Abstract
In contrast to naive T cells, reactivation of memory cells is less dependent on CD28-mediated costimulation. We have shown that circulating beryllium-specific CD4+ T cells from chronic beryllium disease patients remain CD28-dependent, while those present in the lung no longer require CD28 for T cell activation. In the present study, we analyzed whether other costimulatory molecules are essential for beryllium-induced T cell function in the lung. Enhanced proliferation of a beryllium-responsive, HLA-DP2-restricted T cell line was seen after the induction of 4-1BB ligand expression on the surface of HLA-DP2-expressing fibroblasts. Following beryllium exposure, CD4+ T cells from blood and bronchoalveolar lavage of chronic beryllium disease patients up-regulate 4-1BB expression, and the majority of beryllium-responsive, IFN-γ-producing CD4+ T cells in blood coexpress CD28 and 4-1BB. Conversely, a significant fraction of IFN-γ-producing bronchoalveolar lavage (BAL) T cells express 4-1BB in the absence of CD28. In contrast to blood, inhibition of the 4-1BB ligand-4-1BB interaction partially blocked beryllium-induced proliferation of BAL CD4+ T cells, and a lack of 4-1BB expression on BAL T cells was associated with increased beryllium-induced cell death. Taken together, these findings suggest an important role of 4-1BB in the costimulation of beryllium-responsive CD4+ T cells in the target organ.
Optimal activation of naive T cells requires signals through the TCR following engagement with cognate Ag and MHC on the surface of APCs as well as a second signal, termed costimulation (1, 2). This costimulatory signal is most frequently mediated by engagement of the CD28 molecule on the T cell with its ligands, B7-1 or B7-2, on the APC. CD28 is constitutively expressed on almost all human CD4+ T cells. However, in the setting of chronic inflammation or advanced age, a subset of circulating CD4+ T cells lose CD28 expression (3-7). Down-regulation of CD28 expression has also been observed on bronchoalveolar lavage (BAL)3 CD4+ T cells from patients with sarcoidosis (8) and chronic beryllium disease (CBD) (9). In fact, blockade of CD28-B7 interaction with CTLA-4Ig had no effect on either the beryllium-induced proliferation or Th1-type cytokine expression of BAL CD4+ T cells from CBD patients (9). These findings suggest a functional independence of beryllium-responsive CD4+ effector memory T (TEM) cells in lung from CD28 costimulation and raise the possibility that other costimulatory ligand-receptor interactions may assume a critical role in activating and sustaining the T cell response in the lung.
4-1BB is an inducible costimulatory molecule of the TNF receptor family that is expressed on activated CD4+ and CD8+ T cells (10, 11), while its ligand, 4-1BBL, is expressed on activated macrophages, dendritic cells, and B cells (11-13). Although some reports have suggested that 4-1BB is primarily involved in the activation of CD8+ T cells, recent studies have shown that both CD4+ and CD8+ T cells respond to 4-1BBL with enhanced T cell proliferation, effector function, and survival (11, 14). In humans, the interaction of 4-1BBL-4-1BB enhances Th1-type cytokine expression (15, 16). In addition, 4-1BB has been shown to be an important costimulatory molecule on T cells lacking CD28 expression (17, 18), suggesting its potential importance in the activation of lung CD4+ T cells because a large portion of these cells have lost CD28 expression (9). Although the role of 4-1BBL in the activation of human anti-influenza and HIV-specific CD8+ T cells has been studied (19-21), the role of 4-1BBL-4-1BB in the costimulation of Ag-specific CD4+ TEM cells in a target organ has not been addressed.
CBD is characterized by the presence of granulomatous inflammation and progressive fibrosis in the lung (22). Historically, one-third of untreated patients progressed to end-stage respiratory insufficiency (23). Considerable evidence indicates that the accumulation and activation of beryllium-specific CD4+ T cells in the lung is central to the pathogenesis of disease (24-27). The ability of peripheral blood and/or BAL CD4+ T cells to proliferate in the presence of beryllium salts in vitro forms an essential part of the disease definition (28-31). In the lung, large numbers of beryllium-specific CD4+ T cells accumulate (25), with the vast majority expressing an effector-memory phenotype and demonstrating immediate release of IFN-γ, TNF-α, and IL-2 when stimulated with beryllium salts in culture (25, 32). With a known Ag stimulus and access to large numbers of pathogenic T cells from the target organ, CBD is an important organ-specific, immune-mediated disease.
Based on the enhanced proliferation of a beryllium-responsive CD4+ T cell line after 4-1BBL expression on the APC, we examined the role of the 4-1BBL-4-1BB interaction in the costimulation of beryllium-specific T cells in blood and lung. In the present study, the results demonstrate that the majority of beryllium-responsive CD4+ T cells in blood coexpress CD28 and 4-1BB. Inhibition of the 4-1BBL-4-1BB interaction had no effect on beryllium-induced proliferation, suggesting that the dominant costimulatory molecule in blood is CD28. Conversely, a significant fraction of IFN-γ-producing CD4+CD28− T cells in the BAL express 4-1BB, and blockade of 4-1BB costimulation inhibited the proliferation of BAL CD4+ T cells in response to beryllium. In addition, 4-1BB expression on BAL CD4+ T cells prevented activation-induced cell death after stimulation with Ag. These findings confirm the importance of 4-1BB in the beryllium-induced immune response in the lung and further support a transition within the memory CD4+ T cell population from CD28 dependence in blood to a requirement for 4-1BB costimulation in lung.
Materials and Methods
Study population
Seventeen patients with a diagnosis of CBD and eight beryllium-sensitized (BeS) patients were enrolled in this study. Ten healthy, non-beryllium-exposed control subjects were also enrolled. The diagnosis of CBD was established using previously defined criteria, including the presence of granulomatous inflammation on lung biopsy and a positive proliferative response of blood and/or BAL T cells to beryllium sulfate (BeSO4) in vitro (26, 33). The diagnosis of BeS was established based on a positive proliferative response of PBMCs to BeSO4 in vitro, and the absence of granulomatous inflammation or other abnormalities on lung biopsy (30, 31). Active smokers were excluded from enrollment. Informed consent was obtained from each patient and control subject, and the protocol was approved by the Human Subject Institutional Review Boards at the University of Colorado Denver and National Jewish Medical and Research Center (Denver, CO).
Preparation of cells and generation of beryllium-specific T cell lines
PBMCs were isolated from heparinized blood by Ficoll-Hypaque density gradient separation, and BAL was performed as previously described (34, 35). The beryllium-specific T cell lines were derived after initial stimulation of BAL cells in 12-well plates (Costar) with RPMI 1640 supplemented with 10% heat-inactivated human serum (Gemini Bio-Products), 20 mM HEPES, 1 mM sodium pyruvate, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM l-glutamine (all from Invitrogen), and the addition of 100 μM of BeSO4, as previously described (36). After 5 days of culture, cells were washed free of BeSO4 and the lymphoblasts were expanded further in culture medium supplemented with 20 U/ml human rIL-2 (R&D Systems). The T cell lines were maintained in culture by cycles of restimulation (every 2–3 wk) with 100 μM of BeSO4 and autologous, mitomycin C-treated EBV-transformed lymphoblastoid cell lines and further expanded in culture with rIL-2.
Recombinant adenoviruses
Replication-defective adenovirus 5 expressing 4-1BBL (4-1BBL-Adv), B7.1 (B7.1-Adv), OX-40 ligand (OX-40L) (OX-40L-Adv), or no transgene as a control (Control-Adv) were generated and purified as previously described (19, 21). For costimulation, murine DAP.3 L cells expressing HLA-DP2 were plated at 5 × 105 cells per well in a 48-well plate for 1 h to allow attachment of the cells to the well. Control-Adv, 4-1BBL-Adv, B7.1-Adv, or OX-40L-Adv was added at a multiplicity of infection (MOI) of 50 (previously determined to be the optimal MOI), and the plate was centrifuged at 37°C at 1,643 × g for 1 h (19). BeSO4 was added to the wells at a concentration of 100 μM. After overnight incubation, the DP2-expressing fibroblasts were washed twice with PBS. The adherent DP2-fibroblasts were dislodged from the wells with ice cold Versene (Invitrogen) and washed twice in RPMI 1640 supplemented with 10% FBS, 20 mM HEPES, 1 mM sodium pyruvate, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM l-glutamine (all from Invitrogen). Transgene expression on DP2-expressing fibroblasts was confirmed by FACS analysis with each corresponding anti-4-1BBL, anti-B7.1, or anti-OX-40L mAb (all from BD Biosciences).
Lymphocyte proliferation assay
PBMCs (2.5 × 105 cells/well) and BAL cells (1 × 105 cells/well) were cultured in 96-well flat-bottom microtiter plates in the presence of medium alone, 10 μM BeSO4, or 2.5 μg/ml PHA. After 96 h of culture, the wells were pulsed with 1 μCi of [3H]thymidine for an additional 16 h, and incorporation of radioactivity was determined by β-emission spectroscopy. Proliferation assays were performed in triplicate. Inhibition of proliferation was attempted with addition of indicated amounts of mAbs directed 4-1BBL (M730; a gift from Amgen) at the beginning of culture as described above. Proliferation with and without the indicated mAb was determined and the extent of inhibition was calculated. In some experiments, beryllium-specific T cell lines (1 × 105 cells) were cultured in 96-well flat-bottom microtiter plates with 5 × 104 medium or BeSO4-pulsed, mitomycin C-treated, fibroblasts expressing HLA-DP2 and either B7.1, OX-40L, or 4-1BBL. The transfected fibroblast cells were washed three times with HBSS before use as APCs to ensure the absence of free BeSO4 in culture.
Immunofluorescence staining and analysis for intracellular cytokine expression
For cytokine expression, ex vivo PBMCs (2 × 106 cells/tube) and BAL cells (1 × 106 cells/tube) were placed in polypropylene tubes (12 × 75 mm; Fisher Scientific) containing 1 ml of complete culture medium plus one of the following experimental conditions: medium alone, 10 ng/ml staphylococcal enterotoxin B, or 100 μM of BeSO4. Cells were incubated for a total of 20 h at 37°C in a humidified 5% CO2 atmosphere with 10 μg/ml brefeldin A added after six hours of stimulation. This length of stimulation was necessary to allow for the induction of 4-1BB expression on the CD4+ T cell surface. In some experiments, inhibition of Th1-type cytokine expression was attempted with the addition of indicated amounts of 4-1BBL mAb at the beginning of culture. After stimulation, cells were washed and stained with mAbs directed against CD3 (PE-Texas Red, Beckman Coulter) and CD4 (APC-Cy7, BD Biosciences) for 30 min at 4°C, as previously described (9, 25). Cells were washed with PBS containing 1% BSA and placed in fixation medium (Caltag Laboratories) for 15 min at room temperature. Following washing with PBS containing 1% BSA, cells were added to permeabilization medium (Caltag Laboratories), and stained with mAbs directed against IFN-γ (FITC, BD Biosciences), IL-2 (APC, Caltag Laboratories), TNF-α (Alexa Fluor 700, Caltag Laboratories), 4-1BB (PE, BD Biosciences), and CD28 (PE-Cy5, BD Biosciences) for 30 min at 4°C. Fluorescence minus one and isotype controls were used in all staining. Cells were analyzed using a FACSAria flow cytometer (BD Immunocytometry Systems) (37). The number of events collected ranged between 1 and 3 million. Electronic compensation was performed with Ab capture beads (BD Biosciences) that were stained separately with individual mAbs used in the test samples. The data files were analyzed using Diva software (BD Biosciences). Lymphocytes were gated based on their forward and side scatter profiles.
PBMCs or BAL cells were stained with anti-CD14-FITC (BD Biosciences) and PE-labeled anti-4-1BBL (BD Biosciences). Cells were first incubated with FcR-Blocking reagent (Miltenyi Biotec) to reduce nonspecific staining. Because 4-1BBL expression was not bimodal, expression levels on CD14+ T cells were determined by subtracting the mean fluorescence intensity (MFI) of the fluorescence minus one control from the 4-1BBL MFI on the population of interest. Fluorescence intensity was analyzed using a FACSCalibur cytometer (BD Immunocytometry Systems), as previously described (25, 38).
Apoptosis assay
BAL T cell lines from CBD patients were stimulated with medium, 10 μg/ml anti-CD3, or 100 μM of BeSO4 for 24 h. Rates of apoptosis were determined on CD3+CD4+ T cells with and without 4-1BB expression by staining with annexin-V and 7-aminoactinomycin D (7-AAD) (both from Molecular Probes), and the cells were immediately analyzed by flow cytometry as described above.
Statistical analysis
The Mann-Whitney U test and the Kruskal-Wallis ANOVA were used to determine significance of differences between subject groups. A p value of <0.05 was considered statistically significant.
Results
Beryllium-responsive CD4+ T cells in blood produce more IFN-γ per cell than CD4+ T cells in BAL
The MFI of each functional parameter within a given sample is related to the quantitative expression of that function on a per cell basis (39). As a result, the relative amount of each cytokine per cell can be measured for each functional CD4+ T cell population. In addition, previous studies have shown that MFI measured by intracellular cytokine staining directly correlates with cytokine secretion as measured by ELISA (40). We compared IFN-γ MFI on beryllium-responsive CD4+ T cells in blood and in the target organ. Despite a 14-fold greater frequency of beryllium-responsive CD4+ T cells in BAL compared with blood (41), circulating IFN-γ-expressing beryllium-responsive CD4+ T cells were more functional than their counterparts in the lung, expressing 2.5 times more IFN-γ per cell (p = 0.0006) (Fig. 1A).
FIGURE 1.
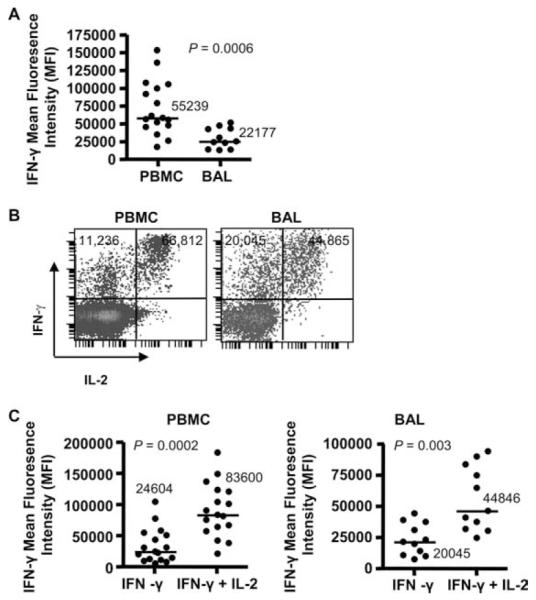
Beryllium-responsive CD4+ T cells in blood are more functional than beryllium-reactive T cells in the BAL. A, IFN-γ expression (MFI) on CD4+ T cells from blood (n = 17) and BAL (n = 11) of CBD patients in response to 100 μM BeSO4 is shown. B, Representative density plots of blood and BAL CD4+ T cells expressing IFN-γ and IL-2 in response to BeSO4 is shown. The MFI of IFN-γ expression per cell on CD4+ T cells producing either IFN-γ/IL-2 or only IFN-γ is depicted. C, The expression of IFN-γ (MFI) on beryllium-responsive CD4+ T cells from blood and BAL of CBD patients in relation to the ability or inability to coexpress IL-2 is shown. Median values are indicated with solid lines, and statistical comparisons were made using the Mann-Whitney U test.
Previous studies have shown that vaccinia-induced, polyfunctional CD8+ T cells (i.e., secrete IFN-γ, IL-2, and TNF-α) produce more IFN-γ on a per cell basis compared with monofunctional cells (39). In response to BeSO4, we had previously identified two predominant populations of beryllium-responsive CD4+ T cells in blood and BAL: cells capable of expressing both IFN-γ and IL-2 and those expressing only IFN-γ (42). Thus, we sought to determine whether beryllium-responsive T cells that retained the ability to secrete IL-2 were more functional than those cells that had lost the ability to express this cytokine. As shown in the representative density plots of BeSO4-induced IFN-γ and IL-2 expression by CD4+ T cells from blood and BAL, beryllium-responsive CD4+ T cells expressing both IFN-γ and IL-2 were more functional than cells expressing only IFN-γ as measured by the MFI of IFN-γ per cell (Fig. 1B). Overall, CD4+ T cells expressing IFN-γ/IL-2 in response to BeSO4 produced 3.4 and 2.2 times more IFN-γ per cell than IFN-γ-only-secreting CD4+ T cells in blood and BAL, respectively (Fig. 1C).
4-1BBL enhances beryllium-induced CD4+ T cell proliferation
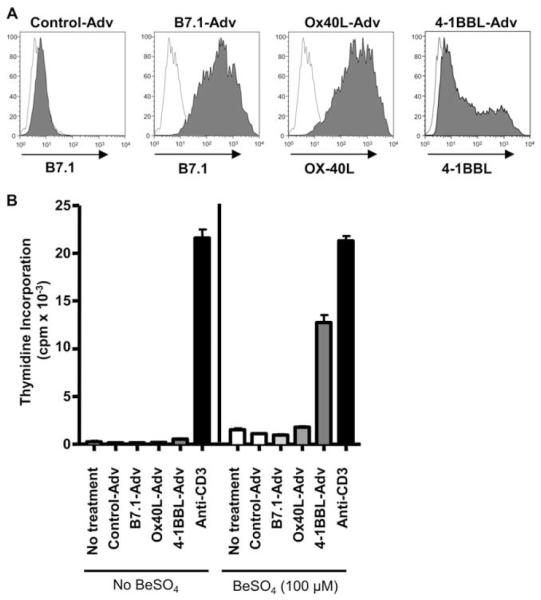
The decreased functional capacity of the beryllium-responsive CD4+ T cell in the lung raised the possibility of different costimulatory requirements existing between the circulating Ag-specific T cells and those present in the target organ. We have previously shown that beryllium-responsive CD4+ T cells in blood remain CD28 costimulation-dependent, while those in the lung no longer require the engagement of CD28 for effector T cell function with a substantial fraction of these cells having lost CD28 expression (9). In the absence of CD28, we sought to determine whether CD4+ T cells in the lung were costimulation-independent or required another costimulatory molecule, such as OX-40 or 4-1BB, for T cell activation. Using replication-defective recombinant adenoviruses expressing full-length 4-1BBL, OX-40L, B7.1, or no transgene (Control-Adv) as previously described (19, 21), expression of 4-1BBL, OX-40L, and B7.1, respectively, on the surface of murine HLA-DP2-expressing DAP3. L cells was documented 24 h after infection (Fig. 2A). The ability of these BeSO4-pulsed fibroblasts expressing either 4-1BBL, OX-40L, or B7.1 to stimulate a beryllium-responsive, HLA-DP2-restricted CD4+ T cell line derived from the lung of a CBD patient was determined. As shown in Fig. 2B, HLA-DP2-expressing fibroblasts were able to initiate beryllium-induced T cell proliferation (1512 ± 135 cpm) compared with background proliferation (236 ± 114 cpm; stimulation index = 6.4). No augmentation of the beryllium-induced T cell proliferative response was seen following the expression of either B7.1 or OX-40L on the HLA-DP2-expressing fibroblasts. Conversely, an 8.4-fold increase in BeSO4-induced T cell proliferation (12,751 ± 803 cpm) was seen after the expression of 4-1BBL on the surface of the HLA-DP2-expressing fibroblasts. No additive or synergistic effects of combining 4-1BBL costimulation with either OX-40L or B7.1 was seen (data not shown). These findings suggest that the binding of 4-1BB on the surface of the beryllium-specific CD4+ TEM cell to 4-1BBL on the APC may represent a critical costimulatory interaction in the lung of CBD patients.
FIGURE 2.
4-1BBL enhances beryllium-specific CD4+ T cell proliferation. A, Adenovirus-infected HLA-DP2-expressing DAP.3 L cells express B7.1, OX-40L, or 4-1BBL. HLA-DP2-expressing fibroblasts were infected with replication-defective adenoviruses (MOI of 50) containing no transgene, B7.1, OX-40L, or 4-1BBL. Twenty-four hours after infection, the surface levels of B7.1, OX-40L, and 4-1BBL were assessed by flow cytometry. B, Proliferation of an HLA-DP2-restricted CD4+ T cell line to 100 μM BeSO4 presented by DP2-expressing DAP.3 L cells with and without the expression of B7.1, OX-40L, or 4-1BBL is shown. A representative example of three separate experiments is shown, and data are expressed as the mean cpm ± SEM.
4-1BB expression on BeSO4-stimulated CD4+ T cells in BAL and blood
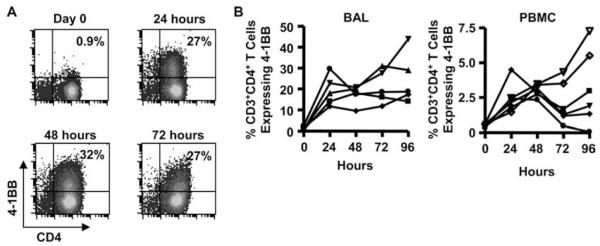
Due to the role of the 4-1BBL-4-1BB interaction in enhancing beryllium-induced proliferation of a beryllium-responsive CD4+ T cell line, we analyzed 4-1BB expression on ex vivo BAL CD4+ T cells before and after exposure to 100 μM BeSO4 in culture. A representative example of the induction of 4-1BB on the surface of BAL CD4+ T cells over a 96-h time period is shown in Fig. 3A. Even though the vast majority of BAL CD4+ T cells express an activated TEM cell phenotype (38), few of these cells express 4-1BB ex vivo (median, 1.0%; range, 0.3–2.9%) (Fig. 3, A and B). After BeSO4 exposure, 4-1BB was rapidly up-regulated, being expressed by a median of 18% of BAL CD4+ T cells at 24 h (range, 12–30%). In addition, 4-1BB expression peaked at 24 h and remained stable over the 96 h time course. For example, at 96 h, 19% (range, 12–30%) of BAL CD4+ T cells expressed 4-1BB (Fig. 3B). Although the frequency of 4-1BB-expressing T cells in blood was dramatically reduced due to a lower precursor frequency of beryllium-responsive cells, similar findings were seen upon exposure of PBMCs to BeSO4. For example, a median of 0.5%, 2.3%, 3.0%, 1.5%, and 2.4% of CD4+ T cells expressed 4-1BB ex vivo and after BeSO4 exposure for 24, 48, 72, and 96 h, respectively (Fig. 3B).
FIGURE 3.
Induction of 4-1BB expression on the surface of CD4+ T cells from blood and BAL of CBD patients after BeSO4 exposure. A, A representative example is shown of the time course of 4-1BB induction on the surface of BAL CD4+ T cells ex vivo and after BeSO4 exposure for 24, 48, 72, and 96 h. B, 4-1BB expression of BAL cells (n = 5) and PBMCs (n = 6) ex vivo and after 100 μM BeSO4 exposure for up to 96 h is shown.
4-1BB expression on beryllium-responsive, IFN-γ-expressing CD4+ T cells on blood and BAL
Next, we analyzed 4-1BB expression on beryllium-responsive, IFN-γ-expressing CD3+CD4+ T cells in the blood and BAL of CBD patients. In the representative histograms shown in Fig. 4A, 84% and 43% of IFN-γ-expressing CD4+ T cells in the blood and BAL, respectively, expressed 4-1BB. Overall, a significantly greater percentage of IFN-γ-producing T cells in blood (median, 80%; range, 48–100%) had up-regulated 4-1BB expression compared with their BAL counterparts (53%; range, 6.5–77%; p = 0.0004) (Fig. 4B). Similar to the decreased expression of IFN-γ per BAL CD4+ T cell compared with blood shown in Fig. 1A, these findings are consistent with a hyporesponsive nature of Ag-specific T cells residing in the target organ.
FIGURE 4.
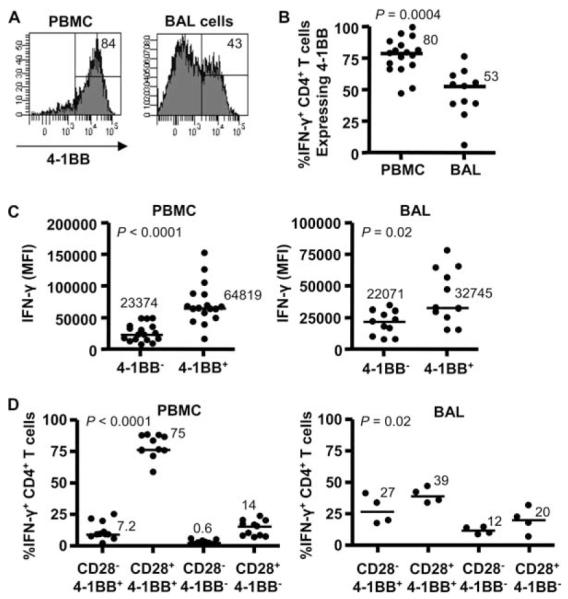
Induction of 4-1BB on the surface of IFN-γ-expressing CD4+ T cells from blood and BAL of CBD patients. A, A representative example of 4-1BB expression on IFN-γ+CD4+ T cells from blood and BAL is shown, with the percentage of 4-1BB+ T cells displayed in the upper right of the histogram. B, Percentage of IFN-γ+CD4+ T cells from blood (n = 17) and BAL (n = 11) expressing 4-1BB is shown. C, The expression of IFN-γ (MFI) on beryllium-responsive CD4+ T cells in blood (left) and BAL (right) with and without the up-regulation of 4-1BB is depicted. Median values for B and C are indicated and shown with solid lines, and statistical comparisons were made using the Mann-Whitney U test. D, The percentage of IFN-γ+CD4+ T cells from blood (left) and BAL (right) in relation to CD28 and 4-1BB expression is shown. Median values are indicated with solid lines, and statistical comparisons were made using the Kruskal-Wallis test.
To determine whether 4-1BB expression enhanced beryllium-induced IFN-γ production, we compared the expression of IFN-γ on a per cell basis in response to 100 μM BeSO4 on blood and BAL CD4+ T cells with and without surface 4-1BB. In both blood and BAL, a significantly increased IFN-γ MFI was seen on beryllium-specific, CD4+4-1BB+ T cells ( p < 0.0001 for blood and p = 0.02 for BAL) (Fig. 4C). Interestingly, beryllium-responsive 4-1BB-expressing CD4+ T cells in blood (median, 64819; range, 39,308–152,300) produced significantly more IFN-γ per cell than those cells in the lung (32,745; range, 15,404–78,067; p = 0.01) (Fig. 4C). Based on the dependence of beryllium-specific T cells in blood on CD28 costimulation for optimal activation (9), these findings raise the possibility that the continued requirement of blood cells for CD28-mediated costimulation enhanced IFN-γ production compared with lung T cells which no longer require costimulation through the B7-CD28 interaction. When analyzing IFN-γ expression in blood, the majority (median, 75%; range, 57–91%; p < 0.0001) of beryllium-responsive CD4+ T cells coexpress CD28 and 4-1BB (Fig. 4D). Conversely, only 39% (range, 34–47%; p = 0.02) of beryllium-responsive, IFN-γ-expressing CD4+ T cells in the BAL coexpressed CD28 and 4-1BB, with the majority of the remaining cells being 4-1BB+CD28−. These findings further emphasize the potential importance of 4-1BB in the costimulation of beryllium-specific TEM cells in the lung.
Increased expression of 4-1BBL in the lung
4-1BB-mediated costimulation of T cells requires that the 4-1BB ligands are expressed on the APC. To examine this, 4-1BB ligand expression was measured by flow cytometry on CD14+ monocyte/macrophages in PBMCs and BAL cells from BeS, CBD, and normal control subjects. There was no difference between groups in 4-1BBL expression on CD14+ cells from blood (Fig. 5). The median 4-1BBL expression (MFI) on CD14+ cells from the peripheral blood of normal control, BeS, and CBD subjects was 25, 7.3, and 11, respectively (Fig. 5). However, 4-1BBL expression on CD14+ cells from the BAL was significantly elevated. Median 4-1BBL expression (MFI) on CD14+ cells in the BAL of BeS (88; range 42–116) and CBD (102; range 55–135) subjects was 12- and 9.2-fold higher than in blood. These data demonstrate that CD14+ APCs in the BAL of BeS and CBD patients have significantly higher levels of 4-1BB ligands than those in blood and suggest that the increased expression of 4-1BBL on CD14+ BAL cells is not specific for CBD patients, but indicative of an activated phenotype of CD14+ cells in the lung.
FIGURE 5.
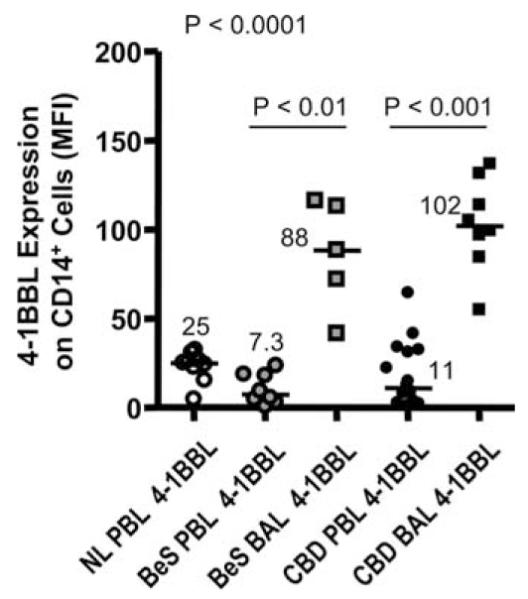
4-1BB ligand expression is elevated on CD14+ cells in the lung of BeS and CBD patients. 4-1BBL expression was evaluated on CD14+ cells from the peripheral blood of normal (n = 11), BeS (n = 8), and CBD (n = 16) patients and the BAL of BeS (n = 5) and CBD (n = 16) subjects. Median values are shown with solid lines. Statistical significance was established using the Kruskal-Wallis test.
Functional dependence of BAL CD4+ T cells on 4-1BB-mediated costimulation
We used inhibitory concentrations of a 4-1BBL mAb to test whether cells from CBD patients require 4-1BB-mediated costimulation for BeSO4-induced T cell activation in culture. PBMCs and BAL cells from 8 CBD patients demonstrated a positive proliferative response (defined as a stimulation index of ≥2.5) in the presence of 10 μM BeSO4. A representative example of beryllium-induced T cell proliferation of PBMCs from a CBD patient is shown in Fig. 6A, with a peak thymidine incorporation of 33,004 ± 803 cpm compared with background proliferation of 223 ± 138 cpm. Following the addition of 30, 3.0, or 0.3 μg/ml 4-1BBL mAb, no inhibition of beryllium-induced proliferation was seen. In contrast, BAL cells from the same individual showed a 45%, 54%, and 0% inhibition of beryllium-induced T cell proliferation in the presence of 30, 3.0, or 0.3 μg/ml 4-1BBL mAb, respectively (Fig. 6B).
FIGURE 6.
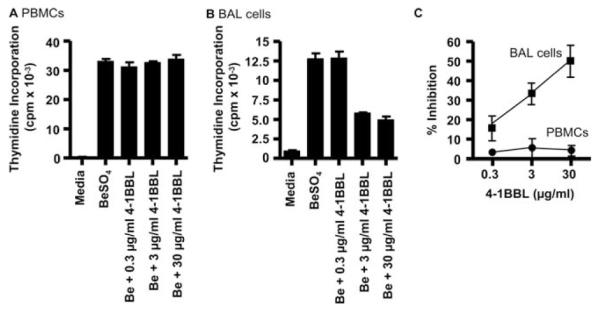
A and B, Proliferative response of PBMCs (A) and BAL cells (B) from CBD patients. Proliferation of cells in medium alone and after the addition of BeSO4 with and without 0.3, 3.0, or 30 μg/ml anti-4-1BBL mAb is shown. The data are expressed as the mean cpm ± SEM. C, Inhibition of the T cell proliferative responses of BAL cells and PBMCs to BeSO4 with various concentrations of 4-1BBL mAb is shown. PBMCs (n = 8) and BAL cells (n = 8) from CBD patients were cultured with 10 μM BeSO4 and increasing concentrations of 4-1BBL mAb. The data are expressed as the mean percentage inhibition of stimulation ± SEM as a function of the concentration of 4-1BBL mAb.
Fig. 6C shows the overall effects of varying concentrations of 4-1BBL mAb on the proliferative responses of PBMCs and BAL cells. These studies again used optimal stimulatory concentrations of BeSO4 (10 μM). Proliferation of BAL cells was partially inhibited with concentrations of 4-1BBL ranging from 0.3 to 30 μg/ml (Fig. 6, B and C). As shown in Fig. 6C, 0.3 μg/ml, 3 μg/ml, and 30 μg/ml 4-1BBL inhibited the beryllium-induced proliferative response of eight CBD patients by 16 ± 6.4%, 33 ± 5.5%, and 47 ± 7.9%, respectively. In contrast, 4-1BBL mAb had little effect on the proliferation of PBMCs in response to BeSO4, with a 3.8 ± 2.7% inhibition when 30 μg/ml 4-1BBL mAb was added to the culture conditions. Thus, as opposed to PBMCs, these studies indicate that the beryllium-induced proliferative response in the lung requires 4-1BB-mediated costimulation. In separate experiments, the addition of OX-40L mAb had no effect on beryllium-induced proliferation, regardless of the source of the cells (data not shown).
Despite increased IFN-γ expression on beryllium-responsive, CD4+4-1BB+ T cells, blockade of the 4-1BBL-4-1BB interaction had no effect on the percentage of IFN-γ+ CD4+ T cells in the BAL after short-term exposure to BeSO4. In the representative example shown in Fig. 7A, ~19% of CD4+ T cells expressed IFN-γ in response to 10 μM BeSO4, irrespective of whether 4-1BBL mAb had been added. Similar findings were seen in two other subjects (Fig. 7B). Thus, despite the link between 4-1BB costimulation and beryllium-induced T cell proliferation, beryllium-stimulated IFN-γ expression in a short-term assay occurs independently of 4-1BB.
FIGURE 7.
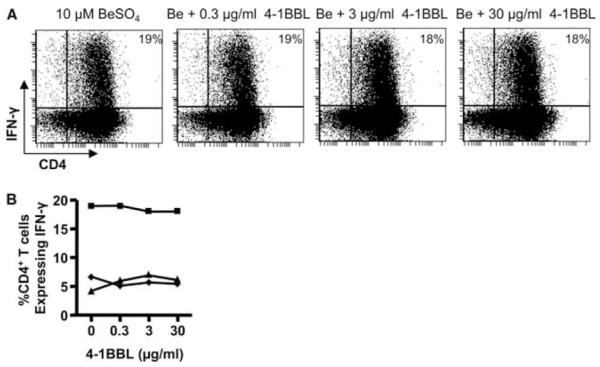
Intracellular IFN-γ expression after BeSO4 stimulation of BAL T cells from CBD patients with and with the addition of anti-4-1BBL mAb. A, A representative experiment is shown for the flow cytometric analysis of BAL CD4+ T cells from a CBD patient stimulated with 10 μM BeSO4 in the presence of varying concentrations of anti-4-1BBL mAb and subsequently stained for surface CD4 and intracellular IFN-γ. The number in the upper right quadrant of each density plot is the percentage of CD4+ T cells that express IFN-γ. B, Percentage of BAL CD4+ T cells from three CBD patients expressing IFN-γ in response to BeSO4 in the absence and presence of 0.3, 3.0, or 30 μg/ml anti-4-1BBL mAb is shown.
Activation-induced apoptosis of BAL CD4+ T cells in relation to 4-1BB expression
We also investigated whether the expression of 4-1BB on beryllium-specific CD4+ T cells protected these cells from undergoing activation-induced cell death. BAL-derived beryllium-specific T cell lines were stimulated with BeSO4 for 24 h, and the CD4+4-1BB+ and CD4+4-1BB− T cell subsets were stained with annexin-V and 7-AAD to determine the fraction of apoptotic cells (a representative experiment is shown in Fig. 8). Using a beryllium-specific CD4+ T cell line, the fraction of cells expressing annexin-V in the absence of 7-AAD (i.e., early apoptosis) increased 5.5-fold on cells lacking 4-1BB. In two other T cell lines, a 2.3- and 3.8-fold increase in beryllium-induced apoptosis was seen in those CD4+ T cells lacking 4-1BB (data not shown). In addition, we observed a 30-fold increase in the percentage of cells expressing annexin-V and 7-AAD (i.e., necrosis) on CD4+4-1BB− T cells compared with T cells that had up-regulated 4-1BB expression (Fig. 8). The increased frequency of apoptotic cell death in activated beryllium-responsive T cells lacking 4-1BB was seen in all three experiments in which it was studied and indicated that the expression of this costimulatory molecule protected from activation-induced cell death.
FIGURE 8.
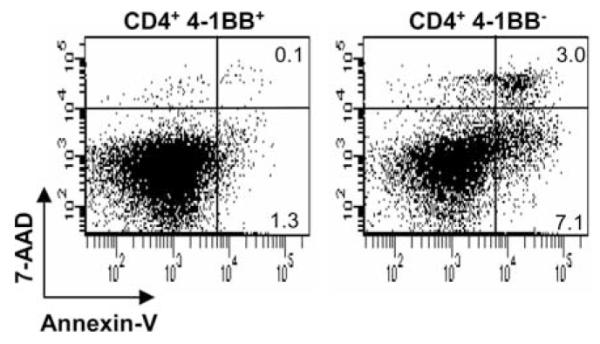
BAL CD4+4-1BB+ T cells are protected from beryllium-induced cell death. Data are shown for representative experiments using lung-derived, beryllium-responsive CD4+ T cell lines stimulated with 100 μM BeSO4. After 24 h of stimulation, cells were stained with CD3, CD4, 4-1BB, annexin-V, and 7-AAD. BAL cells were gated for the expression of CD3 and CD4 and the presence (left) or absence (right) of 4-1BB. The number in the right upper and lower quadrants of each density plot represents the percentage of necrotic and apoptotic cells, respectively. A representative example of three separate experiments using this T cell line is shown.
Discussion
Our previous work suggests that differences in costimulatory requirements exist between beryllium-responsive CD4+ T cells in the circulating pool and those present in the target organ. Although Ag-specific T cells in blood remain dependent on CD28-mediated costimulation, those in lung no longer require CD28 for either proliferation or Th1-type cytokine production. Importantly, despite a >10-fold increase in the frequency of beryllium-responsive CD4+ T cells in BAL compared with blood (42), we show that beryllium-specific, CD28-dependent CD4+ T cells in blood are more functional on a per cell basis than their CD28-independent counterparts in lung. Whether beryllium-responsive, lung T cells are costimulation-independent or -dependent on another costimulatory molecule formed the basis of this study. In this study, we show that the expression of 4-1BBL on HLA-DP2-expressing murine fibroblasts enhanced beryllium-induced T cell proliferation. Blockade of the 4-1BBL-4-1BB interaction using a mAb directed against 4-1BBL partially inhibited the beryllium-induced proliferative response of BAL T cells while having no effect on beryllium-induced proliferation of blood cells. Thus, these studies suggests that CD28 is the key costimulatory molecule for the activation of beryllium-specific memory CD4+ T cells in blood while 4-1BB is required for the beryllium-induced response of TEM cells in lung.
We have previously shown that two populations of beryllium-specific CD4+ T cells exist in blood and BAL of CBD patients: those cells capable of secreting both IFN-γ and IL-2 and those only able to express IFN-γ (25, 42). It is widely accepted that IFN-γ-producing CD4+ T cells are more differentiated than CD4+ T cells that retain the ability to secrete IL-2 (43). However, when we analyzed beryllium-responsive CD4+ T cells from blood and BAL for the expression of markers associated with either T cell senescence (e.g., CD57) or exhaustion (e.g., programmed death-1 (PD-1)), no significant difference in the expression of either PD-1 or CD57 on IFN-γ-only and IFN-γ/IL-2-secreting CD4+ T cells was observed, irrespective of the location of the beryllium-responsive T cell (37, 41). In the present study, we noted that beryllium-specific CD4+ T cells expressing both IFN-γ and IL-2 were more functional (i.e., producing more IFN-γ per cell) than an IFN-γ-only secreting CD4+ T cell in both blood and BAL. In addition, 4-1BB expression in response to beryllium exposure in culture enhanced IFN-γ expression. Thus, polyfunctional (IFN-γ+, IL-2+, and 4-1BB+) Ag-specific CD4+ T cells in blood and BAL express more IFN-γ than their monofunctional counterparts, further supporting the role of 4-1BB costimulation in enhancing the effector function of the beryllium-responsive CD4+ T cell.
In addition to an independence from CD28 costimulation, beryllium-specific CD4+ T cells residing in the target organ are characterized by up-regulation in the expression of negative costimulatory molecules, such as PD-1 (37) and CTLA-4 (9). Furthermore, the percentage of IFN-γ-producing, beryllium-responsive CD4+ T cells expressing 4-1BB in the BAL was significantly diminished compared with IFN-γ-producing cells in blood, and this lung T cell subset was predisposed to undergo activation-induced cell death upon beryllium exposure in culture. Thus, it is likely that the combination of the loss of CD28 expression from the surface of BAL CD4+ T cells, the expression of PD-1 and CTLA-4, and the marginal ability to these cells to up-regulate other costimulatory molecules, such as 4-1BB, contribute to the hyporesponsive state of the beryllium-specific CD4+ T cell in the lung as documented in the current study. Whether the same is true in the target organ of other immune-mediated human diseases is an important unanswered question.
Conversely, there is a continued reliance of memory T cells in blood on CD28 for T cell activation, and the majority of circulating beryllium-specific, 4-1BB-expressing CD4+ T cells coexpress CD28. This is in contrast to CD8+ T cells where 4-1BB is predominantly expressed on CD28− cells (44, 45). In addition, despite the induction of 4-1BB on the surface of beryllium-responsive, IFN-γ-expressing CD4+ T cells in blood, there was no effect of blocking the 4-1BBL-4-1BB interaction on beryllium-induced T cell proliferation. When B7.1 and 4-1BBL were expressed simultaneously on blood monocytes, the kinetics of the anti-influenza CD8+ T cell response paralleled the response with B7.1 alone (19). This is likely due to the constitutive expression of CD28 as opposed to the inducible nature of 4-1BB on the T cell surface. Thus, these findings strongly suggest that despite the induction of 4-1BB on beryllium-responsive CD4+ T cells, CD28 delivers the dominant costimulatory signal in blood.
Interestingly, 4-1BBL blockade had no effect on the percentage of BAL CD4+ T cells expressing IFN-γ after short-term culture in the presence of beryllium salts. This was not entirely surprising given the inducible nature of 4-1BB on activated CD4+ T cells. Despite an increased expression of 4-1BBL on BAL CD14+ cells compared with blood, it is also possible that a greater expression may be required to prime the T cell response. Similar to our findings, 4-1BB has been associated with sustaining as opposed to initiating the immune response, primarily through the induction of Bcl-xL and prevention of apoptosis (11, 14, 46, 47). In the opposite manner, our findings with 4-1BBL blockade are reminiscent of studies in HIV where PDL1-PD-1 blockade had no effect on intracellular Th1-type cytokine expression while enhancing proliferation of HIV-specific CD8+ T cells in blood (48). However, we have previously noted a discordance between proliferation and cytokine-secretion with the maturation state of the beryllium-responsive T cell dictating functional capacity (38). Thus, it remains possible that the beryllium-induced proliferative response of BAL cells is 4-1BB dependent while the Th1-type cytokine response is independent of costimulation.
The partial inhibition of proliferation seen with the 4-1BBL mAb raises the possibility that other inducible costimulatory molecules may be involved in the beryllium-induced immune response. In this regard, the induction of OX-40L expression on APCs had no effect on T cell proliferation, and no synergistic or additive effects were seen when OX-40L was combined with 4-1BBL. In addition, blockade of the OX-40L-OX-40 interaction with an inhibitory OX-40L mAb had no effect on beryllium-induced T cell proliferation, suggesting that OX-40 plays no role in this disease. In preliminary experiments, we have noted that CD40 ligand (CD40L) is up-regulated on BAL CD4+ T cells after BeSO4 exposure in culture, with 32% of BAL CD4+ T cells expressing CD40L at 96 h compared with 0.2% of ex vivo BAL T cells (data not shown). Whether inhibition of the CD40-CD40L interaction also blocks beryllium-induced proliferation of BAL T cells will require additional study.
Due to the presence of beryllium in the lung years after exposure cessation (49), the natural history of CBD is characterized by a gradual decline in lung function, with one-third of untreated patients historically progressing to end-stage respiratory insufficiency (23). Combined with our previous work (9), our results clearly show that beryllium-induced activation of CD4+ TEM cells in the BAL is independent of CD28 costimulation while requiring the interaction of 4-1BBL with 4-1BB for the proliferative response. In addition, the enhanced proliferation and prevention of activation-induced cell death of pathogenic CD4+ T cells upon 4-1BB costimulation suggest that the 4-1BBL-4-1BB interaction contributes to disease severity. Current therapeutic options for CBD patients are limited to corticosteroids which nonspecifically suppress T cell function. Blockade of the 4-1BBL-4-1BB interaction may down-regulate the function of beryllium-specific CD4+ TEM cells, and as a result, diminish the severity of alveolitis. Thus, the development of targeted immunotherapy directed toward inhibiting 4-1BB costimulation in pathogenic TEM cells in the lung has therapeutic potential, especially in diseases characterized by persistent Ag exposure.
In summary, despite the low frequency of circulating beryllium-specific CD4+ T cells, these cells are more functional than their counterparts in the lung. This enhanced functional capacity is more than likely due to the continued reliance of these cells on CD28 costimulation as opposed to beryllium-responsive T cells in the lung which are independent of CD28 and require 4-1BB engagement for an optimal beryllium-induced proliferative response. These findings further the developing paradigm in CBD and other immune-mediated disorders of a costimulatory transition within the memory CD4+ T cell population from CD28 dominance in blood to a requirement for other inducible costimulatory molecules, such as 4-1BB, on TEM cells in a target organ. The induction of 4-1BB on Ag-specific memory T cells allows for enhanced effector T cell function and survival. In the case of CBD, this translates into continued lung inflammation and disease progression, suggesting the potential utility of inhibition of 4-1BB costimulation as a therapeutic target.
Footnotes
This work is supported by National Institute of Health Grants HL62410 and ES11810 to A.P.F., Canadian Institutes of Health Grant MOP-74492 to T.H.W., and the General Clinical Research Center Grant M01-RR-0051 from the Division of Research Resources.
- BAL
- bronchoalveolar lavage
- CBD
- chronic beryllium disease
- 7-AAD
- 7-aminoactinomycin D
- BeS
- beryllium sensitized
- BeSO4
- beryllium sulfate
- CD40L
- CD40 ligand
- MFI
- median fluorescence intensity
- MOI
- multiplicity of infection
- OX-40L
- OX-40 ligand
- PD-1
- programmed death-1
- TEM cells
- effector memory T cells
Disclosures
The authors have no financial conflict of interest.
References
- 1.Bluestone JA. New perspectives of CD28–B7-mediated T cell costimulation. Immunity. 1995;2:555–559. doi: 10.1016/1074-7613(95)90000-4. [DOI] [PubMed] [Google Scholar]
- 2.June CH, Ledbetter JA, Linsley PS, Thompson CB. Role of the CD28 receptor in T-cell activation. Immunol. Today. 1990;11:211–216. doi: 10.1016/0167-5699(90)90085-n. [DOI] [PubMed] [Google Scholar]
- 3.Haffar OK, Smithgall MD, Bradshaw J, Brady B, Damle NK, Linsley PS. Costimulation of T-cell activation and virus production by B7 antigen on activated CD4+ T cells from human immunodeficiency virus type 1-infected donors. Proc. Natl. Acad. Sci. USA. 1993;90:11094–11098. doi: 10.1073/pnas.90.23.11094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lovett-Racke AE, Trotter JL, Lauber J, Perrin PJ, June CH, Racke MK. Decreased dependence of myelin basic protein-reactive T cells on CD28-mediated costimulation in multiple sclerosis patients: a marker of activated/memory T cells. J. Clin. Invest. 1998;101:725–730. doi: 10.1172/JCI1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Markovic-Plese S, Cortese I, Wandinger KP, McFarland HF, Martin R. CD4+CD28− costimulation-independent T cells in multiple sclerosis. J. Clin. Invest. 2001;108:1185–1194. doi: 10.1172/JCI12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmidt D, Goronzy JJ, Weyand CM. CD4+ CD7− CD28− T cells are expanded in rheumatoid arthritis and are characterized by autoreactivity. J. Clin. Invest. 1996;97:2027–2037. doi: 10.1172/JCI118638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vallejo AN, Nestel AR, Schirmer M, Weyand CM, Goronzy JJ. Aging-related deficiency of CD28 expression in CD4+ T cells is associated with the loss of gene-specific nuclear factor binding activity. J. Biol. Chem. 1998;273:8119–8129. doi: 10.1074/jbc.273.14.8119. [DOI] [PubMed] [Google Scholar]
- 8.Katchar K, Wahlstrom J, Eklund A, Grunewald J. Highly activated T-cell receptor AV2S3+ CD4+ lung T-cell expansions in pulmonary sarcoidosis. Am. J. Respir. Crit. Care Med. 2001;163:1540–1545. doi: 10.1164/ajrccm.163.7.2005028. [DOI] [PubMed] [Google Scholar]
- 9.Fontenot AP, Gharavi L, Bennett SR, Canavera SJ, Newman LS, Kotzin BL. CD28 costimulation independence of target organ versus circulating memory antigen-specific CD4+ T cells. J. Clin. Invest. 2003;112:776–784. doi: 10.1172/JCI18317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vinay DS, Kwon BS. Role of 4-1BB in immune responses. Semin. Immunol. 1998;10:481–489. doi: 10.1006/smim.1998.0157. [DOI] [PubMed] [Google Scholar]
- 11.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu. Rev. Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 12.Futagawa T, Akiba H, Kodama T, Takeda K, Hosoda Y, Yagita H, Okumura K. Expression and function of 4-1BB and 4-1BB ligand on murine dendritic cells. Int. Immunol. 2002;14:275–286. doi: 10.1093/intimm/14.3.275. [DOI] [PubMed] [Google Scholar]
- 13.Laderach D, Wesa A, Galy A. 4-1BB-ligand is regulated on human dendritic cells and induces the production of IL-12. Cell. Immunol. 2003;226:37–44. doi: 10.1016/j.cellimm.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Cannons JL, Lau P, Ghumman B, DeBenedette MA, Yagita H, Okumura K, Watts TH. 4-1BB ligand induces cell division, sustains survival, and enhances effector function of CD4 and CD8 T cells with similar efficacy. J. Immunol. 2001;167:1313–1324. doi: 10.4049/jimmunol.167.3.1313. [DOI] [PubMed] [Google Scholar]
- 15.Kim YJ, Kim SH, Mantel P, Kwon BS. Human 4-1BB regulates CD28 co-stimulation to promote Th1 cell responses. Eur. J. Immunol. 1998;28:881–890. doi: 10.1002/(SICI)1521-4141(199803)28:03<881::AID-IMMU881>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 16.Wen T, Bukczynski J, Watts TH. 4-1BB ligand-mediated costimulation of human T cells induces CD4 and CD8 T cell expansion, cytokine production, and the development of cytolytic effector function. J. Immunol. 2002;168:4897–4906. doi: 10.4049/jimmunol.168.10.4897. [DOI] [PubMed] [Google Scholar]
- 17.DeBenedette MA, Shahinian A, Mak TW, Watts TH. Costimulation of CD28− T lymphocytes by 4-1BB ligand. J. Immunol. 1997;158:551–559. [PubMed] [Google Scholar]
- 18.Bukczynski J, Wen T, Watts TH. Costimulation of human CD28-T cells by 4-1BB ligand. Eur. J. Immunol. 2003;33:446–454. doi: 10.1002/immu.200310020. [DOI] [PubMed] [Google Scholar]
- 19.Bukczynski J, Wen T, Ellefsen K, Gauldie J, Watts TH. Costimulatory ligand 4-1BBL (CD137L) as an efficient adjuvant for human antiviral cytotoxic T cell responses. Proc. Natl. Acad. Sci. USA. 2004;101:1291–1296. doi: 10.1073/pnas.0306567101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bukczynski J, Wen T, Wang C, Christie N, Routy JP, Boulassel MR, Kovacs CM, Macdonald KS, Ostrowski M, Sekaly RP, et al. Enhancement of HIV-specific CD8 T cell responses by dual costimulation with CD80 and CD137L. J. Immunol. 2005;175:6378–6389. doi: 10.4049/jimmunol.175.10.6378. [DOI] [PubMed] [Google Scholar]
- 21.Serghides L, Bukczynski J, Wen T, Wang C, Routy JP, Boulassel MR, Sekaly RP, Ostrowski M, Bernard NF, Watts TH. Evaluation of OX40 ligand as a costimulator of human antiviral memory CD8 T cell responses: comparison with B7.1 and 4-1BBL. J. Immunol. 2005;175:6368–6377. doi: 10.4049/jimmunol.175.10.6368. [DOI] [PubMed] [Google Scholar]
- 22.Fontenot AP, Maier LA. Genetic susceptibility and immune-mediated destruction in beryllium-induced disease. Trends Immunol. 2005;26:543–549. doi: 10.1016/j.it.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Newman LS, Lloyd J, Daniloff E. The natural history of beryllium sensitization and chronic beryllium disease. Environ. Health Perspect. 1996;104:937S–943S. doi: 10.1289/ehp.96104s5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fontenot AP, Newman LS, Kotzin BL. Chronic beryllium disease: T cell recognition of a metal presented by HLA-DP. Clin. Immunol. 2001;100:4–14. doi: 10.1006/clim.2001.5053. [DOI] [PubMed] [Google Scholar]
- 25.Fontenot AP, Canavera SJ, Gharavi L, Newman LS, Kotzin BL. Target organ localization of memory CD4+ T cells in patients with chronic beryllium disease. J. Clin. Invest. 2002;110:1473–1482. doi: 10.1172/JCI15846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rossman MD, Kern JA, Elias JA, Cullen MR, Epstein PE, Preuss OP, Markham TN, Daniele RP. Proliferative response of bronchoalveolar lymphocytes to beryllium. Ann. Intern. Med. 1988;108:687–693. doi: 10.7326/0003-4819-108-5-687. [DOI] [PubMed] [Google Scholar]
- 27.Saltini C, Winestock K, Kirby M, Pinkston P, Crystal RG. Maintenance of alveolitis in patients with chronic beryllium disease by beryllium-specific helper T cells. N. Engl. J. Med. 1989;320:1103–1109. doi: 10.1056/NEJM198904273201702. [DOI] [PubMed] [Google Scholar]
- 28.Kreiss K, Newman LS, Mroz M, Campbell PA. Screening blood test identifies subclinical beryllium disease. J. Occup. Med. 1989;31:603–608. doi: 10.1097/00043764-198907000-00011. [DOI] [PubMed] [Google Scholar]
- 29.Kreiss K, Wasserman S, Mroz MM, Newman LS. Beryllium disease screening in the ceramics industry: blood test performance and exposure-disease relations. J. Occup. Med. 1993;35:267–274. [PubMed] [Google Scholar]
- 30.Mroz MM, Kreiss K, Lezotte DC, Campbell PA, Newman LS. Reexamination of the blood lymphocyte transformation test in the diagnosis of chronic beryllium disease. J. Allergy Clin. Immunol. 1991;88:54–60. doi: 10.1016/0091-6749(91)90300-d. [DOI] [PubMed] [Google Scholar]
- 31.Newman LS. Significance of the blood beryllium lymphocyte proliferation test. Environ. Health Perspect. 1996;104:953S–956S. doi: 10.1289/ehp.96104s5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tinkle SS, Kittle LA, Schumacher BA, Newman LS. Beryllium induces IL-2 and IFN-γ in berylliosis. J. Immunol. 1997;158:518–526. [PubMed] [Google Scholar]
- 33.Newman LS, Kreiss K, King TE, Jr., Seay S, Campbell PA. Pathologic and immunologic alterations in early stages of beryllium disease: reexamination of disease definition and natural history. Am. Rev. Respir. Dis. 1989;139:1479–1486. doi: 10.1164/ajrccm/139.6.1479. [DOI] [PubMed] [Google Scholar]
- 34.Fontenot AP, Kotzin BL, Comment CE, Newman LS. Expansions of T-cell subsets expressing particular T cell receptor variable regions in chronic beryllium disease. Am. J. Respir. Cell Mol. Biol. 1998;18:581–589. doi: 10.1165/ajrcmb.18.4.2981. [DOI] [PubMed] [Google Scholar]
- 35.Fontenot AP, Falta MT, Freed BM, Newman LS, Kotzin BL. Identification of pathogenic T cells in patients with beryllium-induced lung disease. J. Immunol. 1999;163:1019–1026. [PubMed] [Google Scholar]
- 36.Fontenot AP, Torres M, Marshall WH, Newman LS, Kotzin BL. Beryllium presentation to CD4+ T cells underlies disease susceptibility HLA-DP alleles in chronic beryllium disease. Proc. Natl. Acad. Sci. USA. 2000;97:12717–12722. doi: 10.1073/pnas.220430797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palmer BE, Mack DG, Martin AK, Gillespie M, Mroz MM, Maier LA, Fontenot AP. Up-regulation of programmed death-1 expression on beryllium-specific CD4+ T cells in chronic beryllium disease. J. Immunol. 2008;180:2704–2712. doi: 10.4049/jimmunol.180.4.2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fontenot AP, Palmer BE, Sullivan AK, Joslin FG, Wilson CC, Maier LA, Newman LS, Kotzin BL. Frequency of beryllium-specific, central memory CD4+ T cells in blood determines proliferative response. J. Clin. Invest. 2005;115:2886–2893. doi: 10.1172/JCI24908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Precopio ML, Betts MR, Parrino J, Price DA, Gostick E, Ambrozak DR, Asher TE, Douek DC, Harari A, Pantaleo G, et al. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8+ T cell responses. J. Exp. Med. 2007;204:1405–1416. doi: 10.1084/jem.20062363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat. Med. 2007;13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 41.Palmer BE, Mack DG, Martin AK, Maier LA, Fontenot AP. CD57 expression correlates with alveolitis severity in subjects with beryllium-induced disease. J. Allergy Clin. Immunol. 2007;120:184–191. doi: 10.1016/j.jaci.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 42.Pott GB, Palmer BE, Sullivan AK, Silviera L, Maier LA, Newman LS, Kotzin BL, Fontenot AP. Frequency of beryllium-specific, TH1-type cytokine-expressing CD4+ T cells in patients with beryllium-induced disease. J. Allergy Clin. Immunol. 2005;115:1036–1042. doi: 10.1016/j.jaci.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 43.Sallusto F, Lanzavecchia A. Exploring pathways for memory T cell generation. J. Clin. Invest. 2001;108:805–806. doi: 10.1172/JCI14005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hurtado JC, Kim SH, Pollok KE, Lee ZH, Kwon BS. Potential role of 4-1BB in T cell activation: comparison with the costimulatory molecule CD28. J. Immunol. 1995;155:3360–3367. [PubMed] [Google Scholar]
- 45.Kim YJ, Brutkiewicz RR, Broxmeyer HE. Role of 4-1BB (CD137) in the functional activation of cord blood CD28−CD8+ T cells. Blood. 2002;100:3253–3260. doi: 10.1182/blood-2001-11-0136. [DOI] [PubMed] [Google Scholar]
- 46.Bertram EM, Lau P, Watts TH. Temporal segregation of 4-1BB versus CD28-mediated costimulation: 4-1BB ligand influences T cell numbers late in the primary response and regulates the size of the T cell memory response following influenza infection. J. Immunol. 2002;168:3777–3785. doi: 10.4049/jimmunol.168.8.3777. [DOI] [PubMed] [Google Scholar]
- 47.Lee HW, Park SJ, Choi BK, Kim HH, Nam KO, Kwon BS. 4-1BB promotes the survival of CD8+ T lymphocytes by increasing expression of Bcl-xL and Bfl-1. J. Immunol. 2002;169:4882–4888. doi: 10.4049/jimmunol.169.9.4882. [DOI] [PubMed] [Google Scholar]
- 48.Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, Precopio ML, Schacker T, Roederer M, Douek DC, Koup RA. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J. Exp. Med. 2006;203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sawyer RT, Abraham JL, Daniloff E, Newman LS. Secondary ion mass spectroscopy demonstrates retention of beryllium in chronic beryllium disease granulomas. J. Occup. Environ. Med. 2005;47:1218–1226. doi: 10.1097/01.jom.0000184884.85325.36. [DOI] [PubMed] [Google Scholar]