Abstract
Evidence for important role of poly(ADP-ribose) polymerase (PARP) activation in diabetic complications is emerging. This study evaluated the role for PARP in rat and mouse models of advanced diabetic neuropathy. The orally active PARP inhibitor 10-(4-methyl-piperazin-1-ylmethyl)-2H-7-oxa-1,2-diaza-benzo[de]anthracen-3-one(GPI-15427, formulated as mesilate salt, 30 mg kg−1d−1 in the drinking water, for 10 weeks after first 2 weeks without treatment) at least partially prevented PARP activation in peripheral nerve and DRG neurons, as well as thermal hypoalgesia, mechanical hyperalgesia, tactile allodynia, exaggerated response to formalin, and, the most important, intraepidermal nerve fiber degeneration in streptozotocin-diabetic rats. These findings are consistent with the lack of small sensory nerve fiber dysfunction in diabetic PARP−/− mice. Furthermore, whereas diabetic PARP+/+ mice displayed ~ 46% intraepidermal nerve fiber loss, diabetic PARP−/− preserved completely normal intraepidermal nerve fiber density. In conclusion, PARP activation is an important contributor to intraepidermal nerve fiber degeneration and functional changes associated with advanced Type 1 diabetic neuropathy. The results support the rationale for development of potent and low toxic PARP inhibitors and PARP inhibitor-containing combination therapies.
Keywords: intraepidermal nerve fiber loss, mechanical hyperalgesia, mechanical hypoalgesia, neuropathic pain, oxidative-nitrosative stress, poly(ADP-ribose) polymerase, tactile allodynia, thermal hypoalgesia
Evidence for a fundamental role of poly(ADP-ribose) polymerase (PARP) (1) in diabetic complications including endothelial (2) and myocardial (3) dysfunction, peripheral (PDN) (4,5) and autonomic (6) neuropathy, retinopathy (7,8), and nephropathy (9,10), is emerging. PARP activation manifest by accumulation of poly(ADP-ribose) polymer has been observed in vascular endothelium, myocardium, peripheral nerve, spinal cord, dorsal root ganglion (DRG) neurons, retinal vasculature, inner neurons and ganglion cells, and renal cortex glomeruli and tubuli in animal models of both Type 1 and Type 2 diabetes (1–5,7–12), as well as cutaneous microvascular endothelium of diabetic patients (13). Furthermore, PARP activation has been identified in peripheral nervous system of high fat diet fed mice, a model of obesity and prediabetes (14), as well as in human subjects at risk of developing type 2 diabetes in whom it was associated with impaired vascular reactivity (13). Thus, PARP activation known to result in NAD+ depletion, energy failure, and profound metabolic abnormalities (1), changes in transcriptional regulation and gene expression (1,7,15), inflammatory response (1,15), altered signal transduction (16), angiogenesis (17), translocation of apoptosis-inducing factor from the mitochondria to the nucleus (18) and cell death signaling (19), is an early phenomenon characteristic for prediabetes and overt diabetes. Several studies with structurally diverse PARP inhibitors (4–6,20) and PARP-deficient mice (4) suggest that this mechanism is implicated in motor and sensory nerve conduction velocity (MNCV and SNCV) deficits, peripheral nerve energy failure, sensory disorders, and impaired nitrergic innervation, known to contribute to gastroparesis and impotence associated with autonomic neuropathy, in rats and mice with short-term STZ-diabetes. The present study employing a novel, orally active PARP inhibitor 10-(4-methyl-piperazin-1-ylmethyl)-2H-7-oxa-1,2-diaza-benzo[de] anthracen-3-one (GPI-15427, MGI Pharma, Baltimore) (21,22) as well as PARP-deficient mice reveals a new role for PARP activation in small sensory nerve fiber degeneration and neuropathic pain associated with advanced PDN.
METHODS
A. Reagents
Unless otherwise stated, all chemicals were of reagent-grade quality, and were purchased from Sigma Chemical Co., St. Louis, MO. The PARP inhibitor 10-(4-methyl-piperazin-1-ylmethyl)-2H-7-oxa-1,2-diaza-benzo[de]anthracen-3-one(GPI-15427, formulated as mesilate salt), was synthesized by MGI Pharma, Baltimore. Mouse monoclonal anti-poly(ADP-ribose) was obtained from Trevigen, Inc., Gaithersburg, MD. Secondary Alexa Fluor 488 goat anti-rabbit and Alexa Fluor 488 goat anti-mouse antibodies, secondary FITS-rabbit anti-goat IgG (H+L) as well as Prolong Gold Antifade Reagent were purchased from Invitrogen, Eugene, OR. Avidin/Biotin Blocking Kit, M.O.M. Basic Kit, VECTASTAIN Elite ABC Kit (Standard*), DAB Substrate Kit, and 3,3’-diaminobenzidine were obtained from Vector Laboratories, Burlingame, CA. Rabbit polyclonal anti-protein gene product 9.5 (ubiquitin c-terminal hydrolase) antibody was purchased from Chemicon International, Inc, Temecula, CA. Other reagents for immunohistochemistry have been purchased from Dako Laboratories, Inc., Santa Barbara, CA.
B. Animals
The experiments were performed in accordance with regulations specified by the National Institutes of Health “Principles of Laboratory Animal Care, 1985 Revised Version” and Pennington Biomedical Research Center Protocol for Animal Studies.
B.1. Studies in STZ-diabetic rats
Male Wistar rats (Charles River, Wilmington, MA), body weight 250–300 g, were fed a standard rat chow (PMI Nutrition Int., Brentwood, MO) and had access to water ad libitum. STZ-diabetes was induced as described (4,20). Blood samples for glucose measurements were taken from the tail vein ~48 h after the STZ injection and the day before the animals were killed. The rats with blood glucose ≥13.8 mM were considered diabetic. The experimental groups comprised control and diabetic rats treated with or without the PARP inhibitor GPI-15427, 30 mg kg−1d−1, in the drinking water. This dose was selected from a preliminary experiment in which it essentially normalized MNCV and SNCV deficits in rats with 4-week duration of STZ-diabetes (MNCV: controls: 54.7 ± 2.0 m/s; controls + GPI-15427: 53.6 ± 2.8 m/s; diabetics: 43.2 ± 1.2 m/s; diabetics+GPI-15427: 51.5 ± 1.7 m/s; SNCV: controls: 40.1 ± 0.6 m/s; controls + GPI-15427: 41.3 ± 1.0 m/s; diabetics: 35.3 ± 0.5 m/s; diabetics + GPI-15427: 39.3 ± 1.2 m/s, Mean ± SEM, n = 6). The agent was given for 10 weeks after initial 2 weeks without treatment, to avoid restoration of normoglycemia or alleviation of hyperglycemia that would occur if a PARP inhibitor administration was started shortly after induction of STZ-diabetes (1). The behavioral tests have been started 24 hours after the last GPI-15427 injection and performed in the following order: tactile responses to flexible von Frey filaments, paw withdrawal test of thermal algesia, tail-flick test, paw pressure Randall-Sellito test, mechanical algesia with rigid von Frey filaments and von Frey anesthesiometer, and formalin flinching responses.
B.1. Studies in STZ-diabetic PARP+/+ and PARP−/− mice
Several breeding pairs of PARP−/− (129S-Parp1tm1Zqw/J) mice were purchased from Jackson Laboratories (Bar Harbor, ME) and their colony was established at Pennington Biomedical Research Center. PARP−/− mice and the corresponding wild-type mice (PARP+/+, 129S1/SvImJ) were fed standard mouse chow (PMI Nutrition International, Brentwood, MO) and had access to water ad libitum. PARP−/− mice do not develop diabetes after a single high-dose injection of STZ (24), and are partially protected after multiple (5) injections (27). Therefore, in our study, PARP+/+ and PARP−/− mice were treated with STZ, 40 mg kg−1d−1, i.p., for, at least, 7 consecutive days for induction of diabetes. Typically, PARP−/− mice required 2–3 more injections than the wild-type mice, to produce similar levels of hyperglycemia. The duration of experiment was 10 weeks. Blood samples for glucose measurements were taken from the tail vein 3 days after the last STZ injection and the day before the animals were killed. The mice with blood glucose >13.8 mM were considered diabetic.
C. Anesthesia, euthanasia and tissue sampling
The animals were sedated by CO2, and sacrificed by cervical dislocation. Sciatic nerves, dorsal root ganglia (DRG), and foot pads were fixed in normal buffered 4% formalin for assessment of poly(ADP-ribose) and intraepidermal nerve fiber density by immunohistochemistry.
D. Specific Methods
D.1. Behavioral tests
Assessment of tactile response thresholds, thermal and mechanical algesia, tactile allodynia, and flinching behavior in the formalin pain test was performed as described [12,20]. The paw withdrawal latency in response to the radiant heat was recorded at a 15% intensity (heating rate of ~1.3° C per s) with a cut-off time 35 s. Tail flick response latencies were determined at a 40% heating intensity (heating rate ~ 2.5° C per s) and with a cut off at 10 s.
D.2. Immunohistochemical studies
All sections were processed and evaluated blindly. Low power observations of skin sections stained for PGP 9.5 were made using a Zeiss Axioskop microscope. Color images were captured with a Zeiss Axiocam HRc CCD camera at 1300 × 1030 resolution. Low power images were generated with a 40X acroplan objective using the automatic capturing feature of the Zeiss Axiovision software (Ver. 3.1.2.1). Low power observations of sciatic nerve and DRG sections stained for poly(ADP-ribose) were made using a Zeiss Axioplan 2 imaging microscope. Color images were captured with a Photometric CoolSNAP™ HQ CCD camera at 1392 × 1040 resolution. Low power images were generated with a 40X acroplan objective using the RS Image™ 1.9.2 software.
D.2.1. Poly(ADP-ribose) immunoreactivity
Poly(ADP-ribose) immunoreactivity was assessed as described (12,20). In the rat study, non-specific binding was blocked in 10% goat serum containing 1% BSA in TBS (DAKO, Carpinteria, CA), for 2 hours. In the mouse study, non-specific binding was blocked with the mouse IG blocking reagent supplied with the Vector M.O.M. Basic Immunodetection Kit. Then mouse monoclonal anti poly(ADP-ribose) antibody was diluted 1:100 in 1% BSA in TBS, and applied overnight at 4°C in the humidity chamber. Secondary Alexa Fluor 488 goat anti-mouse antibody was diluted 1:200 in TBS and applied for 2 hours at room temperature. Sections were mounted in Prolong Gold Antifade Reagent. At least, ten fields of each section were examined to select one representative image. Representative images were microphotographed, and the number of poly(ADP-ribose)-positive nuclei calculated for each microphotograph.
D.2.2. Intraepidermal nerve fiber density (INFD)
INFD was assessed as described (12). Intraepidermal nerve fiber profiles were counted blindly by three independent investigators, under an Olympus BX-41 microscope, and the average values were used. Microphotographs of stained sections were taken on Axioscop 2 microscope (Zeiss) at 4X magnification, and the length of epidermis was assessed with the ImagePro 3.0 program (Media Cybernetics). An average of 2.8 ± 0.3 mm of the sample length was investigated to calculate a number of nerve fiber profiles per mm of epidermis.
E. Statistical analysis
The results are expressed as Mean ± SEM. Data were subjected to equality of variance F test, and then to log transformation, if necessary, before one-way analysis of variance. Where overall significance (p<0.05) was attained, individual between group comparisons were made using the Student-Newman-Keuls multiple range test. Significance was defined at p ≤ 0.05. When between-group variance differences could not be normalized by log transformation (datasets for body weights and plasma glucose), the data were analyzed by the nonparametric Kruskal-Wallis one-way analysis of variance, followed by the Bonferroni/Dunn test for multiple comparisons.
RESULTS
The final body weights were similarly reduced and the final blood glucose concentrations similarly elevated in untreated and GPI-15427-treated diabetic rats compared with the control group (Table 1). PARP inhibition did not affect either weight gain or blood glucose concentrations in non-diabetic rats. Weight gain was similar in non-diabetic wild-type and PARP−/− mice and was lower in diabetic PARP+/+ and diabetic PARP−/− mice than in corresponding untreated groups. Diabetic PARP−/− mice, but not diabetic PARP+/+ mice, demonstrated a weight loss during the course of observation. The final blood glucose concentrations were similarly elevated in diabetic wild-type and PARP−/− mice compared with the non-diabetic groups.
Table 1.
Initial and final body weights and blood glucose concentrations in experimental rats and mice
| Body weight (g) | Blood glucose (mmol/l) | |||
|---|---|---|---|---|
| Initial | Final | Initial | Final | |
| RAT STUDY | ||||
| Control | 291 ± 2.3 | 565 ± 22 | 5.7 ± 0.16 | 5.5 ± 0.4 |
| Control + GPI-15427 | 299 ± 6.6 | 557 ± 19 | 5.4 ± 0.11 | 5.1 ± 0.3 |
| Diabetic | 288 ± 3.8 | 353 ± 13** | 25.4 ± 1.23** | 26.1 ± 1.3** |
| Diabetic + GPI-15427 | 297 ± 4.5 | 359 ± 16** | 26.2 ± 1.1** | 24.5 ± 0.9** |
| MOUSE STUDY | ||||
| PARP+/+ | 26.3 ± 0.27 | 28.9 ± 0.37 | 6.14 ± 0.31 | 6.06 ± 0.26 |
| PARP−/− | 31.3 ± 1.4 | 34.0 ± 1.4 | 6.08 ± 0.42 | 6.40 ± 0.18 |
| Diabetic PARP+/+ | 26.5 ± 0.37 | 26.5 ± 0.32* | 14.6 ± 0.75 | 27.7 ± 1.4** |
| Diabetic PARP−/− | 31.0 ± 1.0 | 25.6 ± 0.53** | 14.0 ±0.98 | 24.6 ± 0.94** |
Data are means ± SEM, n = 6–12 per group in the rat study and n= 13–19 per group in the mouse study.
significantly different from controls (p < 0.05 and < 0.01 vs controls).
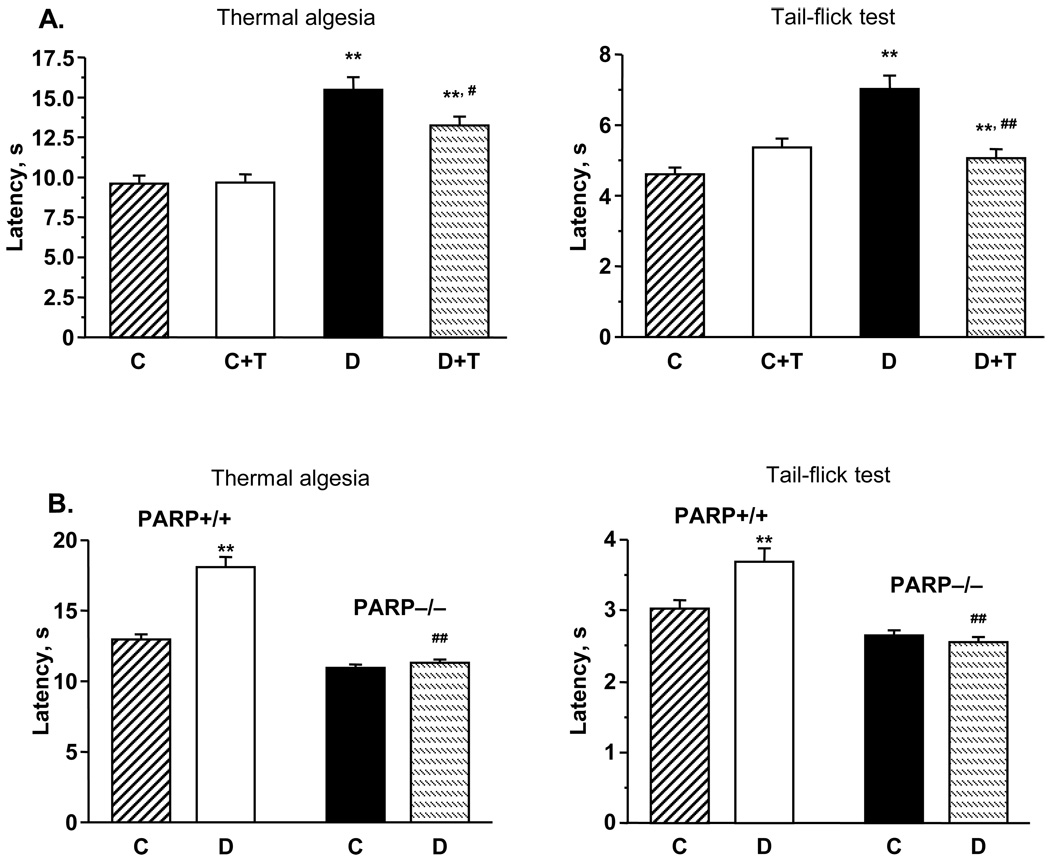
Diabetic rats with 12-wk duration of STZ-diabetes had thermal hypoalgesia detected by both paw withdrawal and tail-flick tests (Fig.1, A). In particular, the latencies of paw withdrawal in response to radiant heat and tail flick response were increased by 61% and 52% in diabetic rats compared with controls (p < 0.01). GPI-14527 partially (paw withdrawal test) and completely (tail flick test) corrected thermal algesia in diabetic rats, without affecting this variable in the control group. The latencies of paw withdrawal and tail-flick responses were increased in diabetic wild-type mice compared with the control group (p < 0.01), consistent with clearly manifest thermal hypoalgesia (Fig.1, B). In contrast, diabetic PARP−/− mice preserved normal paw withdrawal and tail flick response latencies i.e., did not manifest any signs of thermal hypoalgesia.
Fig.1.
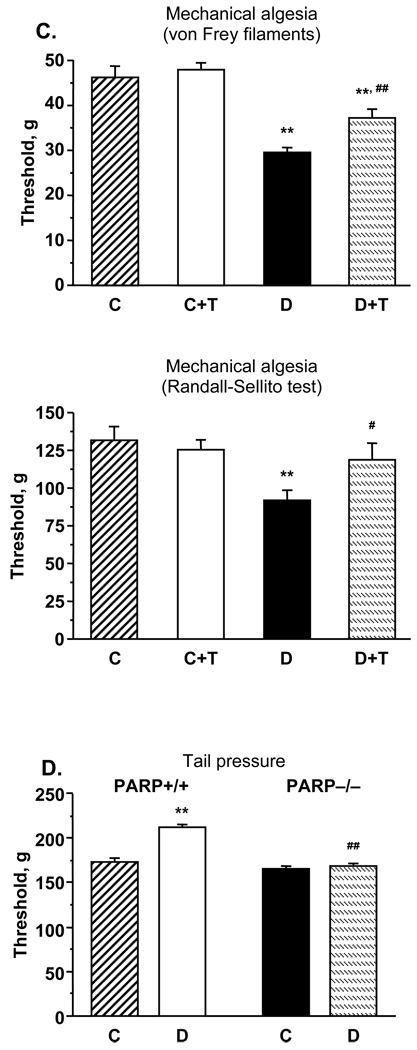
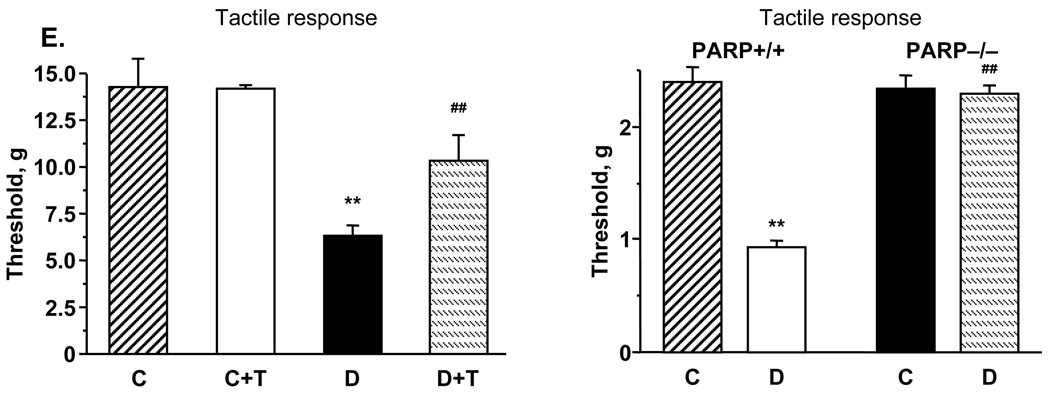
A. Paw withdrawal latencies in response to radiant heat and tail-flick test response latencies in control and diabetic mice with or without the PARP inhibitor GPI-15427 treatment. Mean ± SEM, n = 6–12 per group. C – control rats, D - diabetic rats. T- GPI-15427 treatment. ** – p < 0.01 vs non-diabetic control rats; #, ## – p < 0.05 and < 0.01 vs untreated diabetic rats. B. Paw withdrawal latencies in response to radiant heat and tail-flick test response latencies in control and diabetic PARP+/+ and PARP−/− mice. Mean ± SEM, n = 13–18 per group. C – control mice, D - diabetic mice. ** – p < 0.01 vs non-diabetic control mice; ## – p < 0.01 vs diabetic wild-type mice. C. Mechanical withdrawal thresholds in rigid von Frey filament test and paw-pressure Randall-Selitto test in control and diabetic rats with or without the PARP inhibitor GPI-15427 treatment. Mean ± SEM, n = 6–12 per group. C – control rats, D – diabetic rats. T - GPI-15427 treatment. ** – p < 0.01 vs control rats; #, ## – p < 0.05 and < 0.01 vs untreated diabetic rats. D. Mechanical withdrawal thresholds in tail-pressure Randall-Selitto test in control and diabetic PARP+/+ and PARP−/− mice. Mean ± SEM, n = 13–18 per group. C – control mice, D - diabetic mice. ** – p < 0.01 vs non-diabetic control mice; ## – p < 0.01 vs diabetic wild-type mice. E. Left - tactile response thresholds in flexible von Frey filament test (left) in control and diabetic rats with or without the PARP inhibitor GPI-15427 treatment. Mean ± SEM, n = 6–12 per group. C – control rats, D – diabetic rats. T - GPI-15427 treatment. ** – p < 0.01 vs control rats; ## – p < 0.01 vs untreated diabetic rats. Right - tactile response thresholds in flexible von Frey filament test in control and diabetic PARP+/+ and PARP−/− mice. Mean ± SEM, n = 13–18 per group. C – control mice, D - diabetic mice. ** – p < 0.01 vs non-diabetic control mice; ## – p < 0.01 vs diabetic wild-type mice.
Diabetic rats with 12-wk duration of STZ-diabetes also had mechanical hyperalgesia, detected by 1) measuring paw withdrawal thresholds in response to stimulation with rigid von Frey filaments, and 2) the paw pressure Randall-Sellito test (Fig.1, C). In particular, the paw withdrawal thresholds in response to rigid von Frey filaments and applied pressure were reduced by 36% and 30% in diabetic rats compared with controls (p < 0.01). GPI-15427 partially (von Frey filament test) or essentially (Randall-Selitto test) corrected diabetes-induced decrease in paw withdrawal thresholds, without affecting this variable in control rats. In contrast to diabetic rats, wild-type diabetic mice with 10-wk duration of STZ-diabetes had mechanical hypoalgesia detected with the tail pressure Randall-Sellito test (Fig.1, D). Whereas the tail pressure threshold was increased by 23% in diabetic PARP+/+ mice compared with the control group (p < 0.01), diabetic PARP−/− mice preserved normal sensitivity to mechanical noxious stimulation.
Another sensory abnormality developing in both diabetic rats and diabetic PARP+/+ mice was tactile allodynia. Tactile withdrawal threshold in response to light touch with flexible von Frey filaments was reduced by 56% in diabetic rats compared with controls (p<0.01) (Fig. 1, E, left). GPI-15427 partially (to 72% of the control value, p< 0.01 vs controls and vs untreated diabetic group) corrected diabetes-induced decrease in tactile withdrawal thresholds in diabetic rats, without affecting this variable in the control group. Tactile withdrawal threshold was reduced by 61% in diabetic PARP+/+ mice compared with controls (p < 0.01), consistent with tactile allodynia (Fig. 1, E, right). In contrast, diabetic PARP−/− mice maintained normal tactile withdrawal thresholds.
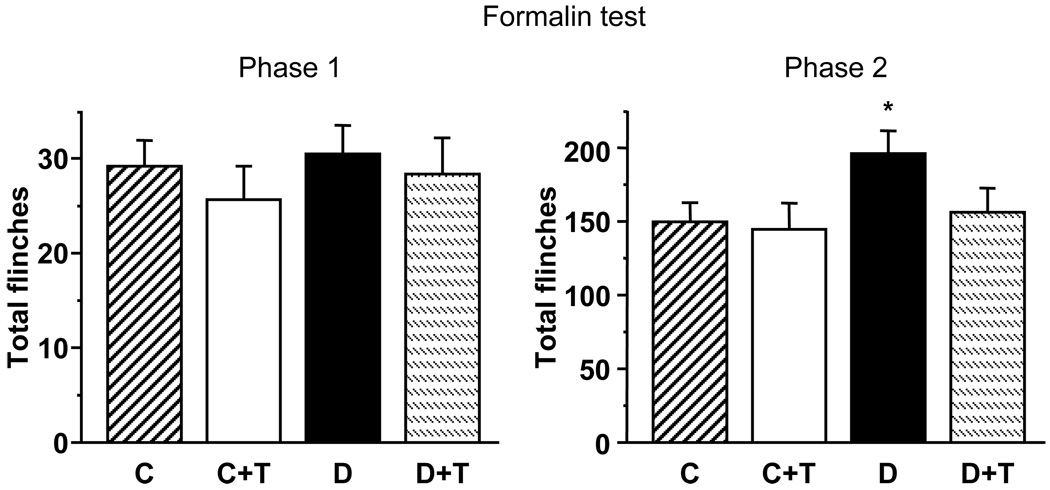
In contrast to rats with 4-week duration of STZ-diabetes that displayed exaggerated flinching behavior in both first and second phases of the formalin pain test (20), rats with 12-week diabetes displayed hyperalgesia in the second phase of formalin test only (Fig. 2). GPI-14527 did not affect the second phase responses to formalin in control rats, but essentially normalized them in diabetic rats.
Fig.2.
Total number of flinches in the first and second phases of the formalin pain test in control and diabetic rats with and without the PARP inhibitor GPI-15427 treatment. C – control rats, D – diabetic rats. T- GPI-15427 treatment. * – p < 0.05 vs control rats.
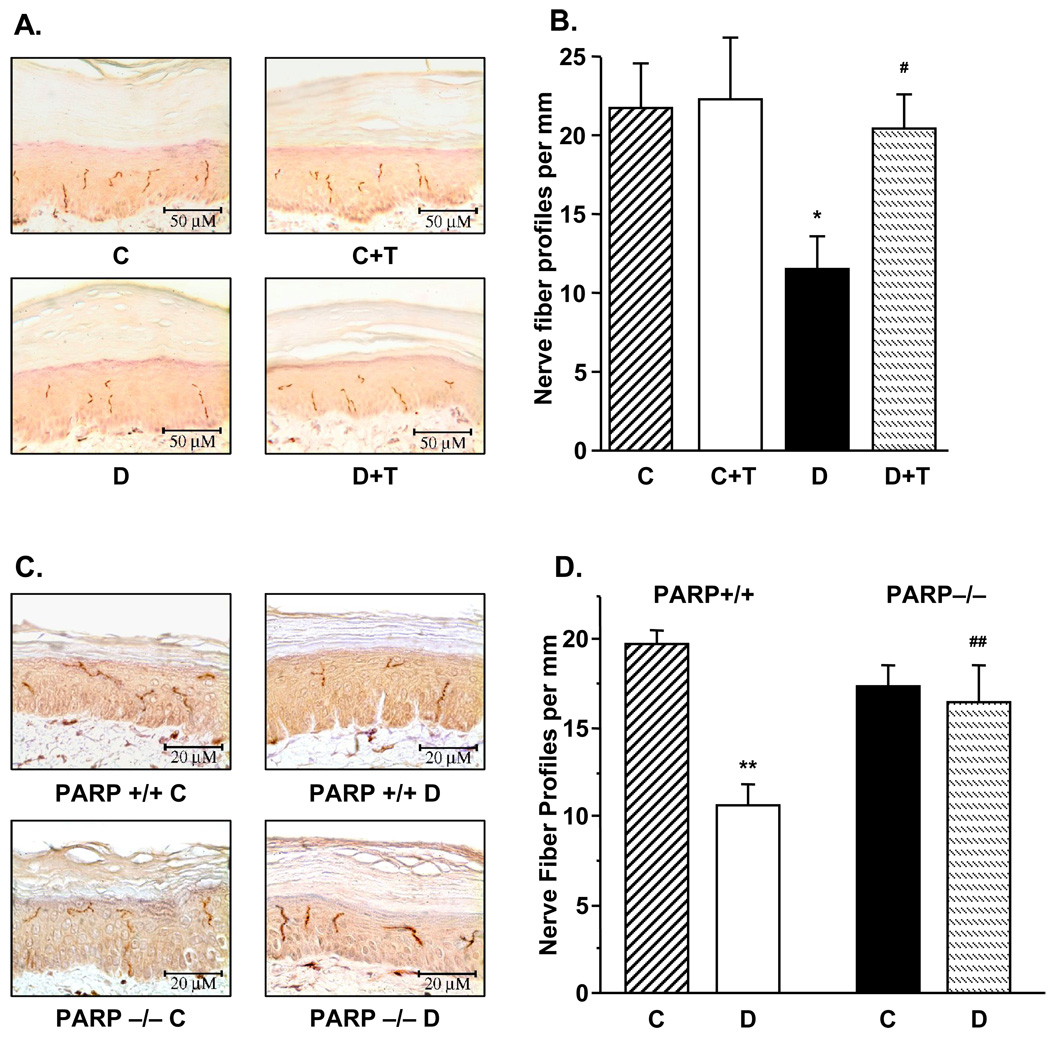
INFD was reduced by 47% in diabetic rats compared with controls (p < 0.01, Fig. 3, A and B), and this reduction was essentially prevented by GPI-15427 (to 94% of the control value, p < 0.05 vs diabetic group). PARP inhibition did not affect INFD in control rats. Whereas diabetic PARP+/+ mice displayed ~46% intraepidermal nerve fiber loss (p < 0.01 vs controls), diabetic PARP−/− preserved normal INFD (Fig. 3, C and D).
Fig.3.
A. Intraepidermal nerve fiber profiles in control and diabetic rats with and without the PARP inhibitor GPI-15427 treatment. Left – representative image of intraepidermal nerve fiber profiles, magnification × 40; Right – skin fiber density. Mean ± SEM, n = 6–9 per group. C – control rats, D – diabetic rats. T- GPI-15427 treatment. * – p < 0.05 vs control rats; # – p < 0.05 vs untreated diabetic rats. B. Intraepidermal nerve fiber profiles in control and diabetic PARP+/+ and PARP−/− mice. Left – representative image of intraepidermal nerve fiber profiles, magnification × 80; Right – skin fiber density. Mean ± SEM, n = 8–11 per group. C – control mice, D – diabetic mice. ** – p < 0.01 vs control mice; ## – p < 0.01 vs diabetic PARP+/+ mice.
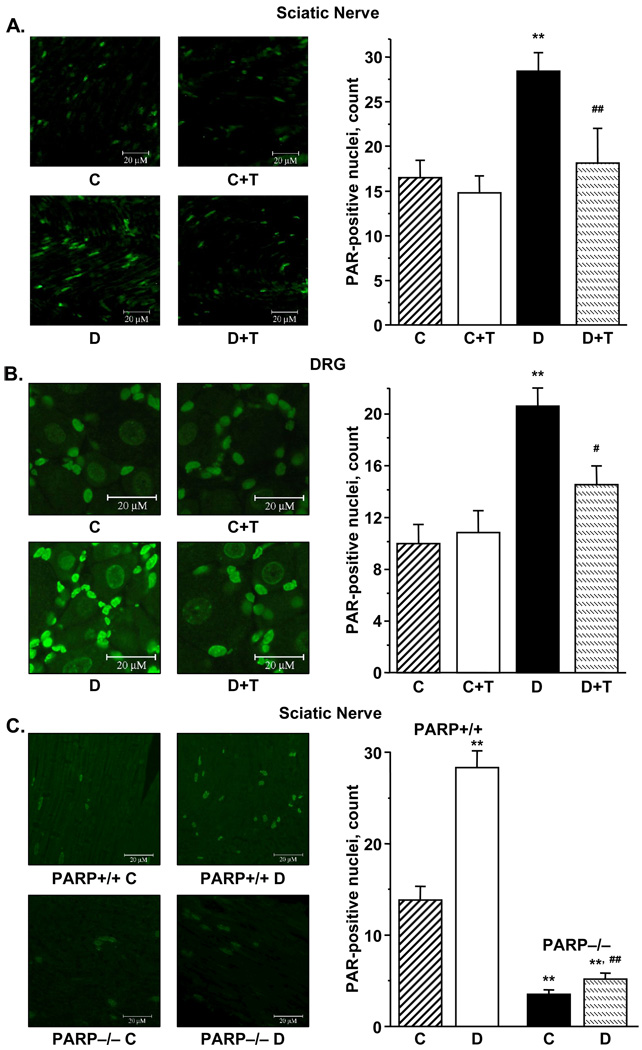
The number of poly (ADP-ribose) positive nuclei was increased in the sciatic nerve and DRG of diabetic rats (Fig. 4, A, B), indicative of PARP-1 activation. GPI-15427 essentially inhibited PARP activation in sciatic nerve and DRG of diabetic rats (p < 0.01 vs diabetic group for all three tissues), without affecting this variable in the control group. The number of poly(ADP-ribose) positive nuclei was increased by 104% in the sciatic nerve of diabetic PARP+/+ mice compared with non-diabetic controls (p < 0.01). Of interest, a small number of poly(ADP-ribose) positive nuclei was identified in the sciatic nerves of control and diabetic PARP−/− mice, probably due to activity of the PARP enzyme isoforms, others than PARP-1. Note, that the number of sciatic nerve poly(ADP-ribose) positive-nuclei was ~50% greater in diabetic PARP−/− mice compared with the non-diabetic controls, but constituted only 18% of the corresponding value in diabetic PARP+/+ mice (Fig. 4, C).
Fig.4.
Left - representative microphotographs of immunofluorescent staining of poly(ADP-ribose) in sciatic nerves (A) and DRG (B) in control and diabetic rats with and without the PARP inhibitor GPI-15427 treatment. C – control rats, D – diabetic rats. T-GPI-15427 treatment. Magnification × 100, and × 200, respectively.
Right - the numbers of poly(ADP-ribose)-positive nuclei in sciatic nerves (A) and DRG (B) of control and diabetic rats with and without the PARP inhibitor GPI-15427 treatment. C – control rats, D – diabetic rats. T - GPI-15427 treatment. Mean ± SEM, n = 6–17 per group. ** – p < 0.01 vs control rats; #, ## – p < 0.05 and < 0.01 vs untreated diabetic rats.
Left - representative microphotographs of immunofluorescent staining of poly(ADP-ribose) in sciatic nerves of control and diabetic PARP+/+ and PARP−/− mice (C). C – control mice, D – diabetic mice. Magnification × 100.
Right - the numbers of poly(ADP-ribose)-positive nuclei in sciatic nerves of control and diabetic PARP+/+ and PARP−/− mice. C – control mice, D – diabetic mice. Mean ± SEM, n = 6–13 per group. ** – p < 0.01 vs control mice; ## – p < 0.01 vs diabetic PARP+/+ mice.
DISCUSSION
Evidence for important role of oxidative-nitrosative stress in small sensory fiber neuropathy, a devastating complication of diabetes mellitus that culminates in total sensation loss and is responsible for foot amputation (25), is emerging (26–28). We have recently reported that peroxynitrite decomposition catalysts, at a very low dose of 5–10 mg kg−1d−1, alleviated small sensory nerve fiber dysfunction and degeneration (29–31). The present study suggests that free radicals and oxidants contribute to diabetes-associated sensory disorders via PARP activation, a factor that apparently plays an important role in small sensory nerve fiber dysfunction and degeneration as well as neuropathic pain associated with advanced PDN. These findings are consistent with other reports suggesting a key role of oxidative-nitrosative stress and PARP in the pathogenesis of diabetic complications (32–34).
Altered thermal perception thresholds with increased or reduced sensitivity to thermal noxious stimuli (heat or cold) are observed in a large proportion of human subjects with diabetes mellitus. Thermal hyperalgesia has been registered in some patients with mild PDN, whereas advanced stage of the disease is typically characterized by increased thermal perception thresholds (hypoalgesia) that progress to sensory loss, occurring in conjunction with degeneration of all types of peripheral nerve fibers (35). Thus, studies of the mechanisms underlying both thermal hyper- and hypoalgesia are clinically relevant. We have previously found that thermal hyperalgesia in rats with the short-term (4-wk) STZ-induced diabetes is alleviated by a PARP inhibitor treatment (20), consistent with beneficial effects of antioxidants in other reports (27,28). In the present study, rats and mice with 12-wk and 10-wk duration of STZ-diabetes, respectively, had clearly manifest thermal hypoalgesia, consistent with findings of others (36). Prevention of thermal hypoalgesia in the PARP inhibitor GPI-15427-treated diabetic rats and preservation of normal thermal response latencies in the paw withdrawal and tail flick tests in diabetic PARP−/− mice reveal a key role of PARP activation in this frequent sensory disorder associated with diabetes.
In contrast to STZ-diabetic rats that display clearly manifest mechanical hyperalgesia (20,27,28,37, and in the present study), STZ-diabetic mice have increased mechanical withdrawal threshold i.e., the condition consistent with sensory loss in human subjects with advanced PDN. Alleviation of mechanical hyperalgesia with the PARP inhibitor GPI-15427 in diabetic rats, and the presence of mechanical hypoalgesia in diabetic PARP+/+, but not PARP−/−, mice, suggest that PARP activation plays an important role in both phenomena associated with experimental Type 1 PDN. These findings are consistent with alleviation of diabetes-induced mechanical hyper- or hypoalgesia by the antioxidants α–lipoic acid, dimethylthiourea, and taurine, as well as a peroxynitrite decomposition catalyst (27,28,30,31), and suggest that contribution of oxidative-nitrosative stress to these sensory abnormalities is, at least, in part mediated via PARP activation.
The second phase of the formalin-induced flinching response occurs despite minimal input to the spinal cord from primary afferent nociceptors. Thus, the test is suitable for studying mechanisms by which innocuous sensory input can be modulated and amplified in the spinal cord and higher CNS to generate a neuropathic pain state, as well as malfunctions of these mechanisms produced by pathological conditions including diabetes. In the present study, diabetic rats displayed exaggerated flinching behavior in the second phase of the formalin test. This behavior was suppressed by a PARP inhibitor treatment which supports the role for PARP activation in diabetic neuropathic pain. In contrast to STZ-diabetic rats, STZ-diabetic mice have blunted (compared with non-diabetic controls) second phase in the formalin pain test (38), and, therefore, the conclusion from a pharmacological study with GPI-15427 could not have been verified by corresponding experiments in PARP-deficient mice.
Painful diabetic neuropathy in human subjects is sometimes complicated by tactile allodynia, a condition where light touch is perceived as painful (39). Reduced tactile response thresholds were observed in both STZ-diabetic rats and mice. Alleviation of tactile allodynia in GPI-15427-treated diabetic rats and preservation of normal tactile response thresholds in PARP−/− diabetic mice suggest an important role for PARP activation in this sensory disorder associated with advanced PDN.
Recent development of the technique for assessment of small-caliber nerve fiber degeneration i.e., skin biopsy with quantitation of epidermal nerve fibers (40), stimulated studies of this phenomenon in animal models of PDN (12,30,31,41,42) and human subjects with diabetes mellitus (40,43). In the present study, rats and wild-type mice with 12-wk and 10-wk durations of STZ-diabetes, respectively, displayed ~44 % and ~ 46 % epidermal nerve fiber loss. Prevention of diabetes-induced intraepidermal nerve fiber loss by GPI-15427 in the rat model as well preservation of normal intraepidermal nerve fiber density in diabetic PARP−/− mice implicate PARP activation in small sensory nerve fiber degeneration in advanced PDN. The underlying mechanisms require further clarification.
In conclusion, PARP activation plays an important role in small sensory nerve fiber dysfunction and degeneration and neuropathic pain associated with advanced PDN. A PARP inhibitor GPI-15427 counteracted small sensory nerve fiber dysfunction and degeneration, without correcting or alleviating diabetic hyperglycemia. These findings provide rationale for development of PARP inhibitors and PARP-inhibitor-containing combination therapies, to prevent and treat this devastating complication of diabetes mellitus.
ACKNOWLEDGMENTS
The study was supported by the Juvenile Diabetes Research Foundation International Grant 1-2005-223, the American Diabetes Association Research Grant 7-05-RA-102, and the National Institutes of Health Grant DK 071566-01 (all to I.G.O.). Drs. Weizheng Xu, Jie Zhang, and Barbara Slusher are employed by MGI Pharma, the company developing PARP inhibitors, and thus have a potential conflict of interests. The authors thank Jeho Shin for expert technical assistance.
LIST OF ABBREVIATIONS
- INFD
intraepidermal nerve fiber density
- MAPK
mitogen-activated protein kinase
- MNCV
motor nerve conduction velocity
- NT
nitrotyrosine
- PARP
poly(ADP-ribose) polymerase
- PDN
peripheral diabetic neuropathy
- ROS
reactive oxygen species
- SNCV
sensory nerve conduction velocity
- STZ
streptozotocin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Jagtap P, Szabo C. Poly(ADP-ribose) polymerase and the therapeutic effects of its inhibitors. Nat. Rev. Drug Discov. 2005;4:421–440. doi: 10.1038/nrd1718. [DOI] [PubMed] [Google Scholar]
- 2.Garcia Soriano F, Virag L, Jagtap P, Szabo E, Mabley JG, Liaudet L, Marton A, Hoyt DG, Murthy KG, Salzman AL, Southan GJ, Szabo C. Diabetic endothelial dysfunction: the role of poly(ADP-ribose) polymerase activation. Nat. Med. 2001;7:108–113. doi: 10.1038/83241. [DOI] [PubMed] [Google Scholar]
- 3.Pacher P, Liaudet L, Soriano FG, Mabley JG, Szabo E, Szabo C. The role of poly(ADP-ribose) polymerase activation in the development of myocardial and endothelial dysfunction in diabetes. Diabetes. 2002;51:514–521. doi: 10.2337/diabetes.51.2.514. [DOI] [PubMed] [Google Scholar]
- 4.Obrosova IG, Li F, Abatan OI, Forsell MA, Komjati K, Pacher P, Szabo C, Stevens MJ. Role of poly(ADP-ribose) polymerase activation in diabetic neuropathy. Diabetes. 2004;53:711–720. doi: 10.2337/diabetes.53.3.711. [DOI] [PubMed] [Google Scholar]
- 5.Li F, Szabo C, Pacher P, Southan GJ, Abatan OI, Charniauskaya T, Stevens MJ, Obrosova IG. Evaluation of orally active poly(ADP-ribose) polymerase inhibitor in streptozotocin-diabetic rat model of early peripheral neuropathy. Diabetologia. 2004;47:710–717. doi: 10.1007/s00125-004-1356-0. [DOI] [PubMed] [Google Scholar]
- 6.Gibson TM, Cotter MA, Cameron NE. Effects of poly(ADP-ribose) polymerase inhibition on dysfunction of non-adrenergic non-cholinergic neurotransmission in gastric fundus in diabetic rats. Nitric Oxide. 2006;15:344–350. doi: 10.1016/j.niox.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Zheng L, Szabo C, Kern TS. Poly(ADP-ribose) polymerase is involved in the development of diabetic retinopathy via regulation of nuclear factor-kappaB. Diabetes. 2004;53:2960–2967. doi: 10.2337/diabetes.53.11.2960. [DOI] [PubMed] [Google Scholar]
- 8.Obrosova IG, Minchenko AG, Frank RN, Seigel GM, Zsengeller Z, Pacher P, Stevens MJ, Szabo C. Poly(ADP-ribose) polymerase inhibitors counteract diabetes- and hypoxia-induced retinal vascular endothelial growth factor overexpression. Int. J. Mol. Med. 2004;14:55–64. [PubMed] [Google Scholar]
- 9.Minchenko AG, Stevens MJ, White L, Abatan OI, Komjati K, Pacher P, Szabo C, Obrosova IG. Diabetes-induced overexpression of endothelin-1 and 15 endothelin receptors in the rat renal cortex is mediated via poly(ADP-ribose) polymerase activation. FASEB J. 2003;17:1514–1516. doi: 10.1096/fj.03-0013fje. [DOI] [PubMed] [Google Scholar]
- 10.Szabo C, Biser A, Benko R, Bottinger E, Susztak K. Poly(ADP-ribose) polymerase inhibitors ameliorate nephropathy of type 2 diabetic Leprdb/db mice. Diabetes. 2006;55:3004–3012. doi: 10.2337/db06-0147. [DOI] [PubMed] [Google Scholar]
- 11.Cheung AK, Fung MK, Lo AC, Lam TT, So KF, Chung SS, Chung SK. Aldose reductase deficiency prevents diabetes-induced blood-retinal barrier breakdown, apoptosis, and glial reactivation in the retina of db/db mice. Diabetes. 2005;54:3119–3125. doi: 10.2337/diabetes.54.11.3119. [DOI] [PubMed] [Google Scholar]
- 12.Drel VR, Mashtalir N, Ilnytska O, Shin J, Li F, Lyzogubov VV, Obrosova IG. The leptin-deficient (ob/ob) mouse: a new animal model of peripheral neuropathy of type 2 diabetes and obesity. Diabetes. 2006;55:3335–3343. doi: 10.2337/db06-0885. [DOI] [PubMed] [Google Scholar]
- 13.Szabo C, Zanchi A, Komjati K, Pacher P, Krolewski AS, Quist WC, LoGerfo FW, Horton ES, Veves A. Poly(ADP-Ribose) polymerase is activated in subjects at risk of developing type 2 diabetes and is associated with impaired vascular reactivity. Circulation. 2002;106:2680–2686. doi: 10.1161/01.cir.0000038365.78031.9c. [DOI] [PubMed] [Google Scholar]
- 14.Obrosova IG, Ilnytska O, Lyzogubov VV, Mashtalir N, Nadler JL, Drel VR. High fat diet-induced neuropathy of prediabetes and obesity: effects of “healthy” diet and aldose reductase inhibition. Diabetes. 2007 doi: 10.2337/db06-1176. (abstract) In press. [DOI] [PubMed] [Google Scholar]
- 15.Ha HC, Hester LD, Snyder SH. Poly(ADP-ribose) polymerase-1 depen- dence of stress-induced transcription factors and associated gene expression in glia. Proc. Natl. Acad. Sci. USA. 2002;99:3270–3275. doi: 10.1073/pnas.052712399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Genovese T, Mazzon E, Di Paola R, Muia C, Threadgill MD, Caputi AP, Thiemermann C, Cuzzocrea S. Inhibitors of poly(ADP-ribose) polymerase modulate signal transduction pathways and the development of bleomycin-induced lung injury. J. Pharmacol. Exp. Ther. 2005;313:529–538. doi: 10.1124/jpet.104.080705. [DOI] [PubMed] [Google Scholar]
- 17.Rajesh M, Mukhopadhyay P, Batkai S, Godlewski G, Hasko G, Liaudet L, Pacher P. Pharmacological inhibition of poly(ADP-ribose) polymerase inhibits angiogenesis. Biochem. Biophys. Res. Commun. 2006;350:352–357. doi: 10.1016/j.bbrc.2006.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu SW, Wang H, Poitras MF, Coombs C, Bowers WJ, Federoff HJ, Poirier GG, Dawson TM, Dawson VL. Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science. 2002;297:259–263. doi: 10.1126/science.1072221. [DOI] [PubMed] [Google Scholar]
- 19.Andrabi SA, Kim NS, Yu SW, Wang H, Koh DW, Sasaki M, Klaus JA, Otsuka T, Zhang Z, Koehler RC, Hurn PD, Poirier GG, Dawson VL, Dawson TM. Poly(ADP-ribose) (PAR) polymer is a death signal. Proc. Natl. Acad. Sci. USA. 2006;103:18308–18313. doi: 10.1073/pnas.0606526103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ilnytska O, Lyzogubov VV, Stevens MJ, Drel VR, Mashtalir N, Pacher P, Yorek MA, Obrosova IG. Poly(ADP-Ribose) Polymerase Inhibition Alleviates Experimental Diabetic Sensory Neuropathy. Diabetes. 2006;55:1686–1694. doi: 10.2337/db06-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tentori L, Leonetti C, Scarsella M, Muzi A, Mazzon E, Vergati M, Forini O, Lapidus R, Xu W, Dorio AS, Zhang J, Cuzzocrea S, Graziani G. Inhibition of poly(ADP-ribose) polymerase prevents irinotecan-induced intestinal damage and enhances irinotecan/temozolomide efficacy against colon carcinoma. FASEB J. 2006;20:1709–1711. doi: 10.1096/fj.06-5916fje. [DOI] [PubMed] [Google Scholar]
- 22.Di Paola R, Mazzon E, Xu W, Genovese T, Ferrraris D, Muia C, Crisafulli C, Zhang J, Cuzzocrea S. Treatment with PARP-1 inhibitors, GPI 15427 or GPI 16539, ameliorates intestinal damage in rat models of colitis and shock. Eur. J. Pharmacol. 2005;527:163–171. doi: 10.1016/j.ejphar.2005.09.055. [DOI] [PubMed] [Google Scholar]
- 23.Pieper AA, Brat DJ, Krug DK, Watkins CC, Gupta A, Blackshaw S, Verma A, Wang ZQ, Snyder SH. Poly(ADP-ribose) polymerase-deficient mice are protected from streptozotocin-induced diabetes. Proc. Natl. Acad. Sci. USA. 1999;96:3059–3064. doi: 10.1073/pnas.96.6.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mabley JG, Suarez-Pinzon WL, Hasko G, Salzman AL, Rabinovitch A, Kun E, Szabo C. Inhibition of poly (ADP-ribose) synthetase by gene disruption or inhibition with 5-iodo-6-amino-1,2-benzopyrone protects mice from multiple-lowdose- streptozotocin-induced diabetes. Br. J. Pharmacol. 2001;133:909–919. doi: 10.1038/sj.bjp.0704156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boulton AJ, Malik RA, Arezzo JC, Sosenko JM. Diabetic somatic neuropathies. Diabetes Care. 2004;27:1458–1486. doi: 10.2337/diacare.27.6.1458. [DOI] [PubMed] [Google Scholar]
- 26.Pertovaara A, Wei H, Kalmari J, Ruotsalainen M. Pain behavior and response properties of spinal dorsal horn neurons following experimental diabetic neuropathy in the rat: modulation by nitecapone, a COMT inhibitor with antioxidant properties. Exp. Neurol. 2001;167:425–434. doi: 10.1006/exnr.2000.7574. [DOI] [PubMed] [Google Scholar]
- 27.Cameron NE, Jack AM, Cotter MA. Effect of alpha-lipoic acid on vascular responses and nociception in diabetic rats. Free Radic. Biol. Med. 2001;31:125–135. doi: 10.1016/s0891-5849(01)00564-0. [DOI] [PubMed] [Google Scholar]
- 28.Cameron NE, Tuck Z, McCabe L, Cotter MA. Effect of the hydroxyl radical scavenger, dimethylthiourea, on peripheral nerve tissue perfusion, conduction velocity and nociception in experimental diabetes. Diabetologia. 2001;44:1161–1169. doi: 10.1007/s001250100626. [DOI] [PubMed] [Google Scholar]
- 29.Obrosova IG, Mabley JG, Zsengeller Z, Charniauskaya T, Abatan OI, Groves JT, Szabo C. Role for nitrosative stress in diabetic neuropathy: evidence from studies with a peroxynitrite decomposition catalyst. FASEB J. 2005;19:401–403. doi: 10.1096/fj.04-1913fje. [DOI] [PubMed] [Google Scholar]
- 30.Vareniuk I, Pavlov IA, Drel VR, Lyzogubov VV, Ilnytska O, Bell SR, Tibrewala J, Groves JT, Obrosova IG. Nitrosative stress and peripheral diabetic neuropathy in leptin-deficient (ob/ob) mice. Exp. Neurol. 2007;205:425–436. doi: 10.1016/j.expneurol.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 31.Drel VR, Pacher P, Vareniuk I, Pavlov. I, Ilnytska O, Lyzogubov VV, Tibrewala J, Groves JT, Obrosova IG. A peroxynitrite decomposition catalyst counteracts sensory neuropathy in streptozotocin-diabetic mice. Eur. J. Pharmacol. 2007;569:48–58. doi: 10.1016/j.ejphar.2007.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pacher P, Szabo C. Role of poly(ADP-ribose) polymerase-1 activation in the pathogenesis of diabetic complications: endothelial dysfunction, as a common underlying theme. Antioxid. Redox Signal. 2005;7:1568–1580. doi: 10.1089/ars.2005.7.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pacher P, Szabo C. Role of peroxynitrite in the pathogenesis of cardiovascular complications of diabetes. Curr. Opin. Pharmacol. 2006;6:136–141. doi: 10.1016/j.coph.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dyck PJ, Dyck PJ, Larson TS, O'Brien PC, Velosa JA. Patterns of quantitative sensation testing of hypoesthesia and hyperalgesia are predictive of diabetic polyneuropathy: a study of three cohorts. Nerve growth factor study group. Diabetes Care. 2000;23:510–517. doi: 10.2337/diacare.23.4.510. [DOI] [PubMed] [Google Scholar]
- 36.Calcutt NA, Freshwater JD, Mizisin AP. Prevention of sensory disorders in diabetic Sprague-Dawley rats by aldose reductase inhibition or treatment with ciliary neurotrophic factor. Diabetologia. 2004;47:718–724. doi: 10.1007/s00125-004-1354-2. [DOI] [PubMed] [Google Scholar]
- 37.Li F, Obrosova IG, Abatan O, Tian D, Larkin D, Stuenkel EL, Stevens MJ. Taurine replacement attenuates hyperalgesia and abnormal calcium signaling in sensory neurons of STZ-D rats. Am. J. Physiol. Endocrinol. Metab. 2005;288:E29–E36. doi: 10.1152/ajpendo.00168.2004. [DOI] [PubMed] [Google Scholar]
- 38.Christianson JA, Riekhof JT, Wright DE. Restorative effects of neurotrophin treatment on diabetes-induced cutaneous axon loss in mice. Exp. Neurol. 2003;179:188–199. doi: 10.1016/s0014-4886(02)00017-1. [DOI] [PubMed] [Google Scholar]
- 39.Vinik AI, Suwanwalaikorn S, Stansberry KB, Holland MT, McNitt PM, Colen LE. Quantitative measurement of cutaneous perception in diabetic neuropathy. Muscle Nerve. 1995;18:574–584. doi: 10.1002/mus.880180603. [DOI] [PubMed] [Google Scholar]
- 40.Sumner CJ, Sheth S, Griffin JW, Cornblath DR, Polydefkis M. The spectrum of neuropathy in diabetes and impaired glucose tolerance. Neurology. 2003;60:108–111. doi: 10.1212/wnl.60.1.108. [DOI] [PubMed] [Google Scholar]
- 41.Toth C, Brussee V, Zochodne DW. Remote neurotrophic support of epidermal nerve fibres in experimental diabetes. Diabetologia. 2006;49:1081–1088. doi: 10.1007/s00125-006-0169-8. [DOI] [PubMed] [Google Scholar]
- 42.Christianson JA, Ryals JM, Johnson MS, Dobrowsky RT, Wright DE. Neurotrophic modulation of myelinated cutaneous innervation and mechanical sensory loss in diabetic mice. Neuroscience. 2007;145:303–313. doi: 10.1016/j.neuroscience.2006.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pittenger G, Burkus N, McNulty P, Basta B, Vinik A. Intraepidermal nerve fibers are indicators of small fiber neuropathy in both diabetic and non-diabetic patients. Diabetes Care. 2004;27:1974–1979. doi: 10.2337/diacare.27.8.1974. [DOI] [PubMed] [Google Scholar]