Abstract
Cell surface lipophosphoglycan (LPG) is commonly regarded as a multifunctional Leishmania virulence factor required for survival and development of these parasites in mammals. In this study, the LPG biosynthesis gene lpg1 was deleted in Leishmania mexicana by targeted gene replacement. The resulting mutants are deficient in LPG synthesis but still display on their surface and secrete phosphoglycan-modified molecules, most likely in the form of proteophosphoglycans, whose expression appears to be up-regulated. LPG-deficient L.mexicana promastigotes show no significant differences to LPG-expressing parasites with respect to attachment to, uptake into and multiplication inside macrophages. Moreover, in Balb/c and C57/BL6 mice, LPG-deficient L.mexicana clones are at least as virulent as the parental wild-type strain and lead to lethal disseminated disease. The results demonstrate that at least L.mexicana does not require LPG for experimental infections of macrophages or mice. Leishmania mexicana LPG is therefore not a virulence factor in the mammalian host.
Keywords: Leishmania/lipophosphoglycan/proteophosphoglycan/macrophage/virulence
Introduction
Leishmania are protozoan parasites that cause a number of important human diseases ranging from cutaneous lesions to fatal visceral infection. These pathogens have a digenetic life cycle that alternates between the colonization of the vector sandfly digestive tract by extracellular flagellated promastigotes, and intracellular parasitism of mammalian macrophages by non-motile amastigotes. Transmission occurs during the bite of a mammal by a sandfly, where infectious metacyclic promastigotes are injected into the skin. After uptake by macrophages, the metacyclic promastigotes transform to amastigotes which multiply within a phagolysosome and, after release by an unknown mechanism, infect other macrophages (Alexander and Russell, 1992). Leishmania are most remarkable in their ability to circumvent or resist various innate and acquired immune mechanisms of the mammalian host (Reiner and Locksley, 1995). Both metacyclic promastigotes and amastigotes are largely resistant to complement lysis (Mosser et al., 1985; Sacks, 1989). Inside the macrophage phagolysosome, the two Leishmania mammalian forms avoid or inhibit the production of toxic NO and/or O2– (Liew et al., 1990; Murray and Nathan, 1999), they resist acidic pH (Antoine et al., 1990) and the action of hydrolytic enzymes (Prina et al., 1990) and other microbiocidal activities of their host cell.
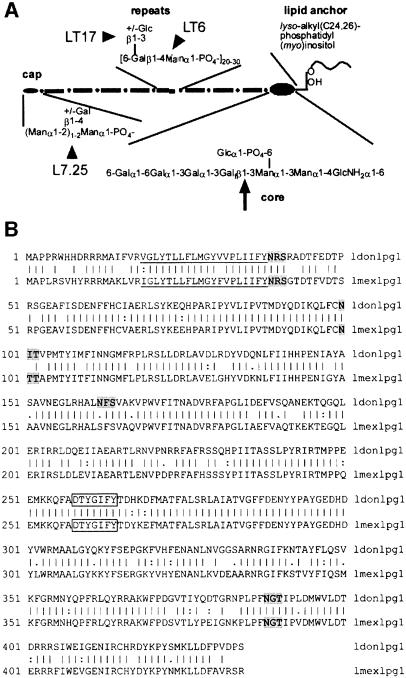
Molecules and mechanisms allowing these parasites not only to survive, but actually to thrive in such hostile environments have been a main topic of interest in Leishmania reseach. In particular, the major cell surface glycoconjugate (1–5×106/cell) of all Leishmania promastigotes, a unique molecule called lipophosphoglycan (LPG), has been a focus of intense research over the past 15 years. This complex glycolipid is organized in four domains: a conserved 1-O-alkyl-2-lyso-phosphatidyl(myo)inositol membrane anchor, a conserved diphosphoheptasaccharide core structure, repeating phosphodisaccharide units carrying species-specific side chains and variable, often mannose-rich cap structures (McConville and Ferguson, 1993; see Figure 1A). In the mammalian host, LPG has been proposed to mediate promastigote complement resistance (Sacks, 1989) as well as attachment and uptake of promastigotes by macrophages (Handman and Goding, 1985; Da Silva et al., 1989; Talamas-Rohana et al., 1990). It has been suggested that LPG may protect the invading promastigotes against macrophage phagolysosome hydrolases (Turco and Descoteaux, 1992) and against the respiratory burst (Chan et al., 1989). LPG modulates macrophage signal transduction pathways by inhibition of protein kinase C (reviewed in Descoteaux and Turco, 1993; Giorgione et al., 1996) and, possibly, by chelation of intracellular calcium (Eilam et al., 1985). The marked suppression of interleukin (IL)-1β and tumour necrosis factor-α gene expression in LPG-treated macrophages (Hatzigeorgiou et al., 1996) may be a consequence of these effects. Furthermore, LPG inhibits phagosome–endosome fusion in the macrophage after invasion of promastigotes (Desjardins and Descoteaux, 1997) and reduces endothelial adhesion and transendothelial migration of monocytes (Ho et al., 1996; Lo et al., 1998), processes believed to be involved in survival and spreading of the parasites. Finally, it was shown that LPG down-regulates expression of inducible NO synthase (iNOS) in macrophages and synthesis of macrophage IL12, which are both agents crucial for control of Leishmania infections (Proudfoot et al., 1996; Piedrafita et al., 1999). This large number of functional studies led to the concept of LPG as a multifunctional virulence determinant required for establishment of Leishmania infections in the mammalian host (Turco and Descoteaux, 1992; Beverley and Turco, 1998; Descoteaux and Turco, 1999). The fact that LPG-deficient mutants either identified fortuitously (L.major LRC-L119, Handman et al., 1986) or obtained by chemical mutagenesis (various L.donovani and L.major lines; King and Turco, 1988; Elhay et al., 1990; McNeely and Turco, 1990) were non-infective to macrophages or mice strengthened this view further. However, the exact defects in some of these mutants are not known, and chemical mutagenesis is likely to lead to multiple mutations. Furthermore, some LPG-deficient L.donovani mutants (Descoteaux et al., 1995, 1998) have defects that affect not only LPG biosynthesis, but also the synthesis of Leishmania proteophosphoglycans (PPGs; reviewed in Ilg et al., 1999a). In addition, it has not yet been reported for any of these mutants that restoration of LPG expression leads to restoration of infectivity to macrophages or mice (Beverley and Turco, 1998).
Fig. 1. (A) Structure of L.mexicana LPG (Ilg et al., 1992). The site of the defect caused by the deletion of lpg1 (Huang et al., 1993) and the putative binding sites of anti-phosphoglycan mAbs used in this study (Ilg et al., 1993) are indicated by an arrow and arrowheads, respectively. (B) Alignment of L.mexicana LPG1 with L.donovani LPG1 (Ryan et al., 1993). The putative membrane anchor sequence is underlined, the region similar to mannose-binding proteins boxed and the potential N-glycosylation sites are shaded in grey.
The biosynthesis of LPG has attracted considerable interest, and several enzymes and transporters have been identified biochemically, genetically or both (reviewed in Mengeling et al., 1997). One of the enzymes essential for LPG biosynthesis is LPG1, a putative β-galactofuranosyl (β-Galf) transferase localized in the Golgi apparatus, that is crucial for the addition of an unusual internal β-Galf residue to the LPG diphosphoheptasaccharide core (Huang and Turco, 1993). The gene encoding LPG1 (lpg1) was isolated by genetic complementation of the L.donovani mutant R2D2 (King and Turco, 1988; Ryan et al., 1993), which partially restored LPG expression in this LPG-deficient parasite line. In L.mexicana, no β-Galf-containing compounds other than the LPG core are known (McConville and Ferguson, 1993). Therefore, targeted deletion of lpg1 in this species is expected to block LPG biosynthesis selectively, while leaving other glycan biosynthesis pathways unaffected. Such a defined mutant should allow a more definite assessment of the significance of LPG for virulence of L.mexicana promastigotes to cultured macrophages and to mice.
In this study, L.mexicana lpg1 mutants were generated by targeted gene deletion. These mutants were unable to synthesize LPG, but still displayed a variety of phosphoglycan structures on their surface, most likely PPGs. Surprisingly, the LPG-deficient mutants were impaired neither in binding and uptake by macrophages, nor in their survival, transformation and multiplication in host cell phagolysosomes. Despite the lack of LPG expression, large amounts of phosphoglycan-modified compounds were still synthesized and released by the parasites in infected host cells. Moreover, in mouse infection experiments, the LPG-deficient L.mexicana mutants were as virulent or even more virulent than their parental wild-type L.mexicana strain. The results suggest that in experimental infections with L.mexicana, LPG is not a virulence factor.
Results
Targeted gene replacement of the L.mexicana lmexlpg1 gene
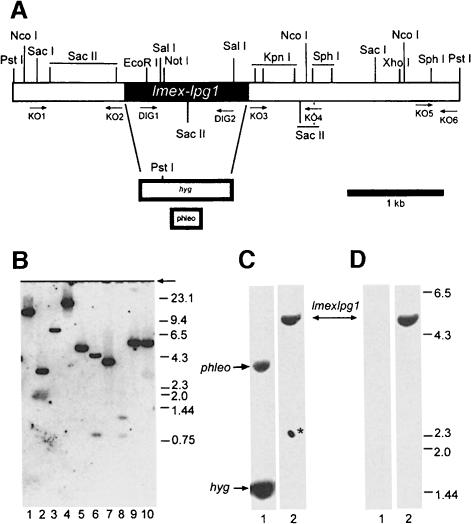
The lmexlpg1 gene was isolated by homology cloning from L.mexicana genomic DNA using a fragment as a probe that was amplified from the same DNA by PCR with primers derived from the known sequence of L.donovani lpg1 (Ryan et al., 1993). The deduced protein sequence of LPG1 from both species is 83% identical and 87% similar, with conservation of the putative 20 residue membrane-spanning sequence, the region with similarity to mannose-binding proteins and three out of four N-glycosylation sites (Figure 1B; Ryan et al., 1993). Southern blot analysis showed that lmexlpg1 is a single copy gene (Figure 2B), which is advantageous for efficient generation of gene deletion mutants. Two rounds of targeted gene replacement (Figure 2A) resulted in several clones lacking the lmexlpg1 open reading frame (ORF) (Figure 2C and D; data not shown). All clones showed normal growth behaviour in culture compared with their parental wild-type strain (data not shown).
Fig. 2. Targeted gene replacement of the lmexlpg1 alleles. (A) Restriction map of the lmexlpg1 locus. Restriction sites relevant for Southern blot analysis, the resistance genes and the primer-binding sites for the construction of gene deletion cassettes and for the generation of DIG-labelled probes are indicated. (B) Southern blot analysis of restriction enzyme-digested L.mexicana chromosomal DNA (5 µg) resolved on a 0.7% agarose gel using a labelled lmexlpg1 gene probe. Lane 1, HindIII; lane 2, NcoI; lane 3, KpnI; lane 4, PvuII; lane 5, PstI; lane 6, SalI; lane 7, SacI; lane 8, SacII; lane 9, SphI; lane 10, XhoI. The size of DNA standards is indicated in kb. (C and D) Southern blot analysis of L.mexicana Δlmexlpg1 mutants. Genomic DNA was digested with PstI, separated on a 0.7% agarose gel, blotted and probed with a labelled lmexlpg1 5′-UTR fragment (C) or an lmexlpg1 ORF probe (D). Lanes 1, L.mexicana Δlmexlpg1, clone I/8D; lanes 2, L.mexicana wild type. The asterisk depicts a background spot due to antibody aggregates.
Expression of phosphoglycans by lmexlpg1 gene deletion mutants
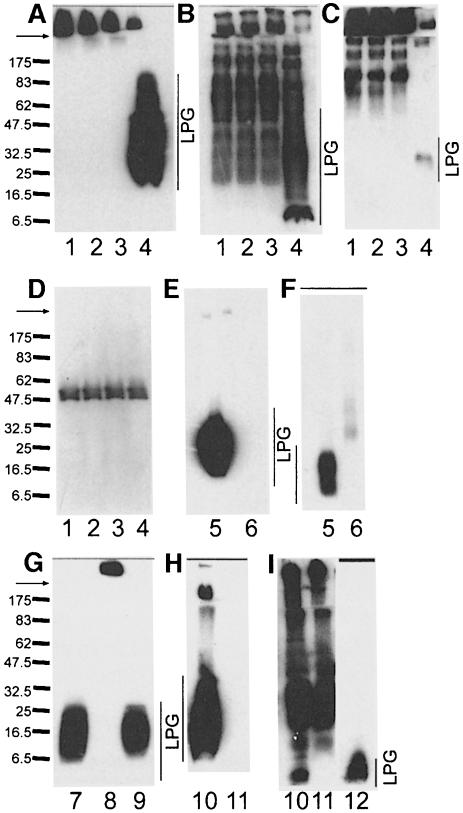
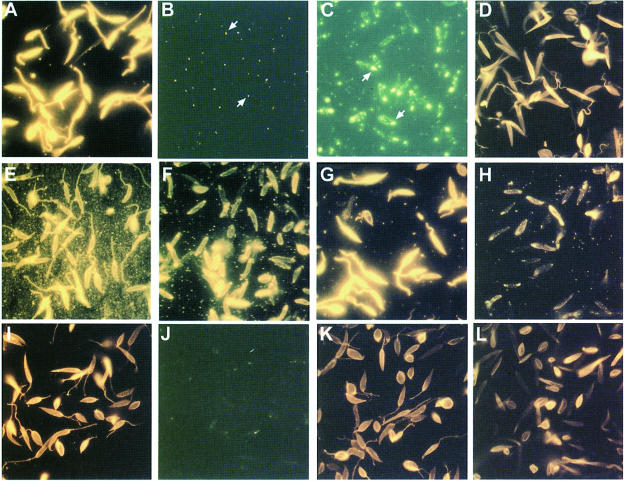
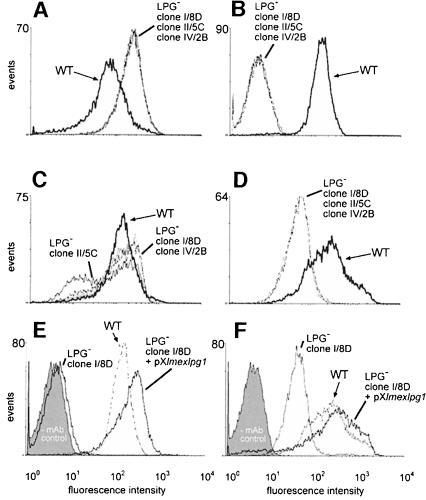
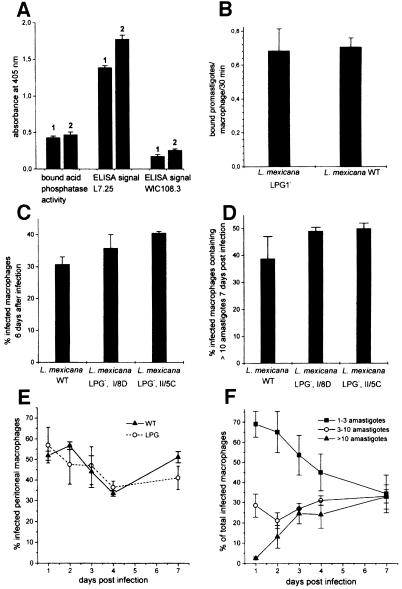
Immunoblots of total cell lysates from three independent lmexlpg1 gene deletion mutants using the anti-[PO4-6Galβ1–4Manα1-]x repeat monoclonal antibody (mAb) LT6 suggested that they lacked LPG (Figure 3A). This was confirmed by attempts to purify LPG from such mutants, which resulted in no enrichment of this glyco lipid, as judged by SDS–PAGE/immunoblots (Figure 3E and F) and chemical staining after SDS–PAGE with the dye Stains-all (not shown). Likewise, the strong L.mexicana promastigote surface labelling by LT6 that can be observed in immunofluorescence was drastically diminished (Figure 4A and B) and, in fluorescence activated cell sorting (FACS) analysis of live cells, the fluorescence intensity was decreased at least 100-fold (Figure 5B). Similar results were obtained in labellings with ricin (not shown), a lectin known to bind strongly to the β-Gal-containing cap structures of many LPGs (King and Turco, 1988; T.Ilg, unpublished results). Episomal addback of the lmexlpg1 gene to mutant promastigotes showed that the LPG-negative phenotype was due to the gene deletion, because it resulted in parasites that re-expressed LPG (Figure 3G) that was displayed on the cell surface at wild-type levels (Figures 4D and 5E). Cell to cell variability of LPG expression in the complemented mutants was higher than in wild-type cells (Figure 4D), a phenomenon previously also observed in pX-plasmid-driven expression of a membrane-bound acid phosphatase in L.major (Ilg et al., 1999b). Possibly, the copy number of the expression vector pX is not uniform in a Leishmania population. However, the LPG-negative mutants were not completely devoid of LT6 epitopes. A compound strongly reactive with this mAb was present in SDS–PAGE/immunoblots in the stacking gel region and appeared to be up-regulated in the LPG-deficient mutants compared with the wild-type parasites (Figure 3A). The electrophoretic migration of this compound was reminiscent of filamentous PPG (fPPG) secreted via the flagellar pocket (Ilg et al., 1999a) and of membrane-bound PPG (mPPG) present on the surface of L.major promastigotes (Ilg et al., 1999b). Indeed, a strong flagellar pocket signal was observed in immunofluorescence labelling of LPG-deficient L.mexicana promastigotes with LT6 (Figure 4B and C), and longer photographic exposure revealed a spotty cell surface signal on many promastigotes that was intense on 10–20% of the cells (Figure 4C). Likewise, in FACS analysis with mAb LT6, the surface signal on LPG-deficient L.mexicana promastigotes was markedly above background fluorescence for a subfraction of the cells (Figure 5E). The high molecular weight phosphoglycan compounds present in LPG-deficient L.mexicana promastigotes could also be detected with the anti-[Manα 1–2]0–2Manα1-PO4] mAb L7.25 (Figure 3B) as well as with mAb LT17 (Figure 3C), most likely directed against [PO4-6(Glcβ1–3)Galβ1–4Manα1-]x epitopes (Figure 1A). These anti-phosphoglycan antibodies, however, also recognized a variety of other L.mexicana proteins whose expression was either unaffected or even up-regulated in lmexlpg1 deletion mutants (Figure 3B and C). At least some of these phosphoglycosylated proteins are displayed on the surface of L.mexicana LPG-deficient promastigotes (Figure 4F and H). FACS analyses suggest that the surface epitope density for L7.25 (Figure 5C) is similar to or even slightly elevated compared with L.mexicana wild type, while for LT17, a decrease of mAb binding by a factor of 5, but still well above background, was noted (Figure 5D). This decrease in LT17 binding could be reversed to wild-type levels by episomal expression of lmexlpg1 (Figure 5F). Taken together these results suggest that in the absence of LPG, L.mexicana promastigotes still display abundant phosphoglycan structures on their surface, in particular manno-oligosaccharide caps, but also, to a lesser degree, repeats. Except for the putative fPPG/mPPG in the stacking gel (Figure 3G), the bulk of these non-LPG phosphoglycans are sensitive to digestion with proteinase K (not shown), suggesting that they are phosphoglycosylated proteins. In FACS analysis, the anti-leishmanolysin/gp63 mAb L3.8 (Ilg et al., 1993) recognizes more epitopes on LPG-deficient L.mexicana promastigotes (Figure 5A) despite the fact that expression levels on immunoblots appeared to be unaltered compared with the wild type (Figure 3D). The absence of the bulky glycolipid LPG may improve the accessiblity of the parasite surface to antibodies (Karp et al., 1991). A remarkable observation is the restriction of surface-exposed phosphoglycosylated proteins to the cell body of the parasites while they appear to be absent from the flagella (Figure 4C, F and H), in contrast to the distribution of LPG (Figure 4A, D, E, G, I and K). Phosphoglycosyl ation of secreted products such as secretory acid phos phatase (SAP) and fPPG (Ilg et al., 1999a) appeared to be normal in L.mexicana LPG-deficient strains, as shown by monoclonal antibody binding in two-site enzyme-linked immunosorbent assay (ELISA) (Figure 6A) and by immunofluorescence of fPPG-containing promastigote aggregates (not shown).
Fig. 3. SDS–PAGE/immunoblot analysis of L.mexicana and L.donovani LPG-deficient mutants. (A–D) Total promastigote lysates (100 µg protein). Lane 1, L.mexicana Δlmexlpg1, clone I/8D; lane 2, L.mexicana Δlmexlpg1, clone II/5C; lane 3, L.mexicana Δlmexlpg1, clone IV/2B; lane 4, L.mexicana wild type. The blots were probed with the mAbs LT6 (A), L7.25 (B) and LT17 (C). Equal loading of lanes 1–4 was confirmed by probing a blot with the anti-leishmanolysin/gp63 mAb L3.8 (D). (E–F) LPG from 108 promastigotes enriched by solvent extraction (McConville et al., 1990). Lane 5, L.mexicana wild type; lane 6, L.mexicana Δlmexlpg1, clone I/8D. The blots were probed with LT6 (E) and L7.25 (F). (G) Total promastigote lysates (100 µg protein) digested with proteinase K in SDS–PAGE sample buffer (200 µg/ml final concentration, 15 min, 37°C). Lane 7, L.mexicana wild type; lane 8, L.mexicana Δlmexlpg1, clone I/8D; lane 9, L.mexicana Δlmexlpg1, clone I/8D + pXlmexlpg1. The blot was probed with mAb LT6. (H and I) Total promastigote lysates (100 µg of protein). Lane 10, L.donovani wild type; lane 11, L.donovani R2D2; lane 12, purified L.donovani LPG (2.5 µg), for reference. The blots were probed with mAb LT6 (H) or L7.25 (I). Arrows mark the border between the stacking and the separating gel. The molecular masses of standard proteins are indicated in kDa.
Fig. 4. Immunofluorescence labelling of L.mexicana and L.donovani LPG-deficient mutants using anti-phosphoglycan mAbs. (A–D) mAb LT6. (A) L.mexicana wild type; (B) L.mexicana Δlmexlpg1, clone I/8D at the same exposure time as in (A). (C) L.mexicana Δlmexlpg1, clone I/8D exposed 10 times longer than in (A). Flagellar pockets are indicated by arrows. (D) L.mexicana Δlmexlpg1, clone I/8D + pXlmexlpg1 exposed for half the time in (A). (E and F) mAb L7.25. (E) L.mexicana wild type; (F) L.mexicana Δlmexlpg1, clone I/8D at the same exposure time as in (E). (G and H) mAb LT17. (G) L.mexicana wild type; (H) L.mexicana Δlmexlpg1, clone I/8D at the same exposure time as in (G). (I and J) mAb LT6. (I) L.donovani wild type; (J) L.donovani R2D2 at 10 times the exposure time in (I). (K and L) mAb L7.25. (K) L.donovani wild type; (L) L.donovani R2D2 at the same exposure time as in (K).
Fig. 5. FACS analysis of live L.mexicana wild-type (WT), LPG-deficient mutant (LPG–) and episomal lmexlpg1 addback (LPG– + pXlmexlpg1) promastigotes. (A) mAb L3.8; (B) mAb LT6; (C) mAb L7.25; (D) mAb LT17; (E) mAb LT6; (F) mAb LT17. The fluorescence signal of the three LPG-deficient mutants overlaps completely in (A), (B) and (D), and partially in (C). The control lacking the primary antibody (–mAb control, shaded grey) is only shown in (E) and (F), but was identical in (A–D).
Fig. 6. Analysis of L.mexicana SAP phosphoglycosylation, macrophage binding and macrophage infection by L.mexicana wild-type and LPG-deficient mutant (LPG–) promastigotes. (A) ELISA of LT8.2-bound SAP released from L.mexicana wild-type (bar 1) and L.mexicana Δlmexlpg1, clone I/8D (bar 2) using the anti-phosphoglycan cap mAb L7.25 and the anti-repeat mAb WIC108.3. (B) Binding of L.mexicana wild-type and an LPG-deficient L.mexicana mutant (clone II/5C) to peritoneal macrophages. The bars represent the average of three experiments. The standard error is indicated. (C and D) Infection of peritoneal macrophages by L.mexicana wild type and LPG-deficient L.mexicana mutants (clones I/8D and II/5C). The ratio of infected to uninfected macrophages 6 days after a challenge with two promastigotes/cell is shown in (C), while (D) shows the percentage of infected macrophages with an amastigote burden >10 after a challenge with two promastigotes/cell of L.mexicana wild-type or LPG-deficient L.mexicana mutants (clone I/8D and clone II/5C) after 7 days in culture. The bars represent the average of duplicate determinations and the standard error is indicated. A representative of three separate experiments with similar results is shown. (E) Time course of infection of peritoneal macrophages after a challenge with two promastigotes/cell of L.mexicana wild-type or an LPG-deficient L.mexicana mutant (clone I/8D). (F) Time course of parasite burden after infection of peritoneal macrophages with two promastigotes/cell of an LPG-deficient L.mexicana mutant (clone I/8D). (E and F) Each time point was determined in duplicate experiments. The standard error is indicated. Identical experiments with clone II/5C gave the same results.
To investigate whether other Leishmania species also display phosphoglycans on their surface in the absence of LPG, immunofluorescence experiments were performed on the LPG-deficient L.donovani mutant strain R2D2 generated by chemical mutagenesis (King and Turco, 1988) that carries an unknown defect in the lpg1 gene (Ryan et al., 1993). mAb LT6, which produces a strong surface signal on the parental wild-type strain (Figure 4I), showed only an occasional weak flagellar pocket labelling in R2D2, presumably due to phosphoglycosylated SAP (Bates et al., 1990; Ilg et al., 1993). This result is in agreement with the virtual absence of staining with this mAb in immunoblots of washed cells (Figure 3H). In contrast, a large number of proteins are recognized by the mAb L7.25 (Figure 3I). This antibody binds to the surface of R2D2 promastigotes and produces a fluorescence signal only slightly weaker than that obtained with wild-type parasites (Figure 4K and L). Up-regulation of PPG expression, as in lpg1-deficient L.mexicana, was not observed.
Binding of LPG-deficient L.mexicana promastigotes to macrophages and multiplication inside their host cell
Earlier publications have reported an inability of LPG-deficient mutants to establish infections in macrophages (Handman et al., 1986; McNeely and Turco, 1990) that was paramount to the proposition that LPG is a key factor for successful parasitism of mammalian host cells by Leishmania.
In macrophage binding experiments, the LPG-deficient L.mexicana promastigotes generated in this study were found attached to the same proportion of host cells as the wild-type promastigotes (45 ± 5% and 42 ± 8%, n = 3, respectively) and also the total number of bound parasites was very similar (Figure 6B). After uptake by their host cells, the L.mexicana Δlmexlpg1 mutants were at least as successful in colonizing macrophages as the parental wild-type strain with respect to both the percentage of infected host cells (Figure 6C) and the fraction of infected host cells carrying >10 parasites (Figure 6D). It has been argued that the presence of LPG in Leishmania may be particularly crucial during the initial phase of macrophage invasion by promastigotes. However, we could not detect any disadvantage of LPG-deficient L.mexicana promastigotes compared with the parental wild-type strain within the first 4 days of infection, because the percentage of parasitized peritoneal macrophages was very similar in both cases (Figure 6E). In addition, the LPG-deficient mutants started to proliferate inside the macrophages within 1 day with no sign of a lag phase (Figure 6F). Similar results were obtained with bone marrow-derived macrophages (data not shown).
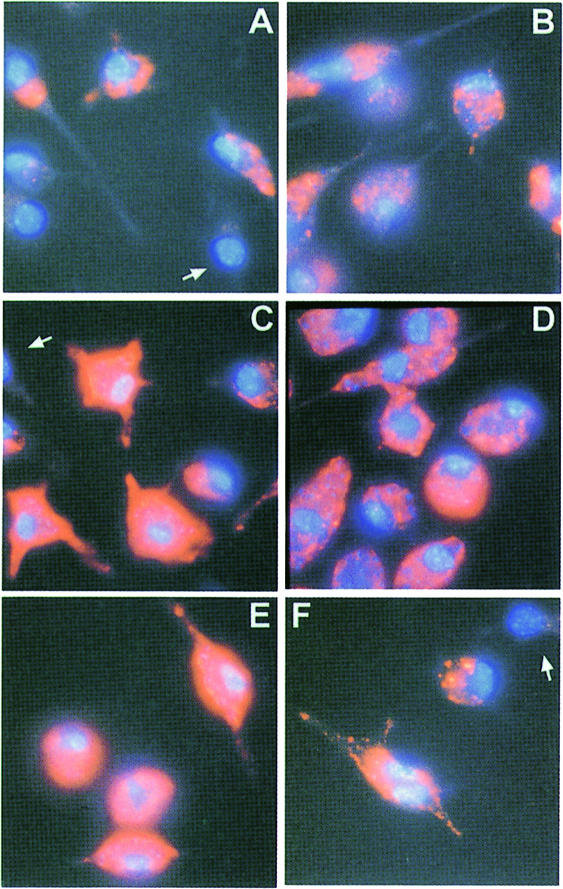
An important argument for the hypothesis that LPG functions as a key molecule in the successful colonization of macrophages by promastigotes is the observation that LPG appeared to be transfered to the host cell plasma and internal membranes during invasion and the early stages of infection (Handman, 1990; Tolson et al., 1990), where it was predicted to have a variety of effects on signal transduction events (reviewed in Descoteaux and Turco, 1993, 1999). Immunofluorescence experiments on macrophages infected 2 days earlier with L.mexicana wild-type promastigotes confirmed the presence of phosphoglycan epitopes. Three anti-phosphoglycan mAbs of different specificity (Figure 1A) all recognized intracellular structures and often also the surface of parasitized cells (Figure 7A, C and E). However, macrophages infected with LPG-deficient Δlmexlpg1 promastigotes also displayed abundant epitopes for all three mAbs, often intracellularly in compartments apparently devoid of parasites; only fluorescence on the host cell surface was observed less frequently than in infections with wild-type strains (Figure 7B, D and E). These data demonstrate that LPG is not the only phosphoglycan-modified compound that is released by the invading promastigotes and distributed in the host macrophage in the early stages of infection.
Fig. 7. Immunofluorescence of saponin-permeabilized peritoneal macrophages infected with L.mexicana wild-type (WT) and LPG-deficient mutant (LPG–) promastigotes. Peritoneal macrophages were infected with five promastigotes of L.mexicana wild-type (A, C and E) or of L.mexicana Δlmexlpg1, clone I/8D per cell (B, D and F). Infected macrophages were labelled after 2 days in culture with the mAbs LT6 (A and B), L7.25 (C and D) and LT17 (E and F) (orange fluorescence). Macrophages and Leishmania nuclei and kinetoplasts were stained with DAPI (blue fluorescence). Uninfected macrophages showing only background fluorescence are indicated by arrows.
Experimental infection of mice with L.mexicana LPG-deficient mutants
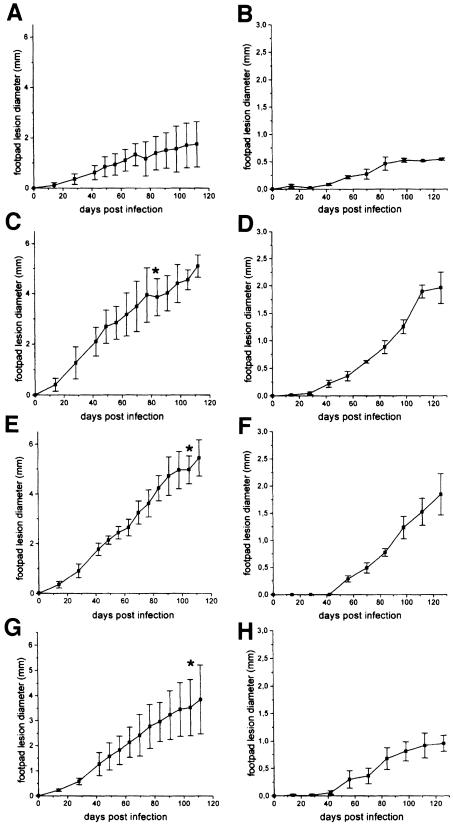
It is widely believed that LPG is required by Leishmania promastigotes for successful infection of mammals. This is largely due to the fact that previously characterized LPG-deficient mutants proved to be unable to infect mice or hamsters and because LPG was thought to protect the parasites against complement lysis in mammalian tissue (reviewed in Sacks, 1989; Turco and Descoteaux, 1992). However, stringent experimental proof for a role of LPG as an essential virulence factor in an animal model has not yet been provided. To investigate this open question for L.mexicana, Balb/c mice were infected with three independent LPG-deficient L.mexicana Δlmexlpg1 mutants and their parental wild-type strain. At a challenge dose of 107 parasites/mouse, footpad lesions developed immediately in all four cases, with extensive swelling visible after 14 days. Disease progressed rapidly in infections with all three LPG-deficient L.mexicana mutants and more slowly in the case of the parental wild-type strains (Figure 8A, C, E and G). It could be argued that the high parasite load swamped the innate defence mechanisms of the mice, such as the complement system. However, a similar picture was obtained when the challenge dose was lowered to 105 parasites/mouse. A lag phase of ∼40 days was followed by rapid disease progression for all three LPG-deficient mutants, while the infection with the parental wild-type strain showed the same lag phase and then a slow increase in footpad swelling (Figure 8B, D, F and H). In sacrificed mice, LPG-deficient parasites could be recovered not only from the lesion and the draining lymph nodes, but also in each case from the spleen, indicating that the Δlmexlpg1 mutants retained the capability to disseminate and to form metastases. The absence of LPG was confirmed in these re-isolated parasites by immunofluorescence using mAb LT6. The three LPG-deficient mutants were also capable of causing lesions in C57/BL6, a mouse strain that is more resistant to L.mexicana infections than Balb/c (Alexander and Kaye, 1985). Disease development was similar or faster than that observed in infections with wild-type L.mexicana and led variably to rapid progression and ulceration, or to a cessation of lesion growth after ∼8 weeks (data not shown). Taken together, these results suggest that LPG is not essential for efficient experimental infections of mice with L.mexicana promastigotes.
Fig. 8. Infection of Balb/c mice with L.mexicana wild-type and LPG-deficient mutant promastigotes. Mice were challenged with either 107 (A, C, E and G) or 105 (B, D, F and H) L.mexicana promastigotes in the right hind footpad. The swellings caused by L.mexicana wild type (A and B), L.mexicana Δlmexlpg1, clone I/8D (C and D), L.mexicana Δlmexlpg1, clone II/5C (E and F) or L.mexicana Δlmexlpg1, clone IV/2B (G and H) were measured. The infection experiments were performed in quadruplicate (107 parasites) or triplicate (105 parasites) and the standard error is indicated. In (C), (E) and (G), at the time points indicated by *, the mouse with the largest lesion was killed for ethical reasons.
Discussion
It is commonly accepted that Leishmania promastigote LPG is a multifunctional virulence factor that is required for parasite survival and development both in sandflies and in mammals. In the latter host organism, LPG has been implicated in promastigote complement resistance, attachment and uptake of promastigotes by macrophages, resistance to and inhibition of several host cell defence mechanisms, and manipulation of signal transduction pathways and of gene expression in macrophages (reviewed in Sacks, 1989; Turco and Descoteaux, 1992; Descoteaux and Turco, 1993, 1999; Beverley and Turco, 1998). The results obtained in this study show that in experimental infections with L.mexicana, all potential functions of LPG listed above are dispensable for the parasites, because LPG-deficient lines are at least as efficient in colonizing macrophages and appear to be even more virulent to mice than their parental wild-type strain. We currently are performing mouse infection studies with re-isolated wild-type and mutant parasites to determine whether this increased virulence of LPG-deficient L.mexicana is a stable phenomenon. In summary, at least for L.mexicana, LPG is not a virulence factor in the mammalian host.
Currently, it is not known whether LPG is required in other Leishmania species for infectivity to mammals. It will be interesting to investigate lpg1-negative mutants in L.major or L.donovani, which were the model organisms for many of the experiments on LPG function. In the case of L.major, it is possible that deletion of lpg1 would affect not only LPG synthesis, because, in contrast to L.mexicana and L.donovani, this organism contains several β-Galf-containing glycosylinositolphospholipids (McConville and Ferguson, 1993). However, it should be noted that there is evidence already that LPG is not absolutely required for infectivity of L.major to mice. Some virulent ricin-resistant L.major mutants previously were believed to synthesize a high molecular weight LPG that replaced wild-type LPG (Cappai et al., 1994). Our recent results suggest that this modified L.major LPG may in fact be the novel surface molecule mPPG (Ilg et al., 1999b; Piani et al., 1999). In this light, it is interesting that a similar molecule appears to be up-regulated in the Δlmexlpg1 mutants generated in this study. It should also be noted that for the disease-causing amastigotes, the findings of this report are not too surprising, because this main mammalian parasite form down-regulates LPG expression to very low or even undetectable levels in L.mexicana, L.donovani and L.major (McConville and Blackwell, 1991; Bahr et al., 1993; Moody et al., 1993).
A series of earlier studies indicated that species-specific LPG has a crucial role in successful colonization of the insect host (reviewed in Sacks et al., 1994). This suggestion has been confirmed recently by the investigation of LPG-deficient L.major mutants that were generated by the same approach as in this study (Sacks et al., 2000). Promastigotes of these mutants showed only a slight reduction of survival and growth in the early stages of development in the sandfly, which suggested that LPG is not required for protection against digestive hydrolases in the bloodfed midgut. However, after bloodmeal excretion, the LPG-deficient mutants were completely lost from the midgut, most likely due to lack of binding to the walls of the insect’s digestive tract. These results demonstrate unequivocally that LPG is a virulence factor for the sandfly stages of the Old World Leishmania species L.major (Sacks et al., 2000). The LPG-deficient mutants and the corresponding lmexlpg1 addback lines generated in this study will now allow the investigation of a similar role for LPG in sandfly infections by the New World species L.mexicana.
It is possible that part of the biological effects identified for LPG may still be of importance for Leishmania parasites in the mammal. In a number of reports, it was shown that the pharmacologically active phosphoglycan repeats and caps of LPG are also attached to a variety of secreted and membrane-bound PPGs of both Leishmania life stages (reviewed in Ilg et al., 1999a). In particular, some of these PPGs are present in macrophages at both an early (Piani et al., 1999; this study) and late stage of Leishmania infection (Ilg et al., 1999a), where they may mediate effects previously attributed to LPG. It is also possible that the up-regulation of PPGs observed in lpg1-deficient L.mexicana promastigotes compensates for the loss of LPG. However, it should also be pointed out that so far, it remains unclear whether PPGs are virulence factors. Deletion mutants for phosphoglycan-modified SAP, for example, retain their infectivity to macrophages and mice (Wiese, 1998). To solve this question, it may be necessary to perform targeted deletions on multiple PPG genes or on genes of biosynthetic enzymes that are selectively involved in assembly of the PPG glycans, such as the GDP-mannose:serine-protein mannose-1-phosphotrans ferase that has been characterized recently in L.mexicana promastigotes (Moss et al., 1999). Further studies will be required to define the role of the PPG family for Leishmania virulence in mammals.
Materials and methods
Parasites and experimental infections of mice and cultured peritoneal macrophages
Promastigotes of the L.mexicana wild-type strain MNYC/BZ/62/M379 and of derived gene knockout mutants were grown at 27°C in semi-defined medium 79 (SDM) supplemented with 4% heat-inactivated fetal calf serum (iFCS) as described previously (Ilg et al., 1993). For mouse infection studies, 50 µl of phosphate-buffered saline (PBS) containing either 107 or 105 stationary phase promastigotes were injected into the right hindleg footpad of either Balb/c or C57/BL6 mice. The course of infections was followed by measuring the swelling relative to the uninfected left hindleg footpad at 7–14 day intervals. Parasites were re-isolated from infected animals by homogenizing footpad lesion tissue, draining lymph nodes and spleens in SDM/5% iFCS and culturing at 27°C. Peritoneal cells were isolated from either Balb/c or C57/BL6 mice by peritoneal lavage, seeded onto glass coverslips (10 mm diameter, 8 × 105 cells each) and incubated overnight in Dulbecco’s modified Eagle’s medium (DMEM), 10% iFCS at 37°C and 5% CO2 in air. Approximately 50% of the cells adhered to the coverslips and were peritoneal macrophages as judged by their morphology. For promastigote binding studies, macrophages were washed twice with serum-free DMEM and incubated with stationary phase promastigotes resuspended in serum-free DMEM at a parasite to macrophage ratio of 5:1 for 30 min at 33°C. Non-adherent parasites were removed by three washings with phosphate-buffered saline (PBS), followed by a fixation step [0.1 M PIPES–NaOH pH 7.2, 2% para-formaldehyde (PAF), 0.05% glutaraldehyde (GA), 30 min, 22°C]. After three washes with PBS and a 20–40 min incubation with 1 µg/ml 4′,6-diamidino-2-phenylindole (DAPI) in PBS, 2% bovine serum albumin (BSA), 50 mM NH4Cl, followed by an additional three washes with PBS, the coverslips were embedded in Mowiol (Stierhof et al., 1999). Macrophages with bound parasites and the total number of bound parasites were counted by inspection with a fluorescence microscope. For macrophage infection studies, peritoneal macrophages were incubated overnight at 33°C with stationary phase promastigotes resuspended in DMEM/10% iFCS at a parasite to macrophage ratio of 2:1, followed by three washes with DMEM/10% iFCS to remove residual free promastigotes. After 6–7 days, the infected macrophages were washed, fixed and stained with DAPI as described above. The number of infected macrophages and the number of L.mexicana amastigotes per host cell were counted by inspection with a fluorescence microscope. In some experiments, a time course of macrophage infection was performed by counting parasitized host cells and intracellular amastigotes at days 1, 2, 3, 4 and 7.
Immunofluorescence microscopy and FACS analysis of Leishmania promastigotes and infected macrophages
Leishmania promastigotes were washed with PBS, fixed to poly-l-lysine-coated glass slides by incubation (30 min, 22°C) with PBS, 2% FA and 0.05% GA, washed again with PBS and incubated with blocking buffer [2% (w/w) BSA, 50 mM NH4Cl in PBS, 30–60 min, 22°C]. The cells were then incubated (60 min, 22°C) with the mAbs (Ilg et al., 1993) LT6, L7.25, LT17 and L3.8 diluted 1:2–1:10 (hybridoma supernatant) or 1:500–1:2000 (ascites fluid) in PBS, 2% BSA, followed by four washings with PBS. Bound mAbs were detected by incubation (45 min, 22°C) with Cy3-labelled goat anti-mouse IgG/IgM (Dianova) diluted 1:500 in PBS, 2% BSA. Subsequently, the cells were incubated for 10 min with 1 µg/ml DAPI in PBS, washed again four times with PBS, embedded in Mowiol and inspected by fluorescence microscopy.
Peritoneal macrophages were infected with L.mexicana promastigotes as described above. After 2 days, the cells were washed three times with PBS, fixed with 2% PAF, 0.05% GA in PBS and subjected to mAb labelling as described above, except for adding 0.1% saponin to all buffers for permeabilization of the host cell membranes.
For FACS analysis, all steps were performed at 0°C with ice-cold and sterile-filtered solutions. Promastigotes were washed three times with PBS, incubated at 5 × 107 cells/ml in PBS, 2% BSA for 1 h and then resuspended for 20 min in PBS, 2% BSA containing hybridoma culture supernatant (LT17 and LT6, at 1:2 dilutions) or ascites fluid (L7.25 and L3.8, diluted 1:1000 and 1:500, respectively). The cells were washed twice with PBS, resuspended in PBS to 5 × 107/ml and then fixed for 45 min by the addition of an equal volume of 0.1 M PIPES–NaOH pH 7.2, 4% PAF, 0.1% GA followed by two washings with PBS, once with PBS, 2% BSA, and a 15 min incubation in PBS, 2% BSA. The fixed samples were incubated for 1 h with fluorescein isothiocyanate (FITC)-labelled goat anti-mouse IgG/IgM antibodies (Dianova, 1:250 in PBS, 2% BSA), washed twice with 2% BSA in PBS, once with PBS, then resuspended to 2 × 107 cells/ml in PBS and subjected to FACS analysis (FACScan, Becton Dickinson).
Cloning of the L.mexicana lpg1 gene and generation of gene knockout mutants
DNA techniques were performed as described previously (Ilg et al., 1999b). Part of the L.mexicana lpg1 gene (lmexlpg1) was obtained from L.mexicana genomic DNA by PCR using the primers AAT GGATCCAATCGCTCGCGCGCAGACAC and AATAAGCTTTCT TCGCCGTAGGCGGGGTA derived from the L.donovani lpg1 gene (Ryan et al., 1993). The PCR product was subcloned into BamHI–HindIII-cut pQE30 (Qiagen). The digoxygenin-labelled PCR product was used to screen a dedicated pBSK+ plasmid library of 4–6 kb PstI fragments derived from L.mexicana genomic DNA. Positive clones were sequenced on both strands by the dideoxy chain termination method using an ALFexpress automated sequencer (Amersham-Pharmacia) as described earlier (Ilg et al., 1999b) and the ORF corresponding to lmexlpg1 was identified by homology to L.donovani lpg1. Double targeted gene replacement was performed by PCR amplification of the 5′-untranslated region (5′-UTR) of lmexlpg1 using the primers KO1 (AAT GCGGCCGCAACGTTCAGGAGTGACGAG) and KO2 (AGTACT AGTGATGCGCTCTCTGTTTCT) and by amplification of the 3′-UTR of lmexlpg1 using the primers KO5 (TCACTAGTGGATCC AGCGCGACATGCCAGT) and KO6 (TGAATTCAACGTTTACGGT CCTGCTCCAG). The NotI–SpeI-cut lmexlpg1 5′-UTR PCR DNA fragment, the BamHI–EcoRI-cut lmexlpg1 3′-UTR PCR DNA fragment and a SpeI–BamHI DNA fragment containing a hygromycin phosphotransferase gene (hyg; Cruz et al., 1991) were ligated consecutively into pBSK+. For the second lmexlpg1 gene replacement cassette, the phleomycin-binding protein gene (phleo) was amplified from pHM-PHLEO (Freedman and Beverley, 1993) using the primers AGTACTAGTCATCCGGGTCCGAGC and ATGGATCCTTGGT CGGCGTCGGTCA. For this construct, a DNA fragment was amplified from the lmexlpg1 3′-UTR region by PCR using the primers KO3 (AGTACTAGTGGATCCCGTTAAGCATTCTGG) and KO4 (TTC GAATTCGGAAGCGCTGTGATGAA). The ligation strategy into pBSK+ was as outlined above. The hyg- and phleo-containing gene replacement cassettes were excised from the plasmids by AclI or AclI–EcoRI digestion, respectively, and transfected into L.mexicana promastigotes as previously described (Ilg et al., 1999b). Selection on 96-well microtitre plates was initiated by the addition of 20 µg/ml hygromycin B (Roche), 2.5 µg/ml phleomycin (Sigma) or both to the growth medium. Positive clones were analysed by Southern blotting using PCR-DIG-labelled DNA fragments (PCR DIG labelling kit, Roche) containing parts of the ORFs of lmexlpg1 (DIG1, GAACACCCA GCCCGAATTCC, and DIG2, ATGACAGCGAATATTCTCGC) or the lmexlpg1 5′-UTR (primers see above) as probes. For gene addback studies, the complete lmexlpg1 ORF was PCR amplified using the primers CCCGGGATCCTCGTAGAAACAGAGAGC and AGATCTAGA ATGCTTAACGGGAGCGA and the lmexlpg1 gene-containing PstI fragment as a template. The BamHI–XbaI-cut PCR fragment was then cloned into pX (LeBowitz et al., 1990). Leishmania mexicana Δlmexlpg1 promastigotes were transfected with this construct as described earlier (Ilg et al., 1999b) and transfectants were selected by growth in SDM/5% iFCS containing 10 µg/ml G418 (Roche). The sequence data for the lmexlpg1-containing PstI fragment have been submitted to the DDBJ/EMBL/GenBank database under accession No. AJ271080.
Analytical procedures
For the production of lysates, late log phase L.mexicana and L.donovani promastigotes were washed twice in PBS and then resuspended at 2 × 109 cells/ml in 50 mM Tris–HCl pH 8.0 containing 10 mM o-phenanthroline, 0.5 mM phenylmethylsulfonyl fluoride (PMSF), 20 µM leupeptin, 0.1% Triton X-100 and 100 U/ml benzonuclease (Merck, Darmstadt, Germany) and sonicated briefly. After a 15 min incubation at 37°C to digest nucleic acids, 1/5 volume 10% SDS was added. Total protein was estimated by the method of Peterson (1983). Discontinuous SDS–PAGE was performed on 4% stacking gels over 7.5–20% separating gels. For electrotransfer of proteins from SDS–PAGE, polyvinylidene difluoride membranes (Millipore) were used (Ilg et al., 1993). Immunodetection of antigens was performed as outlined earlier (Ilg et al., 1999a) using the mAbs LT6, L7.25, LT17 and L3.8. SAP binding experiments with subsequent two-site ELISA detection from culture supernatants from L.mexicana promastigotes were performed using flexibe polvinylchloride ELISA plates (Becton-Dickinson) coated (50 µl/well 10 µg/ml, 50 mM NaHCO3, 100 mM NaCl pH 9.6, 1 h at 25°C) with mAb LT8.2 (directed against a peptide epitope of L.mexicana SAP; Ilg et al., 1993). Bound acid phosphatase activity and the two-site ELISA signal obtained after subsequent incubation with the biotinylated mAbs L7.25 and WIC108.3 were determined as described earlier (Ilg et al., 1999b).
Acknowledgments
Acknowledgements
I would like to thank Professors Steven Beverley and Salvatore Turco for making the various Leishmania expression vectors and L.donovani mutants available to the research community, Suzanne Gokool and Peter Overath for valuable suggestions on the manuscript, and Dorothee Harbecke for her excellent technical assistance.
References
- Alexander J. and Kaye,P.M. (1985) Immunoregulatory pathways in murine leishmaniasis: different regulatory control during Leishmania mexicana mexicana and Leishmania major infections. Clin. Exp. Immunol., 61, 674–682. [PMC free article] [PubMed] [Google Scholar]
- Alexander J. and Russell,D.G. (1992) The interaction of Leishmania species with macrophages. Adv. Parasitol., 31, 175–254. [DOI] [PubMed] [Google Scholar]
- Antoine J.-C., Prina,E., Jouanne,C. and Bongrand,P. (1990) Parasito phorous vacuoles of Leishmania amazonensis-infected macrophages maintain an acidic pH. Infect. Immun., 58, 779–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahr V., Stierhof,Y.-D., Ilg,T., Demar,M., Quinten,M. and Overath,P. (1993) Expression of lipophosphoglycan, high-molecular weight phosphoglycan and glycoprotein 63 in promastigotes and amastigotes of Leishmania mexicana. Mol. Biochem. Parasitol., 58, 107–122. [DOI] [PubMed] [Google Scholar]
- Bates P.A., Hermes,I. and Dwyer,D.M. (1990) Golgi-mediated post-translational processing of secretory acid phosphatase by Leishmania donovani promastigotes. Mol. Biochem. Parasitol., 39, 247–255. [DOI] [PubMed] [Google Scholar]
- Beverley S.M. and Turco,S.J. (1998) Lipophosphoglycan (LPG) and the identification of virulence genes in the protozoan parasite Leishmania. Trends Microbiol., 6, 35–40. [DOI] [PubMed] [Google Scholar]
- Cappai R. et al. (1994) Ricin-resistant mutants of Leishmania major which express modified lipophosphoglycan remain infective for mice. Parasitology, 108, 397–405. [DOI] [PubMed] [Google Scholar]
- Chan J., Fujiwara,T., Brennan,P., McNeil,M., Turco,S.J., Sibille,J.C., Snapper,M., Aisen,P. and Bloom,B.R. (1989) Microbial glycolipids: possible virulence factors that scavenge oxygen radicals. Proc. Natl Acad. Sci. USA, 86, 2453–2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz A., Coburn,C.M. and Beverley,S.M. (1991) Double targeted gene replacement for creating null mutants. Proc. Natl Acad. Sci. USA, 88, 7170–7174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva R.P., Hall,B.F., Joiner,K.A. and Sacks,D.L. (1989) CR1, the C3b receptor, mediates binding of infective Leishmania major metacyclic promastigotes to human macrophages.J. Immunol., 143, 617–622. [PubMed] [Google Scholar]
- Descoteaux A. and Turco,S.J. (1993) The lipophosphoglycan of Leishmania and macrophage protein kinase C. Parasitol. Today, 9, 468–471. [DOI] [PubMed] [Google Scholar]
- Descoteaux A. and Turco,S.J. (1999) Glycoconjugates in Leishmania infectivity. Biochim. Biophys. Acta, 1455, 341–352. [DOI] [PubMed] [Google Scholar]
- Descoteaux A., Luo,Y., Turco,S.J. and Beverley,S.M. (1995) A specialized pathway affecting virulence glycoconjugates of Leishmania. Science, 269, 1869–1872. [DOI] [PubMed] [Google Scholar]
- Descoteaux A., Mengeling,B.J., Beverley,S.M. and Turco,S.J. (1998) Leishmania donovani has distinct mannosylphosphoryltransferases for the initiation and elongation phases of lipophosphoglycan repeating unit biosynthesis. Mol. Biochem. Parasitol., 94, 27–40. [DOI] [PubMed] [Google Scholar]
- Desjardins M. and Descoteaux,A. (1997) Inhibition of phagolysosomal biogenesis by the Leishmania lipophosphoglycan. J. Exp. Med., 185, 2061–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilam Y., El-On,J. and Spira,D.T. (1985) Leishmania major: excreted factor, calcium ions and the survival of amastigotes. Exp. Parasitol., 59, 161–168. [DOI] [PubMed] [Google Scholar]
- Elhay M.J., Kelleher,M., Bacic,A., McConville,M.J., Tolson,D.L., Pearson,T.W. and Handman,E. (1990) Lipophosphoglycan expression and virulence in ricin-resistant variants of Leishmania major. Mol. Biochem. Parasitol., 40, 255–268. [DOI] [PubMed] [Google Scholar]
- Freedman D.J. and Beverley,S.M. (1993) Two more independent selectable markers for stable transfection of Leishmania. Mol. Biochem. Parasitol., 62, 37–44. [DOI] [PubMed] [Google Scholar]
- Giorgione J.R., Turco,S.J. and Epand,R.M. (1996) Transbilayer inhibition of protein kinase C by the lipophosphoglycan from Leishmania donovani. Proc. Natl Acad. Sci. USA, 93, 11634–11639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handman E. (1990) Study of Leishmania major-infected macrophages by use of lipophosphoglycan-specific monoclonal antibodies. Infect. Immun., 58, 2297–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handman E. and Goding,J.W. (1985) The Leishmania receptor for macrophages is a lipid-containing glycoconjugate. EMBO J., 4, 329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handman E., Schnur,L.F., Spithill,T.W. and Mitchell,G.F. (1986) Passive transfer of Leishmania lipopolysaccharide confers parasite survival in macrophages. J. Immunol., 137, 3608–3613. [PubMed] [Google Scholar]
- Hatzigeorgiou D.E., Geng,J., Zhu,B., Zhang,Y., Liu,K., Rom,W.N., Fenton,M.J., Turco,S.J. and Ho,J.L. (1996) Lipophosphoglycan from Leishmania suppresses agonist-induced interleukin 1 β gene expression in human monocytes via a unique promoter sequence. Proc. Natl Acad. Sci. USA, 93, 14708–14713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho J.L., Kim,H.K., Sass,P.M., He,S., Geng,J., Xu,H., Zhu,B., Turco,S.J. and Lo,S.K. (1996) Structure–function analysis of Leishmania lipophosphoglycan. Distinct domains that mediate binding and inhibition of endothelial cell function. J. Immunol., 157, 3013–3020. [PubMed] [Google Scholar]
- Huang C. and Turco,S.J. (1993) Defective galactofuranose addition in lipophosphoglycan biosynthesis in a mutant of Leishmania donovani. J. Biol. Chem., 268, 24060–24066. [PubMed] [Google Scholar]
- Ilg T., Etges,R., Overath,P., McConville,M.J., Thomas-Oates,J., Thomas,J., Homans,S.W. and Ferguson,M.A.J. (1992) Structure of Leishmania mexicana lipophosphoglycan. J. Biol. Chem., 267, 6834–6840. [PubMed] [Google Scholar]
- Ilg T., Harbecke,D., Wiese,M. and Overath,P. (1993) Monoclonal antibodies directed against Leishmania secreted acid phosphatase and lipophosphoglycan. Partial characterization of private and public epitopes. Eur. J. Biochem., 217, 603–615. [DOI] [PubMed] [Google Scholar]
- Ilg T., Handman,E. and Stierhof,Y.-D. (1999a) Proteophosphoglycans from Leishmania promastigotes and amastigotes. Biochem. Soc. Trans, 27, 518–525. [DOI] [PubMed] [Google Scholar]
- Ilg T., Montgomory,J., Stierhof,Y.-D. and Handman,E. (1999b) Molecular cloning and characterization of a novel repeat-containing Leishmania major gene, ppg1, that encodes a membrane-associated form of proteophosphoglycan with a putative glycosylphosphatidylinositol anchor. J. Biol. Chem., 274, 31410–31420. [DOI] [PubMed] [Google Scholar]
- Karp C.L., Turco,S.J. and Sacks,D.L. (1991) Lipophosphoglycan masks recognition of the Leishmania donovani promastigote surface by human kala-azar serum. J. Immunol., 147, 680–684. [PubMed] [Google Scholar]
- King D.L. and Turco,S.J. (1988) A ricin agglutinin-resistant clone of Leishmania donovani deficient in lipophosphoglycan. Mol. Biochem. Parasitol., 28, 285–294. [DOI] [PubMed] [Google Scholar]
- LeBowitz J.H., Coburn,C.M., McMahon-Pratt,D. and Beverley,S.M. (1990) Development of a stable Leishmania expression vector and application to the study of parasite surface antigen genes. Proc. Natl Acad. Sci. USA, 87, 9736–9740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew F.Y., Millott,S., Parkinson,C., Palmer,R.M. and Moncada,S. (1990) Macrophage killing of Leishmania parasites in vivo is mediated by nitric oxide from l-arginine. J. Immunol., 144, 4794–4797. [PubMed] [Google Scholar]
- Lo S.K., Bovis,L., Matura,R., Zhu,B., He,S., Lum,H., Turco,S.J. and Ho,J.L. (1998) Leishmania lipophosphoglycan reduces monocyte transendothelial migration: modulation of cell adhesion molecules, intercellular junctional proteins and chemoattractants. J. Immunol., 160, 1857–1865. [PubMed] [Google Scholar]
- McConville M.J. and Blackwell,J.M. (1991) Developmental changes in the glycosylated phosphatidylinositols of Leishmania donovani. Characterization of the promastigote and amastigote glycolipids. J. Biol. Chem., 266, 15170–15179. [PubMed] [Google Scholar]
- McConville M.J. and Ferguson,M.A.J. (1993) The structure, biosynthesis and function of glycosylated phosphatidylinositols in the parasitic protozoa and higher eukaryotes. Biochem. J., 294, 305–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeely T.B. and Turco,S.J. (1990) Requirement of lipophosphoglycan for intracellular survival of Leishmania donovani within human monocytes. J. Immunol., 144, 2745–2750. [PubMed] [Google Scholar]
- Mengeling B.J., Beverley,S.M. and Turco,S.J. (1997) Designing glycoconjugate biosynthesis for an insidious intent: phosphoglycan assembly in Leishmania parasites. Glycobiology, 7, 873–880. [DOI] [PubMed] [Google Scholar]
- Moody S.F., Handman,E., McConville,M.J. and Bacic,A. (1993) The structure of Leishmania major amastigote lipophosphoglycan. J. Biol. Chem., 268, 18457–18466. [PubMed] [Google Scholar]
- Moss J.M., Reid,G., Mullin,K.A., Zawadzki,J.L., Simpson,R.J. and McConville,M.J. (1999) Characterization of a novel GDP-mannose:serine-protein mannose-1-phosphotransferase from Leishmania mexicana. J. Biol. Chem., 274, 6678–6688. [DOI] [PubMed] [Google Scholar]
- Mosser D.M., Wedgwood,J.F. and Edelson,P.J. (1985) Leishmania amastigotes: resistance to complement-mediated lysis is not due to a failure to fix C3. J. Immunol., 134, 4128–4131. [PubMed] [Google Scholar]
- Murray H.W. and Nathan,C.F. (1999) Macrophage microbicidal mechanisms in vivo: reactive nitrogen versus oxygen intermediates in the killing of intracellular visceral Leishmania donovani. J. Exp. Med., 189, 741–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G.L. (1983) Determination of total protein. Methods Enzymol., 91, 95–119. [DOI] [PubMed] [Google Scholar]
- Piani A., Ilg,T., Elefanty,E.G., Curtis,J. and Handman,E. (1999) Leishmania major proteophosphoglycan is expressed by amastigotes and has an immunomodulatory effect on macrophage function. Microbes Infect., 1, 589–599. [DOI] [PubMed] [Google Scholar]
- Piedrafita D. et al. (1999) Regulation of macrophage IL-12 synthesis by Leishmania phosphoglycans. Eur. J. Immunol., 29, 235–244. [DOI] [PubMed] [Google Scholar]
- Prina E., Antoine,J.-C., Wiederanders,B. and Kirschke,H. (1990) Localization and activity of various lysosomal proteases in Leishmania amazonensis-infected macrophages. Infect. Immun., 58, 1730–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot L., Nikolaev,A.V., Feng,G.-J., Wei,X.-Q., Ferguson,M.A.J., Brimacombe,J.S. and Liew,F.Y. (1996) Regulation of the expression of nitric oxide synthase and leishmanicidal activity by glycoconjugates of Leishmania lipophosphoglycan in murine macrophages. Proc. Natl Acad. Sci. USA, 93, 10984–10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner S.L. and Locksley,R.M. (1995) The regulation of immunity to Leishmania major. Annu. Rev. Immunol., 13, 151–177. [DOI] [PubMed] [Google Scholar]
- Ryan K.A., Garraway,L.A., Descoteaux,A., Turco,S.J. and Beverley,S.M (1993) Isolation of virulence genes directing surface glycosyl-phosphatidylinositol synthesis by functional complementation of Leishmania. Proc. Natl Acad. Sci. USA, 90, 8609–8613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks D.L. (1989) Metacyclogenesis in Leishmania promastigotes. Exp. Parasitol., 69, 100–103. [DOI] [PubMed] [Google Scholar]
- Sacks D.L., Saraiva,E.M., Rowton,E., Turco,S.J. and Pimenta,P.F.P. (1994) The role of the lipophosphoglycan of Leishmania in vector competence. Parasitology, 108, S55–S62. [DOI] [PubMed] [Google Scholar]
- Sacks D.L., Modi,G., Rowton,E., Späth,G., Epstein,L., Turco,S.J. and Beverley,S.M. (2000) The role of phosphoglycans in Leishmania–sandfly interactions. Proc. Natl Acad. Sci. USA, 97, 406–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stierhof Y.-D., Bates,P.A., Jacobson,R., Schlein,Y., Rogers,M.E., Handman,E. and Ilg,T. (1999) Filamentous proteophosphoglycan secreted by Leishmania promastigotes forms gel-like three-dimensional networks that obstruct the digestive tract of infected sandfly vectors. Eur. J. Cell Biol., 78, 675–689. [DOI] [PubMed] [Google Scholar]
- Talamas-Rohana P., Wright,S.D., Lennartz,M.R. and Russell,D.G. (1990) Lipophosphoglycan from Leishmania mexicana promastigotes binds to members of the CR3, p150,95 and LFA-1 family of leukocyte integrins. J. Immunol., 144, 4817–4824. [PubMed] [Google Scholar]
- Tolson D.L., Turco,S.J. and Pearson,T.W. (1990) Expression of a repeating phosphorylated disaccharide lipophosphoglycan epitope on the surface of macrophages infected with Leishmania donovani.Infect. Immun., 58, 3500–3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco S.J. and Descoteaux,A. (1992) The lipophosphoglycan of Leishmania parasites. Annu. Rev. Microbiol., 46, 65–94. [DOI] [PubMed] [Google Scholar]
- Wiese M. (1998) A mitogen-activated protein (MAP) kinase homologue of Leishmania mexicana is essential for parasite survival in the infected host. EMBO J., 17, 2619–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]