated electrodes to achieve accumulation and immobilization of the filaments in solution (Jia et al. 2004; Minoura and Muto 2006; Uppalapati et al. 2008a). Positive dielectrophoresis, under the proper conditions, eliminates the disadvantages of electrophoresis, providing a better avenue for targeted microtubule alignment. In addition to being able to manipulate native microtubules using electric fields, microtubules functionalized with nanoparticles can be aligned or moved in electric or magnetic fields via forces operating on the attached nanoparticles (Platt et al. 2005; Hutchins et al. 2006a).
Storage conditions
Long-term stability is an important design constraint in the construction of hybrid nanoscale devices using the kinesin–microtubule system. In order for nanoscale devices to be feasible for diverse applications, the devices must be able to maintain functionality (i.e., microtubule stability) for adequate time periods to accommodate shipping and handling. An initial approach taken for extending the lifetime of microtubules was to use crosslinking agents to fix polymerized microtubules (Turner et al. 1996). Glutaraldehyde treatment results in chemically fixed microtubules with improved thermo- and chemostability and motility characteristics similar to nonfixed microtubules (Brown and Hancock 2002; Boal et al. 2006). Innovative functionalization techniques, such a posttranscriptional modifications (Pucciarelli et al. 1997) or genetically engineered tubulin (Gupta et al. 2001), may also provide avenues for improved stability of the microtubule polymer.
Other methods used to improve long-term stability of devices incorporating microtubules are freezing, lyophilizing, or critical point drying the intact device (Seetharam et al. 2006; Uppalapati et al. 2008b). Microtubule functionality is best maintained by lyophilization, and devices stored up to 40 days were shown to maintain function when reconstituted with motility solution (Seetharam et al. 2006).
General features of nanoscale devices
Hybrid nanoscale devices are envisioned as miniaturized factories, able to sort complex collections of molecules, deliver specific molecules to targeted areas, or sense environmental agents. Although actualization of these devices is still in the early stages, there are three features that are common in their vision: appropriate guidance of transport, loading/unloading of cargo, and controllable movement (Hess and Vogel 2001). As several recent reviews have focused on features of these devices (Hess et al. 2004; Agarwal and Hess 2010a, b; Korten et al. 2010), we will not present an exhaustive review here. Instead we will briefly describe key features of these systems to better understand how functionalized microtubules play a role in their development.
There are two geometries used for transport in hybrid nanoscale devices: immobilized motor proteins that transport functionalized microtubules and immobilized microtubules that create paths for cargo-transporting motor proteins. Guidance—controlling the direction of transport—can be achieved through either physical or chemical methods (Clemmens et al. 2003). Physical guidance approaches utilize mechanical constraints (most commonly microscale walls created through surface patterning and lithography techniques) to organize, align, and guide microtubules moving on immobilized kinesins (Stracke et al. 2000; Hess and Vogel 2001; Hiratsuka et al. 2001; Hess et al. 2002a; Clemmens et al. 2003; Jia et al. 2004; Cheng et al. 2005; van den Heuvel et al. 2005). This geometrical approach has proven very effective for guiding kinesin-driven microtubule transport.
The alternate approach chemically modifies surfaces to spatially control the immobilization of microtubule tracks or motor proteins (Clemmens et al. 2003, 2004; Jia et al. 2004; van den Heuvel et al. 2005; Lin et al. 2008; Uppalapati et al. 2009). While techniques have been developed to immobilize motors in spatially defined regions, the purely chemical approach to guiding microtubules falls short because the microtubules tend to simply run off of the motor-functionalized region of the surface and diffuse away (Hess et al. 2002b; Verma et al. 2009). As will be discussed below, patterning surface chemistry is an effective way to selectively immobilize microtubule tracks for guiding kinesin transport.
Specific loading and unloading of cargo is another requirement for hybrid nanoscale devices. To facilitate this process, microtubules can be functionalized with specific linkers to bind targeted molecules. Three main linkages are used in this process: biotin/streptavidin bridges, antibody/antigen interactions, and DNA hybridization. In many cases, the latter two mechanisms also utilize biotin–streptavidin interactions to achieve final functionalization of microtubules.
A final requirement for integrating the kinesin–microtubule system into nanoscale devices is the ability to turn transport on and off. One approach to date involves sequestering and releasing ATP to achieve an “on/off” switch (Hess et al. 2001; Yokokawa et al. 2003; Tucker et al. 2008). Greene and coworkers engineered a zinc binding site into conventional kinesin and showed that increasing the zinc concentration inhibited motility (Greene et al. 2008). Tomishige and Vale engineered cysteines into the neck linker domain and showed that the motor can be regulated by reversible disulfide linking (Tomishige and Vale 2000).
Functionalizing microtubules with fluorescent labels
The earliest form of microtubule functionalization involved the addition of fluorophore “labels” (Fig. 1a). Fluorophores are covalently linked to the surface of polymerized tubulin (as opposed to free tubulin subunits in solution), thereby ensuring that proper assembly is not affected by the addition of the label (Keith et al. 1981). Fluorescein was the first fluorochrome used, by means of dichlorotriazinyl fluorescein labeling (Keith et al. 1981; Leslie et al. 1984). Although successful in labeling microtubules, fluorescein photobleaches easily and is problematic for observation (Leslie et al. 1984; Kellogg et al. 1988). Fluorescent labeling with the fluorochrome rhodamine, attached to tubulin using a succinimidyl ester form of the fluorophore, improved visualization and is still a popular form of fluorescent functionalization used today, in addition to the quantum dots described in the next section (Kellogg et al. 1988; Hyman et al. 1991; Uppalapati et al. 2009). Alexa dyes, which can be attached to tubulin in the similar mechanism as rhodamine, have also been adopted due to their improved emission quantity and photostability compared to rhodamine (Panchuk-Voloshina et al. 1999).
Fig. 1.
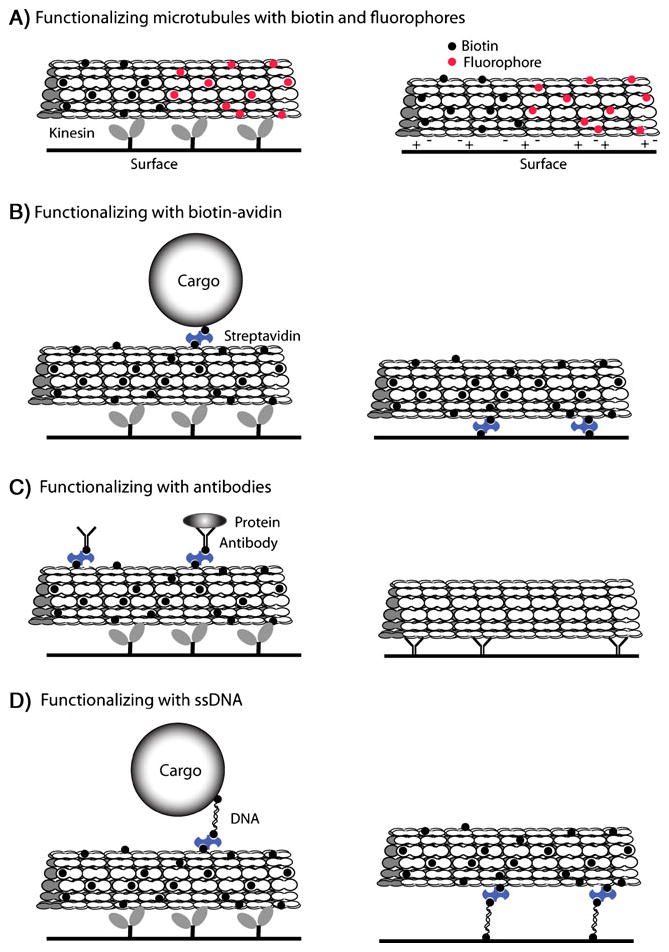
Approaches for functionalizing microtubules. Kinesin–microtubule transport can be integrated into hybrid nanodevices by two different strategies: cargo-carrying microtubules moved by immobilized motors (left column) and cargo-transporting motors moving along immobilized microtubules (right column, motor and cargo not shown). a Microtubules functionalized with biotin and/or fluorophores support transport and can be bound to surfaces through nonspecific electrostatic interactions. b Biotin–avidin can be used to link cargo or to immobilize microtubules to selected regions of a surface. c By linking with antibodies, specific protein targets can be transported, and tubulin antibodies can also be used as a microtubule immobilization strategy. d ssDNA can be used to reversibly link cargo to moving microtubules, or as strategy for reversibly linking microtubules to surfaces
The ability to fluorescently label microtubules allowed for their visualization by fluorescence microscopy, enabling experiments to explore in vivo microtubule function, as well as the use of microtubules in in vitro applications. Moreover, different segments of microtubules (i.e., either the plus end or the minus end) can be labeled to different degrees or by different fluorophores, allowing for the characterization of microtubule growth or visualization of microtubule polarity (Vale et al. 1992).
Functionalizing microtubules with biotin–streptavidin
It is well established that biotin and streptavidin bind with both high affinity and high specificity, making them a popular nano-”adhesive” (Helm et al. 1991; Wong et al. 1999). Streptavidin, a tetrameric protein isolated from Streptomyces avidinii, is evolutionarily unrelated to avidin, another notable biotin-binding protein (Wilchek and Bayer 1989), though it is functionally similar. Streptavidin lacks the carbohydrate modifications seen in avidin; therefore, it leads to less nonspecific binding (Wilchek and Bayer 1989). Additionally, an alternate deglycosylated form of avidin, called NeutrAvidin, is commercially available and functions similarly to avidin and streptavidin (Hiller et al. 1987).
The standard protocol for biotinylating microtubules is to react biotin-XX sulfosuccinimidyl ester with polymerized microtubules, quench the reaction, and separate unreacted biotin by centrifugation (Hyman et al. 1991; Uppalapati et al. 2009). The succinimidyl ester moiety reacts with exposed lysine residues on tubulin and the XX denotes a 14-atom spacer that improves accessibility of the biotin to streptavidin. The ability to bind biotinylated microtubules to streptavidin-coated surfaces has been exploited to deduce fundamental characteristics of the tubulin protein, such as loads necessary for buckling (Gittes et al. 1996), the strength of ligand-receptor interactions (Hess et al. 2002a), motor protein responses to complex intersections (Ross et al. 2008), and patterns of self assembly (Nedelec et al. 1997; Hess et al. 2005). More recently, the same interactions have facilitated selective loading of cargo to biotinylated microtubules in in vitro applications.
Early microtubule shuttles utilized rhodamine-labeled, biotinylated microtubules that were capable of gliding over immobilized kinesin while transporting cargo, such as streptavidin-coated beads (Hess et al. 2001) or biotin–streptavidin functionalized DNA (Diez et al. 2003) (Fig. 1b). An important detail of these experiments was that the microtubules are functionalized with both biotin and rhodamine, enabling both visualization and cargo loading. In a study that points to the possibility of kinesin–microtubule-driven drug delivery systems, Kato et al. used streptavidin to attach biotinylated cyclodextrin molecules to biotinylated microtubules and showed that the cyclodextrin could capture a fluorescent target molecule (Kato et al. 2005).
The transport of streptavidin-coated quantum dots by the kinesin–microtubule system provides an example of biologically based transport of an inorganic cargo (Bachand et al. 2004; Reuther et al. 2006). Magnetic nanoparticles have also been attached to microtubules as a way to direct the movement of kinesin-driven microtubule transport through external magnetic fields (Platt et al. 2005; Hutchins et al. 2007). Because of the unique optical, electrical, and magnetic properties of nanoparticles, these experiments point the way toward future kinesin–microtubule-based approaches for assembling nanoscale materials into more functional geometries. Due to their larger size, binding nanoparticle cargo to microtubules can disrupt normal kinesin/microtubule interactions, particularly when high densities of nanoparticles are used (Hutchins et al. 2006a, b, 2007). One approach to avoiding this problem has been to polymerize segmented microtubules containing one region functionalized with biotin, and other segments made from native or simply fluorescently labeled tubulin; the biotin region is then used to attach cargo and the non-biotinylated region interacts with immobilized kinesin motors (Bachand et al. 2004; Hutchins et al. 2006a).
While biotin–avidin interactions are an efficient way to attach defined cargo to microtubules, this strategy is less effective for preferentially binding specific molecules in a complex mixture because the molecule of interest must be biotinylated. Additionally, the strength of the biotin/streptavidin interaction precludes efficient unloading of cargo. To overcome these obstacles, biotin/streptavidin bridges have been used to functionalize microtubules with antibodies, which allow for binding to diverse targets, and single-stranded DNA, which hybridizes specifically and reversibly to target containing complementary sequences.
Functionalizing microtubules with antibodies
Antibody–antigen binding represents a highly specific interaction based on a combination of noncovalent hydrogen bonds and van der Waals contacts (Davies et al. 1990). The inherent specificity can be harnessed by nanoscale devices to sort molecules with limited, or no, manipulation of the target molecule. The simplest approach is to bind a biotinylated antibody to a biotinylated microtubule through a streptavidin bridge (Ramachandran et al. 2006) (Fig. 1c). As this antibody-functionalized microtubule moves across a kinesin surface, it can pull target molecules out of solution and transport them to a specific site.
A more advanced approach to antibody functionalization is to utilize biotinylated microtubules to create “double-antibody sandwiches” (Ramachandran et al. 2006). In the double-antibody sandwich strategy, a molecular cargo binds to an antibody-functionalized microtubule, and the cargo is then visualized by binding a second, fluorescently tagged antibody that is complementary to a different region of the same target molecule. Microtubules functionalized with double-antibody sandwich structures maintained motility similar to nonfunctionalized microtubules, suggesting this approach does not hinder dynamics of the kinesin/microtubule interaction (Ramachandran et al. 2006). Biotinylated microtubules and streptavidin have also been used to create microtubules functionalized with two biotinylated antibodies, allowing for the targeting of two distinct molecules (Rios and Bachand 2009).
A potential problem of the double-antibody sandwich strategy is that streptavidin can bind to multiple biotin molecules, resulting in the formation of unwanted microtubule–microtubule or microtubule–antibody complexes. To preclude this possibility, microtubules have been directly functionalized with antibodies through covalent crosslinking (Bachand et al. 2006; Soto et al. 2008; Carroll-Portillo et al. 2009). To maximize function, antibodies can be bound to microtubules in a preferred orientation (e.g., through the Fc domain), which orients the antigen-binding Fab region away from the microtubule surface (Bachand et al. 2009).
Although noncovalent bonds mediate antibody–antigen interactions, the interactions are relatively strong and stable due to the fact that multiple noncovalent bonds occur over a large area between the antibody–antigen interface (Davies et al. 1990; Hinterdorfer et al. 1996). Because the ability to unload cargo is a generally a desirable feature in hybrid nanoscale devices, particularly if sorting of complex mixtures is the goal, it is important to be able to dissociate the antigen from the antibody. Conditions promoting dissociation of antibody–antigen bonds include the addition of chaotropic agents, an increase or decrease in the pH, or an increase in the temperature or ionic strength of the buffer (van Oss et al. 1986). These conditions may be detrimental or impossible to successfully achieve in hybrid nanoscale devices. A different approach for cargo attachment to microtubules that is inherently much more reversible is attachment through complementary DNA hybridization.
Functionalizing microtubules with single-stranded DNA
DNA hybridization is an appealing attachment strategy because the interaction of complementary single-stranded DNA (ssDNA) oligonucleotides is highly specific, and the strength of hybridization can be controlled by the length and AT content of the complementary sequence. In a traditional, antiparallel duplex, longer stretches of hybridized DNA require larger forces to separate the strands (i.e., all nucleotide pairs must be broken simultaneously) (Strunz et al. 1999). Alternatively, DNA hybridization can be arranged in a “zipper” geometry, where single nucleotide pairs are broken sequentially, thus requiring lower forces (Albrecht et al. 2003; Kufer et al. 2008).
Biotinylated oligonucleotides can be purchased at low cost; therefore, the principal strategy for functionalizing microtubules has been to bridge biotinylated ssDNA and biotinylated microtubules through streptavidin (Fig. 1d) (Muthukrishnan et al. 2004; Taira et al. 2006; Brunner et al. 2007; Schmidt and Vogel 2010). Oligonucleotides can also be directly linked to microtubules through crosslinking reactions (Hiyama et al. 2009, 2010). DNA hybridization has been used both as a strategy for immobilizing microtubules at defined sites on a surface and for loading or unloading cargo to microtubule shuttles being transported by surface-immobilized kinesins.
The first study to use microtubules functionalized with ssDNA demonstrated that hybridization could be used to immobilize microtubule tracks at defined locations on glass surfaces. Properly oriented microtubule tracks are an important feature of nanoscale devices in which the cargo is directly attached to kinesin motors, as it ensures transport to defined destinations. Microcontact printing with a polydimethylsiloxane stamp was used to pattern neutravidin at specific sites on a surface (Muthukrishnan et al. 2004), and biotinylated ssDNA molecules were then linked to these regions of the surface. Microtubules, functionalized at each end with ssDNA complementary to the surface-immobilized strands, were introduced and allowed to bind through DNA hybridization (Fig. 1d). The microtubules were shown to retain their function as tracks for kinesin motors; hence, by defining the spatial position of immobilized ssDNA on the surface, this approach provides a flexible mechanism for defining microtubule placement on surfaces.
Rather than defining transport tracks, DNA hybridization can also be used to reversibly link specific cargo to functionalized microtubule shuttles (Taira et al. 2006; Hiyama et al. 2010; Schmidt and Vogel 2010). The basic approach is to functionalize microtubules and cargo with complementary ssDNA oligonucleotides, such that when mixed, the cargo binds to the functionalized microtubule shuttle and is transported along the surface (Taira et al. 2006; Hiyama et al. 2010). In principle, heating the solution in specific regions of the device could be used to dissociate the cargo from the microtubule (i.e., melt the DNA double helix).
An improvement to this approach was to create loading and unloading stations on the surface, where cargo is picked up and deposited through hybridization events (Hiyama et al. 2010; Schmidt and Vogel 2010). An important initial demonstration was to show that forces generated by the kinesin motors are sufficient to shear DNA complexes (Brunner et al. 2007). Reversible cargo loading was implemented as follows. First, cargo is functionalized with an oligonucleotide that has two regions of complementary: a distal region complementary to an immobilized surface-oligonucleotide (i.e., the loading or unloading station) and a central region complementary to a sequence on the microtubules. The functionalized microtubules are transported across a surface by immobilized kinesin motors, and they travel though a loading station containing DNA-tethered cargo. At the loading station, the hybridization of the microtubule oligonucleotide to the cargo oligonucleotide competes with surface immobilization and results in the cargo shearing from the surface. Cargo is transported a distance and then unloaded at the unloading station through another hybridization event (Hiyama et al. 2009, 2010; Schmidt and Vogel 2010). Differential hybridization strengths are achieved by using different geometries (i.e., traditional versus zippering) and different lengths of complementarity.
DNA hybridization enables selective loading, transport, and unloading of cargo in hybrid nanoscale devices, an important milestone toward the ultimate goal of creating devices such as “pharmacytes” (Freitas 2006) for industrial or medical applications. The ability to selectively bind ssDNA to moving microtubules also sets the stage for future applications such as sorting of mRNA species for expression profiling studies or binding and transport of viral RNA or DNA for isolation or sensing applications. However, for this system to be of utility, it is important to be able to detect when specific target sequences have bound to the moving microtubules (analogous to the double-antibody sandwich to detect proteins bound to microtubules). Molecular beacons are ssDNA stem-loop structures labeled with fluorophores on each end (Tyagi and Kramer 1995; Raab and Hancock 2008). In the resting state, resonance energy transfer between the donor and acceptor fluorophores quenches fluorescence. However, when a complementary ssDNA oligonucleotide binds to the loop sequence, the donor is unquenched and fluorescence is observed. Hence, the beacons provide both a DNA-targeting function as well as reporting when the target DNA is bound. Microtubules functionalized with molecular beacons retain their kinesin transport properties, and the system can report the presence of specific ssDNA sequences fluorescently (Raab and Hancock 2008), making the system suitable for not just sorting, but also detecting target nucleotide sequences in heterogeneous samples.
A final ssDNA-based approach is the use of DNA or RNA aptamers, oligonucleotides that bind to specific target sequences in a similar mechanism as antibodies (Ellington and Szostak 1990). Aptamers can be bound to dyes, such as malachite green, to create conjugates that function similar to molecular beacons (Hirabayashi et al. 2006). Biotinylated aptamers can be bridged to microtubules with streptavidin, and bound targets can be eluted under conditions that preserve microtubule function, allowing for an unloading mechanism.
Innovative microtubule functionalization—future directions
The techniques discussed thus far represent an array of strategies researchers have used to functionalize microtubules to date. However, this list of chemical modifications is actually rather narrow and there are other innovative avenues of microtubule functionalization that have the potential to propel the field of hybrid nanoscale devices forward. Two such areas include tubulin posttranscriptional modifications (PTMs) and the use of recombinant tubulin.
PTMs are reversible covalent modifications that typically occur on the carboxyl end of α- and β-tubulin monomers, where many proposed protein/protein interactions occur (MacRae 1997; Westermann and Weber 2003). It is thought that PTMs generate functional diversity of microtubules in vivo because different motor proteins and MAPs interact with different subsets of PTM microtubules (Hammond et al. 2008). PTMs of tubulin include detyrosination, polyglutamylation, phosphorylation, and acetylation (Westermann and Weber 2003). Detyrosination, which involves removing the terminal tyrosine, polyglutamylation, which add glutamate to the C-terminal tail, and acetylation, which modifies the side chain of lysine-40, all result in selective binding of MAPs [e.g., plus-tip tracking proteins prefer tyrosinated microtubules (Hammond et al. 2008)]. With continued research concerning these modifications, it is possible to envision nanoscale devices that utilize multiple shuttles or tracks consisting of different posttranslationally modified microtubules, each binding a different kinesin and/or having cargo such as nanoparticles, DNA, or proteins attached through PTM-specific MAPs.
Detyrosination and acetylation, along with phosphorylation, are also thought to enhance the stability of microtubules (Hammond et al. 2008; Janke and Kneussel 2010). Microtubule stability is an important characteristic to consider when seeking to improve storage conditions and longevity of nanoscale devices. Therefore, these modifications could be used to create stable microtubule shuttles or tracks with targeted binding of different sets of MAPs.
Recombinant tubulin offers another possibility for microtubule functionalization, by creating tubulin building blocks with modifications encoded at the DNA level. Recombinant expression could, in principle, enable tubulin isoforms that are naturally fluorescent or biotinylated, or which have reactive side chains in site-specific locations to enhance microtubule functionality and cargo attachment. However, while bacterial expression of tubulin could theoretically produce a vast supply of tubulin, this approach has been hindered to date by low yield, low tubulin solubility, and aggregation in inclusion bodies (MacDonald et al. 2003). In recent work, functional recombinant tubulin from protozoa (MacDonald et al. 2003), nematodes (Oxberry et al. 2001), and plants (Jang et al. 2008) have been bacterially expressed and purified. This recombinant tubulin polymerizes to form microtubules, but whether these microtubules support kinesin motility is yet to be determined. Further research in this direction may pave the way for novel tubulin isoforms engineered for use in hybrid nanoscale devices.
The development of nanodevices powered by biomolecular motors is a true interdisciplinary effort that combines advances in nanoscience and microscale engineering with advances in biophysics and cell biology. Innovations in microtubule functionalization have been a driving force in the development of this field and have enabled key features central to hybrid devices: guidance, cargo loading and unloading, and regulation of movement. Although widely used functionalization strategies such as biotin–streptavidin interactions opened the door to these advancements, further exploration of alternative areas may reveal functionalization possibilities for the next generation of nanoscale devices.
Acknowledgments
J.L.M was supported by NSF grant MCB 0920911 and W.O.H was supported by NIH grant GM083297.
Contributor Information
Jennelle L. Malcos, Department of Biology, The Pennsylvania State University, 208 Muller Lab, University Park, PA 16802, USA
William O. Hancock, Email: wohbio@engr.psu.edu, Department of Bioengineering, The Pennsylvania State University, 205 Hallowell Building, University Park, PA 16802, USA
References
- Agarwal A, Hess H. Biomolecular motors at the intersection of nanotechnology and polymer science. Prog Polym Sci. 2010a;35:252–277. [Google Scholar]
- Agarwal A, Hess H. Molecular motors as components of future medical devices and engineered materials. J Nanotech Eng Med. 2010b;1:1–9. [Google Scholar]
- Albrecht C, Blank K, Lalic-Multhaler M, Hirler S, Mai T, Gilbert I, Schiffmann S, Bayer T, Clausen-Schaumann H, Gaub HE. DNA: a programmable force sensor. Science. 2003;301:367–370. doi: 10.1126/science.1084713. [DOI] [PubMed] [Google Scholar]
- Bachand GD, Rivera SB, Boal AK, Gaudioso J, Liu J, Bunker BC. Assembly and transport of nanocrystal CdSe quantum dot nanocomposites using microtubules and kinesin motor proteins. Nano Lett. 2004;4:817–821. [Google Scholar]
- Bachand GD, Rivera SB, Carroll-Portillo A, Hess H, Bachand M. Active capture and transport of virus particles using a biomolecular motor-driven, nanoscale antibody sandwich assay. Small. 2006;2:381–385. doi: 10.1002/smll.200500262. [DOI] [PubMed] [Google Scholar]
- Bachand GD, Hess H, Ratna B, Satir P, Vogel V. “Smart dust” biosensors powered by biomolecular motors. Lab Chip. 2009;9:1661–1666. doi: 10.1039/b821055a. [DOI] [PubMed] [Google Scholar]
- Boal AK, Tellez H, Rivera SB, Miller NE, Bachand GD, Bunker BC. The stability and functionality of chemically crosslinked microtubules. Small. 2006;2:793–803. doi: 10.1002/smll.200500381. [DOI] [PubMed] [Google Scholar]
- Brown TB, Hancock WO. A polarized microtubule array for kinesin-powered nanoscale assembly and force generation. Nano Lett. 2002;2:1131–1135. [Google Scholar]
- Brunner C, Wahnes C, Vogel V. Cargo pick-up from engineered loading stations by kinesin driven molecular shuttles. Lab Chip. 2007;7:1263–1271. doi: 10.1039/b707301a. [DOI] [PubMed] [Google Scholar]
- Carroll-Portillo A, Bachand M, Greene AC, Bachand GD. In vitro capture, transport, and detection of protein analytes using kinesin-based nanoharvesters. Small. 2009;5:1835–1840. doi: 10.1002/smll.200900491. [DOI] [PubMed] [Google Scholar]
- Cassimeris LU, Walker RA, Pryer NK, Salmon ED. Dynamic instability of microtubules. Bioessays. 1987;7:149–154. doi: 10.1002/bies.950070403. [DOI] [PubMed] [Google Scholar]
- Castoldi M, Popov AV. Purification of brain tubulin through two cycles of polymerization-depolymerization in a high-molarity buffer. Protein Expr Purif. 2003;32:83–88. doi: 10.1016/S1046-5928(03)00218-3. [DOI] [PubMed] [Google Scholar]
- Cheng LJ, Kao MT, Meyhofer E, Guo LJ. Highly efficient guiding of microtubule transport with imprinted CYTOP nanotracks. Small. 2005;1:409–414. doi: 10.1002/smll.200400109. [DOI] [PubMed] [Google Scholar]
- Clemmens J, Hess H, Lipscomb R, Hanein Y, Bohringer KF, Matzke CM, Bachand GD, Bunker BC, Vogel V. Mechanisms of microtubule guiding on microfabricated kinesin-coated surfaces: chemical and topographic surface patterns. Langmuir. 2003;19:10967–10974. [Google Scholar]
- Clemmens J, Hess H, Doot R, Matzke CM, Bachand GD, Vogel V. Motor-protein “roundabouts”: microtubules moving on kinesin-coated tracks through engineered networks. Lab Chip. 2004;4:83–86. doi: 10.1039/b317059d. [DOI] [PubMed] [Google Scholar]
- Davies DR, Padlan EA, Sheriff S. Antibody-antigen complexes. Annu Rev Biochem. 1990;59:439–473. doi: 10.1146/annurev.bi.59.070190.002255. [DOI] [PubMed] [Google Scholar]
- Dennis J, Howard J, Vogel V. Molecular shuttles: directed motion of microtubules along nanoscale kinesin tracks. Nano-technology. 1999;10:232–236. [Google Scholar]
- Diez S, Reuther C, Dinu C, Seidel R, Mertig M, Pompe W, Howard J. Stretching and tranporting DNA molecules using motor proteins. Nano Lett. 2003;3:1251–1254. [Google Scholar]
- Ellington AD, Szostak JW. In vitro selection of RNA molecules that bind specific ligands. Nature. 1990;346:818–822. doi: 10.1038/346818a0. [DOI] [PubMed] [Google Scholar]
- Fischer T, Agarwal A, Hess H. A smart dust biosensor powered by kinesin motors. Nat Nanotechnol. 2009;4:162–166. doi: 10.1038/nnano.2008.393. [DOI] [PubMed] [Google Scholar]
- Freitas RA. Pharmacytes: an ideal vehicle for targeted drug delivery. J Nanosci Nanotech. 2006;6:2769–2775. doi: 10.1166/jnn.2006.413. [DOI] [PubMed] [Google Scholar]
- Gittes F, Meyhofer E, Baek S, Howard J. Directional loading of the kinesin motor molecule as it buckles a microtubule. Biophys J. 1996;70:418–429. doi: 10.1016/S0006-3495(96)79585-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene AC, Trent AM, Bachand GD. Controlling kinesin motor proteins in nanoengineered systems through a metal-binding on/off switch. Biotechnol Bioeng. 2008;101:478–486. doi: 10.1002/bit.21927. [DOI] [PubMed] [Google Scholar]
- Gupta ML, Jr, Bode CJ, Dougherty CA, Marquez RT, Himes RH. Mutagenesis of beta-tubulin cysteine residues in Saccharomyces cerevisiae: mutation of cysteine 354 results in cold-stable micro-tubules. Cell Motil Cytoskeleton. 2001;49:67–77. doi: 10.1002/cm.1021. [DOI] [PubMed] [Google Scholar]
- Hammond JW, Cai D, Verhey KJ. Tubulin modifications and their cellular functions. Curr Opin Cell Biol. 2008;20:71–76. doi: 10.1016/j.ceb.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helm CA, Knoll W, Israelachvili JN. Measurement of ligand-receptor interactions. Proc Natl Acad Sci. 1991;88:8169–8173. doi: 10.1073/pnas.88.18.8169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess H, Vogel V. Molecular shuttles based on motor proteins: active transport in synthetic environments. J Biotechnol. 2001;82:67–85. doi: 10.1016/s1389-0352(01)00029-0. [DOI] [PubMed] [Google Scholar]
- Hess H, Clemmens J, Qin D, Howard J, Vogel V. Light-controlled molecular shuttles made from motor proteins carrying cargo on engineered surfaces. Nano Lett. 2001;1:235–239. [Google Scholar]
- Hess H, Clemmens J, Matzke CM, Bachand GD, Bunker BC, Vogel V. Ratchet patterns sort molecular motors. Appl Phys A. 2002a;75:309–313. [Google Scholar]
- Hess H, Howard J, Vogel V. A piconewton forcemeter assembled from microtubules and kinesins. Nano Lett. 2002b;2:1113–1116. [Google Scholar]
- Hess H, Bachand GD, Vogel V. Powering nanodevices with biomolecular motors. Chemistry. 2004;10:2110–2116. doi: 10.1002/chem.200305712. [DOI] [PubMed] [Google Scholar]
- Hess H, Clemmens J, Brunner C, Doot R, Luna S, Ernst KH, Vogel V. Molecular self-assembly of “nanowires”and “nanospools” using active transport. Nano Lett. 2005;5:629–633. doi: 10.1021/nl0478427. [DOI] [PubMed] [Google Scholar]
- Hiller Y, Gershoni JM, Bayer EA, Wilchek M. Biotin binding to avidin. Oligosaccharide side chain not required for ligand association. Biochem J. 1987;248:167–171. doi: 10.1042/bj2480167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinterdorfer P, Baumgartner W, Gruber HJ, Schilcher K, Schindler H. Detection and localization of individual antibody-antigen recognition events by atomic force microscopy. Proc Natl Acad Sci. 1996;93:3477–3481. doi: 10.1073/pnas.93.8.3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirabayashi M, Taira S, Kobayashi S, Konishi K, Katoh K, Hiratsuka Y, Kodaka M, Uyeda TQ, Yumoto N, Kubo T. Malachite green-conjugated microtubules as mobile bioprobes selective for malachite green aptamers with capturing/releasing ability. Biotechnol Bioeng. 2006;94:473–480. doi: 10.1002/bit.20867. [DOI] [PubMed] [Google Scholar]
- Hiratsuka Y, Tada T, Oiwa K, Kanayama T, Uyeda TQ. Controlling the direction of kinesin-driven microtubule movements along microlithographic tracks. Biophys J. 2001;81:1555–1561. doi: 10.1016/S0006-3495(01)75809-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiyama S, Gojo R, Shima T, Takeuchi S, Sutoh K. Biomolecular-motor-based nano- or microscale particle translocations on DNA microarrays. Nano Lett. 2009;9:2407–2413. doi: 10.1021/nl901013k. [DOI] [PubMed] [Google Scholar]
- Hiyama S, Moritani Y, Gojo R, Takeuchi S, Sutoh K. Biomolecular-motor-based autonomous delivery of lipid vesicles as nano- or microscale reactors on a chip. Lab Chip. 2010;10:2741–2748. doi: 10.1039/c004615a. [DOI] [PubMed] [Google Scholar]
- Hutchins BM, Hancock WO, Williams ME. Magnet assisted fabrication of microtubule arrays. Phys Chem Chem Phys. 2006a;8:3507–3509. doi: 10.1039/b605399h. [DOI] [PubMed] [Google Scholar]
- Hutchins BM, Platt M, Hancock WO, Williams ME. Motility of CoFe2O4 nanoparticle-labelled microtubules in magnetic fields. Micro Nano Lett. 2006b;1:47–52. [Google Scholar]
- Hutchins BM, Platt M, Hancock WO, Williams ME. Directing transport of CoFe2O4-functionalized microtubules with magnetic fields. Small. 2007;3:126–131. doi: 10.1002/smll.200600410. [DOI] [PubMed] [Google Scholar]
- Hyman A, Drechsel D, Kellogg D, Salser S, Sawin K, Steffen P, Wordeman L, Mitchison T. Preparation of modified tubulins. Methods enzymol. 1991;196:478–485. doi: 10.1016/0076-6879(91)96041-o. [DOI] [PubMed] [Google Scholar]
- Jang MH, Kim J, Kalme S, Han JW, Yoo HS, Koo BS, Kim SK, Yoon MY. Cloning, purification, and polymerization of Capsicum annuum recombinant alpha and beta tubulin. Biosci Biotechnol Biochem. 2008;72:1048–1055. doi: 10.1271/bbb.70794. [DOI] [PubMed] [Google Scholar]
- Janke C, Kneussel M. Tubulin post-translational modifications: encoding functions on the neuronal microtubule cytoskeleton. Trends Neurosci. 2010;33:362–372. doi: 10.1016/j.tins.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Jia L, Moorjani SG, Jackson TN, Hancock WO. Microscale transport and sorting by kinesin molecular motors. Biomed Microdevices. 2004;6:67–74. doi: 10.1023/b:bmmd.0000013368.89455.8d. [DOI] [PubMed] [Google Scholar]
- Kato K, Goto R, Katoh K, Shibakami M. Microtubule-cyclodextrin conjugate: functionalization of motile filament with molecular inclusion ability. Biosci Biotechnol Biochem. 2005;69:646–648. doi: 10.1271/bbb.69.646. [DOI] [PubMed] [Google Scholar]
- Keith CH, Feramisco JR, Shelanski M. Direct visualization of fluorescein-labeled microtubules in vitro and in microinjected fibroblasts. J Cell Biol. 1981;88:234–240. doi: 10.1083/jcb.88.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg DR, Mitchison TJ, Alberts BM. Behaviour of microtubules and actin filaments in living Drosophila embryos. Development. 1988;103:675–686. doi: 10.1242/dev.103.4.675. [DOI] [PubMed] [Google Scholar]
- Korten T, Diez S. Setting up roadblocks for kinesin-1: mechanism for the selective speed control of cargo carrying microtubules. Lab Chip. 2006;8:1441–1447. doi: 10.1039/b803585g. [DOI] [PubMed] [Google Scholar]
- Korten T, Mansson A, Diez S. Towards the application of cytoskeletal motor proteins in molecular detection and diagnostic devices. Curr Opin Biotechnol. 2010;21:477–488. doi: 10.1016/j.copbio.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Kufer SK, Puchner EM, Gumpp H, Liedl T, Gaub HE. Single-molecule cut-and-paste surface assembly. Science. 2008;319:594–596. doi: 10.1126/science.1151424. [DOI] [PubMed] [Google Scholar]
- Ledbetter MC, Porter KR. A “microtubule” in plant cell fine structure. J Cell Biol. 1963;19:239–250. doi: 10.1083/jcb.19.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie RJ, Saxton WM, Mitchison TJ, Neighbors B, Salmon ED, McIntosh JR. Assembly properties of fluorescein-labeled tubulin in vitro before and after fluorescence bleaching. J Cell Biol. 1984;99:2146–2156. doi: 10.1083/jcb.99.6.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CT, Kao MT, Kurabayashi K, Meyhofer E. Self-contained, biomolecular motor-driven protein sorting and concentrating in an ultrasensitive microfluidic chip. Nano Lett. 2008;8:1041–1046. doi: 10.1021/nl072742x. [DOI] [PubMed] [Google Scholar]
- Lowe J, Li H, Downing KH, Nogales E. Refined structure of alpha beta-tubulin at 3.5 A resolution. J Mol Biol. 2001;313:1045–1057. doi: 10.1006/jmbi.2001.5077. [DOI] [PubMed] [Google Scholar]
- MacDonald LM, Armson A, Thompson RC, Reynoldson JA. Characterization of factors favoring the expression of soluble protozoan tubulin proteins in Escherichia coli. Protein Expr Purif. 2003;29:117–122. doi: 10.1016/s1046-5928(03)00006-8. [DOI] [PubMed] [Google Scholar]
- MacRae TH. Tubulin post-translational modifications—enzymes and their mechanisms of action. Eur J Biochem. 1997;244:265–278. doi: 10.1111/j.1432-1033.1997.00265.x. [DOI] [PubMed] [Google Scholar]
- Mandelkow E, Mandelkow E-M. Microtubules and microtubule-associated proteins. Curr Opin Cell Biol. 1995;7:72–81. doi: 10.1016/0955-0674(95)80047-6. [DOI] [PubMed] [Google Scholar]
- Minoura I, Muto E. Dielectric measurement of individual microtubules using the electroorientation method. Biophys J. 2006;90:3739–3748. doi: 10.1529/biophysj.105.071324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukrishnan G, Roberts CA, Chen Y, Zahn JD, Hancock WO. Patterning surface-bound microtubules through reversible DNA hybridization. Nano Lett. 2004;2004:2127–2132. [Google Scholar]
- Nedelec FJ, Surrey T, Maggs AC, Leibler S. Self-organization of microtubules and motors. Nature. 1997;389:305–308. doi: 10.1038/38532. [DOI] [PubMed] [Google Scholar]
- Nogales E, Whittaker M, Milligan RA, Downing KH. High-resolution model of the microtubule. Cell. 1999;96:79–88. doi: 10.1016/s0092-8674(00)80961-7. [DOI] [PubMed] [Google Scholar]
- Oxberry ME, Geary TG, Winterrowd CA, Prichard RK. Individual expression of recombinant alpha- and beta-tubulin from Haemonchus contortus: polymerization and drug effects. Protein Expr Purif. 2001;21:30–39. doi: 10.1006/prep.2000.1347. [DOI] [PubMed] [Google Scholar]
- Panchuk-Voloshina N, Haugland RP, Bishop-Stewart J, Bhalgat MK, Millard PJ, Mao F, Leung WY. Alexa dyes, a series of new fluorescent dyes that yield exceptionally bright, photostable conjugates. J Histochem Cytochem. 1999;47:1179–1188. doi: 10.1177/002215549904700910. [DOI] [PubMed] [Google Scholar]
- Platt M, Muthukrishnan G, Hancock WO, Williams ME. Millimeter scale alignment of magnetic nanoparticle functionalized microtubules in magnetic fields. J Am Chem Soc. 2005;127:15686–15687. doi: 10.1021/ja055815s. [DOI] [PubMed] [Google Scholar]
- Pucciarelli S, Ballarini P, Miceli C. Cold-adapted microtubules: characterization of tubulin posttranslational modifications in the Antarctic ciliate Euplotes focardii. Cell Motil Cytoskeleton. 1997;38:329–340. doi: 10.1002/(SICI)1097-0169(1997)38:4<329::AID-CM3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Raab M, Hancock WO. Transport and detection of unlabeled nucleotide targets by microtubules functionalized with molecular beacons. Biotechnol Bioeng. 2008;99:764–773. doi: 10.1002/bit.21645. [DOI] [PubMed] [Google Scholar]
- Ramachandran S, Ernst KH, Bachand GD, Vogel V, Hess H. Selective loading of kinesin-powered molecular shuttles with protein cargo and its application to biosensing. Small. 2006;2:330–334. doi: 10.1002/smll.200500265. [DOI] [PubMed] [Google Scholar]
- Reuther C, Hajdo L, Tucker R, Kasprzak AA, Diez S. Biotemplated nanopatterning of planar surfaces with molecular motors. Nano Lett. 2006;6:2177–2183. doi: 10.1021/nl060922l. [DOI] [PubMed] [Google Scholar]
- Rios L, Bachand GD. Multiplex transport and detection of cytokines using kinesin-driven molecular shuttles. Lab Chip. 2009;9:1005–1010. doi: 10.1039/b816444d. [DOI] [PubMed] [Google Scholar]
- Ross JL, Shuman H, Holzbaur EL, Goldman YE. Kinesin and dynein-dynactin at intersecting microtubules: motor density affects dynein function. Biophys J. 2008;94:3115–3125. doi: 10.1529/biophysj.107.120014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff PB, Fant J, Horwitz SB. Promotion of microtubule assembly in vitro by taxol. Nature. 1979;277:665–667. doi: 10.1038/277665a0. [DOI] [PubMed] [Google Scholar]
- Schmidt C, Vogel V. Molecular shuttles powered by motor proteins: loading and unloading stations for nanocargo integrated into one device. Lab Chip. 2010;10:2195–2198. doi: 10.1039/c005241h. [DOI] [PubMed] [Google Scholar]
- Seetharam R, Wada Y, Ramachandran S, Hess H, Satir P. Long-term storage of bionanodevices by freezing and lyophilization. Lab Chip. 2006;6:1239–1242. doi: 10.1039/b601635a. [DOI] [PubMed] [Google Scholar]
- Soto CM, Martin BD, Sapsford KE, Blum AS, Ratna BR. Toward single molecule detection of staphylococcal enterotoxin B: mobile sandwich immunoassay on gliding microtubules. Anal Chem. 2008;80:5433–5440. doi: 10.1021/ac800541x. [DOI] [PubMed] [Google Scholar]
- Stracke R, Bohm KJ, Burgold J, Schacht H, Unger E. Physical and technical parameters determining the functioning of a kinesin-based cell-free motor system. Nanotechnology. 2000;11:52–56. [Google Scholar]
- Stracke R, Bohm KJ, Wollweber L, Tuszynski JA, Unger E. Analysis of the migration behaviour of single microtubules in electric fields. Biochem Biophys Res Commun. 2002;293:602–609. doi: 10.1016/S0006-291X(02)00251-6. [DOI] [PubMed] [Google Scholar]
- Strunz T, Oroszlan K, Schafer R, Guntherodt HJ. Dynamic force spectroscopy of single DNA molecules. Proc Natl Acad Sci USA. 1999;96:11277–11282. doi: 10.1073/pnas.96.20.11277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taira S, Du YZ, Hiratsuka Y, Konishi K, Kubo T, Uyeda TQ, Yumoto N, Kodaka M. Selective detection and transport of fully matched DNA by DNA-loaded microtubule and kinesin motor protein. Biotechnol Bioeng. 2006;95:533–538. doi: 10.1002/bit.21055. [DOI] [PubMed] [Google Scholar]
- Tomishige M, Vale RD. Controlling kinesin by reversible disulfide cross-linking. Identifying the motility-producing conformational change. J Cell Biol. 2000;151:1081–1092. doi: 10.1083/jcb.151.5.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker R, Katira P, Hess H. Herding nanotransporters: localized activation via release and sequestration of control molecules. Nano Lett. 2008;8:221–226. doi: 10.1021/nl072516n. [DOI] [PubMed] [Google Scholar]
- Turner D, Chang C, Fang K, Cuomo P, Murphy D. Kinesin movement on glutaraldehyde-fixed microtubules. Anal Biochem. 1996;242:20–25. doi: 10.1006/abio.1996.0422. [DOI] [PubMed] [Google Scholar]
- Tyagi S, Kramer FR. Molecular beacons: probes that fluoresce upon hybridization. Nat Biotechnol. 1995;14:303–308. doi: 10.1038/nbt0396-303. [DOI] [PubMed] [Google Scholar]
- Uppalapati M, Huang YM, Jackson TN, Hancock WO. Enhancing the stability of kinesin motors for microscale transport applications. Lab Chip. 2008a;8:358–361. doi: 10.1039/b714989a. [DOI] [PubMed] [Google Scholar]
- Uppalapati M, Huang YM, Jackson TN, Hancock WO. Microtubule alignment and manipulation using AC electrokinetics. Small. 2008b;4:1371–1381. doi: 10.1002/smll.200701088. [DOI] [PubMed] [Google Scholar]
- Uppalapati M, Huang YM, Shastry S, Jackson TN, Hancock WO. Microtubule motors in microfluidics. In: Zahn JD, Lee LP, editors. Methods in bioengineering: microfabrication and microfluidics. Artech House; Boston: 2009. [Google Scholar]
- Vale RD, Malik F, Brown D. Directional instability of microtubule transport in the presence of kinesin and dynein. J Cell Biol. 1992;119:1589–1596. doi: 10.1083/jcb.119.6.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MG, Butcher CT, Smeets RM, Diez S, Dekker C. High rectifying efficiencies of microtubule motility on kinesin-coated gold nanostructures. Nano Lett. 2005;5:1117–1122. doi: 10.1021/nl0506554. [DOI] [PubMed] [Google Scholar]
- van Oss CJ, Good RJ, Chaudhury MK. Nature of the antigen-antibody interaction: primary and secondary bonds: optimal conditions for association and dissociation. J Chromatogr. 1986;376:111–119. [PubMed] [Google Scholar]
- Vassilev P, Kanazirska M. The role of cytoskeleton in the mechanisms of electric field effects and information transfer in cellular systems. Med Hypotheses. 1985;16:93–96. doi: 10.1016/0306-9877(85)90065-9. [DOI] [PubMed] [Google Scholar]
- Verma V, Hancock WO, Catchmark JM. Nanoscale patterning of kinesin motor proteins and its role in guiding microtubule motility. Biomed Microdevices. 2009;11:313–322. doi: 10.1007/s10544-008-9237-9. [DOI] [PubMed] [Google Scholar]
- Westermann S, Weber K. Post-translational modifications regulate microtubule function. Nat Rev Mol Cell Biol. 2003;4:938–947. doi: 10.1038/nrm1260. [DOI] [PubMed] [Google Scholar]
- Wilchek M, Bayer EA. Avidin-biotin technology ten years on: has it lived up to its expectations? Trends Biochem Sci. 1989;14:408–412. doi: 10.1016/0968-0004(89)90289-2. [DOI] [PubMed] [Google Scholar]
- Williams RC, Jr, Lee JC. Preparation of tubulin from brain. Methods enzymol. 1982;85(Pt B):376–385. doi: 10.1016/0076-6879(82)85038-6. [DOI] [PubMed] [Google Scholar]
- Wong J, Chilkoti A, Moy VT. Direct force measurements of the streptavidin-biotin interaction. Biomol Eng. 1999;16:45–55. doi: 10.1016/s1050-3862(99)00035-2. [DOI] [PubMed] [Google Scholar]
- Yokokawa R, Takeuchi S, Kon T, Ohkura R, Edamatsu M, Sutoh K, Fujita H. Transportation of micromachined structures by biomolecular linear motors; Proceedings Micro Electro Mechanical Systems; 2003. pp. 8–11. [Google Scholar]


